Abstract
Objective:
To evaluate whether urothelial carcinoma with sarcomatoid differentiation is associated with a lower pathologic response rate to neoadjuvant chemotherapy and worse oncologic outcomes compared to urothelial carcinoma without variant histology among patients undergoing radical cystectomy.
Patients and Methods:
Patients with urothelial carcinoma undergoing cystectomy from 1995–2018 at Memorial Sloan Kettering were identified. Patients with sarcomatoid differentiation at transurethral resection or cystectomy and patients without variant histology were selected. Downstaging from ≥cT2 to ≤pT1N0 defined partial response; pT0N0 defined complete response. Recurrence-free, cancer-specific, and overall survival were modeled.
Results:
We identified 131 patients with sarcomatoid differentiation and 1,722 patients without variant histology, of whom 25 with sarcomatoid histology on biopsy and 313 without variant histology received neoadjuvant chemotherapy. Those with sarcomatoid differentiation presented with higher consensus tumor stage (94% ≥T2 vs 62%, P <0.001) and were therefore more likely to receive neoadjuvant chemotherapy (29% vs 18%, P = 0.004). We found no evidence to support a difference in partial (24% vs 31%) or complete (20% vs 24%) response between patients with sarcomatoid histology and pure urothelial carcinoma at transurethral resection (p=0.6). Among patients with sarcomatoid differentiation, five-year recurrence-free survival was 55% (95% CI 41%-74%) among patients receiving NAC and 40% (95% CI 31%-52%) among patients undergoing cystectomy alone (p=0.1). Adjusting for stage, nodal involvement, margin status, and receipt of neoadjuvant chemotherapy, sarcomatoid differentiation was associated with worse recurrence-free (HR 1.83, 95% CI 1.39–2.40), disease-specific (HR 1.66, 95% CI 1.24–2.23), and overall survival (HR 1.38, 95% CI 1.06–1.78).
Conclusions:
Sarcomatoid differentiation was associated with higher stage at presentation and independently associated with worse survival. Given similar pathologic response rates if sarcomatoid differentiation is detected at initial resection, and greater survival among patients receiving NAC, treatment with NAC appears warranted. Other drivers of the poor outcomes of this histology must be investigated.
Keywords: Bladder Cancer, Urothelial carcinoma, Sarcomatoid differentiation, Neoadjuvant therapy, Pathologic outcomes, Survival
Introduction
Urothelial carcinoma (UC) with sarcomatoid differentiation is a rare histologic variant found in an estimated 0.6% of bladder cancers [1]. It is associated with higher stage at presentation and worse survival than urothelial carcinoma, not otherwise specified (UC, NOS), even among patients with organ-confined disease [1]. Effective neoadjuvant or adjuvant treatment for patients with UC with sarcomatoid differentiation would represent an important adjunct to radical cystectomy (RC) for these patients; however, the efficacy of neoadjuvant chemotherapy (NAC) in this histologic variant is not well established. Although an analysis of the National Cancer Database (NCDB) demonstrated a lower incidence of non–organ-confined disease at RC among patients with UC with sarcomatoid differentiation receiving NAC compared to those receiving RC alone [2], comparison with patients with UC,NOS was not performed and cancer-specific outcomes are not available in this dataset [3]. Furthermore, long-term survival outcomes in patients with UC with sarcomatoid differentiation treated with radical cystectomy are limited.
Hypothesizing that patients with UC with sarcomatoid differentiation are similarly responsive to NAC, we sought to compare pathologic response rates between patients with UC with sarcomatoid differentiation and UC, NOS. Furthermore, we sought to evaluate whether the presence of sarcomatoid differentiation is associated with worse oncologic outcomes in a contemporary cohort of patients with UC undergoing RC with long-term follow-up.
Patients and Methods
Under an institutional review board–approved waiver, we identified 131 patients with sarcomatoid histology on either transurethral resection (TUR) or RC, and 1,722 patients with UC without variant histology (UC, NOS) on TUR and at RC, who underwent RC between January 5, 1995 and November 16, 2018. For this study, histology was determined by the pathology report from TUR and RC specimens, which at our institution are read by dedicated genitourinary pathologists. The presence of any sarcomatoid differentiation, without a specific threshold of percent involvement of the specimen, was used to categorize patients into the UC with sarcomatoid differentiation group. As general practice, pathology slides from TUR performed at outside institutions are routinely reviewed internally. For this study, pathologic re-review was only performed for patients in whom sarcomatoid differentiation was identified in the RC, but not the TUR, specimen.
Our first aim was to determine whether response to NAC differed between those with and without sarcomatoid histology. This analysis included 338 patients who received NAC and who did not demonstrate variant histology on TUR or at RC (UC, NOS group) or who had UC with sarcomatoid differentiation at TUR (sarcomatoid group). The sarcomatoid comparison group only included those who had sarcomatoid histology on TUR, as these are the patients whose clinical decisions would be influenced by the presence of sarcomatoid histology. Patients were categorized into three groups based on their response to chemotherapy. Complete response (CR) was defined as pathologic stage T0N0 at RC. Partial response (PR) was defined as downstaging from clinical stage ≥T2 to pathologic stage ≤T1N0. Patients who had pathologic stage ≥T2 disease or lymph node involvement were included in the no response group. Fisher’s exact tests were used to test the difference in chemotherapy response between patients with sarcomatoid histology and UC, NOS.
The next aim was to determine whether sarcomatoid histology, present either at TUR or RC, was associated with worse disease characteristics, including pathologic T stage, pathologic N stage, receipt of NAC, receipt of adjuvant chemotherapy, and surgical margin status. Adjuvant chemotherapy was defined as any chemotherapy that occurred within 6 months after RC and before disease recurrence. Disease characteristics were compared using Wilcoxon rank-sum or Fisher’s exact tests between those patients with sarcomatoid histology and those with UC, NOS.
We then assessed whether sarcomatoid features were associated with worse recurrence-free, cancer-specific, or overall survival. Patients who died without recurrence were censored at their date of death. We created univariate Cox proportional hazards models for each outcome with the predictor of interest being presence of any sarcomatoid histology (on TUR or cystectomy). Multivariable models for each outcome were then created, which were adjusted for pathologic T stage (≥ T2 vs < T2), lymph node involvement, receipt of NAC, and surgical margin status. The model for overall survival was also adjusted for age at surgery. All analyses were performed using R version 3.6.0.
Results
Our final cohort consisted of 1,853 patients, 131 with sarcomatoid histology on either TUR or at cystectomy and 1,722 patients with UC, NOS. There were 580 patients who experienced recurrence and 943 patients who died, with 487 of those patients dying from bladder cancer. The median follow-up for survivors was 5.8 years (quartiles 3.6, 10).
There were more male patients in the UC, NOS group than in the sarcomatoid histology group (80% vs 71%, P = 0.027). Detection of sarcomatoid histology appeared to increase with time, with this variant identified in 4.9% of patients undergoing RC before 2005 and 7.8% of patients undergoing RC from 2005 and on. Patients with sarcomatoid histology had higher consensus T stage (94% ≥T2 vs 62%, P <0.001), defined as the highest stage between clinical and pathologic stage, and were more likely to receive NAC (29% vs 18%, P = 0.004) (Table 1). The greater use of NAC among patients with sarcomatoid differentiation was likely due to the higher clinical stage at presentation; among patients with clinical T2 or greater disease, there was no difference in the percentage of patients with sarcomatoid differentiation or UC, NOS who received NAC (34% in each group). Since NAC was not consistently used until about 2005, we performed a sensitivity analysis to investigate the association between sarcomatoid histology and receipt of NAC among patients undergoing surgery from 2005 onwards. After adjusting for age, we found no significant association between sarcomatoid histology and receipt of NAC in these patients (P = 0.10).
Table 1.
Patient and disease characteristics, separately for those patients with sarcomatoid histology and UC, NOS. Results are presented as median (quartiles) and frequency (%)
| Characteristic | UC, NOS n = 1,722 |
Sarcomatoid histology n = 131 |
P |
|---|---|---|---|
| Age at surgery, years | 68 (61–75) | 68 (58–75) | 0.5 |
| Male | 1,370 (80%) | 93 (71%) | 0.027 |
| Neoadjuvant chemotherapy | 313 (18%) | 38 (29%)* | 0.003 |
| Gemcitabine-cisplatin | 274 (88%) | 29 (76%) | |
| MVAC | 13 (4%) | 3 (8%) | |
| Other cisplatin-based regimen | 3 (1%) | 3 (8%) | |
| Carboplatin-based regimen | 23 (7%) | 2 (5%) | |
| Unknown | 0 | 1 (3%) | |
| Neoadjuvant chemotherapy cycles | 0.3 | ||
| ≤3 | 54 (17%) | 3 (8%) | |
| 4 | 212 (68%) | 25 (66%) | |
| ≥5 | 30 (10%) | 4 (11%) | |
| Unknown | 17 (5%) | 6 (16%) | |
| Adjuvant chemotherapy | 126 (7.3%) | 9 (7.0%) | >0.9 |
| Muscle-invasive bladder cancer** | 980 (59%) | 85 (65%) | 0.2 |
| Consensus T stage | <0.001*** | ||
| pTa | 41 (2.4%) | 0 (0%) | |
| pTis | 108 (6.3%) | 0 (0%) | |
| pT1 | 499 (29%) | 8 (6.1%) | |
| pT2 | 548 (32%) | 39 (30%) | |
| pT3 | 323 (19%) | 64 (49%) | |
| pT4 | 203 (12%) | 20 (15%) | |
| Lymph node involvement | 273 (17%) | 26 (21%) | 0.2 |
| Positive margins | 63 (3.7%) | 7 (5.3%) | 0.3 |
| Timing of sarcomatoid histology | |||
| On TUR | 35 (27%) | ||
| At cystectomy | 51 (39%) | ||
| Both TUR and cystectomy | 45 (34%) |
This includes 25 patients with sarcomatoid differentiation identified at TUR and 13 patients who sarcomatoid differentiation identified only in the cystectomy specimen
Clinical stage at time of treatment.
For consensus T stage, the data are presented for all stages, but when comparing consensus T stage between patients with and without sarcomatoid histology, the pTa, pTis and pT1 groups were combined, and a chi-squared test was used.
We then investigated whether there was a difference in chemotherapy response among those patients who received NAC. There were 313 patients with UC, NOS and 25 patients with sarcomatoid histology on TUR included in this analysis. Gemcitabine-cisplatin and methotrexate-vinblastine-doxorubicin-cisplatin (MVAC) were the two most commonly utilized neoadjuvant chemotherapy regimens (Table 1). A cisplatin-based regimen was administered to 290 patients (93%) with UC, NOS and 23 patients (92%) of patients with sarcomatoid differentiation on TUR. At least four cycles of neoadjuvant chemotherapy were administered to 242 patients (77%) and 18 patients (72%) in the UC, NOS and sarcomatoid differentiation groups, respectively. There was no evidence of difference in response to NAC between the two groups, with PR in 31% vs. 24% and CR in 24% vs. 20% of patients with UC, NOS and sarcomatoid differentiation, respectively (Table 2, P = 0.6). Among patients with sarcomatoid differentiation identified at TUR, 33 of 55 patients (60%) who did not receive NAC and 12 of 25 patients (48%) who did receive NAC had sarcomatoid differentiation identified at RC.
Table 2.
Chemotherapy response rates for those patients with muscle-invasive bladder cancer who received neoadjuvant chemotherapy. Patients in the sarcomatoid group are those patients with sarcomatoid histology found on TUR. Patients who had sarcomatoid histology at cystectomy only are excluded from this analysis.
| UC, NOS n = 313 |
Sarcomatoid histology n = 25 |
P | |
|---|---|---|---|
| Response to chemotherapy | 0.6 | ||
| No response | 139 (44%) | 14 (56%) | |
| Partial | 98 (31%) | 6 (24%) | |
| Complete | 76 (24%) | 5 (20%) |
Thirteen patients had sarcomatoid histology identified only at RC, and all 13 of these patients had no response to chemotherapy. On a post hoc analysis which included all patients who had sarcomatoid histology seen at TUR or RC and who received NAC (N = 38), significantly fewer patients responded as compared to patients with UC, NOS (Table 3, P = 0.009). Among the 93 patients with sarcomatoid histology who did not receive NAC, approximately two-thirds had pT3 or higher disease at RC (N = 60), 22% of patients had pT2 disease, and 14% had pathologic stage T1 or lower.
Table 3.
Chemotherapy response rates for those patients with muscle-invasive bladder cancer who received neoadjuvant chemotherapy including patients who had sarcomatoid histology at cystectomy but not on TUR in the sarcomatoid group
| UC, NOS, N = 313 | Sarcomatoid histology N = 38 | P | |
|---|---|---|---|
| Response to chemotherapy | 0.009 | ||
| No response | 139 (44%) | 27 (71%) | |
| Partial | 98 (31%) | 6 (16%) | |
| Complete | 76 (24%) | 5 (13%) |
The presence of any sarcomatoid histology was found to be significantly associated with worse recurrence-free survival (Fig. 1, P <0.001), cancer-specific survival (Fig. 2, P <0.001), and overall survival (Fig. 3, P <0.001). Five-year recurrence-free survival for patients with sarcomatoid histology was 45% (95% CI 37%–55%), with cancer-specific and overall survival estimates at five years of 51% (95% CI 42%–61%) and 45% (95% CI 37%–55%), respectively. At five years, recurrence-free survival among patients with UC, NOS was 71% (95% CI 68%–73%), with cancer-specific survival of 75% (95% CI 73%–78%) and overall survival of 63% (95% CI 61%–66%). After adjusting for pathologic T stage, lymph node involvement, receipt of NAC and surgical margin status, sarcomatoid histology was significantly associated with worse recurrence-free and cancer-specific survival (HR 1.83, 95% CI 1.39–2.40, P <0.001 and HR 1.66, 95% CI 1.24–2.23, P <0.001, respectively). After adjusting for these same covariates as well as age at surgery, there was also a significant association between sarcomatoid histology and poorer overall survival (HR 1.38, 95% CI 1.06–1.78, P = 0.015). Among patients with sarcomatoid differentiation, five-year recurrence-free survival after RC was 55% (95% CI 41%, 74%) among patients who received NAC and 40% (95% CI 31%, 52%) among patients who did not receive NAC (p=0.1).
Figure 1.
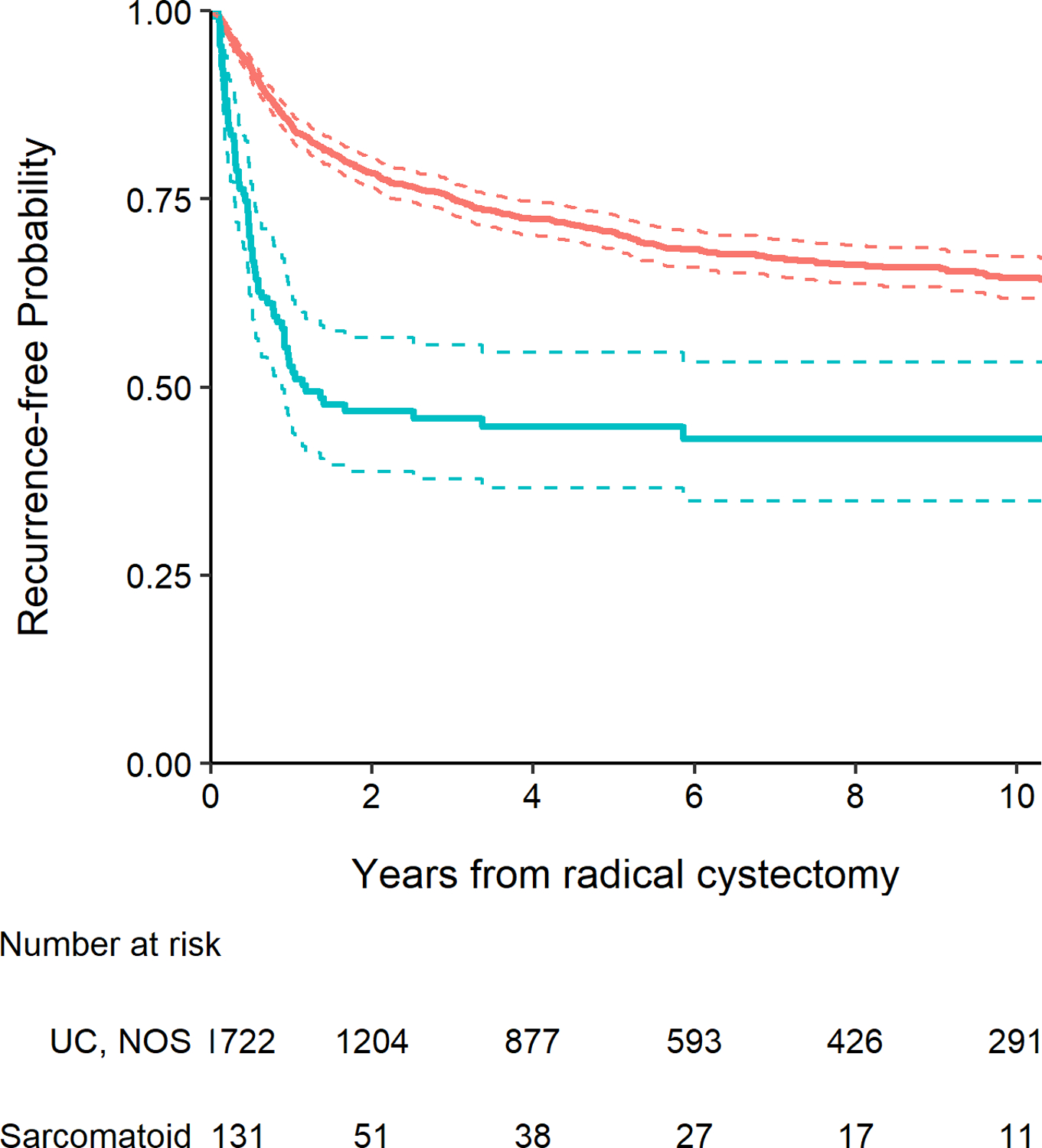
Kaplan-Meier plot for recurrence-free survival after radical cystectomy with 95% confidence intervals (dashed lines), separately for patients with UC, NOS (red line) and those with any sarcomatoid histology (blue line, P <0.001).
Figure 2.
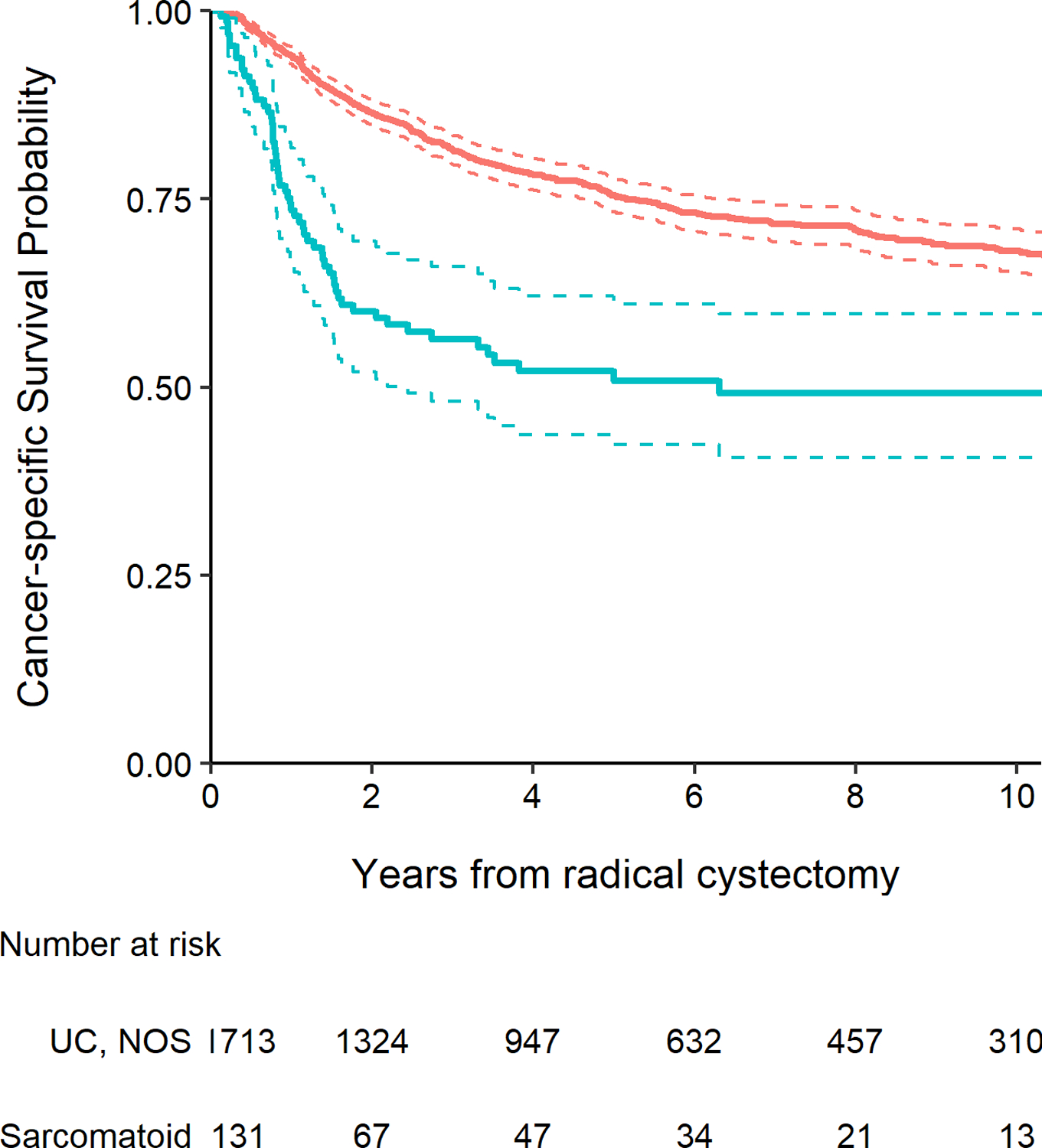
Kaplan-Meier plot for cancer-specific survival after radical cystectomy with 95% confidence intervals (dashed lines), separately for patients with UC, NOS (red line) and those with any sarcomatoid histology (blue line, P <0.001).
Figure 3.
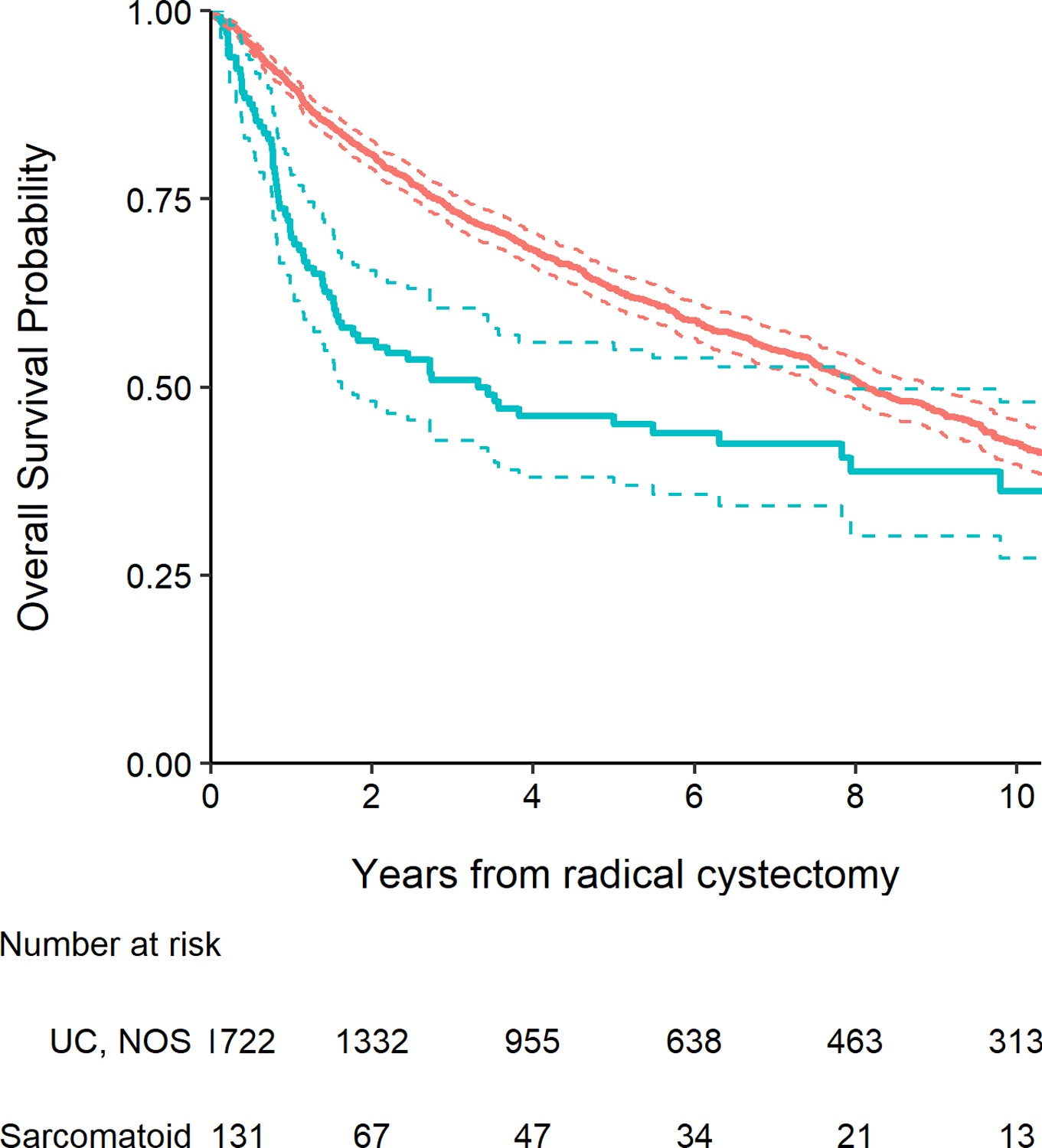
Kaplan-Meier plot for overall survival after radical cystectomy with 95% confidence intervals (dashed lines), separately for patients with UC, NOS (red line) and those with any sarcomatoid histology (blue line, P <0.001).
Discussion
Urothelial carcinoma with sarcomatoid differentiation is a rare histologic variant associated with advanced stage and poor oncologic outcomes. Whether UC with sarcomatoid differentiation is responsive to NAC is not well established. Furthermore, studies assessing long-term outcomes in patients with sarcomatoid differentiation managed with RC are limited. The current study represents the largest reported single-institutional RC experience in patients with sarcomatoid differentiation, and unlike prior studies compares pathologic response and oncologic outcomes between patients with sarcomatoid differentiation to patients with UC, NOS, and reports long-term cancer-specific outcomes. Among patients receiving NAC, similar rates of pathologic downstaging were observed between patients with sarcomatoid differentiation on TUR and UC, NOS (PR: 24% vs. 31%; CR: 20% vs. 24%), suggesting chemoresponsiveness of this variant. Additionally, among patients with sarcomatoid differentiation, greater recurrence-free survival was observed among patients receiving NAC compared to patients treated with cystectomy alone. However, when including patients with sarcomatoid differentiation identified only at time of RC, patients with sarcomatoid differentiation detected at any timepoint had significantly lower rates of response to NAC. When controlling for stage, margin status, and receipt of NAC, the presence of sarcomatoid differentiation was associated with significantly worse recurrence-free, cancer-specific, and overall survival compared to UC, NOS. The worse oncologic outcomes observed among patients with sarcomatoid differentiation highlights the need to identify predictors of response to NAC and additional effective agents to treat this aggressive histologic variant.
There are limited and conflicting data on the effectiveness of NAC in patients with sarcomatoid variant histology. Data from the NCDB demonstrated a lower incidence of non–organ-confined disease among 47 patients with sarcomatoid differentiation receiving NAC compared to 257 patients treated with RC alone [2]. The pathologic response rate among patients with sarcomatoid differentiation was not compared to patients with UC, NOS, while differences in survival between patients who did and did not receive NAC were not statistically significant. Importantly, such analyses are limited by the NCDB dataset, which does not capture important treatment-related variables such as chemotherapeutic regimen, treatment for disease recurrence, or disease-specific outcomes such as recurrence-free or disease-specific survival [3]. In an early report of 11 patients with sarcomatoid differentiation receiving NAC, five (45%) were down-staged to pT0, compared to four of 32 (12%) treated with RC alone [4,5]. Conversely, a separate single-institutional observational study found the presence of any variant histology was associated with a significantly lower rate of pathologic CR [6]. In the current series, we observed a CR rate of 20% in patients receiving NAC with sarcomatoid differentiation identified upfront, which was no different than the rate observed in the conventional UC group. These findings support a role for neoadjuvant therapy. Gemcitabine-cisplatin is the neoadjuvant regimen of choice at our institution [7] and was administered to the majority of patients receiving NAC. Similar rates of pathologic downstaging have been observed with this regimen in our institutional experience when compared to patients receiving MVAC in the SWOG 8710 study (46% vs. 44% <pT2) [8]. Among patients achieving pathologic downstaging to <pT2N0, five-year overall survival was 89%, similar to that of patients achieving a CR in SWOG 8710.
In the present study, we observed differences in response to neoadjuvant therapy depending on when sarcomatoid differentiation was detected. In our review, 13 patients with UC, NOS in the TUR specimen who received NAC had sarcomatoid differentiation identified only in the final pathologic specimen at RC. When examining patients who had sarcomatoid differentiation detected in the TUR or RC specimen, there were lower overall pathologic response rates relative to UC, NOS. There are several possible explanations for this observation. Sarcomatoid differentiation may not have been found initially because it was either not sampled at TUR or not identified on pathologic review. Acknowledging this limitation, our practice is to internally review all outside biopsies and our dedicated genitourinary pathologists are highly attuned to variant histologies. Another possibility is that patients with chemotherapy-resistant disease may have developed sarcomatoid differentiation while treated with NAC. Thus, the presence of this finding at RC may be a marker of treatment effect among surviving cell populations, which could be biologically distinct from sarcomatoid histology seen before chemotherapy. Indeed, prior studies have identified sarcomatoid differentiation in patients with a history of UC, NOS, even after a short interval between TUR and RC [9].9 Importantly, UC with sarcomatoid differentiation spans a broad morphologic spectrum, including bland spindle cells, spindled cells with significant nuclear atypia, and cells with myxoid or rhabdoid features [9]. It is possible that cancers demonstrating certain morphologic patterns may harbor greater sensitivity to cisplatin-based chemotherapy. Alternatively, the percent of sarcomatoid pattern in a given tumor, or presence of specific genomic alterations, may predict response to NAC. Our results suggest that some tumors with sarcomatoid differentiation are chemoresponsive. Further research, including molecular characterization, is needed to identify specific predictors of response in this rare histologic variant.
The significantly worse outcomes in patients with sarcomatoid differentiation, after controlling for stage, margin status, and receipt of NAC, highlights the need to identify additional effective therapies. Neoadjuvant immune checkpoint blockade has shown activity in MIBC, with the phase II PURE-01 study of neoadjuvant pembrolizumab demonstrating pathologic downstaging in 54% of patients [10]. However, among 34 patients with variant histology included in that study, pathologic response was only observed among patients with squamous cell or lymphoepithelioma-like variant [11]. Of note, only two patients with sarcomatoid differentiation were enrolled in the trial. In a subset analysis of a randomized trial of patients with advanced renal cell carcinoma, patients with sarcomatoid features demonstrated high response rate and improved progression-free survival when treated with immune checkpoint inhibitors compared to targeted therapy [12]. Ongoing clinical trials of immune checkpoint blockade in variant histology bladder cancer, such as NCT04383743 which is assessing pathologic response with neoadjuvant pembrolizumab and chemotherapy in patients with variant histology muscle-invasive bladder cancer, will help elucidate whether similar efficacy is observed in patients with bladder cancer. Given the rarity of this histologic variant, molecular profiling may be required to identify patients likely to respond to systemic therapy. The presence of deleterious alterations in DNA damage response genes has been shown to be associated with improved pathologic response and survival in patients with MIBC [13–15] and improved survival in patients with metastatic bladder cancer treated with cisplatin-based chemotherapy [16] or immune checkpoint blockade [17]. Whether such alterations are present in UC with sarcomatoid differentiation, and whether such alterations are associated with treatment response in this setting, remains unknown and is an area of active investigation.
Our study is limited by its retrospective nature and the selection bias inherent to treatment with NAC. Although controlling for important clinical and disease characteristics associated with survival outcomes, it is possible that unmeasured confounders impact the survival analyses conducted in this study. The identification of sarcomatoid differentiation was based on pathologic review at the time of TUR and RC by dedicated genitourinary pathologists at our institution; however, a significant portion of patients undergoing RC at our institution undergo TUR elsewhere before referral to our center. Although representative slides of the TUR specimen are reviewed by genitourinary pathologists at our institution, it is possible that additional patients would have been classified as having sarcomatoid differentiation had full pathologic review of the TUR specimen been conducted at our institution. As previously mentioned, 13 patients in our cohort were identified as having sarcomatoid differentiation in the RC specimen alone, and when these patients were included in post-hoc analysis, lower rates of pathologic response to NAC were evident. Whether sarcomatoid differentiation was present in the TUR specimen but missed on initial pathologic review is unknown, potentially impacting the pathologic response rates observed in this study. Additionally, given the limited number of patients with sarcomatoid differentiation and pathologic response to NAC, we were unable to perform comparative analysis of survival outcomes of pathologic responders between the two histologies, a limitation that has plagued prior studies in this rare histologic variant. Despite these limitations, this study adds to the existing literature on pathologic response of this histologic variant to NAC and the impact of the presence of sarcomatoid differentiation on long-term oncologic outcomes. In adjusted analysis, the presence of sarcomatoid differentiation is associated with significantly worse oncologic outcomes, highlighting the need for further studies to evaluate for predictors of response to NAC and identify other effective therapies for this histologic variant.
Conclusions
The presence of sarcomatoid differentiation is associated with a higher stage at presentation and is independently associated with worse oncologic outcomes among patients undergoing radical cystectomy. The similar pathologic response rates observed between patients with UC, NOS and UC with sarcomatoid differentiation identified at TUR, and the greater survival observed among patients with sarcomatoid differentiation receiving NAC compared to patients undergoing cystectomy alone, suggests that NAC should be considered in these patients. Molecular characterization may identify mechanisms of resistance or predictors of response to neoadjuvant therapy and should be the focus of future studies. The poor outcomes observed among patients with sarcomatoid differentiation highlight the need to identify additional effective treatments.
Acknowledgements
Performed the research: NA, EAV, DDS, NCW, CH, GI, HA, ACG
Designed the research study: NA, EAV, HA, ACG
Contributed essential reagents or tools: EJP, EKC, TFD, GD, BHB, JER, DFB
Analyzed the data: EAV, DDS
Wrote the paper: NA, EAV, ACG
The authors thank Michael McGregor for editorial support.
Funding Sources
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, the Michael and Zena Wiener for Therapeutics Program in Bladder Cancer, Pin Down Bladder Cancer, Cycle for Survival, the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, NIH/NCATS Grant UL1 TR002384, and the NIH/NCI Cancer Center Core Grant P30 CA008748. N.A. received support from the NIH Ruth L. Kirschstein National Research Service Award T32 CA082088.
Abbreviations and Acronyms
- CR
Complete response
- MIBC
Muscle-invasive bladder cancer
- MVAC
Methotrexate, vinblastine, doxorubicin, cisplatin
- NCDB
National Cancer Database
- NAC
Neoadjuvant chemotherapy
- PR
Partial response
- RC
Radical cystectomy
- TUR
Transurethral resection
- UC
Urothelial carcinoma
- UC, NOS
Urothelial carcinoma, not otherwise specified
Footnotes
Conflicts of Interest
Nima Almassi, Emily A. Vertosick, Daniel D. Sjoberg, Nathan C. Wong, Chun Huang, Eugene K. Cha, Timothy F. Donahue, Guido Dalbagni, and Alvin C. Goh have no conflicts of interest to report. Eugene J. Pietzak reports honoraria from UpToDate, consulting or advisory role at Merck and Chugai Pharma. Bernard H. Bochner serves as consultant for Olympus. Gopa Iyer reports personal fees from Mirati Therapeutics and Janssen and research support from Novartis. Jonathan E. Rosenberg reports stock and other ownership Interests in Merck, Illumina; honoraria from UpToDate, Bristol-Myers Squibb, AstraZeneca, Medscape, Vindico, Peerview, Chugai Pharma; consulting or advisory role for Lilly, Merck, Agensys, Roche/Genentech, Sanofi, AstraZeneca/MedImmune, Bristol-Myers Squibb, EMD Serono, Seattle Genetic, Bayer, Inovio Pharmaceuticals, BioClin Therapeutics, QED Therapeutic, Adicet Bio, Sensei Biotherapeutics; patents, royalties, other intellectual property for Predictor of platinum sensitivity; research funding from Genentech, Oncogenex, Agensys, Mirati Therapeutics, Novartis, Viralytics, Genentech/Roche, Incyte, Seattle Genetics, Bayer; travel, accommodations, expenses from Genentech/Roche, Bristol-Myers Squibb. Dean F. Bajorin reports honoraria from the speaker’s bureau of Merck; lecture fees and travel support from Merck; and Consultant/advisory board: Merck, Pfizer, Bristol-Myers Squibb, Urogen, Genentech, Eli Lilly. Hikmat Al-Ahmadie reports personal fees from AstraZeneca and Bristol-Myers Squibb.
References
- 1.Wright JL, Black PC, Brown GA, et al. Differences in survival among patients with sarcomatoid carcinoma, carcinosarcoma and urothelial carcinoma of the bladder. J Urol 2007; 178: 2302–6 [DOI] [PubMed] [Google Scholar]
- 2.Vetterlein MW, Wankowicz SAM, Seisen T, et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 2017; 123: 4346–55 [DOI] [PubMed] [Google Scholar]
- 3.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol 2017; 3: 1722–8 [DOI] [PubMed] [Google Scholar]
- 4.Black PC, Brown GA, Dinney CP. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol 2009; 27: 3–7 [DOI] [PubMed] [Google Scholar]
- 5.Black PC, Kassouf W, Brown GA, et al. 1521: Variant histology in bladder cancer - experience in 1246 patients undergoing cystectomy. J Urol 2007; 177: 502 [Google Scholar]
- 6.Pokuri VK, Syed JR, Yang Z, et al. Predictors of complete pathologic response (pT0) to neoadjuvant chemotherapy in muscle-invasive bladder carcinoma. Clin Genitourin Cancer 2016; 14: e59–65 [DOI] [PubMed] [Google Scholar]
- 7.Almassi N, Cha EK, Vertosick EA, et al. Trends in management and outcomes among patients with urothelial carcinoma undergoing radical cystectomy from 1995 to 2015: the Memorial Sloan Kettering experience. J Urol 2020; 204: 677–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer G, Tully CM, Zabor EC, et al. Neoadjuvant gemcitabine-cisplatin plus radical cystectomy-pelvic lymph node dissection for muscle-invasive bladder cancer: a 12-year experience. Clin Genitourin Cancer 2020; 18: 387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanfrancesco J, McKenney JK, Leivo MZ, et al. Sarcomatoid urothelial carcinoma of the bladder: analysis of 28 cases with emphasis on clinicopathologic features and markers of epithelial-to-mesenchymal transition. Arch Pathol Lab Med 2016; 140: 543–51 [DOI] [PubMed] [Google Scholar]
- 10.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018; 36: 3353–60 [DOI] [PubMed] [Google Scholar]
- 11.Necchi A, Raggi D, Gallina A, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol 2020; 77: 439–46 [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Motzer RJ, Powles T, et al. Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: a prespecified subgroup analysis of the IMmotion151 clinical trial. Eur Urol 2020: doi: 10.1016/j.eururo.2020.06.021 [DOI] [PMC free article] [PubMed]
- 13.Iyer G, Balar AV, Milowsky MI, et al. Multicenter prospective phase II trial of neoadjuvant dose-dense gemcitabine plus cisplatin in patients with muscle-invasive bladder cancer. J Clin Oncol 2018; 36: 1949–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol 2016; 2: 1094–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014; 4: 1140–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teo MY, Bambury RM, Zabor EC, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 2017; 23: 3610–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol 2018; 36: 1685–94 [DOI] [PMC free article] [PubMed] [Google Scholar]


