Abstract
Initiation of DNA replication in eukaryotes is dependent on the activity of protein phosphatase 2A (PP2A), but specific phosphoprotein substrates pertinent to this requirement have not been identified. A novel regulatory subunit of PP2A, termed PR48, was identified by a yeast two-hybrid screen of a human placental cDNA library, using human Cdc6, an essential component of prereplicative complexes, as bait. PR48 binds specifically to an amino-terminal segment of Cdc6 and forms functional holoenzyme complexes with A and C subunits of PP2A. PR48 localizes to the nucleus of mammalian cells, and its forced overexpression perturbs cell cycle progression, causing a G1 arrest. These results suggest that dephosphorylation of Cdc6 by PP2A, mediated by a specific interaction with PR48, is a regulatory event controlling initiation of DNA replication in mammalian cells.
Initiation of chromosomal DNA replication in eukaryotes is a multistep process that requires assembly of prereplicative complexes on chromosomal DNA, followed by unwinding of replication origins and loading of replicative polymerases and accessory proteins that catalyze DNA synthesis (reviewed in references 6, 34, and 35). Stringent control mechanisms, directed by the competing activities of multiple protein kinases and protein phosphatases, ensure that each segment of the genome is replicated once and only once in each cell cycle and that DNA replication is properly timed with respect to cell growth and mitosis.
The serine/threonine protein phosphatase 2A (PP2A) exerts regulatory control over the initiation of DNA replication in yeast, viral, and vertebrate systems (19, 20, 33). This relationship was demonstrated first for replication of simian virus 40 (SV40) DNA, where dephosphorylation of specific serine residues by PP2A within large T antigen promotes DNA binding of T-antigen hexamers and unwinding of SV40 replication origins (33). More recently, immunodepletion of PP2A holoenzyme was observed to prevent chromosomal DNA replication in a Xenopus cell-free replication system (20). Neither assembly of prereplicative complexes nor elongation of replication forks is dependent on PP2A, but firing of replication origins is suppressed in the absence of this enzyme. This requirement for PP2A activity in promoting DNA replication presumably is based on direct or indirect modification of the proteins responsible for priming and/or firing replication origins. However, with the exception of SV40 large T antigen, specific phosphoprotein substrates of PP2A that are pertinent to its role in promoting DNA replication have not been identified.
Cdc6 proteins are highly conserved in organisms as diverse as Saccharomyces cerevisiae, Xenopus laevis, and Homo sapiens (1, 4, 8, 22, 31, 41) and exert unique functions at each of several steps of replication initiation. Cdc6 is required for formation of prereplicative complexes and is rate limiting for initiation of DNA replication in all species in which the function of Cdc6 has been examined (4, 9, 18, 36, 42). In yeast, forced overexpression of wild-type cdc18+ (the CDC6 homologue in Schizosaccharomyces pombe), or certain mutated alleles of S. cerevisiae CDC6, promotes the repeated firing of replication origins within a single cell cycle (17, 26, 28), while maneuvers that diminish function of Cdc6 and/or cdc18+ proteins during S phase promote premature entry into mitosis despite the presence of unreplicated DNA (30). In mammalian cells, transcription of Cdc6 is controlled by E2F transcription factors (10, 16, 42), and expression of Cdc6 is dysregulated in human tumors (40).
To elucidate molecular mechanisms of Cdc6 function in mammalian cells, we used the yeast two-hybrid system to screen a human placental cDNA library for proteins that bind specifically to Cdc6. Here we describe features of a protein, termed PR48, that was identified in this screen and found to be a novel B" regulatory subunit of PP2A. Our findings support the hypothesis that dephosphorylation of Cdc6 by PP2A, mediated by an interaction with PR48 or related B" proteins, is pertinent to the control of DNA replication in mammalian cells.
MATERIALS AND METHODS
Yeast strains and plasmid constructions.
S. cerevisiae CG1945 and Y190 (Clontech) were used for two-hybrid screens and assays. A human placental cDNA library in pACT2 was purchased from Clontech. Human Cdc6 bait plasmids (Fig. 1) and a plasmid for expression of Aα subunit of PP2A were constructed in pAS2-1 with inserts prepared by PCR using the Expand high-fidelity PCR system (Boehringer Mannheim). Appropriate restriction sites were added to ensure inframe insertion.
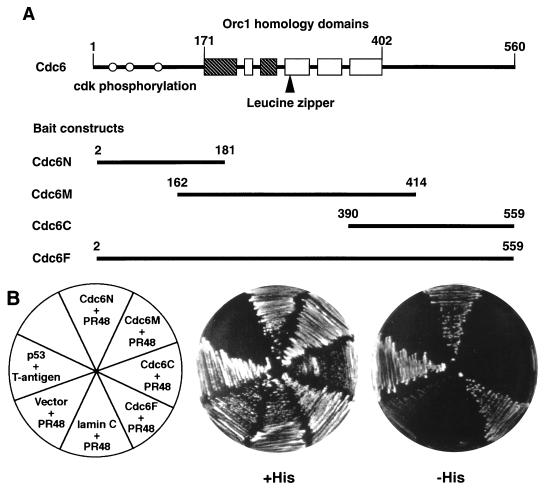
FIG. 1.
Identification of human PR48 from a yeast two-hybrid screen. (A) Schematic representation of Cdc6 segments used as bait proteins. Amino acid positions are numbered relative to the translation initiation codon. Boxes represent highly conserved sequence blocks shared among Cdc6 and ORC1 proteins from different species. The nucleotide binding motifs are shaded. Open circles depict the positions of CDK phosphorylation sites. (B) Two-hybrid assay with pairwise combinations of PR48 (fused to GAL4AD) and various regions of Cdc6 (fused to GAL4BD) in which growth on media lacking histidine (−His) reflects a physical interaction between the two proteins, as seen for PR48 plus Cdc6N and PR48 plus Cdc6F. Negative controls include PR48 plus vector alone or PR48 plus lamin C. The previously described interaction between p53 and SV40 large T antigen provides a positive control.
Yeast two-hybrid screen and assay.
A human placental cDNA library (Clontech) was used for large-scale transformation, using the lithium acetate method, of yeast cells (CG1945) carrying the pCdc6N bait plasmid (Fig. 1A), and interacting cDNA clones were selected by growth on plates lacking histidine. His+ colonies were screened for lacZ expression by a colony lift method (Clontech). Redundant clones were identified by comparing the patterns of products produced by HaeIII digestions of PCR amplification products generated from each candidate colony. Nonredundant clones selected in this manner were characterized further by retransformation of CG1945 yeast cells carrying plasmids encoding GAL4BD (GAL4 DNA binding domain)-Cdc6N, GAL4BD-Cdc6M, GAL4BD-Cdc6C, GAL4BD-Cdc6F, or GAL4BD-lamin and compared to GAL4BD-p53/SV40 T-antigen-GAL4AD as a positive control (Clontech). In addition, to test the interaction between PR48 and the Aα subunit of PP2A, PR48 in pACT2 and Aα in pAS2-1 were cotransformed in S. cerevisiae Y190, and the transformed cells were tested for rapid growth in the absence of histidine and for β-galactosidase activity.
Expression of epitope-tagged or GFP fusions.
To express hemagglutinin (HA) epitope-tagged PR48, the protein coding sequence of PR48 with an HA tag at the amino terminus (HA-PR48) was cloned into pTarget, a cytomegalovirus promoter-driven expression vector (Promega). Construction of expression plasmids for HA-tagged human Cdc6 (HA-Cdc6) and mouse myocyte nuclear factor α (HA–MNF-α) was described previously (41, 43). For coimmunoprecipitation of Cdc6 and PR48 in mammalian cells, a c-Myc tag was fused to the carboxyl terminus of Cdc6 (Cdc6–c-Myc) carried in pTarget vector (Promega). To investigate the subcellular expression and the effect of forced overexpression on cell cycle progression, human Cdc6 was fused in frame to the carboxyl terminus of green fluorescent protein (GFP) in the expression vector pEYFP-C (Clontech). Similarly, cDNAs encoding PR48, the B/PR55α subunit (14), and the B′/B56α1 subunit (23) were fused in frame to the amino terminus of GFP in the expression vector pEGFP-N (Clontech). For transient expression of epitope-tagged or GFP fusions, HeLa (a human epithelial carcinoma cell line) or C2C12 (a mouse myoblast cell line) cells were grown in Dulbecco's modified Eagle's medium (Life Technologies, Inc.) supplemented with 10 and 20% fetal bovine serum, respectively. Cells were transfected by using LipofectaminePlus (Life Sciences) as instructed by the manufacturer.
In vitro protein-protein interaction assays.
GST and GST-Cdc6 fusion proteins were prepared as described previously (41). 35S-labeled PR48 was produced by using pTarget plasmid (Promega), containing cDNA to PR48, in a cell-free transcription-translation reaction with rabbit reticulocyte lysate and [35S]methionine. The formation of protein complexes by using glutathione-agarose beads was assayed as described previously (43). 35S-labeled luciferase protein (Promega) was used as a negative control. For estimation of binding efficiency, 1/10 of the radiolabeled PR48 or luciferase was loaded as an input control (lanes 4 and 5 in Fig. 3).
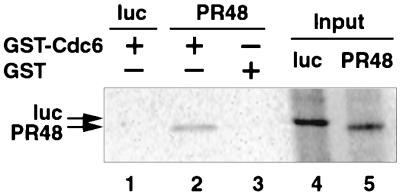
FIG. 3.
Interaction of PR48 and Cdc6 in vitro. PR48 and firefly luciferase (luc) proteins were translated in rabbit reticulocyte lysates and labeled with [35S]methionine (lanes 4 and 5). PR48 copurified with GST-Cdc6 (lane 2) but not with GST (lane 3), as assessed by binding to proteins coupled to glutathione-agarose beads. Luciferase failed to interact with GST-Cdc6 (lane 1), serving as a negative control. Proteins were separated by SDS-PAGE and visualized by autoradiography.
Immunoprecipitation experiments.
Coimmunoprecipitation of transiently expressed Cdc6–c-Myc and HA-PR48 in mammalian cells was performed according to the instructions of the manufacturer of the anti–c-Myc antibody (Boehringer Mannheim). Briefly, 24 h after transfection, HeLa or C2C12 cells were incubated on ice for 20 min in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 0.2% Nonidet P-40, 20% glycerol, 200 mM NaCl, 1 mM EDTA, 1 mM EGTA, and a protease inhibitor cocktail (Boehringer Mannheim). Lysates were centrifuged at 14,000 × g for 10 min, and protein complexes were immunoprecipitated from the supernatant by the addition of 4 μg of a murine monoclonal antibody that recognizes the c-Myc epitope (Boehringer Mannheim) and incubation for 1 h with rotation at 4°C. Fifty microliters of protein A-agarose (Boehringer Mannheim) was added to the immunoprecipitation mixture and rotated at 4°C for 3 h followed by three washes with lysis buffer. The immunoprecipitates were solubilized in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, resolved on an SDS–10% polyacrylamide gel, and transferred to a nitrocellulose membrane. The membrane was then probed with rat anti-HA antibody (3F10; Boehringer Mannheim), and the HA-tagged proteins were visualized by the SuperSignal chemiluminescence system (Pierce). Equivalent amounts of nuclear extracts from duplicate plates were analyzed by Western blotting to estimate the expression levels (Fig. 4).
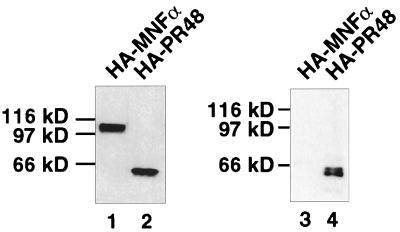
FIG. 4.
Interaction of PR48 and Cdc6 in vivo. C2C12 cells were cotransfected with Cdc6–c-Myc and HA-PR48 or with Cdc6–c-Myc and HA–MNF-α. Proteins were immunoprecipitated from whole-cell extracts (lanes 1 and 2) with anti-Myc antibody and separated by SDS-PAGE, and immunoblots were probed with anti-HA antibody (lane 3 and 4). The loading in lane 1 and 2 is equivalent to the cell extracts used for coimmunoprecipitation and subsequent immunoblotting (lane 3 and 4).
Reconstitution of PP2A holoenzymes in insect cells.
Spodoptera frugiperda (Sf9) cells were grown in suspension cultures in SF-900 II serum-free medium (Life Technologies). Cells (2 × 106 cells/ml) were simultaneously infected at 0.2 PFU per cell with recombinant baculoviruses encoding a hexahistidine-tagged A subunit, the C subunit, and either the Bα/PR55 subunit or HA-tagged human PR48. Cultures were incubated at 27°C, and cells were harvested 68 to 70 h after infection. The cells were washed with Tris-buffered saline (pH 7.0) and stored at −80°C. Expression of PP2A subunits following triple infection of Sf9 cells results in formation of recombinant heterotrimers composed nearly exclusively of the ectopic proteins (14).
PP2A heterotrimers were affinity purified from Sf9 cell lysates, using the hexahistidine tag on the A subunit. All procedures were performed at 4°C. Frozen cell pellets were lysed on ice for 30 min in 1:6 (wt/vol) lysis buffer composed of 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 20 mM imidazole, 10 mM 2-mercaptoethanol, 10% glycerol, 0.5% Triton X-100, 2 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail minus EDTA (Boehringer Mannheim). The lysate was cleared by centrifugation at 30,000 × g for 30 min. The supernatant was applied to a column (1.0 ml) packed with nitrilotriacetic acid (NTA)-nickel resin (Qiagen) equilibrated with lysis buffer. The column was washed with 10 ml of lysis buffer followed by 10 ml of wash buffer (10 mM Tris [pH 8.0], 10% glycerol, 10 mM 2-mercaptoethanol, 20 mM imidazole). Protein was eluted from the NTA-nickel column with wash buffer containing 200 mM imidazole. The elution of HA-PR48 was monitored by immunoblotting with anti-HA antibody. Under these conditions, 60 to 70% of the total Bα/PR55 or HA-PR48 in the lysate was bound to the NTA-nickel column and eluted with 200 mM imidazole. The eluate was loaded onto a Mono Q HR 5/5 column (Pharmacia Biotech) equilibrated in buffer D (50 mM Tris [pH 7.4], 1 mM EDTA, 10% glycerol, 2 mM dithiothreitol, 0.1 mM phenylmethlysulfonyl fluoride). A linear gradient of 0.15 to 0.3 M NaCl in buffer D was used to elute proteins at a flow rate of 0.5 ml/min. Fractions of 0.5 ml were collected, and aliquots were assayed for phosphatase activity, using myosin light chain as a substrate. The fractions were also analyzed by SDS-PAGE and staining with Coomassie brilliant blue and by immunoblotting with anti-HA, anti-A-subunit, and anti-C-subunit antibodies. At this step, all of the HA-PR48 or Bα/PR55 subunit coeluted with a major peak of PP2A activity that also contained the hexahistidine-tagged A subunit and the C subunit. The heterotrimer containing hexahistidine-A, HA-PR48, and the C subunit eluted at a higher NaCl concentration (0.4 M) than the heterotrimer containing the hexahistidine-A, Bα/PR55, and C subunits (0.27 M). Fractions containing active, recombinant PP2A trimers were pooled and concentrated in Amicon-10 concentrators. The concentrated proteins were loaded onto a Superdex 200 10/30 gel filtration column (Pharmacia Biotech) equilibrated in buffer D containing 0.15 M NaCl. Protein was eluted from the column in buffer D–0.15 M NaCl at a flow rate of 0.25 ml/min. Aliquots were assayed for myosin light-chain phosphatase activity and analyzed by SDS-PAGE and immunoblotting. At this stage, all of the HA-PR48 or Bα/PR55 subunit coeluted with the hexahistidine-A subunit and the C subunit in a single symmetrical peak of PP2A activity. The hexahistidine-A-HA-PR48-C heterotrimer eluted from the gel filtration column in the same fractions as the immunoglobulin calibration marker (Mr = 160,000). Fractions containing active, recombinant trimers were pooled, concentrated as described above, and stored at −80°C in buffer D containing 50% glycerol. Column fractions and the purified recombinant holoenzymes were assayed for phosphatase activity by using 32P-labeled myosin light chain as described previously (14).
Flow cytometric analysis of DNA content of transfected cells.
HeLa cells expressing GFP or GFP fusions were trypsinized 24 h posttransfection and fixed with 4% paraformaldehyde on ice for 10 min. Cells were permeabilized with phosphate-buffered saline–0.1% Triton X-100 at 4°C for 10 min followed by incubation in 500 μl of phosphate-buffered saline containing propidium iodide (50 μg/ml) and RNase A (100 U/ml) at room temperature for more than 30 min. Flow cytometric analysis was performed with settings to assess DNA content selectively in GFP-positive cells except for cells transfected with pTarget as a negative control.
Sequencing and alignments.
Complementary DNA clones identified in the yeast two-hybrid screen were sequenced from both directions by using dye terminator chemistry. The PR48 sequence was used to search GenBank with the BLAST algorithm. Highly homologous sequences were compared and aligned by DNASTAR. A dendrogram was generated from the sequence comparison to access the evolutionary relationships among homologous genes.
Nucleotide sequence accession number.
The sequence reported in this paper has been deposited in the GenBank database under accession no. AF135016.
RESULTS
PR48 interacts specifically with an amino-terminal segment of Cdc6 in a yeast two-hybrid assay.
A two-hybrid screen (7) was established in S. cerevisiae CG1945 carrying integrated HIS3 and lacZ genes controlled by the GAL upstream activation sequence (UASGAL). We employed an amino-terminal segment (amino acids 2 to 181) of Cdc6 fused to GAL4BD as the bait protein (Fig. 1A). Several independent clones were selected from a library (constructed by fusion of human placental cDNAs to the transcriptional activation domain of GAL4 [GAL4AD]) based on growth on media without histidine supplementation. The interaction of proteins selected from the library with Cdc6 was confirmed by β-galactosidase assays of colony lifts (not shown), and redundant clones were identified by restriction mapping of PCR-amplified DNA inserts from individual clones (not shown). A cDNA clone selected in this manner encodes a novel protein, which we term PR48, the subsequent analysis of which is reported here.
The interaction of PR48-GAL4AD with Cdc6-GAL4BD was specific to the amino terminus of Cdc6, since only full-length Cdc6 (Cdc6F) or the amino-terminal fragment (Cdc6N), but not other fragments of Cdc6 (Cdc6M and Cdc6C), supported growth in the absence of supplemental histidine when coexpressed with PR48-GAL4AD in the yeast cells (Fig. 1B). PR48-GAL4AD had no intrinsic transactivation function when cotransformed with GAL4BD (pAS2-1) or lamin-GAL4BD, which were used as a negative controls. The well-characterized interaction between p53 and SV40 T antigen was included as a positive control. Specific binding of PR48 to the amino terminus of Cdc6 was also confirmed by β-galactosidase assays of colony lifts (not shown).
PR48 is a novel protein closely related to known B" subunits of PP2A.
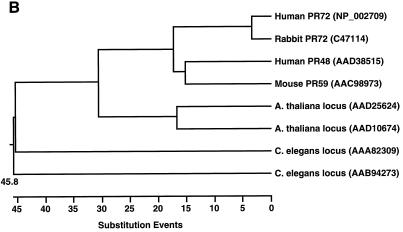
A BLAST search using the predicted amino acid sequence of PR48 based on its nucleotide sequence (GenBank AF135016) identified proteins closely resembling PR48, as shown in the sequence alignment (Fig. 2A). PR48 shares amino acid sequence homology with known B" subunits of PP2A in mammals, invertebrates, and plants, including Arabidopsis thaliana and Caenorhabditis elegans. The highest homology (68% identity and 77% similarity) exists between human PR48 and mouse PR59. PR59 was shown recently to have a functional role in cell cycle control by interacting with and dephosphorylating p107, a pocket protein of the retinoblastoma family (38). PR48 is also homologous to PR72, a splicing variant of PR130 (11). The amino acid sequence of PR48 is sufficiently divergent, however, from sequence of PR59 and PR72/130 to identify PR48 as a product of a separate gene rather than a splicing variant. The presence of closely related proteins in a wide range of species demonstrates strong evolutionary conservation among this class of PP2A subunits (Fig. 2B).
FIG. 2.
Comparison of PR48 to related B" subunits of PP2A and homologous sequences in GenBank. (A) Amino acid sequence alignment of PR48 with previously reported B" proteins and homologous sequences in GenBank. Identical residues are shaded. (B) Dendrogram from analysis by DNASTAR indicating the relative similarities between PR48 and related proteins from nematode, plant, and mammalian species. Units on the horizontal axis indicate the number of substitution events.
The putative initiation codon represents the most 5′ ATG codon sequence of the longest open reading frame within the cDNA clone isolated in the two-hybrid screen. However, since this open reading frame extends further in the 5′ direction, we cannot be certain that translation begins at the indicated site. However, neither a BLAST search of the human expressed sequence tag database nor experimental approaches using PCR-based techniques identified longer cDNAs that would provide additional sequence 5′ to the cloned region. In addition, sequences surrounding this putative initiation codon conform to a consensus Kozak site for translational initiation. We propose, therefore, that the initiation codon predicted from the available sequence is an authentic translation start site.
PR48 binds Cdc6 in vitro and in vivo.
The authenticity of the interaction between PR48 and Cdc6, as observed in the yeast two-hybrid assay, was confirmed by two other techniques. Full-length recombinant Cdc6 fused to glutathione S-transferase (GST) was purified from Escherichia coli and incubated with PR48, which was expressed and labeled with [35S]methionine in a coupled in vitro transcription-translation reaction. PR48 was retained on glutathione beads in the presence of GST-Cdc6 (Fig. 3, lane 2), indicative of complex formation under these cell-free conditions. Based on a comparison of the radioactivity of the retained PR48 to that of the input PR48, which was 1/10 of the amount used in the binding assay (lanes 4 and 5), only a fraction of the expressed protein bound to GST-Cdc6. However, no interaction between the backbone protein GST and PR48 (lane 3) or between the negative control luciferase and GST-Cdc6 (lane 1, Fig. 3) was observed.
To assess binding of Cdc6 to PR48 within intact mammalian cells, murine C2C12 cells were transiently cotransfected to express Cdc6–c-Myc in conjunction with HA-PR48. HA–MNF-α, a nonrelated nuclear transcription factor, was included as a negative control. Following immunoprecipitation with anti-c-Myc antibody, the immunoblots were probed with anti-HA antibody, revealing a selective interaction of Cdc6 with PR48 in this mammalian cell background (Fig. 4, lane 4). Contrary to the in vitro binding results, the interaction between PR48 and Cdc6 in intact cells is much more efficient, based on comparison of the nuclear extract input and immunoprecipitates on the immunoblots (lanes 1 and 2). PR48 interacts specifically with Cdc6, since no interaction was observed between Cdc6–c-Myc and HA–MNF-α (lane 3). This physical association of PR48 and Cdc6 was confirmed by similar experiments using human HeLa cells (not shown).
PR48 localizes to the nuclei of mammalian cells.
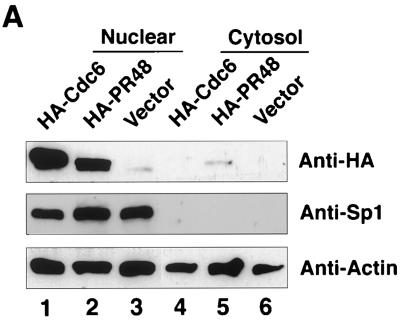
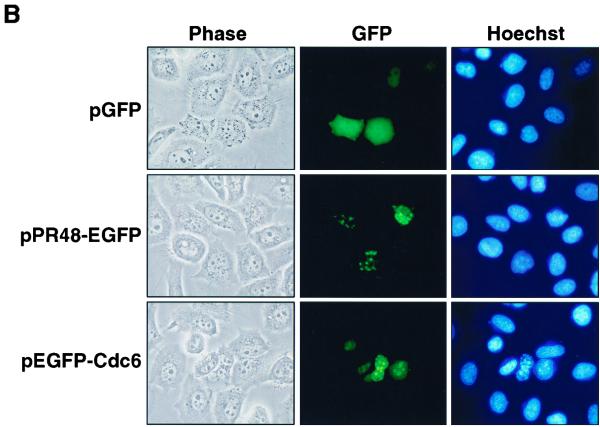
Both HA-PR48 and HA-Cdc6 proteins were noted to partition predominantly to the nuclear compartment when expressed in HeLa cells (Fig. 5A, lanes 1 and 2). Nuclear partitioning of Cdc6 confirmed our previous observations (41, 42). As controls, immunodetection of endogenous Sp1 and actin demonstrated the absence of nuclear material in the cytosolic fraction and the presence of intact protein in all lanes. Nuclear localization of PR48 in mammalian cells was confirmed by expression of PR48-GFP fusion protein followed by fluorescence microscopy (Fig. 5B). In the absence of the PR48 moiety, GFP distributes throughout both the nuclear and cytoplasmic compartments. In contrast, the PR48-GFP fusion concentrates in the nucleus (Fig. 5B). This subcellular distribution supports a potential role for PR48 in recruiting the catalytic subunits of PP2A to nuclear substrates such as Cdc6.
FIG. 5.
Subcellular localization of PR48. (A) Immunoblot of nuclear and cytosolic proteins from human HeLa cells transfected to express HA-Cdc6 (lanes 1 and 4) and HA-PR48 (lanes 2 and 5). Immunodetection of Sp1 and immunodetection of actin provide controls for the cell fractionation procedure and protein loading (30 μg per lane), respectively. (B) Fluorescence of GFP, PR48-GFP, or GFP-Cdc6 after transfection of HeLa cells.
PR48 is an authentic subunit of functional PP2A holoenzymes.
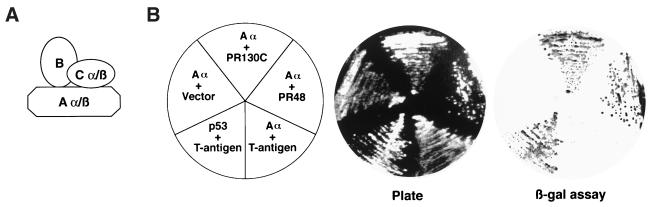
The PP2A holoenzyme is composed of three subunits, A, B, and C (Fig. 6A). The structural A subunit and the catalytic C subunit form a catalytic core, while substrate specificity is directed by B subunits, which include the B/PR55, B′/PR56, and B"/PR72 families. We tested whether PR48 interacted with the Aα subunit of PP2A in a yeast two-hybrid assay. We also compared this interaction with that between Cdc6 and the C-terminal region (amino acids 354 to 1150) of PR130 (PR130C). PR130 is a splice variant of the B"/PR72 subunit of PP2A that shares C-terminal sequence homology with PR48. Using an S. cerevisiae strain with an integrated UASGAL-lacZ gene, we confirmed that both PR48-GAL4AD and PR130C-GAL4AD interacted with Aα-GAL4BD, expressed from a vector in which the human Aα (PR65) cDNA is fused to GAL4DB (Fig. 6B). Inclusion of negative (Aα-GAL4BD plus vector; Aα-GAL4BD plus T antigen-GAL4AD) and positive (p53-GAL4BD plus T antigen-GAL4AD) controls confirmed the specificity of the interactions.
FIG. 6.
PR48 forms a PP2A holoenzyme complex. (A) Schematic depiction of the subunit composition of PP2A. (B) Two-hybrid assay with pairwise combinations of PR48 or PR130C (fused to GAL4AD) and Aα (fused to GAL4BD) reveals a physical interaction between PR48 or PR130C with the Aα subunit of PP2A, as indicated by induction of β-galactosidase activity. Negative controls include Aα plus vector or Aα plus SV40 large T antigen. The interaction between p53 and SV40 large T antigen provides a positive control (Promega).
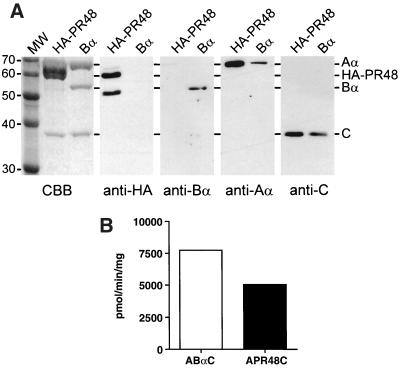
Once interaction between PR48 and the A subunit of PP2A was verified, reconstitution in insect cells was used to determine whether PR48 forms an active PP2A holoenzyme. Triple infection of Sf9 cells with recombinant baculoviruses encoding the A, C, and Bα subunits of PP2A results in formation of recombinant holoenzymes that have activities identical to that of the native enzyme (14). A recombinant baculovirus encoding an HA-tagged version of human PR48 was constructed and used to infect Sf9 cells in a triple infection that also included recombinant viruses encoding a hexahistidine-tagged Aα subunit and the catalytic subunit. In a parallel set of experiments, the HA-PR48 virus was replaced with a virus encoding the Bα subunit. Recombinant PP2A holoenzymes were recovered from Sf9 cell lysates 3 days after infection by NTA-nickel affinity chromatography, using the hexahistidine tag on the Aα subunit. As described previously (14), the A subunit was expressed in a considerable excess over the HA-PR48, Bα, and C subunits. Therefore, the holoenzymes eluted from NTA-nickel columns were further purified by ion-exchange and gel filtration chromatography. The recombinant holoenzymes isolated by this procedure were highly purified, as determined by SDS-PAGE and staining with Coomassie brilliant blue (Fig. 7A). The subunit composition of the purified holoenzymes was determined by immunoblotting with anti-HA or PP2A subunit-specific antibodies. This analysis showed that the major bands present in the purified recombinant holoenzymes corresponded to PR48 and the A, Bα, and C subunits of PP2A (Fig. 7A). The predicted size of HA-PR48 is 60 kDa, which corresponds to an Mr-60,000 band in the purified A-PR48-C preparation that stains intensely with Coomassie blue. This band coincides with the uppermost band detected by immunoblotting with anti-HA antibodies (Fig. 7A). The anti-HA immunoreactive band migrating at 50 kDa in this preparation most likely represents a proteolytic fragment of HA-PR48 that remains associated with the A and C subunits during purification. The presence of this lower-molecular-weight band was variable between preparations; it was not readily observed in the preparation used for Coomassie blue staining.
FIG. 7.
Reconstitution of an active PP2A holoenzyme containing PR48. (A) PP2A holoenzymes were isolated from Sf9 cells that had been triply infected with baculoviruses expressing hexahistidine-tagged Aα subunit, the C subunit, and either HA-PR48 or the Bα subunit. The complexes were resolved by SDS-PAGE and stained with Coomassie brilliant blue (CBB) or immunoblotted with antibodies against the HA epitope (anti-HA), the Bα subunit (anti-Bα), the Aα subunit (anti-Aα), or the catalytic subunit (anti-C); 3 μg of each preparation was loaded onto the gels for Coomassie blue staining, while 0.5 μg was used for immunoblotting. Also shown are Coomassie blue-stained markers (MW) whose molecular masses (in kilodaltons) are indicated on the left. The mobilities of Aα, HA-PR48, Bα, and C are indicated by lines starting at the right. (B) Phosphatase activity of purified PP2A holoenzymes. The purified holoenzymes shown in panel A were assayed for phosphatase activity, using 2 μM 32P-labeled myosin light chain as described in Materials and Methods. Phosphatase activity is expressed as picomoles of phosphate released per minute per milligram of purified enzyme. Background was determined in parallel incubations in which enzyme was omitted and was subtracted from values obtained in the presence of enzyme.
HA-PR48 copurified with the A and C subunits through each step of the purification. During the first step, 60 to 70% of the total HA-PR48 in the Sf9 cell lysates was retained on the NTA-nickel columns, indicating that the bulk of the expressed protein was associated with the hexahistidine-tagged A subunit. This level of recovery of HA-PR48 was identical to the recovery of the Bα subunit at this step, indicating that HA-PR48 forms complexes with the A subunit as readily as Bα, a well-characterized subunit of PP2A. The presence of free HA-PR48 and Bα that is not associated with the ectopic hexahistidine-Aα subunit is probably due to incomplete holoenzyme formation or formation of complexes with the endogenous Sf9 cell A subunit. Like the Bα subunit, HA-PR48 remained tightly associated with the A and C subunits, as there was no evidence of free protein during subsequent steps in the purification. The hexahistidine-Aα-PR48-C complex eluted from the gel filtration column in the same volume as the immunoglobulin calibration marker. This apparent size, 160,000 kDa, is consistent with formation of a 1:1:1 complex of the three proteins. The affinity isolation and copurification of PR48 with the A and C subunits demonstrates that PR48 is capable of forming a stable PP2A holoenzyme in intact cells.
The purified recombinant holoenzymes were tested for enzymatic activity to determine whether the A-PR48-C holoenzyme had phosphatase activity and to compare it with the holoenzyme containing the Bα subunit. Figure 7B shows that the purified A-PR48-C complex had phosphatase activity slightly lower than but comparable to that of the A-Bα-C complex toward myosin light chain, a highly selective substrate for PP2A. This result demonstrates that PR48 forms an active PP2A holoenzyme.
Forced overexpression of PR48 leads to dysregulation of the cell cycle.
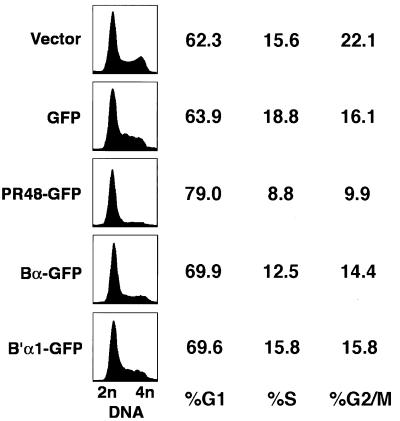
PR48-GFP was overexpressed in HeLa cells under the control of the cytomegalovirus promoter/enhancer, and DNA content was determined selectively in GFP-positive cells by flow cytometry. Cells transfected with empty vector (pTarget), GFP, Bα-GFP, or B′α1-GFP served as controls. Forced overexpression of PR48 disrupted cell cycle progression, causing a decrease in S and G2/M cells and an increase in G1 cells, as indicated by the DNA content of the cells (Fig. 8). The apparent G1 arrest was highly selective for PR48. Similar expression of Bα-GFP had a modest effect, while B′α1-GFP had very little effect on the cell cycle of HeLa cells. This finding is consistent with the notion that PR48 targets protein factors, such as Cdc6, in the DNA replication initiation complex and controls initiation of DNA replication.
FIG. 8.
Overexpression of PR48 alters cell cycle progression. Flow cytometry profiles of DNA content (propidium iodide fluorescence) and estimates of the percentage of cells in G1, S, and G2/M stages of the cell cycle in asynchronously growing HeLa cells under control conditions and following transfection to express either GFP, PR48-GFP, Bα-GFP, or B′α1-GFP. Profiles of cell number versus DNA content (DNA) were constructed from the cells transfected with an empty vector, pTarget (Vector), or selectively within the transfected cell population as identified by direct (GFP) fluorescence.
DISCUSSION
Progression through the cell cycle is controlled by the activities of cyclin-dependent protein kinases (CDKs) and protein phosphatases acting on intermediate or terminal effector proteins that drive molecular processes required for cell growth, DNA replication, and mitosis. With respect to the initiation of DNA replication, several components of the prereplicative complex, including Cdc6, are substrates for CDKs (2, 12, 13, 21, 22, 29, 32, 45), and several discrete events required for assembly of prereplicative complexes and firing of replication origins are controlled by CDK activity. In S. cerevisiae, chromatin binding of Cdc45, an essential component of the prereplicative complex, depends on CDK activity (45). Conversely, the premature activation of CDKs in early G1 can block formation of prereplicative complexes (5). Fully formed prereplicative complexes that are competent for initiation of DNA replication are fired by a process that requires both CDK activity and the Dbf4-Cdc7 protein kinase complex (reviewed in references 6, 15, 27, 31, and 34).
Ubiquitination and proteolysis of the Cdc6 orthologue in S. pombe (cdc18+), a prerequisite for progression from S phase into mitosis, requires prior phosphorylation of cdc18+ at CDK consensus sites (21). Phosphorylation of human Cdc6 by CDK2 during S phase was recently reported to promote translocation of Cdc6 from the nucleus to the cytoplasm of mammalian cells (13, 29). In addition, DNA replication can be blocked by forced expression of a recombinant form of Cdc6 in which serine residues within three amino-terminal CDK phosphorylation sites (Fig. 1A) are mutated (13). Thus, phosphorylation of replication factors, including Cdc6, is pertinent to each of the stepwise events that ensures the proper timing and fidelity of chromosomal duplication: formation of prereplicative complexes, firing of replication origins, prevention of rereplication, and coupling of mitosis to the completion of chromosomal replication.
The exquisitely ordered functional regulation of replication factors acting during the G1-S transition is likely to be determined by a fine balance between the activities of specific protein kinases and phosphatases. Here we present evidence suggesting that a physical interaction between PP2A and Cdc6 is pertinent to cell cycle progression in human cells. This interaction may be mediated by a previously unknown protein termed PR48, the primary amino acid sequence of which is conserved among a class (B") of regulatory subunits of PP2A (11, 38, 39).
PP2A holoenzymes are heterotrimeric proteins comprising three subunits, A, B, and C (Fig. 6A). The A and C subunits form a catalytic core, while B subunits confer regulatory functions with respect to subcellular localization and substrate specificity (25). Higher eukaryotes express a great diversity of PP2A isoforms, based on two variants each for the A and C subunits (α and β), and several families of B subunits (B, B′, and B"), each of which shares sequence homology within the family but not with members of other families. Differentiated cells maintain distinctive profiles of PP2A isoforms, based largely on differences in B-subunit expression (37, 44).
Different B subunits can direct PP2A to dephosphorylate discrete amino acid residues, even within a single protein substrate. PR59 binds and dephosphorylates p107, and forced overexpression of PR59 in mammalian cells perturbs cell cycle progression, causing G1 arrest (38). Among several heterotrimeric forms of PP2A, only a form containing B"/PR72 was capable of stimulating SV40 large T antigen to promote unwinding of replication origins and initiation of viral DNA replication, while other forms of PP2A actively inhibited T-antigen function (3). These effects were correlated with the selective removal of inhibitory phosphoryl groups from serines 120 and 123 by PP2A holoenzyme containing PR72, while other PP2A isoforms promoted dephosphorylation of the activating p34cdc2 target site at threonine 124.
Collectively, B" subunits (PR59 and PR72) have been shown to be related to cell cycle control in mammalian cells or in viral DNA replication. Our findings suggest that PR48 may also participate in control of cell cycle progression by directing PP2A activity to the DNA replication machinery through an interaction with Cdc6.
Our current data demonstrate that PR48 is a bona fide subunit of PP2A holoenzyme and that PR48 forms a complex with Cdc6 through a specific interaction with the amino-terminal region (amino acids 2 to 181) of Cdc6, within which reside sites for CDK phosphorylation (13, 41). Notably, PR48 fails to bind the middle region of Cdc6 (amino acids 172 to 402) that is highly conserved among the extended family of related proteins that includes both Cdc6 and ORC1 (1, 8, 41). Thus, it is likely that ORC1 does not interact with PR48. The authenticity of the physical interaction between PR48 and Cdc6 is supported by consistent results from three different experimental approaches: yeast two-hybrid assay, copurification of recombinant proteins, and immunoprecipitation of a complex containing both proteins from mammalian cells. Other B" subunits of PP2A may be capable of binding Cdc6 in vivo as well, as predicted from the high degree of sequence identity among PR48, PR59, and PR72/130 (Fig. 2), and as observed experimentally in an in vitro interaction between Cdc6 and a C-terminal region of PR130 (amino acids 354 to 1150), the portion of the protein that shares sequence homology with PR48 (not shown).
We propose that the interaction between Cdc6 and PP2A, mediated by PR48 or related B" proteins, is involved in the control of DNA replication in mammalian cells. PR48 is present in the nuclei of proliferating mammalian cells, as indicated by subcellular fractionation experiments and by fluorescence microscopy of cells expressing a PR48-GFP fusion protein. Forced overexpression of PR48 in human cells causes a G1 arrest, consistent with a dominant negative effect on the initiation of DNA replication. Overabundance of monomeric PR48 relative to the A and C subunits of PP2A could reduce the fraction of Cdc6 molecules bound by the holoenzyme and reduce dephosphorylation events catalyzed by PP2A that are required for initiation of S phase. Subunit overexpression leading to a dominant negative effect on function has been documented in the case of other multisubunit protein complexes (24). As to the normal function of PR48/PP2A in DNA replication, we suggest that phosphorylation of Cdc6 by cyclin E/A-CDK2 is necessary both for firing of replication origins and for cycling of Cdc6 out of the nucleus, a notion supported by recent data from other investigators (13, 21, 29, 32). Dephosphorylation of Cdc6 by PR48/PP2A could promote partitioning of Cdc6 to the nucleus and maintaining Cdc6 in a state competent for binding to ORC in the prereplication complex. The presence of PP2A in the complex would ensure that Cdc6 remains inactive with respect to firing of origins until the phosphatase activity is overcome by sufficient amounts of active CDK2. In this model, overexpression of PR48 would lead to excess targeting of PP2A to Cdc6 and prevent origin firing.
To our knowledge, these results are the first to demonstrate a physical interaction between a protein that functions directly in the initiation of chromosomal DNA replication and a protein phosphatase known to be required for firing of replication origins. More detailed characterization of enzyme-substrate relationships between specific PP2A isoforms, specific CDKs, and Cdc6 should advance our understanding of the molecular mechanisms that govern initiation of DNA replication in human cells.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL06296, HL07360, and HL31107.
We thank Caroline Humphries, Michelle Smith, and John Shelton for expert technical contributions, Weiguang Zhu for providing plasmid pEGFP-Cdc6, David Virshup for the B′/B56α1 cDNA, and Rhonda Bassel-Duby for critical reading of the manuscript.
REFERENCES
- 1.Bell S P, Mitchell J, Leber J, Kobayashi R, Stillman B. The multidomain structure of ORC1p reveals similarity to regulators of DNA replication and transcriptional silencing. Cell. 1995;83:563–568. doi: 10.1016/0092-8674(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 2.Brown G W, Jallepalli P V, Huneycutt B J, Kelly T J. Interaction of the S phase regulator cdc18 with cyclin-dependent kinase in fission yeast. Proc Natl Acad Sci USA. 1997;94:6142–6147. doi: 10.1073/pnas.94.12.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cegielska A, Shaffer S, Derua R, Goris J, Virshup D M. Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol Cell Biol. 1994;14:4616–4623. doi: 10.1128/mcb.14.7.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman T R, Carpenter P B, Dunphy W G. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 5.Detweiler C S, Li J J. Ectopic induction of Clb2 in early G1 phase is sufficient to block prereplicative complex formation in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1998;95:2384–2389. doi: 10.1073/pnas.95.5.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta A, Bell S P. Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- 7.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 8.Gavin K A, Hidaka M, Stillman B. Conserved initiator proteins in eukaryotes. Science. 1995;270:1667–1671. doi: 10.1126/science.270.5242.1667. [DOI] [PubMed] [Google Scholar]
- 9.Hartwell L. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Cell Biol. 1976;104:803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- 10.Hateboer G, Wobst A, Petersen B O, Le Cam L, Vigo E, Sardet C, Helin K. Cell cycle-regulated expression of mammalian CDC6 is dependent on E2F. Mol Cell Biol. 1998;18:6679–6697. doi: 10.1128/mcb.18.11.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrix P, Mayer-Jackel R E, Cron P, Goris J, Hofsteenge J, Merlevede W, Hemmings B A. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J Biol Chem. 1993;268:15267–15276. [PubMed] [Google Scholar]
- 12.Jallepalli P V, Tien D, Kelly T J. sud1(+) targets cyclin-dependent kinase-phosphorylated Cdc18 and Rum1 proteins for degradation and stops unwanted diploidization in fission yeast. Proc Natl Acad Sci USA. 1998;95:8159–8164. doi: 10.1073/pnas.95.14.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Wells N J, Hunter T. Multistep regulation of DNA replication by cdk phosphorylation of HsCdc6. Proc Natl Acad Sci USA. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamibayashi C, Estes R, Lickteig R L, Yang S I, Craft C, Mumby M C. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 15.Kelly T J, Nurse P, Forsburg S L. Coupling DNA replication to the cell cycle. Cold Spring Harbor Symp Quant Biol. 1993;58:637–644. doi: 10.1101/sqb.1993.058.01.071. [DOI] [PubMed] [Google Scholar]
- 16.Leone G, DeGregori J, Yan Z, Jakoi L, Ishida S, Williams R S, Nevins J R. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 1998;12:2120–2130. doi: 10.1101/gad.12.14.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang C, Stillman B. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 1997;11:3375–3386. doi: 10.1101/gad.11.24.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang C, Weinreich M, Stillman B. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell. 1995;81:667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- 19.Lin F C, Arndt K T. The role of Saccharomyces cerevisiae type 2A phosphatase in the actin cytoskeleton and in entry into mitosis. EMBO J. 1995;14:2745–2759. doi: 10.1002/j.1460-2075.1995.tb07275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X H, Walter J, Scheidtmann K, Ohst K, Newport J, Walter G. Protein phosphatase 2A is required for the initiation of chromosomal DNA replication. Proc Natl Acad Sci USA. 1998;95:14693–14698. doi: 10.1073/pnas.95.25.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Girona A, Mondesert O, Leatherwood J, Russell P. Negative regulation of Cdc18 DNA replication protein by Cdc2. Mol Biol Cell. 1998;9:63–73. doi: 10.1091/mbc.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madine M A, Khoo C Y, Mills A D, Laskey R A. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature. 1995;375:421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- 23.McCright B, Virshup D M. Identification of a new family of protein phosphatase 2A regulatory subunits. J Biol Chem. 1995;270:26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- 24.Milan M, Cohen S M. Regulation of LIM homeodomain activity in vivo: a tetramer of dLDB and apterous confers activity and capacity for regulation by dLMO. Mol Cell. 1999;4:267–273. doi: 10.1016/s1097-2765(00)80374-3. [DOI] [PubMed] [Google Scholar]
- 25.Mumby M C, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 26.Muzi-Falconi M, Brown G W, Kelly T J. cdc18+ regulates initiation of DNA replication in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1996;93:1566–1570. doi: 10.1073/pnas.93.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasmyth K. Viewpoints: putting the cell cycle in order. Science. 1996;274:1643–1645. doi: 10.1126/science.274.5293.1643. [DOI] [PubMed] [Google Scholar]
- 28.Nishitani H, Nurse P. p65cdc18 plays a major role controlling the initiation of DNA replication in fission yeast. Cell. 1995;83:397–405. doi: 10.1016/0092-8674(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 29.Petersen B O, Lukas J, Sorensen C S, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piatti S, Lengauer C, Nasmyth K. Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a “reductional” anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J. 1995;1141:3788–3799. doi: 10.1002/j.1460-2075.1995.tb00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowles A, Blow J J. Chromatin proteins involved in the initiation of DNA replication. Curr Opin Genet Dev. 1997;7:152–157. doi: 10.1016/s0959-437x(97)80123-2. [DOI] [PubMed] [Google Scholar]
- 32.Saha P, Chen J, Thome K C, Lawlis S J, Hou Z H, Hendricks M, Parvin J D, Dutta A. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol Cell Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheidtmann K H, Mumby M C, Rundell K, Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991;11:1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- 35.Stillman B. Smart machines at the DNA replication fork. Cell. 1994;78:725–728. doi: 10.1016/s0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 36.Stoeber K, Mills A D, Kubota Y, Krude T, Romanowski P, Marheineke K, Laskey R A, Williams G H. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 1998;17:7219–7229. doi: 10.1093/emboj/17.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tehrani M A, Mumby M C, Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J Biol Chem. 1996;271:5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- 38.Voorhoeve P M, Hijmans E M, Bernards R. Functional interaction between a novel protein phosphatase 2A regulatory subunit, PR59, and the retinoblastoma-related p107 protein. Oncogene. 1999;18:515–524. doi: 10.1038/sj.onc.1202316. [DOI] [PubMed] [Google Scholar]
- 39.Wera S, Hemmings B A. Serine/threonine protein phosphatases. Biochem J. 1995;311:17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams G H, Romanowski P, Morris L, Madine M, Mills A D, Stoeber K, Marr J, Laskey R A, Coleman N. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci USA. 1998;95:14932–14937. doi: 10.1073/pnas.95.25.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams R S, Shohet R V, Stillman B. A human protein related to yeast Cdc6p. Proc Natl Acad Sci USA. 1997;94:142–147. doi: 10.1073/pnas.94.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Z, DeGregori J, Shohet R, Leone G, Stillman B, Nevins J R, Williams R S. Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells. Proc Natl Acad Sci USA. 1998;95:3603–3608. doi: 10.1073/pnas.95.7.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Q, Bassel-Duby R, Williams R S. Transient expression of a winged-helix protein, MNF-β, during myogenesis. Mol Cell Biol. 1997;17:5236–5243. doi: 10.1128/mcb.17.9.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zolnierowicz S, Csortos C, Bondor J, Verin A, Mumby M C, DePaoli-Roach A A. Diversity in the regulatory B-subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry. 1994;33:11858–11867. doi: 10.1021/bi00205a023. [DOI] [PubMed] [Google Scholar]
- 45.Zou L, Stillman B. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science. 1998;280:593–596. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]