Abstract
Ameloblastoma is a benign odontogenic tumor characterized by slow growth causing painless facial swelling. The tumor can behave locally aggressively, and may have direct destructive effects on the surrounding soft and hard tissues. This paper reports the unique case of a female patient with giant ameloblastoma of the mandible. Computed tomography (CT) revealed an enormous swelling of the left side of the face, resorption of the affected hemi-mandible, left maxilla, and tissues of the temporal, infratemporal, and pterygopalatine fossae. Pressure from the tumor resulted in displacement and destruction of the facial skeleton, upper aero-digestive tract structures, and some structures of the neck. The patient was treated by radical hemimandibulectomy with removal of the tumorous mass. Precise knowledge of the anatomical structures, and their locations and topographical relationships is required in the diagnosis and treatment plan for each surgical procedure in cases of giant ameloblastoma. CT imaging can be used to determine the extent and exact location of the lesion, revealing other important details that may help in selecting appropriate treatment.
Keywords: Ameloblastoma, mandible, facial skeleton, tissue destruction, computed tomography, hemimandibulectomy
Introduction
Ameloblastoma is a locally aggressive and destructive benign tumor. This tumor has the potential to grow to an enormous size, with resulting bone deformity, facial asymmetry, and displacement of the soft tissues and neurovascular structures. Ameloblastoma usually originates from remnants of the dental lamina and odontogenic epithelium in the mandible and maxilla.1,2 Generally, ameloblastoma typically occurs at approximately equal rates in both sexes between 30 and 60 years of age. The peak age at diagnosis is in the fifth decade of life, in the overall population, while the peak incidence in Europe, specifically, is reported in adults in the fifth and sixth decades of life.3,4 These differences are likely attributable to socioeconomic factors in specific countries.2–4
The objective of this case report was to highlight a giant destructive ameloblastoma. To the best of our knowledge, no similar cases describing such extensive changes in the facial skeleton and soft tissues have previously been reported in the European population.
Case report
A 60-year-old female patient was referred because of a massive swelling of the left side of her face and neck. The size of the swelling had increased over at least 10 to 15 years. The patient suffered no pain or problems with breathing and eating, although there was an obvious obstruction of the upper aero-digestive tract. Medical examination revealed a monstrous swelling (23.0 × 12.8 × 13.4 cm) on the left side of the face affecting the temporal, parotideomasseteric, zygomatic, buccal, and oral regions of the head, and the entire left half of the mandible, spreading to the anterior cervical region. Pathological masses filled most of the oral cavity, spreading from the left part of the mandible. Histopathological examination of a biopsy confirmed the presence of conventional “multicystic” ameloblastoma, with mixed, follicular, acanthomatous, and reticular growth patterns. Three-dimensional (3D) computed tomography (CT) virtual reconstruction and surgical planning were performed using the software program, SimPlant® OMS 10.1 (Materialise®, Leuven, Belgium). CT angiography was performed to examine the relationship of the major neck vessels to the tumor and to evaluate the potential microanastomosis options.
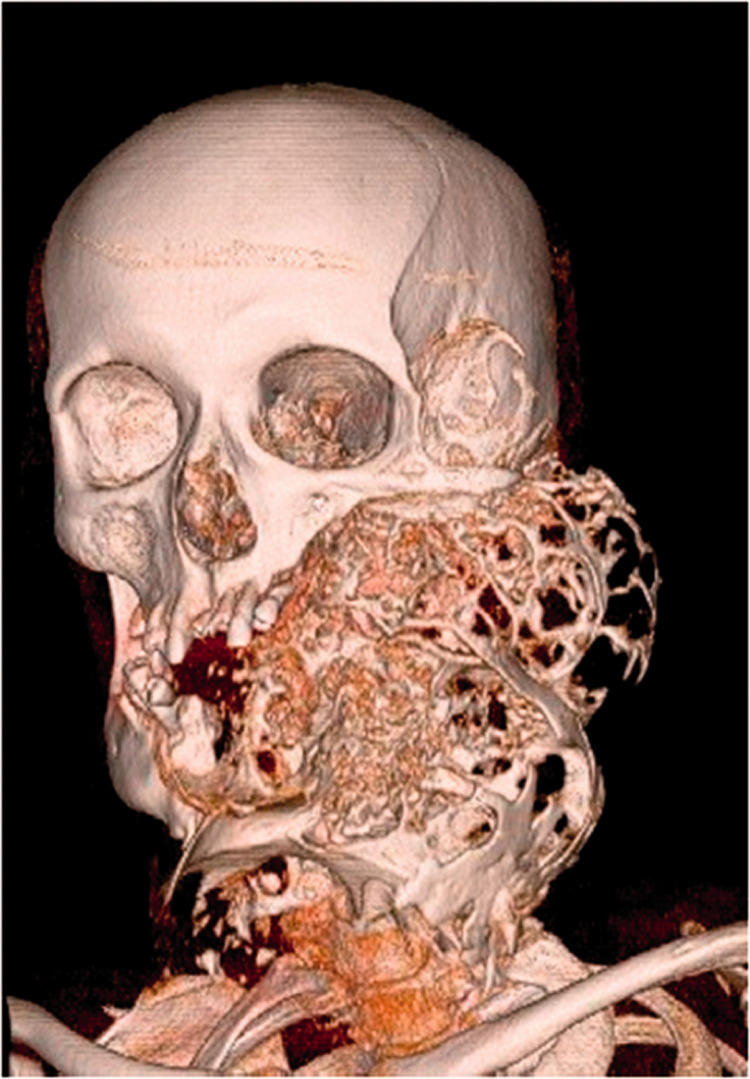
3D CT imaging revealed a large, well-defined lesion affecting the entire left side of the mandible, destroying and pressing on the left maxilla and growing into the infratemporal and temporal fossae (Figure 1). Axial CT images (Figures 2–4) showed the presence of a large multilocular radiolucent area on the left side of the head. Growing from the midline, the tumor formed numerous cavities of various sizes and shapes containing septations. The cavities, presenting as “soap bubbles”, were surrounded by tumorous bony septa with a thickness of up to 2.5 cm (Figure 2a–c). The left half of the mandible was completely consumed by the tumor (Figure 2b). The temporomandibular joint and associated structures on the left side were completely missing (Figure 2c). The alveolar bone of the lateral segment of the maxilla was also completely missing, and the body of the maxilla, including the sinus, was considerably reduced in volume but was not destroyed (Figure 3a, b). The size and shape of the zygomatic bone were severely affected by an abnormal growing tumorous mass extending from the infratemporal to temporal fossae and pushing anteriorly and laterally. The zygomatic arch was 0.09 to 0.12 cm thick compared with 0.17 to 0.41 cm on the healthy right side (Figure 3c). The cranial margin of the tumor was identified 4.15 cm above the zygomatic arch plane extending into the temporal fossa (Figure 3d).
Figure 1.
Three-dimensional (3D) computed tomographic (CT) reconstruction demonstrating a massive multilocular radiolucent lesion measuring 23.0 × 12.8 × 13.4 cm on the left side of the face and neck with a classical “soap-bubble” appearance (anterolateral view).
Figure 2.
Axial computed tomography (CT) images (a–c) showing (a) plane through the 3rd cervical vertebra (C3) and hyoid bone (HB); multicystic ameloblastoma (orange asterisk) with a thick solid septum inside the tumor (green arrow); (b) plane through the 2nd cervical vertebra (C2); body of the mandible (blue arrow), with oropharyngeal (OP) displacement clearly visible; (c) plane through the posterior cranial fossa (PCF) and mastoid cells (brown arrows); missing condylar process (yellow circle) compared with the healthy right side (yellow arrow).
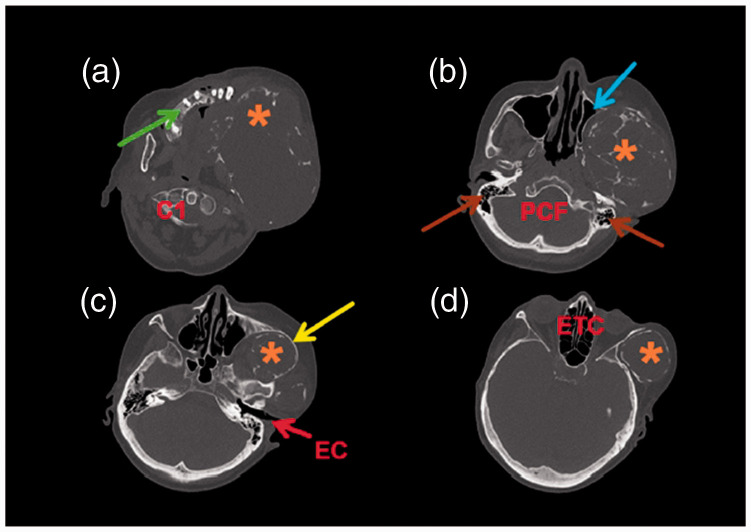
Figure 3.
Axial computed tomography (CT) images (a–d) (a) Plane through the 1st cervical vertebra (C1) showing the maxillary alveolar bone on the right side (green arrow) with the tumor resorbing the alveolar bone (orange asterisk) on the left side; (b) Plane through the posterior cranial fossa (PCF) and mastoid cells (brown arrows) showing the left maxillary sinus with preserved pneumatization (blue arrow) affected by expansion of the tumor (orange asterisk); (c) Plane through the ear canal (EC) showing the thin zygomatic arch (yellow arrow) and the tumor (orange asterisk) growing cranially from the infratemporal fossa. Facial asymmetry is clearly visible; (d) Plane through the ethmoidal cells (ETC) showing a giant tumorous mass (orange asterisk) in the temporal fossa (d).
Figure 4.
Axial computed tomography (CT) images (a–c) (a) Plane through the hyoid bone (HB) at the level of the body showing the external carotid artery (green arrow) and the internal jugular vein (yellow arrow) compressed by expansion of the tumor; (b) Plane through the HB at the level of the greater horn showing the external carotid artery (green arrow) and the internal jugular vein (yellow arrow) severely compressed by expansion of the tumor; (c) Plane through the 4th cervical vertebra (C4) showing the carotid bifurcation (blue arrow) and that the internal jugular vein is completely missing (orange circle).
Muscles as well as vessels and nerves located within the temporal, infratemporal, and pterygopalatine fossae were not identifiable. The tongue, sublingual tissue, and the oropharynx were pushed to the healthy side by the tumor, and the oropharynx was deviated 1.43 cm from the midline (Figure 2b).
CT also revealed morphological changes and compression of the cervical blood vessels. The carotid bifurcation was separated from the tumor by only 0.53-cm-thick soft tissue and a distance of only 1.18 cm from the midline (compared with 3.37 cm on the healthy side). The lumen of the internal carotid artery and the beginning of the external carotid artery (ECA) were preserved. The ECA had merged with the tumor at the level of the C2–C3 vertebrae (hyoid bone). The internal jugular vein was significantly cranially, with its lumen disappearing at the level of the C4 vertebra (Figure 4a–c).
Surgery was performed as radical hemimandibulectomy with removal of the tumorous mass in the left mandible via a submandibular approach. The patient’s postoperative recovery was uneventful and smooth. Neither clinical nor radiological evidence of tumor recurrence was found during the 3.5-year follow-up period. The patient refused mandibular reconstruction owing to the complicated nature and risks of treatment.
Discussion
Ameloblastoma, often referred to, classically, as an intraosseous lesion, is a slow-growing benign epithelial odontogenic tumor. Ameloblastoma accounts for approximately 10% of all odontogenic tumors in the mandible and maxilla, which comprise 90% of all cases of ameloblastoma. 4 Most cases of odontogenic tumors are diagnosed in young adults, with a median age of 10 to 38 years, with no significant sex predilection.5–8 In Asia and North America, the mean (± standard deviation) age of patients with ameloblastoma is 38.27 ± 17.78 years. 6 More specifically, in the Thai population, Kitisubkanchana et al. reported an average age of 34.9 years; 7 Soylu et al. reported an average age of 39.35 years in Turkey (Middle Anatolia); 8 and older sources report a mean of 36 years. 5
Approximately 80% of ameloblastomas occur in the mandible, usually in the posterior region, and represent only 1% of all oral/head and neck tumors.4,7–9 Patients are very often asymptomatic because tumor growth is intermittent, with no evidence of swelling. In cases of massive and rapid growth, aggressive tumors can cause severe disfigurement, facial asymmetry, pathological fractures, and functional impairment of neurovascular structures in affected and surrounding areas. 8 Tumors may erode through the cortical bone into adjacent soft tissues and impair facial expressions, speech, and mouth opening. Paraesthesia and pain are rare. 10 Up to 80% of cases are associated with an un-erupted mandibular third molar, and the remaining 20% occur in the maxilla, causing a grotesque facial appearance if the patient delays seeking treatment. The most common symptom is a painless facial swelling. Other symptoms include malocclusion, and tooth displacement and loosening.7,9,10
Histological examination remains the most sensitive tool for the differential diagnosis. However, clinical and radiological findings are important in the final diagnosis. 11 Many lesions, especially smaller lesions, are asymptomatic and may be detected incidentally during an intra-oral examination or by conventional dental intra-oral or panoramic X-ray. With larger tumors, CT and 3D volume rendering techniques or magnetic resonance imaging are useful and provide precise information in the assessment of the buccolingual expansion of the lesion and cortical bone destruction.9–11 Knowledge of the characteristic radiological imaging features narrows the differential diagnosis and is crucial in planning treatment.
The treatment of ameloblastoma includes various surgical methods, which are divided into two types: a conservative approach (type I), such as enucleation with curettage, and a radical approach (type II), with wide local excision and reconstruction. Considering the lesser aggressiveness of the tumor, enucleation is an adequate treatment for unicystic-type lesions, and radical treatment with bone resection is appropriate for aggressive multicystic ameloblastoma, which has a higher recurrence rate than with the unicystic vari-ant.5,8 Segmental hemimandibulectomy with wide margins and concurrent reconstruction are currently accepted as the treatment of choice in most cases. Segmental resection with 1- to 2-cm margins is therefore favored for solid or multicystic ameloblastoma.8,10 A recent quantitative and epidemiological study revealed that the risk of recurrence is three times higher with conservative treatment compared with resection. Solid ameloblastoma shows a high recurrence rate (60%–90%) with conservative treatment. 12
In conclusion, even though human anato-my has not changed over time, precise knowledge of anatomical structures and their clinical presentation is required in the diagnosis and treatment plan for current surgical procedures. Although ameloblastoma is a locally invasive neoplasm, delayed surgical treatment can lead to severe facial disfigurement; therefore, early referral to a specialist is the best approach.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211050185 for A case of giant ameloblastoma: destructive effect on the facial skeleton and soft tissues of the head and neck by Květuše Lovásová, Branislav Borza, Peter Kizek, Milan Almaši, David Kachlík and Ingrid Hodorová in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211050185 for A case of giant ameloblastoma: destructive effect on the facial skeleton and soft tissues of the head and neck by Květuše Lovásová, Branislav Borza, Peter Kizek, Milan Almaši, David Kachlík and Ingrid Hodorová in Journal of International Medical Research
Authors’ contributions: KL designed the study and created the figures; was responsible for the acquisition, analysis, and interpretation of the data for the work; and had final control and approval of the version to be published – PhD supervisor. BB wrote the manuscript and figure legends – PhD student and the oral surgeon who performed the operation. PK is a maxillofacial surgeon who performed the operation and was actively involved in obtaining the results data. MA is an otorhinolaryngologist who performed the operation and evaluated the patient. DK took part in critical revision of the manuscript and the anatomical relationships descriptions. IH was responsible for acquiring the relevant references and is the corresponding author.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Cultural and Educational Grant Agency of the Ministry of Education, Science, Research, and Sport of the Slovak Republic (MŠVVaŠ SR); grant number: KEGA 018UPJŠ-4/2021.
ORCID iDs: Květuše Lovásová https://orcid.org/0000-0003-0141-9762
Ingrid Hodorová https://orcid.org/0000-0002-7888-7719
Ethics statement
The present study was performed in accordance with the current laws in our country and with the written approval (number: 13N/2020) of the Scientific Ethical Committee of the Faculty of Medicine, Pavol Jozef Šafárik University in Košice, which are based on the World Medical Association Declaration of Helsinki.
Written informed consent was obtained from the patient for publication of this case report prior to submission of the manuscript, including the accompanying images.
The reporting of this study conforms to the CARE guidelines. 13
References
- 1.Effiom OA, Ogundana OM, Akinshipo AO, et al. Ameloblastoma: current etiopathological concepts and management. Oral Dis 2018; 24: 307–316. [DOI] [PubMed] [Google Scholar]
- 2.Rajaonarison Ny Ony N, Randriamarolahy A, Randrianjanahary OME, et al. Giant ameloblastoma. Clin Imaging 2012; 36: 146–148. [DOI] [PubMed] [Google Scholar]
- 3.Hendra FN, Van Cann EM, Helder MN, et al. Global incidence and profile of ameloblastoma: a systematic review and meta-analysis. Oral Dis 2020; 26: 12–21. [DOI] [PubMed] [Google Scholar]
- 4.Kreppel M andZöller J.. Ameloblastoma – clinical, radiological, and therapeutic findings. Oral Dis 2018; 24: 63–66. [DOI] [PubMed] [Google Scholar]
- 5.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol 1995; 31B: 86–99. [DOI] [PubMed] [Google Scholar]
- 6.Dhanuthai K, Chantarangsu S, Rojanawatsirivej S, et al. Ameloblastoma: a multicentric study. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 782–788. [DOI] [PubMed] [Google Scholar]
- 7.Kitisubkanchana J, Reduwan NH, Poomsawat S, et al. Odontogenic keratocyst and ameloblastoma: radiographic evaluation. Oral Radiol 2021; 37: 55–65. [DOI] [PubMed] [Google Scholar]
- 8.Soylu E, Bayındır S, Demirbaş AE, et al . How often are ameloblastomas in middle anatolia? a 13-year retrospective study. Ann Clin Anal Med 2020; doi:10.4328/ACAM.20275. Available at: http://www.bayrakol.org/en/about-acam/publish-online/item/2443-how-often-are-ameloblastomas-in-middle-anatolia-a-13-year-retrospective-study [Google Scholar]
- 9.Alves DBM, Tuji FM, Alves FA, et al. Evaluation of mandibular odontogenic keratocyst and ameloblastoma by panoramic radiograph and computed tomography. Dentomaxillofac Radiol 2018; 47: 20170288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida RAC, Andrade ES, Barbalho JC, et al. Recurrence rate following treatment for primary multicystic ameloblastoma: systematic review and meta-analysis. Int J Oral Maxillofac Surg 2016; 45: 359–367. [DOI] [PubMed] [Google Scholar]
- 11.Meng Y, Zhao YN, Zhang YQ, et al. Three-dimensional radiographic features of ameloblastoma and cystic lesions in the maxilla. Dentomaxillofac Radiol 2019; 48: 20190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarlabous M andPsutka D.. Treatment of mandibular ameloblastoma involving the mandibular condyle: resection and concomitant reconstruction with a custom hybrid total joint prosthesis and iliac bone graft. J Craniofac Surg 2018; 29: e307–e314. [DOI] [PubMed] [Google Scholar]
- 13.Gagnier JJ, Kienle G, Altman DG, et al. CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211050185 for A case of giant ameloblastoma: destructive effect on the facial skeleton and soft tissues of the head and neck by Květuše Lovásová, Branislav Borza, Peter Kizek, Milan Almaši, David Kachlík and Ingrid Hodorová in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211050185 for A case of giant ameloblastoma: destructive effect on the facial skeleton and soft tissues of the head and neck by Květuše Lovásová, Branislav Borza, Peter Kizek, Milan Almaši, David Kachlík and Ingrid Hodorová in Journal of International Medical Research