Abstract
We recently identified a group of chemicals that are misclassified by most, if not all, in vitro alternative ocular irritation tests, suggesting that nonanimal tests may not fully model the ocular environment in which these chemicals interact. To address this, we evaluated the composition of tears, the first defense against foreign substances, and identified the presence of antioxidants that could detoxify reactive chemicals that otherwise may be falsely identified as irritants in alternative irritation tests. In this study, we evaluated the effects of tear antioxidants on the ocular irritation scoring of commonly overclassified chemicals (false positives) using the OptiSafe™ ocular irritation test. Six tear-related antioxidants were individually added to the OptiSafe formulation, and the effects on test outcome were determined. Ascorbic acid, the most abundant water-soluble antioxidant in tears, specifically reduced the OptiSafe false-positive rate. Titration curves showed that this reduction occurs at in vivo concentrations and is specific to chemicals identified either as producing reactive oxygen species or as crosslinkers. Importantly, the addition of tear antioxidants did not impact the detection of true negatives, true positives, or other false positives unassociated with reactive oxygen species or crosslinking. These results suggest that the addition of tear antioxidants to in vitro alternative test systems may substantially reduce the false-positive rate and improve ocular irritant detection.
Keywords: Eye Irritation, False Positive, Antioxidants, Tear
Introduction
Consumer products and chemicals are routinely tested for ocular irritancy potential. In the past, the testing of materials for ocular safety has relied on the in vivo Draize rabbit eye test, which has become the “gold standard” for chemical classification (Draize et al., 1944; Lebrun et al., 2020). This live animal test uses a clinical scoring system that grades the severity and duration of the ocular irritation response that generally occurs from 1 to 21 days after a chemical exposure. The resulting clinical scores and durations of effects are then applied to either the United Nations Globally Harmonized System (GHS; UN, 2011) or the U.S. Environmental Protection Agency (EPA) methods of eye irritation classification (ICCVAM, 2010). Because of animal cruelty concerns, there has been a major effort over the past several decades to develop alternative strategies (nonanimal tests) that could be safely used to classify and label potentially harmful chemicals.
In vitro test methods have been developed and validated for the detection of materials that cause the most severe level of ocular irritation (ocular corrosives, GHS category 1) and the least irritating level [GHS not classified (NC) ocular irritants], but not for the detection of reversible irritants (GHS categories 2B and 2A) (Lebrun et al., 2019). Nonanimal tests cannot detect reversible irritants because the false-positive (FP) rate is too high for the detection of GHS NC, and the false-negative (FN) rate is too high for ocular corrosives; this combination results in inaccurate predictions for the middle classification (reversible irritants). For example, we previously compared the Bovine Corneal Opacity and Permeability, EpiOcular™, Isolated Chicken Eye, Ocular Irritection®, and OptiSafe™ tests and found that they all mispredicted a group of chemicals with FP rates (GHS NC overpredicted as GHS category 2 or 1) around 40% (Lebrun et al., 2020). The finding that all of the nonanimal tests overpredict the same chemicals indicates the current in vitro tests are incomplete models of in vivo eye irritation. “Defined approaches” have been proposed for testing chemicals for eye irritation (GHS categories 2B and 2A) using specific physiochemical properties of the materials. These properties are then used to follow a predetermined flow chart for a theoretically better prediction (OECD, 2017; Alepee et al., 2019a, b). However, defined approaches require knowing specific physiochemical properties (e.g., octanol-water partition coefficient, vapor pressure, water solubility, surface tension) of the material being tested and have only been developed for known liquid chemicals (OECD, 2017; Alepee et al., 2019a, b).
The Unique Redox Environment of the Eye
The cornea consists of the stroma that is protected by the corneal epithelium, while the rest of the eye is covered by the conjunctiva. The cornea and conjunctiva function as barriers to protect the eye from exposure to environmental insults including foreign bodies, microbes, and irritating chemicals. In particular, the corneal epithelium uniquely expresses very high levels of corneal crystallin proteins that are water-soluble enzymes such as aldehyde dehydrogenases 1A1 and 3A1 that can protect against metabolic insults and oxidative injury including ambient oxygen, photo-oxidation, ionizing radiation, and chemicals (Jester, 2008).
Likewise, human tears consist of three layers composed of mucin, water, and lipids (inner to outer) that also contribute to the health and maintenance of the ocular surface (Conrady et al., 2016). Lacrimal glands produce the aqueous layer of the tear film, which is produced at a basal rate of up to 2 μL/minute (Kim et al., 2019) and up to 100-fold higher in response to mechanical, thermal, or chemical exposure (reflex rate). Importantly, these increased aqueous tears have the potential to dilute, clear, and detoxify chemicals. Rabbit tears and human tears are similar (Chen et al., 2009). Proteins present in rabbit tears include lysozyme, β-hexosaminidase, lactate dehydrogenase, angiotensin-converting enzyme, plasminogen activator, and prostaglandin E2 (Thorig et al., 1985). Proteins present in human tears include lysozyme, immunoglobulins (IgG), lactoferrin, albumin, and protein G (Wollensak et al., 1990).
The cornea is exposed to reactive oxygen species (ROS) that include superoxide anion (O22−), hydrogen peroxide (H2O2), hydroxyl radical (HO*), hydroperoxides (ROOH), peroxyl radicals (ROO*), and singlet oxygen (O2) (Nita and Grzybowski, 2016; Ung et al., 2017; Kumari et al., 2018). Cellular phospholipid bilayers are susceptible to ROS-induced damage via lipid peroxidation, which occurs when free radical species including oxyl, peroxyl, and hydroxyl radicals remove electrons from lipids and subsequently produce reactive intermediates that can cause considerable damage via redox cycling (Njie-Mbye et al., 2013; Babizhayev, 2016; Tangvarasittichai, 2018; Su et al., 2019). The oxidation of nucleotides and proteins may also lead to changes in gene expression, mutations, and the formation of insoluble protein aggregates. Sources of ROS include ultraviolet light induction, ozone, cigarette smoke, pollution, and accidental chemical exposure (Wakamatsu et al., 2008; McMonnies, 2014; de Jager et al., 2017; Ung et al., 2017; AlKahtane et al., 2020).
Importantly, the eye is protected against these oxidative stresses not only by the expression of intracellular corneal crystallin proteins that provide enzymatic protection, but also by the presence of nonenzymatic antioxidants in the cornea, aqueous humor, and tears. As shown in Table 1, human tears and aqueous humor have similar concentrations of antioxidants. Tears are the first biological fluid to interact with and potentially detoxify chemicals that contact the eye. Nonetheless, nonanimal tests do not model tears or the tearing process. Aqueous humor production and turnover are also dynamic processes, which like tearing, are not modeled by nonanimal tests. In human tears, ascorbic acid and uric acid account for approximately 50% of the total antioxidant activity, with ascorbic acid being the most abundant (Chen et al., 2009). Other small molecules, including reduced glutathione, L-cysteine, and L-tyrosine, make up the rest. Enzymes include superoxide dismutase (SOD), which has an activity around 3.5 U/mL (Behndig et al., 1998). In the order of abundance in human aqueous humor, nonenzymatic antioxidants include ascorbic acid (530 μM), L-tyrosine (78 μM), uric acid (43 μM), L-cysteine (14.3 μM), and glutathione (5.5 μM). SOD activity is not believed to contribute significantly to the antioxidant defense mechanisms of the aqueous humor (Chen et al., 2009).
Table 1.
Antioxidants Present in Aqueous Humor and Tears
Even though scientists in the area of ophthalmology have characterized the importance of protective antioxidant effects on the eye in disease and during wound healing, current in vitro eye tests are theoretically limited by their ability to model the response to oxidative stress. Based on our literature review, current nonanimal eye irritation tests have not specifically accounted for the antioxidant capabilities and properties of the eye, especially the tear response that occurs when chemicals contact the ocular surface. Since we have noted that in vitro tests overpredict many of the same chemicals (Lebrun et al., 2020), we questioned whether these FPs might have common chemical reactions that involve the formation of ROS. After evaluating the chemistries of commonly missed FPs, it was noticed that a group of chemicals showed reactive chemistries, particularly for ROS and crosslinker (CL) potential. CLs covalently bind molecules via electron transfer and redox cycling between reduced and oxidized molecules that can lead to the generation of ROS (van Amsterdam et al., 2001; Kovacic et al., 2002). Based on observations that chemicals associated with ROS-generating chemistries and CLs are more likely to be FPs, we hypothesized that the addition of antioxidants to nonanimal eye safety tests to more closely resemble the tear defense mechanism might significantly improve (lower) the FP rate. To test this hypothesis, various antioxidants were titrated into the OptiSafe test, and improvements were assessed.
Methods
The OptiSafe method was conducted as previously described (Choksi et al., 2020). Briefly, samples were initially evaluated for solubility, pH, and foaming using a standardized procedure. Based on the outcomes, a decision tree was followed to allow for standardized procedural modifications for substances with the following properties: 1) extreme pH, 2) insolubility, and 3) categorized as a surfactant. The procedure differed for materials with these properties. For substances with an extreme pH, the buffering power was evaluated, and standards were adjusted to match. Insoluble materials were floated instead of placed on membrane discs. Surfactants were diluted. Materials were tested for potential to damage to water-soluble or -insoluble macromolecules. The OptiSafe test is supplied as a complete kit (Lebrun Labs LLC, Anaheim, CA), which includes the OptiSafe “active agent” reagent formulation (see Choksi et al., 2020 for additional details). Test chemicals were added to “ocular discs” to control the delivery of the chemical to be tested as it enters the reagent mixture. The resulting optical density (OD) and pH values were compared with quality controls and a standard curve to generate a score. The score was then applied to a prediction model (≤15 = NC; >15 – 45 = category 2; >45 = category 1) to classify the material tested.
An initial screen of the six major abundant antioxidants present in aqueous humor and tear film (listed in Table 1) was first performed. Antioxidants were added directly to the OptiSafe formulation and mixed; the pH was then adjusted to 6.36. For the pilot studies, the antioxidants evaluated included L-tyrosine (78 μM, Sigma Aldrich, Milwaukee, WI; catalogue number: T3754), uric acid (43 μM, Sigma Aldrich, Milwaukee, WI; catalogue number: U2625), L-ascorbic acid (5.3 × 102 μM, Sigma Aldrich, Milwaukee, WI; catalogue number: A5960), L-cysteine (14 μM, Sigma Aldrich, Milwaukee, WI; catalogue number: 30089), and glutathione (5.5 μM, Sigma Aldrich, Milwaukee, WI; catalogue number: PHR1359. The initial screen was conducted with a representative “true positive” (TP, cyclohexanol; Lebrun et al., 2020), “true negative” (TN, 2,4-pentanediol; Lebrun et al., 2020), and FP (triethylene glycol; Lebrun et al., 2020) associated with ROS generation (Table 2). After an 18-hour incubation at 31 °C, the OD was measured with a spectrophotometer. The resulting OD and pH values were compared with quality controls and a standard curve. Scores are assigned to classifications based on the OptiSafe prediction model. A score of 15 or less predicts GHS NC, and an OptiSafe score greater than 45 predicts a GHS category 1. Scores that fall within these cutoffs are predicted as GHS category 2A/B.
Table 2.
Chemicals Used
| Chemical Name | CASRN | Catalog #b | In Vivo GHSa | OS | ROS/CL | Description | Reference |
|---|---|---|---|---|---|---|---|
| Cyclohexanol | 108-93-0 | 105899 | Cat. 1 | TP | No | No evidence for ROS/CL | Fisher, 2000. Frater, 2009. |
| 2,4-Pentanediol | 625-69-4 | 156019 | NC | TN | No | No evidence for ROS/CL | Tei, 2002. Wang, 2003. |
| Dodecane | 112-40-3 | 297879 | NC | TN | No | No evidence for ROS/CL | Chen, 2019. Huber, 2004. |
| 2-(2-Ethoxyethoxy)ethanol | 111-90-0 | 537616 | NC | FP | ROS | Forms hydrogen peroxide and hydroperoxi des | Adedara, 2013. Bodin, 2003. |
| Triethylene glycol | 112-27-6 | 95126 | NC | FP | ROS | Forms hydrogen peroxide and hydroperoxi des | Zhu, 2012. Mikulas, 2018. |
| Ethylene glycol diethyl ether | 629-14-1 | 224111 | NC | FP | ROS | Forms hydrogen peroxide and hydroperoxi des | Di Tommaso, 2011. Clark, 2001. |
| Styrene | 100-42-5 | S4972 | NC | FP | ROS | Forms styrene oxide | Zhang, 2017. Belvedere, 1981. Carlson, 2006. Niaz, 2017 |
| 1,9-Decadiene | 1647-16-1 | 118303 | NC | FP | CL | Used as a crosslinker to promote polymerization | Palmlof, 2000. Smedberg, 1997. |
| 2-Ethoxyethyl methacrylate | 2370-63-0 | 280666 | NC | FP | CL | Used as a crosslinker to promote polymerization | Chirila, 1991. Garcia, 2002. Faraguna, 2015. |
| Triphenyl phosphite | 101-02-0 | T84654 | NC | FP | No | No evidence for ROS/CL | Schwetlick, 1987, 1995. Kovacs, 1973. Liu, 2019. |
| Ethyl acetate | 141-78-6 | 270989 | NC | FP | No | No evidence for ROS/CL | Suksomtip, 2010. Nakchat, 2014. |
| 2,4-Pentanedione | 123-54-6 | P7754 | NC | FP | No | No evidence for ROS/CL | Mottley, 1991. Rodrigues, 2006. |
| 2,2-Dimethyl-3-pentanol | 3970-62-5 | D173622 | NC | FP | No | No evidence for ROS/CL | Dwarakanath, 1998. Gierke, 1999. Hurley, 2008. |
CASRN = Chemical Abstracts Service Registry Number; GHS = Globally Harmonized System of classification and labelling of chemicals; Cat. = Category; NC = Not classified; ROS = Reactive oxygen species; CL = Crosslinker.
All chemicals were obtained from Sigma Aldrich, Milwaukee, WI.
After the screening of six antioxidants, L-ascorbic acid was selected, and its effect on the OptiSafe score for a broader range of chemicals was assessed (Table 2). A serial 2-fold titration series with four concentrations above and four concentrations below the approximate physiologic level was conducted for L-ascorbic acid (concentrations of 3.3 × 101 μM, 6.6 × 101 μM, 1.3 × 102 μM, 2.7 × 102 μM, 5.3 × 102 μM, 1.1 × 103 μM, 2.1 × 103 μM, 4.2 × 103 μM, and 8.5 × 103 μM) with 5.3 × 102 μM as the physiologic tear concentration. Each concentration was prepared by weighing and solubilizing the material in the OptiSafe test reagent and adjusting the pH to 6.36. Complete standard series and quality assurance chemicals were run in parallel with each condition, and all chemicals tested were purchased from Sigma Aldrich (Milwaukee, WI).
Chemicals that were tested (Table 2) included one TP, two TNs, and 10 FPs that generated either ROS (n=4), CL (n=2), or were nonreactive (n=4).
Results
A limited set of chemicals were used to initially screen different tear antioxidants. Figure 1 shows results for this study, which included a positive control (A. cyclohexanol) with scores of 73.5 for the no antioxidant condition, 71.7 for L-tyrosine, 73.2 for uric acid, 73.1 for ascorbic acid, 71.9 for L-cysteine, and 66.1 for glutathione, which are all above the OptiSafe cutoff (score of 45) for GHS category 1 classification (dashed line at a score of 45). The negative control (B. 2,4-pentanediol), with scores of 5.3 for the no antioxidant condition, 7.7 for L-tyrosine, 6.4 for uric acid, 10.1 for ascorbic acid, 7.1 for L-cysteine, and 7.7 for glutathione) were all below the cutoff (dashed line, OptiSafe score of 15) for GHS NC classification (dashed line). By comparison, the FP (C, triethylene glycol), showed a significant reduction in the OptiSafe score when ascorbic acid was included (23.2 for no antioxidant and 3.3 for ascorbic acid). The effect of this response resulted in a change of classification of triethylene glycol from a FP to a TN (dash line, OptiSafe score below 15). No effects were identified for the remaining tear antioxidants (23.9 with L-tyrosine, 24.3 for uric acid, 25.7 for L-cysteine, and 22.1 for glutathione).
Figure 1.
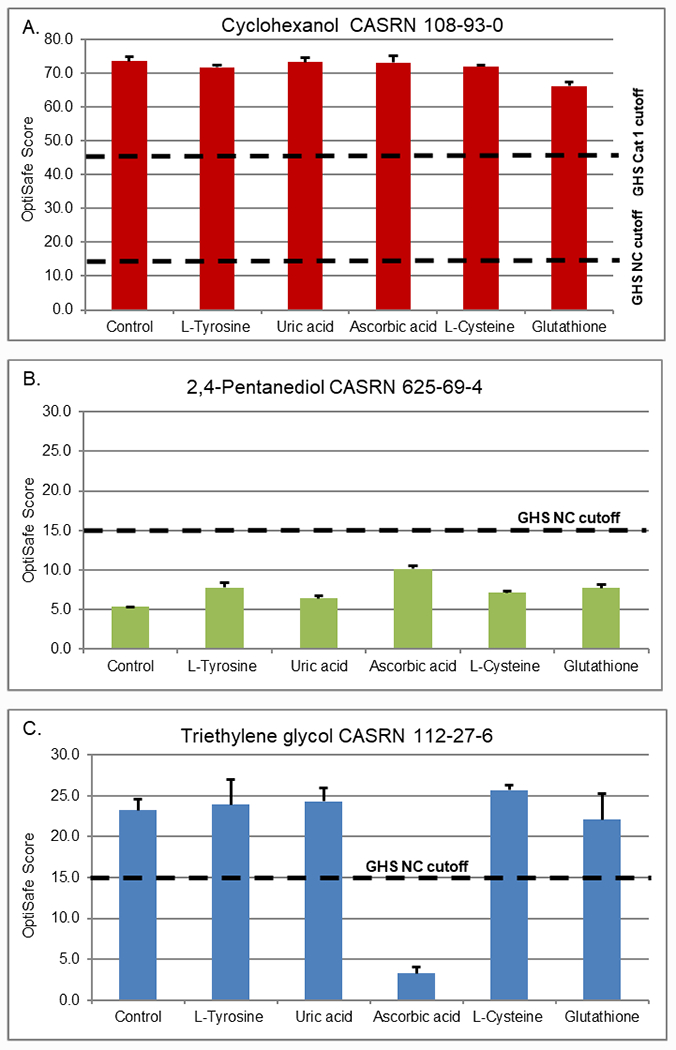
Screening study of antioxidants at in vivo concentrations (control = no antioxidant, L-tyrosine at 78 μM, uric acid at 43 μM, ascorbic acid at 530 μM, L-cysteine at 14.3 μM, and glutathione at 5.5 μM). Three representative chemicals, (A) cyclohexanol GHS category 1; (B) 2,4-pentanediol GHS NC; and (C) triethylene glycol GHS NC, were used to screen the effects of each antioxidant. Dashed lines show the NC and category 1 cut-offs for the OptiSafe prediction model.
Based on these screening results, L-ascorbic acid was selected for more detailed testing to evaluate the effects of different concentrations of the antioxidant on a broader range of chemicals. For this study, the titration of ascorbic acid ranged from 0 to 8.5 × 103 mM, a range that covered one log unit above and below the physiologic concentration of 5.3 × 102 μM in the tears. As shown in Figure 2, the positive control (A. cyclohexanol) ranged from OptiSafe scores of 61.4 to 79.7, which remained above the category 1 irritant cutoff of 45. On the other hand, the negative controls (B, 2,4-pentanediol; C, dodecane) had scores ranging from 5.9–11.1 and 0.0–3.4, respectively, which are all below the score for a NC classification. Of the four FPs not associated with reactive chemistries (Table 2), three showed no effect with triphenyl phosphite (D) ranging from 127.4 to 157.6, ethyl acetate (E) ranging from 25.2 to 15.0, and 2,4-pentanedione (F) ranging from 56.4 to 79.1. All of these remained FP based on their OptiSafe score, while ethyl acetate showed a borderline TN score of 15 at the physiologic ascorbic acid concentration. The fourth FP (G, 2,2-dimethyl-3-pentanol) became a TN, with an initial OptiSafe score of 23.2 that was reduced to 9.7 at the highest concentration of ascorbic acid tested.
Figure 2.
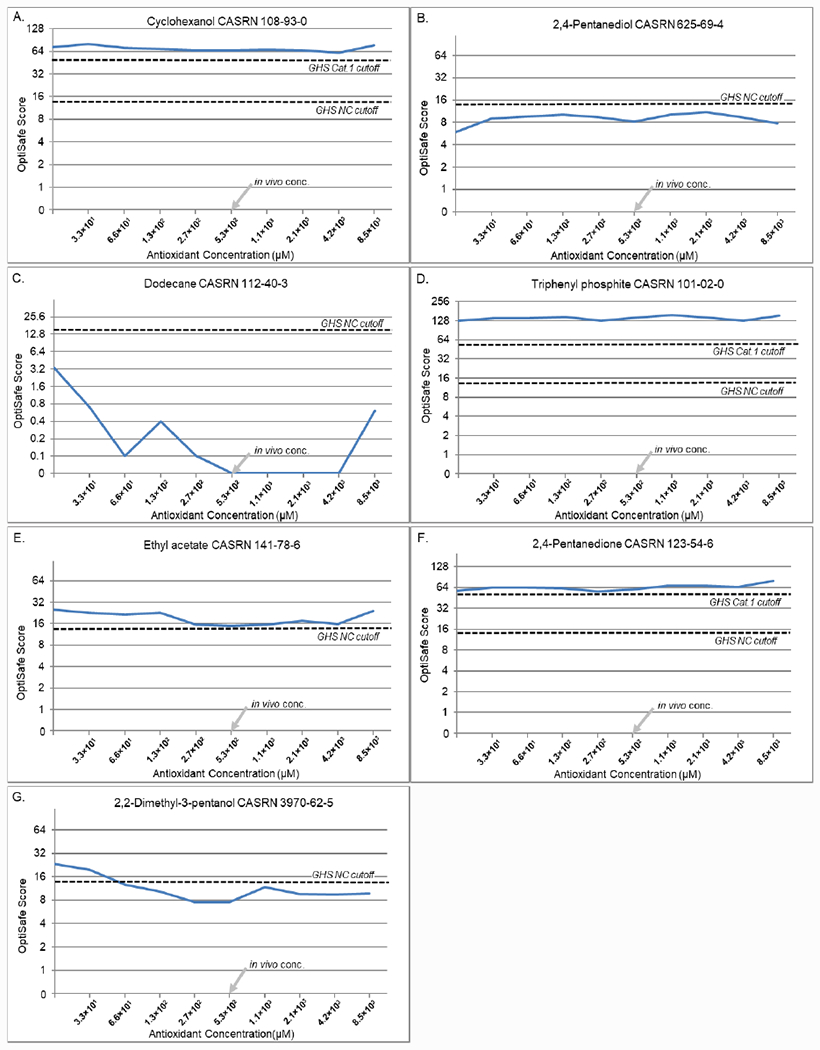
Control titrations. (A) Positive control (cyclohexanol). (B and C) Negative controls (B) 2,4-pentanediol and (C) dodecane. (D–G) FP controls (D) triphenyl phosphite; (E) ethyl acetate; (F) 2,4-pentanedione; and (G) 2,2-dimethyl-3-pentanol. The dashed lines show the GHS NC and category 1 cut-off values.
Of the six FPs that showed reactive chemistries (Figure 3), all showed a marked improvement in the OptiSafe score and five showed an improved GHS category prediction (from FP to TN). For 2-(2-ethoxyethoxy)ethanol (A), the scores ranged from 23.5 to 9.9 with score of 14.4 at the in vivo ascorbic acid concentration, resulting in a TN OptiSafe prediction. Triethylene glycol (B) decreased from 19.8 to 1.7 with the score of 1.7 at the in vivo ascorbic acid concentration. Styrene (D) ranged from 46.9 to 0.8 with a score of 0.8 at the in vivo concentration. The CL 1,9-decadiene (E) ranged from 46.0 to 0.4 with a score of 20.0 at the in vivo concentration, and 2-ethoxyethyl methacrylate (F) ranged from 143.9 to 4.5 with a score of 4.5 at the in vivo concentration. For all five of these formerly FN chemicals, the addition of ascorbic acid lowered the OptiSafe score to below 15, resulting in a reclassification to a TN. The sixth chemical tested, ethylene glycol diethyl ether (C), also showed a reduction in the OptiSafe score from 30.3 to 15.2 and was borderline for the TN OptiSafe classification. Interestingly, the FP chemicals with ROS and CL activity showed a marked reduction in the OptiSafe score at or near the physiological tear concentrations (arrow, in vivo concentration). For all overclassified ROS chemicals, there was an average decrease in the OptiSafe score of 68.9% (comparing no antioxidant to the highest ascorbic acid concentration) and an average decrease of 67.7% (comparing no antioxidant compared to the in vivo ascorbic acid concentration). For the two CL chemicals, there was a marked decrease in the OptiSafe score from 42.8 to 0.9 for 1,9-decadiene (E) and 122.1 to 5.5 for 2-ethoxyethyl methacrylate (F; comparing no antioxidant to the highest ascorbic acid concentration). Overall, for the two CL chemicals there was an average decrease of 74.8% from no antioxidant compared to the in vivo concentration.
Figure 3.
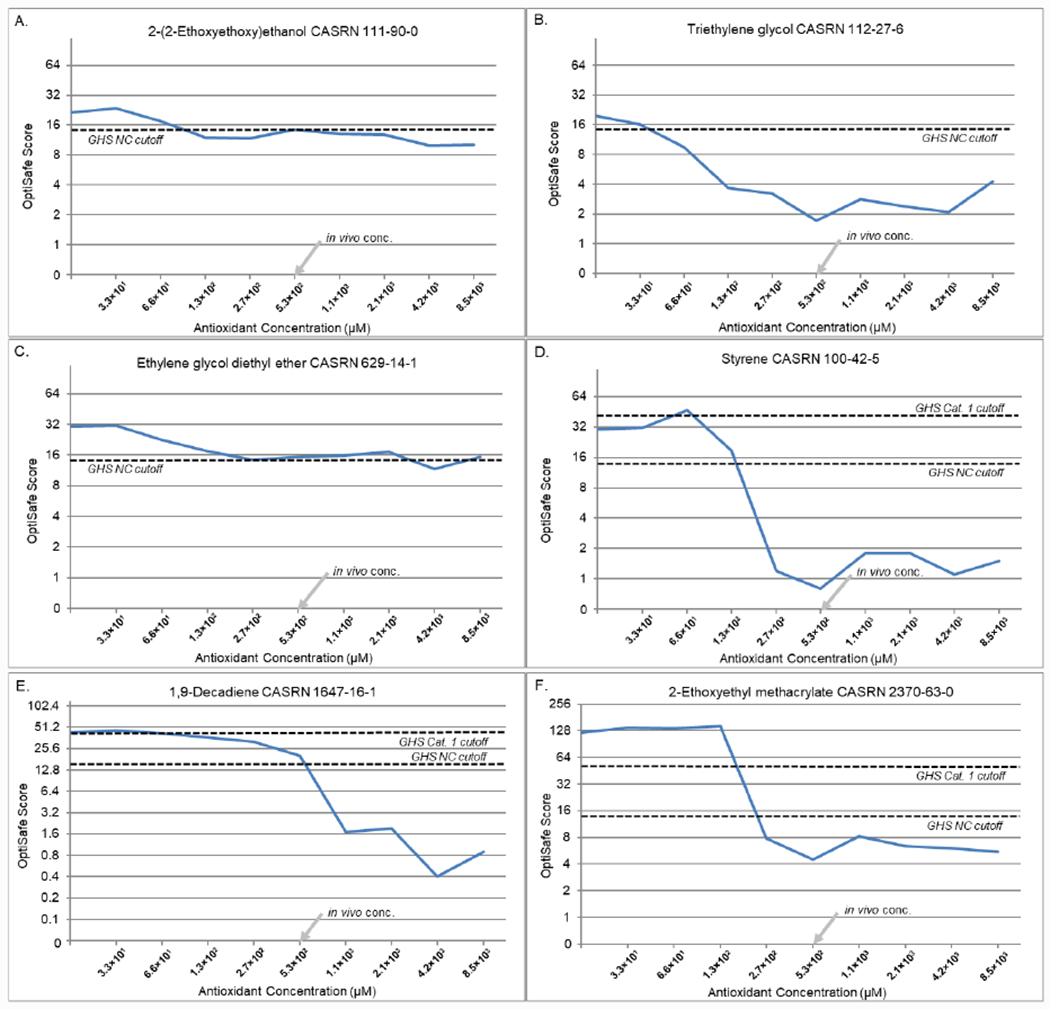
ROS and CL antioxidant titrations. (A–D) ROS chemicals (previously classified as FPs) (A) 2-(2-ethoxyethoxy)ethanol; (B) triethylene glycol; (C) ethylene glycol diethyl ether; and (D) styrene. (E and F) CLs (previously predicted as FPs) (E) 1,9-decadiene and (F) 2-ethoxyethyl methacrylate. The dashed lines show the GHS NC and category 1 cut-off values.
To assess the effect on OptiSafe chemical classification by adding ascorbic acid, triplicate repeats for the formulation without antioxidants (OptiSafe) and with antioxidants (L-ascorbic acid at the approximate in vivo concentration of 5.7 × 102 μM) was performed. This new formulation with antioxidants is called OptiSafe2 (OS2). The results of this test are presented in Table 3 for controls and Table 4 for chemicals with ROS and CL chemistries. As shown, the OptiSafe classification for the control chemicals were essentially unchanged, with the exception of 2,2-dimethyl-3-pentanol, which became a borderline TN with an average FP score of 19.2, improving to an average score of 15.1. On the other hand, as shown in Table 4, all of the chemicals associated with ROS or CL had a reduction in score, and five of the six showed improvements in classification from a FP to TN. For example, the average OptiSafe score for triethylene glycol was 13.2, and the average OS2 score was 5.1 with a standard deviation of 1.7. Significant improvements were seen with styrene, from a score of 53.5 to a score of 3.5, and 2-ethoxyethyl methacrylate, from a score of 97.1 to a score of 14.5; both were previously predicted as GHS category 1 chemicals. While there was a large score reduction for 2-ethoxyethyl methacrylate from an average of 97.1 to 14.5, the consensus classification of the triplicate data predicted this chemical as a category 2.
Table 3.
OptiSafe and OS2 Score Comparison
| Chemical Name | GHS | Condition | OptiSafe Score, AVG ± STD | OS2 Score, AVG ± STD |
|---|---|---|---|---|
| Cyclohexanol (108-93-0) | Cat. 1 | Pos. | 52.6 ± 1.1 | 66.9 ± 4.9 |
| 2,4-Pentanediol (625-69-4) | NC | Neg. | 12.0 ± 1.4 | 10.2 ± 0.7 |
| Dodecane (112-40-3) | NC | Neg. | 0.9 ± 0.8 | 3.0 ± 2.6 |
| Triphenyl phosphite (101-02-0) | NC | NOTROS/CL | 113.3 ± 2.1 | 169.0 ± 9.9 |
| Ethyl acetate (141-78-6) | NC | NOT ROS/CL | 23.9 ± 2.0 | 25.6 ± 2.4 |
| 2,4-Pentanedione (123-54-6) | NC | NOT ROS/CL | 45.3 ± 1.4 | 42.3 ± 6.6 |
| 2,2-Dimethyl-3-pentanol (3970-62-5) | NC | NOT ROS/CL | 19.2 ± 1.2 | 15.1 ± 0.4 |
OptiSafe (without antioxidants), OS2 = OptiSafe2 (OptiSafe with antioxidants added); GHS = Globally Harmonized System of classification and labelling of chemicals; Cat. = Category; NC = Not classified; Pos. = Positive control; Neg. = Negative control; AO = Antioxidant; ROS = Reactive oxygen species; CL = Crosslinker; AVG = Average; STD = Standard deviation.
Table 4.
OptiSafe and OS2 Score Comparison for ROS/CL Antioxidant Titrations
| Chemical Name | GHS | Condition | OptiSafe Score, AVG ± STD | OS2 Score, AVG ± STD |
|---|---|---|---|---|
| 2-(2-Ethoxyethoxy) ethanol (111-90-0) | NC | ROS | 21.7 ± 0.3 | 12.0 ± 0.3 |
| Triethylene glycol (112-27-6) | NC | ROS | 13.2 ± 3.5 | 5.1 ± 1.7 |
| Ethylene glycol diethyl ether (629-14-1) | NC | ROS | 25.4 ± 1.8 | 21.6 ± 2.5 |
| Styrene (100-42-5) | NC | ROS | 53.5 ± 10.1 | 3.5 ± 0.8 |
| 1,9-Decadiene (1647-16-1) | NC | CL | 22.5 ± 3.6 | 11.4 ± 6.4 |
| 2-Ethoxyethyl methacrylate (2370-63-0) | NC | CL | 97.1 ± 18.2 | 14.5 ± 2.7 |
OptiSafe (without antioxidants), OS2 = OptiSafe2 (OptiSafe with antioxidants added); GHS = Globally Harmonized System of classification and labelling of chemicals; NC = Not classified; ROS = Reactive oxygen species; CL = Crosslinker; AVG = Average; STD = Standard deviation.
Discussion
In this study, we show for the first time that when previously identified antioxidants known to be present in tears are added to the formulation for the OptiSafe test, there is a marked reduction in the OptiSafe score that results in an improvement in classification from FP to TN for chemicals that have reactive chemistries. Importantly, this effect seems to be limited to only those FP chemicals with reactive chemistries. The addition of antioxidants has little or no effect on the OptiSafe score or irritancy classification for other chemicals not having reactive chemistries that are classified as a TP, TN, FP, or FN. These findings may have a broad impact not only on our design and interpretation of alternative ocular irritation tests but also on the ocular response to chemical injury.
First, while nonanimal eye tests have been in development for over 30 years (Hartung et al., 2010), they do not take the composition of tears into consideration or specifically add antioxidants to the test matrix or chemical to be tested. On one hand, the prevention of damage by the addition of antioxidants is counterintuitive because nonanimal tests are biased toward high sensitivity; intuitively, the reduction of measured damage by inactivating reactive molecules might be viewed as reducing the sensitivity of the test method. Nonetheless, we show for a limited set of chemicals that the addition of antioxidants does not affect the measured classification of FP for nonreactive chemicals (not identified by literature review as ROS generators or CLs) and, most importantly, does not appear to change the scores or classifications of TPs. However, this finding requires a larger screening of chemicals to establish that tear components do not interfere with the detection of other ocular irritants by the OptiSafe method, which is currently ongoing. Furthermore, further investigation of the effects of reformulating exposure conditions for other alternative tests seems warranted in light of this work. The importance of our findings is also emphasized by the fact that the most effective concentrations of the antioxidant, ascorbic acid, was about the same concentration range as in tears, strongly suggesting that antioxidants play a critical role in mitigating ocular irritation following eye exposure.
Second, it is widely accepted that alternative ocular irritation tests that do not require the use of animals generally fail to identify or model essential components of the live eye and have either poor specificity or sensitivity. Overall, the lack of understanding of the underlying reasons for this limitation has generally hindered the validation and acceptance of nonanimal tests for eye safety testing and chemical classification. In particular, all high-sensitivity alternative ocular irritation tests have a high FP rate (40% or more, Lebrun et al., 2020). It has been suggested that this high FP rate is because nonanimal tests are only able to measure initial damage and do not accurately model the repair/reversibility of damage after initial injury. However, as demonstrated in this study, the FP rate of at least one nonanimal test (OptiSafe) may also be due to a failure to adequately model the ocular chemical defense mechanisms that can potentially mitigate ocular injury during chemical exposure. Our findings appear to argue against the inability of these tests to model repair or reversibility as the reason for their poor performance. Rather, our findings support the contention that a better understanding of the acute and immediate interaction of irritants with the eye could lead to refinement of current alternative tests and produce improvements in both predictability and sensitivity.
Highlights.
Current nonanimal eye irritation tests do not model tear-related antioxidants and have high false-positive rates.
Tear-related antioxidants at physiological concentrations lower the false-positive rate for chemicals associated with reactive oxygen species without impacting the detection of true negatives, true positives, or other false positives not associated with reactive oxygen species.
Refinement of current alternative eye irritation tests to include tear antioxidant chemicals may improve the false-positive rate for these tests.
Acknowledgments
Supported by a NIEHS Small Business Innovative Research Grant and Research to Prevent Blindness (RPB 203478). Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R44ES025501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Adedara IA, Farombi EO (2013). “Kolaviron protects against ethylene glycol monoethyl ether-induced toxicity in boar spermatozoa”. Andrologia. 46(4):399–407. doi: 10.1111/and.12095. [DOI] [PubMed] [Google Scholar]
- Alépée N, Adriaens E, Abo T, Bagley D, Desprez B, Hibatallah J, Mewes K, Pfannenbecker U, Sala À, Van Rompay AR, Verstraelen S, McNamee P (2019a). “Development of a defined approach for eye irritation or serious eye damage for liquids, neat and in dilution, based on cosmetics Europe analysis of in vitro STE and BCOP test methods”. Toxicol In Vitro. 57:154–163. doi: 10.1016/j.tiv.2019.02.019. [DOI] [PubMed] [Google Scholar]
- Alépée N, Adriaens E, Abo T, Bagley D, Desprez B, Hibatallah J, Mewes K, Pfannenbecker U, Sala À, Van Rompay AR, Verstraelen S, McNamee P (2019b). “Development of a defined approach for eye irritation or serious eye damage for neat liquids based on cosmetics Europe analysis of in vitro RhCE and BCOP test methods”. Toxicol In Vitro. 59:100–114. doi: 10.1016/j.tiv.2019.04.011. [DOI] [PubMed] [Google Scholar]
- AlKahtane AA, Ghanem E, Bungau SG, Alarifi S, Ali D, AlBasher G, Alkahtani S, Aleya L, Abdel-Daim MM (2020). “Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues”. Environmental Science and Pollution Research. 27:11663–11670. doi: 10.1007/s11356-020-07736-1. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA (2016). “Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma”. BBA Clin. 6:49–68. doi: 10.1016/j.bbacli.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behndig A,. Svensson B, Marklund SL, Karlsson K (1998). “Superoxide dismutase isoenzymes in the human eye”. Invest Ophthalmol Vis Sci. 39(3):471–5. [PubMed] [Google Scholar]
- Belvedere G, Tursi F (1981). “Styrene oxidation to styrene oxide in human blood erythrocytes and lymphocytes”. Research Communications in Chemical Pathology and Pharmacology. 33(2):273–282. [PubMed] [Google Scholar]
- Bodin A, Linnerbord M, Nilsson JLG, Karlberg AT (2003). “Structure elucidation, synthesis, and contact allergen activity of a major hydroperoxide formed at autoxidation of the ethoxylated surfactant C12E5”. Chem Res Toxicol. 16(5):575–82. doi: 10.1021/tx025609n [DOI] [PubMed] [Google Scholar]
- Carlson GP, Turner M, Mantick NA (2006). “Effects of styrene and styrene oxide on glutathione-related antioxidant enzymes”. Toxicology. October 29;227(3):217–26. doi: 10.1016/j.tox.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang X (2019). “Transport property prediction and inhomogeneity analysis of supercritical n-Dodecane by molecular dynamics simulation”. Fuel. 244:48–60. Doi: doi: 10.1016/j.fuel.2019.01.181. [DOI] [Google Scholar]
- Chen Y, Mehta G, Vasiliou V (2009). “Antioxidant defenses in the ocular surface”. Ocul Surf. 7(4):176–185. doi: 10.1016/s1542-0124(12)70185-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirila TV, Walker LN, Constable IJ, Thompson DE, Barrett GD (1991). “Cytotoxic effects of residual chemicals from polymeric biomaterials for artificial soft intraocular lenses”. J Cataract Refract Surg. 17(2):154–62. doi: 10.1016/s0886-3350(13)80245-3 [DOI] [PubMed] [Google Scholar]
- Choksi N, Lebrun S, Nguyen M, Daniel A, DeGeorge G, Willoughby J, Layton A, Lowther D, Merrill J, Matheson J, Barroso K, Yozzo K, Casey W, Allen D (2020). “Validation of the OptiSafe™ eye irritation test”. Cutan Ocul Toxicol. 39(3):180–192. doi: 10.1080/15569527.2020.1787431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DE (2001). “Peroxides and peroxide-forming compounds”. Chem. Health Saf 8(5):12–22. doi: 10.1021/acs.chas.8b08507 [DOI] [Google Scholar]
- Conrady CD, Joos ZP, Patel BC (2016). “Review: The Lacrimal Gland and Its Role in Dry Eye”. Journal of ophthalmology. 7542929. doi: 10.1155/2016/7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager TL, Cockrell AE, Du Plessis SS (2017). “Ultraviolet Light Induced Generation of Reactive Oxygen Species”. Ultraviolet Light in Human Health, Diseases and Environment, doi: 10.1007/978-3-319-56017-5_2. [DOI] [PubMed] [Google Scholar]
- Di Tommaso S, Rotureau P, Crescenzi O, Adamo C (2011). “Oxidation mechanism of diethyl ether: a complex process for a simple molecule”. Physical Chemistry Chemical Physics. 13:14636–14645. Doi: 10.1039/C1CP21357A [DOI] [PubMed] [Google Scholar]
- Draize JH, Woodward G, Calvery HO (1944). “Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes”. J. Pharmacol. Exp. Ther 82, 377–390. [Google Scholar]
- Dwarakanath V, Pope GA (1998). “New Approach for Estimating Alcohol Partition Coefficients between Nonaqueous Phase Liquids and Water”. Environ. Sci. Technol 32(11):1662–1666. doi: 10.1021/es970744l [DOI] [Google Scholar]
- Faraguna F, Siuc V, Vidović E, Jukic A (2015). “Reactivity ratios and properties of copolymers of 2-ethoxyethyl methacrylate with dodecyl methacrylate or styrene”. J Polym Res. 22(245). doi: 10.1007/s10965-015-0890-4 [DOI] [Google Scholar]
- Fisher WB, VanPeppen JF (2000). “Cyclohexanol and Cyclohexanone”. Kirk-Othmer Encyclopedia of Chemical Technology, doi: 10.1002/0471238961.0325031206091908.a01. [DOI] [Google Scholar]
- Frater R (2009). “Neutralization of Acid in Glycol Methacrylate and the Use of Cyclohexanol as a Plasticizer”. Stain Technology. 56(2):99–101. doi: 10.3109/10520298109067290. [DOI] [PubMed] [Google Scholar]
- Garcia F, Garcia JM, Rubio F, de la Pena JL, Guzman J, Riande E (2002). “Reaction kinetics and gel effect on the polymerization of 2-ethoxyethyl methacrylate and 2(2-ethoxyethoxy) ethyl methacrylate”. Journal of Polymer Science Part A: Polymer Chemistry. 40(22). doi: 10.1002/pola.10480 [DOI] [Google Scholar]
- Gierke JS, Sanders DL, Perram DL (1999). “Laboratory Studies of Aqueous Partitioning Tracer Tests for Measuring Nonaqueous Phase Liquid Volumes”. Water Environment Research. 71(4). doi: 10.2175/106143097X122202 [DOI] [Google Scholar]
- Hartung T, Bruner L, Curren R, Eskes C, Goldberg A, McNamee P, Scott L and Zuang V (2010). “First alternative method validated by a retrospective weight-of-evidence approach to replace the Draize eye test for the identification of non-irritant substances for a defined applicability domain”, ALTEX - Alternatives to animal experimentation, 27(1), pp. 43–51. doi: 10.14573/altex.2010.1.43. [DOI] [PubMed] [Google Scholar]
- Huber ML, Laesecke A, Perkins R (2004). “Transport Properties of n-Dodecane”. Energy Fuels. 18(4):968–975. doi: 10.1021/ef034109e. [DOI] [Google Scholar]
- Hurley MD, Wallington TJ, Bjarrum M, Javadi MS, Nielsen OJ (2008). “Atmospheric Chemistry of 3-Pentanol: Kinetics, Mechanisms, and Products of Cl Atom and OH Radical Initiated Oxidation in the Presence and Absence of NOx”. The Journal of Physical Chemistry 2008. 112(35): 8053–8060. doi: 10.1021/jp803637c. [DOI] [PubMed] [Google Scholar]
- ICCVAM. (2010). “ICCVAM Test Method Evaluation Report: Current Validation Status of a Proposed In Vitro Testing Strategy for U.S. Environmental Protection Agency Ocular Hazard Classification and Labeling of Antimicrobial Cleaning Products”. NIH Publication No. 10-7513. Research Triangle Park, NC: National Institute of Environmental Health Sciences. [Google Scholar]
- Jester JV (2008). “Corneal crystalline and the development of cellular transparency”. Semin Cell Dev Biol. 19(2):82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Graham AD, Li W, Radke CJ, Lin MC (2019). “Human lacrimal production rate and wetted length of modified Schirmer’s tear test strips”. Trans Vis Sci Tech. 8(3):40. doi: 10.1167/tvst.8.3.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P, Sacman A, Wu-Weis M (2002). “Nephrotoxins: Widespread Roles of Oxidative Stress and Electron Transfer”. Current Medicinal Chemistry. 9: 823–847. doi: 10.2174/0929867024606803 [DOI] [PubMed] [Google Scholar]
- Kovacs E, Wolkober Z (1973). “Effectivity of organic phosphites”. Journal of Polymer Science: Polymer Symposia. 40(1):73–78. doi: 10.1002/polc.5070400110. [DOI] [Google Scholar]
- Lantyer-Araujo NL, Lacerda A, Mendonca MA, da Silva A, Dorea Neto F., Portela RD, Oria AP (2020). “Rabbit as an Animal Model for Ocular Surface Disease, Tear Osmolarity, Electrolyte, and Tear Ferning Profiles”. Optometry and Vision Science. 97(10): 847–851. doi: 10.1097/OPX.0000000000001583. [DOI] [PubMed] [Google Scholar]
- Lebrun S, Xie Y, Chavez S, Chan R, Jester JV (2019). “An in vitro depth of injury prediction model of a histopathologic classification of EPA and GHS eye irritants”. Toxicology in Vitro. 2019: 104628. doi: 10.1016/j.tiv.2019.104628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun S, Nguyen L, Chavez S, Chan R, Le D, Nguyen M, Jester JV (2020). “Same-chemical comparison of nonanimal eye irritation test methods: Bovine corneal permeability, EpiOcular™, isolated chicken eye, ocular Irritection®, OptiSafe™, and short time exposure”. Toxicol In Vitro. 2020: 105070. doi: 10.1016/j.tiv.2020.105070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Mabury SA (2019). “Organophosphite Antioxidants in Indoor Dust Represent an Indirect Source of Organophosphate Esters”. Environ Sci Technol. 53(4):1805–1811. doi: 10.1021/acs.est.8b05545. [DOI] [PubMed] [Google Scholar]
- McMonnies CW (2014). “Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications”. Clinical and Experimental Optometry. 98(2):122–125. doi: 10.1111/cxo.12203. [DOI] [PubMed] [Google Scholar]
- Mikulás K, Hermann P, Gera I, Komlódi T, Horváth G, Ambrus A, Tretter L (2018). “Triethylene glycol dimethacrylate impairs bioenergetic functions and induces oxidative stress in mitochondria via inhibiting respiratory Complex I”. Dent Mater. 34(7):e166–e181. doi: 10.1016/j.dental.2018.03.012 [DOI] [PubMed] [Google Scholar]
- Mottley C, Robinson RE, Mason RP (1991). “Free radical formation in the oxidation of malondialdehyde and acetylacetone by peroxidase enzymes”. Archives of Biochemistry and Biophysics. 289(1):153–160. doi: 10.1016/0003-9861(91)90455-R [DOI] [PubMed] [Google Scholar]
- Nakchat O, Nalinratana N, Meksuriyen D, Pongsamart S (2014). “Tamarind seed coat extract restores reactive oxygen species through attenuation of glutathione level and antioxidant enzyme expression in human skin fibroblasts in response to oxidative stress”. Asian Pac J Trop Biomed. 4(5):379–385. doi: 10.12980/APJTB.4.2014C806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaz K, Mabqool F, Khan F, Hassan FI, Baeeri M, Navaei-Nigjeh M, Hassani S, Gholami M, Abdollahi M (2017). “Molecular mechanisms of action of styrene toxicity in blood plasma and liver”. Environmental Toxicology. 32:2256–2266. doi: 10.1002/tox.22441 [DOI] [PubMed] [Google Scholar]
- Nita M, Grzybowski A (2016). “The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie-Mbye YF, Kulkarni-Chitnis M, Opere CA, Barrett A, Ohia SE (2013). “Lipid peroxidation: pathophysiological and pharmacological implications in the eye”. Front Physiol. 4:366. doi: 10.3389/fphys.2013.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Dogru M, Tsubota K (2006). “Laboratory findings in tear fluid analysis”. Clin Chim Acta. 2006 369(1):17–28. doi: 10.1016/j.cca.2005.12.035. [DOI] [PubMed] [Google Scholar]
- OECD. (2017). “Guidance Document on an Integrated Approach to Testing and Assessment (IATA) for Serious Eye Damage and Eye Irritation, Series on Testing & Assessment No. 263”. Available at: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2017)15&doclanguage=en
- Palmlof M, Hjertbeg T (2000). “Chemical and mechanical changes in poly(ethylene-co-1,9-decadiene) following crosslinking induced by peroxides”. Polymer. 41(17): 6497–6505. doi: 10.1016/S0032-3861(99)00881-2 [DOI] [Google Scholar]
- Pathologies of the Anterior and Posterior Eye Segments in Adults”. Oxid Med Cell Longev. 2016:3164734. doi: 10.1155/2016/3164734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AP, da Fonseca LM, de Faria Oliveira OM, Brunetti IL, Ximenes VF (2006). “Oxidation of acetylacetone catalyzed by horseradish peroxidase in the absence of hydrogen peroxide”. Biochim Biophys Acta. 1760(12):1755–61. doi: 10.1016/j.bbagen.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Schwetlick K, Pionteck TJ, Habicher WD (1987). “Organophosphorus antioxidants – VIII. Kinetics and mechanism of the reaction of organic phosphites with peroxy radicals”. European Polymer Journal. 23(5): 383–388. doi: 10.1016/0014-3057(87)90167-4 [DOI] [Google Scholar]
- Schwetlick K, Habicher WD (1995). “Organophosphorus antioxidants action mechanisms and new trends”. Die Angewandte Makromolekular Chemie. 232(1): 239–246. doi: 10.1002/apmc.1995.052320115 [DOI] [Google Scholar]
- Smedberg A, Hjertberg T, Gustafsson B (1997). “Crosslinking reactions in an unsaturated low density polyethylene”. Polymer. 38(16):4127–4138. doi: 10.1016/S0032-3861(96)00994-9 [DOI] [Google Scholar]
- Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY (2019). “Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis”. Oxid Med Cell Longev. 2019:5080843. doi: 10.1155/2019/5080843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksomtip M, Ukrisdawithid S, Bhusawang P, Pongsamart S (2010). “Phenolic compound content, antioxidant and radical-scavenging properties of methanolic extracts from the seed coat of certain thai tamarind culivars”. Journal of Food Biochemistry. 34(5). doi: 10.1111/j.1745-4514.2009.00323.x [DOI] [Google Scholar]
- Tangvarasittichai O, Tangvarasittichai S (2018). “Oxidative Stress, Ocular Disease and Diabetes Retinopathy”. Curr Pharm Des. 24(40):4726–4741. doi: 10.2174/1381612825666190115121531 [DOI] [PubMed] [Google Scholar]
- Tei T, Sato Y, Hagiya K, Tai A, Okuyama T, Sugimura T (2002). “‘Chiral perturbation factor’ approach reveals importance of entropy term in stereocontrol of the 2,4-pentanediol-tethered reaction”. J Org Chem. 67(19):6593–8. doi: 10.1021/jo025937s. [DOI] [PubMed] [Google Scholar]
- Thörig L, Van Agtmaal EJ, Glasius E, Tan KL, Van Haeringen NL (1985). “Comparison of tears and lacrimal gland fluid in the rabbit and guinea pig”. Current Eye Research. 4(8): 913–920. doi: 10.3109/02713688509095259. [DOI] [PubMed] [Google Scholar]
- UN (2011). Globally Harmonized System of Classification and Labelling of Chemicals (GHS): Fourth Revised Edition. Geneva, Switzerland: United Nations Economic Commission for Europe, doi: ST/SG/AC/10/30/Rev.4 [Google Scholar]
- Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, White AJR (2017). “Oxidative stress and reactive oxygen species: a review of their role in ocular disease”. Clin Sci. 131(24): 2865–2883. doi: 10.1042/CS20171246 [DOI] [PubMed] [Google Scholar]
- van Amsterdam IMC, Ubbink M, Einsle O, Messerschmidt A, Merli A, Cavazzini D, Rossi GL, Canters GW (2001). “Dramatic modulation of electron transfer in protein complexes by crosslinking”. Nature Structural Biology. 9: 48–52. doi: 10.1038/nsb736 [DOI] [PubMed] [Google Scholar]
- Wakamatsu TH, Dogru M, Tsubota K (2008). “Tearful relations: oxidative stress, inflammation and eye diseases”. Arq Bras Oftalmol. 71(6):72–79. doi: 10.1590/S0004-27492008000700015. [DOI] [PubMed] [Google Scholar]
- Wang K, Hawley MC, Furney TD (2003). “A selectivity study of 2,4-pentanediol hydrogenolysis combining experiments and computer simulation”. Chemical Engineering Science. 58(18):4271–4285). doi: 10.1016/S0009-2509(03)00285-9. [DOI] [Google Scholar]
- Wollensak G, Mur E, Mayr A, Baier G, Gottinger W, Stoffler G (1990). “Effective methods for the investigation of human tear proteins and lipids”. Graefe’s Arch Clin Exp Opthalmol. 228:78–92. doi: 10.1007/BF02764296 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Z, He X, Zhang F, Zhang Z (2017). “Regulation of the products of styrene oxidation”. Chemical Engineering Research and Design. 120:171–178. doi: 10.1016/j.cherd.2017.02.012 [DOI] [Google Scholar]
- Zhu T, Lim BS, Park HC, Son KM, Yang HC (2012). “Effects of the iron-chelating agent deferoxamine on triethylene glycol dimethacrylate, 2-hydroxylethyl methacrylate, hydrogen peroxide-induced cytotoxicity”. J Biomed Mater Res B Appl Biomater. 100(1):197–205. doi: 10.1002/jbm.b.31939 [DOI] [PubMed] [Google Scholar]


