Abstract
Mitigation of exercise-induced stress is of key interest in determining ways to optimize performance horse health. To test the hypothesis that dietary supplementation of a Saccharomyces cerevisiae fermentation product would decrease markers of exercise-induced stress and inflammation in young horses, Quarter Horse yearlings (mean ± SD; 9 ± 1 mo) were randomly assigned to receive either no supplementation (CON; n = 8) or 21 g/d S. cerevisiae fermentation product (10.5 g/feeding twice daily; SCFP; n = 10) top-dressed on a basal diet of custom-formulated grain as well as ad libitum Coastal bermudagrass hay. After 8 wk of dietary treatments, horses underwent a 2-h submaximal exercise test (SET) on a free-stall mechanical exerciser. Serum was collected before dietary treatment supplementation (week 0), at week 8 pre-SET, and 0, 1, and 6 h post-SET and analyzed for concentrations of cortisol and serum amyloid A (SAA) by commercial enzyme-linked immunosorbent assay (ELISA) and for cytokine concentrations by commercial bead-based ELISA. Data were analyzed using linear models with repeated measures in SAS v9.4. From week 0 to 8 (pre-SET), serum cortisol decreased (P = 0.01) and SAA did not change, but neither were affected by diet. Serum concentrations of all cytokines decreased from week 0 to 8 (P ≤ 0.008), but granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor, and interleukin-8 (IL-8) decreased to a greater extent in CON than in SCFP horses (P ≤0.003). In response to the week 8 SET, serum cortisol increased in all horses (P < 0.0001) but returned to pre-SET levels by 1 h post-SET in horses receiving SCFP. At 6 h post-SET, cortisol concentrations in CON horses returned to pre-SET concentrations, whereas cortisol declined further in SCFP horses to below pre-SET levels (P = 0.0002) and lower than CON (P = 0.003) at that time point. SAA increased at 6 h post-SET in CON (P < 0.0001) but was unchanged through 6 h in SCFP horses. All cytokines except G-CSF increased in response to the SET (P < 0.0001) but showed differing response patterns. Concentrations of IL-1β, IL-6, and tumor necrosis factor-alpha were lesser (P ≤ 0.05), and concentrations of G-CSF and IL-18 tended to be lesser (P ≤ 0.09) in SCFP compared with CON horses throughout recovery from the SET. In summary, 8 wk of dietary supplementation with 21 g/d of SCFP may mitigate cellular stress following a single, prolonged submaximal exercise bout in young horses.
Keywords: equine, exercise stress, performance horse
Introduction
Performance horse health, particularly the health of young horses entering exercise training programs, is of utmost importance to maintaining the quality of life and performance longevity. Various mechanisms to improve longevity of performance horses have been investigated, including the role of stress-induced immune and inflammatory responses. Following a stressor such as exercise, pro- and anti-inflammatory cytokines, acute-phase proteins (APP), and stress hormones are released, which are responsible for many of the cardinal signs of inflammation, such as pyrexia (Pedersen and Hoffman-Goetz 2000; Crisman et al., 2008). Inflammation is the body’s protective response to deviations or challenges to tissue homeostasis. This protective response is necessary for stress-induced cellular adaptations, including adaptations to exercise training. However, the inflammatory response can manifest as swelling, heat, pain, and loss of function (Chovatiya and Medzhito, 2014), and excessive or unresolved inflammation can result in unneccesary cellular damage. Therefore, an appropriate level of post-stress resolution of inflammation is vital to ensure the maintenance of cellular homeostasis (Landskron et al., 2014).
Dietary additives, such as byproducts of yeast fermentation, have been the focus of several animal and human studies because of their potential immune boosting and anti-inflammatory effects (Glade and Campbell-Taylor, 1990; Morgan et al., 2007; Moyad et al., 2010; Paulsen et al., 2010; Evans et al., 2012; Zhu et al., 2017). Supplementation with a Saccharomyces cerevisiae fermentation product in rats appeared to provide both moderate immune-enhancing effects in addition to affecting anti-inflammatory response parameters (Evans et al., 2012); this demonstrates its potential to favorably modulate immune responses without excessive suppression or stimulation of the overall immune activity. In addition, supplementation with the same S. cerevisiae fermentation product decreased the negative effects of heat stress in rats and broiler chickens ( Giblot Ducray et al., 2016; Price et al., 2018). In horses, S. cerevisiae fermentation product supplementation enhanced submaximal exercise performance (Beil et al., 1990; Glade and Campbell-Taylor, 1990; Miller-Graber et al., 1994; Wickler, 2002) and increased the digestibility of low-quality forage (Morgan et al., 2007).
Given the known inflammatory response associated with acute exercise and the favorable reports of yeast fermentation product supplementation on balancing inflammation, the objective of this study was to evaluate the effects of dietary supplementation of an S. cerevisiae fermentation product on systemic inflammation and the cellular stress response following exercise in young horses. We hypothesized that horses receiving the S. cerevisiae fermentation product would have lower concentrations of markers associated with stress and inflammation compared with control horses following a prolonged exercise stressor.
Materials and Methods
This study was reviewed and approved by the Institutional Animal Care and Use Committee at Texas A&M University (2016-0294).
Horses and management
Eighteen Quarter Horses (11 fillies and 7 colts) entering their yearling year (mean ± SD; age: 9 ± 1 mo) with a starting body weight (BW) of 280 ± 31 kg were enrolled in this 8-wk study. Horses originated from two sources: 8 were from the Texas A&M University horse center herd (College Station, TX) and 10 were leased from Birdsong Farms (Hearne, TX). Prior to the beginning of the study, horses from Birdsong Farms were relocated to Texas A&M University’s Freeman Arena (College Station, TX), where all horses remained throughout the 8-wk trial. Horses were group-housed by sex and farm of origin (Texas A&M colts, n = 3; Texas A&M fillies, n = 5; Birdsong colts, n = 5; Birdsong fillies, n = 6) at Freeman Arena in approximately 0.5-hectare dry lots. All horses received a basal diet consisting of a commercial grain mix custom-formulated with no yeast fermentation products at 1.25% BW (dry matter [DM] basis) split into two equal meals per day and ad libitum Coastal bermudagrass hay in dry lots devoid of fresh forage. The basal diet was formulated to meet or exceed the requirements for growing horses (NRC, 2007). Composited hay and grain samples were analyzed for nutrient composition prior to the initiation of the trial (Table 1). All horses received the basal diet for 4 wk prior to the initiation of the trial.
Table 1.
Nutrient composition of custom-formulated concentrate and Coastal bermudagrass hay offered to yearling horses
| Nutrient1 | Concentrate2 | Coastal bermudagrass hay3 |
|---|---|---|
| DE, Mcal/kg | 0.61 | 0.39 |
| CP, % | 18.0 | 13.0 |
| ADF, % | 15.2 | 40.3 |
| NDF, % | 30.4 | 71.6 |
| Starch, % | 18.0 | 1.0 |
| Crude fat, % | 8.4 | 1.7 |
| Ca, % | 1.40 | 0.44 |
| P, % | 1.06 | 0.22 |
| Mg, % | 0.57 | 0.17 |
| K, % | 1.40 | 0.88 |
| Na, % | 0.62 | 0.30 |
| Cl, % | 1.08 | 0.52 |
| S, % | 0.30 | 0.24 |
| Fe, ppm | 813.0 | 184.0 |
| Zn, ppm | 217.0 | 34.0 |
| Cu, ppm | 56.0 | 7.0 |
| Mn, ppm | 189.0 | 241.0 |
| Co, ppm | 2.0 | 0.5 |
1Values presented on a 100% DM basis.
2Concentrate, basal grain diet fed to all horses at 1.25% BW (DM basis) per day.
3Coastal bermudagrass hay fed ad libitum to all horses.
During the study, horses were hand walked into individually assigned 3.2 × 3.2 m stalls twice daily to receive their allotted concentrate grain meal at 0600 and 1700 hours, respectively. Orts were collected and weighed daily to calculate intake. Throughout the study, BW was obtained every 2 wk using a calibrated platform scale (Cardinal Scales, Webb City, MO), and amount of concentrate allowance per day was adjusted accordingly. Body condition scores (BCSs) were also assigned by three independent observers using the 1 to 9 scale described by Henneke et al. (1983).
Dietary treatments
At week 0, horses were stratified by age, sex, BW, and farm of origin and randomly assigned to one of the two groups: 1) no supplementation (CON; n = 8) or 2) supplementation of 21 g/d of S. cerevisiae fermentation product (Original XPC, Diamond V Mills, Inc.; SCFP; n = 10) divided evenly into two feedings. Supplement dosage was determined based on previous work showing improved performance in exercising horses (Glade and Campbell-Taylor, 1990; Biel et al., 1990; Miller-Graber et al., 1994). The ground supplement was top-dressed on the SCFP horses’ grain at each feeding (10.5 g/feeding). A small portion of grain was provided to SCFP horses with the supplement to make sure all of the supplement was consumed. Once all the supplement was consumed, horses were given the remainder of their grain.
Submaximal exercise test
Following 8 wk of dietary supplementation, all horses underwent a 2-h submaximal exercise test (SET) on an 8-horse Panel Walker, measuring approximately 21 m in diameter (Priefert Manufacturing, Mount Pleasant, TX). The exercise test consisted of eight replications of walking at 1.3 m/s for 3 min (24 min total), trotting at 3.1 m/s for 7 min (56 min total), and cantering at 5.4 m/s for 5 min (40 min total). Four replications were completed in one direction then horses were reversed, and the final four replications were completed traveling in the opposite direction (1 h per direction). To allow for accurate sampling, horses were balanced by dietary treatment group, sex, and barn of origin and assigned to one of the three exercise groups (n = 6 per group). The SET was performed over three consecutive days, with one exercise group per day, and started at 0800 hours each day. Ambient temperatures for each day were 12.2, 12.8, and 11.1 °C, respectively, and the relative humidity was 94%, 71%, and 83%, respectively.
Sample collection and analysis
Blood samples were collected at weeks 0 and 8 pre-SET at 0500 hours prior to receiving concentrate. Blood was also collected immediately after the SET (0 h) and 1 and 6 h after the SET at 1000, 1100, and 1600 hours, respectively. Approximately, 30 mL of blood was collected via jugular venipuncture into evacuated tubes devoid of anticoagulant (Vacutainer; Becton, Dickson and Co., Franklin Lakes, NJ). Serum was isolated within 2 h of collection, aliquoted, and stored at −80 °C until analysis. Serum samples were analyzed for cortisol and serum amyloid A (SAA) concentrations using commercially available kits (cortisol: DetectX Cortisol Immunoassay Kit, Arbor Assays, Ann Arbor, MI; SAA: Horse SAA enzyme-linked immunosorbent assay [ELISA] Kit, Innovative Research, Inc., Novi, MI). Each kit provided a pre-prepared 96-well microplate for use with the assay, and standards for each assay were diluted according to the manufacturer’s recommendations. Serum was diluted 1:25 to 1:100 with diluent provided in the kit for cortisol to remain within range of the assay. SAA was diluted at 1:200 with provided diluent. For both assays, the absorbance (450 nm) of each well was determined using a plate reader (Synergy H1; Biotek Instruments, Inc., Winooski, VT). All samples were analyzed in triplicate. The inter-assay and intra-assay coefficient of variations (CVs) were 8.5% and 5.3%, respectively, for cortisol and 2.2% and 4.5%, respectively, for SAA. Serum concentrations of cytokines and chemokines were quantified using a Premixed 10 Plex Magnetic Bead Multiplex assay developed for use in the horse (Millipore Equine Cytokine/Chemokine cat# EQCYTMG-93KPX23; MilliPoreSigma, Burlington, MA). Cytokines and chemokines analyzed included: interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-18, tumor necrosis factor-alpha (TNF-α), fractalkine, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF). The assay was performed in 96-well plates in duplicate following the manufacturer’s protocol, and data acquired on a Luminex FLEXMAP 3D technology (Luminex Corporation, Austin, TX). Intra-assay CVs were 6.2%, 3.7%, 4.2%, 3.7%, 4.1%, 5.5%, 5.3%, 5.2%, and 5.6% and inter-assay CVs were 1.6%, 0.9%, 0.1%, 0.8%, 0.9%, 1.2%, 1.2%, 0.9%, and 0.9% for IL-1 β, IL-6, IL-8, IL-10, IL-18, TNF-α, fractalkine, G-CSF, and GM-CSF, respectively.
Statistical analysis
Data were analyzed using mixed linear models in SAS v9.4 with repeated measures (time). Diet, time, sex, and the diet ×time interaction were included as fixed effects and horse within diet was included as a random effect. No variable was affected by sex, and so sex was removed from all models. Data were tested for normality and log-transformed prior to analysis if not normally distributed. The responses to diet (week 0 to 8 pre-SET) were analyzed separately from the response to the week 8 SET. In cases where week 0 values differed by dietary treatment, week 0 values were included in the model as a covariate. This approach was not utilized when pre-SET values differed by dietary treatment due to the potential physiological relevance of concentrations of a given variable remaining higher or lower throughout the SET in a certain dietary treatment. All data are expressed as least squares means ± SEM. Significance was declared at P ≤ 0.05, and trends declared at P ≤ 0.10.
Results
All horses readily consumed their grain meal (with or without supplement) and intakes did not differ between treatment groups.
Responses to dietary treatment
BW increased in all horses from 280 ± 8 kg at week 0 to 313 ± 8 kg at week 8 (P < 0.0001) concurrent with growth. All horses also increased in BCS from 5.3 ± 0.1 at wk 0 to 5.4 ± 0.1 at wk 8 (P = 0.01). Neither BW nor BCS differed by treatment.
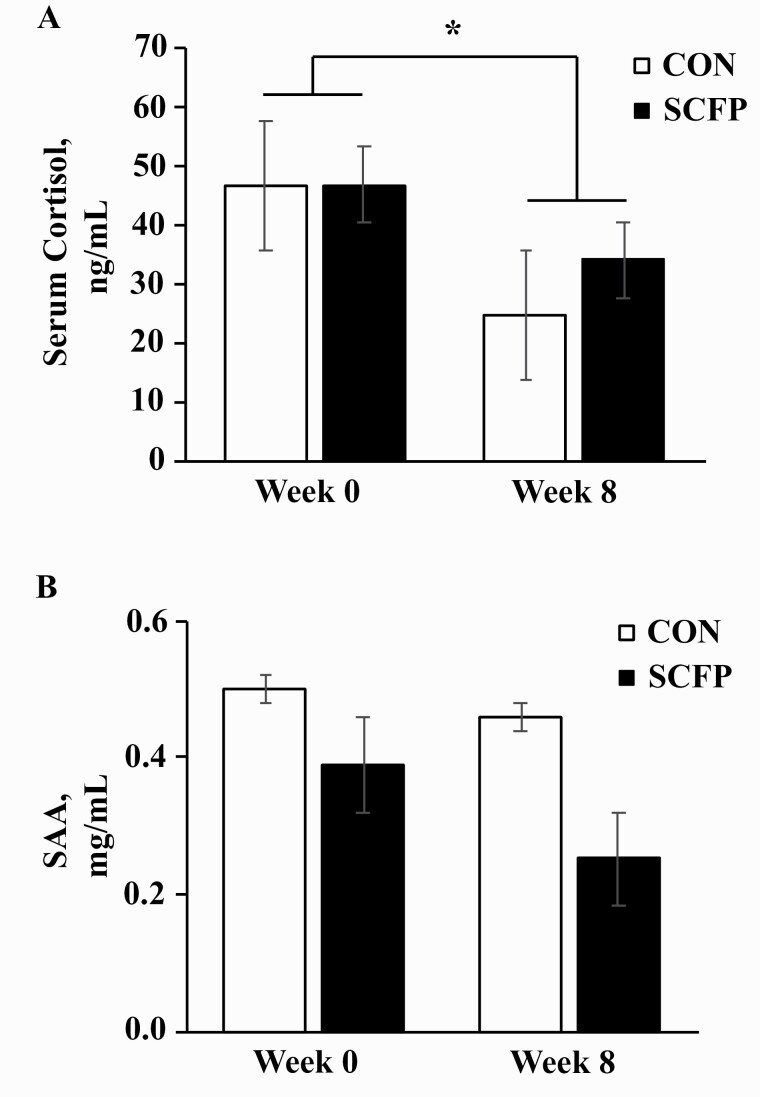
Serum cortisol decreased from week 0 to 8 (P = 0.01) in all horses but was unaffected by diet (Figure 1A). SAA was also similar between dietary groups but did not change between weeks 0 and 8 pre-SET (Figure 1B).
Figure 1.
Serum cortisol (A) and SAA (B) concentrations in yearling horses before (week 0) and after (week 8) 8 wk of receiving either CON (n = 8) or SCFP (n = 10) per day. The overall effects of dietary treatment (P = 0.5, P = 0.2), time (P = 0.01, P = 0.2), and treatment × time (P = 0.5, P = 0.6) for panels A and B, respectively. *Across treatments, week 0 differs from week 8 (P = 0.01).
At week 0, horses receiving SCFP had lesser serum G-CSF, GM-CSF, IL-1β, IL-6, and IL-8 than CON horses (P < 0.05; Table 2). Therefore, these five cytokines were analyzed using week 0 as a covariate. Overall, serum concentrations of all cytokines decreased from week 0 to 8 regardless of dietary treatment (P ≤ 0.008; Table 2). A diet × time interaction was present for G-CSF, GM-CSF, and IL-8 (P = 0.003; Table 2), whereby concentrations of all three cytokines were greater in CON than SCFP horses at week 0 (P ≤ 0.05) but greater in SCFP than CON horses by week 8 (P ≤ 0.05). Conversely, CON horses had greater serum TNF-α concentrations than SCFP horses at week 8 (P = 0.05; Table 2). Concentrations of fractalkine, IL-1β, IL-6, IL-10, and IL-18 were unaffected by dietary treatment from week 0 to 8 pre-SET (Table 2).
Table 2.
Cytokine concentrations in yearling horses before (week 0) and after (week 8) 8 wk of receiving either no supplement (CON, n = 8) or 21 g/d of S. cerevisiae fermentation product
| P-value | |||||||
|---|---|---|---|---|---|---|---|
| Cytokine | Diet | Week 0 | Week 8 | SEM | Diet | Time | Diet × Time |
| Fractalkine, pg/mL | CON | 228.4 | 152.5* | 7.0 | 0.121 | <0.0001 | 0.395 |
| SCFP | 221.3 | 155.5* | |||||
| G-CSF1, pg/mL | CON | 48.5a | 21.3a* | 1.8 | 0.853 | <0.0001 | 0.003 |
| SCFP | 43.6b | 26.9b* | |||||
| GM-CSF1, pg/mL | CON | 1.00a | 0.38a* | 0.04 | 0.909 | <0.0001 | 0.003 |
| SCFP | 0.85b | 0.51b* | |||||
| IL-I β 1, pg/mL | CON | 0.54 | 0.12* | 0.04 | 0.610 | <0.0001 | 0.081 |
| SCFP | 0.40 | 0.20* | |||||
| IL-61, pg/mL | CON | 2.26 | 0.75* | 0.14 | 0.408 | <0.0001 | 0.120 |
| SCFP | 1.89 | 0.85* | |||||
| IL-81, pg/mL | CON | 42.5a | 14.0a* | 3.2 | 0.901 | <0.0001 | 0.003 |
| SCFP | 34.0b | 23.4b* | |||||
| IL-10, pg/mL | CON | 69.2 | 45.2* | 5.6 | 0.261 | 0.007 | 0.699 |
| SCFP | 57.4 | 38.8 | |||||
| IL-18, pg/mL | CON | 6.2 | 3.4* | 4.8 | 0.155 | 0.0001 | 0.718 |
| SCFP | 4.6 | 2.3* | |||||
| TNFα, pg/mL | CON | 1.34 | 0.95a | 0.12 | 0.039 | 0.008 | 0.535 |
| SCFP | 1.03 | 0.57b* | |||||
1Serum concentrations differed between CON and SCFP at week 0 (P < 0.05) and week 0 were included in statistical analyses as a covariate.
a,
bWithin cytokine and column, CON differs from SCFP (P < 0.05).
*Within row, week 0 differs from week 8 (P < 0.05).
Responses to the SET
In response to the week 8 SET, serum cortisol increased in all horses from pre-SET to 0 h post-SET (P < 0.0001) but returned to pre-SET levels in SCFP horses by 1 h post-SET (Figure 2A). At 6 h post-SET, cortisol in CON horses had returned to pre-SET concentrations, whereas cortisol in SCFP horses declined to be lesser than pre-SET concentrations (P = 0.0002) and lesser than CON horses at that time point (P = 0.003; Figure 2A). In CON, SAA increased at 6 h post-SET (P < 0.0001) to be greater than all other time points (P < 0.0001) but was unchanged through 6 h post-SET in SCFP horses (Figure 2B). At 6 h post-SET, SAA concentrations were greater in horses receiving CON compared with SCFP (P < 0.0001; Figure 2B).
Figure 2.
Serum cortisol (A) and SAA (B) concentrations before a 2-h SET (pre-exercise),, immediately post-SET (0 h), and 1 and 6 h post-SET in yearling horses following 8 wk of receiving either CON (n = 8) or SCFP (n = 10) per day. a–c,x–z Within diet, time points with different letters differ (P < 0.05). *Within time, CON differs from SCFP (P < 0.05).
An effect of the interaction of exercise and diet was evident for IL-1β, IL-6, and IL-10 (P ≤ 0.03; Figure 3). In CON horses, serum IL-1β increased from pre-SET to 6 h post-SET (P = 0.0009; Figure 3A). Conversely, serum IL-1β increased in SCFP horses from pre- to 1 h post-SET (P = 0.0001) and then decreased from 1 to 6 h post-SET (P = 0.001), returning to pre-SET concentrations at 6 h post-SET. At 6 h post-SET, serum IL-1β was lesser in SCFP than CON horses (P = 0.001; Figure 3A). All horses showed increased serum IL-6 from pre-SET to 0 h post-SET (P < 0.0001; Figure 3B). Serum IL-6 then decreased from 0 to 1 h (P = 0.05) and from 1 to 6 h post-SET in SCFP horses (P = 0.0003; Figure 3B). Conversely, IL-6 in CON horses continued to increase to 1 h (P = 0.03) and then decreased at 6 h (P < 0.0001) but was greater in CON than SCFP horses at 1 and 6 h post-SET (P ≤ 0.002; Figure 3B). Serum IL-10 increased in all horses from pre- to 1 h post-SET (P < 0.0001); however, SCFP horses tended to have greater serum IL-10 compared with CON horses immediately following exercise (0 h post-SET; P = 0.07; Figure 3C). Additionally, IL-10 decreased from 1 to 6 h post-SET in SCFP horses (P = 0.003) but remained unchanged in CON horses. Neither serum IL-6 nor IL-10 returned to pre-SET concentrations in either group of horses through 6 h post exercise (P < 0.0001; Figure 3B and C).
Figure 3.
Serum concentrations of (A) IL-1β, (B) IL-6, and (C) IL-10 before a 2-h SET (pre-exercise), immediately post-SET (0 h), and 1 and 6 h post-SET in yearling horses following 8 wk of receiving either CON (n = 8) or SCFP (n = 10) per day. a–d,w–zWithin diet, time points with different letters differ (P < 0.05). *Within time, CON differs from SCFP (P < 0.05).
Concentrations of G-CSF, IL-18, and TNF-α were affected by exercise (P < 0.0001) and showed a trend to be greater in CON than SCFP horses throughout recovery from the SET (P ≤ 0.1; Figure 4A–C). In all horses, serum G-CSF was unchanged through 1 h post-SET but decreased from 1 to 6 h post-SET (P < 0.001; Figure 4A). Across dietary treatments, serum IL-18 increased from pre- to 1 h post-SET (P < 0.0001) and then decreased from 1 to 6 h post-SET (P = 0.002) but did not return to pre-SET concentrations (P < 0.0001; Figure 4B). Irrespective of dietary treatment, serum TNF-α increased from 0 to 1 h post-SET (P < 0.0001) and then decreased from 1 to 6 h post-SET (P = 0.0003) but did not return to pre-SET concentrations through 6 h post-SET (P = 0.002; Figure 4C).
Figure 4.
Serum concentrations of (A) G-CSF, (B) IL-18, (C) TNFα, (D) GM-CSF, (E) fractalkine, and (F) IL-8 before a 2-h SET (pre-exercise), immediately post-SET (0 h), and 1 and 6 h post-SET in yearling horses following 8 wk of receiving either CON (n = 8) or SCFP (n = 10) per day. f–iTime points with different letters differ (P < 0.05).
Serum concentrations of GM-CSF, fractalkine, and IL-8 were affected only by exercise (P < 0.0001) but not by diet or the interaction of diet and exercise (Figure 4D–F). Serum GM-CSF increased from pre- to 0 h post-SET (P < 0.0001), then decreased to 1 h post-SET (P < 0.0001) and further decreased to 6 h post-SET (P < 0.0001) but did not return to pre-exercise concentrations by 6 h post-SET (P < 0.001; Figure 4D). Serum fractalkine and IL-8 responded similarly to exercise, increasing from pre- to 0 h post-SET (P < 0.0001), decreasing at 1 h post-SET (P < 0.0001), but remaining greater at 6 h post-SET than pre-SET (P < 0.0001; Figure 4E–F).
Discussion
The present study demonstrated that horses receiving dietary supplementation with an S. cerevisiae fermentation product for 8 wk appeared to respond more favorably to a prolonged submaximal exercise bout than unsupplemented horses. Serum cortisol in SCFP horses returned to pre-SET concentrations more quickly after exercise compared with CON horses. Additionally, SAA, a sensitive marker of inflammation, was unaffected by exercise in supplemented horses but increased in control horses. In general, the SET appeared to induce a cytokine response but the induction of cytokines was either resolved more quickly in horses receiving SCFP (IL-1β, IL-6, and IL-10) or remained lower throughout exercise in SCFP horses than CON horses (G-CSF, IL-18, and TNF-α).
Various mechanisms to improve the longevity of performance horses have been investigated, with increased focus on the role of stress-induced immune and inflammatory responses (Horohov et al., 2012). In human and animal studies, yeast fermentation products have been shown to have potential benefits, from reducing heat stress to enhancing the immune response (Moyad et al., 2010; Evans et al., 2012; Price et al., 2018). The specific mechanisms by which yeast fermentate products affect inflammation and immunity are not fully understood, and it is important to note that the proprietary yeast fermentate used in this study is not a single biological compound with one mode of action. The growing body of evidence from a range of species continues to demonstrate a universality in many aspects of immune response. This is not unexpected given that innate immune responses are highly conserved across species. Various components of yeast fermentate such as cell wall β -glucans are recognized by immune cells within the intestinal mucosa, such as the dectin-1 receptor (Kim et al., 2011) with activation inducing several immune-stimulating effects. Yeast culture-mediated shifts in immune cell numbers have also been observed in canine and porcine models resulting from other oligosaccharide constituents (Swanson et al., 2002; Middelbos et al., 2007) along with upregulation of cell surface markers (CD69 and CD25) on natural killer cells in vitro (Jensen et al., 2008). Increased gut mucosal secretory immunoglobulin A (Gao et. al., 2008) and reductions in deleterious bacteria (Brewer et al., 2014) have also resulted from dietary yeast fermentate supplementation which mechanistically can help reduce inflammatory and immunological reactions.
While minimal research is available documenting the effects of yeast byproduct supplementation in horses, some work has indicated positive outcomes of supplementation. In untrained, young horses, supplementation with a yeast culture decreased plasma lactate levels and delayed increases of plasma triglyceride levels following a treadmill exercise test compared with non-supplemented control horses (Glade and Campbell-Taylor, 1990). Supplemented horses also showed lower heart rates during the first 5 and final 10 min of an exercise test, suggesting enhanced athletic performance in young horses entering a training program (Glade and Campbell-Taylor, 1990). Arabian endurance horses receiving an S. cerevisiae fermentation product (Original XPC; Diamond V Mills, Inc.) for 8 wk showed improvements in submaximal performance as evidenced by increased blood free fatty acids, blood glucose, hemoglobin, and packed cell volume (Wickler, 2002). While these trials demonstrated favorable effects of dietary supplementation with a yeast fermentation product, markers of stress and systemic inflammation in horses have not been thoroughly investigated.
Research exploring the inflammatory response to exercise in human athletes is prevalent; however, in horses, there is significantly less research investigating stress and inflammation in response to exercise, especially in young horses entering a training program. Due to the interplay of inflammation and the immune system and the immune-suppressing effects of long-term or intense exercise, this study aimed to investigate a potential immune-modulating and anti-inflammatory dietary additive for the preventative maintenance of young horses entering into an exercise training program.
Few biomarkers exist to quantitatively capture acute responses to stress. Referred to as the “stress hormone,” cortisol is involved in many biological systems following a stressor and, importantly, serves as a signal for cellular repair following exercise (McKeever et al., 2014). Exercise-induced elevations in cortisol are considered beneficial, as cortisol is responsible for stimulating gluconeogenesis, free fatty acid mobilization, and lipolysis during exercise (Hyyppa, 2005). Additionally, cortisol suppresses insulin release to maximize blood glucose utilization (de Graaf-Roelfsema et al., 2006). However, sustained high levels of cortisol in the blood (hypercortisolism) can result in symptoms, such as high blood pressure (Griffing, 2014), which can put extra strain on the heart and blood vessels. Long-term high blood pressure has been linked to heart and kidney disease in humans (DeMarco et al., 2014). Additionally, excess cortisol is associated with excessive tissue breakdown and increased blood glucose concentrations (hyperglycemia; Griffing, 2014), which, if frequent or ongoing, can cause damage to nerves, blood vessels, and organs. Therefore, timely removal of cortisol from circulation is important to reduce the risk of damage.
In horses, moderate-intensity exercise has been shown to increase circulating cortisol (Ambrojo et al., 2018), which is in agreement with the results of the current study. However, in the current study, serum cortisol in SCFP horses returned to pre-SET levels more quickly and was significantly lower at 6 h post-SET than in CON horses. These results suggest that horses receiving the S. cerevisiae fermentation product may have been more efficient at reducing circulating cortisol levels than unsupplemented horses, which may lead to a decreased degree of potential damage following exercise. While outside the scope of the current study, markers of tissue damage should be considered in future investigations to determine if expedited clearance of cortisol results in a lower degree of cell damage.
The use of APPs, especially SAA, to monitor acute immune responses is becoming more common in equine research as commercial diagnostic kits are being validated (Satoh et al., 1995; Crisman et al., 2008). Low or undetectable SAA levels in healthy horses combined with the large and rapid increase in plasma SAA after an inflammatory stimulus and a short half-life allow SAA to be used to closely monitor the course and resolution of inflammation efficiently (Jacobsen et al., 2005). In horses, SAA increases up to nearly 4-fold in response to various intensities and types of exercise (Cywinska et al., 2013; Witkowska‐Piłaszewicz et al., 2019). In the current study, CON horses showed a significant increase in SAA 6 h after the exercise test, whereas horses receiving SCFP did not experience this increase, suggesting that the S. cerevisiae fermentation product mitigated inflammation after exercise.
This is supported by the difference in systemic cytokine concentrations noted between treatment groups. Systemic cytokine concentrations are an emerging area of interest when investigating exercise-induced inflammation. Cytokines are activated by inflammatory stimuli and affect many different cells and tissues in the body by inducing APP synthesis. Combinations of cytokines on various target cells may have a stimulatory or suppressive effect. For example, the accumulation of SAA generally requires IL-6 and IL-1β or TNF-α, so multiple cytokines must be observed simultaneously to obtain a complete picture of the global inflammatory response (Crisman et al., 2008). In horses, systemic cytokines are present at relatively low concentrations. Due to methodological constraints, concentrations of specific cytokines are rarely quantified in the horse. Recent advances, including bead-based ELISAs, have allowed the investigation of cytokine profiles at the protein rather than mRNA level.
In the current study, serum concentrations of all cytokines decreased from week 0 to 8. There are few studies evaluating circulating cytokine concentrations in response to growth. One study in sheep reported that resting plasma concentrations of IL-6 decreased with maturity, while plasma IL-1β and TNF-α were unaffected by age (Salim et al., 2021). Serum concentrations of cytokines have not been evaluated in young, growing horses. The decrease in systemic cytokine concentrations noted in the current study, then, may be an effect of aging, but age-related changes in circulating cytokines should be further investigated in the young horse. Regardless, after 8 wk of supplementation, horses receiving SCFP had greater serum concentrations of G-CSF, GM-CSF, and IL-8 than CON horses but lesser TNF-α. Interestingly, G-CSF, GM-CSF, and IL-8 are involved with neutrophils, which are necessary for the clearance of bacterial pathogens. G-CSF is the principal cytokine that controls neutrophil development and function (Panopoulos and Watowich, 2008), IL-8 attracts and activates neutrophils in inflammatory regions (Bickel, 1993), and GM-CSF has a profound role in regulating the immune response and maintaining immunological tolerance (Bhattacharya et al., 2015). Elevated levels of these cytokines may be an indication of a strengthened immune system; however, reference ranges of cytokine concentrations in horses have not been established so it is difficult to determine the physiological relevance of these findings. Either way, a perceived enhancement of neutrophil-related cytokines concurrent with a suppression of pro-inflammatory TNF-α certainly seems promising. TNF-α is identified as one of the more significant pro-inflammatory cytokines and is known to play a role in vasodilatation and edema formation, contributes to oxidative stress at sites of inflammation, and indirectly induces fever (Zelová and Hošek, 2013). Additionally, TNFα is responsible for a diverse range of signaling events, including the upregulation of pro-inflammatory cytokines, IL-1β and IL-6 (Idriss and Naismith, 2000). The results of the current study must be further explored to fully understand their implications.
To the authors’ knowledge, this study is the first to detail the impacts of a prolonged bout of submaximal exercise on circulating cytokine concentrations in horses. In humans, strenuous (Pedersen et al., 2000) and submaximal (Starkie et al., 2000; Taghian et al., 2011) exercise results in an increase in concentrations of cytokines, including IL-1β, TNF-α, IL-6 (pro-inflammatory), and IL-10 (anti-inflammatory). Similar increases in cytokine concentrations (IL-1, IL-6, and TNF-α,) have been observed in horses following strenuous exercise (Donovan et al., 2007; Liburt et al., 2010). However, the type, intensity, and duration of physical activity have been shown to affect the cytokine profile differentially (Edwards et al., 2006; Crisman et al., 2008). With the exception of G-CSF, all cytokines examined in this study increased following exercise, though the temporal pattern differed slightly by cytokine. This is expected due to the varying nature and role that each cytokine plays in the body. Both pro- and anti-inflammatory cytokines are activated by inflammatory stimuli.
Exercise is known to induce an inflammatory response and thus activate cytokine mobilization, due to resulting micro-injuries to myofibers and bone (Wright et al., 2017). Interestingly, only G-CSF decreased following exercise in the current study. G-CSF plays a key role in producing neutrophils quickly in response to acute injury or infection (Mak and Saunders, 2006). In humans, G-CSF has been shown to increase in response to exercise (Wright et al., 2017) but G-GSF responses to exercise have not been previously investigated in horses. Thus, the decrease in G-CSF in response to exercise in the current study is unexpected and warrants further investigation into the role of this cytokine in horses.
Fractalkine, GM-CSF, and IL-8 were only affected by exercise so it can be inferred that, at the current supplementation rate and exercise level, there was no dietary effect on these exercise-induced cytokines. GM-CSF is an important hematopoietic growth factor and immune modulator and affects the activities of various leukocytes. It can recruit circulating neutrophils, monocytes, and lymphocytes to enhance their functions in host defense (Shi et al., 2006). Fractalkine mediates functions, including migration, adhesion, and proliferation of monocytes, natural killer cells, and T-cells, both during homeostasis and during times of bodily stress and immune responses (Lopez et al., 2007). The cytokine IL-8 is released from several cell types in response to inflammation and attracts neutrophils, basophils, and T-cells during the inflammatory process to the site of injury or infection (Brennan and Zheng, 2007). In horses, IL-8 is commonly associated with upper respiratory disease (Hansen et al., 2019). However, in humans, plasma IL-8 protein concentrations, as well as skeletal muscle IL-8 mRNA, have been shown to be upregulated following exercise (Chan et al., 2004; Nieman et al., 2004). While IL-8 has been less extensively researched in exercising horses, peripheral blood mononuclear cell IL-8 mRNA was increased in endurance horses at the end of a 90- to 120-km race but returned to basal levels at 24 h post-race (Cappelli et al., 2009). Although few investigations have focused on the specific response of these cytokines to exercise in serum, it is known that they increase in response to stressors and work to circulate and/or activate various immune and inflammatory mediators such as monocytes and neutrophils. Therefore, the increase of these cytokines seen in the current study is most likely due to the stress upon homeostasis of the body induced by exercise.
Throughout the exercise test at week 8, G-CSF, IL-18, and TNF-α tended to be lesser in horses receiving SCFP supplementation compared with non-supplemented control horses. It is interesting that week 0-corrected resting G-CSF concentrations were greater in SCFP compared with CON horses at week 8 but lesser in SCFP horses throughout recovery from the exercise test at week 8. Perhaps a strengthened immune status in the rested state resulted in horses being more immunologically prepared for an acute stressor, which resulted in a mitigated response to exercise in SCFP horses. This quizzical finding must be further investigated. IL-18 exhibits pro-inflammatory characteristics, such as increases in cell adhesion molecules, nitric oxide synthesis, and chemokine production (Dinarello et al., 2013). Additionally, IL-18 has been shown to inhibit the production of IL-10, an anti-inflammatory cytokine, and enhance the production of GM-CSF (also pro-inflammatory), which when elevated can lead to increased global inflammation (Shi et al., 2006). The interaction between IL-18 and strenuous exercise is still being investigated; however, studies in humans have shown significant decreases in IL-18 following exercise (Neumayr et al., 2005, Trøseid et al., 2009). Neumayr et al. (2005) reported a decrease in IL-18 in well-trained amateur cyclists 24 h post-marathon. Other studies have noted similar results (Trøseid et al., 2009; Oda et al., 2013), which is in contrast to the increase seen in the current study. To this author’s knowledge, IL-18 in the exercising horse has not been investigated. The conflicting results of the current study compared with human studies highlight important differences between horses and other species as well as our limited knowledge of the horse’s inflammatory and immune response to exercise. An observation that was congruent with various human and equine studies (Dufaux and Order, 1989; Espersen et al., 1990; Donovan et al., 2007; Liburt et al., 2010) was the exercise-induced increase in the pro-inflammatory cytokine TNF-α. It is notable that SCFP horses maintained lesser TNF-α concentrations both at rest and following submaximal exercise compared with control horses. The tendency for a difference in these three pro-inflammatory cytokines suggests that further investigation into different supplementation rates or varying intensities of exercise/stressors may be needed to yield more conclusive results.
The cytokines IL-1 β, IL-6, and IL-10 all increased in response to exercise but returned to pre-exercise levels more quickly in SCFP compared with control horses. Pro-inflammatory IL-1β has been implicated in pain, inflammation, and autoimmune conditions and is known to induce other pro-inflammatory cytokines and chemokines (Ren and Torres, 2009). It is considered to be a systemic marker of stress and inflammation and has been shown to increase in response to exercise in human studies (Evans et al., 1986; Sprenger et al., 1992). In the current study, not only did supplementation of the Saccharomyces cerevisiae fermentation product result in lower sustained levels of IL-1β during exercise recovery, but also concentrations returned to pre-exercise levels more quickly than in control horses. Similar results were noted for IL-6, which is considered to be an inflammation-responsive cytokine and is a known inflammatory biomarker (Lamprecht et al., 2008). Comparable to the current study, IL-6 increases in response to exercise in humans (Edwards et al., 2006; Febbraio, 2007). Lastly, the anti-inflammatory cytokine, IL-10, increased more quickly following exercise in SCFP supplemented compared with unsupplemented horses. This may indicate a more immediate anti-inflammatory response, which allowed for quicker resolution of inflammation, as evidenced by lower levels of all three cytokines (IL-1β, IL-6, and IL-10) at 6 h post exercise in SCFP horses compared with control horses.
The current study demonstrated that 8 wk of dietary supplementation with SCFP may have a beneficial impact on cellular stress and inflammation following prolonged submaximal exercise in young horses. Decreased levels of circulating SAA and pro-inflammatory cytokines, as well as a prompter return to pre-exercise concentrations, suggest a more favorable circulating inflammatory environment in the supplemented group of young horses compared with their non-supplemented counterparts. Importantly, this study provided foundational information about the cytokine response at the protein level (as opposed to mRNA) to exercise in growing horses. There is much to be learned about the inflammatory response in the horse; however, the results of this study indicate that dietary mitigation of excessive inflammation may be achievable.
Acknowledgment
This project was supported by funds provided by Diamond V.
Glossary
Abbreviations
- APP
acute-phase protein
- BCS
body condition score
- BW
body weight
- DM
dry matter
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL
interleukin
- SAA
serum amyloid A
- SET
submaximal exercise test
- TNF-α
tumor necrosis factor-alpha
Conflict of interest statement
The authors affirmatively acknowledge that they were free from influence by any funding sources or their employees that would result in any conflict of interest.
Literature Cited
- Ambrojo, K., Corzano M., and Poggi J.. . 2018. Action mechanisms and pathophysiological characteristics of cortisol in horsesin corticosteroids. In: Al-kaf, A. G., editor. IntechOpen. [Google Scholar]
- Beil, M., Lawrence L., Novakofski J., Kline K., McLaren D., Moser L., and Powell D.. . 1990. The effect of yeast culture supplementation on exercising horses. J. Anim. Sci. 68(suppl. 1):386. [Google Scholar]
- Bhattacharya, P., Thiruppathi M., Elshabrawy H. A., Alharshawi K., Kumar P., and Prabhakar B. S.. . 2015. GM-CSF: an immune modulatory cytokine that can suppress autoimmunity. Cytokine 75:261–271. doi: 10.1016/j.cyto.2015.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel, M. 1993. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 64(5 suppl):456–460. PMID: 8315568. [PubMed] [Google Scholar]
- Brennan, K., and Zheng J.. . 2007. Intereukin 8. In: Enna, S., and Bylund D., editors. xPharm: the comprehensive pharmacology reference. Amsterdam: (The Netherlands):Elsevier; p. 1–4. [Google Scholar]
- Brewer, M. T., Anderson K. L., Yoon I., Scott M. F., and Carlson S. A.. . 2014. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet. Microbiol. 172:248–255. doi: 10.1016/j.vetmic.2014.05.026 [DOI] [PubMed] [Google Scholar]
- Cappelli, K., Felicetti M., Capomaccio S., Pieramati C., Silvestrelli M., and Verini-Supplizi A.. . 2009. Exercise-induced up-regulation of MMP-1 and IL-8 genes in endurance horses. BMC Physiol. 9:12. doi: 10.1186/1472-6793-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. H., Carey A. L., Watt M. J., and Febbraio M. A.. . 2004. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287:R322–R327. doi: 10.1152/ajpregu.00030.2004 [DOI] [PubMed] [Google Scholar]
- Chovatiya, R., and Medzhitov R.. . 2014. Stress, inflammation, and defense of homeostasis. Mol. Cell 54:281–288. doi: 10.1016/j.molcel.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisman, M. V., Kent-Scarratt W., and Zimmerman K. L.. . 2008. Blood proteins and inflammation in the horse. Vet Clin North Am Equine Pract. 24(2): 285–297. doi: 10.1016/j.cveq.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Cywinska, A., Witkowski L., Szarska E., Schollenberger A., and Winnicka A.. . 2013. Serum amyloid A (SAA) concentration after training sessions in Arabian race and endurance horses. BMC Vet. Res. 9:91. doi: 10.1186/1746-6148-9-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Roelfsema, E., Van Ginneken M., Van Breda E., Wijnberg I., Keizer H., and Van der Kolk J., . 2006. The effect of long-term exercise on glucose metabolism and peripheral insulin sensitivity in standardbred horses. Equine Vet. J. Suppl. 38(S36):221–225. doi: 10.1111/j.2042-3306.2006.tb05543.x [DOI] [PubMed] [Google Scholar]
- DeMarco, V. G., Aroor A. R., and Sowers J. R.. . 2014. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 10:364–376. doi: 10.1038/nrendo.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello, C. A., Novick D., Kim S., and Kaplanski G.. . 2013. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4:289. doi: 10.3389/fimmu.2013.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, D. C., Jackson C. A., Colahan P. T., Norton N., and Hurley D. J.. . 2007. Exercise-induced alterations in pro-inflammatory cytokines and prostaglandin F2alpha in horses. Vet. Immunol. Immunopathol. 118:263–269. doi: 10.1016/j.vetimm.2007.05.015 [DOI] [PubMed] [Google Scholar]
- Dufaux, B., and Order U.. . 1989. Plasma elastase-alpha 1-antitrypsin, neopterin, tumor necrosis factor, and soluble interleukin-2 receptor after prolonged exercise. Int. J. Sports Med. 10:434–438. doi: 10.1055/s-2007-1024939 [DOI] [PubMed] [Google Scholar]
- Giblot Ducray, H. A., Globa L., Pustovyy O., Reeves S., Robinson L., Vodyanoy V., and Sorokulova I.. . 2016. Mitigation of heat stress-related complications by a yeast fermentate product. J. Therm. Biol. 60:26–32. doi: 10.1016/j.jtherbio.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Edwards, K. M., Burns V. E., Ring C., and Carroll D.. . 2006. Individual differences in the interleukin-6 response to maximal and submaximal exercise tasks. J. Sports Sci. 24:855–862. doi: 10.1080/02640410500245645 [DOI] [PubMed] [Google Scholar]
- Espersen, G. T., Elbaek A., Ernst E., Toft E., Kaalund S., Jersild C., and Grunnet N.. . 1990. Effect of physical exercise on cytokines and lymphocyte subpopulations in human peripheral blood. APMIS 98:395–400. doi: 10.1111/j.1699-0463.1990.tb01049.x [DOI] [PubMed] [Google Scholar]
- Evans, W., Meredith C., Cannon J., Dinarello C., Frontera W., Hughes V., Jones B., and Knuttgen H.. . 1986. Metabolic changes following eccentric exercise in trained and untrained men. J. Appl. Physiol. 61(5):1864–1868. doi: 10.1152/jappl.1986.61.5.1864 [DOI] [PubMed] [Google Scholar]
- Evans, M., Reeves S., and Robinson L. E.. . 2012. A dried yeast fermentate prevents and reduces inflammation in two separate experimental immune models. Evid. Based Complement. Alternat. Med. 2012:973041. doi: 10.1155/2012/973041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio, M. A. 2007. Exercise and inflammation. J. Appl. Physiol. (1985). 103:376–377. doi: 10.1152/japplphysiol.00414.2007 [DOI] [PubMed] [Google Scholar]
- Gao, J., Zhang H. J., Yu S. H., Wu S. G., Yoon I., Quigley J., Gao Y. P., and Qi G. H.. . 2008. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 87:1377–1384. doi: 10.3382/ps.2007-00418 [DOI] [PubMed] [Google Scholar]
- Glade, M., and Campbell-Taylor M.. . 1990. Effects of dietary yeast culture supplementation during the conditioning period on equine exercise physiology. J. Equine Vet. Sci. 10(6):434–443. doi: 10.1016/S0737-0806(06)80140-1 [DOI] [Google Scholar]
- Griffing, G. 2014. Serum cortisol. Available from https://emedicine.medscape.com/article/2088826-overview#a1 [accessed May 26, 2019].
- Hansen, S., Otten N. D., Fjeldborg J., Baptiste K. E., and Horohov D. W.. . 2019. Age-related dynamics of pro-inflammatory cytokines in equine bronchoalveolar lavage (BAL) fluid and peripheral blood from horses managed on pasture. Exp. Gerontol. 124:110634. doi: 10.1016/j.exger.2019.110634 [DOI] [PubMed] [Google Scholar]
- Henneke, D., Potter G., Kreider J., and Yeats B. F.. . 1983. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 15(4):371–372. doi: 10.1111/j.2042-3306.1983.tb01826.x [DOI] [PubMed] [Google Scholar]
- Horohov, D., Sinatra S., Chopra R., Jankowitz S., Betancourt A., and Bloomer R.. . 2012. The effect of exercise and nutritional supplementation on proinflammatory cytokine expression in young racehorses during training. J. Equine Vet. Sci. 32(12):805–815. doi: 10.1016/j.jevs.2012.03.017 [DOI] [Google Scholar]
- Hyyppa, S. 2005. Endocrinal responses in exercising horses. Livest. Prod. Sci. 92(2):113–121. doi: 10.1016/j.livprodsci.2004.11.014 [DOI] [Google Scholar]
- Idriss, H. T., and Naismith J. H.. . 2000. TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 50(3):184–195. doi: [DOI] [PubMed] [Google Scholar]
- Jacobsen, S., Jensen J. C., Frei S., Jensen A. L., and Thoefner M. B.. . 2005. Use of serum amyloid A and other acute phase reactants to monitor the inflammatory response after castration in horses: a field study. Equine Vet. J. 37:552–556. doi: 10.2746/042516405775314853 [DOI] [PubMed] [Google Scholar]
- Jensen, G. S., Patterson K. M., and Yoon I.. . 2008. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immunol. Microbiol. Infect. Dis. 31:487–500. doi: 10.1016/j.cimid.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Kim, H. S., Hong J. T., Kim Y., and Han S. B.. . 2011. Stimulatory effect of β-glucans on immune cells. Immune Netw. 11:191–195. doi: 10.4110/in.2011.11.4.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht, E., Bagnell C., and Williams C.. . 2008. Inflammatory responses to three modes of intense exercise in standardbred mares – a pilot study. Comp. Exerc. Physiol. 5(3–4): 115–125. doi: 10.1017/S1478061509294448 [DOI] [Google Scholar]
- Landskron, G., De la Fuente M., Thuwajit P., Thuwajit C., and Hermoso M. A.. . 2014. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014:149185. doi: 10.1155/2014/149185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liburt, N., Adams A., Betancourt A., Horohov D., and McKeever K.. . 2010. Exercise-induced increases in inflammatory cytokines in muscle and blood of horses. Equine Vet. J. 42(s38):280–288. doi: 10.1111/j.2042-3306.2010.00275.x [DOI] [PubMed] [Google Scholar]
- Lopez, A., Yunlong H., and Zheng J.. . 2007. Fractalkine. In: Enna, S., and Bylund D., editors. xPharm: the comprehensive pharmacology reference. Amsterdam; (The Netherlands):Elsevier; p. 1–3. [Google Scholar]
- Mak, T., and Saunders M.. . 2006. Cytokines and cytokine receptors. In: Mak, T., and Saunders M., editors. The immune response. Cambridge (MA):Academic Press; p. 463–516. [Google Scholar]
- McKeever, K., Arent S., and Davitt P.. . 2014. Endocrine and immune responses to exercise and training. In: Hodgson, D. R., McKeever K. H., and McGowan C. M., editors. The athletic horse. 2nd ed. Philadelphia; (PA):Saunders; p. 88–107. [Google Scholar]
- Middelbos, I. S., Godoy M. R., Fastinger N. D., and G. C.Fahey, Jr. 2007. A dose-response evaluation of spray-dried yeast cell wall supplementation of diets fed to adult dogs: effects on nutrient digestibility, immune indices, and fecal microbial populations. J. Anim. Sci. 85:3022–3032. doi: 10.2527/jas.2007-0079 [DOI] [PubMed] [Google Scholar]
- Miller-Graber, P. A., Thompson J., and Martinez T.. . 1994. Effect of yeast culture on the physiological response to submaximal exercise in horses. J. Anim. Sci. 72(suppl. 1):252. [Google Scholar]
- Morgan, L., Coverdale J., Froetschel M., and Yoon I.. . 2007. Effect of yeast culture supplementation on digestibility of varying forage quality in mature horses. J. Equine Vet. Sci. 27(6):260–265. doi: 10.1016/j.jevs.2007.04.009 [DOI] [Google Scholar]
- Moyad, M. A., Robinson L. E., Zawada E. T., Kittelsrud J., Chen D. G., Reeves S. G., and Weaver S.. . 2010. Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J. Altern. Complement. Med. 16:213–218. doi: 10.1089/acm.2009.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutrient requirements of horses. 2007. 6th ed. Washington, DC, National Academies Press. [Google Scholar]
- Neumayr, G., Ludwiczek O., Hoertnagl H., Pfister R., Mitterbauer G., Moschen A., Novick D., Rubinstein M., and Tilg H.. . 2005. The impact of prolonged strenuous endurance exercise on interleukin 18 and interleukin 18 binding protein in recreational cyclists. Int. J. Sports Med. 26:836–840. doi: 10.1055/s-2005-837466 [DOI] [PubMed] [Google Scholar]
- Nieman, D. C., Davis J. M., Brown V. A., Henson D. A., Dumke C. L., Utter A. C., Vinci D. M., Downs M. F., Smith J. C., Carson J., . et al. 2004. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J. Appl. Physiol (1985). 96:1292–1298. doi: 10.1152/japplphysiol.01064.2003 [DOI] [PubMed] [Google Scholar]
- Oda, K., Miyatake N., Sakano N., Saito T., Miyachi M., Tabata I., and Numata T.. . 2013. Serum interleukin-18 levels are associated with physical activity in Japanese men. PLoS One. 8:e81497. doi: 10.1371/journal.pone.0081497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos, A. D., and Watowich S. S.. . 2008. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42:277–288. doi: 10.1016/j.cyto.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen, G., Egner I., Drange M., Langberg H., Benestad H. B., and Fjeld J. G.. . 2010. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand. J. Med. Sci. Sports 20:195–207. doi: 10.1111/j.1600-0838.2009.00947.x [DOI] [PubMed] [Google Scholar]
- Pedersen, B. 2000. Exercise and cytokines. Immunol. Cell Biol. 78(5):532–535. doi: 10.1111/j.1440-1711.2000.t01-11-.x [DOI] [PubMed] [Google Scholar]
- Pedersen, B. K., and Hoffman-Goetz L.. . 2000. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055 [DOI] [PubMed] [Google Scholar]
- Price, P. T., Byrd J. A., Alvarado C. Z., Pavlidis H. O., McIntyre D. R., and Archer G. S.. . 2018. Utilizing original XPC™ in feed to reduce stress susceptibility of broilers. Poult. Sci. 97:855–859. doi: 10.3382/ps/pex386 [DOI] [PubMed] [Google Scholar]
- Ren, K., and Torres R.. . 2009. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 60(1): 57–64. doi: 10.1016/j.brainresrev.2008.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim, M. N., Durmus I., Basegmez M., Küçükkurt I., and Eryavuz A.. . 2021. Effects of age on the concentrations of plasma cytokines and lipid peroxidation in sheep. Kocatepe Vet. J. 14(1): 37–44. doi: 10.30607/kvj.798623 [DOI] [Google Scholar]
- Satoh, M., Fujinaga T., Okumura M., and Hagio M.. . 1995. Sandwich enzyme-linked immunosorbent assay for quantitative measurement of serum amyloid A protein in horses. Am. J. Vet. Res. 56(10): 1286–1291. [PubMed] [Google Scholar]
- Shi, Y., Liu C., Roberts A., Das J., Xu G., Ren G., Zhang Y., Zhang L., Yuan Z., Tan H., . et al. 2006. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 16(2):126–133. doi: 10.1038/sj.cr.7310017 [DOI] [PubMed] [Google Scholar]
- Shi, Y., Liu C., Roberts A., . et al. , 2006. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 16:126–133. doi: 10.1038/sj.cr.7310017 [DOI] [PubMed] [Google Scholar]
- Sprenger, H., Jacobs C., Nain M., Gressner A. M., Prinz H., Wesemann W., and Gemsa D.. . 1992. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clin. Immunol. Immunopathol. 63:188–195. doi: 10.1016/0090-1229(92)90012-d [DOI] [PubMed] [Google Scholar]
- Starkie, R. L., Angus D. J., Rolland J., Hargreaves M., and Febbraio M. A.. . 2000. Effect of prolonged, submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J. Physiol. 528(Pt 3):647–655. doi: 10.1111/j.1469-7793.2000.t01-1-00647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, K. S., Grieshop C. M., Flickinger E. A., Merchen N. R., and Fahey G. C.. . 2002. Effects of supplemental fructooligosaccharides and mannanoligosaccarides on colonic microbial populations, immune function and fecal odor components in the canine. J. Nutr. 132:1717S–1719S. doi: 10.1080/0003942021000019126 [DOI] [PubMed] [Google Scholar]
- Taghian, F., Esteki Ghashghaei F., Badami R., and Esteki Ghashghaei S.. . 2011. Comparison the effect of one session submaximal exercise on plasma levels of IL6 and TNF-a in obese and non-obese women. ARYA Atheroscler. 6:153–156. PMID: 22577435. [PMC free article] [PubMed] [Google Scholar]
- Trøseid, M., Lappegård K., Mollnes T., Arnesen H., and Seljeflot I.. . 2009. The effect of exercise on serum levels of interleukin-18 and components of the metabolic syndrome. Metab. Syndr. Relat. Disord. 7(6): 579–584. doi: 10.1089/met.2009.0003 [DOI] [PubMed] [Google Scholar]
- Wickler, S. J. 2002. Effect of diamond V XP yeast culture on sub-maximal exercise performance of Arabian horses. California State Polytechnic and University. EQ007-s. (Research Summary). Cedar Rapids, IA: Diamond V Mills, Inc. [Google Scholar]
- Witkowska‐Piłaszewicz, O. D., Żmigrodzka M., Winnicka A., Miśkiewicz A., Strzelec K., and Cywińska A.. . 2019. Serum amyloid A in equine health and disease. Equine Vet. Sci. 51(3):293–298. doi:0.1111/evj.13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, C. R., Ward A. C., and Russell A. P.. . 2017. Granulocyte colony-stimulating factor and its potential application for skeletal muscle repair and regeneration. Mediators Inflamm. 2017:7517350. doi: 10.1155/2017/7517350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelova, H., and Hosek J.. . 2013. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 62(7): 641–651. doi: 10.1007/s00011-013-0633-0 [DOI] [PubMed] [Google Scholar]
- Zhu, W., Wei Z., Xu N., Yang F., Yoon I., Chung Y., Liu J., and Wang J.. . 2017. Effects of saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J. Anim. Sci. Biotechnol. 8(36) doi: 10.1186/s40104-017-0167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]