Abstract
We evaluated whether indications for liver transplantation (LT) have changed among people with/without human immunodeficiency virus (HIV) infection and compared LT outcomes and trends by HIV serostatus. LT recipients (2008–2018) from the United Network for Organ Sharing and Organ Procurement and Transplantation Network (UNOS/OPTN) were identifed. Among 62 195 LT recipients, 352 (0.6%) were HIV-infected. The proportion of HIV-infected patients increased over time (P trend = .001), as did the number of transplant centers performing LT for HIV-infected recipients; average annual percentage change of 9.2% (p < .001). Nonviral causes became the leading indication in 2015 for HIV-uninfected and in 2018 for HIV-infected (P trend < .001). Three-year cumulative patient survival rates were 77.5%, for HIV-infected and 84.6%, for HIV-uninfected (p = .15). Over time, graft and patient survival rates improved for both HIV-infected and uninfected (p < .001). Among HCV-infected LT recipients, 3-year patient survival rates were 72.5% for HIV-infected and 81.8% for HIV-uninfected (p = .02). However, in a subanalysis restricted to 2014–2018, differences in graft and patient survival by HIV serostatus were no longer observed (3-year patient survival rates were 81.2% for HIV-infected and 86.4% for HIV-uninfected, p = .34). In conclusion, in the United States, nonviral liver disease is now the leading indication for LT in HIV-infected patients, and posttransplant outcomes have improved over time.
Keywords: HIV/HCV coinfection, HCV, MELD, NASH, trends, UNOS
1 |. INTRODUCTION
Indications for liver transplantation (LT) have changed over the past decade. Nonalcoholic steatohepatitis (NASH) is rising as a cause of end-stage liver disease and represents the most rapidly increasing indication for LT in the United States.1–3 In 2009, NASH was the third leading indication for LT in the United States, and after only five years, became the second in 2013, surpassing alcohol-related liver disease (ALD) and reflecting a waiting list increase of 170% from 2004 to 2013.1,2 Moreover, in 2016, NASH was the leading indication for LT registrants born in the 1945–1965 birth cohort.4 Concurrent with the rise of NASH cirrhosis, the proportion of LT for ALD in the United States also increased 100% from 2002 to 2016.5 These changes in waiting list composition reflect not only rises in NASH and ALD but also the impact of widespread availability of direct-acting antiviral (DAA) drugs to treat hepatitis C virus (HCV). Indeed, in the early DAA era, the number of people on the waiting list due to HCV-related complications decreased by 32% in the United States.6 Further reductions are anticipated with increased access to DAA therapy.
For people living with HIV (PLWH), HCV coinfection has been highly prevalent and an important cause of morbidity and mortality after effective antiretroviral therapy (ART).7 However, a recent study combining registry data from the United States and Europe showed that HCV decreased as an indication for LT for HIV-infected patients (from 76% to 52%) between 2008 and 2015, but the proportion of LTs performed in HIV-infected patients remained unchanged.8 Whether an increasing burden of end-stage liver disease due to NASH and ALD, as seen in HIV-uninfected patients, is occurring in PLWH is not known. We aimed to evaluate changes in indications for LT among persons with and without HIV infection in the United States from 2008 to 2018, with a focus on temporal trends in nonviral indications for LT in HIV-infected patients, and to evaluate graft and patient outcomes during the study period.
2 |. PATIENTS AND METHODS
2.1 |. Data source
Patients were identified from the United Network for Organ Sharing and Organ Procurement and Transplantation Network (UNOS/OPTN). We identified all adults who underwent a LT from 2008 to 2018. HIV serostatus was recorded as negative, positive, undetermined, unknown, or missing. Only patients with a negative or positive HIV serostatus were included in this analysis. Patients who received multiorgan transplantation (other than liver-kidney), retransplantation, or missing Model for End-Stage Liver Disease (MELD) were excluded.
Diagnosis regarding etiology of liver disease was identified based on coded and text diagnosis fields. Etiology was then assigned hierarchically to HCV, hepatitis B virus (HBV), ALD, NASH, cryptogenic liver disease, and other, such that subjects with multiple etiologies were assigned to the first etiology in the list. For example, subjects with HCV and ALD were assigned to HCV. To identify all recipients likely to have NASH, and because a large proportion of patients with cryptogenic liver disease are expected to have NASH, this diagnosis was assigned if the recipient had a diagnosis of cryptogenic liver disease and either diabetes mellitus or body mass index (BMI) >30 kg/m2.1,2 Cryptogenic cirrhosis (CC) was defined as a diagnosis of cryptogenic liver disease with BMI ≤30 and no indication of diabetes. Subsequently, NASH, ALD, and CC were grouped as nonviral causes. The remaining patients who did not have an HCV, HBV, NASH, ALD, or CC diagnosis were grouped under “Other” diagnosis.
2.2 |. Statistical analysis
Recipient factors were described with frequency distributions and median and interquartile range (IQR). Comparisons between groups used Chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables. The primary outcome of interest was changes in indication for LT, and HIV was the primary predictor of interest. Time trends in (1) proportions of transplants were evaluated using the Cochran–Armitage trend test and (2) counts were evaluated using joinpoint regression. For trends in counts, the average annual percentage change (AAPC) was estimated and tested for joinpoints (inflection points) to identify significant changes in slope during the study period. The secondary outcome was 1- and 3-year graft and patient survival. For this analysis, the study period was divided into two periods based on arrival of DAAs: 2008–2013 and 2014–2018. Graft and patient survival were computed using Kaplan–Meier methods and compared by HIV serostatus and transplant period using the log-rank test with 95% confidence intervals (95% CI) provided. Statistical analyses were conducted using SAS v. 9.4, STATA v13, and Joinpoint Regression Version 4.8.0.1 (April 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute) for all statistical analyses. This study was approved by the University of California San Francisco Institutional Review Board.
3 |. RESULTS
3.1 |. Cohort of study
Among the 69 820 patients transplanted during the study period, we excluded 4273 because of previous LT, multiorgan transplantation (other than SLK), or lack of MELD. An additional 3352 were excluded because HIV serostatus was not available. Thus, the final study population included 62 195 individuals who underwent LT, among whom 352 (0.6%) were HIV-infected. Compared with HIV-uninfected recipients, HIV-infected recipients were more likely to be male (80.4% vs. 66.1%, p < .001), were younger (median 54 years vs. 57 years, p < .001), and had lower BMI (median 25.3 vs. 28.0, p < .001).
HIV-infected patients also had lower MELD at the time of LT (23 [IQR 17–32] compared with HIV-uninfected patients 25 [IQR 18–33], p = .049) (Table 1). These differences in recipient characteristics by HIV serostatus persisted after stratifying by viral and nonviral indications for LT. There was no difference comparing the proportion with hepatocellular carcinoma (HCC) (36.4% for HIV-infected patients and 33.7% for HIV-uninfected patients, p = .3).
TABLE 1.
Main characteristics of the entire cohort by HIV status
| Variable | Total (n = 62 195) |
HIV-infected (n = 352) 0.6% |
HIV-uninfected (n = 61 843) 99.4% |
|---|---|---|---|
| Male, n (%) | 41 168 (66.2) | 283 (80.4) | 40 885 (66.1) |
|
| |||
| Age at LT (years), median (IQR) | 57 (51–63) | 54 (49–59) | 57 (51–63) |
| HCV+ (years), median (IQR) | 58 (54–62) | 55 (50–59) | 58 (54–62) |
| HCV− (years), median (IQR) | 57 (48–63) | 54 (48–58) | 57 (48–63) |
| Viral (years), median (IQR) | 58 (53–62) | 55 (50–59) | 58 (53–62) |
| Nonviral (years), median (IQR) | 57 (48–63) | 54 (49–58) | 57 (48–63) |
|
| |||
| Race/ethnicity | |||
| White | 44 235 (71.1) | 198 (56.3) | 44 037 (71.2) |
| AA | 5821 (9.4) | 84 (23.9) | 5737 (9.3) |
| Hispanic | 8632 (13.9) | 53 (15.1) | 8579 (13.9) |
| Other | 3507 (5.6) | 17 (4.8) | 3490 (5.6) |
|
| |||
| MELD at LT, median (IQR) | 21 (14–30) | 20 (12–29) | 21 (14–30) |
|
| |||
| MELD at LT excluding HCC, median (IQR) | 25 (18–33) | 23 (17–32) | 25 (18–33) |
|
| |||
| BMI (kg/m2), median (IQR) | 27.9 (24.4–32.2) | 25.3 (23.2–29.4) | 28.0 (24.5–32.2) |
| NASH, median (IQR) | 31.6 (27.6–35.7) | 27.1 (23.9–31.0) | 31.6 (27.6–35.7) |
| Non-NASH, median (IQR) | 27.2 (24.0–31.2) | 25.1 (23.1–29.0) | 27.2 (24.0–31.2) |
|
| |||
| Diabetes mellitus, n (%) | 16 971 (27.3) | 81 (23.0) | 16 890 (27.3) |
|
| |||
| Hepatocellular carcinoma, n (%) | 20 961 (33.7) | 128 (36.4) | 20 833 (33.7) |
|
| |||
| NASH, n (%) | 11 587 (18.6) | 40 (11.4) | 11 547 (18.7) |
|
| |||
| Etiology | |||
| HCV, n (%) | 22 584 (36.3) | 195 (55.4) | 22 389 (36.2) |
| HBV, n(%) | 2622 (4.2) | 53 (15.1) | 2569 (4.2) |
| NASH/ALD/CC, n (%) | 23 956 (38.5) | 68 (19.3) | 23 888 (38.6) |
| Other, n (%) | 13 033 (21.0) | 36 (10.3) | 12 997 (21.0) |
Abbreviations: ALD, alcoholic liver disease; BMI, body mass index; CC, cryptogenic cirrhosis; HBV, hepatitis B virus; HCV, hepatitis C virus, HIV, human inmmunodeficiency virus; IQR, interquartile range; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatosis liver disease.
Overall, throughout the study period, the proportion of LTs performed by etiology differed by HIV serostatus. Among HIV-infected recipients, HCV was the leading indication for LT (55.4%), followed by NASH/ALD/CC (19.3%), and HBV (15.1%). By contrast, among HIV-uninfected recipients, NASH/ALD/CC was the leading indication (38.6%), followed by HCV (36.2%) and HBV (4.2%) (p < .001 across groups comparing HIV-infected and HIV-uninfected). Within the NASH/ALD/CC group, NASH accounted for 47.1% in the HIV-infected group and 43.6% in the HIV-uninfected group (p = .57).
3.2 |. Changes in liver transplant indications in HIV-infected and HIV-uninfected liver transplant recipients over time
Among all LT recipients, the proportion with HIV increased significantly over time (P trend = .001). The number of LTs for HIV-infected patients increased from 22 LTs in 2008 to 63 LTs in 2018, reflecting a statistically significant AAPC of 9.7% (95%CI, 4.6–15.0, p < .001). The number of LTs for HIV-uninfected patient increased from 4 538 in 2008 to 7 154 in 2018, showing a statistically significant AAPC of 5.0% (95%CI, 4.0–6.0, p < .001). In addition, the number of transplant centers performing LT for HIV-infected recipients also increased with an AAPC of 9.2% (95%CI, 6.3–12.2, p < .001); a potential inflection point was identified in 2015, but it was nonsignificant (p = .32) (Figure 1). For HIV-infected recipients, HCV was the leading indication for LT until 2017, with an increase in the absolute number of patients from 16 recipients in 2008 to 29 in 2017 and 23 in 2018, with an AAPC of 6.9% (95%CI, 0.1–14.2, p < .001). The relative proportion of LT for nonviral causes increased over time and became the leading indication for LT in 2018 (P trend < .001). In HIV-uninfected recipients, HCV was the leading indication for LT in 2008, but the proportion of transplants for HCV decreased with time (P trend < .001). The absolute number of patients with HCV infection changed from 2022 in 2008 to 1548 in 2018, with a negative AAPC of 2.3% (95%CI, 3.8–0.8, p < .001) with an inflection point in 2014 (p < .001) (Figure 2A,B). From 2008 to 2018, NASH as indication for LT increased from 4.5% to 19.1% in HIV-infected patients (P trend = .002) and from 12.1% to 23.3% in HIV-uninfected patients (P trend < .001).
FIGURE 1.
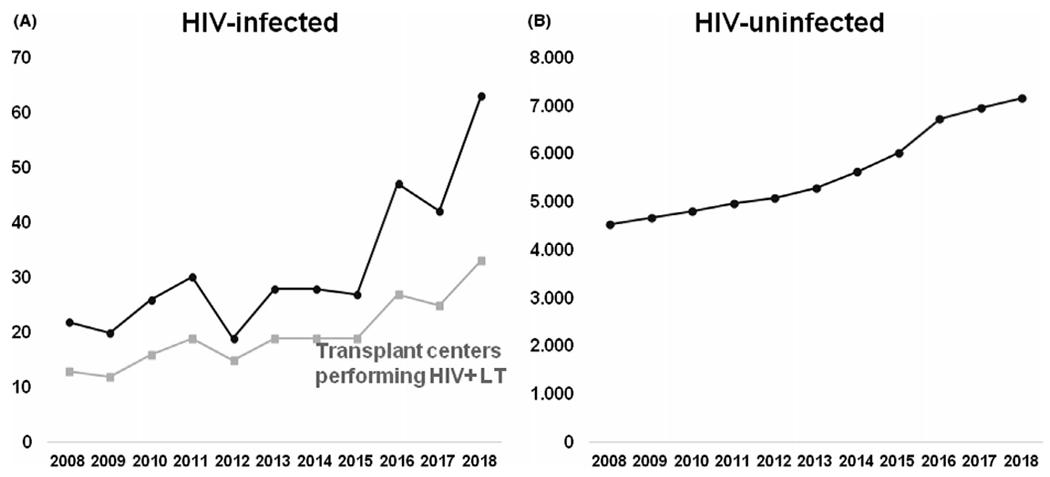
(A) Total number of HIV-infected LT recipients over time and number of centers transplanting HIV-infected patients. (B) Total number of HIV-uninfected LT recipients over time. HIV, human inmmunodeficiency virus; LT, liver transplantation
FIGURE 2.
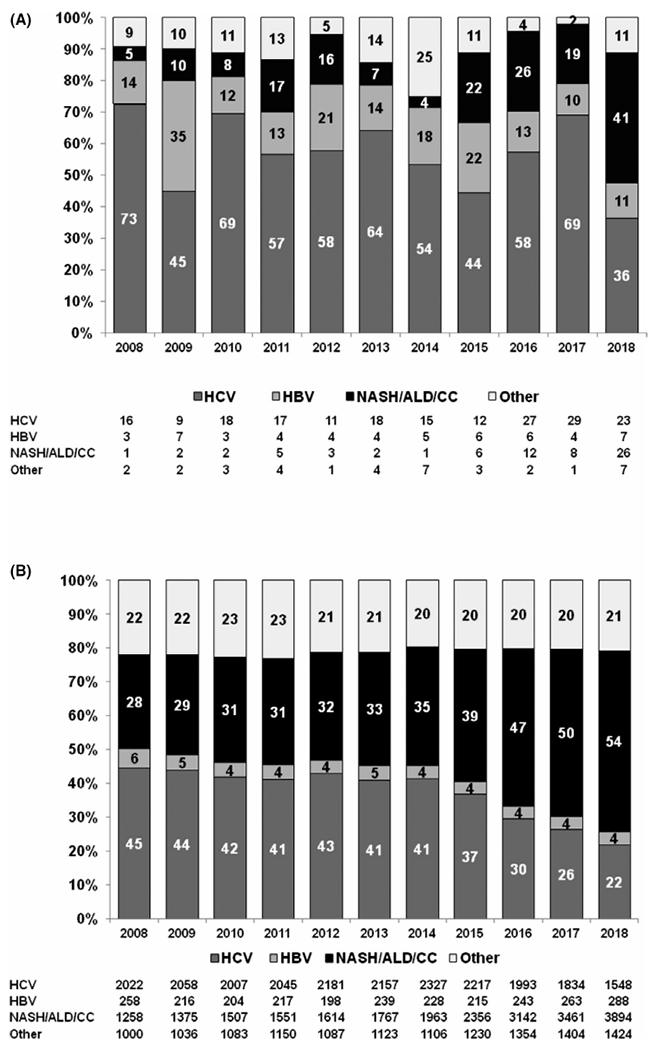
(A) Frequency (in columns) and total number (in table) of indications for liver transplantation among HIV-infected adults in the United States. Time trend for HCV (P trend = 0.9), for HBV (P trend = 0.2), and for NASH/ALD/CC (P trend = 0.02). (B) Frequency (in columns) and total number (in table) of indications for liver transplantation among HIV-uninfected adults in the United States. Time trend for HCV (P trend < .001), for HBV (P trend < .001), and for NASH/ALD/CC (P trend < .001). ALD, alcoholic liver disease; CC, cryptogenic cirrhosis; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatosis liver disease
3.3 |. Patient and graft survival in HIV-infected and HIV-uninfected liver transplant recipients
One- and three-year unadjusted cumulative graft survival rates were 85.5% (95%CI, 81.3–88.9) and 73.0% (95%CI, 67.1–78.1) for HIV-infected LT recipients, respectively, and 89.7% (95%CI, 89.4–89.9) and 81.9% (95%CI, 81.5–82.2) for HIV-uninfected LT recipients, respectively (p = .50 and p = .29). Similarly, 1- and 3-year unadjusted cumulative patient survival rates were comparable. One- and three-year patient survival rates were 87.9% (95%CI, 83.8–91.0) and 77.5% (95%CI, 69.6–80.4) for HIV-infected LT recipients, respectively, and 91.9% (95%CI, 91.7–92.1) and 84.6% (95%CI, 84.2–84.8) in HIV-uninfected recipients, respectively (p = .31 and p = .15) (Figure 3A,B). Over time, graft and patient survival rates improved for both HIV-infected and HIV-uninfected groups (p < .001).
FIGURE 3.
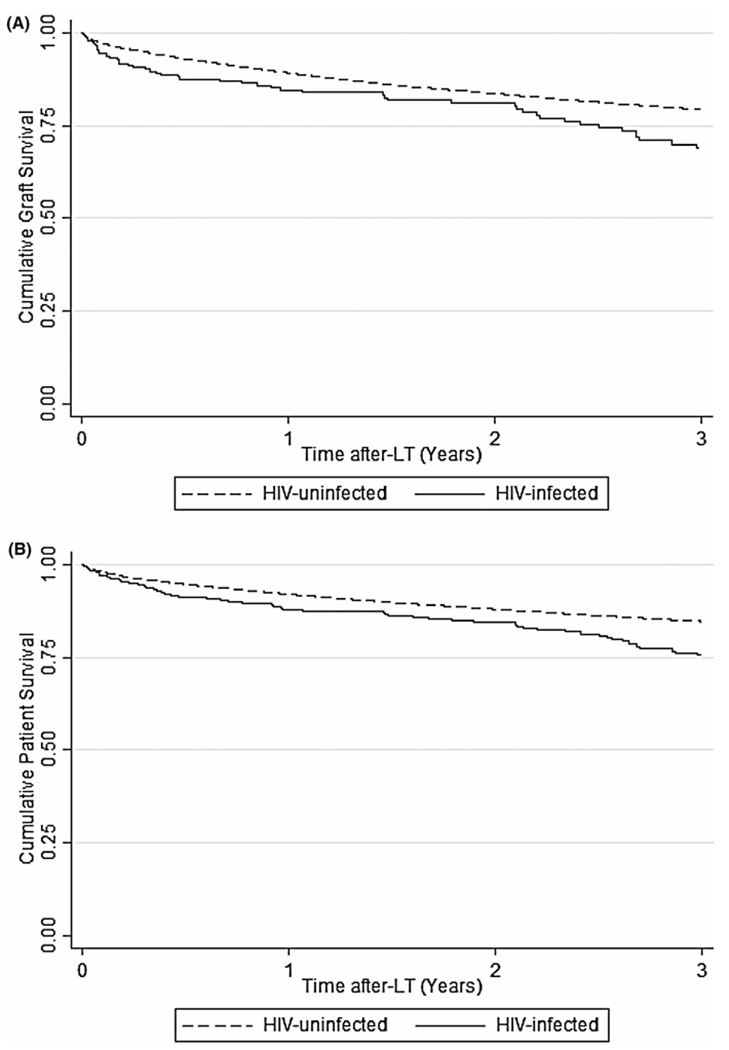
Kaplan–Meier probability of post-liver transplant (LT) graft and patient survival by HIV status overtime. (A) Graft survival. The unadjusted 1- and 3-year graft cumuative survival rates were 85.5% (95%CI, 81.3–88.9) and 73.0% (95%CI, 67.1–78.1), respectively, for the HIV-infected patients and 89.7% (95%CI, 89.4–89.9) and 81.9% (95%CI, 81.5–82.2), respectively, for the HIV-uninfected patients (p = .50 and p = .29). (B) Patient survival. The unadjusted 1- and 3-year cumulative patient survival rates were 87.9% (95%CI, 83.8–91.0) and 77.5% (95%CI, 69.6–80.4), respectively, for the HIV-infected patients and 91.9% (95%CI, 91.7–92.1) and 84.6% (95%CI, 84.2–84.8), respectively, for the HIV-uninfected patients (p = .31 and p = .15). CI, confidence interval; HIV, human inmmunodeficiency virus; LT, liver transplantation
In an analysis stratified by HCV status, for the HCV-infected cohort, 1- and 3-year graft unadjusted survival rates were 84.6% (95%CI, 78.5–89.0) and 69.0%, respectively, (95%CI, 60.7–76.0) for HIV-infected patients and 89.1% (95%CI, 88.7–89.5) and 79.3% (95%CI, 78.7–79.9) for HIV-uninfected patients, respectively (p = .85 and p = .98). One- and three-year patient unadjusted survival rates in the HCV-infected cohort were 87.8% (95%CI, 82.1–91.8) and 72.5% (95%CI, 64.0–79.3), respectively, for HIV-infected patients and 91.2% (95%CI, 90.8–91.5) and 81.8% (95%CI, 81.2–82.3), respectively, for HIV-uninfected patients (p = .97 and p = .02) (Figure S1A,B). In a subanalysis restricted to the period 2014–2018, the difference in patient survival by HIV serostatus was no longer observed. The 1- and 3-year graft unadjusted survial rates were 89.4% (95%CI, 84.0–93.0) and 79.5% (95%CI, 70.7–85.9), respectively, for HIV-infected patients and 91.1% (95%CI, 90.8–91.4) and 84.2% (95%CI, 83.8–84.8), respectively, for the HIV-uninfected patients (p = .81 and p = .46). The 1- and 3-year patients’ unadjusted survival rates were 91.3% (95%CI, 86.1–94.6) and 81.2% (95%CI, 72.2–87.4), respectively, for the HIV-infected patients and 93.0% (95%CI, 92.7–93.3) and 86.4% (86.0–86.9), respectively, for the HIV-uninfected patients (p = .72 and p = .34).
For the HCV-uninfected cohort, the 1- and 3-year graft unadjusted survival rates were 86.8% (95%CI, 80.0–91.4) and 78.3% (95%CI, 69.6–84.8), respectively, for the HIV-infected patients and 89.9% (95%CI, 89.7–90.3) and 83.6% (95%CI, 83.1–84.0), respectively, for the HIV-uninfected patients (p = .98 and p = .62). The 1- and 3-year unadjusted patient survival rates were 88.0% (95%CI, 81.3–92.3) and 79.4% (95%CI, 70.7–85.8), respectively, for the HIV-infected patients and 92.3% (95%CI, 92.1–92.6) and 86.4% (95%CI, 86.0–86.8), respectively, for the HIV-uninfected patients (p = .45 and p = .21) (Figure S1A,B).
3.4 |. Patients with nonalcoholic steatohepatitis by HIV infection
We conducted an exploratory analysis in the subgroup of patients with NASH, to compare their characteristics and outcomes between HIV-infected and HIV-uninfected patients. Among the whole cohort, 11 587 (18.6%) were transplanted with a diagnosis of NASH, including 40 in the group of HIV-infected patients and 11 547 in the HIV-uninfected group (Table 2). Similar to the entire cohort comparisons by HIV serostatus, HIV-infected patients were predominantly male (82.5% vs. 56.8%, p = .001) and younger (median 56 years vs. 61 years, p = .002). Although there were no differences in MELD, race, or proportion with diabetes, the HIV-infected patients had lower BMI (median 27.1 vs. 31.6, p < .001) and a lower proportion with HCC (5.0% vs. 22.1%, p = .009) compared with the HIV-uninfected patients. The 3-year graft unadjusted survival rates were 82.8% (95%CI, 88.6–89.8) for the HIV-infected patients and 83.0 (95%CI, 82.2–83.7) for the HIV-uninfected patients (p = .93), and the 3-year patient unadjusted survival rates were 84.9% (95%CI, 67.2–93.6) for the HIV-infected patients and 85.3% (95%CI, 84.5–86.0) for the HIV-uninfected patients (p = .96).
TABLE 2.
Main characteristics of NASH patients by HIV status
| Variable | Total (n = 11 587) |
HIV-infected (n = 40) 0.4% |
HIV-uninfected (n = 11 547) 99.6% |
|---|---|---|---|
| Male, n (%) | 6590 (56.9) | 33 (82.5) | 6557 (56.8) |
|
| |||
| Age at LT (years), median (IQR) | 61 (54–65) | 56 (53–59) | 61 (54–65) |
|
| |||
| Race/ethnicity | |||
| White | 9203 (79.5) | 28 (70.0) | 9175 (79.5) |
| AA | 280 (2.4) | 0 | 280 (2.4) |
| Hispanic | 1707 (14.7) | 11 (27.5) | 1696 (14.7) |
| Other | 397 (3.4) | 1 (2.5) | 396 (3.4) |
|
| |||
| MELD at LT, median (IQR) | 23 (16–31) | 24 (19–30) | 23 (16–31) |
|
| |||
| MELD at LT excluding HCC, median (IQR) | 24 (18–32) | 24 (20–31) | 24 (18–32) |
|
| |||
| BMI (kg/m2), median (IQR) | 31.6 (27.6–35.7) | 27.1 (23.9–31.0) | 31.6 (27.6–35.7) |
|
| |||
| Diabetes mellitus, n (%) | 6632 (57.2) | 23 (57.5) | 6609 (57.2) |
|
| |||
| Hepatocellular carcinoma, n (%) | 2554 (22.0) | 2 (5.0) | 2552 (22.1) |
Abbreviations: BMI, body mass index; HIV, human inmmunodeficiency virus; IQR, interquartile range; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatosis liver disease.
4 |. DISCUSSION
In this study of HIV-infected and HIV-uninfected individuals who underwent LT from 2008 to 2018 in the United States, we found that the number of PLWH who underwent LT, and the number of transplant centers performing LT for HIV-infected recipients increased over time. The number of LT increased for HIV-infected and HIV-uninfected cohorts, but the AAPC in the HIV-infected cohort was almost double than in the HIV-uninfected cohort (9.7% vs. 5.0%). In HIV-uninfected LT recipients, nonviral indications surpassed HCV as the leading indication for LT in 2015; for HIV-infected LT recipients the same occurred later in 2018. Three-year cumulative graft and patient survival rates for PLWH receiving an LT improved over time.
Recently, several studies have demonstrated the beneficial impact of achieving sustained virological response (SVR) with DAA’s in HCV-monoinfected patients with decompensated cirrhosis,9 even reversing the course of the decompensated disease and enabling delisting in 20% of patients at 60 weeks.10 Consequently, waiting list registration in the United States for decompensated HCV cirrhosis has decreased in the DAA era.6 Similar trends have been described in Europe.10 We confirmed a steady decrease in LT for HCV in the DAA era among HIV-uninfected recipients. In contrast, although a previous study found a decrease in HCV as an indication for LT among HIV-infected recipients in the United States and Europe from 2008 to 2015,8 we observed an increasing number of LT for HCV among the HIV-infected recipients. There are several potential explanations for this difference. In the pre-DAA era, post-LT outcomes among HIV/HCV coinfected recipients were poorer compared with HCV-monoinfected recipients, prompting several LT centers to consider HIV/HCV coinfection a contraindication to LT.11,12 Our observed trends of increased LT for HCV among PLWH may reflect more LT centers transplanting HIV/HCV coinfected patients due to alleviation of the concerns of poorer outcomes in the setting of highly effective DAAs.13–18 The steep reduction in 2018 might reflect a true decrease, similar to the HIV-uninfected patients, but delayed. However, it is important to recognize that despite many promising studies showing favorable response to DAAs both before and after LT,19 there are recent data showing that in HIV/HCV coinfected patients, MELD did not improve in all patients achieved SVR, and 4-year transplant-free survival was 42.8%.20 In addition, restrictions to DAAs remain for some PLWH, including HIV viral load suppression, CD4 thresholds or minimum fibrosis requirements.21,22 Those restraints impact survival,21 and HCV coinfection remains a significant cause of death among PLWH.23
Importantly, in the 2014–2018 period, graft and patient outcomes were similar among LT recipients with HCV regardless of HIV serostatus. This represents a significant change compared with the outcomes of pre-DAA cohorts.11,12,24,25 Moreover, a prior study found that although graft and patient survival among HIV-infected LT recipients improved in the early DAA (2012–2015) versus pre-DAA (2008–2011) period, both were still significantly lower compared with the HIV-uninfected recipients.8 It is reassuring that in the later DAA era, LT outcomes are similar in HCV-infected LT recipients regardless of HIV serostatus. This likely is a result of the similarly high SVR rates in LT recipients with HIV/HCV coinfection and HCV monoinfection.18
We observed a significant increase in LT for nonviral liver disease in HIV-infected patients in recent years. The relatively limited number of HIV-infected LT recipients precluded us from performing a trend analysis based on the specific category of nonviral liver disease. The proportion of LT for NASH is clearly rising in the HIV-uninfected population.1–4 Based on our results, because NASH accounted for nearly half of the nonviral causes of LT in the HIV-infected recipients, we expect that NASH is also increasing as a cause of end-stage liver disease in PLWH. Indeed, the prevalence of metabolic risk factors for NASH are increasing for both HIV-infected and HIV-uninfected persons.26,27 In addition, PLWH have unique conditions thad can lead to an increased risk of NASH, including HIV-related immune activation and gut microbial translocation as well as unfavorable metabolic sideeffects of ART.28–31 Thus, we anticipate that NASH will become a leading indication for LT in PLWH in the decade ahead. Reassuringly, we observed acceptable graft and patient survival among HIV-infected patients transplanted for nonviral cirrhosis etiologies.
We made an attempt to independently evaluate the group of patients with NASH, and no differences in survival were observed comparing between HIV-infected and HIV-uninfected patients, reinforcing the data obtained in the non-HCV cohort analysis. Other differences like the proportion of patients with HCC, not observed in the entire cohort have to be taken with caution, given the small number of HIV-infected patients in the analysis.
Although we found a significant increase in the number of centers transplanting patients with HIV infection over time, barriers to LT for PLWH remain. A recent survey study found that among 57 surveyed LT programs in the United States, 28.1% still consider HIV infection an absolute contraindication for LT, and 31.6% consider it a relative contraindication.32 Our study adds clinical data from a large recent cohort showing that LT survival differences no longer exist between HIV-infected and HIV-uninfected patients and contraindication based on HIV status cannot be supported.8
Registry data offer the availability of a relatively standardized prospectively collected database, with a significant size, that gives the opportunity to gather data from a large number of patients. This is of particular relevance when the population involved represents a small proportion of all LTs. However, there are some limitations, and the relatively small number of HIV-infected LT recipients limits our capacity to develop trends by all the individual categories. We were unable to evaluate trends in LT registration because HIV serostatus is captured in UNOS after LT rather than at the time of listing. Analysis to identify the factors associated with patient and graft survival was not performed, due to the reduced number of events by period. Another limitation, inherent to registry data studies is the assignment of primary liver disease based on UNOS/OPTN codes such that patients with more than one diagnosis are only assigned to the considered primary category. Therefore, misclassification is possible, but it is likely nondifferential by HIV serostatus, and its impact is limited. Also, we might have understimated the numbers of patients with NASH in the HIV-infected group, as these patients are at higher risk of NAFLD at lower BMI, and one criterion to categorize CC cirrhosis as NASH was based on BMI. However, this would not have impacted the comparison of nonviral versus viral etiologies as NASH and CC were categorized together. Finally, information regarding antiviral treatment use is not collected by UNOS, so we were unable to account for the impact of DAA treatment on LT listings and outcomes.
In summary, in the United States, the number of HIV-infected LT recipients has increased in the recent years, as has the number of centers performing LT in PLWH. This may reflect increased confidence in LT for HIV/HCV coinfected patients with the availability of DAA therapy. Indications for LT are also changing, and nonviral causes are now the leading indication for PLWH receiving an LT.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all the centers that contribute to United Network for Organ Sharing (list available at https://optn.transplant.hrsa.gov). Isabel Campos-Varela’s research activity is funded by grant PI19/00330 from Instituto de Salud Carlos III. CIBERehd is supported by Instituto de Salud Carlos III. This work was also supported by an American College of Gastroenterology Junior Faculty Development Award and the University of California San Francisco Liver Center (P30 DK026743) (both to Jennifer C. Price). The work was independent of all funding.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. N.A. reports institutional grant support from Gilead Sciences, GSK, and Roche/Genetech. J.C.P. reports research support from Gilead and Merck, consulting for Theratechnologies, and ownership interest (spouse) in AbbVie, Bristol-Myers Squibb, Johnson and Johnson, and Merck. The other authors have no conflicts of interest to disclose.
Abbreviations:
- 95%CI
95% confidence interval
- AAPC
average annual percentage change
- ALD
alcohol liver disease
- ART
antiretroviral therapy
- BMI
body mass index
- CC
cryptogenic cirrhosis
- DAA
direct-acting antiviral
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NASH
nonalcoholic steatohepatitis
- PLWH
people living with human immunodeficiency virus
- SVR
sustained virological response
- UNOS/OPTN
United Network for Organ Sharing and Organ Procurement and Transplantation Network
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. [DOI] [PubMed] [Google Scholar]
- 2.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. [DOI] [PubMed] [Google Scholar]
- 3.Cholankeril G, Wong RJ, Hu M, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci. 2017;62(10):2915–2922. [DOI] [PubMed] [Google Scholar]
- 4.Shirazi F, Wang J, Wong RJ. Nonalcoholic steatohepatitis becomes the leading indication for liver transplant registrants among US adults born between 1945 and 1965. J Clin Exp Hepatol. 2020;10(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA. National the United States. JAMA Intern Med. 2019;179(3):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. [DOI] [PubMed] [Google Scholar]
- 8.Campos-Varela I, Dodge JL, Berenguer M, et al. temporal trends and outcomes in liver transplantation for recipients with HIV infection in europe and United States. Transplantation. 2020;104(10):2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster GR, Irving WL, Cheung MCM, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. [DOI] [PubMed] [Google Scholar]
- 10.Belli LS, Berenguer M, Cortesi PA, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: a European study. J Hepatol. 2016;65(3):524–531. [DOI] [PubMed] [Google Scholar]
- 11.Terrault NA, Roland ME, Schiano T, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transplant. 2012;18(6):716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miro JM, Montejo M, Castells L, et al. Outcome of HCV/HIV-coinfected liver transplant recipients: a prospective and multicenter cohort study. Am J Transplant. 2012;12(7):1866–1876. [DOI] [PubMed] [Google Scholar]
- 13.Campos-Varela I, Straley S, Agudelo EZ, Carlson L, Terrault NA. Sofosbuvir, simeprevir, and ribavirin for the treatment of hepatitis C virus recurrence in human immunodeficiency virus/hepatitis C virus-coinfected liver transplant recipients. Liver Transplant. 2015;21(2):272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos-Varela I, Moreno A, Morbey A, et al. Treatment of severe recurrent hepatitis C after liver transplantation in HIV infected patients using sofosbuvir-based therapy. Aliment Pharmacol Ther. 2016;43(12):1319–1329. [DOI] [PubMed] [Google Scholar]
- 15.Grant JL, Hawkins C, Brooks H, et al. Successful sofosbuvir-based therapy in HIV/hepatitis C virus coinfected liver transplant recipients with recurrent hepatitis C virus infection. AIDS. 2016;30(1):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castells L, Llaneras J, Campos-Varela I, et al. Sofosbuvir and daclatasvir in mono- and HIV-coinfected patients with recurrent hepatitis C after liver transplant. Ann Hepatol. 2017;16(1):86–93. [DOI] [PubMed] [Google Scholar]
- 17.Antonini TM, Coilly A, Rossignol E, et al. Sofosbuvir-based regimens in HIV/HCV Coinfected patients after liver transplantation: results from the ANRS CO23 CUPILT study. Transplantation. 2018;102(1):119–126.SSSSS [DOI] [PubMed] [Google Scholar]
- 18.Manzardo C, Londoño MC, Castells LLuís, et al. Direct-acting antivirals are effective and safe in HCV/HIV-coinfected liver transplant recipients who experience recurrence of hepatitis C: A prospective nationwide cohort study. Am J Transplant. 2018;18(10):2513–2522. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya D, Belperio PS, Shahoumian TA, et al. Effectiveness of all-oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus Genotype 1-coinfected patients treated in routine practice. Clin Infect Dis. 2017;64(12):1711–1720. [DOI] [PubMed] [Google Scholar]
- 20.Peters MG, Kottilil S, Terrault N, et al. Retrospective-Prospective Study of Safety and Efficacy of Sofosbuvir based Direct Acting Antivirals in HIV/HCV Coinfected Participants with Decompensated Liver Disease Pre or Post Liver Transplant. Am J Transplant. 2020. 10.1111/ajt.16427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breskin A, Westreich D, Hurt CB, et al. The effects of hepatitis C treatment eligibility criteria on all-cause mortality among people with human immunodeficiency virus. Clin Infect Dis. 2019;69(9):1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooka K, Connolly JJ, Lim JK. Medicaid reimbursement for oral direct antiviral agents for the treatment of chronic hepatitis C. Am J Gastroenterol. 2017;112(6):828–832. [DOI] [PubMed] [Google Scholar]
- 23.Breskin A, Westreich D, Cole SR, et al. The effects of hepatitis C infection and treatment on all-cause mortality among people living with human immunodeficiency virus. Clin Infect Dis. 2019;68(7):1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castells L, Rimola A, Manzardo C, et al. Pegylated interferon plus ribavirin in HIV-infected patients with recurrent hepatitis C after liver transplantation: a prospective cohort study. J Hepatol. 2015;62(1):92–100. [DOI] [PubMed] [Google Scholar]
- 25.Terrault N, Reddy KR, Poordad F, et al. Peginterferon and ribavirin for treatment of recurrent hepatitis C disease in HCV-HIV coinfected liver transplant recipients. Am J Transplant. 2014;14(5):1129–1135. [DOI] [PubMed] [Google Scholar]
- 26.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology. 2018;29(3):431–441. [DOI] [PubMed] [Google Scholar]
- 28.Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. 2015;41(4):368–378. [DOI] [PubMed] [Google Scholar]
- 29.Verna EC. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with HIV. Lancet Gastroenterol Hepatol. 2017;2(3):211–223. [DOI] [PubMed] [Google Scholar]
- 30.Price JC, Seaberg EC, Latanich R, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109(5):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez E, Gonzalez-Cordon A, Ferrer E, et al. Differential body composition effects of protease inhibitors recommended for initial treatment of HIV infection: a randomized clinical trial. Clin Infect Dis. 2015;60(5):811–820. [DOI] [PubMed] [Google Scholar]
- 32.Wall A, Lee GH, Maldonado J, Magnus D. Medical contraindications to transplant listing in the USA: a survey of adult and pediatric heart, kidney, liver, and lung programs. World J Surg. 2019;43(9):2300–2308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


