Abstract
Background: Sepsis has been redefined recently as life-threatening organ dysfunction caused by dysregulated host responses to infection and septic shock. Soluble urokinase plasminogen activator receptor (SuPAR) and plasminogen activator inhibitor-1(PAI-1) concentration positively correlate to the activation level of the immune system, and are markers of disease severity and aggressiveness. Objective: The study aimed to identify the blood level of plasminogen activator inhibitor-1 (PAI-1) and soluble urokinase plasminogen activator receptor (SuPAR) in sepsis and its association with mortality. Patient and methods: This is an observational prospective study that enrolled 60 adult patients with sepsis (according to SOFA), admitted to Menoufia and Zagazig university hospitals during the period from December 2019 till October 2020. Plasminogen activator inhibitor-1 (PAI-1) and soluble urokinase plasminogen activator receptor (SuPAR) were checked in all participants. Results: SuPAR and PAI.1 were significant independent predictors of hospital mortality. SuPAR showed sensitivity 100%, specificity 95.9%, and accuracy 94% for prediction of early mortality at a cutoff value of 13.4(pg/ml). While, PAI-1 demonstrated sensitivity 100%, specificity 93.9%, and accuracy of 95% at a cutoff value of 122.5 for predicting mortality. Conclusion: PAI-1 and suPAR were significant predictors of hospital mortality among sepsis patients. The sample size was relatively small, which may have decreased the statistical power of the results of the present study. Hence, additional studies with large sample sizes are required for further validation of the present results.
Keywords: plasminogen activator inhibitor-1, soluble urokinase plasminogen activator receptor, sepsis
Introduction
In 1992, sepsis was defined as a systemic inflammatory response syndrome (SIRS) to infection that results from an activation of the innate immune response, regardless of the cause. 1 Sepsis has been redefined again as life-threatening organ dysfunction caused by dysregulated host responses to infection and septic shock as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. 2 Globally, sepsis is common, with an estimated population incidence of 270 cases per-100,000 person yearly and acute mortality of 26.0%. Many reasons suggest even this underestimate the magnitude of sepsis-associated mortality and morbidity. 3
The biomarkers of sepsis can be classified as markers of acute-phase protein (C-reactive protein [CRP], procalcitonin [PCT], and lipopolysaccharide-binding protein), cytokine/chemokine biomarkers (IL-6, IL-8), and markers of other pathophysiologic processes (coagulation factors and soluble cell surface receptors). Also, complement factors (C3a, C5a, and the soluble form of the C5a receptor, sC5aR) have been defined as early markers of sepsis and sepsis severity. CRP and PCT are the most practically used for the detection of bloodstream infections. 4
Plasminogen activator inhibitor-1(PAI-1) is a protein that in humans is encoded by the SERPINE 1 gene, elevated PAI-1 is a risk factor for thrombosis and atherosclerosis. 5 Soluble urokinase plasminogen activator receptor (SuPAR) is a soluble protein form; SuPAR concentration positively correlates to the activation level of the immune system and is present in plasma, urine, blood, serum, and CSF. SuPAR is an indicator of disease severity and aggressiveness. 6 The use of plasma suPAR level enhanced the efficiency of sepsis diagnosis, and the combination of plasma suPAR and APACHE II score improved mortality prediction. 7 Studying long-term outcomes of sepsis is that poor functional status is a risk factor for becoming critically ill as well as a frequent consequence. Many co-morbidities, age, and chronic diseases are risk factors both for sepsis and for impaired quality of life. Therefore, studies work to distinguish between the potentially causal effects of sepsis and that simply describe morbidity and mortality events. 8
Aim of the study
This study aimed to identify the blood level of plasminogen activator inhibitor-1 (PAI-1) and soluble urokinase plasminogen activator receptor (SuPAR) in sepsis and its association with mortality.
Patient and methods
This is an observational prospective study that included 60 adult patients with sepsis admitted to Menoufia and Zagazig university hospitals during the period from December 2019 till October 2020.
All participants were volunteers, and all of them signed written informed consent with explaining the aim of this study before the study initiation.
Approval of the study protocol was obtained by the local Ethical Scientific Committee of Menoufia University’s institutional review board under number (MNF112/2019).
Patients with sepsis were diagnosed based on both of the following criteria
Patients with suspected infection (thorough history taking with clinical status, routine laboratory tests, blood or urine cultures if possible, and radiological images as by (pelvi-abdominal ultrasound, plain chest X-ray, and CT or MRI if possible) who are likely to have a prolonged ICU stay or to die in the hospital identified SOFA score. 2
All subjects were selected according to inclusion and exclusion criteria
Inclusion criteria
Adult patients with sepsis, both sexes, and age more than 30 years.
Exclusion criteria
Pregnant women, cardiac patients, and chronic renal disease.
For all subjects, the following procedures were performed: personal history, past history, present history, and family history. Also, thorough clinical examination: complete general and local examination. In addition, laboratory examination including: Complete blood picture, Kidney functions, and Liver function test.
Specific investigations including: Plasminogen activator inhibitor-1 (PAI-1) and soluble urokinase plasminogen activator receptor (SuPAR).
Methods
3 mL of blood were collected from all patients and a small quantity of plasma (0.2 mL) was isolated within 30 min and stored at −80°C for measurement of SuPAR and PAI-1 levels. Human PAI-1 ELISA Kit (Chongqing Biospes Co, Ltd, China) with a range of 156 pg/ml-10,000 pg/mL and sensitivity < 10 pg/mL. 9 . Human SuPAR ELISA Kit (Chongqing Biospes Co, Ltd, China) with a range of 30 pg/mL—360 pg/mL and Sensitivity of 3 pg/mL. 9
Statistical analysis
Method of calculation and justification for sample size
The sample size calculation
Sample size was calculated using G*power version 3.1.9.2 based on previous studies and our experience, we expected to find large effect size (d = 0.8) between group I/group II. With a power of 80% (using t-test and alpha of 0.05). The allocation ratio N2/N1 is 2/1. The sample needed for the study was estimated to be 48 patients taking in our consideration 25% drop off. Finally, the total sample size was estimated 60 patients ( 40 patients for group I, 20 patients for group II ). 10
N = 60 patients.
Confidence interval = 95%
Alpha = 0.05
Power = 80%
The clinical data were recorded on a report form. These data were tabulated and analyzed using the computer program SPSS (Statistical package for social science) version 21((SPSS Inc, Chicago, IL, USA). The variables were tested using Chi-Squared (χ2) test for qualitative data, Mann Whitney U test for testing quantitative data, correlation coefficient test (Pearson test), multivariate logistic regression analysis, and the ROC (receiver operating characteristic) curves to detect validity of different markers for prediction of early hospital mortality. When p value was less than 0.05, it was considered significant.
Results
The mean age of the included patients was 62.1±10.7 years with 51.7% of them were females. Diabetes mellitus was reported in 30.0% of the included patients. The pulmonary primary site of infection was found in 56.7%. Also, the mean BMI of the studied patients was 24.9 ± 2.9 Kg/m2. The mean CRP, hemoglobin, and WBCs count were (105.8 ± 70.7, 10.3 ± 0.6, and 17.32 ± 9.6) respectively. The mean creatinine and urea levels were 3.4 ± 2.3 and 158.3 ± 124.9, respectively. Additionally, mean AST, ALT, PT, PAI-1, and SuPAR were 65.7 ± 32.4, 62.4 ± 24.5, 13.6 ± 7.9, 74.9± 6 1.3, and 11.3 ± 5.7, respectively, as shown in Table 1.
Table 1.
Baseline characteristics of studied patients (n=60).
| Item | Patients (n = 60) | |
|---|---|---|
| N | % | |
| Sex: no, % Male Female |
29 31 |
48.3 51.7 |
| Mean ± SD | Min-Max | |
| Age/Years | 64.90 ± 7.40 | 30–74 |
| BMI (kg/m2) | 24.91 ± 2.92 | 21–34 |
| No | % | |
| Comorbidities: Diabetes mellitus Hypertension |
18 14 |
30.0 23.3 |
| Primary site of infection: Pulmonary Cutaneous Digestive Urinary Articular |
34 11 10 41 |
56.7 18.3 16.7 6.7 1.7 |
| Mean ± SD | Min-Max | |
| Vital signs: MABP (mmhg) HR (beats/minutes) Temp. (oC) RR (cycles/minutes) |
79.1 ± 5.5 107.5 ± 8.5 37.3 ± 1.4 22.3 ± 2.6 |
67–98 95–120 27.8–38.4 19–28 |
| SOFA score | 7.2 ± 2.25 | 4–12 |
| CRP (mg/L) | 105.8 ± 70.7 | 2–454 |
| Hemoglobin (g/dl) | 10.3 ± 0.6 | 9.3–11.5 |
| WBCs (*103)/ml | 17.3 ± 9.6 | 2.8–43.8 |
| Creatinine (mg/dl) | 3.42 ± 2.30 | 0.2–8.5 |
| Urea (mg/dl) | 158.30 ± 124.93 | 8.3–434 |
| AST (u/l) | 65.72 ± 32.38 | 28–239 |
| ALT (u/l) | 62.37 ± 24.46 | 35–186 |
| PT (second) | 13.60 ± 1.29 | 12–16 |
| PAI-1 (pg/ml) | 80.75 ± 61.86 | 26–412 |
| SuPAR (pg/ml) | 11.29 ± 5.84 | 3.2–45 |
MABP: Mean arterial blood pressure is defined as the average pressure in the patients arteries during one cardiac cycle, MABP= SBP + 2 (DBP)/3; HR: heart rate; Temp.: temperature; RR: respiratory rate.
Respirator rate, SuPAR, PAI-1, and SOFA score were significantly higher among the non-survivor group than the survivor one (p < 0.05), all other variables were insignificant between the two groups as shown in Table 2.
Table 2.
Comparison between survivors and non-survivors regarding baseline characteristics.
| Variable | Survivors (n = 49) | Non - survivors (n = 11) | U Test | p Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Age/year | 64.24 ± 7.81 | 67.81 ± 4.31 | 1.57 | 0.12 | ||
| N | % | N | % | |||
| Sex Male Female |
25 24 |
51.0 49.0 |
4 7 |
36.4 63.6 |
X2 0.77 |
0.38 |
| BMI (kg/m2) | 25.03 ± 3.14 | 24.37 ± 1.66 | 0.26 | 0.80 | ||
| Blood pressure | 78.97 ± 5.82 | 79.63 ± 3.96 | 0.49 | 0.63 | ||
| Heart rate | 106.73 ± 8.42 | 110.64 ± 8.42 | 1.40 | 0.16 | ||
| Respiratory rate | 21.90 ± 2.46 | 23.91 ± 2.88 | 2.19 | 0.03* | ||
| Temperature | 37.29 ± 1.48 | 37.49 ± 0.51 | 0.05 | 0.96 | ||
| SOFA score | 6.49 ± 1.72 | 10.36 ± 1.50 | 4.66 | <0.001* | ||
| CRP (mg/L) | 103.39 ± 73.47 | 116.64 ± 58.42 | 0.34 | 0.35 | ||
| HB (g/dl) | 10.33 ± 0.55 | 10.18 ± 0.76 | 0.72 | 0.47 | ||
| WBCs (*103)/ml | 17.22 ± 10.39 | 17.74 ± 4.86 | 0.72 | 0.47 | ||
| Creatinine (mg/dl) | 3.49 ± 2.47 | 3.14 ± 1.32 | 0.17 | 0.86 | ||
| Urea (mg/dl) | 159.64 ± 135.82 | 152.35 ± 59.11 | 0.65 | 0.52 | ||
| AST (U/L) | 65.94 ± 35.08 | 64.73 ± 16.71 | 0.60 | 0.55 | ||
| ALT (U/L) | 62.57 ± 26.64 | 61.45 ± 11.07 | 0.59 | 0.55 | ||
| PT (second) | 13.47 ± 1.26 | 14.18 ± 1.33 | 1.66 | 0.10 | ||
| SuPAR (pg/ml) | 9.60 ± 3.13 | 18.8 ± 8.85 | 4.95 | 0.001* | ||
| PAI-1 (pg/ml) | 59.55 ± 28.98 | 175.18 ± 80.84 | 4.98 | <0.001* | ||
U = Mann Whitney U test, (*) significant.
BMI: body mass index; CRP: C-reactive protein; HB: hemoglobin; WBC: white blood cells; AST: Aspartate transaminase; ALT: Alanine transaminase; PT: prothrombin time; PAI-1: Plasminogen activator inhibitor-1; SuPAR: Soluble urokinase plasminogen activator receptor.
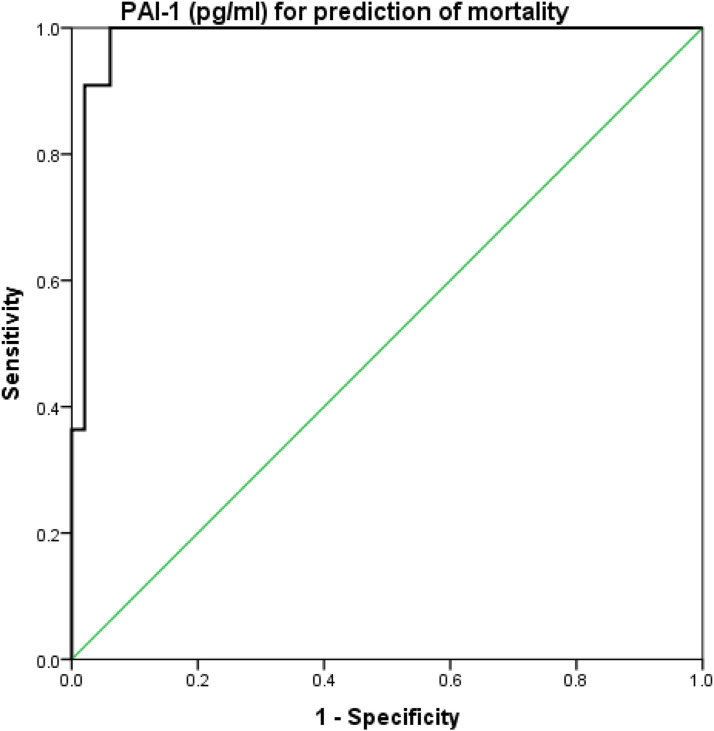
The SOFA score had a sensitivity of 90.9%, specificity of 87.8%, and accuracy of 0.90 at a cutoff value of ≥8.5 for predicting mortality (Figure 1). suPAR had a sensitivity of 100%, specificity of 95.9%, and accuracy of 0.94 at a cutoff value of ≥13.4 for predicting mortality (Figure 2). Finally, PAI-1 had a sensitivity of 100%, specificity of 93.9%, and accuracy of 0.95 at a cutoff value of ≥122.5 for predicting mortality (Figure 3), as shown in Table 3.
Figure 1.
ROC curve analysis of SOFA score for prediction of mortality.
Figure 2.
ROC curve analysis of suPAR for prediction of mortality.
Figure 3.
ROC curve analysis of PAI-1 for prediction of mortality.
Table 3.
Cutoff levels of SOFA score, SuPAR, and PAI-1 for predicting early sepsis mortality/7 days.
| Variable | AUC | Cutoff value | 95% CI | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % |
|---|---|---|---|---|---|---|---|---|
| SOFA score | 0.947 | ≥8.5 | 0.89–1.0 | 90.9 | 87.8 | 62.5 | 97.7 | 88.3 |
| SuPAR (pg/ml) | 0.981 | ≥13.4 | 0.94–1.0 | 100 | 95.9 | 84.6 | 100 | 94 |
| PAI-1 (pg/ml) | 0.983 | ≥122.5 | 0.95–1.0 | 100 | 93.9 | 78.6 | 100 | 95 |
Sens: sensitivity; Spec.: specificity; PPV: positive predictive value; NPV: negative predictive value.
The correlation coefficient between PAI-1 and other parameters, as shown in Table 4. There was a significant correlation according to PT (as shown also in Figure 4) and also there were significant positive correlations between PAI-1 and each of SOFA score and SuPAR level (as shown in Figure 5). Other parameters’ correlations to PAI-1 were insignificant.
Table 4.
Pearson’s correlation coefficient between PAI-1, SuPAR, and other parameters.
| Variable | PAI-1 (pg/ml) | suPAR (pg/ml) | ||
|---|---|---|---|---|
| (r) | p value | (r) | p value | |
| Age/year | 0.004 | 0.970 | −0.086 | 0.417 |
| BMI (kg/m2) | 0.140 | 0.184 | 0.051 | 0.630 |
| CRP (mg/L) | 0.191 | 0.070 | −0.033 | 0.756 |
| Hemoglobin (g/dl) | 0.0123 | 0.246 | −0.171 | 0.104 |
| WBCs (103)/ml a | 0.074 | 0.486 | 0.022 | 0.836 |
| Creatinine (mg/dl) | −0.145 | 0.169 | −0.005 | 0.959 |
| Urea (mg/dl) | −0.063 | 0.552 | 0.047 | 0.655 |
| AST (U/L) | 0.001 | 0.992 | −0.061 | 0.564 |
| ALT (UL) | 0.137 | 0.194 | −0.031 | 0.771 |
| PT (second) | 0.346 | 0.001 a | 0.483 | <0.001 a |
| SOFA score | 0.400 | <0.001 a | 0.389 | <0.001 a |
| SuPAR (pg/ml) | 0.327 | 0.002 a | --- | --- |
| PAI-1 (pg/ml) | ---- | ---- | 0.327 | 0.002 a |
asignificant.
BMI: body mass index CRP: C-reactive protein WBC: white blood cells; AST: Aspartate transaminase ALT: Alanine transaminase PT: prothrombin time PAI-1: Plasminogen activator inhibitor-1 SuPAR: Soluble urokinase plasminogen activator receptor.
Figure 4.
Correlation between PAI-1 and PT.
Figure 5.
Correlation between PAI-1and SuPAR.
SuPAR level had a significant correlation with each of PT (Figure 6) and SOFA score. Other parameters’ correlations to SuPAR were insignificant, as shown in Table 4.
Figure 6.
Correlation between SuPAR and PT.
Finally Table 5 shows the logistic regression for risk, and from that table PAI-1 and SuPAR blood levels had a significant statistical prediction for early sepsis mortality/7 days.
Table 5.
Logistic regression analysis for independent risk of early sepsis mortality/7 days.
| Variable | Wald X2 | p Value | OR | 95% CI |
|---|---|---|---|---|
| SOFA score | 1.12 | 0.33 | 1.19 | 0.67–3.91 |
| SuPAR (pg/ml) | 2.66 | 0.009 a | 2.10 | 1.05–12.8 |
| PAI-1 (pg/ml) | 2.3 | 0.02 a | 1.92 | 0.72–8.68 |
asignificant.
PAI-1: Plasminogen activator inhibitor-1; SuPAR: Soluble urokinase plasminogen activator receptor.
Discussion
Plasminogen activator inhibitor type 1 (PAI-1) is a 50-kDa glycoprotein of the serine protease inhibitor family. The primary role of PAI-1 in vivo is the inhibition of both tissue- and urokinase-type plasminogen activators. In addition to this function, PAI-1 acts as an acute-phase protein during acute inflammation. PAI1 is a pivotal player in the pathogenesis of sepsis, a complex clinical syndrome that results from a systemic inflammatory response. 11
The present study showed the mean age of the included patients was 62.1 ± 1 0.7 years and this agreed with the study by Mohamed et al., 12 who found (71.25%) of patients were males and (28.75%) were females. Maximum patients had belonged to the age group of 50–80 years of age. The mean age of the study population by Ref. 12 was 60.97 years. The study by Kim et al., 13 found the mean SOFA score was 8.0 ± 2.8. In addition, mean MPV was 8.64 fL at baseline and 8.96 fL at 72 h after ED admission. The main infection sites were the urinary tract (25.2%) and lung (24.1%) followed by the intra-abdominal cavity (22.0%). But, we found the pulmonary primary site of infection was in 56.7%, followed by the cutaneous site in 18.3%, and while the digestive site was reported in 16.7%. The mean BMI of the studied patients was 24.9 ± 2.9 Kg/m2.
Mohamed and his colleagues 12 found that type 2 diabetes mellitus and systemic hypertension were the major comorbidities present in the study population, both being present in 37 patients each (46.25%). Respiratory comorbidities, chronic liver, and kidney diseases along with heart diseases were also present in a significant number of patients. Fever was the most common presenting feature (72.50%) followed by breathlessness (43.75%), cough (32.50%), abdominal, and neurologic symptoms. Based on the presenting symptoms and clinical examination findings, the majority of the patients (66.25%) had respiratory tract as the suspected source of sepsis. For us, we had found diabetes mellitus was 30.0% of the included patients while 23.3% of them had hypertension.
According to the inflammatory labs’ assessment (mean CRP, hemoglobin, and WBCs count), mean creatinine and urea levels, mean AST, ALT, and PT showed that we are similar also to Mohamed et al., 12 who found that, 67.5% mortality among the patients with severe sepsis. Low platelet count, high CRP, and elevated levels of serum lactate along with need for invasive mechanical ventilation were found to be a clear predictor of mortality in severely septic patients. SOFA score of more than 8.5, at the time of admission to the ICU. Also, with Ghany et al., 14 found that, forty-four (19%) of 232 patients with baseline AST/ALT ratio >0.8 experienced clinical decompensation compared to 16 (6.7%) of 238 with baseline AST/ALT ratio ≤0.8. Within each stratum of baseline AST/ALT ratio, patients who had severe worsening (>15% increase between month 24 and baseline) had a higher rate of clinical decompensation.
Our study showed that respiratory rate, SuPAR, PAI-1, and SOFA score were significantly higher among the non-survivor group than the survivor one. While there was no significant difference between the two groups regarding age, sex, heart rate, temperature, SOFA score, CRP, HB, WBCs, creatinine, urea, AST, ALT, and PT. These results agreed with that reported by Kim et al., 13 as the non-survivors exhibited significantly higher SOFA score than did the survivors. Also according to they there were no significant differences in age, mean arterial pressure, WBC, Hb, serum creatinine, total bilirubin, RBC transfusion, and heparin use between the two groups. Also, Mohamed et al., 12 found that, none of the difference in mean values of liver enzymes, serum bilirubin, serum albumin, and international normalized ratio between the mortality and survivor groups was statistically significant.
While, the current findings disagreed with the study by Li et al., 15 who had found the patients who survived were more likely to have higher baseline levels of hemoglobin and serum albumin and lower breathing rates, lactate levels, platelet (PLT) counts, urea nitrogen, creatinine, eGFR, and cystatin-C (Cys-C) values than the patients who died. Also, Kim et al., 13 revealed that non-survivors exhibited significantly higher C-reactive protein (CRP) and lactate levels than did survivors, whereas body mass index (BMI); platelet count; estimated glomerular filtration rate (eGFR); and albumin, total cholesterol, and pH levels in non-survivors were significantly lower than those in survivors.
The current study revealed that the sensitivity of SOFA score for predicting early sepsis mortality was 90.9%, specificity of 87.8%, and accuracy of 0.90 at a cutoff value of ≥8.5. Also, the sensitivity of SuPAR for predicting mortality was 100%, specificity of 95.9%, and accuracy of 0.94 at a cutoff value of ≥13.4. While the sensitivity of PAI.1 was 100%, specificity of 93.9%, and accuracy of 0.95 at a cutoff value of ≥122.5. In this line, two studies by Koch et al. 16 and Loonen et al. 17 evaluated diagnostic accuracy of suPAR have shown specificity from 64–77%. Also, the current findings agreed with the study by López-Izquierdo et al. 18 found that for 28-day mortality, the qSOFA presented a cut-off of two points, with a sensitivity of 74.3 and specificity of 73.1. The SOFA score presented a cut-off of three points for 30-day mortality, with a sensitivity of 81.6 and a specificity of 76.5.
The current study revealed that, there was a significant positive correlation between SOFA score and each of suPAR and PAI-1. Also, there was a significant positive correlation between SuPAR level with PT, PAI-1. In addition, PAI-1 level significantly positively correlated with PT. This agreed with the study by Jalkanen et al., 19 found that the SuPAR and PAI-1 concentrations were higher in critically ill patients compared to healthy volunteers. SuPAR and PAI-1 concentrations were higher in critically ill patients compared to healthy volunteers. Another study by Silvestre et al., 20 found that, SOFA was independently associated with a higher risk of in-hospital mortality, 28-day mortality and 90-day mortality.
As mentioned above our study showed that SuPAR, PAI-1, and SOFA score were significant predictors to hospital mortality. This agreed with the study by Wingeyer et al., 21 found that in total, 76.4% of deaths but only 55.6% of surviving patients could be predicted with a SOFA score greater than 4 at time 0, with a global prediction of 65.4%. However, when they combined a SOFA of ≥ 4 and the presence of the PAI-1, in combination with plasma levels of PAI-1 ≥ 16 (UA/l), the global prediction rose to 71.9%, with a prediction of survival of 74.1% and a prediction of death of 69.4%. Another study by Vincent, 22 found that, invasive mechanical ventilation in patients with severe sepsis was identified to be an independent predictor of mortality. Previously, Prabhakaran et al., 23 and Sapru et al., 24 reported PAI-1 has been considered valuable in prognostication in patients with ARF. Previous reports indicate that PAI-1 levels are elevated in sepsis and VAP, and predict mortality and MOF.
On the other hand, Jalkanen et al., 19 reported that like other biomarkers, suPAR as a single biomarker is not strong enough for clinical decision-making. Also, PAI-1 was a poor prognostic marker for mortality or development of sepsis. The highest quartile of PAI-1 concentrations did not have predictive value for 90-day mortality or association with ALI/ARDS. There was a marked variation in suPAR in the healthy volunteers, Koch et al., 16 found the highest suPAR concentrations in healthy volunteers were lower than the concentrations of patients with an increased risk of poor outcome. PAI-1 levels in healthy volunteers were stable and low. Varied results may be due to different inclusion and exclusion criteria and different study samples.
Limitations
One limitation of this study is the limited number of patients as a developing country we have not simply had a registry for all patients and due to environmental and cultural reasons, many critically ill patients from old age not seeking hospital consultation.
Another limitation is the PAI-1 and SuPAR serum levels had been studied a lot in critically ill patients and not a novel hypothesis but to our knowledge, it is the first Egyptian study to elicit its level with early mortality of sepsis and we consider it a trial to use these markers as prognostic predictors not only a diagnostic.
Conclusion
Our study concluded that SuPAR and PAI-1 both can be used for predicting early mortality. Also, SOFA score, PAI-1, and suPAR were significant predictors of hospital morbidity and mortality. The sample size was relatively small, which may have decreased the statistical power of the results of the present study. Hence, additional studies with large sample sizes are required for further validation of the present results.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Approval of the study protocol was obtained by local Ethical Scientific Committee of Menoufia university institutional review board.
Informed consent: All participants were volunteers, and all of them signed a written informed consent with explaining the aim of study before the study initiation
ORCID iDs
Alaa Efat https://orcid.org/0000-0001-8148-3443
Aly Elkholy https://orcid.org/0000-0001-9927-7277
References
- 1.American College of Chest Physicians (1992) American college of chest physicians/society of critical care medicine consensus conference. Critical Care Medicine 20: 864–874. [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8): 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NKJ, et al. (2016) Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. American Journal of Respiratory and Critical Care Medicine 193(3): 259–272. [DOI] [PubMed] [Google Scholar]
- 4.Charchaflieh J, Rushbrook J, Worah S. et al. (2015) Activated complement factors as disease markers for sepsis. Disease Markers 2015: 382463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gawaz M, Vogel S, Pfannenberg C, et al. (2014) Implications of glycoprotein VI for theranostics. Thrombosis and Haemostasis 112(07): 26–31. [DOI] [PubMed] [Google Scholar]
- 6.Elias ACGP, Matsuo T, Grion CMC, et al. (2012) Incidence and risk factors for sepsis in surgical patients: a cohort study. Journal of Critical Care 27(2): 159–166. [DOI] [PubMed] [Google Scholar]
- 7.Zeng M, Chang M, Zheng H, et al. (2016) Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. The American Journal of Emergency Medicine 34(3): 375–380. [DOI] [PubMed] [Google Scholar]
- 8.Shankar-Hari M, Ambler M, Mahalingasivam V, et al. (2016) Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Critical care (London, England) 20(1): 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H, Zhou L-X, Ma H, et al. (2017) Soluble urokinase-type plasminogen activator receptor and urokinase-type plasminogen activator receptor contribute to chemoresistance in leukemia. Oncology Letters 14(1): 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdfelder E, Faul F, Buchner A. (1996) GPOWER: a general power analysis program. Behavior Research Methods, Instruments, & Computers 28(1): 1–11. [Google Scholar]
- 11.Bocskei RM, Meszaros M, Tarnoki AD, et al. (2020) Circulating soluble urokinase-type plasminogen activator receptor in obstructive sleep apnoea. Medicina 56(2): 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed AKS, Mehta AA, James P. (2017) Predictors of mortality of severe sepsis among adult patients in the medical intensive care unit. Lung India: Official Organ of Indian Chest Society 34(4): 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CH, Kim SJ, Lee MJ, et al. (2015) An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PloS one 10(3): e0119437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghany MG, Kim H-Y, Stoddard A, et al. (2011) Predicting clinical outcomes using baseline and follow-up laboratory data from the hepatitis C long-term treatment against cirrhosis trial. Hepatology 54(5): 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Wang M, Zhu B, et al. (2020) Prediction of median survival time in sepsis patients by the SOFA score combined with different predictors. Burns & Trauma 8: tkz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch A, Voigt S, Kruschinski C, et al. (2011) Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Critical care (London, England) 15(1): R63–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loonen AJM, de Jager CPC, Tosserams J, et al. (2014) Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PloS one 9(1): e87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Izquierdo R, Brio-Ibañez Pd, Martín-Rodríguez F, et al. (2020) Role of qSOFA and SOFA scoring systems for predicting in-hospital risk of deterioration in the emergency department. International Journal of Environmental Research and Public Health 17(22): 8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalkanen V, Yang R, Yang R, et al. (2013) SuPAR and PAI-1 in critically ill, mechanically ventilated patients. Intensive Care Medicine 39(3): 489–496. [DOI] [PubMed] [Google Scholar]
- 20.Silvestre J, Coelho L, Pereira JG, et al. (2018) suPAR in the assessment of post intensive care unit prognosis: a pilot study. Revista Brasileira de terapia intensiva 30(4): 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perés Wingeyer SD, Cunto ER, Nogueras CM, et al. (2012) Biomarkers in sepsis at time zero: intensive care unit scores, plasma measurements and polymorphisms in Argentina. Journal of infection in developing countries 6(07): 555–562. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Sakr Y, Reinhart K, et al. (2005) Is albumin administration in the acutely ill associated with increased mortality? Results of the SOAP study. Critical Care (London, England) 9: R745–R754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhakaran P, Ware LB, White KE, et al. (2003) Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology 285(1): L20–L28. [DOI] [PubMed] [Google Scholar]
- 24.Sapru A, Curley MAQ, Brady S, et al. (2010) Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive care medicine 36(1): 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]