Abstract
Chronic wounds remain a substantial source of morbidity worldwide. An emergent approach that may be well suited to inducing the complex, multicellular processes such as angiogenesis that are required for wound repair is the use of extracellular vesicles (EVs). EVs contain a wide variety of proteins and nucleic acids that enable multifactorial signaling. Here, we leverage the capability of EVs to be engineered via producer cell modification to investigate the therapeutic potential of EVs from mesenchymal stem/stromal cells (MSCs) transfected to overexpress long non-coding RNA HOX transcript antisense RNA (HOTAIR). HOTAIR was previously shown by our group to be critical in mediating angiogenic effects of endothelial cell EVs, and MSCs were chosen as EV producer cells for this study due to their widely reported intrinsic angiogenic properties. The results indicate that MSCs overexpressing HOTAIR (HOTAIR-MSCs) produce EVs with increased HOTAIR content that promote angiogenesis and wound healing in a diabetic (db/db) mice. Further, endothelial cells exposed to HOTAIR-MSC EVs exhibit increased HOTAIR content correlated with upregulation of the angiogenic protein vascular endothelial growth factor (VEGF). Thus, this study supports EV-mediated HOTAIR delivery as a strategy for further exploration towards healing of chronic wounds.
Keywords: exosomes, lncRNAs, MSCs, diabetic wounds, excisional wounds
1. Introduction
Approximately 8.2 million Medicare beneficiaries were found to have a chronic wound in a recent study in the United States, with management of these wounds incurring over $28.1-$96.8 billion in healthcare expenses.[1] These numbers are only expected to rise along with increasing prevalence of diabetes and an aging population.[1] Additional human and financial costs are added when accounting for wounds created by surgical interventions as well as those resulting from burns and trauma.[2], [3] Thus, while there are several products for wound repair currently available to patients, new approaches are still needed.[4], [5]
While wound healing is a complex, multicellular process, induction of angiogenesis within the wound bed is thought to be requisite for long-term productive healing.[6], [7] In recognition of this, many approaches to promote angiogenesis therapeutically have been tried, including delivery of angiogenic genes and proteins using a variety of strategies.[8]–[10] However, clinical translation of such efforts has been limited for a number of reasons, some of which owe to the fact that the complexity of angiogenesis is difficult to control via regulation of a small number of molecular pathways.[11]
As a result, new approaches aimed at stimulating angiogenesis more broadly have emerged. One of these involves the use of extracellular vesicles (EVs), which can carry a variety of cargo molecules – such as proteins, lipids, and nucleic acids of many types – capable of actuating numerous signaling nodes within recipient cells.[12] This multifunctional potential distinguishes EVs from nanoscale synthetic drug carriers, for which loading of a multitude of different cargo classes and molecules presents significant challenges.[13]–[15] EVs are produced by cells and have been implicated as key agents driving angiogenesis resulting from cell-based therapies.[16], [17] Additionally, EVs present a more favorable risk profile when compared to whole cells, as EVs cannot divide or differentiate after administration and have been reported to have low toxicity and immunogenicity in immunocompetent animals[18], [19] as well as in human patients.[20] Crucially, the use of EVs leverages the potential of molecular biology and genetic engineering, as producer cell modifications can be used to engineer EV properties to enhance therapeutic potency.
In this study, we sought to apply the ability to engineer EV properties via producer cell modification in a specific manner, building on prior work from our group that revealed potential pro-vascularization roles for long non-coding RNAs (lncRNAs) in EV-mediated angiogenesis.[21] In particular, upregulation of the lncRNA HOX transcript antisense RNA (HOTAIR) was shown to correspond with increased angiogenesis induced by endothelial cell-derived EVs, while knockdown of HOTAIR expression substantially reduced the effect. HOTAIR is among the most well-characterized lncRNAs and has been shown to promote angiogenesis in several settings.[22]–[25] Therefore, we hypothesized that EVs containing HOTAIR could be effective at promoting vascularization and wound healing. We chose to utilize human bone marrow-derived mesenchymal stem/stromal cells (MSCs) as EV producer cells, as MSC EVs have been shown to intrinsically promote angiogenic effects in a number of animal models, including in wound repair.[26]–[30] Here, we report the generation and characterization of EVs from MSCs overexpressing HOTAIR (HOTAIR-MSCs), as well as their application in two excisional wound healing models. The results demonstrate that HOTAIR-MSC EVs have therapeutic potential in enhancing angiogenesis and promoting wound repair.
2. Results
2.1. EV characterization and HOTAIR loading
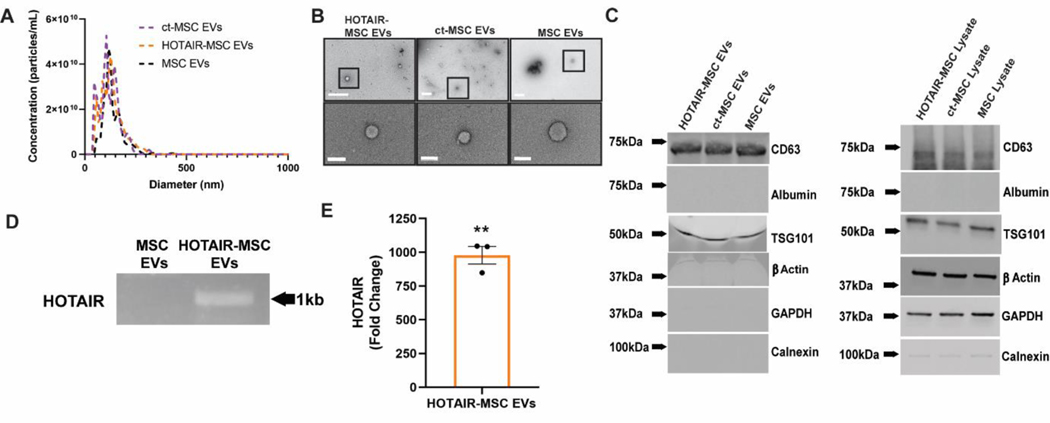
EVs isolated from MSCs overexpressing HOTAIR (HOTAIR-MSC EVs), MSCs transfected with only the plasmid backbone (control transfected-MSC EVs, ct-MSC EVs), and MSCs that were not altered (MSC EVs) were independently produced for each of the following characterizations and run in triplicate. EVs were first assessed via nanotracking analysis (NTA). No significant differences in EV mean diameter or size distribution between groups were observed, with the diameter measurement in the expected range of 100–120 nm (Figure 1A). Additionally, total protein present in the EV preparation was assessed by BCA and compared with vesicle count via both nanoparticle tracking analysis using a NanoSight LM10 and CD63+ ELISA. No significant differences were found between HOTAIR-MSC EVs and native MSC EVs in any of these metrics (Figure S1), and so protein level was used for normalization in further experiments based on that method being the most practical and reproducible. Typical EV morphology was observed via TEM (Figure 1B). Immunoblot analyses confirmed the association of TSG101 and CD63 with EVs. EV samples also showed negligible levels of endoplasmic reticulum (ER) marker calnexin and serum transport protein albumin (Figure 1C). The content of HOTAIR within isolated native MSC EVs and HOTAIR-MSC EVs was visually confirmed using gel electrophoresis of PCR products for a 1 kb segment of HOTAIR after reverse transcriptase of RNA isolated from EVs (Figure 1D). Levels of HOTAIR with EVs were also quantified using RT-qPCR, showing that HOTAIR-loaded MSC EVs had 974-fold increase in HOTAIR compared to ct-MSC EVs (Figure 1E). Additionally, EV preparations were screened for soluble VEGFA using a Western blot, as it has been shown that VEGFA may be a potential contaminant during MSC EV isolation that could impact angiogenic bioactivity.[31] In this case, there were no detectable levels of VEGFA protein (Figure S2).
Figure 1. EV characterization and HOTAIR loading confirmation.
(A) Nanotracking analysis of HOTAIR-transfected MSC EVs, control-transfected (ct)-MSC EVs, and native MSC EVs. (B) Transmission electron microscopy (TEM) of the same groups reveal characteristic EV morphology (top row, scale bar = 500nm; bottom row, scale bar = 100nm). (C) Immunoblots of EV-associated markers as well as endoplasmic reticulum (ER) marker (calnexin), serum transport protein albumin, β-Actin, and GAPDH as controls. (D) Gel electrophoresis of a PCR reaction of a 1kb region of HOTAIR-MSC EVs and native MSC EVs from cDNA constructed from EV RNA. (E) Quantification of HOTAIR in HOTAIR-loaded MSC EVs by RT-qPCR compared to ct-MSC EVs. Statistical significance was calculated using one sample t test with hypothetical value equal to 0 (** p<0.01,) (n=3).
3.2. In vitro validation of HOTAIR-MSC EV activity
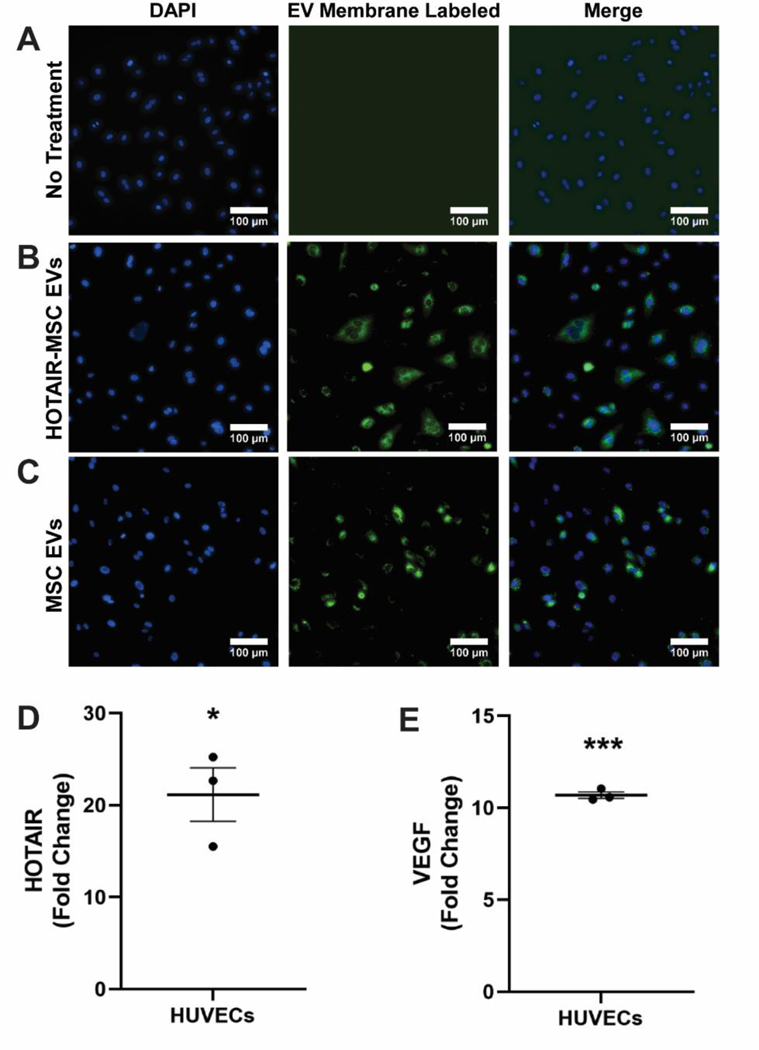
To investigate the angiogenic potential of delivery of HOTAIR via HOTAIR-MSC EVs, the lipid bilayer of EVs was labeled with a fluorescent tag and then administered to human umbilical vein endothelial cells (HUVECs). Cellular association of both MSC EVs and HOTAIR-MSC EVs was evident when compared to untreated cells (Figures 2A-C). Additionally, HOTAIR levels were found to be increased by ~21 fold in HUVECs exposed to HOTAIR-MSC EVs as compared to untreated cells, suggestive of successful HOTAIR delivery (Figure 2D; *p<0.05). Further, prior studies demonstrated that HOTAIR can upregulate VEGFA via a number of angiogenic pathways.[32]–[34] Here, an ~11-fold increase in VEGFA in HUVECs treated with HOTAIR-MSC EVs when compared to ct-MSC EVs was observed (Figure 2E; ***p<0.001).
Figure 2. HOTAIR Delivery to HUVECs via HOTAIR-MSC EVs.
Images of (A) untreated; (B) HOTAIR-MSC EV-treated; and (C) native MSC EV-treated HUVECs in which EV-membrane was labeled and HUVEC nuclei counterstained with Hoechst33372. (D) Quantification of HOTAIR in HUVECs treated with HOTAIR-MSC EVs by RT-qPCR compared to untreated HUVECs. (E) Quantification of VEGFA in HUVECs treated with HOTAIR-MSC EVs by RT-qPCR compared to ct-MSC EVs. Statistical significance was calculated using one sample t test with hypothetical value equal to 0 (*p<0.05, ***p<0.001,) (n=3).
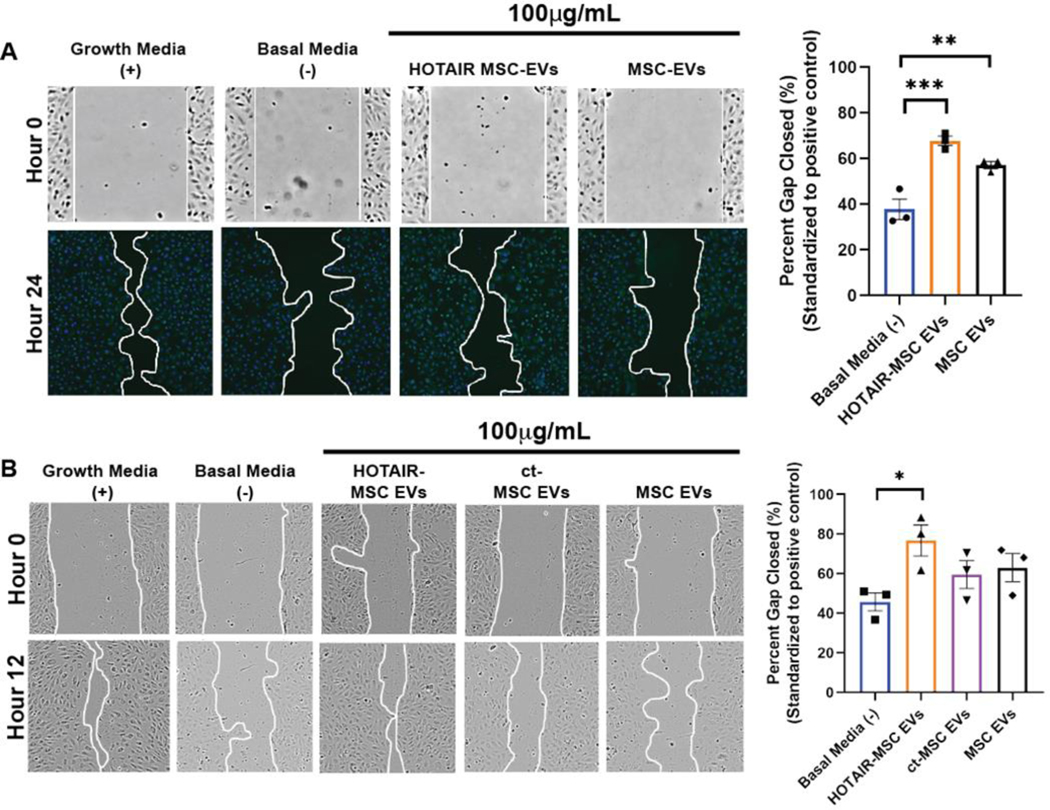
Endothelial cell gap closure assays utilizing both human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs) were utilized to assess in vitro bioactivity of HOTAIR-MSC EVs. Passage 4 (P4) BDMSCs were cultured for EV isolation based on prior data indicating EV bioactivity is retained from cells at this passage.[35] When applied to HDMECs, both HOTAIR-MSC EVs and MSC EVs induced significant increases in gap closure compared to the basal media control (Figure 3A). When applied to HUVECs over three independent experiments (each in triplicate), only HOTAIR-MSC EVs induced significant improvement in gap closure (Figure 3B), although MSC EVs and control transfected-MSC EVs showed a trend towards increased closure.
Figure 3. In vitro assessment of HOTAIR-MSC EV activity.
(A) HDMECs were treated with growth media only (EGM; positive control), basal media only (EBM; negative control), 100 μg/mL HOTAIR-MSC EVs in basal media, or 100 μg/mL native MSC EVs in basal media. Images were taken at time 0 (brightfield) and 24h post wounding of the cell layer (DAPI-stained). Representative images for each group and timepoint are displayed and the gap area outlined with a white line. (B) HUVECs were treated with growth media (EGM; positive control), basal media (EBM; negative control), 100 μg/mL HOTAIR-MSC EVs in basal media, 100 μg/mL ct-MSC EVs in basal media, or 100 μg/mL native MSC EVs in basal media. Brightfield images were taken at time 0 (brightfield) and 12h post wounding of the cell layer. Representative images for each group and timepoint are displayed and the gap area outlined with a white line. For both A and B, data are compared to growth media positive control values (set to 100%) and statistical significance was calculated using one-way ANOVA with Tukey’s test (* p<0.05, **p<0.01, ***p<0.001) (n=3).
2.3. In vivo excisional wound healing models and histological analysis
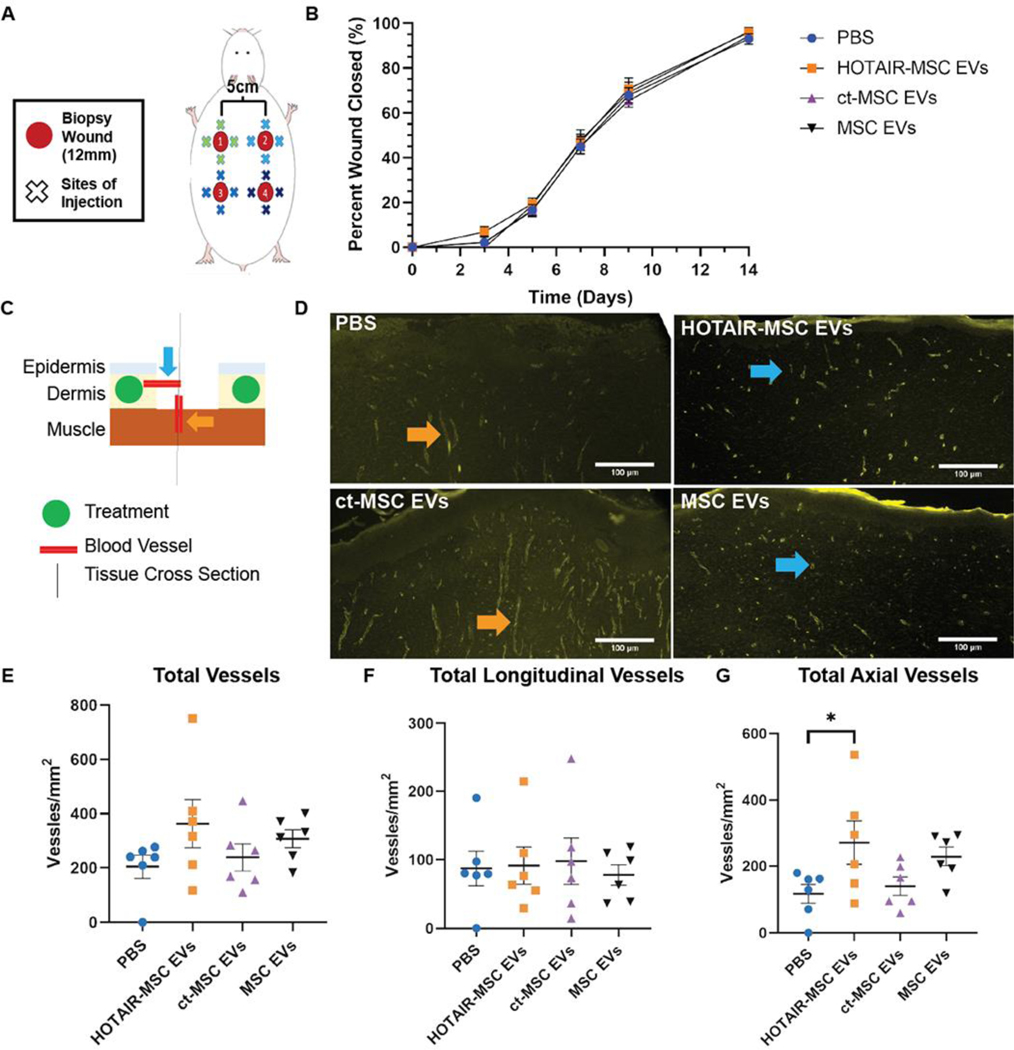
With in vitro bioactivity confirmed, we then assessed effects in rodent wound healing models. First, a full-thickness excisional model employing healthy retired breeder Sprague-Dawley rats was utilized. Four 12mm excisional wounds were created on the dorsum of each rat as indicated (Figure 4A). 50 μg (based on BCA protein content) of either HOTAIR-MSC EVs, control transfected-MSC EVs, native MSC EVs, or PBS as a vehicle control, were administered around the wound four times in a cross pattern on day 3. Since the wounds were placed on different regions of the dorsum, the treatments were rotated in a clockwise manner to account for potential differences in caudal versus cephalic healing. As expected based on the healthy status of the animals used, we found no statistically significance difference in wound closure among any of the groups (Figure 4B, Figure S3). However, given the reported pro-angiogenic properties of HOTAIR, we did expect a local vascularization effect. To assess this, we harvested tissue of 6 rats on day 21 when the wounds were fully healed for H&E and CD31+ immunohistochemical analysis to analyze blood vessel composition. Tissue sections across the center of the wound were created so that the cross section included both the epidermis and dermis. Using this sectioning method, we made the following two assumptions regarding blood vessel origination in our quantitative analysis of new blood vessel formation: 1) blood vessels that would be originating from the dermis would typically be viewed as axial cuts of the vessels, and 2) blood vessels originating from the underlying muscle wound typically be viewed as longitudinal cuts progressing vertically (Figure 4C). Analysis of stained slides (Figure 4D) revealed no significant differences in the total number of blood vessels (Figure 4E) and the number of longitudinal vessels (Figure 4F) among the groups. However, the number of axial vessels (Figure 4G) induced by HOTAIR-MSC EVs was found to be significantly higher when compared to the PBS vehicle control group (Figure 4E; 271 ± 66 vessels/mm2 vs. 117 ± 28 vessels/mm2, p<0.05).
Figure 4. HOTAIR-MSC EVs do not improve healing of excisional wounds in healthy Sprague-Dawley rats but do induce local angiogenic effects.
(A) Schematic of 12 mm excisional wound healing model in Sprague-Dawley rats. (B) Wound closure as assessed using digital planimetry for wounds treated with HOTAIR-MSC EVs, ct-MSC EVs, native MSC EVs, or PBS. Statistical significance was calculated using two-way ANOVA with Holm-Sidak’s multiple comparison test (n=11). (C) Schematic of day 21 tissue processing indicating blood vessel perspective based on the sectioning of tissue with axial vessels originating from the dermis and vertical, longitudinal vessels originating from the underlying muscle. Treatments were injected within the dermis. (D) CD31+ immunohistochemistry of tissue highlighting newly formed blood vessels in the center of the healed wound. Color-coded arrow heads correspond to (C). (E) Total, (F) longitudinal, and (G) axial vessel were counted and displayed as number of vessels per square millimeter of tissue. Statistical significance was calculated using one-way ANOVA with Holm-Sidak’s multiple comparison test (* p<0.05; n=6).
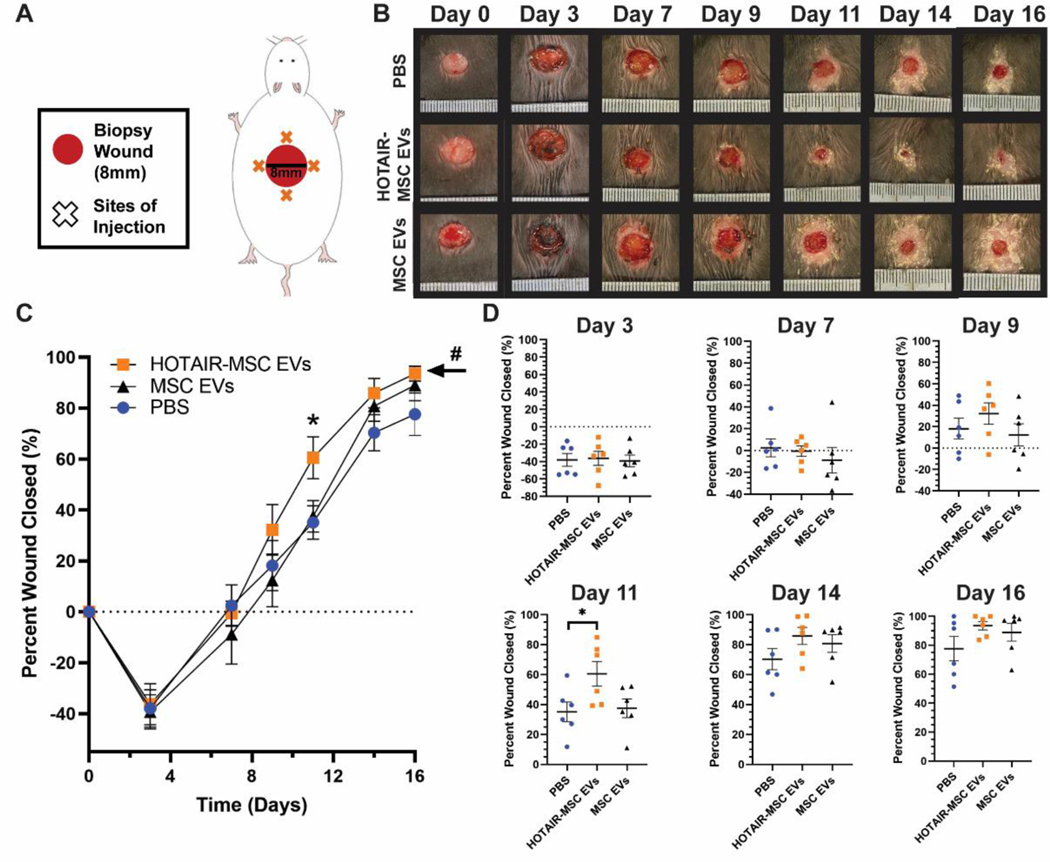
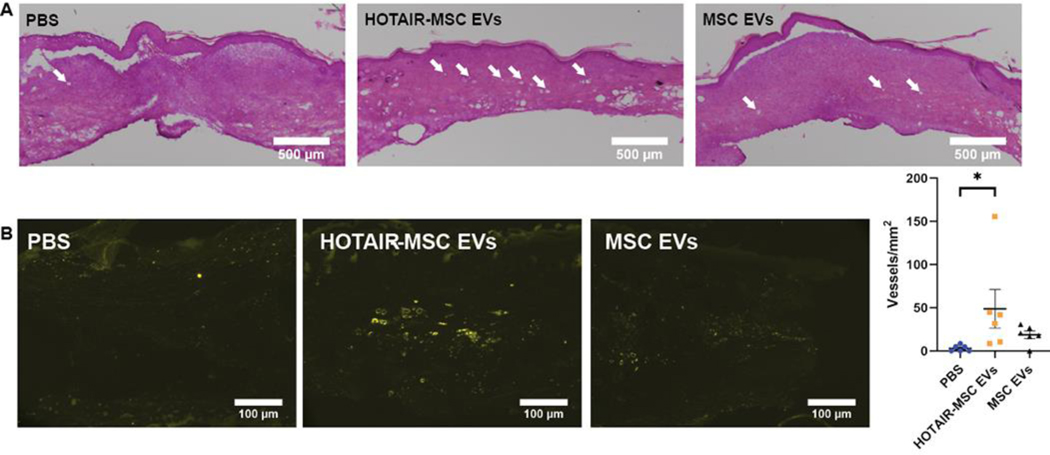
To further assess if this potential angiogenic effect of HOTAIR-MSC EVs could induce functional benefits in wound repair, we utilized a diabetic db/db mouse model where significant impairment in wound healing is expected. Each mouse had an 8 mm punch biopsy excisional wound placed on its dorsum (Figure 5A). Similar to the rat model, treatments of 50 μg of EVs were administered four times in a cross pattern on day 3. The experimental groups were HOTAIR-MSC EVs, native MSC EVs, and PBS vehicle control. In this model, wounds on day 3 still remained larger than the wounds on day 0 due to edema that occurs during inflammation post-wounding[36] that is exacerbated by the chronic inflammatory state associated with diabetes.[37] HOTAIR-MSC EVs induced a statistically significant improvement in healing overall when compared to both PBS vehicle control and native MSC EVs as assessed by two-way ANOVA (Figure 5B,C; p<0.05), whereas native MSC EVs did not induce any significant changes compared to PBS. Additionally, the data indicate that HOTAIR-MSC EVs may increase the rate of wound closure as evidenced by the significantly increased closure observed at day 11 (Figure 5C,D; 61 ± 8% compared to 31 ± 7% for PBS vehicle control, p<0.05). To assess angiogenesis, tissue from all 6 mice per group was collected on day 21 and H&E staining and CD31 immunohistochemistry were performed (Figure 6A,B). Animals treated with HOTAIR-MSC EVs were found to have significantly more CD31+ vessel structures than animals treated with PBS vehicle controls (Figure 6B; 49 ± 22 vessels/mm2 versus 3 ± 1 vessels/mm2; p<0.05), while animals treated with native MSC EVs did not exhibit significantly increased vessel numbers.
Figure 5. HOTAIR-MSC EVs improve wound healing in db/db mice.
(A) Schematic of 8 mm excisional wound healing model in diabetic (db/db) mice. (B) Representative images of PBS-, HOTAIR-MSC EVs-, and native MSC EVs-treated wounds. (C,D) Wound closure as assessed using digital planimetry for wounds treated with HOTAIR-MSC EVs, native MSC EVs, or PBS. # indicates statistical significance (p<0.05) of overall improved wound healing of HOTAIR-MSC EVs when compared to both native MSC EVs and PBS. Statistical significance was calculated using two-way (C) and one-way (D) ANOVA with Holm-Sidak’s multiple comparison test (* p<0.05; n=6).
Figure 6. HOTAIR-MSC EVs induced increased angiogenesis in healed tissue of db/db mice.
(A) Representative H&E staining of day 21 healed tissue of animals treated with PBS, HOTAIR-MSC EVs, and native MSC EVs. White arrowheads indicate blood vessels within the tissue. (B) CD31+ immunohistochemistry of tissue highlighting newly formed blood vessels. Only axial cuts of vessels were visible throughout all tissue sections analyzed. Vessels were counted and displayed as number of vessels per square millimeter of tissue. Statistical significance was calculated using one-way ANOVA with Holm-Sidak’s multiple comparison test (* p<0.05; n=6).
3. Discussion
Enhancing the therapeutic potential of EVs via cargo loading has been an active area of research for our group and others.[19], [38], [47], [39]–[46] In particular, EVs have been investigated as delivery vehicles for small RNAs such as miRNA and siRNA owing to the physiological roles of EVs in intercellular communication via RNA transfer.[19], [38], [41], [44], [47] In this study, we examined the potential of a different class of nucleic acid cargo, lncRNA, as therapeutic EV cargo. We showed that MSC EVs containing significantly enhanced amounts of lncRNA HOTAIR were effective in promoting vascularization of a wound bed and enhancing wound repair in diabetic mice, whereas native MSC EVs were not statistically superior to control treatments (Figures 4–6). These results support the findings of the small number of other studies on EV-mediated lncRNA therapeutic effects in identifying lncRNAs as a potentially important class of therapeutic molecules worthy of further study.[48] Specifically, HOTAIR delivery by non-MSC EV sources could be investigated to determine if HOTAIR is sufficient to induce therapeutic effects.
To load HOTAIR into EVs for these experiments, we utilized non-viral plasmid transfection to promote overexpression within the producer MSCs resulting in ~1000-fold increased EV HOTAIR content (Figure 1). This method is similar to that described by Li et. al. in which lncRNA H19 was loaded into adipose-derived MSC EVs that were subsequently shown to accelerate wound healing in a mouse model by upregulating PTEN via miR-152–3p.[30] Our study with HOTAIR-MSC EVs showed a similar magnitude of overall wound healing improvement as seen with h19-MSC EVs at the terminal point of each study (~25% compared to control). Interestingly, in both cases, control MSC EVs did not induce significant improvement compared to control, in contrast to several prior reports. There are many potential explanations for this disparity, however one notable possibility is that of contamination of some MSC EV preparations with pro-angiogenic proteins such as VEGFA, as rigorously identified in a recent report.[31] We did not observe detectable levels of VEGFA in the EV preparations used in these studies (Figure S2). Regardless, both of these studies identified different miRNA-protein axes as potential regulators of the reported outcomes, suggesting that co-delivery of H19 and HOTAIR may yield additive or synergistic effects. This possibility is supported by prior work from our group identifying potential additive effects between lncRNA MALAT1 and HOTAIR in endothelial cell-derived EV vascularization effects.[21] Indeed, depletion of MALAT1 from adipose-derived stem cell-derived EVs reduced their anti-inflammatory therapeutic properties in a model of traumatic brain injury.[49] Thus, delivery of multiple lncRNAs via EVs may have promise over a wide range of applications.
Due to current limitations in generating synthetic lncRNAs, EVs represent one of the only practical options for lncRNA delivery at present. Beyond overexpression, other methods have also been used to incorporate specific therapeutic lncRNAs into EVs, including control of the EV production environment and strategic selection of native producer cells based on lncRNA expression[21], [48], [50]–[52] Small RNAs such as miRNAs and siRNAs have been loaded into EVs utilizing exogenous methods, such as electroporation, sonication, and pH gradients.[19], [38], [43], [44] However, such approaches all depend fundamentally on diffusion and thus may not be viable for lncRNAs given their relatively large size even if synthetic lncRNAs become widely available.
Several limitations to our study should also be acknowledged. Analysis of angiogenesis, which is a three-dimensional process, using immunohistochemistry on two-dimensional tissue sections has limitations. To further verify the location from which newly formed blood vessels initially sprouted, a computational reconstruction model or another three-dimensional approach could be utilized. Additionally, transfection of EV-producing cells for the purpose of loading specific cargo (e.g. HOTAIR) may also induce other convoluting changes in EV content and bioactivity that were not studied here. Further, in these studies EVs were delivered on day 3 post-wounding, when cells were in the early stages of proliferation. Taking in to account that lncRNAs are capable of acting both in the nucleus and cytoplasm,[53]–[55] this delivery scheme may have enabled nuclear effects that would be less likely to occur in treatment of non-proliferating cells. However, it is notable that lncRNA transport to the nucleus would require a nuclear signaling complex within the RNA,[56] nuclear envelope invagination associated with late endosomes,[57] or possible entrance during cell division when the nuclear envelope breaks down. Moreover, there are no established dosing or scheduling protocols for EVs in general, and these factors would certainly be expected to impact efficacy outcomes. There are, of course, practical limitations on the doses and schedules of EVs that can be tested in any model, but development of further general knowledge in these areas may necessitate re-examination of many studies of therapeutic EVs in the future, including this one.
In summary, we report the potential of HOTAIR-MSC EVs for therapeutic angiogenesis and wound healing. In addition to accelerating and enhancing wound closure in a diabetic db/db mouse model, HOTAIR-MSC EVs induced an increase in the number of vessels in healed tissue. This suggests that eventual translation of such a treatment would be beneficial especially for those with compromised healing, such as the elderly and diabetic, or for those undergoing reconstructive procedures where revascularization is key for survival of skin flaps and grafts. Increasing the therapeutic arsenal associated with EVs via loading of lncRNAs can help promote their eventual successful use in the clinic.
4. Experimental Section
4.1. Cell culture
Bone marrow-derived mesenchymal stem cells (BDMSCs) were obtained from ATCC and cell passage was designated as P1 from time of arrival. BDMSCs were plated in a T175 polystyrene flask and cultured in BDMSC media, consisting of Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids, and 1% penicillin/streptomycin. Cells were grown to 80–90% confluency and replated at 250,000 cells/T175 flask until P4 when the cells were stored in liquid nitrogen within media supplemented with 10% DMSO as a cryoprotectant for all experimental purposes. Human dermal microvascular endothelial cells (HDMECs) and human umbilical cord endothelial cells (HUVECs) were obtained commercially from Promocell and cell passage was again designated as P1 from the time of arrival. HDMECs and HUVECs were cultured in Endothelia Growth Medium-2 (EGM2) (Promocell, C-22221 and C-39221). Cells were cyclically grown to 80–90% confluency and replated at 250,000 cells/T175 flask until P4 when the cells were stored in liquid nitrogen in media supplemented with 10% of DMSO as a cryoprotectant for all experimental purposes. For EV collection, BDMSCs were cultured in EV-depleted FBS media. FBS (VWR) was heat inactivated (HI) at 56oC for 30 min with intermittent bottle inversions, and then chilled on ice for 30 min. HI-FBS was then centrifuged at 100,000 × g for at least 16 h and the supernatant was filtered through a 0.20 μm bottle top filter (VWR) for subsequent use in BDMSC media.
4.2. Plasmid assembly and transfection
pCMV-GFP was a gift from Connie Cepko (Addgene plasmid # 11153 ; http://n2t.net/addgene:11153 ; RRID:Addgene_11153). LZRS-HOTAIR was a gift from Howard Chang (Addgene plasmid # 26110 ; http://n2t.net/addgene:26110 ; RRID:Addgene_26110). HOTAIR from LZRS-HOTAIR was subcloned into the backbone of pCMV-GFP using KOD DNA Polymerase (EMD Millipore, 71086–3) for amplification and Gibson Assembly Cloning Kit (New England BioLabs, E5510S) for assembly to create pCMV-HOTAIR. The GFP from the pCMV-GFP plasmid was also deleted to obtain the pCMV-Backbone plasmid using KOD DNA polymerase and the Gibson Assembly Cloning Kit. Plasmids were sent for Sanger sequencing to Genewiz to verify appropriate sequences. Maxiprep Kits (Qiagen, 12162) were used to amplify the plasmids for use in transfections.
For transfection, 500,000 P4 BDMSCs were plated in a T175 in BDMSC media and left to adhere overnight. Cells were then transfected with either pCMV-HOTAIR plasmid or pCMV-Backbone plasmid using Lipofectamine 3000 Transfection Reagent (Thermo Fisher Scientific) using its corresponding protocol. Briefly, plasmid (23,000 ng) was added to OPTI-MEM (1.7 mL) (VWR) in a 15 mL tube. After vortexing to ensure homogenous composition of plasmid in OPTI-MEME, P3000 reagent (45 μL) was added and the solution was vortexed a second time. In another 15 mL tube, lipofectamine reagent (97 μL) was added to OPTI-MEM (1.7mL). The lipofectamine solution was added to the plasmid/P3000 solution and vigorously pipetted up and down at least 10 times and then left at room temperature for at least 5 min. The lipofectamine solution with the DNA plasmid of interest was then added to BDMSC media (20 mL) in a 50 mL conical tube and inverted at least 10 times. The old media over the BDMSCs was aspirated and the new media, containing the transfection reagents and plasmids, was placed over the cells. The flask was left to incubate undisturbed for 1 h at 37oC. After 1 h, the media was aspirated from the cells. The cells were washed thoroughly with warm, sterile PBS (20 mL) 3X, making sure to wash all areas the previous plasmid-containing media came into contact with. After washing, EV-depleted BDMSC media (30 mL), consisting of DMEM supplemented with 10% EV-depleted FBS, 1% non-essential amino acids, and 1% penicillin/streptomycin, was placed over the cells. Media was collected on days 3, 4, 5, and 6 post-transfection, with new EV-depleted media (30 mL) being placed over the cells after each collection.
4.3. EV separation and characterization
After day 6 of collecting EV-rich media (120 mL), EVs were isolated using multiple rounds of centrifugation. First, media was centrifuged at 1000 × g for 10 min to remove any cells that had detached from the flask. The supernatant from this centrifugation was then centrifuged at 2000 × g to remove any large, cellular debris. The following supernatant was then centrifuged at 10,000 × g for 30 min to remove smaller organelles that may have been present. Finally, to collect the EVs, the supernatant was centrifuged at 100,000 × g for 2 h in 8 tubes, each capable of holding roughly 26 mL. The supernatant was discarded, and the remaining pellets containing EVs were resuspended in sterile PBS (500 μL), transferring the PBS from each of the 8 tubes. EVs were washed 3X with sterile PBS using four Nanosep Centrifugal Devices with 300 kDa MWC Omega Membranes (Pall, OD300C35). EVs were resuspended in PBS (150 μL) and stored at −20oC for up to one month with less than 2 freeze/thaw cycles.
Negative staining of EVs was performed to obtain transmission electron microscopy (TEM) images. Briefly, 4% paraformaldehyde (20 μL) was added to washed EVs (15 μL). The paraformaldehyde/EV solution was placed on parafilm and a resulting droplet formed. A carbon film grid was placed on the droplet for 20 min. The grid was gently picked up with tweezers and excess liquid was blotted off by touching the side at a 45oC Whatman paper. The grid was then transferred to droplet of sterile, PBS to quickly wash. The grid was then floated on a droplet (50 μL) of 1% glutaraldehyde for 5 min.
Molecular characterization of EVs was accomplished by immunoblot. Approximately 100 ug of EVs and 25ug of BDMSC lysates determined by protein content (BCA assay) were used for immunoblots as previously described.[58] Levels of exosome markers CD63 and TSG101, as well as intracellular marker Calnexin and serum protein marker Albumin were assessed, along with β-actin and GAPDH as controls following the standards recommended by the International Society for Extracellular Vesicles.[59] VEGFA presence was also screened for in EV preparations as well, using a positive control of 50ug protein isolated from skin in Sprague-Dawley rats biopsied at the edge of a healing wound and a negative control of PBS. Primary antibodies for CD63 (Proteintech, 25682–1-AP), TSG101 (Abcam, ab125011), and Calnexin(Cell Signaling, C5C9), and VEGFA (Abcam, ab46154) were used at 1:1000 dilution, while albumin (Abcam, ab207327), β-actin (Cell Signaling, 497OS ) and GAPDH (Cell Signaling, 14C10) were used at 1:2000 dilution. Secondary goat anti-rabbit IRDye 800CW (LICOR, 925–32210) was used at a dilution of 1:10,000. Bands were detected with a LI-COR Odyssey CLX Imager.
4.4. EV quantification
Quantification of EVs was performed by two methods. Nanosight tracking analysis (NTA) using a Nanosight LM10 (Malvern Instruments; Malvern, UK) was used to obtain both size distribution as well as particle/mL concentration measurements. Isolated and washed EVs were diluted 1 to 100 for a total volume of 500uL for analysis. To capture and analyze the data, NanoTracking Analysis (NTA) analytical software 3.4 version was used. Each sample was measured three times by advancing the sample through the imaging chamber for 30s of video acquisition time each. Detection threshold was set to 4 for all measurements. Outputted data was “particles/mL” and total particles were calculated based on the initial dilution factor of 100 and total volume of EV sample collected per T175 flask.
EV concentration was also assessed via quantification of the amount of total immunoreactivity CD63 present in the same representative sample used for EV quantification by NTA. The ExoELISA-ULTRA Complete Kit (CD63 Detection; System Biosciences, Mountain View, CA) was used. Briefly, washed EVs (50 μg; determined by BCA) used in the above NTA were combined with the kit’s Coating Buffer and added to a 96-well plate. The plate was left to incubate for 1 h at 37oC in order for the EVs to bind to the surface. The plate was then washed three times for 5 min each using the wash buffer the kit provided. After this, the plate was incubated with the primary CD63 antibody (1:100 dilution) at room temperature for 1 h under agitation. Plates were again washed 3X for 5 min each with the Wash Buffer. The secondary antibody provided was added (1:5000 dilution) at room temperature for 1 h. The plates were washed and incubated with the kit’s Supersensitive TMP ELISA Substrate at room temperature for 15 min, and the reaction was terminated using the kit’s Stop Buffer Solution. Absorbance was measured at 450 nm using a plate reader. The number of CD63+ EVs/mL was obtained using an exosomal CD63 standard curve produced with the kit’s exosome standards. The total number of EVs was calculated based on the total volume of EVs initially collected.
4.5. Assessment of HOTAIR incorporation into EVs
BDMSC EVs were assessed for HOTAIR content by gel electrophoresis and RT-qPCR, and HOTAIR function was examined via an in vitro gap closure assay. For gel electrophoresis, RNA isolation was carried out using the RNeasy Mini Kit (Qiagen, 74134). The starting materials for isolation were the final isolated and washed EVs. The EVs were concentrated using the 300 kDa cutoff nanosep filter and resuspended in Qiazol (700 μL) for resuspension and lysis. After the RNA was isolated using a miRNeasy Kit (Qiagen) following the manufacturers protocol, RNA (1 μg) from each sample was converted to complementary DNA (cDNA) using iScript cDNA Synthesis Kit (Biorad, 1708891) following its corresponding protocol. Primers to amplify a 1 kB segment of HOTAIR were designed (Serial Cloner) and purchased (Integrated DNA Technologies). Polymerase chain reaction using cDNA (10 ng), HOTAIR primers, and KOD DNA Polymerase was performed. The reaction cycle was 95oC for 2 min, followed by 40 cycles of 90oC for 2 min, 60oC for 10 s, and 70oC for 2 min. Then, the PCR product (5 μL) and 6X gel loading dye (1 μL) (New England BioLabs) were run through an agarose gel (1%) (Thermoscientific, R2801) containing Gelred (0.02%) (Biotium, 41003) for approximately 1 h at 120 V. The gel was imaged using a FluorChem Gel Imaging System.
Primer sequences were as follows:
1 kB Fragment of HOTAIR:
Forward: 5’-GCC TAA GCC AGT ACC GAC CTG G-3’
Reverse: 5’-TGG GTG CTG AAC CTT CAA GAG CTT-3’
For HOTAIR quantification by RT-qPCR, 10 ng from the cDNA described above were used for qPCR reactions. SsoAdvanced Universal SYBR Green Supermix PCR (Biorad, 172–5270) master mix was used according the manufacturer’s protocol. A RealTime PCR System (Applied Biosystems 7900HT) was used to detect lncRNA levels specific for HOTAIR and β-Actin was used as a control. The reaction cycle was 95oC for 30 s, followed by 40 cycles of 90oC for 15 s and 60oC for 30 s. HOTAIR fold change was standardized to β-Actin and fold change was thus calculated as 2^(-ΔΔCt) as previously described.[60] Primer sequences were as follows:
qPCR of HOTAIR:
Forward: 5′-GCA GTA GAA AAA TAG ACA TAG GAG A-3′
Reverse: 5′-ATA GCA GGA GGA AGT TCA GGC ATT G-3′
qPCR of β-Actin:
Forward: 5′-ACT TAG TTG CGT TAC ACC CTT-3′
Reverse: 5′-GTC ACC TTC ACC GTT CCA-3′
To assess HOTAIR activity in vitro, an endothelial gap closure assay was used. P5 HDMECs or HUVECs were seeded in gelatin-coated 96 well (200 μL total volume/well) or 24 well plate (500 μL total volume/well) at 15,000 cells/well or 200,000 cells/well in EGM2 and allowed to grow until a uniform monolayer was formed (24 h). Medium was replaced with endothelial basal media (EBM2) (Promocell, C-22221) supplemented with 0.5% EV-depleted FBS for 24 h. The cell monolayer was then scratched using an Autoscratch (BioTek) for the 96 well plate or a 200 μL pipette tip (Rainin, 30389243) for the 24 well plate. Media was then replaced using the same serum starving conditions, but this time supplemented with 100 μg/mL of respective EVs based on BCA quantification of EV-associate protein. EV-depleted EGM-2 and EV-depleted EBM-2 were used as positive and negative controls, respectively. The cell gap was imaged at 0 h and 24 h for HDMECs, and 0 h and 12 h for HUVECs. Overall gap closure was determined as the percentage of area covered by endothelial cells versus the gap remaining after the latter time point image using ImageJ as previously described.[58]
4.6. In vivo functional studies of HOTAIR-loaded EVs
HOTAIR-loaded BDMSC EVs were assessed in two different rodent wound healing models. In one case, an excisional wound model utilizing retired breeder Sprague-Dawley rats (400–600 g) from Charles River (Wilmington, MA) was employed. All procedures involving rats were approved by The Johns Hopkins University Animal Care and Use Committee, and all procedures followed the Johns Hopkins University ACUC. Rats were anesthetized with 3% isoflurane (Baxter Healthcare Corporation, Deerfield, IL) and had their entire dorsum shaved. Four, 12 mm punch biopsies (Integra, Plainsboro, NJ) were performed on the dorsum of each rat. Wounds were administered in a square pattern, each 5 cm apart. Buprenorphine (0.05 mg/kg) was administered on days 0, 1, and 3 subcutaneously. Wounds were matched based on day 0 wound size. A total of 11 animals were used. Treatments were administered four times around the wound in a cross pattern on day 3. Each injection contained 50 μg (determined by BCA) in a total volume of 50 μL of PBS. Photographs with a ruler showing units of millimeter and tracings of the wound were taken on day 0, 3, 5, 7, 9, and 14, and wound size was measured using digital planimetry. Wounds were debrided of the eschar on days 5, 7, 9, and 14 to clearly visualize the underlying wound. The eschar was able to be removed intact once the wounds started to decrease in size after day 3 to allow for a free edge of the eschar to be grasped and then the eschar easily removed from the underlying wound. Wound size was determined as the percentage of area of the wound versus the wound size on day 0.
A second animal model utilizing db/db mice (40–50g) from Jackson Laboratory (Bar Harbor, ME) were used. All procedures involving mice were approved by The Johns Hopkins University Animal Care and Use Committee, and all procedures followed the Johns Hopkins University ACUC (Protocol RA18M86). Mice were anesthetized with 1.5% isoflurane (Baxter Healthcare Corporation, Deerfield, IL) and had their entire dorsum shaved. One 8 mm punch biopsy (Integra, Plainsboro, NJ) was performed on the dorsum of each mouse. Buprenorphine (0.05 mg/kg) was administered on the days 0, 1, and 3 subcutaneously. Wounds were matched base on day 0 wound size. A total of 18 mice were used (6 per group). Treatments were administered four times around the wound in a cross pattern on day 3. Each injection contained 50 μg (determined by BCA) in a total volume of 50 μL of PBS. Photographs with a ruler showing units of millimeter and tracings of the wound were taken on days 0, 3, 7, 9, 11, 14, and 16, and wound size was measured using digital planimetry. Wounds were debrided of the eschar using forceps on days 7, 9, 11, 14, and 16 to clearly visualize the underlying wound. The eschar was able to be removed intact once the wounds started to decrease in size after day 3 to allow for a free edge of the eschar to be grasped and then the eschar easily removed from the underlying wound. Wound size was determined as the percentage of area of the wound versus the wound size on day 0.
Healed tissues on day 21 for both rats and mice were biopsied using a 12 mm punch biopsy. The tissue was cut down the center of the wound, and placed in a cryomold (Tissue Tek, 4557) and covered with OCT medium (Leica, 3801480). The tissue was positioned so that the cut edge was sitting at the bottom of the mold. The cassette was gently transferred onto a metal surface cooled with dry ice and ethanol until the OCT medium solidified. Tissue was stored at −80oC for no longer than 1 week before sectioning. A CM1950 Cryostat (Leica, Wetzlar, Germany) was used to section 10 μm of tissue. Generally, tissue sections were obtained from the first five 10 μm sections after the OCT medium was trimmed to expose the full line of tissue. Tissue was fixed and permeabilized in 1:1 methanol:acetone solution at a −20oC
To assess general tissue architecture, slides containing 10 μm tissue sections were stained with H&E using the following protocol: deionized water wash for 2 min, hematoxylin (VWR, 75810–352) for 3 min, deionized water wash for 1 min, differentiator in 4% HCl in 95% ethanol for 1 min, deionized water wash for 1 min, bluing for 1 min in 1% NaCO3, deionized water wash for 1 min, 95% ethanol for 1 min, eosin (VWR, 75810–354) for 45 s, 95% ethanol for 1 min, 100% ethanol for 2X 1 min, and xylene for 2X 2 min. Sub-X Mounting Medium (Leica, 3801740) was added to a No. 1.5 micro cover slip (VWR, 48393–195) and placed over the tissue section. Clear fingernail polish was used to seal the coverslip to the slide. Brightfield images were obtained using an Eclipse Ti2 Microscope (Nikon, Minato City, Tokyo, Japan) at 10X magnification.
Additionally, slides containing 10 μm tissue sections underwent CD31 staining to identify newly formed blood vessels using the following protocol: wash with tris-buffered saline (TBS) for 2 min, pre-block in 1% Bovine Serum Albumin (Sigma Aldrich, A2058) / 5% Donkey Serum (Sigma Aldrich, D9663) in TBS for 30 min, incubate CD31 primary antibody (Abcam, 28364) at 1:50 in blocking solution for 60 min at room temperature, wash with TBS for 2X 5min, incubate Alexa Flour 647 donkey anti-rabbit secondary antibody (Invitrogen, A31573)) for 60 min at room temperature shielded from light, wash with TBS for 2X 5min. Vectashield Antifade Mounting Media (Vector Laboratories, H-1200) was added to a No. 1.5 micro cover slip (VWR, 48393–195) and placed over the tissue section. Clear fingernail polish was used to seal the coverslip to the slide. Fluorescent images for Cy5 were obtained using an Eclipse Ti2 Microscope (Nikon, Minato City, Tokyo, Japan) at 10X magnification. The numbers of vessels were counted and recorded as either longitudinal or axial, and the area of tissue was quantified using ImageJ. The number of vessels/mm2 of tissue was recorded.
4.7. In vitro molecular analysis of HOTAIR-MSC EV-treated HUVECs
EVs were labeled with ExoGlow™ Membrane EV Labeling Kit (System Biosciences, 200306–007). Briefly, 4 μL of Labeling Dye were added to 24 μL of Reaction buffer to create the Labeling Reaction buffer. Then, 200 μg of EVs were added to the Labeling Reaction buffer and incubated at room temperature for 30 min shielded from light. After the labeling process, EVs were washed with PBS 3x using Nanosep Centrifugal Devices with 300 kDa MWC Omega Membranes (Pall, OD300C35) and then resuspended in 50 μL PBS. HUVECs at P4 were plated in gelatin-coated 6 well plates at a density of 200,000 cells/well. Cells were left to adhere overnight in Endothelial Growth Medium-2 (EGM2) (Promocell, C-22221 and C-39221). Cells were treated with the following membrane-labeled EVs: 100 μg/mL HOTAIR-MSC EVs, 100 μg/mL MSC EVs, or no treatment. After 24 h post-treatment, fluorescent images for GFP were obtained using an Eclipse Ti2 Microscope (Nikon, Minato City, Tokyo, Japan) at 10X magnification. In a subsequent experiment, P4 HUVECs were plated in gelatin-coated 6 well plates at a density of 200,000 cells/well cells and left to adhere overnight in EGM2. The cells were then serum starved for 24 h in Endothelial Cell Basal Medium (EBM2) (Promocell, C-22221) with 0.5% EV-depleted FBS. Cells were washed 3x with PBS and new serum starving media was added. Cells were treated with non-labeled EVs in serum staring media. HUVEC treatment groups included: 100 μg/mL HOTAIR-MSC EVs, 100 μg/mL ct-MSC EVs, or no treatment. Cells were collected after 24 h and RNA was isolated using miRNeasy kit as described above. HOTAIR and VEGFA were measured using RT-qPCR as described above and each standardized to β-Actin. Fold change was determined as 2^(-ΔΔCt). Primer sequences for qPCR of HOTAIR is stated above.
Primer sequences for qPCR of VEGF were as follows:
F: CACCCACCCACATACATACAT
R: AGTCTCTCATCTCCTCCTCTT
4.8. Statistical analysis
Data are presented as mean ± SEM. One-way ANOVAs with Holm-Sidak’s multiple comparison test or Tukey’s test were used to determine statistical significance (p<0.05) in EV characterizations measurements, in vitro scratch assays, individual days for in vivo wound healing experiments, and in vivo vessel density data. One sample t tests were used to determine statistical significance (p<0.05) against baseline value (1) for RT-qPCR fold change graphs. Two-way ANOVAs with Holm-Sidak’s multiple comparison test was used to determine statistical significance (p < 0.05) among treatment groups in in vivo wound healing experiments over time. All statistical analysis was performed with Prism 7 (GraphPad Software, La Jolla, CA). Notation for significance in figures are as follows: ns = P > 0.05, * = P < 0.05; ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health (HL141611 to SMJ) and the National Science Foundation (1750542 to SMJ). JWH was supported by the NIH (HL141611) and the Hendrix Burn/Wound Fund of Johns Hopkins University. LJB was supported by an A. James Clark Doctoral Fellowship from the University of Maryland.
Conflicts of Interest
JWH is Founder of Canton Biotechnologies Inc, in Baltimore, Maryland, a biotechnology company embedded within Johns Hopkins University that develops gene delivery technologies for wound repair.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author..
There is no direct conflict of interest with the work included in this manuscript.
Contributor Information
Louis J. Born, Fischell Department of Bioengineering, University of Maryland, College Park, MD, USA
Kai-Hua Chang, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Pouria Shoureshi, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Frank Lay, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Sameer Bengali, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Angela Ting Wei Hsu, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Sanaz Nourmohammadi Abadchi, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
John W. Harmon, Hendrix Burn and Wound Healing Laboratory, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Steven M. Jay, Fischell Department of Bioengineering, University of Maryland, College Park, MD, USA Program in Molecular and Cell Biology, University of Maryland, College Park, MD, USA.
References
- [1].Sen CK, “Human Wounds and Its Burden: An Updated Compendium of Estimates,” Advances in Wound Care, vol. 8, no. 2. Mary Ann Liebert Inc., pp. 39–48, Feb. 01, 2019, doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Żwierełło W., Styburski D., Maruszewska A., Piorun K., Skórka-Majewicz M., Czerwińska M., Maciejewska D., Baranowska-Bosiacka I., Krajewski A., and Gutowska I., “Bioelements in the treatment of burn injuries – The complex review of metabolism and supplementation (copper, selenium, zinc, iron, manganese, chromium and magnesium),” Journal of Trace Elements in Medicine and Biology, vol. 62. Elsevier GmbH, Dec. 01, 2020, doi: 10.1016/j.jtemb.2020.126616. [DOI] [PubMed] [Google Scholar]
- [3].Nagoba BS, Punpale AS, Ayachit R., Gandhi RC, and Wadher BJ, “Citric acid treatment of postoperative wound in an operated case of synovial sarcoma of the knee,” Int. Wound J, vol. 8, no. 4, pp. 425–427, Aug. 2011, doi: 10.1111/j.1742-481X.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saghazadeh S., Rinoldi C., Schot M., Kashaf SS, Sharifi F., Jalilian E., Nuutila K., Giatsidis G., Mostafalu P., Derakhshandeh H., Yue K., Swieszkowski W., Memic A., Tamayol A., and Khademhosseini A., “Drug delivery systems and materials for wound healing applications,” Advanced Drug Delivery Reviews, vol. 127. Elsevier B.V., pp. 138–166, Mar. 01, 2018, doi: 10.1016/j.addr.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dreifke MB, Jayasuriya AA, and Jayasuriya AC, “Current wound healing procedures and potential care,” Materials Science and Engineering C, vol. 48. Elsevier Ltd, pp. 651–662, Mar. 01, 2015, doi: 10.1016/j.msec.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Veith AP, Henderson K., Spencer A., Sligar AD, and Baker AB, “Therapeutic strategies for enhancing angiogenesis in wound healing,” Advanced Drug Delivery Reviews, vol. 146. Elsevier B.V., pp. 97–125, Jun. 01, 2019, doi: 10.1016/j.addr.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eming SA, Martin P., and Tomic-Canic M., “Wound repair and regeneration: Mechanisms, signaling, and translation,” Science Translational Medicine, vol. 6, no. 265. American Association for the Advancement of Science, p. 265sr6, Dec. 03, 2014, doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chang KH, Shoureshi P., Lay F., Sebastian R., Alikhassy Habibabady Z., Born LJ, Marti GP, Meltzer SJ, Abraham JM, and Harmon JW, “Preconditioning of surgical pedicle flaps with DNA plasmid expressing hypoxia-inducible factor-1α (HIF-1α) promotes tissue viability,” Gene Ther, 2020, doi: 10.1038/s41434-020-00199-6. [DOI] [PubMed] [Google Scholar]
- [9].Zhou YL, Yang QQ, Yan YY, Zhang L., Wang QH, Ju F., and Tang JB, “Gene-Loaded Nanoparticle-Coated Sutures Provide Effective Gene Delivery to Enhance Tendon Healing,” Mol. Ther, vol. 27, no. 9, pp. 1534–1546, Sep. 2019, doi: 10.1016/j.ymthe.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gianni-Barrera R., Di Maggio N., Melly L., Burger MG, Mujagic E., Gürke L., Schaefer DJ, and Banfi A., “Therapeutic vascularization in regenerative medicine,” Stem Cells Transl. Med, vol. 9, no. 4, pp. 433–444, Apr. 2020, doi: 10.1002/sctm.19-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eelen G., Treps L., Li X., and Carmeliet P., “Basic and Therapeutic Aspects of Angiogenesis Updated,” Circulation Research. Lippincott Williams and Wilkins, pp. 310–329, Jul. 03, 2020, doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- [12].Murphy DE, de Jong OG, Brouwer M., Wood MJ, Lavieu G., Schiffelers RM, and Vader P., “Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking,” Experimental and Molecular Medicine, vol. 51, no. 3. Nature Publishing Group, Mar. 01, 2019, doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sarfraz M., Afzal A., Yang T., Gai Y., Raza SM, Khan MW, Cheng Y., Ma X., and Xiang G., “Development of dual drug loaded nanosized liposomal formulation by a reengineered ethanolic injection method and its pre-clinical pharmacokinetic studies,” Pharmaceutics, vol. 10, no. 3, Sep. 2018, doi: 10.3390/pharmaceutics10030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gubernator J., “Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity,” Expert Opinion on Drug Delivery, vol. 8, no. 5. Expert Opin Drug Deliv, pp. 565–580, May 2011, doi: 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- [15].Chen ZJ, Yang SC, Liu XL, Gao Y., Dong X., Lai X., Zhu MH, Feng HY, Di Zhu X., Lu Q., Zhao M., Chen HZ, Lovell JF, and Fang C., “Nanobowl-Supported Liposomes Improve Drug Loading and Delivery,” Nano Lett, vol. 20, no. 6, pp. 4177–4187, Jun. 2020, doi: 10.1021/acs.nanolett.0c00495. [DOI] [PubMed] [Google Scholar]
- [16].Hu H., Wang B., Jiang C., Li R., and Zhao J., “Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21–5p to modulate Thrombospondin-1 expression,” Clin. Sci, vol. 133, no. 14, pp. 1629–1644, Jul. 2019, doi: 10.1042/CS20190188. [DOI] [PubMed] [Google Scholar]
- [17].Sahoo S., Klychko E., Thorne T., Misener S., Schultz KM, Millay M., Ito A., Liu T., Kamide C., Agrawal H., Perlman H., Qin G., Kishore R., and Losordo DW, “Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity,” Circ. Res, vol. 109, no. 7, pp. 724–728, Sep. 2011, doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu X., Badawi M., Pomeroy S., Sutaria DS, Xie Z., Baek A., Jiang J., Elgamal OA, Mo X., La Perle K., Chalmers J., Schmittgen TD, and Phelps MA, “Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells,” J. Extracell. Vesicles, vol. 6, no. 1, p. 1324730, Dec. 2017, doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jeyaram A., Lamichhane TN, Wang S., Zou L., Dahal E., Kronstadt SM, Levy D., Parajuli B., Knudsen DR, Chao W., and Jay SM, “Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles,” Mol. Ther, vol. 28, no. 3, pp. 975–985, Mar. 2020, doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kordelas L., Rebmann V., Ludwig AK, Radtke S., Ruesing J., Doeppner TR, Epple M., Horn PA, Beelen DW, and Giebel B., “MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease,” Leukemia, vol. 28, no. 4. Nature Publishing Group, pp. 970–973, Apr. 09, 2014, doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- [21].Lamichhane TN, Leung CA, Douti LY, and Jay SM, “Ethanol Induces Enhanced Vascularization Bioactivity of Endothelial Cell-Derived Extracellular Vesicles via Regulation of MicroRNAs and Long Non-Coding RNAs,” Sci. Rep, vol. 7, no. 1, Dec. 2017, doi: 10.1038/s41598-017-14356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fu WM, Lu YF, Hu BG, Liang WC, Zhu X., Di Yang H., Li G., and Zhang JF, “Long noncoding RNA hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways,” Oncotarget, vol. 7, no. 4, pp. 4712–4723, 2016, doi: 10.18632/oncotarget.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma X., Li Z., Li T., Zhu L., Li Z., and Tian N., “Long non-coding RNA HOTAIR enhances angiogenesis by induction of vegfa expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles,” Am. J. Transl. Res, vol. 9, no. 11, pp. 5012–5021, 2017, Accessed: Nov. 04, 2020. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/29218099/. [PMC free article] [PubMed] [Google Scholar]
- [24].Botti G., Collina F., Scognamiglio G., Aquino G., Cerrone M., Liguori G., Gigantino V., Malzone MG, and Cantile M., “LncRNA HOTAIR Polymorphisms Association with Cancer Susceptibility in Different Tumor Types,” Curr. Drug Targets, vol. 19, no. 10, pp. 1220–1226, Jul. 2018, doi: 10.2174/1389450118666170622091940. [DOI] [PubMed] [Google Scholar]
- [25].Bhan A. and Mandal SS, “LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer,” Biochimica et Biophysica Acta - Reviews on Cancer, vol. 1856, no. 1. Elsevier, pp. 151–164, Aug. 01, 2015, doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Casado-Díaz A., Quesada-Gómez JM, and Dorado G., “Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing,” Frontiers in Bioengineering and Biotechnology, vol. 8. Frontiers Media S.A., Mar. 03, 2020, doi: 10.3389/fbioe.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu W., Yu M., Xie D., Wang L., Ye C., Zhu Q., Liu F., and Yang L., “Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway,” Stem Cell Res. Ther, vol. 11, no. 1, pp. 1–15, Jun. 2020, doi: 10.1186/s13287-020-01756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang X., Jiao Y., Pan Y., Zhang L., Gong H., Qi Y., Wang M., Gong H., Shao M., Wang X., and Jiang D., “Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating Notch signaling,” Stem Cells Int, vol. 2019, 2019, doi: 10.1155/2019/2402916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., Jin Y., Yuan L., Li B., and Bolontrade MF, “MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing,” Stem Cells Int, vol. 2019, 2019, doi: 10.1155/2019/7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li B., Luan S., Chen J., Zhou Y., Wang T., Li Z., Fu Y., Zhai A., and Bi C., “The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152–3p,” Mol. Ther. - Nucleic Acids, vol. 19, pp. 814–826, Mar. 2020, doi: 10.1016/j.omtn.2019.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Whittaker TE, Nagelkerke A., Nele V., Kauscher U., and Stevens MM, “Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles,” J. Extracell. Vesicles, vol. 9, no. 1, Jan. 2020, doi: 10.1080/20013078.2020.1807674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fu WM, Lu YF, Hu BG, Liang WC, Zhu X., Di Yang H., Li G., and Zhang JF, “Long noncoding RNA hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways,” Oncotarget, vol. 7, no. 4, pp. 4712–4723, 2016, doi: 10.18632/oncotarget.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao D., Zhao Y., Wang J., Wu L., Liu Y., Zhao S., Guo F., Ma X., Zhang H., Li Z., Meng D., Xu L., Zhang L., Liu J., and Qin G., “Long noncoding RNA Hotair facilitates retinal endothelial cell dysfunction in diabetic retinopathy,” Clin. Sci, vol. 134, no. 17, pp. 2419–2434, Sep. 2020, doi: 10.1042/CS20200694. [DOI] [PubMed] [Google Scholar]
- [34].Liu L., Bi N., Wu L., Ding X., Men Y., Zhou W., Li L., Zhang W., Shi S., Song Y., and Wang L., “MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma,” Mol. Cancer, vol. 16, no. 1, p. 50, Feb. 2017, doi: 10.1186/s12943-017-0620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patel DB, Gray KM, Santharam Y., Lamichhane TN, Stroka KM, and Jay SM, “Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles,” Bioeng. Transl. Med, vol. 2, no. 2, pp. 170–179, Jun. 2017, doi: 10.1002/btm2.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonzalez ACDO, Andrade ZDA, Costa TF, and Medrado ARAP, “Wound healing - A literature review,” Anais Brasileiros de Dermatologia, vol. 91, no. 5. Sociedade Brasileira de Dermatologia, pp. 614–620, Sep. 01, 2016, doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao R., Liang H., Clarke E., Jackson C., and Xue M., “Inflammation in chronic wounds,” International Journal of Molecular Sciences, vol. 17, no. 12. MDPI AG, Dec. 11, 2016, doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kooijmans SAA, Stremersch S., Braeckmans K., De Smedt SC, Hendrix A., Wood MJA, Schiffelers RM, Raemdonck K., and Vader P., “Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles,” J. Control. Release, vol. 172, no. 1, pp. 229–238, 2013, doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- [39].Dewitt MR and Rylander MN, “Tunable Collagen Microfluidic Platform to Study,” Methods Mol. Biol, vol. 1831, no. August, pp. 159–178, 2018, doi: 10.1007/978-1-4939-8661-3. [DOI] [PubMed] [Google Scholar]
- [40].Kojima R., Bojar D., Rizzi G., El Hamri GC, El-Baba MD, Saxena P., Ausländer S., Tan KR, and Fussenegger M., “Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment,” Nat. Commun, vol. 9, no. 1, 2018, doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., and Wood MJA, “Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes,” Nat. Biotechnol, vol. 29, no. 4, pp. 341–345, 2011, doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- [42].O’Brien K., Breyne K., Ughetto S., Laurent LC, and Breakefield XO, “RNA delivery by extracellular vesicles in mammalian cells and its applications,” Nat. Rev. Mol. Cell Biol, vol. 21, no. 10, pp. 585–606, 2020, doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lamichhane TN, Raiker RS, and Jay SM, “Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery,” Mol. Pharm, vol. 12, no. 10, pp. 3650–3657, 2015, doi: 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lamichhane TN, Jeyaram A., Patel DB, Parajuli B., Livingston NK, Arumugasaamy N., Schardt JS, and Jay SM, “Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication,” Cell. Mol. Bioeng, vol. 9, no. 3, pp. 315–324, Sep. 2016, doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li YJ, Wu JY, Wang JM, Bin Hu X., Cai JX, and Xiang DX, “Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer,” Acta Biomater, vol. 101, pp. 519–530, Jan. 2020, doi: 10.1016/j.actbio.2019.10.022. [DOI] [PubMed] [Google Scholar]
- [46].Haney MJ, Klyachko NL, Zhao Y., Gupta R., Plotnikova EG, He Z., Patel T., Piroyan A., Sokolsky M., Kabanov AV, and Batrakova EV, “Exosomes as drug delivery vehicles for Parkinson’s disease therapy,” J. Control. Release, vol. 207, pp. 18–30, Jun. 2015, doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hung ME and Leonard JN, “A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery,” J. Extracell. Vesicles, vol. 5, no. 1, p. 31027, Jan. 2016, doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Born LJ, Harmon JW, and Jay SM, “Therapeutic potential of extracellular vesicle-associated long noncoding RNA,” Bioengineering and Translational Medicine, vol. 5, no. 3. Blackwell Publishing Ltd, Sep. 01, 2020, doi: 10.1002/btm2.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Patel NA, Moss LD, Lee JY, Tajiri N., Acosta S., Hudson C., Parag S., Cooper DR, Borlongan CV, and Bickford PC, “Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury,” J. Neuroinflammation, vol. 15, no. 1, p. 204, Jul. 2018, doi: 10.1186/s12974-018-1240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shyu KG, Wang BW, Pan CM, Fang WJ, and Lin CM, “Hyperbaric oxygen boosts long noncoding RNA MALAT1 exosome secretion to suppress microRNA-92a expression in therapeutic angiogenesis,” Int. J. Cardiol., vol. 274, pp. 271–278, Jan. 2019, doi: 10.1016/j.ijcard.2018.09.118. [DOI] [PubMed] [Google Scholar]
- [51].Liu Y., Zou R., Wang Z., Wen C., Zhang F., and Lin F., “Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis,” Biochem. J, vol. 475, no. 22, pp. 3629–3638, Nov. 2018, doi: 10.1042/BCJ20180675. [DOI] [PubMed] [Google Scholar]
- [52].Liu Y., Lin L., Zou R., Wen C., Wang Z., and Lin F., “MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis,” Cell Cycle, vol. 17, no. 21–22, pp. 2411–2422, Nov. 2018, doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Noh JH, Kim KM, McClusky WG, Abdelmohsen K., and Gorospe M., “Cytoplasmic functions of long noncoding RNAs,” Wiley Interdisciplinary Reviews: RNA, vol. 9, no. 3. Blackwell Publishing Ltd, p. e1471, May 01, 2018, doi: 10.1002/wrna.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yu B. and Shan G., “Functions of long noncoding RNAs in the nucleus,” Nucleus, vol. 7, no. 2. Taylor and Francis Inc., pp. 155–166, Apr. 25, 2016, doi: 10.1080/19491034.2016.1179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dong Y., Yoshitomi T., Hu JF, and Cui J., “Long noncoding RNAs coordinate functions between mitochondria and the nucleus,” Epigenetics and Chromatin, vol. 10, no. 1. BioMed Central Ltd., Aug. 23, 2017, doi: 10.1186/s13072-017-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang B., Gunawardane L., Niazi F., Jahanbani F., Chen X., and Valadkhan S., “A Novel RNA Motif Mediates the Strict Nuclear Localization of a Long Noncoding RNA,” Mol. Cell. Biol, vol. 34, no. 12, pp. 2318–2329, Jun. 2014, doi: 10.1128/mcb.01673-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rappa G., Santos MF, Green TM, Karbanová J., Hassler J., Bai Y., Barsky SH, Corbeil D., and Lorico A., “Nuclear transport of cancer extracellular vesicle-derived biomaterials through nuclear envelope invagination-associated late endosomes,” Oncotarget, vol. 8, no. 9, pp. 14443–14461, 2017, doi: 10.18632/oncotarget.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Patel DB, Luthers CR, Lerman MJ, Fisher JP, and Jay SM, “Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system,” Acta Biomater, vol. 95, pp. 236–244, Sep. 2019, doi: 10.1016/j.actbio.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Théry C. et al. , “Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines,” J. Extracell. Vesicles, vol. 7, no. 1, Jan. 2018, doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Livak KJ and Schmittgen TD, “Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method,” Methods, vol. 25, no. 4, pp. 402–408, 2001, doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.