Abstract
Background
Frailty is associated with lower mean activity; however, hourly activity is highly variable among older individuals. We aimed to relate frailty to hourly activity variance beyond frailty’s association with mean activity.
Method
Using the 2010–2011 National Social Life, Health and Aging Project wrist accelerometry data (n = 647), we employed a mixed-effects location scale model to simultaneously determine whether an adapted phenotypic frailty scale (0–4) was associated with the log10-mean hourly counts per minute (cpm) and between-and within-subject hourly activity variability, adjusting for demographics, health characteristics, season, day-of-week, and time-of-day. We tested the significance of a Frailty × Time-of-day interaction and whether adjusting for sleep time altered relationships.
Results
Each additional frailty point was associated with a 7.6% (10–0.0343, β = −0.0343; 95% CI: −0.05, −0.02) lower mean hourly cpm in the morning, mid-day, and late afternoon but not evening. Each frailty point was also associated with a 24.5% (e0.219, β = 0.219; 95% CI: 0.09, 0.34) greater between-subject hourly activity variance across the day; a 7% (e0.07, β = 0.07; 95% CI: 0.01¸ 0.13), 6% (e0.06, β = 0.06; 95% CI: 0, 0.12), and 10% (e0.091, β = 0.091; 95% CI: 0.03, 0.15) greater within-subject hourly activity variance in the morning, mid-day, and late afternoon, respectively; and a 6% (e−0.06, β = −0.06; 95% CI: −0.12, −0.003) lower within-subject hourly activity variance in the evening. Adjusting for sleep time did not alter results.
Conclusions
Frail adults have more variable hourly activity levels than robust adults, a potential novel marker of vulnerability. These findings suggest a need for more precise activity assessment in older adults.
Keywords: Accelerometry, Frailty, Geriatric assessment, Physical activity, Physical performance
The rapid development and broad adoption of wearable sensors have created an opportunity to improve our understanding of frailty (1–4). Wearable activity monitors (accelerometers) are capable of generating high-resolution logs of activity in the free-living environment over extended windows of time, information that may compliment traditional brief (15–30 minutes) clinical assessments (5,6). The literature is now ripe with studies associating accelerometry summary measures of activity volume or intensity (eg, mean counts per minute [cpm], time spent in sedentary, light, or moderate-vigorous activity) to frailty, related biomarkers, and disability in older adults (1,2,7–10). Early, promising prospective studies have also used these intensity and volume accelerometry measures to predict risk for longer term, frailty-related outcomes including mortality (11) and hospitalization (12,13).
Increasing research in frailty and accelerometry has suggested frailty does not just affect how much (14,15) one moves but how and when (16) one moves. Fragmentation of physical activity, modeled as an “active-to-sedentary transition probability” and defined as the reciprocal of the average active bout duration, is associated with gait speed, fatigability, and combined measures of physical function (chair stands, gait, balance), and is more predictive of mortality than traditional measures of physical activity volume (16). Accelerometry-based gait characteristics (amplitude, periodicity, and stride-to-stride variability) extracted from subsecond level accelerometry data from older adults are associated with measures of mobility, fatigability, and fitness (17,18). Activity timing may help distinguish frailty. The hourly activity of frail adults is most reduced in the morning relative to pre- and nonfrail adults while the activity level of all groups converge later in the day (3); in contrast, increasing age is associated with hourly activity reduction in the afternoon (19).
While frail adults exhibit lower average activity than robust adults, hourly activity is highly variable across individuals (19). But, we know little about the association of hourly activity variance with frailty. We hypothesized that activity variance itself may reflect underlying alterations in exhaustion or energetic reserve and that frailty would be associated with greater between- and within-subject hourly activity variance beyond a reduction in mean hourly activity. To relate frailty to hourly activity variance, we employed a mixed-effects location scale (MELS) model (20), a novel statistical model that allows the simultaneous identification of predictors of change in outcome mean, between-subject outcome variance, and within-subject outcome variance.
This study is the first reported application of a MELS regression to accelerometry activity data in older adults to our knowledge. This methodology has been applied to other longitudinal data, including ecological momentary analysis data (21–25), for which the variance of measures or responses within groups or within individuals is as crucial as the change in mean. The high resolution nature of accelerometry data lends itself well to a study of activity variance. It would be possible to study activity variance at multiple levels of the data, such as minute-to-minute, day-to-day, or week-to-week activity variance. We chose to study daytime activity variance at the hourly level to reflect daily activity patterns. The MELS model allows the study of average hourly activity, the heterogeneity of hourly levels between individuals, and the consistency of hourly levels within individuals across hours and days of wear in parallel, each conditional on the other. This methodologic approach has potential application in many datasets of repeated clinical measures from continuous glucose monitoring to daily symptom monitoring.
Method
Study Design
Data for this analysis were from the National Social Life, Health and Aging Project (NSHAP) dataset. NSHAP is a nationally representative, longitudinal survey study that collects extensive information on physical, mental, cognitive, and social health in U.S. older adults (26). The first wave of NSHAP was in 2005–2006 which included a nationally representative probability sample of community-dwelling adults born between 1920 and 1947 (aged 57–85) and over-sampled for African-Americans, Hispanics, and men. Three-thousand and five respondents participated, corresponding to a weighted response rate of 75.5%. Five years later (2010–2011), respondents were re-interviewed as were their cohabiting spouse or partner, for a total sample size of 3,377. Interviews were conducted in the homes of each respondent by professional interviewers from NORC at the University of Chicago. The study was approved by NORC’s Institutional Review Board. The current secondary analysis of de-identified data was deemed exempt from further review from the University of Chicago Institutional Review Board. The NSHAP data are stored at the National Archive of Computerized Data on Aging (http://www.icpsr.umich.edu/icpsrweb/NACDA/studies/34921) and are available to the public after completing a Data Use Agreement.
Wrist Accelerometry Substudy
Wrist accelerometry data were collected from a randomly selected subset of 793 respondents in the 2010–2011 data collection wave. Fifty-five of these were spouses or partners not born between 1920 and 1947 and were, therefore, excluded from this analysis (27). Furthermore, any respondents missing data for the variables considered in this analysis were excluded leaving 647 for the current sample. Respondents self-identifying as being Black/African-American and those with more comorbidities were less likely to agree to participate in the accelerometry substudy (3). The 2010–2011 accelerometry protocol has been previously described in detail (Supplementary Text 1) (27). Briefly, randomly selected respondents in the 2010–2011 data collection wave were asked to wear an ActiWatch Spectrum on their nondominant wrist continuously for 72 consecutive hours (including during bathing or swimming) (27). The filtered accelerometer signal was sampled at a frequency of 32 Hz. The maximum absolute value within each second was summed over 15-second epochs. Data were preprocessed using the Actiware software available from the manufacturer (28). Wear time was recorded using a highly sensitive, built-in galvanic heat sensor that identified when the device was being worn; nonwear periods were excluded. Once put on, the devices were rarely removed (only 0.17% of epochs across all wake data were classified as off-wrist), therefore, missing data were not imputed. Only days with at least 10 hours of daytime monitoring were deemed “valid” and included in analyses. Sundays were also excluded due to routinely lower activity on this day compared to other days among older adults (29). The primary rest intervals (one per 24-hour period) were identified using event markers, changes in ambient light data, sleep logs, and independent review by at least 2 study investigators as previously described in detail and were excluded from all analyses (30). To further standardize measurement across respondents, we excluded hours of the day having wake time activity data from less than 45% of the sample (before 7:00 am and after 10:59 pm).
Hourly Activity
We summed consecutive 15-second epoch activity counts to obtain the total cpm, and then calculated hourly mean cpm by summing the activity cpm separately for each hour of the day and dividing by 60 minutes per hour. Hours with <60 minutes of accelerometry measurements (~13%), such as when a respondent went to bed or awoke mid-hour, were excluded. The hourly cpm were logarithm10-transformed to improve symmetry and stabilize the variance.
Frailty
We used an adapted 4-point phenotypic frailty scale ranging from 0 to 4 points including weakness, slow gait, exhaustion, and low average activity (14,27). Weakness was identified using performance on a timed chair stands exercise. Respondents were asked to stand up and sit down from a chair 5 times as quickly as possible, and were considered weak if they required ≥16.7 seconds to complete the exercise, were wheelchair bound, or could not complete the task safely (27). Slow gait was measured directly by asking respondents to walk 3 m at their “usual” pace. The task was preformed twice and the fastest of the 2 attempts scored. Those requiring ≥5.7 seconds (or 0.53 m/s) to complete the faster of 2 timed walks—as well as those who were wheelchair bound or could not complete the exercise safely—were determined to have slow gait (27). The chair stand and timed gait cut-points were determined from the original Short Physical Performance Battery scoring (31). Exhaustion was determined using 2 modified Center for Epidemiologic Studies-Depression (CES-D) scale questions, similar to those used in the original frailty phenotype (32). Respondents were asked how often over the last week they felt that everything was an effort and how often they felt that they could not get going. Answer categories for both questions were: (a) rarely or none of the time, (b) some of the time, (c) occasionally, or (d) most of the time. A point for exhaustion was given if respondents answered “occasionally” or “most of the time” to either question (27). Finally, low physical activity was assessed by asking respondents the following question: “On average over the last 12 months, how often have you participated in vigorous physical activity or exercise? By vigorous physical activity, we mean 30 minutes or more of things like sports, exercise classes, heavy housework, or a job that involves physical labor.” Answer categories were: (a) 5 or more times per week, (b) 3 or 4 times per week, (c) 1–2 times per week, (d) 1–3 times per month, (e) less than 1 time per month, or (f) Never. Respondents were given a point for low physical activity if they reported engaging in 3 or fewer vigorous activities per month (answers d–f) (27). Self-reported physical activity and accelerometry-measured mean activity (mean count/15 s) are only modestly correlated (r = .27) in this dataset (1). Respondents were identified as frail if they scored ≥3 points, prefrail if they scored 1–2 points, and nonfrail if they scored 0 points.
Covariates
We considered demographic and health covariates. Age (centered, continuous) was calculated using reported date of birth and interview date. Sex (female vs male), race (White/Caucasian, Black/African American, other), Hispanic ethnicity, and employment status (currently working versus not working) were self-reported. Measured weight and height were used to calculate body mass index (BMI) (33). Cognitive function was assessed using the survey-adapted Montreal Cognitive Assessment (MoCA-SA, range 0–30, continuous) as previously described in detail (34,35). A modified Charlson Comorbidity Index (range 0–16, continuous) was constructed using self-reported comorbidity data in the 2010–2011 data collection wave. Respondents were asked whether they had ever been told by a doctor that they had any of the following conditions (number of points given in parentheses): congestive heart failure (1), heart attack (1), coronary procedure (1), stroke (1), diabetes (1), rheumatoid arthritis (1), asthma, emphysema, chronic obstructive pulmonary disease, or chronic bronchitis (1), dementia (1), nonmetastatic cancer excluding skin cancer (2), or metastatic cancer excluding skin cancer (6) (36).
Statistical Analysis
Descriptive statistics summarizing the demographic, health, and activity characteristics of the sample were calculated. We then fit a MELS model using the MIXREGLS program (20) (Supplementary Text 2) to determine whether the adapted phenotypic frailty scale was significantly associated with the mean log10-hourly cpm and between- and within-subject hourly activity variance, adjusting for demographic and health characteristics, month-of-wear (categorical), day-of-week (categorical), and time-of-day (time bins: 7:00–11:00 am, 11:01 am–2:00 pm, 2:01–5:00 pm, 5:01–11:00 pm).
An interaction between frailty and time-of-day was tested using likelihood ratio and Wald tests in the mean log10-hourly cpm and within-subject variance submodels. An interaction between frailty and time-of-day was also tested in the between-subject variance submodel which was found to be nonsignificant (Wald test: Frailty × Morning: reference, Frailty × Mid-day p = .53; Frailty × Late afternoon p = .11; Frailty × Evening p = .27; likelihood ratio test comparing model with and without Frailty × Time-of-day interaction p = .08). Therefore, this interaction is not included in the final between-subject variance submodel. We further explored whether frailty exhibited a nonlinear relationship with mean log10-hourly cpm, between-subject variance, and within-subject variance. A fully adjusted MELS model using a categorical frailty variable (0 = nonfrail, 1–2 = prefrail, ≥3 = frail) rather than a continuous frailty variable did not improve model fit (likelihood ratio test p = .98) (37,38). Thus, we did not find evidence for a nonlinear relationship for frailty in this analysis and report results for the continuous frailty variable models only. We further conducted a sensitivity analysis excluding the 22.1% of the sample of older adults who were still working (Supplementary Table 1; Supplementary Figure 1) which revealed similar findings; therefore, all participants regardless of working status are included in the final model. We subsequently tested whether adjusting for total sleep time (hours, continuous) changed the effect of frailty on mean hourly activity or hourly activity variance.
The changes in mean hourly activity, between- and within-subject variance associated with a 1-point increase in frailty were converted to percentage changes for comparability and plotted using Stata 15 (39). Specifically, we exponentiated each coefficient (10β for the mean submodel; eβ for the variance submodels) to obtain the percent change in the hourly mean cpm and the percent change in the variance of the log10-hourly cpm (see Supplementary Text 2 for detailed methodology).
Results
Descriptive Characteristics
Characteristics of the analytic sample (n = 647) with nonmissing data for all variables are shown in Table 1. The sample included 338 females (52.2%), and the mean age was 72.5 (7.2 SD). The mean hourly cpm was 218.5 (88.0 SD) and the mean log10-mean hourly cpm was 2.2 (0.2 SD). The sample included a broad representation of frailty status (frail = 14.7%, prefrail = 50.7%). Compared to the nonfrail adults, frail adults were older (mean age: nonfrail = 70.9, prefrail = 72.7, frail = 75.4 years), had a greater proportion of minority older adults (proportion Black/African American: nonfrail = 4.9%, prefrail = 11.6%, frail = 22.1%; proportion Hispanic (non-Black): nonfrail = 7.1%, prefrail = 12.2%, frail = 12.6%), a smaller proportion of working older adults (proportion working: nonfrail = 31.3%, prefrail = 19.5%, frail = 9.5%), and a higher BMI (mean BMI: nonfrail = 27.4, prefrail = 29.8, frail = 31.1 kg/m2) on average.
Table 1.
Descriptive Statistics (n = 647)
| Total Sample | Nonfrail | Prefrail | Frail | |
|---|---|---|---|---|
| Estimate | ||||
| Sex | ||||
| Female | 338 (52.2%) | 114 (50.9%) | 169 (51.5%) | 55 (57.9%) |
| Age | ||||
| Mean (years) | 72.5 (7.2 SD) | 70.9 (6.8 SD) | 72.7 (6.8 SD) | 75.4 (8.2 SD) |
| 62–70 | 302 (46.7%) | 124 (55.4%) | 148 (45.1%) | 30 (31.6%) |
| 71–80 | 239 (36.9%) | 75 (33.5%) | 130 (39.6%) | 34 (35.8%) |
| 81+ | 106 (16.4%) | 25 (11.2%) | 50 (15.2%) | 31 (32.6%) |
| Race/ethnicity | ||||
| Black/African-American | 70 (10.8%) | 11 (4.9%) | 38 (11.6%) | 21 (22.1%) |
| White/Caucasian | 486 (75.1%) | 187 (83.5%) | 239 (72.9%) | 60 (63.2%) |
| Hispanic (non-Black) | 68 (10.5%) | 16 (7.1%) | 40 (12.2%) | 12 (12.6%) |
| Other | 23 (3.6%) | 10 (4.5%) | 11 (3.4%) | 2 (2.1%) |
| Employment | ||||
| Working | 143 (22.1%) | 70 (31.3%) | 64 (19.5%) | 9 (9.5%) |
| Body mass index | ||||
| Mean (kg/m2) | 29.2 (6.1 SD) | 27.4 (4.2 SD) | 29.8 (6.1 SD) | 31.3 (8.7 SD) |
| 18–24 | 136 (21.0%) | 59 (26.3%) | 61 (18.6%) | 16 (16.8%) |
| 25–30 | 286 (44.2%) | 113 (50.5%) | 140 (42.7%) | 33 (34.7%) |
| 31+ | 225 (34.8%) | 52 (23.2%) | 127 (38.7%) | 46 (48.4%) |
| Modified Charlson Comorbidity Indexa | 1.0 (1.4 SD) | 0.7 (1.2 SD) | 1.1 (1.4 SD) | 1.5 (1.6 SD) |
| Montreal Cognitive Assessment – Survey Adaptedb | 22.9 (4.1 SD) | 24.2 (3.3 SD) | 22.6 (4.2 SD) | 20.8 (4.6 SD) |
| Hourly counts per minute (cpm) during wake hours between 7:00 am and 10:59 pm | ||||
| Hourly cpm (mean) | 218.5 (88.0 SD) | 243.7 (89.1 SD) | 212.2 (86.1 SD) | 180.8 (76.7 SD) |
| Log-transformed hourly cpm (mean) | 2.2 (0.2 SD) | 2.3 (0.2 SD) | 2.2 (0.2 SD) | 2.1 (0.3 SD) |
| Frailtyc | ||||
| Mean | 1.2 (1.2 SD) | – | – | – |
| Nonfrail | 224 (24.6%) | – | – | – |
| Prefrail | 328 (50.7%) | – | – | – |
| Frail | 95 (14.7%) | – | – | – |
Notes: aModified Charlson Comorbidity Index: range 0–16. 1 point each: congestive heart failure, prior heart attack, prior coronary procedure, stroke, diabetes, rheumatoid arthritis, asthma/emphysema/chronic obstructive pulmonary disease/chronic bronchitis, and dementia; 2 points: nonmetastatic cancer (non-skin); 6 points: metastatic cancer (non-skin). bSurvey-adapted Montreal Cognitive Assessment (MoCA-SA): an 18-item version of the original 28-item MoCA, adapted for use in large scale, survey-based studies. MoCA scores are predicted from MoCA-SA scores, range 0–30. cAdapted Phenotypic Frailty Scale: range 0–4, point assigned for slow gait, slow chair stands, self-reported exhaustion, and low self-reported physical activity.
Mixed-Effects Location Scale Model
We found that frail adults demonstrated higher hourly activity heterogeneity and had less consistent hourly activity patterns across days of wear than nonfrail adults, indicated by the significantly higher between- and within-person hourly activity variance (Table 2). The mean and within-subject variance submodel findings did significantly vary by time-of-day and are therefore reported after including the Frailty × Time-of-day interaction terms (mean hourly activity submodel interaction term Wald test—morning: reference; mid-day: β < 0.001, p = 1.00; late afternoon: β < 0.001, p = .93; evening: β = 0.02, p < .001). Within-subject hourly activity submodel interaction term Wald test—morning: reference; mid-day: β = −0.006, p = .84; late afternoon: β = 0.02, p = .44; evening: β = −0.13, p < .001). Results from the fully adjusted MELS model of hourly activity data including a Frailty × Time-of-day interaction term in the mean and within-subject submodels (Table 2) revealed that each additional frailty point was associated with a 7.6% (10–0.0343, β = −0.0343; 95% CI: −0.05, −0.02) lower mean hourly cpm in the morning, mid-day, and late afternoon but not evening. Each additional frailty point was also associated with a 24.5% (e0.219, β = 0.219; 95% CI: 0.09, 0.34) greater between-subject hourly activity variance across the day. Each additional frailty point was further associated with a 7% (e0.07, β = 0.07; 95% CI: 0.01¸ 0.13) greater within-subject hourly activity variance in the morning; a 6% greater within-subject hourly activity variance in the mid-day (e0.06, β = 0.06; 95% CI: 0, 0.12); a 10% (e0.091, β = 0.091; 95% CI: 0.03, 0.15) greater within-subject hourly activity variance in the late afternoon and a 6% (e-0.06, β = −0.06; 95% CI: −0.12, −0.003) lesser within-subject hourly activity variance in the evening. Adjusting for total sleep time did not alter results.
Table 2.
Mixed-Effects Location Scale Model Simultaneously Regressing log10-Hourly Counts per Minute, Between-Subject Hourly Counts per Minute Variance and Within-Subject Hourly Counts per Minute Variance on Frailtya Including Frailty × Time-of-Day Interaction With 7:00 am–11:00 am as Reference Group
| log10−Mean Hourly cpm Submodelc | Between−Subject Variance Submodelc | Within−Subject Variance Submodelc | |
|---|---|---|---|
| β (95% CI; p value) | β (95% CI; p value) | β (95% CI; p value) | |
| Frailtyb (range 0–4) | −0.0343 (−0.05, −0.02; <.001) | 0.219 (0.09, 0.34; <.001) | 0.07 (0.01¸0.13; .03) |
| Female | 0.09 (0.06, 0.12; <.001) | 0.13 (−0.14, 0.40; .35) | −0.15 (−0.26, −0.04; .006) |
| Age (per decade) | −0.06 (−0.08, −0.04; <.001) | −0.06 (−0.25, 0.12; .50) | 0.11 (0.03, 0.19; .008) |
| Race/ethnicity | |||
| Caucasian/White | Ref | Ref | Ref |
| African American/Black | −0.02 (−0.08, 0.03; .38) | −0.02 (−0.47, 0.43; .93) | 0.21 (0.02, 0.39; .03) |
| Hispanic | 0.02 (−0.03, 0.07; .38) | −0.31 (−0.72, 0.11; .15) | 0.08 (−0.11¸0.27; .43) |
| Other | 0.04 (−0.02, 0.10; .17) | −0.55 (−1.26, 0.17; .14) | −0.05 (−0.33, 0.24; .74) |
| Currently working | 0.03 (−0.004, 0.06; .09) | −0.12 (−0.45, 0.22; .49) | −0.09 (−0.22, 0.05; .22) |
| Body mass index (centered) | −0.005 (−0.007, −0.002; <.001) | −0.004 (−0.020, 0.013; .66) | 0.008 (−0.001, 0.17; .08) |
| Montreal Cognitive Assessment – SA (centered) | −0.003 (−0.007, 0.001; .11) | −0.03 (−0.06, 0.01; .10) | 0.02 (0.001, 0.03; .03) |
| Modified Charlson Comorbidity Scale | −0.02 (−0.03, −0.01; <.001) | 0.08 (−0.02, 0.17; .11) | 0.04 (−0.001, 0.08; .06) |
| Frailtyb × Time-of-day interaction | |||
| Morning (7:00 am–11:00 am) | Ref | – | Ref |
| Mid-day (11:01 am–2:00 pm) | <0.001 (−0.010, 0.010; 1.0) | – | −0.006 (−0.063, 0.051; .84) |
| Late afternoon (2:01 pm–5:00 pm) | <0.001 (−0.009, 0.010; .93) | – | 0.02 (−0.04, 0.08; .44) |
| Evening (5:01 pm–11:00 pm) | 0.02 (0.01, 0.03; <.001) | – | −0.13 (−0.19, −0.08; <.001) |
Notes: aModel additionally adjusted for: hour-of-day, day-of-week, and month-of-wear. bFrailty scale: range 0–4, higher score indicates worse frailty. cMean hourly activity was log10-transformed to satisfy normality assumptions. However, the mixed-effects location scale model estimates the between-subject and within-subject variance and their effects on the natural log scale. Values in bold type indicates p value ≤ .05.
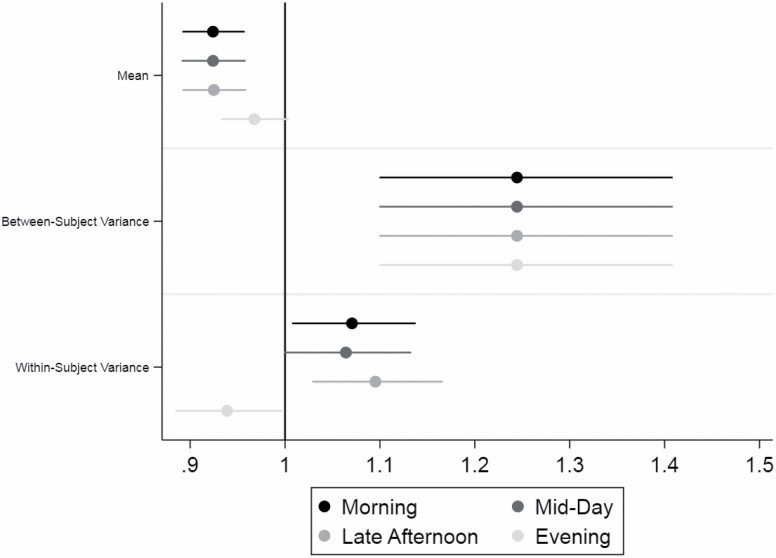
Plots of the percentage change in hourly activity and variance per frailty point are shown with 95% confidence intervals in Figure 1. The association between frailty and mean hourly cpm and within-subject hourly activity variance were similar in the morning (7:00 am–11:00 am), mid-day (11:01 am–2:00 pm), and late afternoon (2:01 pm–5:00 pm) but were different in the evening (5:01 pm–11:00 pm). Each additional frailty point was significantly associated with a 7.6% lower mean hourly cpm in the morning (proportion change = 0.924; 95% CI: 0.89, 0.96), mid-day (proportion change = 0.924; 95% CI: 0.89, 0.96), and late afternoon (proportion change = 0.925; 95% CI: 0.89, 0.96) but was not significantly associated with a proportion change in mean hourly cpm in the evening (proportion change = 0.97; 95% CI: 0.93, 1.00). Each additional frailty point was significantly associated with a 24.4% higher between-subject variance of the log10-hourly cpm across the day (proportion change = 1.244; 95% CI: 1.10, 1.41). Each additional frailty point was significantly associated with a 7% higher within-subject variance of the log10-hourly cpm in the morning (proportion change = 1.07; 95% CI: 1.01, 1.14), 6% higher within-subject variance in the mid-day (proportion change = 1.06; 95% CI: 1.00, 1.13), and 10% higher within-subject variance in the late afternoon (proportion change = 1.10; 95% CI: 1.03, 1.17) and a 6% lower within-subject variance of the log10-hourly cpm in the evening (proportion change = 0.94; 95% CI: 0.88, 0.997).
Figure 1.
Proportion change in mean, between-subject variance and within-subject variance for each additional frailty point across the day. The association between frailty (range 0–4) and mean hourly counts per minute (cpm), between-subject hourly activity variance, and within-subject hourly activity variance are plotted as proportions of change for each additional frailty point. The proportion of change for the mean was expressed as a percent change in the actual mean hourly cpm (10β). The proportion of change for between- and within-subject variance was expressed as a percent change in the variance of the log10-hourly cpm (eβ). The association between frailty and mean hourly cpm and within-subject hourly activity variance were similar in the morning (7:00 am–11:00 am), mid-day (11:01 am–2:00 pm), and afternoon (2:01 pm–5:00 pm) but were different in the evening (5:01 pm–11:00 pm). The association between frailty and between-subject hourly activity variance is estimated over the entire day as the time-of-day interaction was not significant. Each additional frailty point was significantly associated with a 7.6% lower mean hourly cpm in the morning (proportion change = 0.924; 95% CI: 0.89, 0.96), mid-day (proportion change = 0.924; 95% CI: 0.89, 0.96), and afternoon (proportion change = 0.925 95% CI: 0.89, 0.96) but was not significantly associated with a proportion change in mean hourly cpm in the evening (proportion change = 0.97; 95% CI: 0.93, 1.00). Each additional frailty point was significantly associated with a 24.4% higher between-subject variance of the log10-hourly cpm across the day (proportion change = 1.244; 95% CI: 1.10, 1.41). Each additional frailty point was significantly associated with a 7% higher within-subject variance of the log10-hourly cpm in the morning (proportion change = 1.07; 95% CI: 1.01, 1.14), a 6% higher within-subject variance in the mid-day (proportion change = 1.06; 95% CI: 0.999562, 1.13), a 10% higher within-subject variance in the late afternoon (proportion change = 1.10; 95% CI: 1.03, 1.17), and a 6% lower within-subject variance of the log10-hourly cpm in the evening (proportion change = 0.94; 95% CI: 0.88, 0.997).
Discussion
While we confirmed that increasing frailty is associated with lower mean hourly activity (3), for the first time, we found that increasing frailty is significantly associated with increased between-subject hourly activity variability. This finding implies that as frailty increases, the hour-to-hour activity level variability also increases and may reflect a broadening range of mobility capabilities or energy reserves as frailty advances. We did not find that the most frail adults were consistently inactive across the day which would have suggested that high frailty scores reflect homogenously inactive older adults. Instead, we found that the most frail adults in this sample exhibited heterogeneous hourly activity levels. The heterogeneity in hourly activity among increasingly frail adults might mean that frail adult activity depends on a much broader range of factors than robust adults not explored in this analysis such as social context (9), the walkability of the neighborhood (40), intermittent napping as a result of exhaustion (41), wavering cognitive processes (42), and physiologic dysregulation of cortisol and circadian rhythm (43). The heterogeneous nature of frail adult hourly activity levels also suggests a potential clinical role for more precisely assessing and monitoring activity as frailty advances. These assessments could help distinguish the potential mobility or energetic heterogeneity among those with more advanced frailty and could help direct tailored activity interventions, the most promising frailty intervention to date (44), when a “one size” prescription inevitably does not fit all. For example, wrist accelerometry could be used to determine personalized physical activity goals based on activity levels across the day. For a frail older adult who is active in the morning but less active in the afternoon, one might recommend an increase in moderate activity in the afternoon. For the frail older adult who is inactive all day, one might recommend breaking up prolonged inactive bouts throughout the day with light activity.
We also found that increasing frailty was associated with increasing within-subject activity variance across hours and days of wear during the morning and afternoon but not evening. The individual frail adults in this study were less likely to have the same hourly activity levels across hours and day-to-day. Because this study is cross-sectional, this finding is strictly an association. However, it suggests loss of hourly activity routine consistency may be an early frailty indicator. A future study assessing hourly activity variance changes over time would help answer this question. With the increasing ease and acceptability of wearable wrist sensors (45), the potential translation of accelerometry monitoring to augment the in-person frailty assessment is becoming feasible (46). This type of monitoring might help detect and quantify a patient’s “good” mobility days from “bad” days, triggering social or medical check-ins. Early translational accelerometry studies have shown promise in monitoring post-acute care and stroke recovery (12,47), hospitalization risk (48), and to assist chronic disease management (49). The use of accelerometry to augment the clinical frailty and geriatric assessment has received little attention to date but the noninvasive, remote evaluation of one of the most critical aspects of older adult health—mobility—is appealing (50).
While these findings offer new insight into frailty activity heterogeneity, this study has several limitations that may have impacted the findings. The accelerometry protocol in NSHAP was brief, and study participants typically contributed only 2–3 valid days of wear over which to assess activity variance. While we have shown that estimates of mean daily activity volume calculated over just 2 or 3 days of wear versus over 7 days of wear are highly correlated in older adults (Lin’s correlation coefficient r = 0.93, 0.96 for 2 vs 7 and 3 vs 7 day calculations, respectively (29)), it is likely that hourly activity variance would be higher among all individuals if additional wear days were included. The identification of a significant relationship between frailty and between- and within-subject hourly activity variance over such a short wear time is noteworthy, but repeating this analysis over longer wear periods would be valuable. An additional limitation of this study is that the MELS model is not able to incorporate survey weights at this time. Therefore, our study findings cannot be used to make inferences about the U.S. population of adults born between 1920 and 1947 but does reflect a random sample of community-dwelling, U.S. older adults. Future extensions of this model may allow incorporation of survey weights. It is of great clinical and research relevance that older adults were less likely to participate in the wrist accelerometry substudy if they self-identified as Black/African-American or had a high burden of comorbidities in this study, particularly since adults who have a high burden of comorbidities are also at increased risk of frailty. However, NSHAP over-sampled for older adults self-identifying as one of the racial/ethnic minorities, and we, therefore, have preserved their representation in this analysis.
In summary, this study demonstrated the application of a new methodology, a MELS model, to study the relationship of frailty to between- and within-subject hourly activity variance in older adults using wrist accelerometry data while accounting for the association between frailty and mean hourly activity. Increasing frailty was associated with more hourly activity level heterogeneity and less consistency across hours and days of wear than more robust adults, confirming our hypotheses. These findings suggest a potential role for more precise activity monitoring as frailty advances. Replication of this analysis in a study with longer wear protocols is needed.
Supplementary Material
Funding
This work was supported by the National Institute of Aging R37 AG03048, R01 AG0339031, and R01 AG043538 to NSHAP PI, L.W.; and K23 AG049106 to M.H.-S. The National Social Life, Health and Aging Project was supported by the National Institute on Aging of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None declared.
References
- 1. Huisingh-Scheetz MJ, Kocherginsky M, Magett E, Rush P, Dale W, Waite L. Relating wrist accelerometry measures to disability in older adults. Arch Gerontol Geriatr. 2016;62:68–74. doi: 10.1016/j.archger.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huisingh-Scheetz M, Kocherginsky M, Dugas L, et al. Wrist accelerometry in the health, functional, and social assessment of older adults. J Am Geriatr Soc. 2016;64(4):889–891. doi: 10.1111/jgs.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, et al. The relationship between physical activity and frailty among U.S. older adults based on hourly accelerometry data. J Gerontol A Biol Sci Med Sci. 2018;73(5):622–629. doi: 10.1093/gerona/glx208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunlop D, Song J, Arnston E, et al. Sedentary time in U.S. older adults associated with disability in activities of daily living independent of physical activity. J Phys Act Health. 2015;12:93–101. doi:101123./jpah.2013-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46(1):99–106. doi: 10.1249/MSS.0b013e3182a0595f [DOI] [PubMed] [Google Scholar]
- 6. Asch DA, Muller RW, Volpp KG. Automated hovering in health care—watching over the 5000 hours. N Engl J Med. 2012;367(1):1–3. doi: 10.1056/NEJMp1203869 [DOI] [PubMed] [Google Scholar]
- 7. Draganidis D, Jamurtas AZ, Stampoulis T, et al. Disparate habitual physical activity and dietary intake profiles of elderly men with low and elevated systemic inflammation. Nutrients. 2018;10(5):566. doi: 10.3390/nu10050566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ortlieb S, Gorzelniak L, Nowak D, et al. Associations between multiple accelerometry-assessed physical activity parameters and selected health outcomes in elderly people—results from the KORA-age study. PLoS One. 2014;9(11):e111206. doi: 10.1371/journal.pone.0111206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho EC, Hawkley L, Dale W, Waite L, Huisingh-Scheetz M. Social capital predicts accelerometry-measured physical activity among older adults in the U.S.: a cross-sectional study in the National Social Life, Health, and Aging Project. BMC Public Health. 2018;18(1):804. doi: 10.1186/s12889-018-5664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mankowski RT, Anton SD, Axtell R, et al. ; LIFE Research Group . Device-measured physical activity as a predictor of disability in mobility-limited older adults. J Am Geriatr Soc. 2017;65(10):2251–2256. doi: 10.1111/jgs.15037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. Br Med J. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chawla H, Bulathsinghala C, Tejada JP, Wakefield D, ZuWallack R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(8):1203–1209. doi: 10.1513/AnnalsATS.201405-198OC [DOI] [PubMed] [Google Scholar]
- 13. Dohrn IM, Welmer AK, Hagströmer M. Accelerometry-assessed physical activity and sedentary time and associations with chronic disease and hospital visits—a prospective cohort study with 15 years follow-up. Int J Behav Nutr Phys Act. 2019;16(1):125. doi: 10.1186/s12966-019-0878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 15. Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci. 2016;71(9):1184–1194. doi: 10.1093/gerona/glw043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schrack JA, Kuo PL, Wanigatunga AA, et al. Active-to-sedentary behavior transitions, fatigability, and physical functioning in older adults. J Gerontol A Biol Sci Med Sci. 2019;74:560–567. 10.1093/gerona/gly243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Urbanek JK, Zipunnikov V, Harris T, Crainiceanu C, Harezlak J, Glynn NW. Validation of gait characteristics extracted from raw accelerometry during walking against measures of physical function, mobility, fatigability, and fitness. J Gerontol A Biol Sci Med Sci. 2018;73(5):676–681. doi: 10.1093/gerona/glx174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urbanek JK, Zipunnikov V, Harris T, et al. Prediction of sustained harmonic walking in the free-living environment using raw accelerometry data. Physiol Meas. 2018;39(2):02NT02. doi: 10.1088/1361-6579/aaa74d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–979. doi: 10.1093/gerona/glt199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hedeker D, Nordgren R. MIXREGLS: a program for mixed-effects location scale analysis. J Stat Softw. 2013;52(12):1–38. doi: 10.18637/jss.v052.i12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nordgren R, Hedeker D, Dunton G, Yang CH. Extending the mixed-effects model to consider within-subject variance for Ecological Momentary Assessment data. Stat Med. 2020;39(5):577–590. doi: 10.1002/sim.8429 [DOI] [PubMed] [Google Scholar]
- 22. Lin X, Mermelstein RJ, Hedeker D. A 3-level Bayesian mixed effects location scale model with an application to ecological momentary assessment data. Stat Med. 2018;37(13):2108–2119. doi: 10.1002/sim.7627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rast P, Hofer SM, Sparks C. Modeling individual differences in within-person variation of negative and positive affect in a mixed effects location scale model using BUGS/JAGS. Multivariate Behav Res. 2012;47(2):177–200. doi: 10.1080/00273171.2012.658328 [DOI] [PubMed] [Google Scholar]
- 24. Schmeer KK, Tarrence J, Browning CR, Calder CA, Ford JL, Boettner B. Family contexts and sleep during adolescence. SSM Popul Health. 2019;7. doi: 10.1016/j.ssmph.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerhart JI, Burns JW, Bruehl S, et al. Variability in negative emotions among individuals with chronic low back pain: relationships with pain and function. Pain. 2018;159(2):342–350. doi: 10.1097/j.pain.0000000000001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Muircheartaigh C, English N, Pedlow S, Kwok PK. Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl. 2):S15–S26. doi: 10.1093/geronb/gbu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huisingh-Scheetz M, Kocherginsky M, Schumm PL, et al. Geriatric syndromes and functional status in NSHAP: rationale, measurement, and preliminary findings. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl. 2):S177–S190. doi: 10.1093/geronb/gbu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. PhilipsRespironics. Actiwatch: Koninklijke Philips Electronics N.V. 19 February, 2013. http://www.healthcare.philips.com/main/homehealth/sleep/actiwatch/default.wpd#&&/wEXAQUOY3VycmVudFRhYlBhdGgFCUVkdWNhdGlvbrs7D4d8dwFrxbRmM0TsUP60b3xr
- 29. Kocherginsky M, Huisingh-Scheetz M, Dale W, Lauderdale DS, Waite L. Measuring physical activity with hip accelerometry among U.S. older adults: how many days are enough? PLoS One. 2017;12(1):e0170082. doi: 10.1371/journal.pone.0170082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lauderdale DS, Philip Schumm L, Kurina LM, et al. Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl. 2):S125–S133. doi: 10.1093/geronb/gbu092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 32. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 33. World Health Organization. WHO: Global Database on Body Mass Index [July 28, 2011]. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html
- 34. Kotwal AA, Schumm P, Kern DW, et al. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 2015;29(4):317–324. doi: 10.1097/WAD.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shega JW, Sunkara PD, Kotwal A, et al. Measuring cognition: the Chicago Cognitive Function Measure in the National Social Life, Health and Aging Project, Wave 2. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl. 2):S166–S176. doi: 10.1093/geronb/gbu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vasilopoulos T, Kotwal A, Huisingh-Scheetz MJ, Waite LJ, McClintock MK, Dale W. Comorbidity and chronic conditions in the National Social Life, Health and Aging Project (NSHAP), Wave 2. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl. 2):S154–S165. doi: 10.1093/geronb/gbu025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gertheiss J, Oehrlein F. Testing linearity and relevance of ordinal predictors. Electron J Statist. 2011;5:1935–1959. doi: 10.1214/11-EJS661 [DOI] [Google Scholar]
- 38. Rice JA. Mathematical statistics and data analysis. 3rd ed. ed. Belmont, CA: Duxbury Press; 2007. [Google Scholar]
- 39. StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 40. Mayne DJ, Morgan GG, Jalaludin BB, Bauman AE. The contribution of area-level walkability to geographic variation in physical activity: a spatial analysis of 95,837 participants from the 45 and Up Study living in Sydney, Australia. Popul Health Metr. 2017;15(1):38. doi: 10.1186/s12963-017-0149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl. 1):S7–11. doi: 10.1016/j.sleep.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 42. Pickering TA, Huh J, Intille S, Liao Y, Pentz MA, Dunton GF. Physical activity and variation in momentary behavioral cognitions: an ecological momentary assessment study. J Phys Act Health. 2016;13(3):344–351. doi: 10.1123/jpah.2014-0547 [DOI] [PubMed] [Google Scholar]
- 43. Johar H, Emeny RT, Bidlingmaier M, et al. Blunted diurnal cortisol pattern is associated with frailty: a cross-sectional study of 745 participants aged 65 to 90 years. J Clin Endocrinol Metab. 2014;99(3):E464–E468. doi: 10.1210/jc.2013-3079 [DOI] [PubMed] [Google Scholar]
- 44. Fried LP. Interventions for human frailty: physical activity as a model. Cold Spring Harb Perspect Med. 2016;6(6):a025916. doi: 10.1101/cshperspect.a025916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O’Brien T, Troutman-Jordan M, Hathaway D, Armstrong S, Moore M. Acceptability of wristband activity trackers among community dwelling older adults. Geriatr Nurs. 2015;36(Suppl. 2):S21–S25. doi: 10.1016/j.gerinurse.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 46. Yetisen AK, Martinez-Hurtado JL, Unal B, Khademhosseini A, Butt H. Wearables in medicine. Adv Mater. 2018:30(33):e1706910. doi: 10.1002/adma.201706910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gebreuers N, Vanyor C, Truijen S, Engelborghs S, Deyn PPD. Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil. 2010;91(2):288–297. doi: 10.1016/j.apmr.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 48. Durheim MT, Smith PJ, Babyak MA, et al. Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of Global Initiative for Chronic Obstructive Lung Disease 2011 Group. Ann Am Thorac Soc. 2015;12(3):349–356. doi: 10.1513/AnnalsATS.201408-365OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922–932. doi: 10.1016/j.jchf.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 50. Razjouyan J, Naik AD, Horstman MJ, et al. Wearable sensors and the assessment of frailty among vulnerable older adults: an observational cohort study. Sensors (Basel). 2018;18(5):1336. doi: 10.3390/s18051336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.