Abstract
Objectives
Numerous studies have elucidated that circulating tumor cells (CTCs) have significant prognostic value in various solid tumors. However, the prognostic value of CTCs in small cell lung cancer (SCLC) remains controversial. The current study was performed to investigate the prognostic significance of different time points of CTCs in SCLC.
Methods
PubMed, EMBASE, Web of Science, and Cochrane Library databases were retrieved for eligible studies. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to investigate the association between CTCs level and overall survival (OS) and progression-free survival (PFS) in SCLC. Furthermore, subgroup analyses, sensitivity analysis, Begg’s and Egger’s tests were also conducted.
Results
Sixteen cohort studies with 1103 participants were eligible for this meta-analysis. Our results revealed that higher pretreatment CTCs level was significantly correlated with worse OS in SCLC no matter CellSearch (HR, 2.95; 95%CI, 1.56-5.58; P = .001) or other methods (HR, 2.37; 95%CI, 1.13-4.99; P = .023) was used to detect CTCs. Higher pretreatment CTCs status detected by CellSearch was associated with shorter PFS (HR, 3.75; 95%CI, 2.52-5.57; P < .001), while there was no significant association when other methods were adopted to CTC detection (HR, 2.04; 95%CI, .73-5.68; P = .172). Likewise, we observed that higher post-therapy CTCs level detected by both CellSearch (HR, 2.99; 95%CI, 1.51-5.93; P = .002) and other methods (HR, 4.79; 95%CI, 2.03-11.32; P < .001) was significantly correlated with decreased OS in SCLC. However, higher post-therapy CTCs count detected by CellSearch was not correlated with worse PFS (HR, 1.80; 95%CI, .83-3.90; P = .135). Sensitivity analysis demonstrated that the pooled data were still stable after eliminating studies one by one. However, significant publication bias was observed between pretreatment CTCs level detected by CellSearch and OS of SCLC.
Conclusion
Dynamic monitoring of CTCs level could be a non-invasive and effective tool to predict the disease progression and prognosis in patients with SCLC.
Keywords: circulating tumor cells, small cell lung cancer, prognosis, meta-analysis
Introduction
As a high-grade neuroendocrine carcinoma, small cell lung cancer (SCLC) consists of about 15% of lung cancer cases. 1 SCLC is characterized by an exceptionally rapid doubling-time, strong predilection for early metastasis, and bleak prognosis. 1 Despite the advances that have been made in cancer screening, early diagnosis, and precise management in recent years, especially the promising antitumoral effect of immune checkpoint inhibitors (ICIs) in clinical utility, SCLC remains a more lethal disease than other solid tumors.2,3 Most SCLC patients have metastatic diseases at diagnosis, resulting in a median survival duration of less than one year for these individuals, and the 5-year survival rate staggering at 1% ∼ 5%.1,4-6 Therefore, it is crucial to explore early and effective biomarkers to predict tumor prognosis and monitor disease progression in these patients.
In recent decades, various techniques called “liquid biopsy” have emerged as novel tool and played pivotal roles in tracking evolutionary dynamics and heterogeneity of tumors, detecting the emergence of treatment resistance, and predicting disease recurrence.1,7 Circulating tumor cells (CTCs) are malignant cells that originated from either primary tumors or metastases and then migrate into the bloodstream, 8 represent the most prominent liquid biopsy marker. Because CTCs could represent a sampling of the patient’s live tumor cells, they serve as a unique biomarker, which is different from any existing cancer biomarkers. Numerous studies have reported that CTCs have significant prognostic value in various solid tumors, including breast cancer, colorectal cancer, head and neck cancer, esophageal cancer, and lung cancer.9-14 According to previously published studies, it is reported that the concentration of CTCs in SCLC is the highest among all solid tumors, which can mirror the high metastatic tendency of SCLC.1,15
Recently, although a growing number of original studies have evaluated the prognostic value of CTCs at different time points in SCLC, their results are inconsistent, and a vast majority of studies have a small sample size. For example, in a Phase II clinical trial conducted by Salgia et al, they found that pretreatment CTCs count detected by CellSearch was not significantly correlated with overall survival (OS) and progression-free survival (PFS) in SCLC. 16 Besides, Shen et al. also indicated that pretreatment folate receptor-positive CTCs detected by ligand-targeted polymerase chain reaction (LT-PCR) were not associated with OS in SCLC patients treated with first-line chemotherapy. 17 Thus, we performed this meta-analysis to comprehensively investigate the prognostic significance of CTCs in SCLC and provide evidence support to clinical practice in accordance with the Primary Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist. 18
Methods
Search Strategy
The present meta-analysis was performed according to the PRISMA reporting checklist. 18 We conducted a comprehensive literature search in PubMed, EMBASE, Web of Science, and Cochrane Library to identify potential studies, with research language being limited as English and research time being limited from inception to Feb 7, 2021. Both MeSH terms and free words were used to construct a search strategy. The MeSH terms for CTCs and SCLC were “Neoplastic Cells, Circulating” and “Small Cell Lung Carcinoma.” Ultimately, we used the following strategy searched for Title/Abstract in electronic databases: (((((((((((Neoplasm Circulating Cells) OR (Circulating Neoplastic Cells)) OR (Cells, Circulating Neoplastic)) OR (Neoplastic Cells, Circulating)) OR (Circulating Tumor Cells)) OR (Cells, Circulating Tumor)) OR (Tumor Cells, Circulating)) OR (Cells, Neoplasm Circulating)) OR (Circulating Cells, Neoplasm)) OR (CTCs)) OR (Neoplastic Cells, Circulating[MeSH])) AND (((((((Small Cell Lung Cancer) OR (Oat Cell Lung Cancer)) OR (Small Cell Cancer Of The Lung)) OR (Carcinoma, Small Cell Lung)) OR (Oat Cell Carcinoma of Lung)) OR (SCLC)) OR (Small Cell Lung Carcinoma[MeSH])). The protocol for this meta-analysis was also prospectively registered in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO), and the registration number for this article is CRD42021236128.
Eligibility Criteria
To keep our study accurate and reliable, the included studies had to meet the following prespecified inclusion criteria in accordance with PICO(S) principle. (a) Participant: patients were pathologically/cytologically diagnosed with SCLC; (b) Exposure: high/positive CTCs status at different time points of treatment (pretreatment, post-therapy, or at the time of disease progression); (c) Comparison: low/negative CTCs status at different time points of treatment; (d) Outcome: the association between CTCs level and OS/PFS in SCLC. Hazard ratios (HRs) and 95% confidence intervals (CIs) of OS and PFS were calculated using multivariate Cox regression analysis; and (e) Study design: retrospective or prospective cohort studies. Reviews, case reports, conference abstracts, and other irrelevant studies were excluded. Besides, studies were also excluded if the relationship between CTCs level and clinical outcome of SCLC was not reported or the survival difference between different CTCs level groups were compared using Kaplan-Meier survival curve or univariate Cox regression analysis. Furthermore, studies with less than 20 patients were excluded as well.
Data Extraction and Quality Assessment
Two investigators (AJ and HZ) independently extracted essential data into a previously designed standardized Excel sheet. The extracted data mainly including (a) study details (first author’s name, year of publication, study location, and study design); (b) participants characteristics of the included studies (total sample size, gender composition, age, disease stage, and treatment); (c) CTCs data (sampling time, detection methods, markers, and cut-off value of CTC), and (d) survival data (survival outcomes and HRs and their corresponding 95% CIs in multivariate Cox regression analysis).
The Newcastle-Ottawa Scale (NOS) was exploited for quality assessments for included studies in our meta-analysis as previously described.19,20 Studies with a total score of not less than seven were considered high-quality studies. Two investigators (NL and YM) conducted quality assessments independently. Any discrepancies regarding data extraction and quality assessment were resolved by discussion and consulting with another investigator (XF) for a consensus.
Statistical Analysis
In the current study, the Stata 12.0 software (Stata Corporation, College Station, Texas, USA) was adopted for statistical analysis, including pooled data analysis, assessment of heterogeneity, sensitivity analysis, and publication bias detection. The pooled HRs and 95% CIs were calculated to estimate the association between different time points of CTCs levels and the OS/PFS of patients with SCLC. Heterogeneity among included studies was assessed using Cochran’s Q test and I 2 test, with I 2 > 50% and P ≤ .05 being considered existing statistical heterogeneity. The random-effect model was utilized to pooled data analysis and forest plots generating. Besides, subgroup analyses were conducted to explore the potential sources of heterogeneity as well. The stability of the pooled HRs was further evaluated by sensitivity analysis, which by omitting each study one by one from the meta-analysis. Ultimately, Begg’s and Egger’s tests were adopted to detect whether there was publication bias.
Results
Study Selection and Study Characteristics
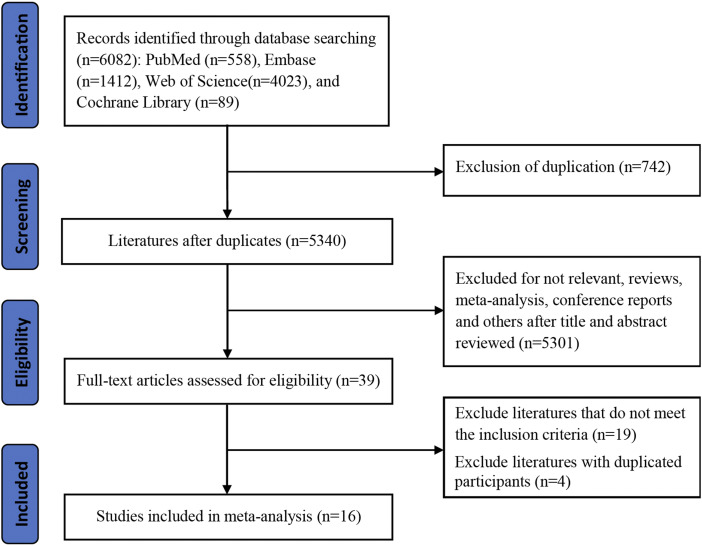
A total of 6082 studies were identified using the predefined search terms. After excluding duplicated records and irrelevant studies, 39 studies were selected into full-text reading. There were 19 studies unsatisfied with inclusion criteria and excluded after reading the full-text carefully. In addition, four studies with duplicated participants were also excluded. Overall, 16 studies with 1103 SCLC patients were eligible for this meta-analysis. The detailed information of the flow diagram of this study is presented in Figure 1.
Figure 1.
Flow chart of literature selection.
All of the included studies were published from 2012 to 2020. Of these, five studies were conducted in China,17,21-24 three studies were conducted in America,16,25,26 and there were two studies each from Japan,27,28 the Netherlands,29,30 the United Kingdom (UK),31,32 and Greece.33,34 The majority of the included studies (13, 81.3%) were prospective cohort studies. The sample size of the included studies varied from 25 to 112 (median value: 62.5). 62.2% (686) of patients were male, and all subjects ranged from 28 to 92 years old. Eleven studies enrolled patients with both limited-stage (LS) and extensive-stage (ES) disease,17,21,24,25,27-30,32-34 two studies enrolled patients with IIIB stage disease,22,23 and only one study enrolled LS-SCLC patients. 31 Regarding treatment regimen for the included SCLC patients, most studies administered chemotherapy/concurrent chemoradiotherapy (CCRT). Patients in one study received pazopanib for targeted therapy. 33 Sixteen studies evaluated the association between pretreatment CTCs level and the prognosis of SCLC,16,17,21-34 nine studies assessed the relationship between post-therapy CTCs level and the prognosis of SCLC,16,22-24,28,30,32-34 and only two studies detected CTCs level at the time of disease progression.33,34 Among the included studies, CellSearch platform was the most predominant method to detect CTCs,16,22,23,25,26,28-34 other applicable methods also included immunofluorescence (IF),21,33,34 PCR,17,24 Flow cytometry, 16 fluorescence in situ hybridization (FISH), 21 and OBP-401 assay (TelomeScan®). 27 When the CellSearch platform was used to detect CTCs level, 5 CTCs/7.5 mL was commonly adopted as CTCs cut-off value. Table 1 summarized the detailed characteristics of the included studies. After a rigorous quality assessment, we identified 12 high-quality studies (≥7 scores, Table 2).
Table 1.
Baseline characteristics of the included studies.
| Study | Year | Country | Study design | Sample size | Gender (M/F) | Age (range) | Disease stage | Treatment | Sampling time | Detection methods | Markers | CTCs Cut-off value | Survival outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang PP 21 | 2020 | China | RO | 108 | 97/11 | 65.3±10.1 | LS/ES | C/R/S | Baseline | FISH/IF | CEP8, CD45, DAPI | 25 | OS |
| Tay RY 31 | 2019 | UK | PO | 75 | 40/35 | 63 (77-45) | LS | C+R | Baseline | CellSearch | EpCAM, CK, CD45 | 15 CTCs/7.5 mL | OS/PFS |
| Messaritakis I 33 | 2018 | Greece | PO | 56 | 46/10 | 66 (39-82) | LS/ES | T | Baseline, Post-therapy, PD | CellSearch, IF | EpCAM, CK, Ki-67, Vimentin, M30, DAPI | 5 CTCs/7.5 mL; Positive/negative | OS/PFS |
| Messaritakis I 34 | 2018 | Greece | PO | 66 | 55/11 | 65 (44-82) | LS/ES | C/C+R | Baseline, Post-therapy, PD | CellSearch, IF | Bcl-2, CK, CD45, Vimentin, M30 | 5 CTCs/7.5 mL; Positive/negative | OS/PFS |
| Fu L 22 | 2018 | China | RO | 112 | 67/45 | 59 (49-69) | IIIB | C+R | Baseline, Post-therapy | CellSearch | EpCAM, CK, CD45 | 19.5 CTCs/7.5 mL | OS/PFS |
| Shen J 17 | 2017 | China | PO | 80 | 69/11 | 62 (41-81) | LS/ES | C | Baseline | LT-PCR | Folate receptor | 14 FU/3 mL | OS/PFS |
| Salgia R 16 | 2017 | America | PO | 89 | NA | NA | ES | C | Baseline, Post-therapy | CellSearch, Flow cytometry | CK, CD45, CXCR4, DAPI | 6 CTCs/7.5 mL; Positive/negative | OS/PFS |
| Aggarwal C 25 | 2017 | America | PO | 50 | 20/30 | 64 (43-80) | LS/ES | C/C+R | Baseline | CellSearch | EpCAM | 5 CTCs/7.5 mL | OS/PFS |
| Pore M 29 | 2016 | Netherlands | RO | 38 | 20/18 | 65 (60-71) | LS/ES | C/C+R | Baseline | CellSearch | EpCAM, CK, CD45, DAPI | 2 CTCs/7.5 mL | OS |
| Igawa S 27 | 2014 | Japan | PO | 30 | 28/2 | 69 (51-85) | LS/ES | C/C+R | Baseline | OBP-401 assays | GFP | 2 CTCs/7.5 mL | OS |
| Huang CH 26 | 2014 | America | PO | 25 | 19/6 | 63 (50-79) | ES | C | Baseline | CellSearch | EpCAM, CK, CD45, DAPI | 5 CTCs/7.5 mL | OS |
| Fu L 23 | 2014 | China | PO | 112 | 67/45 | 58.5 (49-69) | IIIB | C+R | Baseline, Post-therapy | CellSearch | EpCAM, CK, CD45 | 218 CTCs/7.5 mL | PFS |
| Shi WL 24 | 2013 | China | PO | 55 | 36/19 | 59 (41-75) | LS/ES | C/C+R | Baseline, Post-therapy | RT-PCR | CK19 | Positive/negative | OS/PFS |
| Naito T 28 | 2012 | Japan | PO | 51 | 44/7 | 67 (34-92) | LS/ES | C/C+R | Baseline, Post-therapy | CellSearch | EpCAM, CD45 | 8 CTCs/7.5 mL | OS |
| Hou JM 32 | 2012 | UK | PO | 97 | 43/54 | 68 (28-84) | LS/ES | C | Baseline, Post-therapy | CellSearch | EpCAM, CK, CD45, DAPI | 50 CTCs/7.5 mL | OS/PFS |
| Hiltermann TJN 30 | 2012 | Netherlands | PO | 59 | 35/24 | 64 (47-84) | LS/ES | C/C+R | Baseline, Post-therapy | CellSearch | EpCAM, CK, CD45, DAPI | 2 CTCs/7.5 mL | OS |
Abbreviations: UK, United Kingdom; RO, retrospective study; PO, prospective study; M, male; F, female; LS, limited-stage; ES, extensive-stage; C, chemotherapy; R, radiotherapy; S, surgery; T, targeted therapy; PD, progression disease; FISH, fluorescence in situ hybridization; IF, immunofluorescence; LT-PCR, ligand-targeted polymerase chain reaction; RT-PCR, reverse transcription-polymerase chain reaction; DAPI, 4′,6-diamidino-2-phenylindole; EpCAM, epithelial cell adhesion molecule; CK, Cytokeratin; CTC, circulating tumor cell, FU, folate receptor unit; NA, not available; OS, overall survival; PFS, progression-free survival.
Table 2.
Quality assessment conducted according to the NOS for all included studies.
| Selection | Comparability |
Outcome | Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | Score |
| Wang PP | ★ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 7 |
| Tay RY | ★ | ★ | ★ | ★ | ★★ | ★ | ☆ | ☆ | 7 |
| Messaritakis I | ☆ | ★ | ★ | ★ | ★★ | ★ | ☆ | ☆ | 6 |
| Messaritakis I | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Fu L | ★ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 7 |
| Shen J | ☆ | ★ | ★ | ★ | ★★ | ★ | ☆ | ★ | 7 |
| Salgia R | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Aggarwal C | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 6 |
| Pore M | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Igawa S | ☆ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 6 |
| Huang CH | ★ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ☆ | 6 |
| Fu L | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 8 |
| Shi WL | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Naito T | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ☆ | 7 |
| Hou JM | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Hiltermann TJN | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 8 |
Abbreviations: NOS, Newcastle-Ottawa Scale; ★, represents 1 score; ☆, represents no score.
Pretreatment CTCs Level and OS
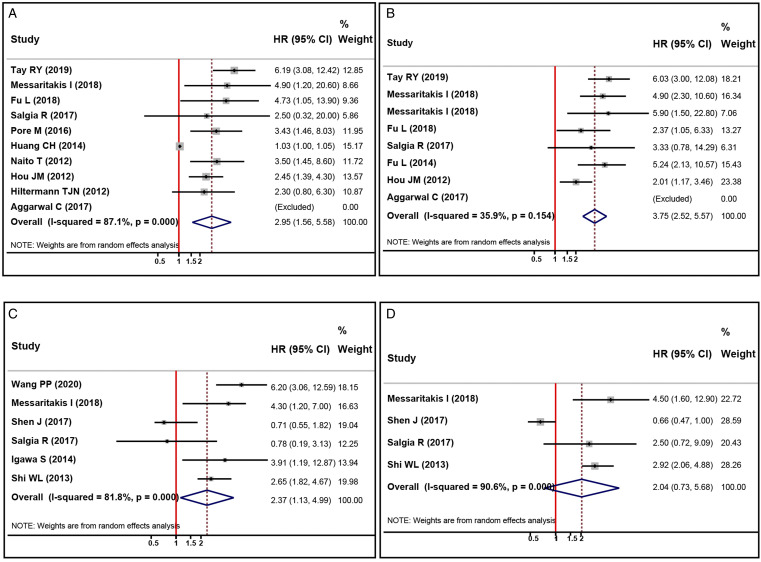
Among the included studies, 10 studies reported HRs and 95%CIs of pretreatment CTCs level detected by CellSearch and OS of SCLC,.16,22,25,26,28-32,34 In comparison, six studies provided the relationship between pretreatment CTCs status detected by other methods and OS of SCLC.16,17,21,24,27,34 Therefore, we performed pooled data analysis to evaluate the association between pretreatment CTCs level and OS of SCLC according to different CTCs detection methods (CellSearch vs. other methods). The pooled results indicated that elevated/positive pretreatment CTCs status was significantly correlated with worse OS in SCLC no matter CellSearch (HR, 2.95; 95%CI, 1.56-5.58; P=.001, Figure 2(A)) or other methods (HR, 2.37; 95%CI, 1.13-4.99; P=.023, Figure 2(B)) was adopted to detect CTCs. We also observed significant statistical heterogeneity between different studies in the above analyses (I 2 for CellSearch: 87.1%, I 2 for other methods: 81.8%, respectively). Subsequently, subgroup analyses were exploited to explore the potential sources of heterogeneity and evaluate the prognostic significance of CTCs status in different subgroups. First, we conducted subgroup analyses (including publication year, study design, region, sample size, treatment, CTCs cut-off value, and NOS score) to assess the potential effect of pretreatment CTCs detected by CellSearch on OS in SCLC (Table 3). We observed that the statistical heterogeneity was reduced after subgroups were stratified by region and NOS score, indicating that the results in these subgroups are stable (Table 3). Besides, the results showed that higher CTCs level was correlated with worse OS in SCLC treated with CCRT (HR, 5.83; 95%CI, 3.15-10.76; P<.001, Table 3) and chemotherapy/CCRT (HR, 3.29; 95%CI, 2.00-5.40; P<.001, Table 3), while there was no significant association between elevated CTCs level and OS in SCLC who treated with chemotherapy alone (HR, 1.59; 95%CI, .74-3.40; P=.231, Table 3). Furthermore, the subgroup analysis results also revealed that higher CTCs level was not associated with worse OS in SCLC when 5 CTCs/7.5 mL (HR, 1.90; 95%CI, .43-8.11; P=.323, Table 3) was set as the cut-off value. However, higher CTCs level was significantly correlated with decreased OS in SCLC when >5 CTCs/7.5 mL (HR, 3.62; 95%CI, 2.42-5.40; P<.001, Table 3) and 2 CTCs/7.5 mL (HR, 2.92; 95%CI, 1.51-5.63; P=.001, Table 3) were used as cut-off values. The results of other subgroups confirmed that higher pretreatment CTCs level detected by CellSearch was significantly correlated with unfavorable OS in SCLC, as summarized in Table 3.
Figure 2.
Forest plots of the association between pretreatment CTCs status and prognosis in patients with SCLC. (A, B) the impact of higher pretreatment CTCs level detected by CellSearch (A) and other methods (B) and OS; (C, D) the impact of higher pretreatment CTCs level detected by CellSearch (C) and other methods (D) on PFS. CTCs, circulating tumor cells; SCLC, small cell lung cancer; OS, overall survival; PFS, progression-free survival.
Table 3.
Subgroup analyses of the potential effects of pretreatment CTCs detected by CellSearch on OS and PFS in SCLC patients.
| Variables | OS | PFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test of association | Test of heterogeneity | Test of association | Test of heterogeneity | |||||||
| No. of studies | Pooled-HR (95% CI) | P-value | I 2 | P-value | No. of studies | Pooled-HR (95% CI) | P-value | I 2 | P-value | |
| Published year | ||||||||||
| ≥2017 | 5 | 5.36 (3.11-9.23) | <.001 | .0% | .864 | 6 | 4.47 (2.97-6.72) | <.001 | .0% | .558 |
| <2017 | 5 | 2.19 (1.13-4.23) | .020 | 84.7% | <.001 | 2 | 3.10 (1.12-7.89) | .018 | 73.5% | .052 |
| Study design | ||||||||||
| PO | 8 | 2.72 (1.33-5.58) | .006 | 87.8% | <.001 | 7 | 4.05 (2.60-6.31) | <.001 | 40.9% | .132 |
| RO | 2 | 3.78 (1.86-7.70) | <.001 | .0% | .684 | 1 | 2.37 (.97-5.82) | .060 | — | — |
| Region | ||||||||||
| Europe | 5 | 3.46 (2.30-5.19) | <.001 | 19.0% | .294 | 4 | 3.99 (2.15-7.41) | <.001 | 60.9% | .053 |
| Asia | 2 | 3.86 (1.85-8.03) | <.001 | .0% | .707 | 2 | 3.62 (1.67-7.87) | .001 | 40.2% | .196 |
| North America | 3 | 1.03 (1.01-1.06) | .017 | .0% | .401 | 2 | 3.33 (.78-14.25) | .105 | — | — |
| Sample size | ||||||||||
| <63 | 5 | 2.15 (.96-4.79) | .011 | 82.6% | .061 | 2 | 4.90 (2.28-10.52) | <.001 | — | — |
| ≥63 | 5 | 3.77 (2.42-5.88) | .011 | 12.4% | <.001 | 6 | 3.59 (2.26-5.69) | <.001 | 41.8% | .127 |
| Treatment | ||||||||||
| C+R | 2 | 5.83 (3.15-10.76) | <.001 | .0% | .719 | 3 | 4.45 (2.60-7.63) | <.001 | 27.9% | .250 |
| C/C+R | 5 | 3.29 (2.00-5.40) | <.001 | .0% | .851 | 2 | 5.90 (1.51-23.00) | .011 | — | — |
| T | — | — | — | — | — | 1 | 4.90 (2.28-10.52) | <.001 | — | — |
| C | 3 | 1.59 (.74-3.40) | .231 | 79.5% | .008 | 2 | 2.14 (1.29-3.55) | .003 | .0% | .524 |
| CTCs cut-off value | ||||||||||
| >5 CTCs/7.5 mL | 5 | 3.62 (2.42-5.40) | <.001 | 9.1% | .355 | 5 | 3.42 (2.06-5.67) | <.001 | 49.3% | .096 |
| 5 CTCs/7.5 mL | 3 | 1.90 (.43-8.11) | .323 | 79.4% | .032 | 3 | 5.12 (2.63-9.97) | <.001 | .0% | .816 |
| 2 CTCs/7.5 mL | 2 | 2.92 (1.51-5.63) | .001 | .0% | .558 | — | — | — | — | — |
| NOS score | ||||||||||
| ≥7 | 8 | 3.47 (2.53-4.75) | <.001 | .0% | .628 | 6 | 3.59 (2.26-5.69) | <.001 | 41.8% | .127 |
| <7 | 2 | 1.03 (1.01-1.06) | .018 | — | — | 2 | 4.90 (2.28-10.52) | <.001 | — | — |
| Total | 10 | 2.95 (1.56-5.58) | .001 | 87.1% | .001 | 8 | 3.75 (2.52-5.57) | <.001 | 35.9% | .154 |
Abbreviations: SCLC, small cell lung cancer; CTC, circulating tumor cell; OS, overall survival; PFS, progression-free survival; HR, Hazard ratio; CI, confidence interval; PO, prospective study; RO, retrospective study; C, chemotherapy; R, radiotherapy; T, targeted therapy; NOS, Newcastle-Ottawa Scale.
We then performed subgroup analyses to assess the potential effects of pretreatment CTCs detected by other methods on OS in SCLC (Table S1). Unlike the previous subgroup stratification, we added CTCs’ detection methods as a new subgroup to explore the effects on OS when different methods were used to detect pretreatment CTCs. We observed that except for subgroup analysis performed by treatment, there was still higher heterogeneity in other subgroups (Table S1). Regarding the detailed CTCs’ detection methods, the results indicated that positive CTCs status at baseline detected by IF was significantly correlated with unfavorable OS in SCLC (HR, 5.37; 95%CI, 3.09-9.33; P<.001, Table S1). Nevertheless, there was no statistical difference between pretreatment CTCs status and OS of SCLC when PCR (HR, 1.40; 95%CI, .39-5.04; P=.611, Table S1) and other methods (HR, 1.82; 95%CI, .38-8.84; P=.455, Table S1) were used to detect CTCs.
Pretreatment CTCs Level and PFS
A total of eight studies evaluated the relationship between pretreatment CTCs level detected by CellSearch and PFS of SCLC.16,22,23,25,31-34 The heterogeneity test suggested mild heterogeneity among these studies (I 2 = 35.9%, Figure 2(C)). The pooled data indicated that elevated pretreatment CTCs level detected by CellSearch was correlated with worse PFS in SCLC (HR, 3.75; 95%CI, 2.52-5.57; P<.001, Table 3). Furthermore, we also performed subgroup analyses to evaluate the potential effect of pretreatment CTCs detected by CellSearch on PFS in SCLC (Table 3). The results of subgroup analyses suggested that higher pretreatment CTCs level detected by CellSearch was not significantly associated with worse PFS in SCLC in retrospective studies (HR, 2.37; 95%CI, .97-5.82; P=.060, Table 3) and studies that were conducted in North America (HR, 3.33; 95%CI, .78-14.25; P=.105, Table 3). However, the results in other subgroups indicated that SCLC patients with higher pretreatment CTCs levels detected by CellSearch also had decreased PFS, as summarized in Table 3.
Four studies investigated the relationship between pretreatment CTCs level detected by other methods and PFS of SCLC.16,17,24,34 We observed that there was significant heterogeneity among these studies (I 2 = 90.6%, Figure 2(D)). The random-effect model was adopted for pooled data analysis. However, the results indicated no significant association between pretreatment CTCs level detected by other methods and PFS of SCLC (HR, 2.04; 95%CI, .73-5.68; P=.172, Figure 2(D)).
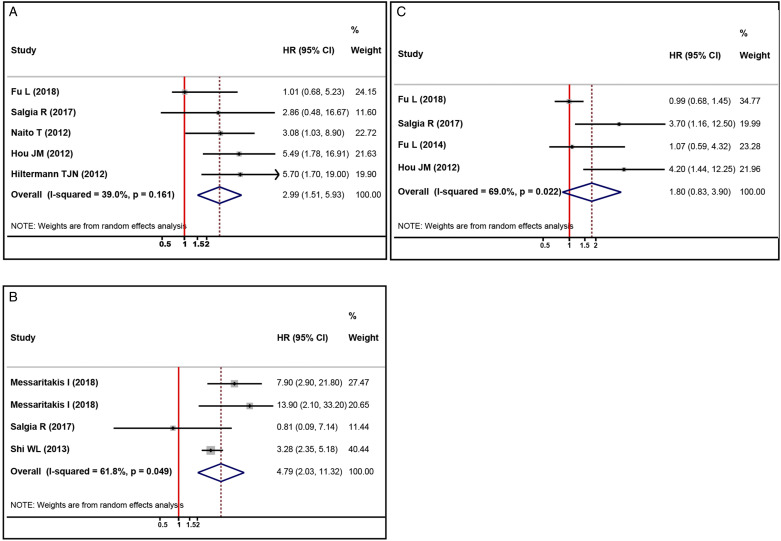
Post-Therapy CTCs Level and OS
Five studies provided HRs and 95%CIs of post-therapy CTCs level detected by CellSearch and OS of SCLC.17,28,30,32,34 We observed that there was mild heterogeneity among these studies (I 2 = 39.0%, Figure 3(A)). The pooled data suggested that higher post-therapy CTCs level detected by CellSearch was correlated with worse OS in SCLC (HR, 2.99; 95%CI, 1.51-5.93; P=.002, Figure 3(A)). Likewise, we performed subgroup analyses to evaluate the prognostic significance of CTCs status in different subgroups (Table S2). Consistent with the pooled data analysis, we found that higher post-therapy CTCs level detected by CellSearch was correlated with poor OS in SCLC no matter >5 CTCs/7.5 mL (HR, 2.55; 95%CI, 1.17-5.55; P=.019) or 2 CTCs/7.5 mL (HR, 5.70; 95%CI, 1.70-19.06; P=.005) was used as cut-off value. Nevertheless, inconsistent results were observed in other main subgroups (Table 3).
Figure 3.
Forest plots of the association between post-therapy CTCs status and prognosis in patients with SCLC. (A, B) the impact of higher post-therapy CTCs level detected by CellSearch (A) and other methods (B) on OS; (C) the impact of higher post-therapy CTCs level detected by CellSearch on PFS. CTCs, circulating tumor cells; SCLC, small cell lung cancer; OS, overall survival; PFS, progression-free survival.
Four studies reported the association between post-therapy CTCs status detected by other methods and OS in SCLC,16,24,33,34 with significant heterogeneity being observed among these studies (I 2 = 61.8%, Figure 3(B)). The pooled data revealed that positive post-therapy CTCs status detected by other methods was significantly correlated with decreased OS in SCLC (HR, 4.79; 95%CI, 2.03-11.32; P<.001, Figure 3(B)).
Post-Therapy CTCs Level and PFS
Among the included studies, four studies evaluated the relationship between post-therapy CTCs levels detected by CellSearch and PFS of SCLC.16,22,23,32 The heterogeneity test result suggested significant heterogeneity among these studies (I 2 = 69.0%, Figure 3(C)). The pooled data analysis indicated that post-therapy CTCs level detected by CellSearch was not significantly correlated with PFS of SCLC (HR, 1.80; 95%CI, .83-3.90; P=.135, Figure 3(C)). Because only two studies reported the HRs and 95%CIs between post-therapy CTCs status detected by other methods and PFS of SCLC,16,24 pooled data analysis was not performed.
CTCs After PD and OS
We found only two studies evaluated the association between CTCs level at the time of disease progression (PD) and OS of SCLC. Therefore, we did not perform pooled data analysis to further investigate their relationship.
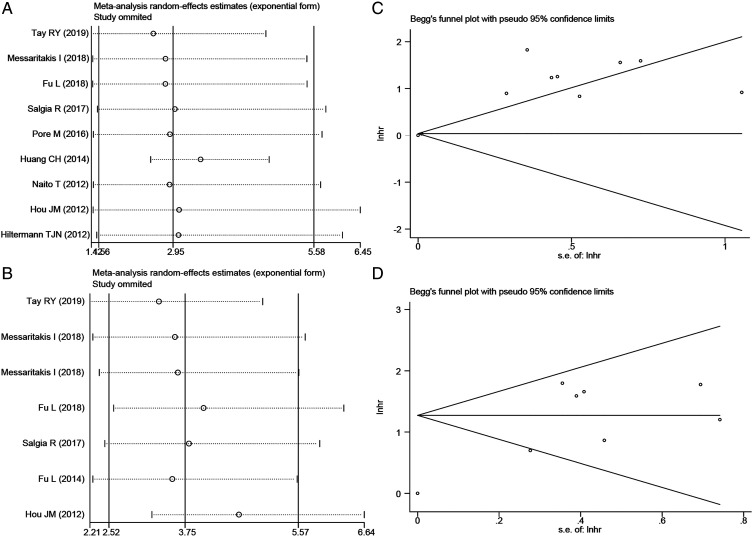
Sensitivity Analysis and Publication Bias
Sensitivity analysis was adopted to assess the impact of a single study on the overall results after studies were removed one by one. It demonstrated that the pooled data were still stable after eliminating studies one by one (Figure 4(A) and (B), Figure S1A, and Figure S2A). Furthermore, Begg’s and Egger’s tests were adopted to detect whether there was significant publication bias. Accordingly, we observed a significant publication bias between pretreatment CTCs level detected by CellSearch and OS of SCLC (P = .175 for Begg’s test, P = .001 for Egger’s test, respectively) (Figure 4(C)). However, we did not find any publication bias in other analyses (Figure 4(D), Figure S1B, and Figure S2B).
Figure 4.
Sensitivity analyses and funnel plots of the impact of higher pretreatment CTCs status detected by CellSearch on prognosis in patients with SCLC. (A, B) sensitivity analyses of the impact of higher pretreatment CTCs level detected by CellSearch on OS (A) and PFS (B); (C, D) funnel plots of the impact of higher pretreatment CTCs status detected by CellSearch on OS (C) and PFS (D). CTCs, circulating tumor cells; SCLC, small cell lung cancer; OS, overall survival; PFS, progression-free survival.
Discussion
Although SCLC consists of 15% of all lung cancer cases, most patients were diagnosed with ES disease and had a bleak prognosis. 1 Even though SCLC patients are initially exceptionally responsive to cytotoxic therapies, they are predisposed to early recurrence and widespread metastasis due to the highly aggressive characteristic of SCLC, thus resulting in an overall 5-year survival rate of less than 8%. Therefore, it is urgent to explore early and effective biomarkers that can monitor disease progression and early recurrence and predict clinical outcomes in these patients. Over recent years, numerous studies have elucidated that CTCs have pivotal roles in cancer early detection, recurrence monitoring, and prognosis stratification. 8 According to a meta-analysis performed by Zhang and his colleagues in 2014, they indicated that the presence of CTCs is significantly correlated with shorter OS and PFS in SCLC. 35 However, they did not consider the dynamic change and detection methods of CTCs in the analysis. Besides, they also included studies that investigated the association of CTCs level and prognosis of SCLC through univariate analysis, which may make pooled data unreliable. Recently, a similar study conducted by Foy et al. showed that higher pretreatment CTC counts are a negative independent prognostic factor in SCLC when considered as a continuous variable or dichotomised counts of ≥15 or ≥50. 36 Nevertheless, this study only included studies from four European cancer centers and they only investigated the relationship between pretreatment CTCs level and patients’ clinical outcomes. Therefore, we conducted the current meta-analysis to comprehensively investigate the prognostic significance of CTCs in SCLC and provide evidence support to clinical practice.
In the present study, a total of 16 studies with 1103 SCLC patients were included in the final pooled data analysis after rigorous literature screening. First, we investigated the association between pretreatment CTCs level and OS in SCLC. We observed that elevated/positive CTCs status prior treatment was significantly correlated with shorter OS in SCLC, no matter the CTCs were detected by CellSearch (HR, 2.95; 95%CI, 1.56-5.58; P=.001) or other methods (HR, 2.37; 95%CI, 1.13-4.99; P=.023). Second, we explored the correlation between pretreatment CTCs level and PFS in SCLC, which indicated that elevated pretreatment CTCs level detected by CellSearch was significantly correlated with worse PFS in SCLC (HR, 3.75; 95%CI, 2.52-5.57; P<.001). However, we observed no significant association between pretreatment CTCs status detected by other methods and PFS in SCLC (HR, 2.04; 95%CI, .73-5.68; P=.172). Third, we assessed whether post-therapy CTCs level was associated with OS in SCLC. The results demonstrated that elevated/positive CTCs status after treatment was significantly correlated with decreased OS in SCLC, no matter the CTCs were detected by CellSearch (HR, 2.99; 95%CI, 1.51-5.93; P=.002) or other methods (HR, 4.79; 95%CI, 2.03-11.32; P<.001). Subsequently, we performed pooled data analysis to evaluate the association between post-therapy CTCs level and PFS in SCLC, which indicated no significant association between post-therapy CTCs level detected by CellSearch and PFS in SCLC (HR, 1.80; 95%CI, .83-3.90; P=.135). To our regret, due to only two studies assessed the relationship between CTCs level at the time of disease progression and OS in SCLC, we did not conduct pooled data analysis. Ultimately, sensitivity analysis demonstrated that the pooled data were still stable after eliminating studies one by one. However, we observed a significant publication bias between pretreatment CTCs level detected by CellSearch and OS of SCLC. This may be because some studies that reported no correlation between CTCs and OS may not have been published.
CTCs were initially mentioned by Prof. Ashworth in 1869. 37 As the most predominant liquid biopsy marker among the analytes in the peripheral blood of cancer patients, CTCs play crucial roles in tracking evolutionary dynamics and heterogeneity of tumors, detecting the emergence of treatment resistance, and predicting disease recurrence.1,7 Unlike conventional tissue biopsy, CTCs detection is a non-invasive test that can provide a “moving picture” of longitudinal tumor progression and obtain a comprehensive understanding of the heterogeneous tumor cells throughout the body.8,38 As mentioned before, although numerous studies have indicated that higher CTCs levels were correlated with worse prognosis and early disease recurrence among the vast majority of solid tumors, the prognostic value of CTCs in SCLC remains controversial. It could be attributed to the fact that different detection methods, different sampling times, and unstandardized cut-off values were used in previous studies. Regulation of tumor biomarker tests is a sophisticated process, and even tests approved by the Food and Drug Administration (FDA) may not have established clinical utility. As so far, the CellSearch platform, a CTCs detection method based on immunological assay with the epithelial cell adhesion molecule (EpCAM), is the only one to date that is approved by FDA for CTC detection in metastatic breast cancer, prostate cancer, and colorectal cancer. 38 In our study, it was the most common method for CTC enumeration in SCLC. We observed that except for pooled data analysis of the association between post-therapy CTCs detected by CellSearch and PFS, higher CTCs count (baseline and after treatment) was significantly correlated with shorter OS/PFS in SCLC. It suggested that dynamic monitoring of CTCs level via CellSearch could predict clinical outcome in SCLC effectively. As we all know, there was no standardized cut-off value for survival analysis when CTCs were detected by the CellSearch platform. According to the currently included studies, 5 CTCs/7.5 mL and >5 CTCs/7.5 mL were the main cut-off values for survival analysis in SCLC. In this study, we also performed subgroup analyses based on different CTCs cut-off values to investigate its effect on pooled data analysis. Interestingly, we observed that higher pretreatment CTCs level detected by CellSearch was not correlated with shorter OS in SCLC while 5 CTCs/7.5 mL was used as the cut-off point. On the contrary, higher pretreatment CTCs level detected by CellSearch was significantly correlated with shorter OS in SCLC while 2 CTCs/7.5 mL and >5 CTCs/7.5 mL were used as cut-off points. Considering the small sample size and significant heterogeneity between these studies, further large-scale and prospective studies are urgently needed to explore the optimal cut-off values for survival analysis when CellSearch was adopted to detect CTCs in patients with SCLC.
Although it has passed more than a decade since FDA approved the clinical application of the CellSearch platform for CTC detection, it has not been widely utilized in clinical practice, especially in recent tumor-resistant studies. 38 This can be explained by the fact that the platform cannot detect mesenchymal CTCs with downregulated EpCAM and/or cytokeratin (CK) expression. Recently, a growing number of studies have emerged to address the above issue by detecting CTCs with different phenotypes, such as proliferating phenotype (CK+/Ki67+), apoptotic phenotype (CK+/M30+), and Epithelial-to-Mesenchymal Transition (EMT; CK+/Vim+ or CK+/TWIST+) phenotype. 33 Furthermore, other methods were also exploited to detect CTCs in SCLC in recent years. For example, in a previously published study, Shen et al. reported that higher pretreatment CTCs level detected by LT-PCR was significantly correlated with shorter PFS in patients with SCLC who received chemotherapy. 17 Besides, Igawa et al also investigated the prognostic value of CTCs detected by the OBP.401 assay in SCLC. They observed that patients with >2 CTCs/7.5 mL at baseline had unfavorable OS than those with lower CTCs count. 27 In the present study, we evaluated the association between different time points of CTCs detected by other methods and prognosis in SCLC. We found that higher/positive pretreatment CTCs status was significantly correlated with worse OS in SCLC. Subsequently, subgroup analyses also demonstrated that pretreatment CTCs could be a prognostic biomarker in SCLC when IF was adopted to detect CTCs. At the same time, there was no statistical significance when PCR and other methods were used. Besides, we also observed that higher/positive CTCs status after treatment was also associated with decreased OS in SCLC when other methods were adopted to detect CTCs. However, there was no significant correlation between pretreatment CTCs and PFS in SCLC when other methods were adopted to detect CTCs.
This meta-analysis systematically evaluated the prognostic value of different time points of CTCs and the prognosis in patients with SCLC. Furthermore, we also evaluated the impact of CTCs detected by different methods on the prognosis of SCLC. Despite the advantages of this study, there are some inevitable limitations in our study. First, publication bias was detected in our study when we assessed the relationship between pretreatment CTCs level detected by CellSearch and OS in SCLC. Second, among the included studies, most studies are single-center studies and have a small sample size. Therefore, large-scale, prospective, and multicenter studies are warranted in the future to verify our results. Third, we only included studies published in English, the potential risk of selection bias may exist in the present study. Forth, to our regret, only two studies assessed the relationship between CTCs level at the time of disease progression and OS in SCLC, so we did not perform pooled data analysis. Therefore, more studies are needed to address this issue. Last but not least, although the pooled data analysis demonstrated that different time points of CTCs have significant prognostic role in SCLC, higher heterogeneity was observed in different groups, and it may lead to the results unreliable. Hence, it further supports that we need well-designed studies to assess the truly impact of CTCs on the prognosis of these individuals.
Conclusions
To sum up, in this meta-analysis, we systematically investigated the impact of CTCs status on the prognosis of SCLC. Besides, we also took the effect of sampling time and detection methods into consideration when evaluated the prognostic value of CTCs status in SCLC. Our findings suggest that dynamic monitoring of CTCs level could be a non-invasive and effective tool to predict the disease progression and prognosis in these individuals. Furthermore, prospective, large-scale, well-designed, and multicenter studies are urgently needed in the future to verify our results.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211050581 for Assessment of the Clinical Utility of Circulating Tumor Cells at Different Time Points in Predicting Prognosis of Patients With Small Cell Lung Cancer: A Meta-Analysis by Ai-Min Jiang, Hao-Ran Zheng, Na Liu, Rui Zhao, Yu-Yan Ma, Shu-Heng Bai, Tao Tian, Xuan Liang, Zhi-Ping Ruan, Xiao Fu and Yu Yao in Cancer Control
Appendix
Abbreviations
- CTCs
circulating tumor cells
- CCRT
concurrent chemoradiotherapy
- CK
cytokeratin
- CIs
confidence intervals
- EpCAM
epithelial cell adhesion molecule
- EMT
Epithelial-to-mesenchymal transition
- ES
extensive-stage
- FISH
fluorescence in situ hybridization
- FDA
Food and Drug Administration
- HRs
hazard ratios
- ICIs
immune checkpoint inhibitors
- IF
immunofluorescence
- LT-PCR
ligand-targeted polymerase chain reaction
- LS
limited-stage
- NOS
Newcastle-Ottawa Scale
- OS
overall survival
- PFS
progression-free survival
- PRISMA
primary reporting items for systematic reviews and meta-analyses
- PD
disease progression
- SCLC
small cell lung cancer
- UK
the United Kingdom
Author Contributions: (I) Conception and design: YY and XF.
(II) Administrative support: YY, XF, TT, XL, and ZR.
(III) Provision of study materials or patients: AJ, HZ, and NL.
(IV) Collection and assembly of data: AJ, RZ, YM, and SB.
(V) Data analysis and interpretation: AJ, HZ, and NL.
(VI) Manuscript writing: All authors
(VII) Final approval of manuscript: All authors
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement: As our study is a meta-analysis, ethics statement is not required.
Supplementary Material: Supplementary material for this article is available online.
ORCID iD
Ai-Min Jiang https://orcid.org/0000-0002-4092-342X
References
- 1.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepherd FA, Crowley J, Van Houtte P, et al. The international association for the study of lung cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (Seventh) Edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067-1077. [DOI] [PubMed] [Google Scholar]
- 3.Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumor Biol. 2015;36:3389-3397. [DOI] [PubMed] [Google Scholar]
- 4.Zhou T, Zhao Y, Zhao S, et al. Comparison of the prognostic value of systemic inflammation response markers in small cell lung cancer patients. J Canc. 2019;10:1685-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14:549-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell HA, Tata LJ, Baldwin DR, et al. Treatment decisions and survival for people with small-cell lung cancer. Br J Canc. 2014;110:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548. [DOI] [PubMed] [Google Scholar]
- 8.Ahn JC, Teng PC, Chen PJ, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology. 2021;73:422-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang SS, Deng B, Feng YG, Qian K, Tan QY, Wang RW. Circulating tumor cells prior to initial treatment is an important prognostic factor of survival in non-small cell lung cancer: a meta-analysis and system review. [DOI] [PMC free article] [PubMed]
- 10.Bidard FC, Michiels S, Riethdorf S, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. JNCI (J Natl Cancer Inst). 2018;110:560-567. [DOI] [PubMed] [Google Scholar]
- 11.Tan Y, Wu H. The significant prognostic value of circulating tumor cells in colorectal cancer: A systematic review and meta-analysis. Curr Probl Canc. 2018;42:95-106. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Zou K, Zheng L, Xiong B. Prognostic and clinicopathological significance of circulating tumor cells detected by RT-PCR in non-metastatic colorectal cancer: A meta-analysis and systematic review. BMC Cancer. 2017;17:725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xun Y, Cao Q, Zhang J, Guan B, Wang M. Clinicopathological and prognostic significance of circulating tumor cells in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2020;104:104638. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Wu G, Yang W, et al. Prognostic value of circulating tumor cells detected with the CellSearch system in esophageal cancer patients: a systematic review and meta-analysis. BMC Cancer. 2020;20:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol : Off J Am Soc Clin Oncol. 2012;30:525-532. [DOI] [PubMed] [Google Scholar]
- 16.Salgia R, Weaver RW, McCleod M, et al. Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study. Invest N Drugs. 2017;35:334-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen J, Zhao J, Jiang T, et al. Predictive and prognostic value of folate receptor-positive circulating tumor cells in small cell lung cancer patients treated with first-line chemotherapy. Oncotarget. 2017;8:49044-49052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-Maxillofacial Surg. 2011;39:91-92. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [DOI] [PubMed] [Google Scholar]
- 20.Jiang AM, Zhao R, Liu N, et al. The prognostic value of pretreatment prognostic nutritional index in patients with small cell lung cancer and it's influencing factors: a meta-analysis of observational studies. J Thorac Dis. 2020;12:5718-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang PP, Liu SH, Chen CT, et al. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J Canc. 2020;11:2113-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu L, Zhu Y, Jing W, Guo D, Kong L, Yu J. Incorporation of circulating tumor cells and whole-body metabolic tumor volume of (18)F-FDG PET/CT improves prediction of outcome in IIIB stage small-cell lung cancer. Chin J Cancer Res. 2018;30:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu L, Liu F, Fu H, et al. Circulating tumor cells correlate with recurrence in stage III small-cell lung cancer after systemic chemoradiotherapy and prophylactic cranial irradiation. Jpn J Clin Oncol. 2014;44:948-955. [DOI] [PubMed] [Google Scholar]
- 24.Shi WL, Li J, Du YJ, et al. CK-19 mRNA-positive cells in peripheral blood predict treatment efficacy and survival in small-cell lung cancer patients. Med Oncol. 2013;30:755. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal C, Wang X, Ranganathan A, et al. Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Canc. 2017;112:118-125. [DOI] [PubMed] [Google Scholar]
- 26.Huang CH, Wick JA, Sittampalam GS, et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front Oncol. 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igawa S, Gohda K, Fukui T, et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncology Letters. 2014;7:1469-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol : Official Publication of the International Association for the Study of Lung Cancer. 2012;7:512-519. [DOI] [PubMed] [Google Scholar]
- 29.Pore M, Meijer C, de Bock GH, et al. Cancer stem cells, epithelial to mesenchymal markers, and circulating tumor cells in small cell lung cancer. Clin Lung Canc. 2016;17:535-542. [DOI] [PubMed] [Google Scholar]
- 30.Hiltermann TJN, Pore MM, Van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann Oncol. 2012;23:2937-2942. [DOI] [PubMed] [Google Scholar]
- 31.Tay RY, Fernandez-Gutierrez F, Foy V, et al. Prognostic value of circulating tumour cells in limited-stage small-cell lung cancer: analysis of the concurrent once-daily versus twice-daily radiotherapy (CONVERT) randomised controlled trial. Ann Oncol: Official Journal of the European Society for Medical Oncology. 2019;30:1114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525-532. [DOI] [PubMed] [Google Scholar]
- 33.Messaritakis I, Politaki E, Koinis F, et al. Dynamic changes of phenotypically different circulating tumor cells sub-populations in patients with recurrent/refractory small cell lung cancer treated with pazopanib. Sci Rep. 2018;8:2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messaritakis I, Nikolaou M, Politaki E, et al. Bcl-2 expression in circulating tumor cells (CTCs) of patients with small cell lung cancer (SCLC) receiving front-line treatment. Lung Canc. 2018;124:270-278. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wang HT, Li BG. Prognostic significance of circulating tumor cells in small--cell lung cancer patients: a meta-analysis. Asian Pac J Cancer Prev APJCP : Asian Pac J Cancer Prev APJCP. 2014;15:8429-8433. [DOI] [PubMed] [Google Scholar]
- 36.Foy V, Lindsay CR, Carmel A, et al. EPAC-lung: European pooled analysis of the prognostic value of circulating tumour cells in small cell lung cancer. Transl Lung Cancer Res. 2021;10:1653-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkinson DR, Dracopoli N, Petty BG, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labib M, Kelley SO. Circulating tumor cell profiling for precision oncology. Mol Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211050581 for Assessment of the Clinical Utility of Circulating Tumor Cells at Different Time Points in Predicting Prognosis of Patients With Small Cell Lung Cancer: A Meta-Analysis by Ai-Min Jiang, Hao-Ran Zheng, Na Liu, Rui Zhao, Yu-Yan Ma, Shu-Heng Bai, Tao Tian, Xuan Liang, Zhi-Ping Ruan, Xiao Fu and Yu Yao in Cancer Control