Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA loads in patient specimens may act as a clinical outcome predictor in critically ill patients with coronavirus disease 2019 (COVID-19).
Methods
We evaluated the predictive value of viral RNA loads and courses in the blood compared with the upper and lower respiratory tract loads of critically ill COVID-19 patients. Daily specimen collection and viral RNA quantification by reverse transcription quantitative polymerase chain reaction were performed in all consecutive 170 COVID-19 patients between March 2020 and February 2021 during the entire intensive care unit (ICU) stay (4145 samples analyzed). Patients were grouped according to their 90-day outcome as survivors (n=100) or nonsurvivors (n=70).
Results
In nonsurvivors, blood SARS-CoV-2 RNA loads were significantly higher at the time of admission to the ICU (P=.0009). Failure of blood RNA clearance was observed in 33/50 (66%) of the nonsurvivors compared with 12/64 (19%) survivors (P<.0001). As determined by multivariate analysis, taking sociodemographic and clinical parameters into account, blood SARS-CoV-2 RNA load represents a valid and independent predictor of outcome in critically ill COVID-19 patients (odds ratio [OR; log10], 0.23; 95% CI, 0.12–0.42; P<.0001), with a significantly higher effect for survival compared with respiratory tract SARS-CoV-2 RNA loads (OR [log10], 0.75; 95% CI, 0.66–0.85; P<.0001). Blood RNA loads exceeding 2.51×103 SARS-CoV-2 RNA copies/mL were found to indicate a 50% probability of death. Consistently, 29/33 (88%) nonsurvivors with failure of virus clearance exceeded this cutoff value constantly.
Conclusions
Blood SARS-CoV-2 load is an important independent outcome predictor and should be further evaluated for treatment allocation and patient monitoring.
Keywords: kinetics, SARS, CoV, 2, SARS, CoV, 2 RNA load, viremia
Risk assessment and stratification of coronavirus disease 2019 (COVID-19) patients are challenging, notably in intensive care units (ICUs), as it is still unclear which factors correlate with severe courses or fatal outcomes. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA load in blood and respiratory tract specimens as detected by reverse transcription quantitative polymerase chain reaction (RT-qPCR) has been suggested to correlate with disease severity and mortality. However, previous studies on the impact of viremia on patient outcomes were mostly limited to single-point measurements and did not consider the level and course of viral loads [1–7]. By analyzing the course of viremia in a small cohort of critically ill patients with hemato-oncologic disorders, we recently reported that failure to clear SARS-CoV-2 RNA from the bloodstream is associated with a high risk of death [8, 9], as confirmed in small patient cohorts [10, 11].
To investigate the prognostic value of viral load in a mixed patient population for the present study, we evaluated the level and course of viral RNA load in the upper respiratory tract (URT), lower respiratory tract (LRT), and bloodstream of 170 critically ill patients. Multivariate analysis, considering primary sociodemographic data and relevant clinical parameters, was performed.
METHODS
Patients and Ethics
All patients (n=170) were hospitalized at the Department of Intensive Care Medicine (ICU), University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany, between March 2020 and March 2021. Patients were hospitalized for COVID-19 and/or COVID-19-associated conditions. Patients were grouped according to their 90-day outcome status as survivors (n=100) or nonsurvivors (n=70). Readmissions of patients were counted as 1 intensive care unit stay. Relevant covariables evaluated were age, sex, body mass index, preexisting medical conditions (ie, prior myocardial infarction, congestive heart failure, peripheral vascular disease, rheumatologic disease, peptic ulcer disease, mild, moderate, or severe liver disease, diabetes mellitus, cerebrovascular [hemiplegia] event, moderate to severe renal disease, diabetes with chronic complications, cancer without metastases, leukemia, lymphoma, metastatic solid tumor, acquired immune deficiency as part of the Charlson comorbidity index, chronic lung diseases, and arterial hypertension), immunosuppression due to preexisting medical conditions, time from COVID-19 diagnosis to ICU admission, presence and degree of acute respiratory distress syndrome (ARDS) according to the Berlin definition, disease severity according to Simplified Acute Physiology Score II (SAPS II) and Sepsis-related Organ Failure Assessment Score (SOFA), need for mechanical ventilation (MV), need for extracorporeal membrane oxidation (ECMO), and need for COVID-19-related treatments (ie, dexamethasone, remdesivir, monoclonal antibodies, therapeutic plasma exchange [TPE]).
The Ethics Committee of the Hamburg Chamber of Physicians was informed about the study. Due to the retrospective nature of the study, the need for informed consent was waived (WF-094/21). Partial data of a subset of the cohort (30 out of 170) have been previously analyzed and published elsewhere [8, 9].
Sampling, Molecular Diagnostics, and Epidemiology
For the upper respiratory tract (URT), nasopharyngeal swabs in UTM (MANTACC, Shenzhen, China) or Amies Medium (E-swab, Copan, Brescia, Italy) were collected. Sputum, bronchial fluid samples, or bronchial lavage samples (all native) were assessed for the lower respiratory tract (LRT). EDTA plasma samples (Sarstedt, Nümbrecht, Germany) were obtained to analyze the blood RNA load. Samples were collected regularly during the ICU stay. All samples were obtained as part of routine clinical practice. In total, 4145 samples were analyzed by qPCR (621 upper respiratory tract, 1455 lower respiratory tract, and 2069 EDTA-plasma samples).
SARS-CoV-2 RNA in respiratory specimens (URT and LRT) was quantified and detected as described previously using the commercially available assays Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA), cobas SARS-CoV-2 (Roche, Mannheim, Germany), and laboratory-developed assays (LDTs) run on the cobas6800 system (Roche), the NeuMoDx system (Qiagen, Germantown, MD, USA), or the Light Cycler 480 II (Roche) [12–15]. Standard RNA reference material (obtained from INSTAND, Düsseldorf, Germany) was used for quantification. To calculate log10 RNA copies/mL (y) based on ct values (x), the following targets and conversion formulae were used for respiratory samples: y= –0.29x + 12.83 (Xpert Xpress SARS-CoV-2, target E2) y=–0.308x + 13.81 (cobas SARS-CoV-2, target T2), y=–0.291x + 12.97 (SARS-CoV-2_UCT (LDT), E-gene), y=–0.425x + 14.8 (NeuMoDx (LDT), E-gene), y=–0.318x + 13.32 (LightCycler 480 II, E-gene). For plasma EDTA samples, the cobas SARS-CoV assay was used with the conversion formula y=–0.247x + 12.27 (cobas SARS-CoV-2, target T2). A threshold of 1×103 copies/mL was set for quantification; RNA loads below this cutoff were excluded from the quantitative analysis. For all patients, initial respiratory samples were analyzed in a multiplex typing PCR, identifying and distinguishing SARS-CoV-2 spike variants [16].
Statistical Analysis
Estimation of Virus RNA Clearance
Successful RNA clearance was defined as the absence of SARS CoV-2 RNA (in RT-qPCRs) from the respective compartment for at least 3 days.
Multivariate Analysis
Assuming nonparametric data distribution, the Wilcoxon-Mann-Whitney U test was used to compare viral loads between 2 groups. Categorical variables were compared using the 2-sided Fisher exact test or 2-sided chi-square test. The survival distribution of 2 groups was compared using the log-rank test. A generalized mixed model with logistic regression and Firth approximation was used to identify predictors of adverse outcomes in the URT, LRT, and blood. The patients’ 90-day outcome status served as the dependent variable. Fixed effects were age [years; metric variable], sex [male=0; female=1], body mass index [kg/m2; metric variable], Charlson comorbidity index [1–13; pseudo-metric variable], the need for mechanical ventilation [no=0; yes=1], the presence of ARDS [no=0; yes=1], the need for extracorporeal membrane oxygenation [no=0; yes=1], and the viral RNA load in the particular compartment [1 log10 level; copies/mL; metric variable]. The patient (correlation structure: compound symmetry) and time (correlation structure: first order autoregression) were set as random factors. The initial selection of statistically independent variables was performed on a clinical and scientific basis. Top-down variable selection was made in the form of a stepwise Akaike Information Criterion (AIC)–guided elimination of predictors. Model optimization was done for the LRT, and the model was subsequently transferred without further adjustments to the other compartments to ensure comparability. Results < limit of detection (<LOD; eg, negative) and < the threshold of 1×103 copies/mL were excluded from the generalized mixed model analyses. P values <.05 were considered significant.
Statistics were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). GraphPad Prism software, version 9.0.0 (GraphPad Software, San Diego, CA, USA), was used for data illustration.
RESULTS
Patients Characteristics
The median patient age (IQR) was 63 (55–73) years, with 35% being female. The overall case fatality rate in our single-center cohort was 41% (70/170), with a median observation time (IQR) of 22 (11–34) days. Baseline characteristics of survivors and nonsurvivors are illustrated in Table 1. The presence of ARDS and, accompanying this, the need for mechanical ventilation (MV) and ECMO were predominantly observed in the nonsurvivor group (90% vs 50%, 93% vs 57%, and 46% vs 17%, respectively).
Table 1.
Patient Characteristics of ICU Patients of the University Medical Center Hamburg-Eppendorf, Hamburg, Germany
| Survivors, No. (%) or Median (IQR) (n=100) | Nonsurvivors, No. (%) or Median (IQR) (n=70) | Comparative Statistics, P Value | Total, No. (%) or Median (IQR) (n=170) | |
|---|---|---|---|---|
| Age, y | 60 (51–72) | 67 (59–76) | .01 | 63 (55–73) |
| Sex | Male: 63 (63) | Male: 48 (69) | .45 | Male:111 (65) |
| Female:37 (37) | Female: 22 (31) | Female: 59 (35) | ||
| Body mass index, kg/m2 | 28 (25–32) | 26 (24–32) | .22 | 27 (25–32) |
| Charlson comorbidity index at ICU admission | 1 (1–3) | 2 (1–4) | .05 | 2 (1–3) |
| Comorbidities | ||||
| Chronic lung disease | 13 (13) | 11 (16) | .62 | 24 (14) |
| Type II diabetes mellitus | 35 (35) | 23 (33) | .77 | 58 (34) |
| Arterial hypertension | 55 (55) | 43 (61) | .40 | 98 (58) |
| Immunosupression | 23 (23) | 28 (40) | .02 | 51 (30) |
| Duration of illness/time from COVID-19 diagnosis until ICU admission, d | 3 (1–8) | 7 (1–13) | .14 | 4 (1–11) |
| COVID-19 disease severity | ||||
| Clinically diagnosed ARDS | 50 (50) | 63 (90) | <.0001 | 113 (67) |
| Sepsis-related Organ Failure Assessment Score | 5 (3–11) | 10 (5–13) | .001 | 7 (3–12) |
| Simplified Acute Physiology Score II | 37 (30–43) | 42 (37–52) | <.0001 | 40 (32–48) |
| ICU-specific treatment | ||||
| Mechanical ventilation | 57 (57) | 65 (93) | <.0001 | 122 (72) |
| ECMO | 17 (17) | 32 (46) | <.0001 | 49 (29) |
| COVID-19-related treatment | ||||
| Dexamethasone | 39 (39) | 35 (50) | .16 | 74 (44) |
| Remdesivir | 22 (22) | 11 (16) | .31 | 33 (19) |
| Monoclonal antibodies | 0 (0) | 3 (4) | .07 | 3 (2) |
| Therapeutic plasma exchange | 3 (3) | 3 (4) | .69 | 6 (4) |
The groups are divided according to survival.
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
In the survivor group, 23% of patients were considered immunosuppressed, while in the nonsurvivor group this proportion was significantly higher at 40%. The proportions of patients receiving COVID-19-related antiviral therapy were comparable, meaning 39/100 (39%) survivors received dexamethasone, compared with 35/70 (50%) nonsurvivors. Remdesivir was administered to 22/100 (22%) survivors compared with 11/70 (16%) nonsurvivors. None of the survivors received monoclonal antibody therapy, and only 3/70 (4%) nonsurvivors were treated with monoclonal antibodies. Therapeutic plasma exchange was performed in 3/100 (3%) survivors and 3/70 (4%) nonsurvivors.
The presence of mutant spike variants of concern (VOC) was ruled out in all patients by multiplex typing PCR [16]. Of note, this is in line with the GSAID database, which documented the first entries of SARS-CoV-2 VOCs (such as B.1.1.7) in Northern Germany only by the end of the entire observation period.
Viral Loads at ICU Admission
Median blood viral loads at the time of ICU admission (IQR) were significantly different between the 2 groups, with 3.56×103 (<limit of detection [LoD]—1.93×104) SARS-CoV-2 RNA copies/mL in nonsurvivors compared with <1.00×103 (<LoD—2.79×103) SARS-CoV-2 RNA copies/mL in survivors (P=.0009) (Figure 1A).
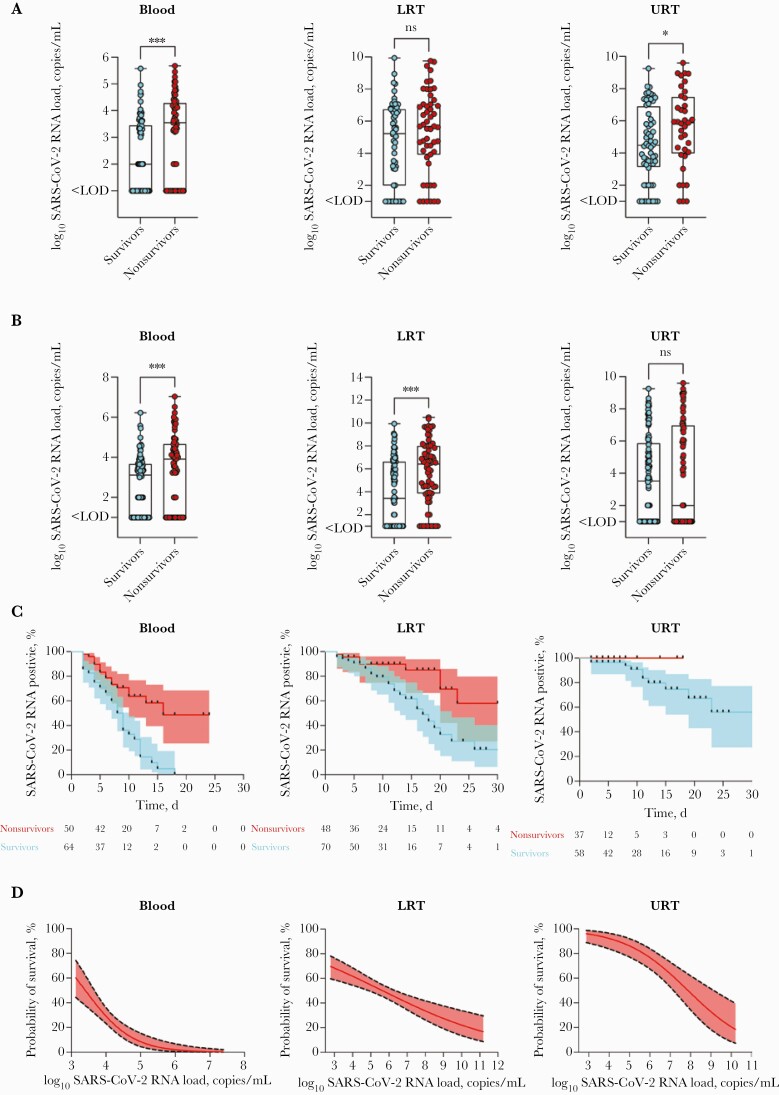
Figure 1.
A, SARS-CoV-2 RNA loads at the time of ICU admission in blood, LRT, and URT. B, Maximum loads of SARS-CoV-2 RNA in blood, LRT, and URT during the course of disease differ significantly between groups. Samples < LOD were set to 1×101, and samples < LOQ were set to 1×102 to allow for logarithmic presentation. C, Kaplan-Meier curves illustrate the probability of virus RNA elimination in the blood, LRT, and URT. The y-axis displays the proportion of patients with continuous detection of SARS-CoV-2 RNA. Successful RNA clearance was assumed at negative RT-qPCR results >3 days. D, Multivariate analysis (generalized linear logistic mixed model) (Table 1) reveals blood RNA level rather than LRT/URT RNA level as a strong predictor of outcome, with a 50.0% probability of death at blood RNA levels exceeding 2.51×103 (=3.40 log10) copies/mL. The red line represents the estimated effect, and the dotted black lines represent the 95% CI. Model estimators for the GLMM (blood) are AIC, 393.28; c-c, 0.86, for the GLMM (LRT) AIC, 923.81; c-c, 0.84, and for the GLMM (URT) AIC, 337.44; c-c, 0.92. P values are displayed as follows: ∗∗∗∗P < .0001; ∗∗∗P = .0002; ∗∗P = .0021; ∗P = .0332; ns = 0.1234. Abbreviations: AIC, Akaike Information Criterion; GLMM, generalized linear mixed model; ICU, intensive care unit; LOD, limit of detection; LOQ, limit of quantification; LRT, lower respiratory tract; RT-qPCR, reverse transcription quantitative polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; URT, upper respiratory tract.
Median LRT RNA loads at the time of admission showed no significant differences, with median LRT RNA loads (IQR) of 4.77×105 (8.38×103–1.11×107) SARS-CoV-2 RNA copies/mL in nonsurvivors compared with 1.70×105 (<LoD—5.39×106) SARS-CoV-2 RNA copies/mL (P=.14) in survivors (Figure 1A).
Median URT RNA loads at the time of admission were found to be different, with median URT viral RNA loads (IQR) of 7.56×105 (9.66×103–3.04×107) SARS-CoV-2 RNA copies/mL compared with 3.03×104 (1.37×103–7.90×106) SARS-CoV-2 RNA copies/mL (P=.04) in nonsurvivors vs survivors (Figure 1A).
Maximum Viral Loads
The maximum blood RNA loads during the course of the disease were significantly higher in nonsurvivors compared with survivors (median [IQR], 8.11×103 [<LoD—4.60×104] SARS-CoV-2 RNA copies/mL; vs median [IQR], 1.32×103 [<LoD—4.67×103] SARS-CoV-2 RNA copies/mL; P=.0009) (Figure 1B). Maximum LRT RNA loads during the course of the disease were significantly higher among nonsurvivors compared with survivors (median [IQR], 2.63×106 [7.26×103–9.81×107] SARS-CoV-2 RNA copies/mL; vs median [IQR], 2.61×103 [<LoD—4.12×106] SARS-CoV-2 RNA copies/mL; P<.0001) (Figure 1B).
During the course of the disease, maximum URT levels showed no significant differences between the 2 groups (median RNA load [IQR], <1.00×103 [<LoD—9.27×106] SARS-CoV-2 RNA copies/mL in nonsurvivors; vs median RNA load [IQR], 3.30×103 [<LoD– 7.36×105] SARS-CoV-2 RNA copies/mL in survivors; P=.73) (Figure 1B).
Viral Load Kinetics and Clearance
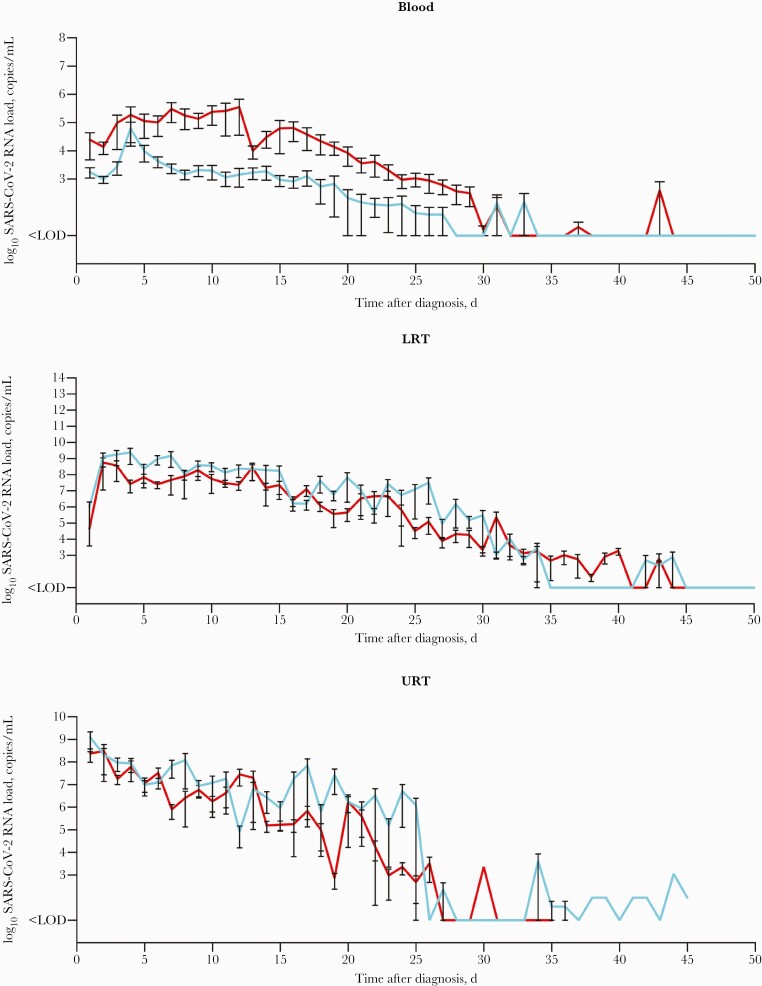
Mean SARS-CoV-2 RNA loads in the 3 compartments normalized to COVID-19 diagnosis are illustrated in Figure 2 (for individual patient kinetics, refer to Supplementary Figure 1). Analyses of viral load kinetics revealed significant differences in viral clearance rates between nonsurvivors and survivors in the blood (median time to clearance, 16 days vs 9 days; P<.0001) and LRT samples (P=.01) (Figure 1C), but not for URT samples (P=.13) (Figure 1C). The Kaplan-Meier curves show the proportion of patients with continuous SARS-CoV-2 detection throughout the observation period. Accordingly, failure of viral RNA clearance from the bloodstream was observed in the majority of nonsurvivors (33/50 [66.0%]) compared with survivors (12/64 [18.8%]; P<.0001).
Figure 2.
Courses of the SARS-CoV-2 RNA loads as determined by RT-qPCR throughout the disease from the time of diagnosis in blood (A), LRT (B), and URT (C). The respective mean and SEM of SARS-CoV-2 RNA loads are illustrated. Red lines refer to nonsurvivors; light blue lines refer to survivors. Samples < LOD were set to 1×101 copies/mL, and samples < threshold of 1×103 copies/mL were set to 1×102 copies/mL to allow for logarithmic presentation. Abbreviations: LOD, limit of detection; LOQ, limit of quantification; LRT, lower respiratory tract; RT-qPCR, reverse transcription quantitative polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEM, standard error of the mean; URT, upper respiratory tract.
Multivariate Analysis
The generalized linear logistic mixed models incorporating blood or respiratory tract (URT and LTR) viral loads in addition to relevant clinical covariables (age, sex, body mass index, Charlson comorbidity index, ARDS, the need for mechanical ventilation [MV], and ECMO) confirmed blood viral RNA load as a strong independent predictor of adverse outcomes (odds ratio [OR; unit: 1 log10], 0.23; 95% CI, 0.12–0.42; P<.0001), with a significantly higher effect for survival if compared with SARS-CoV-2 RNA loads in the LRT and URT (Figure 1D; details of the analysis are given in Table 2). For the URT model, refer to Figure 1D and Table 2.
Table 2.
Multivariate Analysis
| Generalized Linear Logistic Mixed Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blood | LRT | URT | |||||||
| Parameter | Estimator | SE | P Value | Estimator | SE | P Value | Estimator | SE | P Value |
| Intercept | 8.82 | 1.88 | <.0001 | 6.84 | 1.81 | .0002 | 10.63 | 2.27 | <.0001 |
| Age, y | –0.06 | 0.02 | <.0001 | –0.07 | 0.01 | <.0001 | –0.15 | 0.02 | <.0001 |
| Sex (ref: Male) | 0.78 | 0.32 | .01 | 1.01 | 0.21 | <.0001 | 1.15 | 0.40 | .004 |
| BMI, kg/m2 | 0.11 | 0.02 | <.0001 | 0.10 | 0.01 | <.0001 | 0.25 | 0.06 | <.0001 |
| Charlson comorbidity index (1–13) | –0.40 | 0.10 | .0001 | –0.22 | 0.05 | <.0001 | –0.07 | 0.09 | .45 |
| SARS-CoV-2 RNA load (unit: 1 log10 level), copies/mL | –1.49 | 0.31 | <.0001 | –0.29 | 0.07 | <.0001 | –0.64 | 0.14 | <.0001 |
| Clinically diagnosed ARDS | –2.17 | 1.75 | .21 | –0.57 | 0.97 | .56 | 0.85 | 0.64 | .18 |
| Mechanical ventilation | 0.74 | 1.84 | .69 | –1.12 | 1.88 | .55 | –3.10 | 0.82 | .0001 |
| ECMO | –2.87 | 0.49 | <.0001 | –3.41 | 0.35 | <.0001 | –5.77 | 1.15 | <.0001 |
| Viremia (ref: none) | a | a | a | –1.52 | 0.25 | <.0001 | –1.90 | 0.45 | <.0001 |
The patients’ 90-day survival status served as the dependent variable. Multivariate analysis (generalized linear logistic mixed model) reveals blood and LRT RNA levels as predictors of adverse outcomes. The patient (correlation structure: compound symmetry) and time (correlation structure: first order autoregression) were set as random factors to account for repeated measurements. Firth approximation was used to improve the model’s fitness. Model estimators for the GLMM (blood) were AIC, 393.28; c-c, 0.86. For the GLMM (LRT), they were AIC, 923.81; c-c, 0.84. For GLMM (LRT), they were AIC, 337.44; c-c, 0.92.
Abbreviations: AIC, Akaike Information Criterion; ARDS, acute respiratory distress syndrome; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; GLMM, generalized linear mixed model; LRT, lower respiratory tract; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; URT, upper respiratory tract.
Not applicable.
In our model, patients with blood SARS-CoV-2 RNA loads exceeding 2.51×103 SARS-CoV-2 RNA copies/mL had a probability of death exceeding 50% (95% CI, 37.8%–62.3%) (Figure 1D). Here, 40 out of 50 viremic patients (80%) in the nonsurvivor group exceeded this value at least once during the ICU stay, compared with 32 out of 64 viremic patients (50%) in the survivor group (P=.0016). Consistently, in 29/33 (88%) of the nonsurvivors with failure of virus clearance, this cutoff value was constantly exceeded, compared with 2/12 (17%) of the survivors with failure of virus clearance.
DISCUSSION
In the present study, we evaluated the prognostic value of SARS-CoV-2 RNA levels and kinetics in blood and upper and lower respiratory tract samples by daily molecular analyses of all 3 compartments in 170 critically ill patients. This in-depth look at the course of virological data contrasts with previous studies that analyzed single-point measurements [1, 2, 4, 5, 7] or only focused on individual compartments without quantifying viral loads [7, 17]. Two studies on blood SARS-CoV-2 RNA loads found increased mortality in viremic patients, yet their findings were based on smaller cohorts and lower sampling frequencies [10, 11]. A recent large study focused on respiratory specimens and highlighted the association of viral RNA load and infectivity in outpatients compared with inpatients, but without addressing their predictive value for mortality and disease progression [18].
Notably, and consistent with previous studies [6, 10, 11], blood RNA loads on admission to the ICU were significantly elevated in patients with fatal outcomes (P=.0009), while no significant difference in admission viral loads could be shown for the lower respiratory tract. Furthermore, the maximum blood and LRT viral RNA loads during the disease were significantly higher in nonsurvivors compared with survivors. Again, no such differences were observed for maximum URT viral RNA levels during the course of the disease.
Clearance of viral RNA from the bloodstream occurred more frequently (P<.0001) and more rapidly (median time, 9 days) in survivors than nonsurvivors. Likewise, and similar to recently published data [8, 9, 11, 17], survivors were able to clear the virus successfully from the respiratory tract, yet time to clearance took considerably longer. These data indicate that persistently elevated blood RNA levels can serve as an early indicator of severe courses of the disease.
Most essentially, the multivariate analysis confirmed blood SARS-CoV-2 load to be an important outcome predictor independent of other clinically relevant covariables such as primary sociodemographic data, comorbidities, the presence of ARDS, and the need for mechanical ventilation or extracorporeal membrane oxygenation. Importantly, the blood model revealed a significantly higher effect for survival if compared with the URT and LRT models (blood: OR, 0.23; 95% CI, 0.12–0.42; LRT: OR,0.75; 95% CI, 0.66–0.85; and URT: OR,0.53; 95% CI, 0.40–0.69).
According to the model presented here, blood RNA levels exceeding 2.51×103 SARS-CoV-2 RNA copies/mL reflect a 50% probability of death. Considering this blood RNA load as a critical cutoff value, half of all patients in the survivor group compared with the majority in the nonsurvivor group exceeded this value at least once during the ICU stay. Notably, in 88% of the nonsurvivors, blood RNA loads remained constantly elevated above that cutoff value, while in all but 2 of the survivors, blood RNA loads declined below that threshold during the course of the disease.
The proportion of patients receiving COVID-19-related antiviral therapy (dexamethasone, remdesivir, or monoclonal antibodies) was comparable in survivors and nonsurvivors, though monoclonal antibody therapy was initiated in 4 of the nonsurvivors and none of the survivors. Altogether, the difference in successful viral blood clearance seems not to be attributable to specific therapeutic interventions.
However, the proportion of immunocompromised patients was higher among nonsurvivors in our study. Thus, our data prove evidence of an increased risk of SARS-CoV-2 viremia and associated mortality in this particular patient population [8–11]. However, it is not yet possible to conclude whether immunosuppression promotes viremia or whether, conversely, viremia exacerbates immunosuppression in critically ill patients.
Previous studies have shown that SARS-CoV-2 affects different organs besides the respiratory tract and that high viral loads in the affected organs correlate with increased mortality [19].
Although our study does not identify viremia itself as the cause of death, our data indicate that patients with high levels of viremia and delayed virus clearance represent a vulnerable subgroup. This subgroup might particularly benefit from specific therapy such as monoclonal antibodies or direct antiviral substances. Moreover, monitoring of viremia could thus be useful for future patient management in the ICU.
However, it is currently difficult for diagnostic laboratories to offer reliable quantitative molecular SARS-CoV-2 diagnostics for specimens other than respiratory tract samples. Food and Drug Administration (FDA)– or Conformitè Europëenne (CE)-approved molecular assays are missing for this purpose. As the importance of virologic blood diagnostics is highlighted, indicating a high prognostic value for patient outcomes in this study, such diagnostics might help clinicians in patient management, in assessing each patient’s prognosis. Thus, there is an urgent need for the rapid evaluation and approval of blood RT-qPCRs for SARS-CoV-2 [10, 15].
We are aware that our study has limitations. The intermittent shortage of reagents and supplies led to the use of a variety of assays for quantification. Blood samples were almost exclusively analyzed with the cobas 6800 system, but slight deviations in the quantification in URT and LRT cannot be completely ruled out given the multitude of assays. Also, the URT sampling frequency is lower than for LRT and blood because nasopharyngeal swabs were waived in some of the severely ill mechanically ventilated patients. RNA quantification in respiratory tract samples has significant variability, and therefore its clinical implementation is challenging. Swab samples, in particular, are dependent on the collection technique and intra-individual fluctuations (eg, the detection of false high RNA loads by coughing up RNA-positive material in the URT). However, in this study, RNA load variabilities should largely be compensated by close longitudinal sampling. Furthermore, according to the epidemiological situation at patient enrollment, no patient in our study was infected with a recently emerging spike mutant variant (VOC); virus RNA loads in VOC-infected patients might have exceeded the loads measured here.
In summary, our data indicate that SARS-CoV-2 viremia is a better predictor of outcome than respiratory tract viral RNA load, and clearance of SARS-CoV-2 RNA from the bloodstream is strongly associated with survival. Thus, reliable quantification of SARS-CoV-2 RNA in the blood as part of clinical practice seems mandatory to assess patients’ risk of fatal outcomes. Moreover, monitoring of viremia could be an important surrogate marker of the effectiveness of antiviral therapies. FDA-approved assays are required for this purpose.
Supplementary Material
Acknowledgments
Financial support. This study did not receive external funding.
Potential conflicts of interest. M.L. received speaker honoraria and related travel expenses from Roche Diagnostics. S.K. reports grants and personal fees from Pfizer, personal fees from Biotest, personal fees from Cytosorbents, personal fees from Gilead, personal fees from MSD, personal fees from Bayer, personal fees from Astellas, personal fees from Baxter, and personal fees from Fresenius, outside the submitted work. All other authors declare that they have no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The Ethics Committee of the Hamburg Chamber of Physicians was informed about the study. Due to the retrospective nature of the study, the need for informed consent was waived (WF-094/21).
References
- 1. Liu Y, Yan LM, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maltezou HC, Raftopoulos V, Vorou R, et al. . Association between upper respiratory tract viral load, comorbidities, disease severity and outcome of patients with SARS-CoV-2 infection. J Infect Dis 2021; 223:1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bitker L, Dhelft F, Chauvelot L, et al. . Protracted viral shedding and viral load are associated with ICU mortality in Covid-19 patients with acute respiratory failure. Ann Intensive Care 2020; 10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bermejo-Martin JF, González-Rivera M, Almansa R, et al. . Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care 2020; 24:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hagman K, Hedenstierna M, Gille-Johnson P, et al. . Severe acute respiratory syndrome coronavirus 2 RNA in serum as predictor of severe outcome in coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis 2020; ciaa1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fajnzylber J, Regan J, Coxen K, et al. ; Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutmann C, Takov K, Burnap SA, et al. . SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat Commun 2021; 12:3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghandili S, Pfefferle S, Roedl K, et al. . Challenges in treatment of patients with acute leukemia and COVID-19: a series of 12 patients. Blood Adv 2020; 4:5936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roedl K, Heidenreich S, Pfefferle S, et al. . Viral dynamics of SARS-CoV-2 in critically ill allogeneic hematopoietic stem cell transplant recipients and immunocompetent patients with COVID-19. Am J Respir Crit Care Med 2021; 203:242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colagrossi L, Antonello M, Renica S, et al. . SARS-CoV-2 RNA in plasma samples of COVID-19 affected individuals: a cross-sectional proof-of-concept study. BMC Infect Dis 2021; 21:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L, Wang G, Long X, et al. . Dynamics of blood viral load is strongly associated with clinical outcomes in coronavirus disease 2019 (COVID-19) patients: a prospective cohort study. J Mol Diagn 2021; 23:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nörz D, Fischer N, Schultze A, et al. . Clinical evaluation of a SARS-CoV-2 RT-PCR assay on a fully automated system for rapid on-demand testing in the hospital setting. J Clin Virol 2020; 128:104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfefferle S, Reucher S, Norz D, Lutgehetmann M.. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Euro Surveill 2020; 25:2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nörz D, Hoffmann A, Aepfelbacher M, Pfefferle S, Lütgehetmann M.. Clinical evaluation of a fully automated, laboratory-developed multiplex RT-PCR assay integrating dual-target SARS-CoV-2 and influenza A/B detection on a high-throughput platform. J Med Microbiol 2021; 70:001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nörz D, Frontzek A, Eigner U, et al. . Pushing beyond specifications: evaluation of linearity and clinical performance of the cobas 6800/8800 SARS-CoV-2 RT-PCR assay for reliable quantification in blood and other materials outside recommendations. J Clin Virol 2020; 132:104650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nörz D, Grunwald M, Olearo F, et al. . Evaluation of a fully automated high-throughput SARS-CoV-2 multiplex qPCR assay with built-in screening functionality for del-HV69/70- and N501Y variants such as B.1.1.7. J Clin Virol 2021; 141:104894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu Y, Li Y, Guo E, et al. . Dynamics and correlation among viral positivity, seroconversion, and disease severity in COVID-19: a retrospective study. Ann Intern Med 2021; 174:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones TC, Biele G, Mühlemann B, et al. . Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021; 373:eabi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braun F, Lütgehetmann M, Pfefferle S, et al. . SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020; 396:597–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.