Abstract
Rationale: The feasibility of a large, multicenter, randomized controlled trial comparing the risks and benefits of early-use speaking valve after tracheostomy is not clear.
Objectives: To investigate the feasibility of accelerated (⩽24 h) versus standard (⩾48 h) one-way speaking valve (“speaking valve”) placement after percutaneous tracheostomy.
Methods: Twenty awake patients (Glasgow Coma Scale score ⩾9) were randomized to accelerated or standard timing of speaking valve placement. Outcomes included patient identification and recruitment, adherence to protocol-defined time windows for valve placement, experimental separation in time to first speaking valve placement between groups, effectiveness of speech and swallowing (Sentence Intelligibility Test score, patient-reported quality of life), and clinical outcomes (safety events, speaking valve tolerance, decannulation, length of stay, and mortality).
Results: Of 161 patients undergoing percutaneous tracheostomy, 20 of 36 meeting eligibility criteria were randomized. The median time to speaking valve placement was 22 (interquartile range [IQR], 21–23) hours in the accelerated arm versus 45.5 (IQR, 43–50) hours for the standard arm. No aspiration, hypoxemia, or other safety events occurred in either arm as a result of the speaking valve. Sentence intelligibility test scores were not different between arms but correlated with quality of life. After three sessions, patients in the accelerated arm tolerated longer speaking valve trials than those in the standard arm [median, 65 (IQR, 45–720) min vs. median, 15 (IQR, 3–20) min]. Seven patients in the accelerated arm were decannulated before hospital discharge versus one patient in the standard arm.
Conclusions: Speaking valve placement within 24 hours of percutaneous tracheostomy is feasible. A multicenter randomized controlled trial should be conducted to evaluate the safety of this strategy and compare important clinical outcomes, including time to speech and swallow recovery after tracheostomy.Clinical trial registered with ClinicalTrials.gov (NCT03008174).
Keywords: tracheostomy, quality of life, communication, one-way speaking valve, feasibility
In the United States, assessment of patient candidacy for one-way speaking valve (hereafter “speaking valve;” Figure 1) is routinely deferred until 48 hours after percutaneous tracheostomy (1, 2). Although some speech–language pathologists (SLPs) in Europe and Australia introduce speaking valves within 4 hours of the procedure (3), data supporting the clinical benefits and safety of this approach are from observational studies with limited generalizability. Earlier speaking valve placement may expedite speech rehabilitation, thereby improving communication and quality of life (4–6). In addition, speaking valve use may improve end-expiratory lung volume, alveolar recruitment, and diaphragm mobility—all factors that can facilitate liberation from mechanical ventilation (7–11). However, the risks of earlier speaking valve placement require further assessment. Indeed, early placement could potentially trigger untoward outcomes because of immature tracheostomy tracts. Examples include subcutaneous air, pneumomediastinum, pneumothorax, and accidental decannulation.
Figure 1.
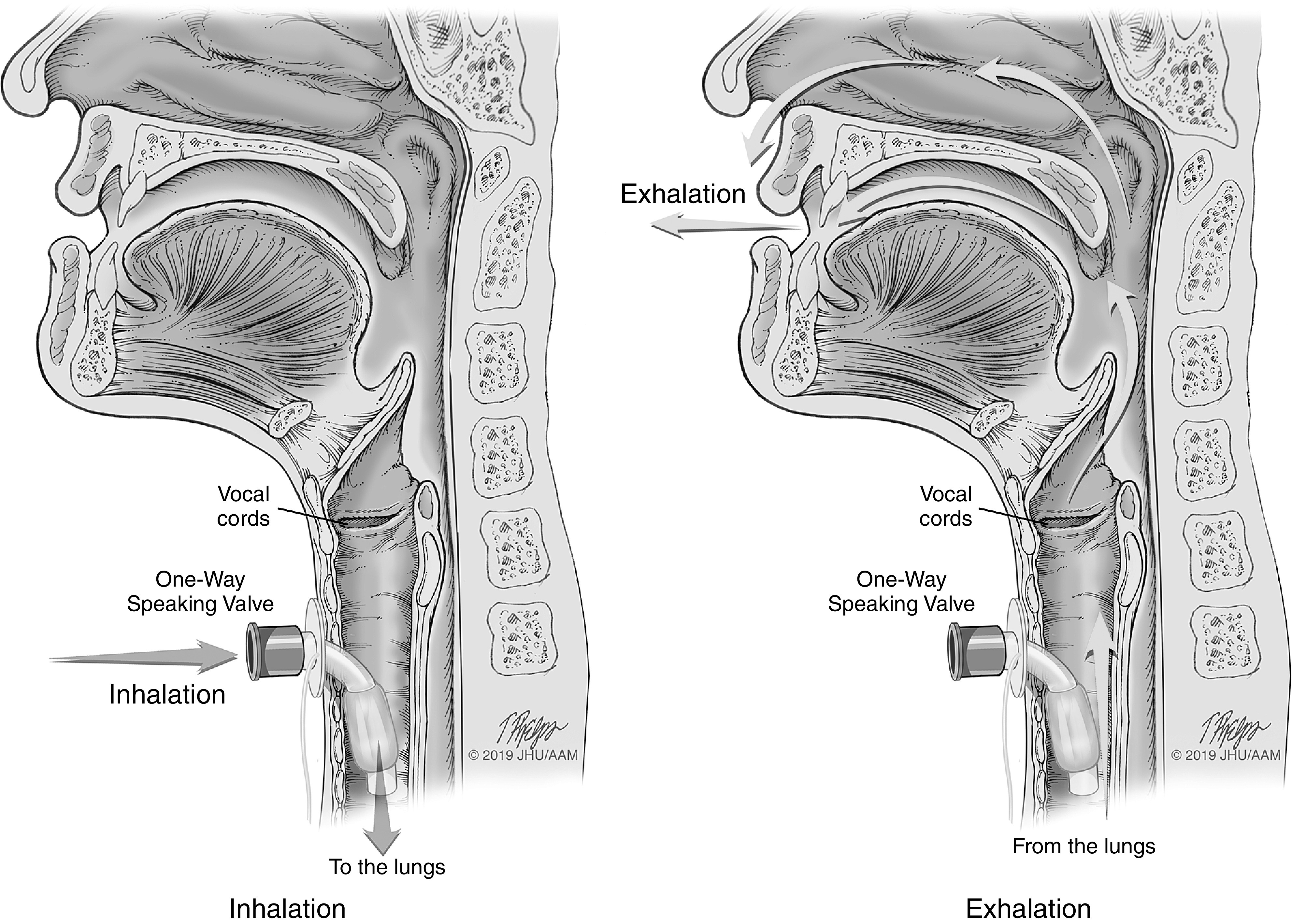
Anatomical depiction of the use of one-way speaking valve. This figure represents two cross-sectional views of the upper airways with a tracheostomy tube in situ. In addition, a one-way speaking valve is present on the tracheostomy tube. In the panel on the left, the picture depicts airflow during inhalation and how the air is directed to the lungs. In the panel on the right, the picture depicts airflow during exhalation and how the air is directed to the upper airways (around the tracheostomy tube) through the vocal cords to the nasopharynx and oropharynx. Illustrations: Tim Phelps 2019 JHU AAM, Department of Art as Applied to Medicine, The Johns Hopkins University School of Medicine.
Although a large, multicenter, randomized controlled trial comparing the risks and benefits of accelerated speaking valve placement after tracheostomy would provide more meaningful guidance than the existing observational studies, the feasibility of such a trial is not clear. Potential barriers to completion include 1) the identification and recruitment of participants, 2) the ability to achieve meaningful temporal separation between accelerated valve placement versus that in a standard care control group, 3) uncertain clinician acceptance of accelerated speaking valve use, and 4) as yet undemonstrated ability of available instruments to detect speech rehabilitation endpoints in this novel study population. We therefore conducted a prospective randomized controlled feasibility trial to investigate these issues and to identify other methodological, safety, and operational factors that would inform the design of a larger study.
Methods
This trial was approved by the Johns Hopkins University Institutional Review Board (protocol number IRB00080981) and is registered at ClinicalTrials.gov (NCT03008174).
Study Design
In this prospective randomized controlled trial, the first placement of a speaking valve between 12 and 24 hours after percutaneous tracheostomy (intervention group; accelerated arm) is compared with first placement between 48 and 60 hours after percutaneous tracheostomy (control group; standard care arm). Enrolled participants were randomly assigned to each group in a 1:1 ratio.
Participants
All adult patients admitted to any of three participating intensive care units (ICUs) at Johns Hopkins Hospital and undergoing percutaneous tracheostomy were screened for enrollment. To be included in the trial, patients had to exhibit an adequate level of consciousness and stable mental status (Glasgow Coma Scale [GCS] score ⩾ 9, Confusion Assessment Method-ICU [CAM-ICU] negative, and Richmond Agitation–Sedation Scale [RASS] of −1 to +1) and had to speak English (Table E1 in the online supplement). Patients were excluded if they exhibited upper airway obstruction, had a diagnosis of a difficult airway per anesthesia assessment, or had complications from tracheostomy (i.e., bleeding requiring transfusion, air leak around tracheostomy cuff compromising ventilation, subcutaneous emphysema, or pneumothorax).
Randomization and Blinding
Participants were randomized to accelerated speaking valve placement versus standard timing using the RAND (randomization) function in Microsoft Excel software (Microsoft Office Systems). This assignment was stored in a password-protected file. After each patient's consent, the speech–language pathology (SLP) team would be notified of whether the patient had been randomized to the accelerated or standard arm of the study. Given the intervention, group assignment was not blinded, with the exception of an assessment of speech intelligibility, which is discussed below.
Intervention
Participants in the accelerated speaking valve group were assessed by SLP for valve placement between 12 and 24 hours after tracheostomy. Provided there were no contraindications at the time of assessment, a speaking valve was placed, and performance metrics (see below) were assessed (session 1; Figure 2). The standard care group was only assessed for quality of life during session 1, with no speaking valve placement. Between 48 and 60 hours after tracheostomy, both study groups were assessed for speaking valve placement and performance metrics (session 2). A third assessment was conducted 2 weeks after tracheostomy (session 3). For all sessions, speaking valve trials required coordination between SLPs, respiratory care practitioners (RCPs), bedside nurses, and provider teams. This coordination ensured adequate monitoring and safety during the trials and optimization of ventilator settings for patient comfort (online supplement, Table E2). For mechanically ventilated patients, in-line Passy Muir Speaking Valves (PMV 007, Aqua Color; Passy Muir, Inc.) were used; for nonventilated patients, Passy Muir Speaking Valves (PMV 2001, Purple Color; Passy Muir, Inc.) were used (2, 12–18).
Figure 2.
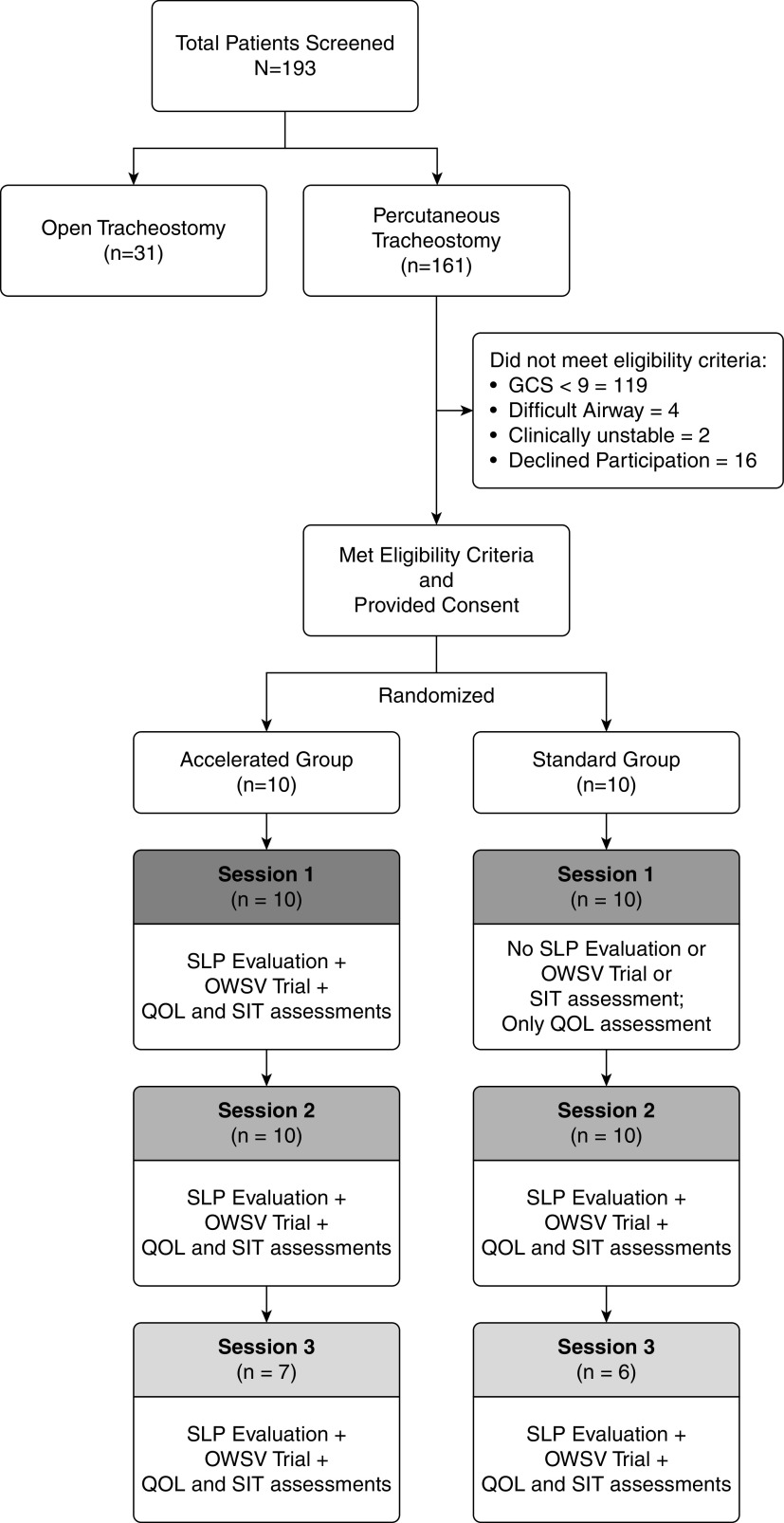
Study protocol. The study protocol showing the randomization into two groups and the follow-up sessions are portrayed in this picture. The assessments and interventions provided during each session are listed. In addition, data regarding the number of patients who did not meet the eligibility criteria are presented. GCS = Glasgow Coma Scale; OWSV = one-way speaking valve; QOL = quality of life; SIT = Sentence Intelligibility Test; SLP = speech–language pathologist.
Outcomes
As a feasibility trial, the key outcomes of interest were pace of participant recruitment and adherence to the protocol, which included achieving adequate temporal separation in initial speaking valve use between patients in accelerated versus standard groups. A separation of 24 hours between groups was sought. Speech-related outcomes included performance on the Sentence Intelligibility Test (SIT), which recorded participant speech during speaking trials (19). Recordings were subsequently scored by experienced SLPs blinded to study group assignment. The SIT uses a 1 to 100 scale, with higher scores indicating more intelligible speech. Speech quality was also evaluated, but in this case, listeners were not blinded. Other speech-related outcomes measured included speaking valve tolerance (minutes of speaking valve use without distress; parameters detailed in online supplement) and independence (need for SLP, RCP, nurse, or family supervision during speaking valve use; Table 1).
Table 1.
Baseline data comparing standard and accelerated groups
| Patient characteristics | Standard (n = 10) | Accelerated (n = 10) | P Value | |
|---|---|---|---|---|
| Age, yr | 48 (38–64) | 40 (35–74) | 0.78 | |
| Sex | Female | 5 (50) | 6 (60) | 0.65 |
| Male | 5 (50) | 4 (40) | ||
| Race/ethnicity | White | 3 (30) | 6 (60) | 0.26 |
| African American | 6 (60) | 3 (30) | ||
| Hispanic | 1 (10) | 0 (0) | ||
| Other | 0 (0) | 1 (10) | ||
| Indication for admission | Pulmonary | 5 (50) | 7 (70) | 0.27 |
| Neurological | 5 (50) | 2 (20) | ||
| Cardiac | 0 (0) | 1 (10) | ||
| Intubation duration (days) | 9.5 (7–16) | 16.5 (9–19) | 0.25 | |
| Indication for tracheostomy | Chronic ventilator dependence | 10 (100) | 10 (100) | |
| Initial tracheostomy tube size | 6.0 cuffed | 7 (70) | 9 (90) | 0.26 |
| 8.0 cuffed | 3 (30) | 1 (10) | ||
| Tracheal suctioning requirement | ||||
| Suctioning frequency (every × h) | 2 (2–4) | 2 (1–3) | 0.68 | |
| Amount | Small | 3 (30) | 4 (40) | 0.79 |
| Moderate | 5 (50) | 5 (50) | ||
| Copious | 2 (20) | 1 (10) | ||
| Consistency | Thin | 4 (40) | 1 (10) | 0.12 |
| Thick | 6 (60) | 9 (90) | ||
| Color | Clear | 1 (10) | 0 (0) | 0.45 |
| Creamy | 0 (0) | 1 (10) | ||
| Blood-tinged | 4 (40) | 4 (40) | ||
| Tan | 2 (20) | 4 (40) | ||
| White | 3 (30) | 1 (10) | ||
| Sequential Organ Failure Assessment score | 6 (5–6) | 6 (4–7) | 0.77 | |
Age, days intubated, and suction frequency are reported in median (interquartile range); remaining variables are reported in frequencies (percentages).
Other outcomes of interest included Quality of Life (QOL) of Mechanically Ventilated Patients (QOL-MV; score 1 to 120; higher scores indicate better QOL) score, patient-reported satisfaction, progression of swallow (based on a modified barium swallow study or fiber optic endoscopic evaluation), time to decannulation, ICU and hospital lengths of stay, and mortality. We monitored several safety endpoints, including bleeding, subcutaneous air, pneumothorax, accidental decannulation, tracheal–esophageal fistula, inadvertent occlusion of tracheostomy, abnormalities of gas exchange, and any changes in status that triggered rapid response events (see online supplement).
Statistical Methods
All analyses adhered to an intention-to-treat approach. Descriptive statistics for continuous variables are presented as medians with interquartile range (IQR), and frequencies and percentages are presented for categorical variables. As a feasibility trial, sample size calculations were deferred; however, a minimum enrollment of 10 patients to each study arm was planned to identify barriers to implementing the protocol. Between group comparisons were assessed using the Wilcoxon rank-sum test or Fisher’s exact test as appropriate. The proportion of patients who met feasibility targets are described with percentages. For exploratory purposes, a multivariate, stepwise, backward linear regression analysis was conducted to identify potential predictors of SIT and QOL, controlling for potential confounding factors such as age, sex, race, intubation duration, indications for admission, and illness severity.
Results
Recruited Population
Between February 2016 and November 2019, 161 patients underwent percutaneous tracheostomy in the participating ICUs. Thirty-six met eligibility criteria, and the 20 (55.5%) patients who provided consent to participate in the study were randomized to accelerated speaking valve (n = 10) or standard care (n = 10) (Figure 2). Sixteen were invited to participate in the study but did not provide consent. Patients who failed to meet enrollment criteria included patients with low GCS score of less than 9 (n = 119), difficult airway anatomy (n = 4), or clinical instability (n = 2). The median age of enrolled participants was 45 (IQR, 35–67.5) years, and 55% were women. The median baseline Sequential Organ Failure Assessment score for all participants was 6 (IQR, 5–6.5) and did not differ between randomization groups. Other baseline characteristics were also similar between groups (Tables 1 and E3).
Protocol Implementation
All participants underwent speaking valve evaluation according to the protocol (e.g., ⩽24 h for the accelerated group and ⩾48 h for standard care group) at session 1, although one patient in the accelerated group, discussed subsequently, did not pass the 1-minute finger occlusion test because of laryngeal edema, and a speaking valve was not attempted. The speech rehabilitation required a concerted effort by nursing staff, SLPs, and RCPs to align schedules. All participants had session 2 speech rehabilitation assessments, but seven participants (three in the accelerated group and four in the standard care group) did not complete session 3. Of these seven individuals, four died, two were discharged from the institution, and one developed neurocognitive impairment. All clinical assessments, including swallow function, time to decannulation, ICU and hospital lengths of stay, and mortality, were able to be collected as per protocol.
Speech Rehabilitation Assessments
Tolerance to speaking valve and independence with speaking valve
Median time to speaking valve placement was 22 (IQR, 21–23) hours in the accelerated arm versus 45.5 (IQR, 43–50) hours in the standard arm from the time of tracheostomy. Participants in the accelerated arm tolerated the speaking valve for a median of 10 (IQR, 5–35) minutes during session 1 (Table 2); participants in the standard arm did not receive a speaking valve evaluation during session 1 per protocol. During session 2, the accelerated group tolerated the speaking valve for a median of 7.5 (IQR, 5–60) minutes versus 20 (IQR, 7–35) minutes in the standard group (P = 0.84).
Table 2.
Comparison of clinical outcomes between standard and accelerated groups at each session
| Clinical outcomes | Session Number | Standard | Accelerated | P Value |
|---|---|---|---|---|
| Speech Intelligibility Test score, median (IQR) | 1 | 0 | 9.4 (0–53.9) | 0.03 |
| 2 | 32 (0–58.7) | 4.2 (0–63.2) | 0.09 | |
| 3 | 24.4 (0–78.9) | 73.6 (56.8–92.3) | 0.16 | |
| Voice quality, n (%) | ||||
| Hoarse | 1 | — | 1 (10) | — |
| 2 | 4 (40) | 3 (30) | 0.79 | |
| 3 | 1 (17) | 2 (33) | 0.35 | |
| Rough | 1 | — | 0 | — |
| 2 | 0 | 0 | 0.79 | |
| 3 | 1 (17) | 0 | 0.35 | |
| Strained | 1 | — | 2 (20) | — |
| 2 | 3 (30) | 2 (20) | 0.79 | |
| 3 | 0 | 1 (17) | 0.35 | |
| Breathy | 1 | — | 2 (20) | — |
| 2 | 2 (20) | 2 (20) | 0.79 | |
| 3 | 1 (17) | 2 (33) | 0.35 | |
| Dysphonic | 1 | — | 2 (20) | — |
| 2 | 0 | 1 (10) | 0.79 | |
| 3 | 1 (17) | 0 | 0.35 | |
| Aphonic | 1 | — | 3 (30) | — |
| 2 | 1 (10) | 2 (20) | 0.79 | |
| 3 | 2 (33) | 0 | 0.35 | |
| Duration of speaking valve tolerance, median (IQR), min | 1 | — | 10 (5–35) | — |
| 2 | 20 (7–35) | 7.5 (5–60) | 0.84 | |
| 3 | 15 (3–20) | 65 (45–720) | 0.03 | |
| Independence with speaking valve, n (%) | ||||
| With speech–language pathologist only | 1 | — | 7 (70) | — |
| 2 | 6 (60) | 6 (60) | 1.0 | |
| 3 | 3 (50) | 3 (42.9) | 0.61 | |
| Trained staff | 1 | — | 2 (20) | — |
| 2 | 4 (40) | 3 (30) | 1.0 | |
| 3 | 2 (33.3) | 1 (14.3) | 0.61 | |
| Independent or with family | 1 | — | 1 (10) | — |
| 2 | 0 | 1 (10) | 1.0 | |
| 3 | 1 (16.7) | 3 (42.9) | 0.61 | |
| QOL-MV score, median (IQR) | 1 | 46.9 (35.4–51.5) | 40.6 (34.8–51.5) | 0.80 |
| 2 | 43 (40.3–51.4) | 50.1 (40.9–70.9) | 0.36 | |
| 3 | 43 (33.9–59.8) | 57.6 (36.3–73.7) | 0.76 | |
| Reported satisfaction, n (%) | 1 | — | 7 (70) | — |
| 2 | 7 (70) | 7 (70) | 1.0 | |
| 3 | 3 (50) | 6 (85.7) | 0.22 | |
Definition of abbreviations: IQR = interquartile range; QOL-MV = Quality of Life in Mechanically Ventilated Patients questionnaire.
By session 3, the accelerated group tolerated the speaking valve for a significantly longer duration (P = 0.03) (65 min; IQR, 45–720 min) compared with the standard group (15 min; IQR, 3–20 min). One patient in the accelerated group did not tolerate cuff deflation at any of the three sessions (Table E4) and was, therefore, given a duration of 0 minutes for all data analyses. Upon further evaluation by an otolaryngologist using a flexible laryngoscopy after session 3, the patient was found to have upper airway edema. During session 1 (intervention group) and session 2 (both groups), most participants required supervision from a SLP, nurse, or RCP to use their speaking valves (Table 3). By the end of session 3, three (43%) of the accelerated arm participants were using their speaking valves independently or with family. In contrast, only one participant (16.7%) in the standard arm was a candidate for independent use of the speaking valve.
Table 3.
Levels of independence
| Tier | Level of Independence | Does Not Tolerate Cuff Deflation (Change in Vital Signs/Impaired Airway Clearance) | Aphonic Vocal Quality | Requires Ventilator Adjustments | Lack of Tolerance to In-Line Speaking Valve | Requires Frequent Tracheal or Oral Suctioning | Cognitive Impairment Affecting Ability to Successfully Seek Attention | Physical Impairment Affecting Ability to Use Call Bell for Help |
|---|---|---|---|---|---|---|---|---|
| I | Supervision required by speech–language pathologist | Y | Y | Y | Y | Y | Y | Y |
| II | Supervision required by respiratory care practitioners/physiotherapists | N | N | Y | Y | Y | Y | Y |
| III | Supervision required by trained staff (nurses and occupational/physical therapists) | N | N | N | N | Y | Y | Y |
| IV | Independent or assistance sought from trained family member/caregiver | N | N | N | N | N | N | N |
Definition of abbreviations: N = no; Y = yes.
Speech intelligibility, voice quality, QOL, and satisfaction
All instruments were successfully administered to all study participants. The median session 1 SIT score for the accelerated group was 9.4 (IQR, 0–53.9). The median session 1 SIT score for the standard care groups was 0, as they did not receive a speaking valve per protocol. At subsequent sessions, SIT scores did not differ between arms. Voice quality was also similar between experimental arms at sessions 2 and 3, although there was significant heterogeneity in voice quality within groups (Table 2). Patient-reported QOL did not differ between groups (session 1 = 40.6 [IQR, 34.8–51.5], session 2 = 50.1 [IQR, 40.9–70.9], and session 3 = 57.6 [IQR, 36.3–73.7]), although SIT scores correlated with participant perception of QOL; after controlling for session and experimental arm, each 1-point increase in SIT score corresponded with a 0.20-point increase in on the QOL-MV (P = 0.03).
Swallowing, decannulation, and length of stay
All participants in our study were nil per os at sessions 1 and 2. By session 3, two in each group were able to swallow orally (Table E3). Seven participants in the accelerated arm and one in the standard care arm were decannulated while still in the hospital (P = 0.006). However, the median time to decannulation, ICU length of stay, and hospital length of stay did not differ between groups (Table E5).
Safety and Mortality Outcomes
Prospective standardized safety monitoring protocols revealed no adverse events, near misses, or mortality attributable to speaking valve trials. However, subcutaneous air was noted in one participant in the accelerated arm during session 1 and in another participant in the standard arm during session 2. In each case, a retrospective focused review of chest radiographs by the physicians revealed that the subcutaneous air was visible after tracheostomy procedure but before speaking valve placement. Both participants were observed without any additional interventions. Other postoperative complications were minor (Table E5). Two deaths occurred in each study group (Figure E3); all were attributed to progression of underlying disease with transitions to comfort measures (see online supplement). Before death, one participant noted the speaking valve permitted her to say “I love you” to family members.
Discussion
We randomized 20 patients to accelerated speaking valve trials versus standard care after percutaneous tracheostomy to assess protocol feasibility, with the long-term goal of conducting a larger multicenter study to assess efficacy and safety of early speaking valve placement. In our study, a minority of patients undergoing percutaneous tracheostomy qualified for enrollment, with most patients being excluded because of a low GCS score. However, among those that qualified for enrollment, more than half were willing to participate, and the majority of enrolled participants were able to complete the protocol. Those not completing the protocol were either discharged before the final assessment or experienced progression of disease that prohibited further participation. In no case was disease progression believed to be related to the timing of the first use of speaking valve. Of note, it took 3 years to enroll the planned 20 patients. These findings suggest a larger study of similar patients is feasible but would require a multicenter, collaborative approach. Modifications to the inclusion and exclusion criteria could mitigate some of the challenges of enrollment.
Recruitment and Retention
Recruitment was primarily limited by stringent eligibility criteria designed to facilitate effective participation in speech assessments and mitigate the risk of adverse events. Most subjects (n = 119) were excluded because of alterations in mental status. However, although a person with a higher GCS score who is alert and calm may be more likely to effectively participate in assessments of speech intelligibility, a low GCS score does not necessarily increase the risk of early placement of a speaking valve. Indeed, an argument can be made that the ability to communicate earlier may decrease agitation and even delirium (20). A much smaller number of patients were excluded because of the presence of a difficult airway, clinical instability, or ventilator requirements not conducive to speaking valve placement. We also excluded patients who had undergone an open (surgical) tracheostomy, as there is a perception that these patients may be at higher risk for adverse events such as bleeding or subcutaneous emphysema. However, it is not clear that these concerns are valid, an issue that warrants further investigation. Lack of readiness to consent (n = 16) was another barrier to enrollment for several otherwise eligible patients. Having a discussion of opportunity for enrollment in this trial before the tracheostomy—rather than shortly after, when many patients and families are preoccupied with tracheotomy care—may have increased participation, given the narrow window for enrollment into the study. One barrier we did not encounter was resistance from the clinical team to the accelerated placement of a speaking valve. We also did not encounter instances in which patients in the accelerated group requested a delay in speaking valve placement, but two patients in the standard group sought speaking valve placement before 48 hours.
Protocol Implementation
Timing of speaking valve
All participants randomized to accelerated speaking valve placement underwent session 1 speaking valve evaluation between 12 and 24 hours after tracheostomy. One patient could not tolerate a 1-minute finger occlusion test, and a speaking valve was not attempted. The underlying etiology of upper airway edema was not established until weeks later, whereas upper airway assessment using endoscopy at the time of failed finger occlusion test could have excluded this patient from the study. For this reason, future studies may consider an upper airway endoscopy when a SLP evaluation is abnormal. The 12–24-hour interval after tracheostomy presented no implementation challenges for the remaining nine patients. Although a few observational studies have reported successful placement of speaking valves within 4 hours of tracheostomy (21), such early speaking valve placement would likely increase the hurdles to achieving adequate recruitment, particularly if the GCS and RASS thresholds used for this feasibility study were unchanged in future studies, as residual sedation is most likely to affect these measures in the initial hours after surgery. Importantly, our window of 12–24 hours for the accelerated group was late enough to identify 36 candidates who met GCS and RASS thresholds yet early enough to give us clear temporal separation between groups.
Assessment of Speech Rehabilitation
The SIT
The SIT was administered successfully to all patients, but it may be subject to floor effects, lacking discriminative power in patients who have a meaningful but limited ability to communicate with a speaking valve. For example, anecdotally, several nurses reported instances in which patients demonstrated improved communication with clinicians and family after speaking valve placement, even when the SIT scores were sufficiently low to suggest no improvement. This finding suggests a need for instruments that are more sensitive for capturing patient communication. Therefore, tools that assess verbal communication not discernible using the SIT repository may be considered in future studies to capture organized thinking. Despite this limitation, the computerized assessment of intelligibility of speech using the SIT allows for more objectivity than face-to-face judgments of comprehensibility. The SIT computer analysis is not influenced by contextual factors and provides a nonbiased assessment of speech intelligibility; however, the SIT relies on preset words and sentences that may not be applicable or of interest to the patients, thereby making the tool potentially restrictive.
The QOL-MV
The QOL-MV was successfully administered to all study participants, but scores did not differ between study groups. Although the QOL-MOV is not specific to communication and speaking, it is valid for ventilator-dependent populations. The caveat with QOL-MOV is that its domains that are not related to speech, such as overall comfort, sleep, and pain, may be affected by the severity of illness, diagnosis, and medications, thereby dampening speech-related signals. A similar limitation has been noted when using the EuroQol-5D to assess speech-related QOL (22). For these reasons, future studies should consider using instruments that specifically measure patient-reported quality of life relevant to voice, such as the voice-related QOL score (23).
Other assessments of rehabilitation
The other assessments of speech and airway rehabilitation, specifically duration of speaking valve tolerance, independence with valve in place, progression of swallow, and time to decannulation, were all straightforward to monitor and useful outcomes to include in subsequent larger trials.
Safety and Clinical Outcomes
Although this trial was not designed or powered to measure safety, all patients were carefully monitored throughout the study period to detect complications, medical errors, or other adverse events that would inform a larger trial design or require specific monitoring in that setting. The only safety events we identified were a case of airway edema that precluded speaking valve placement (not known before randomization) and two occurrences of subcutaneous emphysema. These safety-related data suggest that an endoscopic evaluation may be warranted if patients fail speaking valve trial after meeting clinical readiness criteria. The presence of subcutaneous emphysema was first detected after the speaking valve evaluation, but a review of the chest X-ray shortly after the tracheostomy demonstrated that the air was present before the speaking valve evaluation. Future study designs should include careful inspection of chest X-ray subjects to avoid enrollment of subjects with subcutaneous emphysema.
Participants in our accelerated arm had a longer duration of speaking valve use at session 3, with the caveat of small sample size and wide IQR. Three participants in the accelerated arm were decannulated compared with only one in the standard arm. Although this observation is also limited by the sample size, the structured approach to capping and decannulating used at our institution gives us confidence all patients were assessed for decannulation in a timely fashion (24). Larger studies should follow a similar strategy.
Limitations
There are important limitations of our study protocol. First, members of the research and clinical teams were not blinded, with the exception of SLP assessment of speech intelligibility. Although creative approaches could be considered in future studies, such as covering the speaking valves or using sham valves, this would significantly misinform providers on important clinical endpoints such as the potential readiness of participants for ventilator weaning. Second, there was likely a practice effect in the accelerated group relative to the standard group (25, 26). The accelerated group's first evaluation involved speaking valve placement, whereas the standard group did not have a speaking valve placed until the second visit. Although the experimental design allowed for assessment of speaking valve tolerance at matched time points, an alternative design may have inserted an additional evaluation or practice session for the standard group to allow for a more balanced comparison between groups at the third evaluation time point. Third, our experience and use of the protocols are from a single institution, and it is unknown whether the implementations of protocols would be similarly successful elsewhere.
Conclusions
Speaking valve placement within 24 hours after percutaneous tracheostomy appears feasible in patients who meet clinical readiness criteria. Pilot data indicate modifiable barriers to consent, possible floor effect of SIT, and utility of other metrics of speech rehabilitation. The data also reveal potential limitations of our measure of QOL, the need for additional tools to capture organized thinking, and new triggers for safety assessments of airway edema and subcutaneous air. With these changes, a large-scale multicenter trial of accelerated versus standard timing of first speaking valve use is likely to provide a valuable comparison of meaningful outcomes in this population.
Acknowledgments
Acknowledgment
The authors thank their fellow speech–language pathologists, RCPs, nurses, physicians, and advanced practice providers. Speech–language pathologists who assisted with this project include Colleen McElroy, Gabrielle Miller, Nicole Langton, Kathleen Holden, and Christine Smith in recruiting, administering assessments, and grading Sentence Intelligibility Tests. RCPs were instrumental in informing the authors of potential participants and assisting with the completion of in-line speaking valve assessments while the patients were on ventilators. Nurses, physicians, and advanced practice providers offered their interdisciplinary expert opinions as needed. The authors also thank their participants, who—while processing and dealing with critical illness—were eager and willing to participate in their research. They also thank Martin Blair for helping with editorial support.
Footnotes
Supported by National Institutes of Health R01 5-R017433 (V.P.), on laryngotracheal injury and rehabilitation after translaryngeal intubation in intensive care unit settings.
Author Contributions: K.A.M.: data acquisition, interpretation of the data, drafting of the manuscript, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. T.D.K.C. and C.M.P.: conception and design of study, data acquisition, critical revision of the manuscript for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. N.A.: data acquisition, critical revision of the manuscript for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. K.M., D.N.H., and M.J.B.: interpretation of the data, critical revision of the manuscript for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. V.P.: conception and design of the study, data analysis, interpretation of the data, drafting the manuscript, critical revision of the manuscript for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. The Joint Commission. Safer care for patients with tracheostomies. Jt Comm Pers Pat Saf. 2010;10:1–11. [Google Scholar]
- 2.Passy-muir tracheostomy and ventilator speaking valve resource guide. Irvine, CA: Passy-Muir, Inc; 2003. pp. 1–54. [Google Scholar]
- 3.Sutt AL.Global Tracheostomy Collaborative. [Google Scholar]
- 4. Carroll SM. Silent, slow lifeworld: the communication experience of nonvocal ventilated patients. Qual Health Res. 2007;17:1165–1177. doi: 10.1177/1049732307307334. [DOI] [PubMed] [Google Scholar]
- 5. Foster A. More than nothing: the lived experience of tracheostomy while acutely ill. Intensive Crit Care Nurs. 2010;26:33–43. doi: 10.1016/j.iccn.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6. Freeman-Sanderson AL, Togher L, Elkins M, Kenny B. Quality of life improves for tracheostomy patients with return of voice: a mixed methods evaluation of the patient experience across the care continuum. Intensive Crit Care Nurs. 2018;46:10–16. doi: 10.1016/j.iccn.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 7. Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868; discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- 8. Rose L, Istanboulian L, Smith OM, Silencieux S, Cuthbertson BH, Amaral ACK, et al. Feasibility of the electrolarynx for enabling communication in the chronically critically ill: the EECCHO study. J Crit Care. 2018;47:109–113. doi: 10.1016/j.jcrc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 9. Tuinman PR, Ten Hoorn S, Aalders YJ, Elbers PW, Girbes AR. The electrolarynx improves communication in a selected group of mechanically ventilated critically ill patients: a feasibility study. Intensive Care Med. 2015;41:547–548. doi: 10.1007/s00134-014-3591-2. [DOI] [PubMed] [Google Scholar]
- 10. Zaga CJ, Berney S, Vogel AP. The feasibility, utility, and safety of communication interventions with mechanically ventilated intensive care unit patients: a systematic review. Am J Speech Lang Pathol. 2019;28:1335–1355. doi: 10.1044/2019_AJSLP-19-0001. [DOI] [PubMed] [Google Scholar]
- 11. Hoit JD, Banzett RB, Lohmeier HL, Hixon TJ, Brown R. Clinical ventilator adjustments that improve speech. Chest. 2003;124:1512–1521. doi: 10.1378/chest.124.4.1512. [DOI] [PubMed] [Google Scholar]
- 12. Bell SD. Use of Passy-Muir tracheostomy speaking valve in mechanically ventilated neurological patients. Crit Care Nurse. 1996;16:63–68. [PubMed] [Google Scholar]
- 13. Byrick RJ. Improved communication with the Passy-Muir valve: the aim of technology and the result of training. Crit Care Med. 1993;21:483–484. doi: 10.1097/00003246-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 14. Fröhlich MR, Boksberger H, Barfuss-Schneider C, Liem E, Petry H. [Safe swallowing and communicating for ventilated intensive care patients with tracheostoma: implementation of the Passy Muir speaking valve] Pflege. 2017;30:387–394. doi: 10.1024/1012-5302/a000589. [DOI] [PubMed] [Google Scholar]
- 15. Jackson D, Albamonte S. Enhancing communication with the Passy-Muir valve. Pediatr Nurs. 1994;20:149–153. [PubMed] [Google Scholar]
- 16. Kaut K, Turcott JC, Lavery M. Passy-Muir speaking valve. Dimens Crit Care Nurs. 1996;15:298–306. doi: 10.1097/00003465-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 17. Passy V. Passy-Muir tracheostomy speaking valve. Otolaryngol Head Neck Surg. 1986;95:247–248. doi: 10.1177/019459988609500224. [DOI] [PubMed] [Google Scholar]
- 18. Passy V, Baydur A, Prentice W, Darnell-Neal R. Passy-Muir tracheostomy speaking valve on ventilator-dependent patients. Laryngoscope. 1993;103:653–658. doi: 10.1288/00005537-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Yorkston K, Beukeman D, Tice R. Sentence Intelligibility Test. 1st ed. Lincoln, NE: Tice Technology Services; 1996. [Google Scholar]
- 20. Eghbali-Babadi M, Shokrollahi N, Mehrabi T. Effect of family-patient communication on the incidence of delirium in hospitalized patients in cardiovascular surgery ICU. Iran J Nurs Midwifery Res. 2017;22:327–331. doi: 10.4103/1735-9066.212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brodsky MB, Levy MJ, Jedlanek E, Pandian V, Blackford B, Price C, et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Crit Care Med. 2018;46:2010–2017. doi: 10.1097/CCM.0000000000003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman-Sanderson AL, Togher L, Elkins MR, Phipps PR. Return of voice for ventilated tracheostomy patients in ICU: a randomized controlled trial of early-targeted intervention. Crit Care Med. 2016;44:1075–1081. doi: 10.1097/CCM.0000000000001610. [DOI] [PubMed] [Google Scholar]
- 23. Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL) J Voice. 1999;13:557–569. doi: 10.1016/s0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 24. Pandian V, Miller CR, Schiavi AJ, Yarmus L, Contractor A, Haut ER, et al. Utilization of a standardized tracheostomy capping and decannulation protocol to improve patient safety. Laryngoscope. 2014;124:1794–1800. doi: 10.1002/lary.24625. [DOI] [PubMed] [Google Scholar]
- 25. Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donovan JJ, Radosevich DJ. A meta-analytic review of the distribution of practice effect: now you see it, now you don’t. J Appl Psychol. 1999;84:795–805. [Google Scholar]