Abstract
Background
Pregnant women with coronavirus disease 2019 (COVID-19) may be at greater risk of poor maternal and pregnancy outcomes. This retrospective analysis reports clinical and pregnancy outcomes among hospitalized pregnant women with COVID-19 in the United States.
Methods
The Premier Healthcare Database—Special Release was used to examine the impact of COVID-19 among pregnant women aged 15–44 years who were hospitalized and who delivered compared with pregnant women without COVID-19. Outcomes evaluated were COVID-19 clinical progression, including the use of supplemental oxygen therapy, intensive care unit admission, critical illness, receipt of invasive mechanical ventilation/extracorporeal membrane oxygenation, maternal death, and pregnancy outcomes, including preterm delivery and stillbirth.
Results
Overall, 473 902 hospitalized pregnant women were included, 8584 (1.8%) of whom had a COVID-19 diagnosis (mean age = 28.4 [standard deviation = 6.1] years; 40% Hispanic). The risk of poor clinical and pregnancy outcomes was greater among pregnant women with COVID-19 compared with pregnant women without a COVID-19 diagnosis in 2020; the risk of poor clinical and pregnancy outcomes increased with increasing age. Hispanic and Black non-Hispanic women were consistently observed to have the highest relative risk of experiencing poor clinical or pregnancy outcomes across all age groups.
Conclusions
Overall, COVID-19 had a significant negative impact on maternal health and pregnancy outcomes. These data help inform clinical practice and counseling to pregnant women regarding the risks of COVID-19. Clinical studies evaluating the safety and efficacy of vaccines against severe acute respiratory syndrome coronavirus 2 in pregnant women are urgently needed.
Keywords: clinical outcomes, COVID-19, hospitalization, pregnancy outcomes
In the United States, COVID-19 has resulted in poor maternal health and pregnancy outcomes, especially among certain race/ethnicity groups and in older women. Educational outreach efforts on infection risks and studies evaluating SARS-CoV-2 vaccines in pregnant women are urgently needed.
As of 15 July 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused >188 million confirmed cases of coronavirus disease 2019 (COVID-19) and >4 million COVID-19-attributed deaths worldwide [1]. The burden of COVID-19 has been particularly high in the United States; as of 15 July 2021, there have been >33 million COVID-19 cases resulting in >600 000 COVID-19-attributed deaths [1].
Pregnant women with COVID-19 may be at greater risk of pregnancy and maternal complications [2–4]. Physiologic changes to the immune and respiratory systems during pregnancy significantly increase the risk of more severe illness from respiratory infections [5–7]. Furthermore, increased oxygen consumption and edema of the respiratory mucosa during pregnancy [8] heighten susceptibility to severe respiratory pathogens. During the 2009 H1N1 influenza A virus pandemic, pregnancy increased the risk of admission for H1N1 influenza A almost 4-fold [9]. During the SARS-CoV and Middle Eastern Respiratory Syndrome outbreaks in 2003 and 2012, respectively, reports indicated that pregnant women with these coronavirus infections experienced poor maternal and fetal outcomes [10, 11]. Data concerning pregnancy outcomes and the ongoing COVID-19 pandemic are limited [12]; reviews are often based on case series and case studies, which may be inherently biased [13].
The objective of this retrospective database analysis was 2-fold: to examine the impact of COVID-19 on (1) worsening COVID-19 clinical progression and (2) inpatient pregnancy outcomes, specifically preterm delivery and stillbirth, among pregnant women and women who delivered in the United States by age and race/ethnic categories.
METHODS
Data Source
This retrospective study used the Premier Healthcare Database ([PHD] Charlotte, NC) and PHD Special Release (PHD-SR) specially designed for COVID-19 research [14, 15]. The PHD is a US hospital-based, service-level, all-payer database containing administrative data on healthcare encounters at nonprofit, nongovernmental community and teaching hospitals, and health systems from rural and urban areas in all 9 US Census divisions; the data lag between discharge and inclusion in PHD is approximately 6 months. The PHD-SR contains data from a subset of the facilities included in the PHD that submit data on a monthly or more frequent basis with an approximate data lag time of 1 to 3 weeks from date of discharge. Data were extracted from the PHD for 2019 and PHD-SR for 2020. Institutional review board approval was not required for this study because the PHD is deidentified and Health Insurance Portability and Accountability Act compliant.
Study Population
The study included pregnant women aged 15–44 years who were hospitalized and who delivered between April and November 2019 and 2020. Hospitalizations involving pregnancy in the previous year (ie, April to November 2019) were included to allow comparison of pregnant women without COVID-19 in 2020 versus 2019. Pregnancy status was ascertained using diagnosis and procedure codes from MacDonald et al [16]; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were converted to the Tenth Revision (ICD-10-CM) by a professional medical coder. Coronavirus disease 2019 infection was ascertained using ICD-10-CM diagnosis code U07.1, which became effective on April 1, 2020, and indicates laboratory-confirmed infection with SARS-CoV-2 [17, 18]. For patients with COVID-19, the first hospitalization with evidence of both pregnancy and COVID-19 was retained for analysis. For patients without COVID-19, the first hospitalization involving pregnancy was retained for analysis. For evaluation of pregnancy outcomes (ie, preterm delivery and stillbirth), a subcohort was created and limited to women with evidence of livebirth or stillbirth during hospitalization.
Clinical and Pregnancy Outcomes
Coronavirus disease 2019 clinical progression outcomes included the following: use of supplemental oxygen therapy; intensive care unit (ICU) admission; critical illness, defined as a composite outcome of any of respiratory failure (including acute respiratory distress syndrome), shock, or multiorgan dysfunction syndrome; receipt of invasive mechanical ventilation/extracorporeal membrane oxygenation (ECMO); and maternal death [19]. Coronavirus disease 2019 outcomes were examined among all pregnant women and additionally among women who delivered. Definitions of COVID-19-related outcomes used code lists specified in the FDA Sentinel COVID-19 Master Protocol [20] and a search of standard chargemaster codes. Preterm delivery was identified using ICD-10-CM codes O60.10-O60.14 and O42.919 [21]. Identification of stillbirth used diagnosis and procedure codes from MacDonald et al [16], including ICD-9-CM codes converted to ICD-10-CM.
Statistical Analysis
P values were calculated for (1) categorical variables using χ 2 tests and (2) continuous variables using 2-sample t tests (Welch-Satterthwaite for unequal variances, pooled t test for equal variances). Risk ratios (RRs) and 95% confidence intervals (CIs) using log-normal distribution were calculated using the PROC FREQ procedure in SAS (v9.4; SAS Institute Inc., Cary, NC) to evaluate the impact of COVID-19 on clinical disease progression and pregnancy outcomes among hospitalized pregnant women overall, by age group (15–24, 25–34, and 35–44 years), race (Asian, Black, White), and ethnicity (Hispanic and non-Hispanic). Race and ethnicity were collected via self-report, and individuals identifying as >1 race/ethnicity were counted as “Other.” Due to the large sample sizes, statistical significance observed between values with similar magnitude may have limited practical significance.
RESULTS
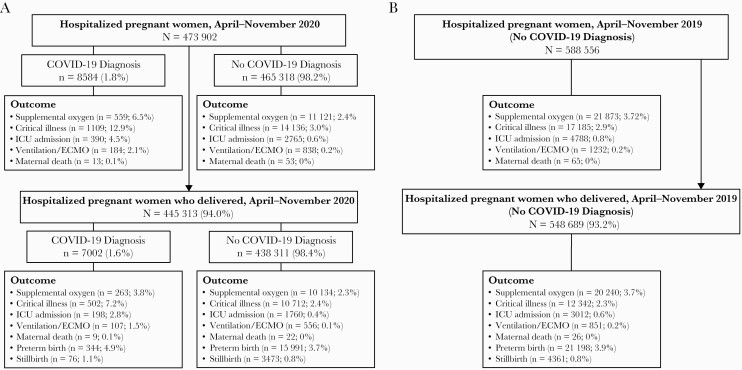
Among 473 902 pregnant women who were hospitalized between April and November 2020 in 720 hospitals, 8584 (1.8%) had a COVID-19 diagnosis (Figure 1). Mean age was similar between patients with and without COVID-19 (28.4 vs 29.1 years, respectively), and patients with COVID-19 were more likely to be Hispanic (Table 1). Overall, 445 313 pregnant women were included in the 2020 pregnancy outcomes analysis. The most common primary diagnoses among pregnant women who were hospitalized in 2020 but did not have evidence of a livebirth or stillbirth during the hospitalization (n = 28 362) were (1) third trimester preterm labor without delivery (8.8%; ICD-10 O60.03), (2) hyperemesis gravidarum with metabolic disturbance (3.3%; ICD-10 O21.1), and (3) missed abortion (3.1%; ICD-10 O02.1). The distribution of age, race, and ethnicity (Table 1) and clinical and pregnancy outcomes (Figure 1) was similar between patients without COVID-19 in 2020 compared with the historical control group comprising pregnant women without COVID-19 from 2019. Among pregnant women and women who delivered, a higher percentage of women with COVID-19 diagnoses had poor clinical (0.1%–12.9%) and pregnancy outcomes (1.1%–4.9%) compared with pregnant women without a COVID-19 diagnosis (clinical outcomes: 0%–3.0%; pregnancy outcomes: 0.8%–3.7%) in 2020 (Figure 1).
Figure 1.
Clinical outcomes for hospitalized pregnant women and women who delivered, April–November 2020 (A) and 2019 (B). COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Table 1.
Demographics for Hospitalized Pregnant Women Aged 15–44 Years April to November 2019 and 2020
| April–November 2020 | April–November 2019 | |||||
|---|---|---|---|---|---|---|
| Demographic | Total | COVID-19 | No COVID-19 | COVID-19 vs No COVID-19 | Total | No COVID-19 in 2020 vs Total in 2019 |
| N = 473 902 | n = 8584 | n = 465 318 | P Value | N = 588 556 | P Value | |
| Mean age (SD), y | 29.1 (5.7) | 28.4 (6.1) | 29.1 (5.7) | <.0001 | 28.9 (5.7) | <.0001 |
| Age group, y, n (%) | ||||||
| 15–24 | 110 985 (23.4) | 2492 (29.0) | 108 493 (23.3) | <.0001 | 142 135 (24.1) | <.0001 |
| 25–34 | 275 890 (58.2) | 4561 (53.1) | 271 329 (58.3) | 341 025 (57.9) | ||
| 35–44 | 87 027 (18.4) | 1531 (17.8) | 85 496 (18.4) | 105 396 (17.9) | ||
| Race/ethnicity, n (%) | ||||||
| Hispanic | 80 095 (16.9) | 3437 (40.0) | 76 658 (16.5) | <.0001 | 95 223 (16.2) | <.0001 |
| Asian non-Hispanic | 17 353 (3.7) | 222 (2.6) | 17 131 (3.7) | <.0001 | 20 184 (3.4) | <.0001 |
| Black non-Hispanic | 62 973 (13.3) | 1375 (16.0) | 61 598 (13.2) | <.0001 | 78 504 (13.3) | .13 |
| White non-Hispanic | 206 452 (43.6) | 1769 (20.6) | 204 683 (44.0) | <.0001 | 248 948 (42.3) | <.0001 |
| Other non-Hispanica | 20 581 (4.3) | 327 (3.8) | 20 254 (4.4) | .01 | 27 073 (4.6) | <.0001 |
| Race or ethnicity unknown | 97 337 (20.5) | 1956 (22.8) | 95 381 (20.5) | <.0001 | 126 550 (21.5) | <.0001 |
Abbreviations: COVID-19, coronavirus disease 2019; SD, standard deviation; y, years.
aIncludes Native Hawaiian and Other Pacific Islander, American Indian and Alaska Native, and Some Other race.
Maternal Outcomes
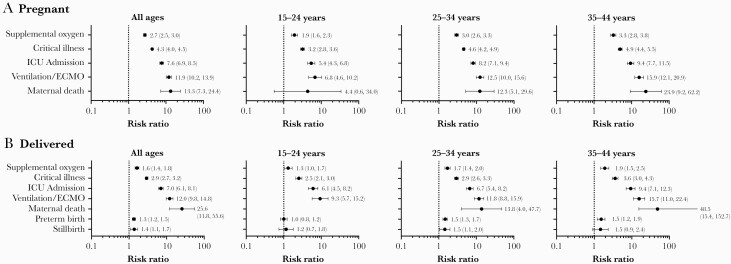
Pregnant women with COVID-19 and those who delivered were substantially more likely to need supplemental oxygen (pregnant: RR = 2.7 [95% CI, 2.5–3.0]; delivered: RR = 1.6 [95% CI, 1.4–1.8]), be critically ill (pregnant: RR = 4.3 [95% CI, 4.0–4.5]; delivered: RR = 2.9 [95% CI, 2.7–3.2]), be admitted to the ICU (pregnant: RR = 7.6 [95% CI, 6.9–8.5]; delivered: RR = 7.0 [95% CI, 6.1–8.1]), need invasive mechanical ventilation or ECMO (pregnant: RR = 11.9 [95% CI, 10.2–13.9]; delivered: RR = 12.0 [95% CI, 9.8–14.8]), or die (pregnant: RR = 13.3 [95% CI, 7.3–24.4]; delivered: RR = 25.6 [95% CI, 11.8–55.6]) compared with hospitalized pregnant women without COVID-19 (Figure 2). The risk of needing supplemental oxygen, critical illness, and ICU admission was greater among all pregnant women with COVID-19 versus when limited to women who delivered, whereas risk of maternal death was higher among women who delivered (Figure 2). The risk ratios for poor clinical outcomes increased with increasing age; similar trends with respect to poor clinical and maternal outcomes among pregnant women versus those who delivered were generally observed across age groups (Figure 2).
Figure 2.
Risk ratios (95% confidence intervals)* for outcomes among hospitalized pregnant women (A) and women who delivered (B) by age group (coronavirus disease 2019 [COVID-19] diagnosis vs no COVID-19 diagnosis) April to November 2020. *Risk ratios were not calculated for outcomes having a count of zero in either group (COVID-19 diagnosis vs no COVID-19 diagnosis). ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
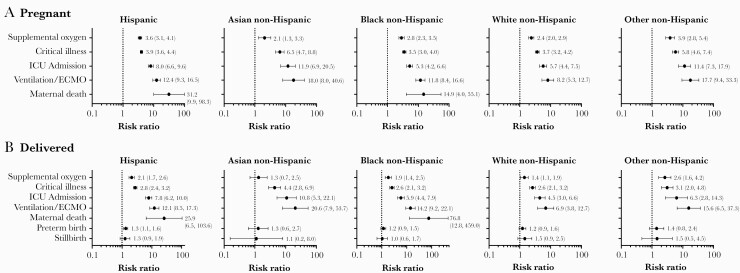
An analysis of maternal outcomes by race and ethnicity revealed that the relative risk of poor clinical outcomes varied by race and ethnicity. Pregnant Hispanic women and Other non-Hispanic women with COVID-19 had the highest RR for needing supplemental oxygen (RR = 3.6 [95% CI, 3.1–4.1] and 3.9 [95% CI, 2.8–5.4], respectively). Pregnant Hispanic women with COVID-19 had among the highest RR for (1) needing ventilation/ECMO (RR = 12.4; 95% CI, 9.3–16.5) and (2) maternal death (RR = 31.2; 95% CI, 9.9–98.3) compared with pregnant Hispanic women without COVID-19 (Figure 3A). Asian non-Hispanic pregnant women had the highest risk of being critically ill (RR = 6.5; 95% CI, 4.7–8.8) and admitted to the ICU (RR = 11.9; 95% CI, 6.9–20.5) compared with Asian non-Hispanic women without COVID-19 (Figure 3A). Among pregnant women with COVID-19 who delivered, Hispanic women had the highest risk of needing supplemental oxygen (RR = 2.1; 95% CI, 1.7–2.6) compared with Hispanic women without COVID-19 who delivered (Figure 3B); Asian non-Hispanic women had the highest risk of being critically ill (RR = 4.4; 95% CI, 2.8–6.9), being admitted to the ICU (RR = 10.8; 95% CI, 5.3–22.1), or needing mechanical ventilation/ECMO (RR = 20.6; 95% CI, 7.9–53.7). Finally, black non-Hispanic women with COVID-19 who delivered had a substantially greater risk of maternal death (RR = 76.8; 95% CI, 12.8–459.0) compared with Black non-Hispanic women without COVID-19 who delivered (Figure 3B).
Figure 3.
Risk ratios (95% confidence intervals)* for outcomes among hospitalized pregnant women (A) and women who delivered (B) by race (coronavirus disease 2019 [COVID-19] diagnosis vs no COVID-19 diagnosis) April to November 2020. *Risk ratios were not calculated for outcomes having a count of zero in either group (COVID-19 diagnosis vs no COVID-19 diagnosis). Other non-Hispanic includes Native Hawaiian and Other Pacific Islander, American Indian and Alaska Native, and Some Other race. ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Within individual race and ethnicity groups, the overall risk of needing supplemental oxygen and being critically ill was generally higher among all pregnant women versus when limited to women who delivered for all race and ethnicity groups (Figure 3). It is worth noting that Asian non-Hispanic and Black non-Hispanic women who delivered had higher risk of needing mechanical ventilation/ECMO versus all pregnant women. Furthermore, pregnant Hispanic women with COVID-19 and Black non-Hispanic women with COVID-19 who delivered were among those at greatest risk of maternal death (Figure 3). Overall, for all race and ethnicity groups, the risk ratios for poor clinical outcomes increased with age (Supplementary Figures 1–3).
Pregnancy Outcomes
Pregnant women with COVID-19 had a higher relative risk of preterm birth (RR = 1.3; 95% CI, 1.2–1.5) and stillbirth (RR = 1.4; 95% CI, 1.1–1.7), compared with pregnant women without COVID-19; the relative risk of preterm birth or stillbirth increased with age and was highest among women aged ≥25 years (Figure 2B). When pregnancy outcomes were assessed by race and ethnicity, only Hispanic women had evidence of a significant association between COVID-19 infection and increased risk of preterm birth (RR = 1.3; 95% CI, 1.1–1.6); no significant associations were observed for stillbirth within race and ethnicity subgroups (Figure 3B). When these data were further stratified by age group and race/ethnicity, COVID-19 was associated with increased risk of preterm birth among Hispanic women aged ≥25 years (25–34: RR = 1.4 [95% CI, 1.1–1.8]; 35–44: RR = 1.5 [95% CI, 1.0–2.1]) and with stillbirth among White non-Hispanic women aged 25–34 (RR = 2.0; 95% CI, 1.1–3.7) and Black non-Hispanic women aged 35–44 (RR = 2.6; 95% CI, 1.2–5.4) (Supplementary Figures 1–3). It is notable that consistent increases for the risk of preterm birth with increasing age were observed for Hispanic and Black non-Hispanic women with COVID-19 (Supplementary Figures 1–3).
DISCUSSION
To our knowledge, this is the largest study to date that investigates the impact of COVID-19 on pregnancy and maternal health in the United States. In this study, higher proportions of pregnant women with COVID-19 experienced poor clinical and pregnancy outcomes compared with pregnant women without COVID-19. The relative risk of poor clinical outcomes among pregnant women with COVID-19 largely depended on age; no uniform trends were observed with respect to poor clinical outcomes and race/ethnicity. Coronavirus disease 2019 infection was also associated with poor pregnancy outcomes (preterm birth and stillbirth); notably, Hispanic and Black non-Hispanic women experienced consistent increases in the risk of poor pregnancy outcomes with increasing age.
Coronavirus disease 2019 was associated with an overall ≥2-fold increased risk of needing supplemental oxygen, critical illness, ICU admission, receipt of invasive mechanical ventilation/ECMO, and death among pregnant women. In addition, older pregnant women had a higher risk of worse clinical outcomes. The findings of this study are consistent with a large nationwide survey (N = 406 446) that reported that pregnant women with COVID-19 were more likely to be admitted to the ICU, need mechanical ventilation/ECMO, and die compared with pregnant women without COVID-19 [22]. Another nationwide study (N = 461 825) reported that pregnant women with COVID-19 had up to 70% increased risk for death compared with nonpregnant women with COVID-19 [23]. The study also reported that pregnant women with COVID-19 aged 25–34 and 35–44 years were more frequently admitted to the ICU, required mechanical ventilation, and died, compared with nonpregnant women with COVID-19 aged 15–24 years [23]. Furthermore, pregnant women aged 35–44 years with COVID-19 had an approximate 4- and 2-fold greater likelihood to require mechanical ventilation and die, respectively, compared with nonpregnant women with COVID-19 [23]. These reports, combined with the data observed in this study, show that pregnant women with COVID-19 may be at greater risk of poor pregnancy and maternal outcomes, especially with increasing age.
During the early stages of the pandemic (March–May 2020), a study of 105 hospitalized pregnant women reported that the prevalence of stillbirths was 4-fold higher among women with SARS-CoV-2 infection compared with the baseline rate during the analysis period [24]. Likewise, the risk of adverse birth outcomes in pregnant women with COVID-19 in this study was found to be significantly higher compared with COVID-19 negative controls, with a 30% increased risk for preterm delivery and a 40% increased risk of stillbirth. Furthermore, although the risk of adverse birth events increased with increasing age, adverse birth events were not substantially higher among older women overall. Likewise, a long-term nationwide survey conducted by the US Centers for Disease Control and Prevention in pregnant women with COVID-19 between March and October 2020 found that among pregnant women with confirmed SARS-CoV-2 infection (N = 5252), the percentage of preterm live births in 2020 was only slightly higher among this population than in the general population in 2019 (12.9% vs 10.2%) [25], similar to the observations reported in this study. Similarly, a nationwide study conducted in pregnant women (N = 406 446) between April and November 2020 reported that COVID-19 only marginally increased the risk of preterm birth and stillbirth [22]. The low overall prevalence and risk of preterm birth and stillbirths among pregnant women with COVID-19 reported in this study may be a factor of the large and therefore more representative sample size and improved clinical management of COVID-19 during the latter half of 2020.
In this study, the risk of needing supplemental oxygen, critical illness, and ICU admission was greater among all pregnant women with COVID-19 versus when limited to women who delivered, whereas risk of maternal death was higher among women who delivered. Of note, hospitalized pregnant women included in this study likely encompassed all pregnancy trimesters, whereas those who delivered were presumably in their late second trimester or third trimester; in addition, most hospitalizations (94%) were for delivery. To our knowledge, this study is the first to show differences in clinical outcomes between pregnant women with COVID-19 and those who delivered during their hospitalization. The data from this study indicate that there may be differences in how COVID-19 affects women at different trimesters and that women earlier in their pregnancy may be at greater risk of negative outcomes compared with women whose pregnancies have progressed further. These data could be crucial to inform the ongoing care of pregnant women with COVID-19.
Stark racial disparities were observed in this study for both maternal and pregnancy outcomes. Among pregnant women with COVID-19, only Hispanic and Black non-Hispanic women experienced maternal death, and only Hispanic women had a significant association between COVID-19 and preterm delivery. These observations mirror those reported in other national surveillance studies. Studies have reported that Hispanic and Black non-Hispanic women are not only disproportionately represented among SARS-CoV-2-positive cohorts, they also face higher risk and rates of mortality from COVID-19 [23, 25, 26]. To our knowledge, this study is the first to report the risk of poor pregnancy outcomes stratified by age and race/ethnicity. The data show that the risk of preterm birth increases with increasing age among Hispanic and non-Hispanic Black women. A 2015–2017 survey reported racial and ethnic disparities in pregnancy outcomes in the United States with Black women facing twice the stillbirth rate compared with White women [27]. Furthermore, in certain regions of the United States, high stillbirth rates were observed among Hispanic women [27]. Many studies have reported that racial and ethnic minorities have been disproportionately affected by COVID-19 [28–31]. In addition, long-standing systemic and social inequities, such as (1) healthcare access and utilization; (2) occupation; (3) educational, income, and wealth gaps; and (4) housing, have put certain racial and ethnic minority groups at increased risk of infection with SARS-CoV-2 and associated illness and death [32]. Thus, there remains a critical need to address the potential reasons for poor pregnancy and maternal outcomes and to ensure pregnant women are better protected from COVID-19 and concomitant pregnancy complications.
This analysis used retrospective administrative data, which are not collected for research purposes and rely on the accuracy of self-reporting (eg, age, race/ethnicity) and coding of diagnoses and billable services; any reporting errors could introduce bias into the analysis. Information on geography was limited to a resolution of US Census region or division and was not considered in this analysis. Moreover, in the Premier Healthcare Database, patients can be tracked at the hospital level across the inpatient and hospital-based outpatient setting, but only in the same hospital. As a result, longitudinal information (ie, before admission, postdischarge) is not universally available, which prevents a comprehensive assessment of underlying medical conditions, prenatal care utilization, and previous history of adverse pregnancy outcomes. This study examined hospitalizations among pregnant women and did not examine telehealth or outpatient visits. Because almost all hospitalizations in this study involved childbirth, the results of this study are expected to be generalizable to hospital deliveries.
CONCLUSIONS
In conclusion, in this large US cohort of hospitalized pregnant women from April to November 2020, COVID-19 had a significant negative impact on maternal health and pregnancy outcomes. Understanding the severe risk of SARS-CoV-2 among pregnant women can help inform clinical practice. Pregnant women should be counseled regarding the risk of severe clinical and pregnancy outcomes associated with COVID-19 and on taking appropriate measures to prevent infection with SARS-CoV-2. In addition, in light of the disproportionate risk of poor outcomes faced by pregnant women of minority races and ethnicities, outreach efforts are needed to overcome existing barriers to healthcare access, thus ensuring that minority women are better protected from SARS-CoV-2. Finally, given the high risk of morbidity and mortality from COVID-19 among pregnant women, targeted clinical studies evaluating the safety and efficacy of vaccines against SARS-CoV-2 in pregnant women are urgently needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Editorial/medical writing support was provided by Drs. Sheena Hunt and Srividya Ramachandran (ICON plc, North Wales, PA).
Financial support. This work and the article processing charges were funded by Pfizer Inc.
Potential conflicts of interest. J. L. N., M. R., B. E., M. B., D. M., and J. H. are employees of Pfizer Inc and may hold stock or stock options. J. C. reports unrelated funding from a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (R01-HL150065). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 15 July 2021.
- 2. Lai PH, Lancet EA, Weiden MD, et al. . Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiology 2020; 5:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Narang K, Enninga EAL, Gunaratne MDSK, et al. . SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc 2020; 95:1750–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prabhu M, Cagino K, Matthews KC, et al. . Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 2020; 127:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramsey PS, Ramin KD. Pneumonia in pregnancy. Obstet Gynecol Clin North Am 2001; 28:553–69. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen SA, Kissin DM, Yeung LF, et al. . Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol 2011; 204:S13–20. [DOI] [PubMed] [Google Scholar]
- 7. Littauer EQ, Esser ES, Antao OQ, et al. . H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog 2017; 13:e1006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwaiberger D, Karcz M, Menk M, et al. . Respiratory failure and mechanical ventilation in the pregnant patient. Crit Care Clin 2016; 32:85–95. [DOI] [PubMed] [Google Scholar]
- 9. Jamieson DJ, Honein MA, Rasmussen SA, et al. ; Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451–8. [DOI] [PubMed] [Google Scholar]
- 10. Maxwell C, McGeer A, Tai KFY, Sermer M; Maternal Fetal Medicine Committee; Infectious Disease Committee. Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). J Obstet Gynaecol Can 2009; 31:358–64.19497157 [Google Scholar]
- 11. Di Mascio D, Khalil A, Saccone G, et al. . Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020; 2:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellos I, Pandita A, Panza R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2021; 256:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ang XL, Chonkar SP, Chua MSQ, et al. . Problems with early systematic reviews: the case of coronavirus disease 2019 (COVID-19) in pregnancy. Matern Child Health J 2020; 25:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premier Applied Sciences® PI. Premier healthcare database (COVID-19): data that informs and performs; April 10, 2020. Available at: http://offers.premierinc.com/rs/381-NBB-525/images/PHD_COVID-19_White_Paper.pdf. Accessed 13 January 2021.
- 15.Premier Applied Sciences® PI. Premier healthcare database: data that informs and performs; March 2, 2020. Available at: https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf. Accessed 23 February 2021.
- 16. MacDonald SC, Cohen JM, Panchaud A, et al. . Identifying pregnancies in insurance claims data: methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf 2019; 28:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. New ICD-10-CM code for the 2019 novel coronavirus (COVID-19). Available at: https://www.cdc.gov/nchs/data/icd/Announcement-New-ICD-code-for-coronavirus-3-18-2020.pdf. Accessed 4 November 2020.
- 18. World Health Organization. COVID-19 coding in ICD-10. Available at: https://www.who.int/classifications/icd/COVID-19-coding-icd10.pdf?ua=1. Accessed 4 November 2020.
- 19. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed 11 January 2021.
- 20. US Food and Drug Administration. Master protocol development: COVID-19 natural history. Available at: https://www.sentinelinitiative.org/methods-data-tools/methods/master-protocol-development-covid-19-natural-history. Accessed 16 November 2020.
- 21. Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Available at: https://icd10cmtool.cdc.gov/. Accessed 11 January 2021.
- 22. Jering KS, Claggett BL, Cunningham JW, et al. . Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med 2021; 181:714–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zambrano LD, Ellington S, Strid P, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panagiotakopoulos L, Myers TR, Gee J, et al. . SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics - Eight U.S. Health Care Centers, March 1-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodworth KR, Olsen EO, Neelam V, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delahoy MJ, Whitaker M, O’Halloran A, et al. ; COVID-NET Surveillance Team. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 States, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pruitt SM, Hoyert DL, Anderson KN, et al. . Racial and ethnic disparities in fetal deaths - United States, 2015-2017. MMWR Morb Mortal Wkly Rep 2020; 69:1277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stokes EK, Zambrano LD, Anderson KN, et al. . Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan D, Sze S, Minhas JS, et al. . The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine 2020; 23:100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med 2020; 382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention. COVID-19: health equity considerations and racial and ethnic minority groups. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Accessed 27 October 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.