Abstract
Background
The outcome of patients with muscle-invasive bladder cancer (MIBC) remains poor, despite aggressive treatments. Inadequate primary staging, classically performed by computed tomography (CT)-imaging, could lead to inappropriate treatment and might contribute to these poor results. Although not (yet) adapted by international guidelines, several reports have indicated the superiority of 18F-fluorodeoxyglucose-positron emission tomography-CT (18F-FDG-PET-CT) compared to CT in the detection of lymph node and distant metastases. Thereby the presence of extra-vesical disease on 18F-FDG-PET-CT has been correlated with a worse overall survival. This supports the hypothesis that 18F-FDG-PET-CT is useful in stratifying MIBC patients and that adapting the treatment plan accordingly might result in improved outcome.
Methods
EFFORT-MIBC is a multicentric prospective phase II trial aiming to include 156 patients. Eligible patients are patients with histopathology-proven MIBC or ≥ T3 on conventional imaging treated with MIBC radical treatment, without extra-pelvic metastases on conventional imaging (thoracic CT and abdominopelvic CT/ magnetic resonance imaging (MRI)). All patients will undergo radical local therapy and if eligible neo-adjuvant chemotherapy. An 18F-FDG-PET-CT will be performed in addition to and at the timing of the conventional imaging. In case of presence of extra-pelvic metastasis on 18F-FDG-PET-CT, appropriate intensification of treatment with metastasis-directed therapy (MDT) (in case of ≤3 metastases) or systemic immunotherapy (> 3 metastases) will be provided. The primary outcome is the 2-year overall survival rate. Secondary endpoints are progression-free survival, distant metastasis-free survival, disease-specific survival and quality of life. Furthermore, the added diagnostic value of 18F-FDG-PET-CT compared to conventional imaging will be evaluated and biomarkers in tumor specimen, urine and blood will be correlated with primary and secondary endpoints.
Discussion
This is a prospective phase II trial evaluating the impact of 18F-FDG-PET-CT in stratifying patients with primary MIBC and tailoring the treatment accordingly. We hypothesize that the information on the pelvic nodes can be used to guide local treatment and that the presence of extra-pelvic metastases enables MDT or necessitates the early initiation of immunotherapy leading to an improved outcome.
Trial registration
The Ethics Committee of the Ghent University Hospital (BC-07456) approved this study on 11/5/2020. The trial was registered on ClinicalTrials.gov (NCT04724928) on 21/1/2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08861-x.
Keywords: Muscle-invasive bladder cancer, Primary staging, 18F-FDG-PET-CT, Distant metastasis, Oligometastasis, Neo-adjuvant chemotherapy, Metastasis-directed therapy, Stereotactic body radiation therapy, Immunotherapy, Overall survival
Background
Bladder cancer is the 10th most frequently diagnosed cancer and ranks 14th in causes of cancer-related death worldwide [1]. As the incidence of bladder cancer increases steadily with age and life expectancy improves, the number of bladder cancer patients of whom approximately 30% are diagnosed with muscle-invasive bladder cancer (MIBC) [2], is expected to increase in the future [3]. Despite an aggressive treatment with neo-adjuvant chemotherapy [4] followed by either radical cystectomy (RC) or trimodality therapy (TMT) [5], the outcome of MIBC patients remains poor with 2- to 5-year overall survival (OS) rates of ±60 and 50%, respectively [3, 4] and a 2-year disease free survival rate of ±64% [3]. Inadequate primary staging probably contributes to these poor results as adequate staging and appropriate treatment are closely linked.
Staging is classically done by computed tomography (CT) of the chest, abdomen and pelvis [6]. Although several reports indicate the superiority of 18F-fluorodeoxyglucose-positron emission tomography-CT (18F-FDG-PET-CT) compared to CT in the detection of lymph node as well as distant metastases [7], 18F-FDG-PET-CT is currently not recommended in international guidelines [6].
The presence of metastases on 18F-FDG-PET-CT has been correlated with survival rates. When compared to patients without metastatic lesions on 18F-FDG-PET-CT, MIBC patients with metastases on 18F-FDG-PET-CT had inferior OS and disease-specific survival (DSS). At 2 years the difference increased to 35% for both OS and DSS in favor of 18F-FDG-PET-CT-negative MIBC patients [8]. This suggests that there is a role for 18F-FDG-PET-CT in risk stratification to guide optimal treatment strategy.
For patients with metastatic disease on 18F-FDG-PET-CT, we hypothesize that treatment intensification improves outcome. In analogy with other tumor types [9–11], trials -although limited in patient number- have demonstrated that metastasis-directed therapy (MDT) of a limited number of metastases results in improved and durable disease control [12]. Similarly, patients with multiple metastases on 18F-FDG-PET-CT can benefit from earlier initiation of systemic immunotherapy [13].
To our knowledge, there is currently no prospective trial evaluating the impact of 18F-FDG-PET-CT implementation in the staging of patients with MIBC, to stratify patients and guide further treatment decisions in order to improve the outcome of patients with primary MIBC.
Methods/design
This study is approved by the Ethics Committee of the Ghent University Hospital (BC-07456) and the Belgian Federal Agency for Nuclear Control (PK-0061377). The trial is registered on ClinicalTrials.gov (NCT04724928).
In this multicentric prospective phase II trial, patients with MIBC will be offered 18F-FDG-PET-CT in addition to and at the timing of the conventional imaging (thoracic CT and abdominopelvic CT/ magnetic resonance imaging (MRI)), to guide further treatment after radical local therapy. In case of presence of metastasis on 18F-FDG-PET-CT, appropriate intensification of treatment with MDT or immunotherapy will be provided. A flowchart presenting the different steps from inclusion until follow-up (as described below) is presented in Fig. 1. Items from the World Health Organization Trial Registration Data Set are addressed in an additional file (see additional file 1).
Fig. 1.
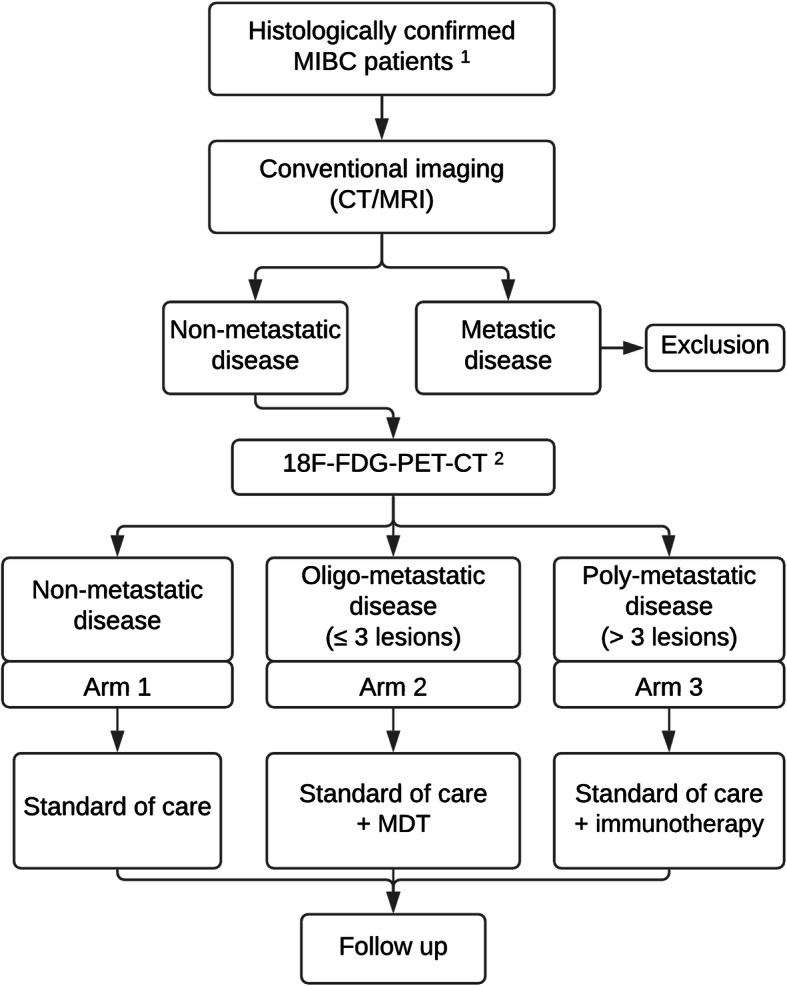
Overview of the EFFORT-MIBC study design. (1) Inclusion and exclusion criteria need to be fulfilled to be included in the study. (2) Stratification into treatment arms is based on the 18F-FDG-PET-CT result. In case of neo-adjuvant chemotherapy, stratification is based on the results of both 18F-FDG-PET-CT’s (e.g. prior to and after neo-adjuvant chemotherapy). Abbreviations: MIBC: muscle-invasive bladder cancer; CT: computed tomography; MRI: magnetic resonance imaging; 18F-FDG-PET-CT: 18F-fluorodeoxyglucose-positron emission tomography-computed tomography; MDT: metastasis-directed therapy
Objectives
The primary endpoint is the 2-year overall survival (OS) rate defined as the percentage of patients alive at 2 years after diagnosis of MIBC. OS is calculated from time of diagnosis until death due to MIBC or other causes.
Secondary endpoints are progression-free survival (PFS, defined as time of diagnosis until progression: i.e. local (T2-T4 in case of of TMT)/locoregional recurrence or extra-pelvic metastases), distant metastasis-free survival (DMFS, defined as time of diagnosis until occurrence of distant metastasis on repeated imaging), disease-specific survival (DSS, defined as time of diagnosis until death due to MIBC) and quality of life (QOL) evaluated by the general EORTC QOL questionnaire QLQ-C30 (version 3) and the bladder cancer specific module QLQ-BLM30. The added diagnostic value of 18F-FDG-PET-CT compared to conventional imaging will be evaluated. If neo-adjuvant chemotherapy is administered, the treatment response will be evaluated on the repeated 18F-FDG-PET-CT. A biopsy specimen of the bladder, obtained after transurethral resection of the bladder (TURb) as well as urine and blood samples will be collected for validation of predictive biomarkers by evaluating the correlation between response to therapy and outcome (PFS, DMFS, DSS and OS) with in literature reported biomarkers determined on biopsy specimen of the bladder, obtained after TURb.
Inclusion criteria
Histopathology-proven MIBC on TURb or ≥ T3 on conventional imaging treated with MIBC radical treatment
T1–4 N0–3 M0 MIBC on conventional imaging (thoracic CT and abdominopelvic CT/ MRI)
Age > 18 years
WHO 0–2
Willingness to undergo 18F-FDG-PET-CT
Willingness to undergo MDT or immunotherapy, in case of diagnosis of oligometastatic or polymetastatic disease on 18F-FDG-PET-CT, respectively
Willingness and ability to provide a signed informed consent according to ICH/GCP and national/local regulations
Exclusion criteria
Presence of distant metastasis on conventional imaging (thoracic CT and abdominopelvic CT/ MRI)
Refusal of or having contraindications to 18F-FDG-PET-CT
Refusal of MDT or immunotherapy
Prior radiotherapy unabling MDT
Contraindications to radiotherapy (including active inflammatory bowel disease)
Contraindications to immunotherapy
Other primary tumor diagnosed < 5 years ago and for which treatment is still required, except for diagnosis of non-metastatic prostate cancer at time of diagnosis of MIBC or non-melanoma skin cancer.
Evaluation and inclusion
Patients who were recently diagnosed with MIBC on TURb and who are considered for curative treatment, will be informed of this clinical study if eligible at the departments of urology, radiation oncology, medical oncology or at the multidisciplinary consultations as carried out in some participating centers. The decision to participate will be entirely voluntary. Eligible patients who decide not to participate will be offered standard of care treatment.
Intervention
Imaging
Conventional imaging, consisting of standard thoracic CT and abdominopelvic CT (or MRI), will be performed prior to the radical local therapy. In case of neo-adjuvant chemotherapy, conventional imaging will be performed prior to and after neo-adjuvant chemotherapy. After the second cycle of chemotherapy, MRI bladder will be performed to evaluate provisional therapy response. In addition to and at timing of the conventional imaging patients will receive an 18F-FDG-PET-CT. We aim to perform the 18F-FDG-PET-CT within 1 month of the thoracic CT and abdominopelvic CT (or MRI).
The 18F-FDG-PET-CT procedure is described in detail in an additional file (see Additional file 2).
Conventional imaging images will be evaluated by experienced radiologists in uro-oncology. 18F-FDG-PET-CT images will be reviewed and analyzed by a nuclear medicine physician and a radiologist experienced in reading PET-CT images. Evaluation of the 18F-FDG-PET-CT images will occur blinded to the conventional imaging or vice versa in case the 18F-FDG-PET-CT is performed first.
After ruling out presence of distant metastases on conventional imaging and evaluation of the 18F-FDG-PET-CT’s, patients will be allocated to the appropriate treatment arm:
Arm 1: 18F-FDG-PET-CT ➜ T1–4 N0–3 M0
Arm 2: 18F-FDG-PET-CT ➜ T1–4 N0–3 M1 (≤3 metastases)
Arm 3: 18F-FDG-PET-CT ➜ T1–4 N0–3 M1 (> 3 metastases)
If no neo-adjuvant chemotherapy is administered, group allocation is based on the findings of the single 18F-FDG-PET-CT. In case of neo-adjuvant chemotherapy, group allocation is based on 18F-FDG-PET-CT with the highest number of distant metastases. Thus, patients who show a decrease of the total number of metastases or a complete response on the second 18F-FDG-PET-CT, will be allocated based on the result of their first 18F-FDG-PET-CT.
Treatment
All included patients will receive a standard of care approach which involves either RC with extended pelvic lymph node dissection (ePLND) or TMT consisting of a visible complete TURb and radiochemotherapy. Radiochemotherapy consists of moderately hypofractionated radiotherapy to a dose of 55 Gy in 20 fractions to the bladder, in combination with a radiosensitizer. In case of clinically node-positive disease, the pelvic nodal areas are included in the radiotherapy field. Decision of local therapy is at the discretion of the patient unless there is a contraindication for one treatment option. If the patient is eligible, neo-adjuvant chemotherapy will be administered.
18F-FDG-PET-CT information concerning the pelvic lymph nodes will be used to guide local treatment i.e. adapting the ePLND template or radiotherapy field, if feasible.
According to the risk-group allocation, treatment will be intensified by adding MDT or immunotherapy, in treatment arm 2 or 3, respectively. Patients will not be randomized. Both surgery and stereotactic body radiation therapy (SBRT) can be applied as MDT for oligometastases. The SBRT procedure is described in detail in an additional file (see Additional file 3). In treatment arm 3, systemic immunotherapy will be initiated in case of 18F-FDG-PET-CT detected polymetastatic disease. The systemic immunotherapy protocol can be adjusted following changes in guidelines and/or reimbursement criteria.
In case of diagnosis of metastases on the conventional imaging before local treatment is started, the patient is excluded from the trial and metastatic bladder cancer standard of care is initiated.
Follow up and data collection
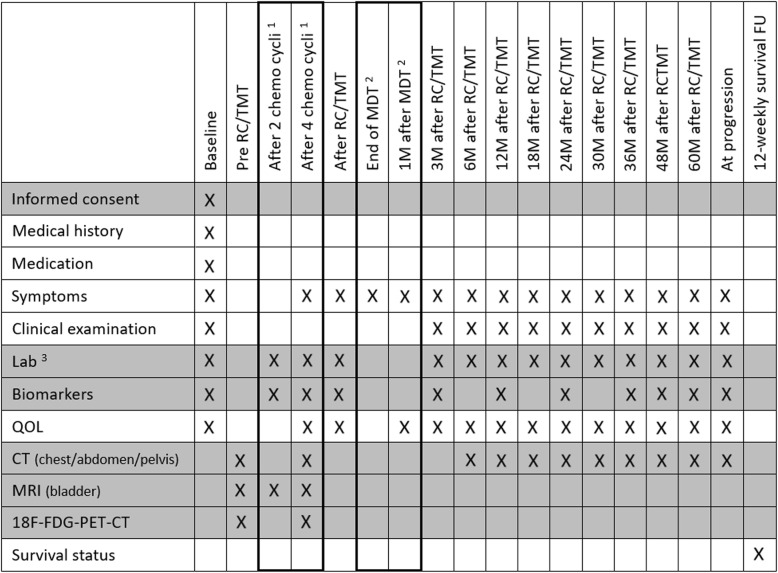
An overview of the different follow-up moments and associated assessments and investigations is presented in Fig. 2. Adverse events (AE) will be assessed using the Common Terminology Criteria for Adverse Events version (CTCAE) 5.0 [14].. Patients will be instructed by the investigator to report the occurrence of any AE. The investigator assesses and records all AE observed during the AE reporting period from inclusion until 5 years after inclusion. QOL will be assessed using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 [15] and QLQ-BLM30 [16] questionnaires. Imaging studies are done conform standard of care. In case of TMT a routine cystoscopy is advocated every 3 months during the first year of follow-up and 6-monthly thereafter until 5 years. Patients will be followed up until death or disease progression defined as per: Response Evaluation Criteria in Solid Tumors (RECIST)-criteria [17]. Once disease progression has been confirmed, survival status will be assessed 3-monthly until death, withdrawal of consent or the end of the study, whichever occurs first. Patients who are no longer followed in the center of inclusion or who refuse follow-up visits but are willing to have telephone follow-up will be contacted by phone at the scheduled follow-up time points.
Fig. 2.
Schedule of the follow-up moments and associated assessments and investigations. (1) Follow-up specific for patients receiving neo-adjuvant chemotherapy. (2) Follow-up specific for patients in treatment arm 2. (3) At predefined follow-up visits a standard blood control (including erythrocytes, leucocytes (including formula), thrombocytes, sedimentation, creatinine, electrolytes, liver/renal and inflammatory parameters) is performed. Abbreviations: QOL: quality of life; CT: computed tomography; MRI: magnetic resonance imaging; 18F-FDG-PET-CT: 18F-fluorodeoxyglucose-positron emission tomography-computed tomography; RC: radical cystectomy; TMT: trimodality treatment; MDT: metastasis-directed therapy; M: month(s); FU: follow-up
Data management and confidentiality
All study data will be handled in accordance with the law on General Data Protection Regulation (GDPR) and institutional rules. The collection and processing of personal data from subjects enrolled in this study will be limited to those data that are necessary to fulfill the objectives of the study. Study-related data of the patient will be provided in a coded manner to Ghent University Hospital. A sequential unique and coded study ID number will be attributed to each patient included into the trial, to maintain participants confidentiality. Identification of patients must be guaranteed at the center of inclusion. In order to avoid identification errors, the year of birth and the unique Study ID Number need to be provided on the case report form. All data will be collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Ghent University Hospital, a secure web-based application designed to support data capture for research studies [18]. Patient data will be stored for 25 years.
Statistical analyse
Sample size
This is a multicentric prospective phase II study in which 156 patients will be enrolled. A confidence interval for the 2-year OS was estimated using the Kaplan Meier survival estimates and corresponding confidence intervals (type ‘log-log’). These Kaplan Meier survival estimates are based on binomial proportions. When the true proportion of survival is 63% for arm 2 + 3, a sample size of 35 patients (39 when taking into account 10% drop-out rate) in group 2 + 3 yields 80% power to show that the proportions of patients surviving at 2 years, is 38% or more using an exact binomial test at alpha level 5% [12]. We expect 1/4 patients to have a positive 18F-FDG-PET-CT, based on the unpublished observations of Fonteyne et al.
Subsequent analyses
Descriptive statistics will be used to summarize patient characteristics and toxicity per treatment group. Progression and survival are defined as mentioned above and calculated from time of diagnosis to disease progression or death. Survival analysis will be compared between groups using the log-rank test. Kaplan-Meier estimates of 2-year PFS, DMFS, DSS and OS will be provided for each treatment group and as a post-hoc subgroup analysis based on patient characteristics described above. Median follow-up time will be derived using both complete and incomplete follow-up times. Cox proportional hazards regression will be used to provide hazard ratio estimates. A p-value of less than 0.05 will be considered statistically significant. For the evaluation of biomarkers on one time point, differences between groups will be tested using the Mann-Whitney U test. For the evaluation of biomarkers over time, differences between groups will be tested using the Wilcoxon signed-rank test. To evaluate correlations, Spearman correlation coefficients will be calculated. A p-value of less than 0.05 will be considered statistically significant.
All statistical analyses will be performed using SPSS (SPSS Inc., Chicago, Il, USA).
Discussion
After diagnosis of non-metastatic MIBC, patients undergo primary staging followed by neo-adjuvant chemotherapy (if eligible) and radical local treatment. The prognosis of MIBC remains poor, despite this aggressive treatment [3, 4]. Several reports indicate the superiority of 18F-FDG-PET-CT compared to CT in the detection of lymph node and distant metastases [7]. Furthermore, there is evidence that the 18F-FDG-PET-CT result is correlated with OS and DSS [8]. We hypothesize that the additional information of 18F-FDG-PET-CT can be used to guide local treatment in case of presence of pelvic nodes metastases and that the presence of extra-pelvic metastases on 18F-FDG-PET-CT enables MDT or necessitates the early initiation of immunotherapy. The aim of this prospective phase II trial is to evaluate the impact of implementing a 18F-FDG-PET-CT in stratifying patients with primary MIBC and tailoring the treatment accordingly in order to improve the patient’s outcome.
Supplementary Information
Additional file 1. Items from the World Health Organization Trial Registration Data Set.
Additional file 2. 18F-FDG-PET-CT procedure. Description of the 18F-FDG-PET-CT procedure.
Additional file 3. Stereotactic body radiation therapy (SBRT) procedure, conducted as metastasis directed therapy (MDT) in treatment arm 2.
Acknowledgements
Not applicable.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- AAPM
The American Association of Physicists in Medicine
- AE
Adverse events
- CBCT
Cone beam computed tomography
- CT
Computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DMFS
Distant metastasis-free survival
- DSS
Disease-specific survival
- EORTC
European Organisation for Research and Treatment of Cancer
- GTV
Gross tumor volume
- Gy
Gray
- IMRT
Intensity-modulated radiotherapy
- mCi
Millicurie
- MDT
Metastasis-directed therapy
- MIBC
Muscle-invasive bladder cancer
- MRI
Magnetic resonance imaging
- OAR
Organs at risk
- OS
Overall survival
- PET-CT
Positron emission tomography-computed tomography
- PFS
Progression-free survival
- PRV
Planning risk volume
- PTV
Planning target volume
- QOL
Quality of life
- RC
Radical cystectomy
- RECIST
Response Evaluation Criteria in Solid Tumors
- SBRT
Stereotactic body radiation therapy
- TMT
Trimodality therapy
- TURb
Transurethral resection of the bladder
- VMAT
Volumetric modulated arc therapy
Authors’ contributions
Study Conception: VF. Initial study design: VF, PO, SR, DDM and KD. Revision of study design and protocol: all authors’ (e.g. FV, LP, SV, PD, MA, GDM, CB, PO, GV, PDV, KDM, DDM, SR, CVP, KD and VF). Study coordination: VF, FV and LP. Participating centers: VF, PD and GDM. All authors’ read and approved the final manuscript.
Authors’ information
Not applicable.
Funding
Research project funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish cancer society (projectID: 12146)”. The funding body provided peer-review and is not involved in the design of the study and collection, management, analysis, and interpretation of data and in writing or submitting of the manuscript.
Availability of data and materials
The data set used and/or analyzed during the current study are available from the corresponding author on reasonable request. Not all data are obtained yet since the study is still ongoing.
Declarations
Ethics approval and consent to participate
This study is approved by the Ethics Committee of the Ghent University Hospital on 11/5/2020 (BC-07456). Any significant change or addition to the protocol will only be made in a written protocol amendment that is approved by the Ethics Committee. All patients will give their written informed consent before inclusion. This will be obtained after a clear and thorough discussion between the patient and the consenting physician.
Consent for publication
Not applicable.
Competing interests
VF has received grant funding from Ipsen and has consulted for Ipsen and Janssen, all unrelated to this research project. PO has received grants from Ferring Pharmaceuticals, Merck, Varian and Bayer, has consulted for Ferring Pharmaceuticals, Bayer, Janssen, Curium and Novartis, all unrelated to this research project. VPC has consulted for Astellas, unrelated to this project. The other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cumberbatch MGK, Noon AP. Epidemiology, aetiology and screening of bladder cancer. Transl Androl Urol. 2019;8(1):5–11. doi: 10.21037/tau.2018.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janisch F, Yu H, Vetterlein MW, Dahlem R, Engel O, Fisch M, Shariat SF, Soave A, Rink M. Do younger patients with muscle-invasive bladder Cancer have better outcomes? J Clin Med. 2019;8(9):1459. doi: 10.3390/jcm8091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, White RWV, Sarosdy MF, Wood DP, Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, Bostrom PJ, Kuk C, Li K, Templeton AJ, Sridhar SS, van der Kwast T, Chung P, Bristow RG, Milosevic M, Warde P, Fleshner NE, Jewett MAS, Bashir S, Zlotta AR. Propensity score analysis of radical cystectomy versus bladder-sparing Trimodal therapy in the setting of a multidisciplinary bladder Cancer clinic. J Clin Oncol. 2017;35(20):2299–2305. doi: 10.1200/JCO.2016.69.2327. [DOI] [PubMed] [Google Scholar]
- 6.EAU: Guidelines Muscle-invasive and Metastatic Bladder Cancer. https://uroweb.org (2021). Accessed 16 Jun 2021.
- 7.Fonteyne V, De Man K, Decaestecker K, De Visschere P, Dirix P, De Meerleer G, et al. PET–CT for staging patients with muscle invasive bladder cancer: is it more than just a fancy tool? Clin Transl Imaging. 2021;9(1):83–94. doi: 10.1007/s40336-020-00397-7. [DOI] [Google Scholar]
- 8.Mertens LS, Mir C, Scott AM, Lee ST, Bruining A, Vegt E, et al. PET/CT predicts mortality in muscle invasive bladder cancer. BJU Int. 2013;111:71. doi: 10.1111/bju.12138. [DOI] [Google Scholar]
- 9.Shimada Y, Saji H, Kakihana M, Kajiwara N, Ohira T, Ikeda N. Survival outcomes for oligometastasis in resected non-small cell lung cancer. Asian Cardiovasc Thorac Ann. 2015;23(8):937–944. doi: 10.1177/0218492315596463. [DOI] [PubMed] [Google Scholar]
- 10.Decaestecker K, De Meerleer G, Ameye F, Fonteyne V, Lambert B, Joniau S, et al. Surveillance or metastasis-directed therapy for OligoMetastatic prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer. 2014;14(1):671. doi: 10.1186/1471-2407-14-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67(5):852–863. doi: 10.1016/j.eururo.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Abe T, Minami K, Harabayashi T, Sazawa A, Chiba H, Kikuchi H, Miyata H, Frumido J, Matsumoto R, Osawa T, Junji I, Tango M, Satoshi C, Tomoshige A, Masashi M, Naoto M, Kunihiko T, Satoru M, Murai S, Shinohara N. Prognostic impact of local radiotherapy on metastatic urothelial carcinoma patients receiving systemic chemotherapy. Jpn J Clin Oncol. 2020;50(2):206–213. doi: 10.1093/jjco/hyz152. [DOI] [PubMed] [Google Scholar]
- 13.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 14.NIH National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. [Google Scholar]
- 15.Fau AN, Ahmedzai S, Fau AS, Bergman B, Fau BB, Bullinger M, Fau BM, Cull A, Fau CA, Duez NJ, Fau DN, Filiberti A, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16.EORTC Quality of Life: QLQ-BLM30 muscle invasive bladder cancer. https://qol.eortc.org/questionnaire/qlq-blm30. Accessed 10 Jun 2021.
- 17.Eisenhauer EA, Fau TP, Bogaerts J, Fau BJ, Schwartz LH, Fau SL, Sargent D, Fau SD, Ford R, Fau FR, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Fau TR, Thielke R, Fau TR, Payne J, Fau PJ, Gonzalez N, Fau GN, Conde JG, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Items from the World Health Organization Trial Registration Data Set.
Additional file 2. 18F-FDG-PET-CT procedure. Description of the 18F-FDG-PET-CT procedure.
Additional file 3. Stereotactic body radiation therapy (SBRT) procedure, conducted as metastasis directed therapy (MDT) in treatment arm 2.
Data Availability Statement
The data set used and/or analyzed during the current study are available from the corresponding author on reasonable request. Not all data are obtained yet since the study is still ongoing.