Abstract
We demonstrate a novel fiber endface photoacoustic (PA) generator using infrared (IR) 144 laser dye dispersed within an ultraviolet adhesive. The generator provides a wide acoustic bandwidth in the transducer frequency range of 2–7 MHz, high thermal conversion efficiency (>90%), good PA signal controllability (well-controlled IR 144 concentration), and high feasibility (simple procedures). Through a series of experimental validations, we show that this fiber-based endface PA generator can be a useful tool for a broad range of biomedical applications such as calibrating the local absorption coefficient of biological tissue for quantitative PA tomography.
Ultrasound (US) generators have long been studied for use in biomedical US imaging [1], focused US-assisted drug delivery for treatment of tumors [2], and US non-destructive testing (NDT) [3]. However, the traditional piezo transducers are bulky, and fabricating these transducers with millimeter-scale lateral dimensions is both challenging and expensive.
Alternatively, optical US generators, especially fiber optic US generators, are attractive candidates for many US applications [4,5]. The most common optical US generation method exploits the photoacoustic (PA) effect, in which the generating material is excited by pulses of laser energy that cause transient heating and localized temperature rises. The temperature rise will cause contraction and expansion of the PA material due to a thermal expansion effect. As a result, the periodic compression and expansion will generate acoustic waves. The PA wave emission intensity is proportional to the light fluence and the absorption coefficient of the material. Due to the short pulse width of an ultrafast laser, the pulse width of the generated acoustic signals can be very short, and the corresponding frequency bandwidth of acoustic signals can be broad.
Taking advantage of the submillimeter diameters of optical fibers and the PA effect, we developed a fiber-based PA wave generator (called a “PA generator” hereafter) that features a compact size and wide bandwidth. The system is useful for a variety of applications such as quantitative evaluation of biological tissue absorption coefficients using PA imaging (PAI).
Accurately estimating functional parameters of biological tissue (e.g., total hemoglobin concentration and oxygen saturation) using PAI is challenging, because the detected signals are a convolution of a product of local fluence and tissue optical absorption with the system response. Computing the total hemoglobin concentration depends on the accurate estimation of the local absorption profile of the tissue which is coupled with the local fluence. Nevertheless, by positioning the fiber endface of the calibrated PA generator close to the target tissue and assuming identical local fluence for the generator and tissue, we can assume that the mean PA signal ratio of the target tissue and the generator can approximate the ratio of their absorption coefficients, provided the absorption coefficients of PA generator and target tissue are linearly related. As a result, the absorption coefficient profile of the target tissue can be estimated by using the fiber endface PA generator as a reference. Such PA generators can be used with biopsy needles or surgical implantable devices for quantitative PAI. Additionally, these generators can also be embedded inside the brain as broadband acoustic sources to activate neurons.
The PA wave generation material is an important factor. Recently, various studies have described developing and applying novel materials to improve PA wave generation efficiency. Several papers have reported that polymer-based materials show higher PA generation efficiency than metallic materials [6,7]. For instance, Biagi et al. demonstrated that, compared to metallic materials, graphite powder mixed with epoxy resin could increase the conversion efficiency by two orders of magnitude [8]. Zou et al. synthesized a gold nanocomposite by directly mixing gold nanoparticles with polydimethylsiloxane (PDMS), then coated it on the optical fiber tip for PA emission [9]. Baac et al. used a nano-composite film of carbon nanotubes (CNTs) and elastomeric polymer as an efficient PA source [7,10]. Shi et al. combined multi-wall carbon nanotubes (MWCNTs) and PDMS to achieve a high light absorption coefficient and high thermal expansion coefficient [11]. However, all of these PA generation materials involve complicated designs and demanding fabrication protocols. Moreover, they all lack a high optical absorption within 700–900 nm. The materials in reference [11] were designed for narrowband and sub-MHz frequency emission which are not suitable for most biomedical applications. The materials in references [7,10] required growing a multi-layer CNT on a fused silica substrate which has difficult-to-control quality and is extremely expensive. Additionally, a PDMS-based curing agent makes the manufacturing process time-consuming and difficult for precisely controlling the fiber tip generator dimension[6–11].
In this Letter, we report a fiber endface PA generator with a wide acoustic bandwidth, high thermal conversion efficiency, good PA single controllability, and high feasibility (simple procedures). This novel generator could be a useful tool for calibrating the local absorption coefficient of biological tissue for quantitative PAI.
The fiber endface PA generator, as illustrated in Figs. 1(a) and 1(b), was fabricated by applying IR 144 dye (Exciton, Lockbourne, OH, U.S.) on the fiber tip. A piece of multimode fiber (FP1000ERT, 0.50 NA, THORLABS) with a core diameter of 1000 μm was stripped and cleaved, and the end face was polished. Then it was dipped into a mixture of IR 144 and ultraviolet (UV) adhesive (Norland Optical Adhesive 63, EDMUNDOPTICS) and gradually pulled out to form a partial spherical tip due to the high viscosity and surface tension of the adhesive [12]. The tip was exposed to UV light for several seconds for fast curing; then it was placed under a microscope for inspection. Any tip with a non-spherical shape was re-fabricated. After the tip passed the inspection, it went through a UV exposure of several minutes to fully cure the UV adhesive.
Fig. 1.
(a) Microphotograph of the fiber endface PA generator; (b) schematic of the generator; (c) frequency response of the PA generator and 200 μm diameter carbon fiber.
IR 144 is a tricarbocyanine dye widely used in various biomedical applications employing near-infrared (NIR) imaging due to its high-absorption cross section at around 750 nm and strong fluorescent emission in the NIR regions. However, NIR-emitting dyes often have relatively low quantum yields (commonly in the range of 2–10%[13]), compared to the yields of traditional red-emitting fluorescent dyes (up to 96% [14]). The reported quantum yield value of IR 144 in DMSO is 5% [15], and the rest of the absorbed energy could lead to high thermal and thus PA conversion efficiency. Additionally, due to the presence of long extended conjugation in the chemical structure, IR 144 shows a high light energy conversion rate[16]. To the best of knowledge, this is the first time that IR 144 has been used for PA wave generation.
We acquired the PA emission signals from the fiber endface PA generator and 200 μm diameter carbon fiber (approximated as a point source) using our previously described co-registered PA/US imaging system [17]. The detected PA signals for each target were processed with fast Fourier transform (FFT), and the spectra are shown as a function of frequency [Fig. 1(c)]. To briefly describe the PAI/US system, a Ti:sapphire laser (Symphotics TII, LS-2122) optically pumped with a Q-switched Nd:YAG laser (Symphotics TII, LS-2134) was employed to deliver pulsed laser light (10–30 ns pulse duration, 15 Hz pulse repletion rate). A commercial US system (EC-12R, Alpinion Medical Systems) and a linear US transducer (L312H, center frequency of 8.8 MHz and a −6 dB US bandwidth ≥ 60%) were used. As shown in Fig. 1(c), the PA generator spectrum (brown color) in a 2–7 MHz range closely follows the carbon fiber spectrum (blue) indicating a broad PA signal bandwidth of the IR 144 dye.
The absorbance was calculated by log10(I0/I), where I0 and I are the intensities of incident and transmitted light, respectively. By measuring the absorbance at different wavelengths, the absorption spectrum of IR 144 was obtained with a Beckman DU-640 spectrophotometer (Beckman Coulter, Inc., Indianapolis, IN, U.S.).A stock solution of IR 144 was prepared in DMSO (1 mg/ml) from which IR 144 samples with different concentrations were diluted. The samples were placed in quartz cuvettes, and their UV-Vis absorption spectra were recorded.
Figure 2(a) shows the absorbance versus wavelength plots for IR 144 concentrations from 0.3125 to 20 μg/ml. The peak absorbance occurs at around 745 nm. Additionally, the plots suggest that absorbance increases with IR144 concentration. Figure 2(b) shows the peak absorbance value occurring at 745 nm as a function of IR 144 concentrations. The linear relationship of the peak absorbance and the IR144 concentrations indicate an R2 of 0.9963. We also calculated the mean PA signal from the recorded PA generator signals. We selected an appropriate region of interest (ROI) based on the US image and calculated the mean value of the PAT image envelopes within the ROI. Then we normalized the mean value to the corresponding fiber tip power measured by a power meter to obtain the normalized mean PA signal at each wavelength. Figure 2(c) shows the normalized mean PA signal and the corresponding absorbance for wavelengths from 730 to 850 nm. The plots of the PA signal and absorbance follow similar trends, which are in accord with the PA principle in which the profile of the acoustic signals is similar to the profile of the absorption coefficient for a constant fluence.
Fig. 2.
(a) Absorption plots of IR 144 as a function of wavelength for concentrations from 0.3125 to 20 μg/ml (solvent: DMSO); (b) peak absorbance value at 745 nm as a function of IR144 concentrations; (c) normalized mean PA signal and the corresponding absorbance as a function of wavelength.
Figure 3(a) shows the experimental setup for validating the calibration accuracy of the fiber endface PA generator. Three generators with three concentrations were buried with equal lateral distance inside a calibrated phantom (made of intralipid and gelatin, absorption coefficient μa, 0.02 cm−1; reduced scattering coefficient μns, 4 cm−1) at a depth of 20 mm. Guided by co-registered US images, the three generators were oriented perpendicular to the transducer imaging plane. However, small angles might occur, and the PA signal variation was less than 3% which would not affect the results.
Fig. 3.
(a) Experimental setup to validate the calibration accuracy of the fiber endface PA generator. (The light was delivered through the fiber itself to its endface.) (b) and (c) Corresponding 1-D profiles across three different fiber endface PA generators (averaged from 10 consecutive envelopes along the depth) with respect to two sets of IR 144concentrations(2.5,5,and10μg/ml;and1.25,2.5,and5μg/ml). The PA signal ratios of the generators with higher to lower IR 144 concentrations are also provided in each profile.
Figures 3(b) and 3(c) show the corresponding 1-D profiles across the three fiber endface PA generators for two sets of concentrations (2.5, 5, and 10 μg/ml; and 1.25, 2.5, and 5 μg/ml) acquired from the PA images at 745 nm. Based on the linear relationship of the IR 144 absorbance and concentration at 745 nm [Fig. 2(b)], the true PA signal ratio of the generators with adjacent higher to lower IR 144 concentrations is around 2, which is consistent with the experimental ratios: 1.74 and 1.78 marked in Fig. 3(b), and 2.27 and 1.87 in Fig. 3(c).
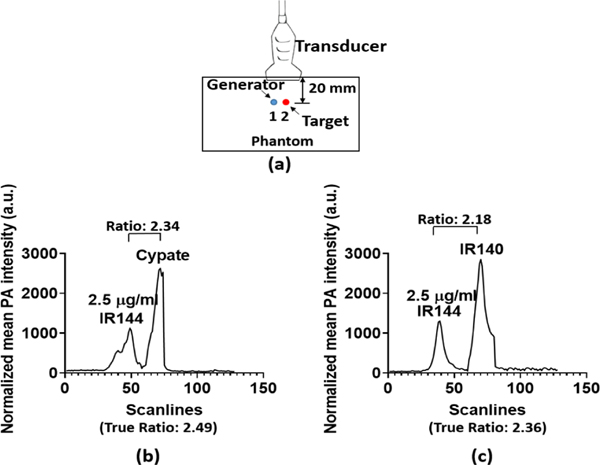
Figure 4(a) shows the experimental setup of the PA generator calibration with either of two targets: cypate (Optical Radiology Lab, Washington University in St. Louis) and IR 140 (Exciton, Lockbourne, OH, U.S.). As in Fig. 3(a), one fiber endface generator and one target were buried inside the calibrated phantom perpendicular to the transducer imaging plane at a depth of 20mm.
Fig. 4.
(a) Experimental setup of the PA generator target calibration. (The light was delivered through the fiber itself to its endface.) (b) and (c) Corresponding 1-D profiles (averaged from 10 consecutive envelopes along the depth) across one PA generator (left, IR 144 with a concentration of 2.5 μg/ml) and one target (right, cypate or IR 140). The ratios of the PA signal of the target to that of the generator are also provided in each profile.
The laser wavelength was 745 nm, and the IR 144 concentration was 2.5 μg/ml. Cypate and IR 140 are two frequently used NIR dyes. The measured absorbance of the cypate and IR 140 samples at 745 nm were 0.92 and 0.97. Based on the results in Fig. 2(a), the measured absorbance of the fiber endface PA generator (2.5 μg/ml) at 745 nm was 0.39. As a result, the true PA signal ratios of cypate and IR 140 to the signal of the generator were 2.49 and 2.36, which match well with the experimental ratios:2.34 in Fig. 4(b) and 2.18 in Fig. 4(c).
Figure 5(a) shows the experimental setup of the fiber endface PA generator calibration of a mouse blood sample. The mouse blood samples were collected from a C57BL/KaLwRij mouse via cardiac puncture under anesthesia and transferred into EDTA tubes to prevent blood coagulation. The blood samples were diluted with saline water to achieve different absorbances. Then the samples were injected into a 5 mm diameter tube which was then tightly capped. The fiber generator and blood tube were submerged inside a tank filled with a 0.4% calibrated intralipid solution perpendicular to the transducer imaging plane at a depth of 20 mm. The intralipid solution had a μa of 0.02 cm−1 and of 4 cm−1. The laser wavelength used here was 745 nm, and the IR 144 concentration was 2.5 μg/ml. As shown in Fig. 5(b), strong PA signals (yellow) were detected from the generator and blood tube area (gray). Meanwhile, second reflection PA artifacts were observed underneath the blood PA signals at a depth roughly two times that of the blood PA signals due to transducer face absorption. The measured absorbance of the fiber endface PA generator (2.5 μg/ml) and blood sample (diluted 20 times) at 745 nm were 0.39 and 2.04, respectively.
Fig. 5.
(a) Experimental setup of the fiber endface PA generator calibration of a mouse blood sample. (The light was delivered from the top of the water tank perpendicular to the intralipid solution.) (b) Co-registered PAI/US images of the generator and mouse blood sample (dynamic range: 18 dB, color bar refers to normalized PA signal). (c) Corresponding 1-D profile (averaged over 2 mm in depth) across one fiber endface generator (left, IR 144 with a concentration of 2.5 μg/ml) and one mouse blood sample (right, injected into a 5 mm diameter tube). The PA signal ratio of the blood sample’s signal to the generator’s signal is also provided. (d) Mean PA signal value of the blood sample at 745 nm as a function of the measured blood sample absorbance with respect to different dilution rates. (e) Comparison between the experimental absorption coefficient of the blood sample based on the mean PA signal ratio and the measured absorption coefficient based on the spectrophotometer measurement. (The concentrations 1–4 refer to four dilution rates of the blood sample.)
As a result, the true ratio of the PA signals of the blood sample and the generator was 5.22, which agrees well with the experimental ratio in Fig. 5(c). Figure 5(d) shows the mean PA signal value of the blood sample at 745 nm as a function of the measured blood sample absorbance (1.09, 1.67, 2.1, 2.59) with respect to different dilution rates (∼ 30, 25, 20, 15 times). The linear relationship of the mean PA signal and the blood sample absorbance indicate an R2 of 0.98. We also compared the experimental blood sample absorption coefficients with respect to different dilution rates and the measured ones, and they showed a good match with a difference less than 0.5 cm−1 in Fig. 5(e).
In this Letter, we have demonstrated a novel fiber endface PA generator made of a mixture of IR 144 and UV adhesive. IR 144 has been widely used in various biomedical applications. With a high-absorption cross section and low quantum yield (5%), it achieved a high thermal conversion efficiency (>90%) and thus high PA conversion efficiency. The PA emission frequency exhibited abroad bandwidth as the point-like carbon fiber in the frequency range of 2–7 MHz evaluated. The IR 144 absorbance at 745 nm was linearly correlated with its concentration. By experimentally validating the generator’s calibration accuracy with different concentrations and different target materials, we demonstrated good consistency between the experimental and calibration results. Finally, we used the generator to calibrate the absorption coefficient of a mouse blood sample. Again, there was a good match between the experimental and calibration results with a difference less than 0.5 cm−1. IR 144 dye is commercially available and low in cost which can facilitate clinical translation of such fiber-based acoustic generators. Moreover, dual modality fluorescent and PA imaging can be achieved by utilizing the IR 144 moderate quantum yield in the NIR region and the good PA conversion efficiency.
Acknowledgments
Funding. National Cancer Institute (R01CA237664).
Footnotes
Data Availability. Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
Disclosures. The authors declare no conflicts of interest.
REFERENCES
- 1.Baerwald A, Dauk S, Kanthan R, Singh J, and Singh J, Ultrasound Obstet. Gynecol 34, 201 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Wang TY, and Willmann JK, Adv. Exp. Med. Biol 880, 263 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Sposito G, Ward C, Cawley P, Nagy PB, and Scruby C, NDT&E Int. 43, 7 (2010). [Google Scholar]
- 4.Zou X, Wu N, Tian Y, Zhang Y, and Wang X, Appl. Opt 52, 6239 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Tian Y, Wu N, Zou X, Felemban H, Cao C, and Wang X, Opt. Eng 52, 065005 (2013). [Google Scholar]
- 6.Wu N, Tian Y, Zou X, Silva V, Chery A, and Wang X, J. Opt. Soc. Am B29, 8 (2012). [Google Scholar]
- 7.Baac HW, Ok JG, Park HJ, Ling T, Chen SL, Hart AJ, and Guo LJ, Appl. Phys. Lett 97, 234104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biagi E, Margheri F, and Menichelli D, IEEE Trans. Ultrason. Ferroelectr. Freq. Control 48, 1669 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Zou X, Wu N, Tian Y, and Wang X, Opt. Express 22, 15 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Baac HW, Ok JG, Maxwell A, Lee KT, Chen YC, Hart AJ, Xu Z, Yoon E, and Guo LJ, Sci. Rep 2, 989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Jiang Y, Zhang Y, Lan L, Huang Y, Cheng JX, and Yang C, Photoacoustics 20, 100208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H, Yang G, and Zhu Q, Opt. Lett 45, 632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P, Kang F, Yang W, Zhang M, Dang R, Jiang P, and Wang J, Nanoscale 12, 5084 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Kubin RF and Fletcher AN, J. Lumin 27, 455 (1982). [Google Scholar]
- 15.Mohanty J, Palit DK, and Mittal JP, Proc. Natl. Acad. Sci. India A66, 303 (2000). [Google Scholar]
- 16.Castro FA, Faes A, Geiger T, Graeff CF, Nagel M, Nuesch F, and Hany R, Synth. Met 156, 973 (2006). [Google Scholar]
- 17.Yang G, Amidi E, Nandy S, Mostafa A, and Zhu Q, Photoacoustics 13, 66 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]