Abstract
Background
In the CARD study (NCT02485691), cabazitaxel significantly improved clinical outcomes versus abiraterone or enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel and the alternative androgen-signalling-targeted inhibitor. However, some patients received docetaxel or the prior alternative androgen-signalling-targeted inhibitor in the metastatic hormone-sensitive (mHSPC) setting. Therefore, the CARD results cannot be directly translated to a Japanese population.
Methods
Patients (N = 255) received cabazitaxel (25 mg/m2 IV Q3W, prednisone, G-CSF) versus abiraterone (1000 mg PO, prednisone) or enzalutamide (160 mg PO) after prior docetaxel and progression ≤12 months on the alternative androgen-signalling-targeted inhibitor. Patients who received combination therapy for mHSPC were excluded (n = 33) as docetaxel is not approved in this setting in Japan.
Results
A total of 222 patients (median age 70 years) were included in this subanalysis. Median number of cycles was higher for cabazitaxel versus androgen-signalling-targeted inhibitors (7 versus 4). Clinical outcomes favoured cabazitaxel over abiraterone or enzalutamide including, radiographic progression-free survival (rPFS; median 8.2 versus 3.4 months; P < 0.0001), overall survival (OS; 13.9 versus 11.8 months; P = 0.0102), PFS (4.4 versus 2.7 months; P < 0.0001), confirmed prostate-specific antigen response (37.0 versus 14.4%; P = 0.0006) and objective tumour response (38.9 versus 11.4%; P = 0.0036). For cabazitaxel versus androgen-signalling-targeted inhibitor, grade ≥ 3 adverse events occurred in 55% versus 44% of patients, with adverse events leading to death on study in 2.7% versus 5.7%.
Conclusions
Cabazitaxel significantly improved outcomes including rPFS and OS versus abiraterone or enzalutamide and are reflective of the Japanese patient population. Cabazitaxel should be considered the preferred treatment option over abiraterone or enzalutamide in this setting.
Keywords: clinical trials, urology, chemo-urology
Cabazitaxel significantly improved radiographic progression-free survival and overall survival versus abiraterone or enzalutamide in patients who had not received combination therapy for metastatic hormone-sensitive prostate cancer. These data are reflective of a Japanese patient population.
Introduction
Several therapies have been approved in the US and EU over the past decade for the treatment of metastatic castration-resistant prostate cancer (mCRPC), including taxanes (docetaxel, cabazitaxel), androgen-signalling-targeted inhibitors (abiraterone, enzalutamide), immunotherapy (sipuleucel-T), poly (ADP-ribose) polymerase inhibitor (olaparib, rucaparib) and a radiopharmaceutical (radium-223) (1–3). The optimal treatment sequence of these agents remains unclear, with retrospective studies suggesting that overall survival (OS) is related to the number of different life-extending therapies received (4–6). There is also increasing evidence from randomized studies that patients progressing with abiraterone respond poorly to enzalutamide and vice versa (7–9). Cabazitaxel can retain activity in patients progressing on prior docetaxel, abiraterone or enzalutamide (10–12). Despite this, many patients receive androgen-signalling-targeted inhibitors in sequence before receiving cabazitaxel therapy (13, 14).
Recently, a large European randomized trial (CARD) demonstrated that cabazitaxel more than doubled radiographic progression-free survival (rPFS) and significantly improved OS versus abiraterone or enzalutamide in patients with mCRPC who had previously received docetaxel and progressed within 12 months on the alternative androgen-signalling-targeted inhibitor (15). When looking at the applicability of the CARD study results to the Japanese treatment landscape, a criticism of CARD was that it included some patients treated with a combination of androgen deprivation therapy (ADT) and docetaxel for newly diagnosed metastatic prostate cancer. Although such a combination is recommended by US and European guidelines (1–3), it is not licenced in Japan (16, 17).
Therefore, US and European treatment guidelines for prostate cancer may not be applied in all countries, such as Japan. For example, in the US, the 2020 National Comprehensive Cancer Network guidelines recommend that newly diagnosed patients first receive ADT. Depending on disease severity, ADT may also be delivered in combination with docetaxel, abiraterone, enzalutamide or apalutamide. Once a patient is classified as castration resistant, they may, in selected cases, receive a second androgen-signalling-targeted inhibitor or cabazitaxel. Targeted therapies, such as olaparib, pembrolizumab or radium-223 can be considered for patients who meet specific criteria (2). The latest European guidelines from European Society for Medical Oncology recommend continuous ADT as first-line treatment of metastatic hormone-sensitive prostate cancer (mHSPC) or ADT plus docetaxel in patients fit enough for chemotherapy. In patients with mCRPC in the post-docetaxel setting, abiraterone, enzalutamide, cabazitaxel and radium-223 (in those without visceral disease) are recommended options (3, 18). As per European Association of Urology guidelines, ADT alone is no longer the standard of care for newly diagnosed metastatic prostate cancer. Such patients should receive either ADT plus docetaxel or ADT plus an androgen-signalling-targeted inhibitor (3). In Japan, ADT is a treatment option for patients with any stage of prostate cancer (19), however, combination with docetaxel is not currently recommended for the treatment of patients with mHSPC (16, 17).
As patients in the CARD study were enrolled from Europe and eligibility criteria followed European guidelines, some patients may have received docetaxel or the prior alternative androgen-signalling-targeted inhibitor in the mHSPC setting (3, 15). Consequently, the CARD results cannot be directly translated to the Japanese population. The post hoc analysis of the CARD study presented here excluded patients treated with a combination therapy for newly diagnosed metastatic prostate cancer to evaluate the efficacy of cabazitaxel versus abiraterone or enzalutamide in patients with mCRPC who had only received docetaxel and abiraterone or enzalutamide in the castrate-resistant setting, in order to be more relevant to the Japanese mCRPC population.
Methods
Study design and population
The CARD trial was a multicentre, randomized, open-label, clinical trial conducted at 62 sites across 13 European countries. The trial was designed to compare cabazitaxel with either abiraterone or enzalutamide in patients with mCRPC who had previously received docetaxel and who had disease progression within 12 months while they had been receiving an androgen-signalling-targeted inhibitor (abiraterone or enzalutamide). Overall, 255 patients were randomly assigned 1:1 to receive cabazitaxel (25 mg/m2 every 3 weeks plus prednisone daily and granulocyte colony-stimulating factor [G-CSF]) versus abiraterone (1000 mg plus prednisone daily) or enzalutamide (160 mg daily) until radiographic progression, unacceptable toxicity, start of subsequent treatment, or patient request to discontinue treatment.
Full details of the CARD study design can be found in the primary manuscript (15). Briefly, eligible patients had histologically confirmed prostate cancer, castrate levels of serum testosterone (<0.5 ng/ml), were previously treated with docetaxel, had progressed within 12 months of treatment with a prior androgen-signalling-targeted inhibitor (either before or after docetaxel), had disease progression (measurable disease using Response Evaluation Criteria in Solid Tumors (RECIST) criteria and/or appearance of ≥2 new bone lesions using Prostate Cancer Working Group 2 (PCWG2) criteria, and/or rising prostate-specific antigen [PSA] as per PCWG2 criteria), and had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 1 (patients with an ECOG PS of two were allowed if related to prostate cancer). In May 2018, the protocol was amended to allow the use of abiraterone or docetaxel in the context of mHSPC (in combination with ADT) to comply with European guidelines (1, 3). For this post hoc analysis, these patients were excluded.
The type of progression at enrollment was classified as the following: PSA progression only, imaging-based progression (defined as objective tumour progression according to RECIST, version 1.1, or progression of bone lesions according to PCWG2 criteria—with or without PSA progression and without pain), or pain progression (defined as a Brief Pain Inventory-Short Form score > 1 [on an 11-point scale, with higher numbers indicating greater pain] or a World Health Organization cancer pain analgesic level of 2–3 [on a 3-point scale, with higher numbers indicating use of stronger analgesic agents]—with or without PSA or imaging-based progression) (15, 20).
The CARD trial was approved by the institutional review board at each centre and was conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The trial was sponsored by Sanofi. The sponsor and the members of the steering committee contributed to the trial design, data analysis and interpretation, and critical review of the manuscript. All authors had full access to the trial data, were responsible for the content of the manuscript, and made the decision to submit the manuscript for publication. The authors developed the first draft of the manuscript with editorial assistance funded by Sanofi. The authors vouch for the accuracy and completeness of the data and for the adherence of the trial to the protocol.
Statistical analysis
All analyses were performed by the sponsor using data obtained at a cut-off date of 27 March 2019. The CARD trial was designed to have 80% power to detect a hazard ratio (HR) of 0.67 (cabazitaxel versus abiraterone or enzalutamide) in the analysis of rPFS, with the use of a stratified log-rank test at a two-sided alpha level of 5%. If an imaging-based progression event or death did not occur during the trial, then the data on rPFS were censored at the last tumour assessment or at the cut-off date, whichever occurred first. If no valid tumour assessment was available, data were censored at the date of randomization. No interim analysis was performed. Precise definitions of study endpoints are provided in Table S1.
The efficacy analysis included all the patients who had undergone randomization. Stratified log-rank tests were used to analyse time-to-event data. The primary analysis compared rPFS between the two treatment groups with the use of a stratified log-rank test. Survival curves were generated with the use of Kaplan–Meier estimates. HRs and associated 95% confidence intervals (CIs) were estimated with the use of a stratified Cox proportional-hazards model. Stratified Cochran– Mantel–Haenszel chi-square tests were used to analyse categorical data. Descriptive statistics were used to summarize the characteristics of the patients. The safety population, which included all randomized patients who had received at least one dose of trial treatment, was used for all safety analyses.
To control for type I error due to multiple comparisons, a hierarchical testing procedure was applied for the primary and key secondary endpoints. Only if rPFS differed significantly between two treatment groups would key secondary endpoints be tested in the following order: OS, progression-free survival (PFS), PSA response and tumour response. Further tests were stopped once a comparison was found not to be significant at a two-sided alpha level of 0.05.
Results
Baseline and treatment characteristics
From November 2015 through November 2018, 255 patients were randomized to the CARD study. Of these, five patients were randomized but did not receive treatment, 33 patients were treated with a combination of ADT and docetaxel (n = 32) or ADT and abiraterone (n = 1) for newly diagnosed metastatic disease and were excluded, leaving 217 patients for the analysis (112 with cabazitaxel and 105 with abiraterone or enzalutamide) (Fig. 1). Of the 105 patients who received an androgen-signalling-targeted inhibitor, 52 received abiraterone and 53 received enzalutamide.
Figure 1.
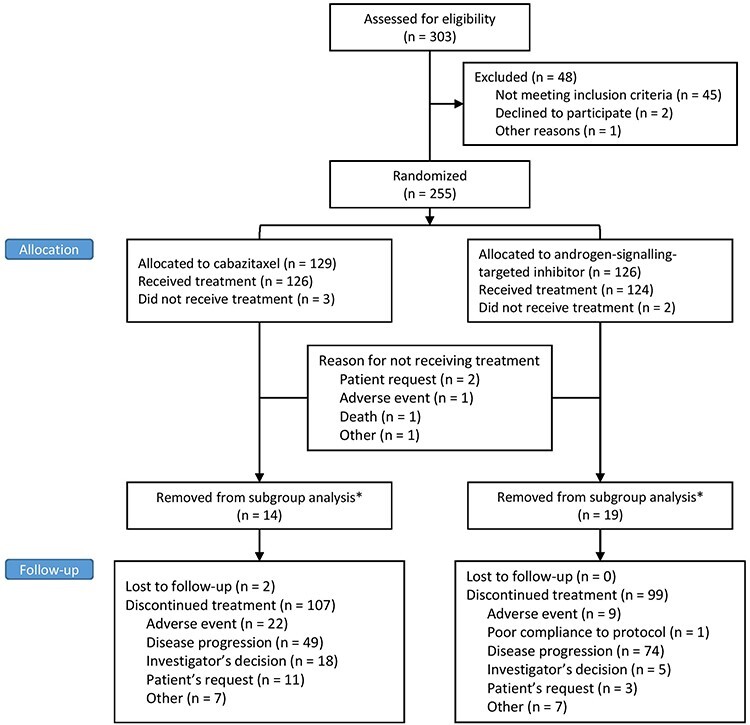
CONSORT flow diagram. *Patients were removed from subgroup analysis because they received either ADT and docetaxel or ADT and abiraterone for newly diagnosed mHSPC. ADT, androgen deprivation therapy; mHSPC, metastatic hormone-sensitive prostate cancer.
The median age of patients in this subgroup was 70 years, with 31.1% aged 75 years or older (Table 1). Most patients had pain progression with or without PSA or imaging-based progression at randomization (149 [67.1%]), 34 patients (15.3%) had imaging-based progression with or without PSA progression and 20 patients (9.0%) had PSA progression only. Liver or lung metastases were present at diagnosis in 17.1% of patients. Overall, 56 patients (48.7%) in the cabazitaxel arm and 51 patients (47.7%) in the enzalutamide or abiraterone arm progressed ≤6 months from the start of androgen-signalling-targeted inhibitor prior to enrollment.
Table 1.
Baseline characteristics
| Characteristics | Cabazitaxel (n = 115) | Abiraterone or enzalutamide (n = 107) | Total (N = 222) |
|---|---|---|---|
| Median age, years | 70 | 70 | 70 |
| Aged ≥75 years, n (%) | 42 (36.5) | 27 (25.2) | 69 (31.1) |
| ECOG PS 0 or 1, n (%) | 111 (96.5) | 104 (97.2) | 215 (96.8) |
| Liver or lung metastases, n (%) | 18 (15.7) | 20 (18.7) | 38 (17.1) |
| Median PSA, ng/mL | 61.02 | 68.04 | 62.7 |
| Median neutrophil count, mm3 | 4500 | 4660 | 4540 |
| Median haemoglobin, g/L | 121 | 121 | 121 |
| Median alkaline phosphatase, IU/L | 125 | 115.5 | 120.5 |
| Median LDH, IU/L | 241.5 | 251 | 243 |
| Type of progression at trial entry, n (%) | |||
| PSA only (no radiological progression, no pain) | 11 (9.6) | 9 (8.4) | 20 (9.0) |
| Radiological progression (±rising PSA) and no pain | 20 (17.4) | 14 (13.1) | 34 (15.3) |
| Pain status at study entry | 75 (65.2) | 74 (69.2) | 149 (67.1) |
| Missing | 9 (7.8) | 10 (9.3) | 19 (8.6) |
| Disease history | |||
| Metastatic (M1) disease at diagnosis, n (%) | 35 (30.4) | 41 (38.3) | 76 (34.2) |
| Gleason score 8–10 at diagnosis, n (%) | 63 (54.8) | 66 (61.7) | 129 (58.1) |
| Median duration of first ADT, months | 14.9 | 15 | 14.9 |
| Duration ≥12 months, n (%) | 67 (58.3) | 65 (60.7) | 132 (59.5) |
| Previous life extending therapy, n (%) | |||
| Docetaxel | 115 (100) | 107 (100) | 222 (100) |
| Abiraterone | 49 (42.6) | 54 (50.5) | 103 (46.4) |
| Enzalutamide | 65 (56.5) | 53 (49.5) | 118 (53.2) |
| Missing | 1 (0.9) | 0 | 1 (0.5) |
| Timing of androgen-signalling-targeted inhibitor before docetaxel, n (%) | 50 (43.5) | 48 (44.9) | 98 (44.1) |
| Median time from androgen-signalling-targeted inhibitor to progression, months | 8.0 | 7.1 | 7.6 |
| ≤6 months on first androgen-signalling-targeted inhibitor, n (%) | 56 (48.7) | 51 (47.7) | 107 (48.2) |
ADT, androgen deprivation therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; PSA, prostate-specific antigen.
Patients received cabazitaxel longer than abiraterone or enzalutamide (median treatment duration 22.0 versus 12.3 weeks), and the median number of treatment cycles received was higher in patients receiving cabazitaxel than in those receiving abiraterone or enzalutamide (7 versus 4) (Table S2). The principal reasons for the discontinuation of treatment with cabazitaxel or the androgen-signalling-targeted inhibitor were disease progression (42.6% versus 69.2%) or an adverse event (19.1% versus 8.4%) (Table S2).
Primary endpoint
In line with the primary analysis, the median rPFS was significantly longer for cabazitaxel versus abiraterone or enzalutamide (8.2 versus 3.4 months; HR [95% CI] 0.54 [0.39–0.74]; P ≤ 0.0001) (Figs. 2A and S1). Cabazitaxel improved rPFS regardless of the androgen-signalling-targeted inhibitor received (HR [95% CI] 0.51 [0.31–0.86]; HR [95% CI] 0.44 [0.28–0.68]) (Fig. S2). The treatment effect regarding rPFS was consistent across all the prespecified subgroups (Figs. 2B and S1). An analysis of rPFS by treatment sequence also showed that patients who received docetaxel–androgen-signalling-targeted inhibitor–cabazitaxel had a longer median rPFS compared with patients who received androgen-signalling-targeted inhibitor–docetaxel–cabazitaxel (9.0 versus 6.3 months; HR [95% CI] 2.08 [1.33–3.24]; P = 0.0010) (Fig. 3).
Figure 2.
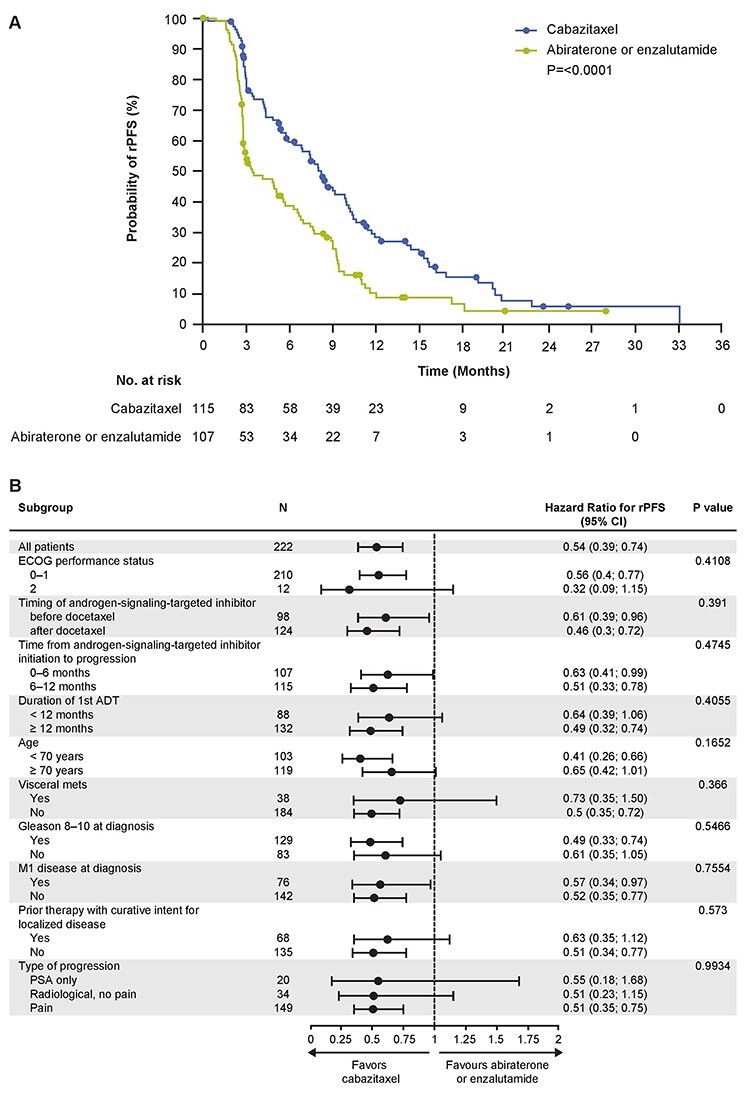
(A) Kaplan–Meier estimate for rPFS and (B) subgroup analysis. P values presented are for interactions. ADT, androgen deprivation therapy; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen; rPFS, radiographic profession-free survival.
Figure 3.
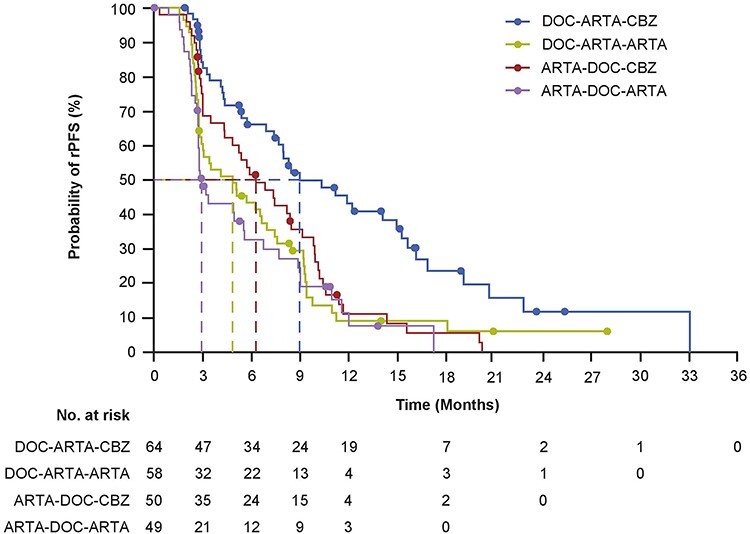
Kaplan–Meier estimate for rPFS by treatment sequence. ARTA, enzalutamide or abiraterone; DOC, docetaxel; CABA, cabazitaxel; rPFS, radiographic profession-free survival.
Key secondary efficacy endpoints
All key secondary endpoints were improved with cabazitaxel compared with abiraterone or enzalutamide (Fig. 4). Median OS was significantly longer with cabazitaxel compared with abiraterone or enzalutamide (13.9 versus 11.8 months; HR [95% CI] 0.64 [0.45–0.90]; P = 0.0102). PFS was also significantly longer for cabazitaxel compared with abiraterone or enzalutamide (4.4 versus 2.7 months; HR [95% CI] 0.55 [0.41–0.74]; P ≤ 0.001). A reduction of ≥50% from baseline in the PSA level, confirmed by a second value obtained at least 3 weeks later, was observed in 37.0% of the patients in the cabazitaxel group and in 14.4% of those in the androgen-signalling-targeted inhibitor group (P = 0.0006) (Figs. 4 and S3). Among patients with measurable disease at baseline, the percentage of patients with a tumour response was 39% with cabazitaxel and 11% with an androgen-signalling-targeted inhibitor (P = 0.0036) (Fig. 4).
Figure 4.
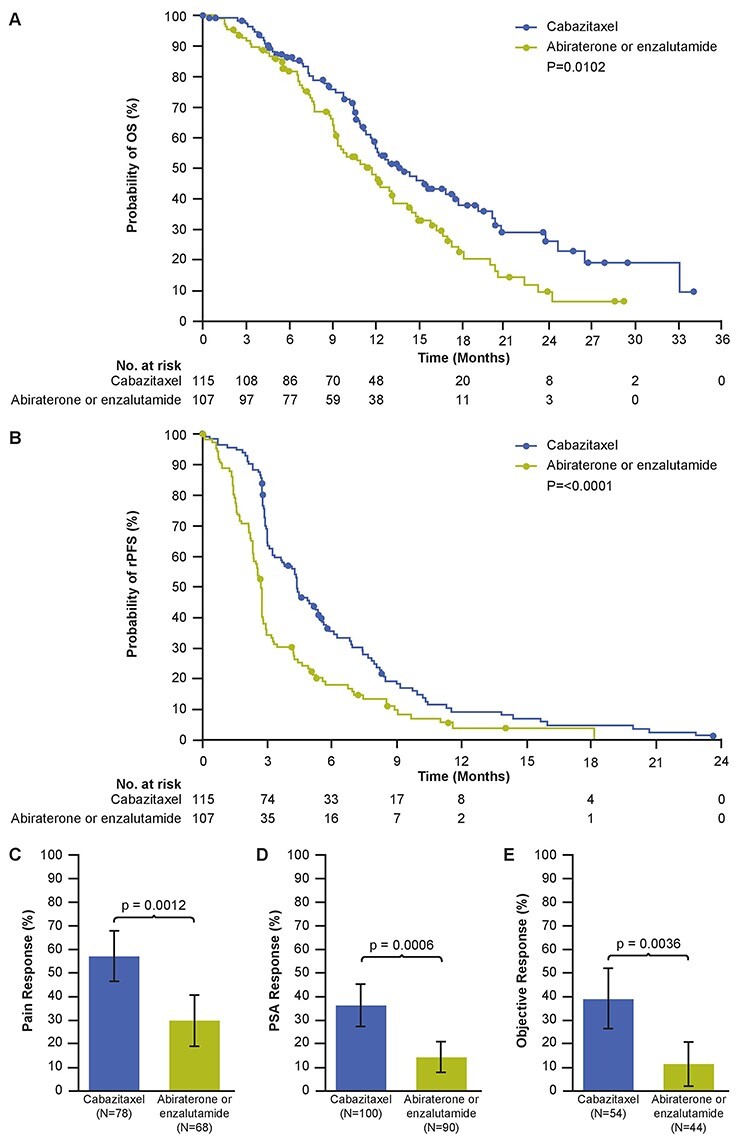
Kaplan–Meier estimates for (A) OS and (B) PFS, (C) pain response rate, (D) PSA response rate, and (E) objective tumour response rate. Error bars represent 95% confidence intervals. OS, overall survival; PFS, progression-free survival; PSA, prostate-specific antigen.
Other endpoints also favoured cabazitaxel. Cabazitaxel was associated with a significantly increased rate of pain response compared with abiraterone or enzalutamide (56.4% versus 29.4%; P = 0.0012) (Fig. 4). Median time to symptomatic skeletal events (SSEs) was not reached in either group (HR [95 CI%] 0.70 [0.39–1.27]; P = 0.2368) (Fig. S4). At 18 months, 25.6% of patients in the cabazitaxel group were estimated to have had a SSE compared with 47.3% of those in the androgen-signalling-targeted inhibitor group. Post hoc multivariate analyses of the primary endpoint confirmed the robustness of the treatment effect seen in the primary analysis (Table S3).
Safety
Almost all patients had an adverse event of any grade (98.2% for cabazitaxel versus 94.3% for abiraterone or enzalutamide) (Tables 2 and 3). The incidence of serious adverse events of any grade was similar for cabazitaxel (39.3%) and abiraterone or enzalutamide (37.1%). Adverse events leading to treatment discontinuation occurred more frequently with cabazitaxel (19.1%) than with abiraterone or enzalutamide (8.4%). However, adverse events leading to death during the assessment period (from randomization to 30 days after the last treatment administration) occurred less frequently with cabazitaxel (seven patients [6.3%]) than with abiraterone or enzalutamide (12 patients [11.4%]) (Table S4).
Table 2.
Haematological abnormal laboratory tests by all grade or grade ≥ 3 during the treatment period
| Laboratory abnormalities*, n (%) | Cabazitaxel (n = 112) | Abiraterone or enzalutamide (n = 105) | ||
|---|---|---|---|---|
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Anaemia | 110/111 (99.1) | 7/111 (6.3) | 101/105 (96.2) | 4/105 (3.8) |
| Thrombocytopenia | 47/111 (42.3) | 3/111 (2.7) | 17/105 (16.2) | 2/105 (1.9) |
| Leukopenia | 84/111 (75.7) | 39/111 (35.1) | 28/105 (26.7) | 1/105 (1.0) |
| Neutropenia | 74/109 (67.9) | 51/109 (46.8) | 4/105 (3.8) | 2/105 (1.9) |
| Lymphopenia | 79/109 (72.5) | 29/109 (26.6) | 57/105 (54.3) | 15/105 (14.3) |
* Laboratories abnormalities were graded using National Cancer Institute Common Terminology Criteria version 4.03.
Table 3.
Non-haematological adverse events occurring in ≥5% of patients for any grade and ≥3% of patients for grade ≥ 3
| Preferred term n (%) | Cabazitaxel (n = 112) | Abiraterone or enzalutamide (n = 105) | ||
|---|---|---|---|---|
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Asthenia or fatigue | 62 (55.4) | 5 (4.5) | 37 (35.2) | 1 (1.0) |
| Diarrhoea | 45 (40.2) | 4 (3.6) | 6 (5.7) | 0 |
| Infection | 36 (32.1) | 7 (6.3) | 23 (21.9) | 7 (6.7) |
| Musculoskeletal pain or discomfort* | 32 (28.6) | 2 (1.8) | 38 (36.2) | 4 (3.8) |
| Nausea or vomiting | 30 (26.8) | 0 | 21 (20.0) | 1 (1.0) |
| Peripheral neuropathy† | 23 (20.5) | 4 (3.6) | 3 (2.9) | 0 |
| Haematuria | 19 (17.0) | 1 (0.9) | 6 (5.7) | 1 (1.0) |
| Constipation | 16 (14.3) | 0 | 10 (9.5) | 0 |
| Decreased appetite | 16 (14.3) | 1 (0.9) | 15 (14.3) | 3 (2.9) |
| Dysgeusia | 12 (10.7) | 0 | 4 (3.8) | 0 |
| Abdominal pain | 10 (8.9) | 1 (0.9) | 3 (2.9) | 1 (1.0) |
| Bladder or urethral symptom‡ | 10 (8.9) | 0 | 6 (5.7) | 0 |
| Peripheral edema | 10 (8.9) | 0 | 8 (7.6) | 1 (1.0) |
| Cancer pain | 9 (8.0) | 2 (1.8) | 8 (7.6) | 1 (1.0) |
| Stomatitis | 9 (8.0) | 0 | 1 (1.0) | 0 |
| Pain | 8 (7.1) | 0 | 5 (4.8) | 1 (1.0) |
| Renal disorder§ | 7 (6.3) | 3 (2.7) | 12 (11.4) | 9 (8.6) |
| Alopecia | 6 (5.4) | 0 | 0 | 0 |
| Arthralgia | 6 (5.4) | 0 | 14 (13.3) | 1 (1.0) |
| Dyspepsia | 6 (5.4) | 0 | 2 (1.9) | 0 |
| Fall | 6 (5.4) | 0 | 0 | 0 |
| Paraesthesia | 6 (5.4) | 0 | 2 (1.9) | 0 |
| Pyrexia | 6 (5.4) | 0 | 9 (8.6) | 0 |
| Spinal cord or nerve-root disorder|| | 6 (5.4) | 3 (2.7) | 5 (4.8) | 3 (2.9) |
| Hypertensive disorder¶ | 5 (4.5) | 3 (2.7) | 9 (8.6) | 2 (1.9) |
| Psychiatric disorder†† | 5 (4.5) | 0 | 13 (12.4) | 0 |
| Weight decreased | 5 (4.5) | 0 | 7 (6.7) | 0 |
| Cardiac disorder | 4 (3.6) | 1 (0.9) | 8 (7.6) | 5 (4.8) |
| Disease progression | 4 (3.6) | 4 (3.6) | 8 (7.6) | 7 (6.7) |
| Febrile neutropenia | 4 (3.6) | 4 (3.6) | 0 | 0 |
| Bone fracture | 2 (1.8) | 1 (0.9) | 6 (5.7) | 2 (1.9) |
| Dizziness | 1 (0.9) | 0 | 6 (5.7) | 0 |
*Musculoskeletal pain or discomfort included ‘Back pain’, ‘Flank pain’, ‘Musculoskeletal discomfort’, ‘Musculoskeletal pain’, ‘Discomfort’,
‘Neck pain’, ‘Pain in extremity’, ‘Growing pains’, ‘Musculoskeletal chest pain’.
†Peripheral Neuropathy included, ‘Neuropathy peripheral’, ‘Peripheral motor neuropathy’, ‘Peripheral sensorimotor neuropathy’, ‘Peripheral sensory neuropathy’, ‘Polyneuropathy’.
‡Bladder or urethral symptom included ‘Dysuria’, ‘Pollakiuria’, ‘Lower urinary tract symptoms’, ‘Micturition urgency’, ‘Urinary incontinence’, ‘Urinary retention’.
§Renal disorder included, ‘Acute kidney injury’, ‘Renal failure’, ‘Renal impairment’, ‘Hydronephrosis’, ‘Pyelocaliectasis’.
||Spinal cord or nerve-root disorder included ‘Sciatica’, ‘Radiculopathy’, ‘Spinal cord compression’.
¶ Hypertensive disorder included ‘Hypertension’, ‘Hypertensive crisis’.
††Psychiatric disorder included ‘Anxiety’, ‘Depression’, ‘Confusional state’, ‘Disorientation’, ‘Persistent depressive disorder’, ‘Insomnia’.
Note: System organ class was used for infection and cardiac disorders.
Haematological grade ≥ 3 laboratory abnormalities occurring more frequently with cabazitaxel than with abiraterone or enzalutamide were anaemia (6.3% versus 3.8%), platelet counts decreased (2.7% versus 1.9%), white blood cells decreased (35.1% versus 1.0%), neutrophil counts decreased (46.8% versus 1.9%) and lymphocyte counts decreased (26.6% versus 14.3) (Table 2).
Non-haematological grade ≥ 3 adverse events occurring more frequently with cabazitaxel than with abiraterone or enzalutamide were asthenia or fatigue (4.5% versus 1.0%), diarrhoea (3.6% versus 0), peripheral neuropathy (3.6% versus 0), and febrile neutropenia (3.6% versus 0). Grade ≥ 3 adverse events occurring more frequently with abiraterone or enzalutamide were renal disorders (8.6% versus 2.7%), musculoskeletal pain or discomfort (3.8% versus 1.8%) and cardiac disorders (4.8% versus 0.9%) (Table 3). In the cabazitaxel group, haematuria of any grade was reported in 19 patients (17.0%), and mild alopecia was reported in six patients (5.4%); no nail disorders were reported in the cabazitaxel group. No new safety signals were reported (Table S5).
First subsequent anticancer treatment
Of 107 patients in the androgen-signalling-targeted inhibitor group, 38 (35.5%) crossed over to receive cabazitaxel. Of 115 patients in the cabazitaxel group, 27 (23.5%) crossed over to receive abiraterone or enzalutamide. Anticancer therapies that were received as the first subsequent treatment after the trial treatments are listed in Table S6.
Discussion and conclusions
The results presented here unambiguously confirm that after exclusion of patients treated with chemohormonal therapy for newly diagnosed metastatic disease, cabazitaxel remains superior to abiraterone or enzalutamide in patients with mCRPC who had previously received docetaxel and progressed within 12 months on the alternative androgen-signalling-targeted inhibitor. Cabazitaxel more than doubled the rPFS (median rPFS 8.2 versus 3.4 months; HR [95% CI] 0.54 [0.39–0.74]) compared with abiraterone or enzalutamide. This benefit was observed across all the prespecified subgroups, regardless of the timing of the previous alternative androgen-signalling-targeted inhibitor (before or after docetaxel). Cabazitaxel also reduced the risk of death by 36% versus abiraterone or enzalutamide. Other secondary endpoints (PFS, PSA response, tumour response, pain response and SSEs) also favoured cabazitaxel. A longer rPFS benefit was observed in patients who received the sequence docetaxel–androgen-signalling-targeted inhibitor–cabazitaxel compared with the sequence androgen-signalling-targeted inhibitor–docetaxel–cabazitaxel. In the large retrospective registry CATS, patients who received the sequence docetaxel–androgen-signalling-targeted inhibitor–cabazitaxel also had a longer rPFS with cabazitaxel (median 15.0 months) versus those who received the sequence androgen-signalling-targeted inhibitor–docetaxel–cabazitaxel (median 10.7 months) (6). Since docetaxel may lose activity after an androgen-targeted inhibitor (6, 21, 22), one could hypothesize that resistant clones continued to develop with docetaxel leading to more aggressive disease at the time of cabazitaxel initiation. Another hypothesis is that sequential use of taxanes might favour some degree of cross-resistance (21), although preclinical data and the TROPIC study have clearly established the activity of cabazitaxel in tumours resistant to docetaxel (10, 23–25).
In current practice, chemotherapy is often used after androgen-signalling-targeted inhibitors. Our findings, as well as previous evidence, suggest that patients might benefit from receiving chemotherapy earlier (22, 26).
In daily clinical practice, many patients receive androgen-signalling-targeted inhibitors in sequence, possibly driven by fear of chemotherapy (13, 27). The CARD study demonstrated that patients already treated with an androgen-signalling-targeted inhibitor respond poorly to a second one, regardless of docetaxel timing (before or after the first androgen-signalling-targeted inhibitor) and androgen-signalling-targeted inhibitor sequence (abiraterone followed by enzalutamide or vice versa) (15).
Our findings agree with other prospective randomized studies evaluating the treatment sequence in mCRPC (7–9). In the PLATO study, good responders to enzalutamide who developed disease progression had a poor response to abiraterone, alone or in combination with enzalutamide (9). In a prospective cross-over study of patients with newly diagnosed mCRPC randomized to abiraterone followed by enzalutamide, or the inverse sequence, median time to progression after receiving the second androgen-signalling-targeted inhibitor was <3 months in either arm (8). Additionally, in the PROfound study of olaparib versus abiraterone or enzalutamide in mCRPC patients with DNA repair abnormalities, the second androgen-signalling-targeted inhibitor (control arm) was also associated with a very short rPFS (3.6 months compared with 3.7 months in CARD), despite no eligibility restrictions in relation to time to progression with the first androgen-signalling-targeted inhibitor (7). Cross-resistance between abiraterone and enzalutamide was also reported in retrospective studies conducted in Japan (28). This is likely because androgen-signalling-targeted inhibitors target the same pathway, albeit by different mechanisms, and thus share common mechanisms of resistance. Taxanes, due to their different mechanism of action, are able to overcome several mechanisms of resistance to androgen-signalling-targeted inhibitors, such as increased androgen receptor signalling and phosphatase and tensin homologue loss (29–32). Although some studies suggest that docetaxel loses some activity in tumours that are resistant to androgen-signalling-targeted inhibitors (33), this does not appear to be the case for cabazitaxel. Prospective and retrospective data show that cabazitaxel retains its activity in tumours resistant to androgen-signalling-targeted inhibitors, which may be attributed to the increased intratumoural penetration of cabazitaxel versus docetaxel (11, 12, 34, 35).
The results of this subgroup analysis are consistent with similar studies that focus on Japanese patient populations. For example, in a retrospective analysis of Japanese patients with mCRPC, cabazitaxel was consistently effective in patients with aggressive disease and those who had poor prior response to androgen-signalling-targeted inhibitors (36). Additionally, in a study of 660 Japanese patients with mCRPC treated in the real-world setting, cabazitaxel showed a consistent safety profile with that reported in pivotal clinical studies and was also effective in terms of PSA response, OS, and time to treatment failure (37). In another retrospective analysis of Japanese patients with mCRPC, cabazitaxel was active irrespective of prior treatment with docetaxel, and it was suggested that introducing cabazitaxel to mCRPC patients with a good performance status may be preferable to maximize prognosis (38).
Trends in adverse events were consistent with the primary analysis. Incidence of grade ≥ 3 adverse events was higher in the cabazitaxel group compared with abiraterone or enzalutamide (55.4% versus 43.8%). However, the incidence of adverse events leading to death during the trial was almost twice as high with abiraterone or enzalutamide versus cabazitaxel, mainly related to disease progression.
The CARD trial differed from previous Phase III studies conducted with cabazitaxel by the fact that prophylactic G-CSF was administered at every cycle. This was justified by the fact that patients had been heavily pretreated and it was previously shown, in a large compassionate use program conducted in Europe, that the risk of severe neutropenia and/or neutropenic complications was maximum at Cycle 1, especially when the baseline neutrophil count was below 4000 mm3 (39). With G-CSF, the incidence of febrile neutropenia with cabazitaxel was 3.6% which is much lower than in TROPIC (8.0%), FIRSTANA (12.0%) and PROSELICA (9.2%) trials, which did not allow prophylactic G-CSF at Cycle 1 (10, 40, 41). Prospective studies conducted with cabazitaxel in Japan, also evidenced that the risk of neutropenic complications was maximum at Cycle 1 and was dramatically reduced by G-CSF prophylaxis (37, 42).
There are some limitations of this analysis that should be acknowledged. Firstly, this is a post hoc analysis. Therefore, the results should be interpreted with caution. However, the strong similarity between this analysis and the primary CARD results further support the conclusions initially made. Secondly, the critiques of the CARD study design still stand, whereby the cabazitaxel starting dose of 20 mg/m2 was not tested. In the prospective, non-inferiority, Phase III PROSELICA trial, it was reported that the 20 mg/m2 dose maintained at least 50% of the survival benefit of the 25 mg/m2 dose and was associated with a lower incidence of grade ≥ 3 adverse events (41). The 25 mg/m2 dose was used for the CARD trial as the trial was conducted in Europe and the European label was used as a reference (1, 3, 43). This is also the recommended dose in Japan (16).
Finally, the CARD trial enrolled patients who had progressed within 12 months with abiraterone or enzalutamide, which may question the generalizability of our results to daily practice. Importantly, patients experiencing PSA progression within 12 months were eligible, even if treatment was continued for a longer period. Among these 44 patients who continued the first androgen-signalling-targeted inhibitor for >12 months following PSA progression, the rPFS and OS benefit was identical to the total patient population (44). In pivotal Phase III studies of abiraterone and enzalutamide in patients with asymptomatic chemotherapy-naive mCRPC, median time to PSA progression was <12 months (45, 46). Median time to progression with a first androgen-signalling-targeted inhibitor was also <8 months in a randomized cross-over study of abiraterone followed by enzalutamide (or inverse sequence) (8). Median duration of first-line enzalutamide in chemotherapy-naïve mCRPC was 9.1 months in the PLATO study (9). However, data are currently lacking for patients receiving androgen-signalling-targeted inhibitors in the non-metastatic setting.
In conclusion, this post hoc analysis excluding patients treated with ADT and docetaxel or an androgen-signalling-targeted inhibitor in mHSPC confirms that the results of the CARD study can be applied to patients in Japan. Cabazitaxel should be considered the preferred treatment option over abiraterone or enzalutamide in patients with mCRPC who have previously received docetaxel and an alternative androgen-signalling-targeted inhibitor.
Conflict of interest statement
HS has received institutional research grants from Astellas Pharma Inc, Bayer, Chugai, Daiichi-Sankyo, Kissei, Nippon Kayaku, Nihon Shinyaku, Pfizer, Sanofi, Taiho Pharmaceutical Co. Ltd, and Takeda Pharmaceutical Co.; honoraria from Astellas Pharma Inc, AstraZeneca, Bayer, Daiichi-Sankyo, Fuji Film, Janssen Pharmaceutical K.K., MSD, Ono, Pfizer, Sanofi and Takeda Pharmaceutical Co. and provides an advisory role for Bayer, Daiichi-Sankyo, Janssen Pharmaceutical K.K., MSD, Nihon, Medi-Physics, Roche and Takeda Pharmaceutical Co.
DC received personal fees from Pfizer, Roche, Sanofi, Janssen, Astellas, Bayer, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Pierre Fabre, AstraZeneca and Lilly.
JdB has received honoraria from AstraZeneca, Sanofi, Astellas Pharma, Pfizer, Genentech/Roche, Janssen Oncology, Menarini Silicon Biosystems, Daiichi Sankyo, Sierra Oncology and BioXcel Therapeutics, and provided an advisory role for AstraZeneca, Sanofi, Genentech/Roche, Astellas Pharma, Bayer, Pfizer, Merck Sharp & Dohme, Merck Serono, Boehringer Ingelheim, Sierra Oncology, Menarini Silicon Biosystems, Celgene, Taiho Pharmaceutical, Daiichi Sankyo, Janssen Oncology, Genmab, GlaxoSmithKline, Orion Pharma GmbH, Eisai and BioXCel Therapeutics.
CNS has received honoraria from Janssen, AstraZeneca, Sanofi and Astellas, received consultancy fees from Sanofi, Bayer and Pfizer, and received institutional funding from Genentech/Roche, Bayer, Sanofi Genzyme, Janssen, Medivation, Merck Sharp & Dohme and Exelixis.
KF has received honoraria and provided an advisory role for Astellas, AAA, Bayer, Essa, Janssen, Orion, Sanofi, CureVac, Sanofi and Endocyte.
BT has received personal fees and research grants from Astellas, Janssen, Sanofi Genzyme, Amgen and Ferring, and received non-financial support from Sanofi Genzyme.
CW reported no conflicts of interest.
MF and AO are employees, and AO is a stockholder, of Sanofi.
CG-R is an employee of Sanofi.
RdW provided an advisory role for Sanofi, Janssen, Merck, Bayer, Clovis and Roche, received honoraria from Sanofi and Merck, and received institutional grants from Sanofi and Bayer.
Supplementary Material
Acknowledgements
Research and analysis were supported by Sanofi. The authors were responsible for all content and editorial decisions and received no honoraria for development of this manuscript. Editorial support was provided by Michael Patan and Amber Wood of MediTech Media, funded by Sanofi.
Related papers: Dr Daniel Castellano MD, Prof. Johann De Bono MD, Dr Cora N. Sternberg MD, Prof. Karim Fizazi MD, Prof. Bertrand Tombal MD, Prof. Christian Wülfing MD, Dr Ayse Ozatilgan MD, Dr Christine Geffriaud-Ricouard MD, Prof. Ronald de Wit MD were all authors for the original CARD trial.
Contributor Information
Hiroyoshi Suzuki, Department of Urology, Toho University Sakura Medical Center, Chiba, Japan.
Daniel Castellano, Medical Oncology Department, 12 de Octubre University Hospital, Madrid, Spain.
Johann de Bono, Drug Development Unit, The Institute of Cancer Research and the Royal Marsden Hospital, London, UK.
Cora N Sternberg, Division of Hematology and Medical Oncology, Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY, USA.
Karim Fizazi, Department of Cancer Medicine, Gustave Roussy Institute and Paris Sud University, Villejuif, France.
Bertrand Tombal, Division of Urology, Université Catholique de Louvain, Louvain, Belgium.
Christian Wülfing, Department of Urology, Asklepios Klinik Altona, Hamburg, Germany.
Meredith C Foster, Global Medical Affairs Oncology, Sanofi, Cambridge, MA, USA.
Ayse Ozatilgan, Global Medical Affairs Oncology, Sanofi, Cambridge, MA, USA.
Christine Geffriaud-Ricouard, Europe Medical Affairs Oncology, Sanofi, Paris, France.
Ronald de Wit, Department Medical Oncology, Erasmus University Hospital, Rotterdam, the Netherlands.
References
- 1. Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119–34. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology Prostate Cancer (Version 2.2020). 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed December 2020).
- 3. Mottet N, Bergh RCN, Briers E, et al. EAU - ESTRO - ESUR - SIOG guidelines on prostate cancer 2020. In: European Association of Urology Guidelines, Vol. 2020. Edition. Arnhem, the Netherlands: European Association of Urology Guidelines Office, 2020. [Google Scholar]
- 4. Maines F, Caffo O, Veccia A, Bria E. Sequential use of new agents (NAs) after docetaxel (DOC) first line in metastatic castration-resistant prostate cancer (mCRPC) patients (pts): a pooled-analysis of the published studies. J Clin Oncol 2015;33:Abstract 258. [Google Scholar]
- 5. Angelergues A, Efstathiou E, Gyftaki R, et al. Results of the FLAC European database of metastatic castration-resistant prostate cancer patients treated with docetaxel, cabazitaxel, and androgen receptor-targeted agents. Clin Genitourin Cancer 2018;16:e777–e84. [DOI] [PubMed] [Google Scholar]
- 6. Delanoy N, Hardy-Bessard AC, Efstathiou E, et al. Sequencing of taxanes and new androgen-targeted therapies in metastatic castration-resistant prostate cancer: results of the international multicentre retrospective CATS database. European Urology Oncology 2018;1:467–75. [DOI] [PubMed] [Google Scholar]
- 7. Bono JS, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;382:2091–102. [DOI] [PubMed] [Google Scholar]
- 8. Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol 2019;20:1730–9. [DOI] [PubMed] [Google Scholar]
- 9. Attard G, Borre M, Gurney H, et al. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol 2018;36:2639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147–54. [DOI] [PubMed] [Google Scholar]
- 11. Soest RJ, Morree ES, Kweldam CF, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol 2015;67:981–5. [DOI] [PubMed] [Google Scholar]
- 12. Soest RJ, Nieuweboer AJ, Morree ES, et al. The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur J Cancer 2015;51:2562–9. [DOI] [PubMed] [Google Scholar]
- 13. Oh WK, Miao R, Vekeman F, et al. Real-world characteristics and outcomes of patients with metastatic castration-resistant prostate cancer receiving chemotherapy versus androgen receptor-targeted therapy after failure of first-line androgen receptor-targeted therapy in the community setting. Clin Genitourin Cancer 2017;S1558-7673:30170–2. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi T, Terada N, Kimura T, et al. Sequential use of androgen receptor axis-targeted agents in chemotherapy-naive castration-resistant prostate cancer: a multicenter retrospective analysis with 3-year follow-up. Clin Genitourin Cancer 2020;18:e46–54. [DOI] [PubMed] [Google Scholar]
- 15. Wit R, Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 2019;381:2506–18. [DOI] [PubMed] [Google Scholar]
- 16. Kakehi Y, Sugimoto M, Taoka R. Committee for establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition). Int J Urol 2017;24:648–66. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki H, Shin T, Fukasawa S, et al. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: final subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, phase 3 study. Jpn J Clin Oncol 2020;50:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker C, Gillessen S, Heidenreich A, Horwich A. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v69–77. [DOI] [PubMed] [Google Scholar]
- 19. Hinotsu S, Namiki M, Ozono S, Akaza H. NCCN Asia consensus statement prostate cancer. Jpn J Clin Oncol 2018;48:964–5. [DOI] [PubMed] [Google Scholar]
- 20. Atkinson TM, Mendoza TR, Sit L, et al. The brief pain inventory and its "pain at its worst in the last 24 hours" item: clinical trial endpoint considerations. Pain Med 2010;11:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soest RJ, Royen ME, Morree ES, et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer 2013;49:3821–30. [DOI] [PubMed] [Google Scholar]
- 22. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morrée ES, Böttcher R, Soest RJ, et al. Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br J Cancer 2016;115:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vrignaud P, Semiond D, Lejeune P, et al. Preclinical antitumor activity of cabazitaxel, a semi-synthetic taxane active in taxane-resistant tumors. Clin Cancer Res 2013;19:2973–83. [DOI] [PubMed] [Google Scholar]
- 25. Oprea-Lager DE, Bijnsdorp IV, Van Moorselaar RJ, Eertwegh AJ, Hoekstra OS, Geldof AA. ABCC4 decreases docetaxel and not cabazitaxel efficacy in prostate cancer cells in vitro. Anticancer Res 2013;33:387–91. [PubMed] [Google Scholar]
- 26. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyake H, Hara T, Terakawa T, Ozono S, Fujisawa M. Comparative assessment of clinical outcomes between abiraterone acetate and enzalutamide in patients with docetaxel-naive metastatic castration-resistant prostate cancer: experience in real-world clinical practice in Japan. Clin Genitourin Cancer 2017;15:313–9. [DOI] [PubMed] [Google Scholar]
- 28. Matsubara N, Yamada Y, Tabata KI, et al. Comparison of sequential treatment with androgen receptor-targeted agent followed by another androgen receptor-targeted agent versus androgen receptor-targeted agent followed by docetaxel in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 2017;15:e1073–e80. [DOI] [PubMed] [Google Scholar]
- 29. Fitzpatrick JM, Wit R. Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol 2014;65:1198–204. [DOI] [PubMed] [Google Scholar]
- 30. Antonarakis ES, Lu C, Wang H, et al. Androgen receptor splice variant, AR-V7, and resistance to enzalutamide and abiraterone in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2014;32:Abstract 5001. [Google Scholar]
- 31. Rescigno P, Lorente D, Dolling D, et al. Docetaxel treatment in PTEN- and ERG-aberrant metastatic prostate cancers. European Urology Oncology 2018;1:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conteduca V, Castro E, Wetterskog D, et al. Plasma AR status and cabazitaxel in heavily treated metastatic castration-resistant prostate cancer. Eur J Cancer 2019;116:158–68. [DOI] [PubMed] [Google Scholar]
- 33. Mezynski J, Pezaro C, Bianchini D, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol 2012;23:2943–7. [DOI] [PubMed] [Google Scholar]
- 34. Al Nakouzi N, Le Moulec S, Albiges L, et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol 2015;68:228–35. [DOI] [PubMed] [Google Scholar]
- 35. Beer TM, Hotte SJ, Saad F, et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. Lancet Oncol 2017;18:1532–42. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto T, Ishizuka O, Oike H, et al. Safety and efficacy of cabazitaxel in Japanese patients with castration-resistant prostate cancer. Prostate Int 2020;8:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suzuki K, Matsubara N, Kazama H, Seto T, Tsukube S, Matsuyama H. Safety and efficacy of cabazitaxel in 660 patients with metastatic castration-resistant prostate cancer in real-world settings: results of a Japanese post-marketing surveillance study. Jpn J Clin Oncol 2019;49:1157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyake H, Sugiyama T, Aki R, et al. No significant impact of prior treatment profile with docetaxel on the efficacy of cabazitaxel in Japanese patients with metastatic castration-resistant prostate cancer. Med Oncol (Northwood, London, England) 2017;34:141. [DOI] [PubMed] [Google Scholar]
- 39. Heidenreich A, Bracarda S, Mason M, et al. Safety of cabazitaxel in senior adults with metastatic castration-resistant prostate cancer: results of the European compassionate-use programme. Eur J Cancer 2014;50:1090–9. [DOI] [PubMed] [Google Scholar]
- 40. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol 2017;35:3189–97. [DOI] [PubMed] [Google Scholar]
- 41. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol 2017;35:3198–206. [DOI] [PubMed] [Google Scholar]
- 42. Kosaka T, Uemura H, Sumitomo M, et al. Impact of pegfilgrastim as primary prophylaxis for metastatic castration-resistant prostate cancer patients undergoing cabazitaxel treatment: an open-label study in Japan. Japanese J Clin Oncol 2019;49:766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanofi . Jevtana Summary of Product Characteristics. 2020. https://products.sanofi.us/jevtana/jevtana.html (accessed December 2020).
- 44. Wit R, Tombal B, Freedland S. Use of chemotherapy and androgen signaling-targeted inhibitors in patients with metastatic prostate cancer. Eur Urol 2020;79:170–2. [DOI] [PubMed] [Google Scholar]
- 45. Ryan CJ, Smith MR, Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


