Abstract
Background
Elucidating the relationship between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load and clinical outcomes is critical for understanding coronavirus disease 2019 (COVID-19).
Methods
The SARS-CoV-2 levels were analyzed by quantitative real-time polymerase chain reaction (RT-qPCR) of nasopharyngeal or oropharyngeal swab specimens collected at baseline, and clinical outcomes were recorded over 60 days from 1362 COVID-19 hospitalized patients enrolled in a multicenter, randomized, placebo-controlled phase 2/3 trial of sarilumab for COVID-19 (ClinicalTrials.gov NCT04315298).
Results
In post hoc analyses, higher baseline viral load, measured by both RT-qPCR cycle threshold and log10 copies/mL, was associated with greater supplemental oxygenation requirements and disease severity at study entry. Higher baseline viral load was associated with higher mortality, lower likelihood of improvement in clinical status and supplemental oxygenation requirements, and lower rates of hospital discharge. Viral load was not impacted by sarilumab treatment over time versus placebo.
Conclusions
These data support viral load as an important determinant of clinical outcomes in hospitalized patients with COVID-19 requiring supplemental oxygen or assisted ventilation.
Keywords: COVID-19, hospital, outcomes, severity, viral load
Post hoc analyses of a multicenter, randomized, placebo-controlled phase 2/3 trial of hospitalized COVID-19 patients demonstrated that high viral load is associated with higher mortality, lower likelihood of clinical improvement, and lower rates of hospital discharge.
Since the association of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with coronavirus disease 2019 (COVID-19) was established, studies have examined the kinetics and biological compartments of viral shedding across disease presentations [1–4]. Data demonstrate that the virus persists in the upper respiratory tract, particularly in the absence of an antiviral therapeutic [5]. Viral shedding may have different clinical associations between outpatient and hospital settings. Furthermore, viral persistence is associated with seroconversion status; patients seronegative for endogenous anti-SARS-CoV-2 antibodies have higher viral loads in nasopharyngeal specimens than seropositive patients [1, 6]. In hospitalized patients, higher SARS-CoV-2 viral load was associated with intubation risk and mortality [7, 8]. To explore the relationship between viral load and disease severity, baseline viral load, serology, supplemental oxygenation requirements, survival, and recovery were evaluated.
METHODS
Study Population
In this adaptive, phase 2/3, randomized, double-blind, placebo-controlled trial, subjects aged ≥18 years, hospitalized with laboratory-confirmed SARS-CoV-2 infection (within 2 weeks of study) and COVID-19 pneumonia requiring supplemental oxygen and/or assisted ventilation were treated between March and July 2020 with intravenous (IV) sarilumab or placebo (ClinicalTrials.gov NCT04315298) [9]. Local institutional review boards or ethics committees at each center oversaw trial conduct and documentation. All patients provided written informed consent.
Quantification of Severe Acute Respiratory Syndrome Coronavirus 2 Virus
Specimen collection included nasopharyngeal (N = 1047) and oropharyngeal swabs (N = 315). Baseline refers to predose collections poststudy randomization, required as part of the protocol but were missing in some randomized subjects. Subsequent testing was optional. Nucleic acid extraction and quantitative real-time polymerase chain reaction (RT-qPCR) was performed at Eurofins Viracor, Inc. laboratory (Lee’s Summit, MO). Details of the Emergency Use Authorization assay have been previously described [6]; “Not Detected” results were transformed to 1, “Detected <714” results were transformed to half the lower limit of quantification of the assay, and results greater than the upper limit of quantification (ULOQ) were transformed to the ULOQ before log10 transformation for analysis.
Serology Testing
See Supplementary Appendix for details.
Clinical Outcomes Definitions
For all outcomes, patients who died were censored at day 60; patients who were alive were censored at day 60 or last follow-up date, whichever was earlier.
Time to All-Cause Mortality
Time to all-cause mortality is the number of days to death (any cause) minus the first dose date + 1 (assumed that patients were alive on first dose date and alive on date of death until death).
Time to Clinical Status Improvement
Time to clinical status improvement is the number of days to achieve ≥1-point increase in clinical status + 1 using the 7-point ordinal scale [10]: 1, death; 2, hospitalized, requiring invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation; 3, hospitalized, requiring noninvasive ventilation or high-flow oxygen devices; 4, hospitalized, requiring supplemental oxygen; 5, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (any reason); 6, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; and 7, not hospitalized.
Time to Hospital Discharge
Time to hospital discharge is the date of discharge minus the first dose date + 1 day.
Time to Improvement in Oxygenation
Time to improvement in oxygenation is the number of days from first dose to first improvement in oxygenation (SpO2/FiO2 ratio ≥nadir + 50), lasting ≥48 hours or until discharge, whichever was sooner.
Post Hoc Statistical Analysis
Descriptive statistics grouped by disease severity are reported as mean (standard deviation) for normally distributed continuous variables, median (interquartile range [IQR]) for nonnormally distributed continuous variables, and frequency (%) for categorical variables. Baseline viral load across disease strata were compared using Kruskal-Wallis and post hoc Dunn tests. Wilcoxon tests were used to compare baseline viral load between 2 groups, and Spearman correlation was performed to assess relationships between baseline continuous demographic, clinical, laboratory, and virology variables. Survival analysis was performed for binary outcome variables with censored time-to-event information. Patients were grouped into viral load tertiles using baseline measurements. Hazard ratios (HRs) for middle and high viral load groups, relative to low, were calculated. Since all-cause mortality is a competing risk with other outcomes, subdistribution HR were calculated.
Covariates for all analyses included age, sex, race, ethnicity, baseline steroid use, duration of pneumonia prebaseline, body mass index (BMI), diabetes, and hypertension. Treatment arm was included as a covariate in all analyses of longitudinal outcomes. A Type-I error rate of α = 0.05 was used as the threshold for statistical significance, with Bonferroni adjustment for multiple comparisons. Analyses were conducted using R version 3.6.1.
RESULTS
Enrollment and Inclusion Criteria
The phase 2/3 study included 1912 randomized (not all treated) patients from 62 sites with COVID-19 pneumonia who either required supplemental oxygen, were admitted in the intensive care unit (ICU), were immunocompromised, or had evidence of multisystem organ dysfunction (MSOD). The post hoc analysis included 1362 patients (70% of randomized) with baseline virology measurements (after enrollment but before dosing) and disease strata at randomization. Disease strata included hospitalized patients who were receiving low-flow oxygen (severe), critically ill patients who were receiving high-flow oxygen (critical without IMV) or mechanical ventilation (critical with IMV), and patients with MSOD receiving extracorporeal life support, renal replacement therapy, or vasopressors (MSOD). The phase 3 immunocompromised stratum (n = 36) and patients enrolled in phase 3 cohorts receiving 800 mg of sarilumab or placebo (N = 78) were excluded (Supplementary Figure 1).
Demographics and Medical History
Baseline demographics and clinical variables were similar to the overall patient population (Table 1), except for BMI (higher in the MSOD stratum). A minority of patients (27%) were receiving concomitant corticosteroids at randomization.
Table 1.
Demographic Variables and Medical History by Disease Severitya
| Demographic and Medical History | Severe (n = 352) |
Critical Without IMV (n = 414) |
Critical With IMV (n = 334) |
MSOD (n = 262) |
|---|---|---|---|---|
| Demographic | ||||
| Sex | ||||
| Female | 131 (37.2) | 129 (31.2) | 101 (30.2) | 94 (35.9) |
| Male | 221 (62.8) | 285 (68.8) | 233 (69.8) | 168 (64.1) |
| Age, years (SD) | 60.5 (14.8) | 60.5 (14.5) | 58.1 (14.3) | 60.1 (13.2) |
| BMI, kg/m2 (SD) | 31.5 (7.7) | 31.3 (7.4) | 31.9 (7.8) | 33.2 (10.5) |
| Ethnicity, Hispanic or Latino | 66 (18.8) | 130 (31.4) | 109 (32.6) | 73 (27.9) |
| Race | ||||
| American Indian or Alaska Native | 2 (0.6) | 2 (0.5) | 1 (0.3) | 1 (0.4) |
| Asian | 29 (8.2) | 20 (4.8) | 21 (6.3) | 17 (6.5) |
| Black or African American | 72 (20.5) | 75 (18.1) | 56 (16.8) | 57 (21.8) |
| Native Hawaiian or Other Pacific Islander | 4 (1.1) | 1 (0.2) | 0 (0) | 0 (0) |
| White | 141 (40.1) | 144 (34.8) | 136 (40.7) | 70 (26.7) |
| Other | 41 (11.6) | 64 (15.5) | 34 (10.2) | 30 (11.5) |
| Not reported | 63 (17.9) | 108 (26.1) | 86 (25.7) | 87 (33.2) |
| Medical History | ||||
| Days between diagnosis and study enrollment (IQR) | 3 (1–5) | 3 (1–6) | 4 (2–7) | 4 (2–7) |
| Duration of pneumonia before enrollment, days (IQR) | 8 (4–11) | 9 (5–12) | 8 (5–12) | 8 (5–12) |
| Duration of hospitalization before enrollment (days) (IQR) | 3 (2–5) | 4 (3–6) | 5 (3–8) | 5 (3–8) |
| Number of patients admitted to ICU before enrollment | 15 (4.3) | 30 (7.2) | 107 (32.0) | 80 (30.5) |
| Fever | 152 (43.2) | 164 (39.6) | 202 (60.5) | 149 (56.9) |
| Obesity | 164 (46.6) | 184 (44.4) | 163 (48.8) | 134 (51.1) |
| Hypertension | 182 (51.7) | 219 (52.9) | 147 (44.0) | 145 (55.3) |
| Diabetes | 70 (19.9) | 77 (18.6) | 57 (17.1) | 60 (22.9) |
| Corticosteroid use | 74 (21.0) | 147 (35.5) | 78 (23.4) | 67 (25.6) |
| Vasopressor use | 13 (3.7) | 6 (1.4) | 90 (26.9) | 180 (68.7) |
| Immunocompromised | 10 (2.8) | 6 (1.4) | 12 (3.5) | 11 (4.2) |
Abbreviations: BMI, body mass index; ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range; MSOD, multisystem organ dysfunction; SD, standard deviation.
aData are presented as n (%), mean (SD), or median (Q1–Q3). Demographic and medical history information from patients included in the analysis.
The median duration of COVID-19 pneumonia symptom before baseline was 8 days (IQR, 5–12), median time between positive diagnosis and enrollment was 3 days (IQR, 2–6), and median duration of hospitalization was 4 days (IQR, 3–7). Medical history variables were well matched except for admission to ICU, baseline fever, obesity, and vasopressor use (Table 1). Within the critical stratum, IMV patients had more vasopressor use (27% vs 1%) and fever (61% vs 40%) at baseline versus those not on IMV. More patients with MSOD were on vasopressors at baseline versus other disease strata.
Association Between Viral Load and Disease Characteristics
Baseline Virology and Clinical Characteristics
Information regarding baseline viral load, serology, oxygen device, clinical outcomes, and symptom duration is summarized in Table 2.
Table 2.
Virological and Clinical Outcomes Grouped by Baseline Disease Severity Strata
| Virological and Clinical Outcomes | Severe (n = 352) |
Critical Without IMV (n = 414) |
Critical With IMV (n = 334) |
MSOD (n = 262) |
|---|---|---|---|---|
| Baseline Virology | ||||
| Log10 copies/mL (IQR) | 3.75 (2.55–5.11) | 3.72 (2.55–5.09) | 4.58 (3.33–5.97) | 5.01 (3.76–6.17) |
| Above ULOQa | 8 (2.3) | 4 (1.0) | 15 (4.5) | 15 (5.7) |
| Below LLOQb | 47 (13.4) | 41 (9.9) | 24 (7.2) | 13 (5.0) |
| Not detected | 60 (17.0) | 91 (22.0) | 35 (10.5) | 22 (8.4) |
| Baseline SARS-COV-2 Serology (N = 384) | ||||
| Positive N/n (%) | 88/100 (88.0) | 103/109 (94.5) | 70/77 (90.9) | 89/98 (90.8) |
| Serology index | 66 (8–153) | 90 (20–170) | 86 (24–160) | 68 (18–138) |
| Baseline Oxygen Device | ||||
| Nonec | 4 (1.6) | 2 (0.5) | – | 1 (0.4) |
| Nasal cannula | 265 (75.3) | 36 (8.7) | – | 5 (1.9) |
| Simple face mask | 18 (5.1) | 13 (3.1) | – | 0 (0) |
| Non-rebreather facemask | 45 (12.8) | 170 (41.1) | – | 1 (0.4) |
| High-flow nasal cannula | 11 (3.1) | 160 (38.6) | – | 2 (0.8) |
| Noninvasive ventilation | 1 (0.3) | 29 (7.0) | – | 2 (0.8) |
| IMV | 7 (2.0) | – | 334 (100) | 229 (87.4) |
| Extracorporeal life support | 1 (0.3) | 4 (1.0) | – | 22 (8.4) |
| Clinical Outcomes | ||||
| All-cause mortality at day 60 | 39 (11.1) | 118 (28.5) | 135 (40.4) | 107 (40.8) |
| Clinical status improvement (≥1 point) at day 29 | 294 (83.5) | 261 (63.0) | 166 (49.7) | 118 (45.0) |
| Improvement in oxygenation at day 29 | 219 (62.2) | 263 (63.5) | 181 (54.2) | 139 (53.1) |
| Hospital discharge at day 29 | 291 (82.7) | 246 (59.4) | 121 (36.2) | 71 (27.1) |
| Symptom Duration (Days Post randomization) | ||||
| Fever (IQR) | 1 (1–2) | 1 (1–3) | 5 (2–10) | 5 (2–11) |
| Tachypnoea (IQR) | 2 (1–5) | 3 (1–8) | 7 (3–12) | 10 (4–16) |
| Hypoxemia (IQR) | 6 (4–10) | 11 (7–20) | 21 (13–30) | 27 (15–35) |
| Supplemental oxygen (IQR) | 6 (3–9) | 11 (7–20) | 20 (13–29) | 26 (14–34) |
Abbreviations: IMV, invasive mechanical ventilation; IQR, interquartile range; LLOQ, lower limit of quantification; MSOD, multisystem organ dysfunction; SARS-COV-2, severe acute respiratory syndrome coronavirus 2; ULOQ, upper limit of quantification.
NOTES: Data are presented as n (%) or median (Q1–Q3), except for serology index (SI), which is median (range). For longitudinal clinical outcomes, all-cause mortality was assessed at day 60, and clinical status improvement, improvement in oxygenation, and hospital discharge were assessed at day 29. For symptom duration, the data shown are for survivors only. Serology index values ≥1.1 were considered positive, SI values ≤0.8 were considered negative, SI values of 0.9 and 1.0 were considered borderline.
aULOQ corresponds to 7.1 × 107 copies/mL or 7.85 log10 copies/mL.
bLLOQ corresponds to 714 copies/mL or 1.85 log10 copies/mL.
cAll but 1 subject who enrolled into the study without supplemental oxygen requirements at randomization but who received supplemental oxygen by day 1 (baseline).
Supplemental oxygen devices varied across disease strata as defined in the protocol-based randomization. Patients in the severe stratum had low oxygen requirements, and a simple oxygen face mask was the predominant device (75%). Patients in the critical stratum not on IMV received oxygen primarily by a non-rebreather face mask (41%) and high-flow nasal cannula (39%). Most patients with MSOD were on IMV (87%). Mortality was higher in critical patients on IMV and patients with MSOD, consistent with the primary study results.
Differences in clinical outcomes and symptom duration were observed across disease severity (Table 2). Our study cohort overall had 29% mortality and 42% of patients on IMV at baseline, suggesting a critically ill population. Greater disease severity in critical patients on IMV and patients with MSOD was associated with higher rates of all-cause mortality and lower rates of clinical status improvement ≥1 point, improvement in oxygenation, and hospital discharge. Among survivors, greater disease severity was also associated with more days with symptoms, including fever, tachypnoea, hypoxemia, and requiring supplemental oxygen.
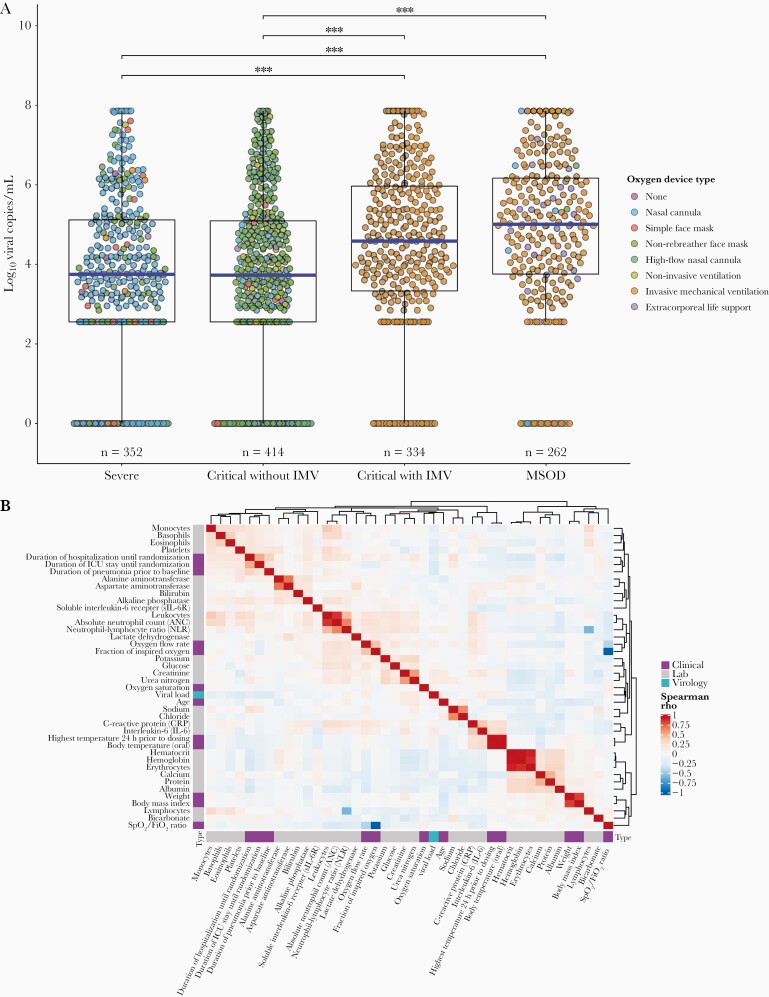
Of 1362 patients analyzed, 208 (15%) had undetectable viral levels at baseline. Baseline viral load was significantly higher in critical patients on IMV and patients with MSOD, versus severe patients or critical patients not on IMV (Figure 1A). Seropositivity rates (qualitative) and serology index ([SI] quantitative) did not differ between disease strata (Table 2).
Figure 1.
Boxplot of baseline viral load grouped by disease severity* (A) and correlation matrix of baseline virology, clinical, and laboratory values** (B). *Each point represents an individual patient’s log10 viral copies/mL at baseline, and the corresponding color depicts the type of oxygen delivery device. The horizontal blue lines represent each group’s median viral load. Significant Dunn pairwise comparisons are shown above the boxplots; ***, P < .001. **, Spearman correlation matrix of all continuous baseline clinical, virological (quantitative polymerase chain reaction log10 copies/mL), and laboratory values with data collected on ≥50% of subjects in this study. Spearman correlations range from –1 to 1, with 1 indicating a perfect positive correlation and –1 indicating a perfect negative correlation. Variables are clustered together based on Spearman rho value using unsupervised hierarchical clustering with Euclidian distances. IMV, invasive mechanical ventilation; MSOD, multisystem organ dysfunction.
Baseline viral load was significantly higher in patients aged ≥60 years (4.42 log10 copies/mL; IQR, 3.04–6.04) versus patients aged <60 years (3.93 log10 copies/mL; IQR, 2.55–5.28; P < .001). Viral loads were similar across other variables, including sex, obesity, diabetes, and hypertension status.
See Supplementary Appendix and Supplementary Table 1 for results on relationships between viral, clinical, and laboratory measures and cytokine and inflammatory marker profiling.
Association Between Baseline Viral Load and Clinical Outcomes
To assess whether baseline viral load was predictive of longitudinal outcomes (all-cause mortality, clinical score improvement ≥1 point, improvement in oxygenation, and hospital discharge), we analyzed the contribution of treatment allocation and found that it did not result in significantly different rates of these outcomes. Therefore, we combined all subjects and grouped into tertiles to assess the prognostic value of baseline viral load. In addition, 384 patients were selected randomly from the low and high tertiles and had serostatus and SI evaluated. Seropositivity significantly varied between the low (98%) and high (85%) tertiles. The SI in the low tertile (128; IQR, 54–193) was significantly higher versus the high tertile (28; IQR, 3–89) (P < .001) (Supplemental Figure 2).
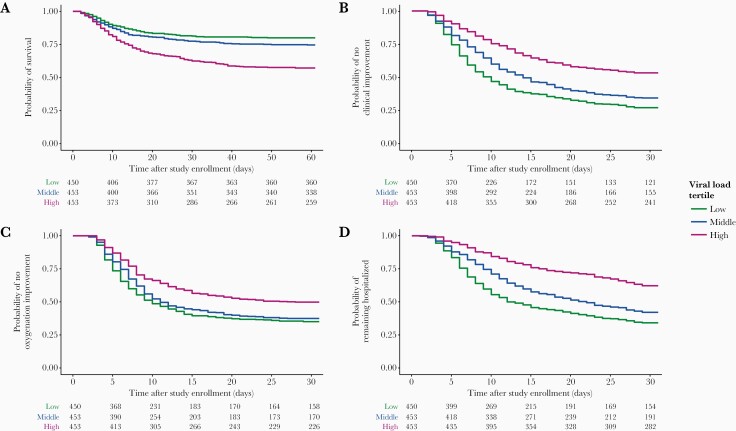
Survival analysis was also performed for outcome variables with censored time-to-event information. Cumulative incidence plots are shown in Figure 2, and event rates and HR are presented in Table 3. The results of the analysis described below were not substantially different if subjects with undetectable baseline viral load (N = 208) were excluded from the analysis.
Figure 2.
Survival curves grouped by viral load tertiles for probability of survival (A), clinical improvement (1 point) (B), improvement in oxygen requirements (C), and hospitalization (D). Viral load tertiles were defined as follows: low (<3.32 log10 copies/mL), middle (3.32–5.09 log10 copies/mL), and high (>5.09 log10 copies/mL). Tables of number of patients at risk at particular timepoints after baseline are shown below each plot.
Table 3.
Event Rates and Survival Analysis Results for Longitudinal Clinical Outcomes
| Event Rates and Survival Analysis | Low Viral Load (<3.32 log10 copies/mL) |
Middle Viral Load (3.32–5.09 log10 copies/mL) |
High Viral Load (>5.09 log10 copies/mL) |
|---|---|---|---|
| Participantsa | 450 | 453 | 453 |
| All-Cause Mortality | |||
| Events | 90 (20.0) | 115 (25.4) | 194 (42.8) |
| Person-days | 22 932 | 21 966 | 18 732 |
| Event ratea,b | 3.92 (3.17–4.80) | 5.24 (4.34–6.26) | 10.36 (8.98–11.89) |
| Unadjusted HR | 1 (ref) | 1.31 (0.99–1.73) | 2.42 (1.89–3.11) |
| Adjusted HR | 1 (ref) | 1.22 (0.92–1.65) | 2.13 (1.62–2.82) |
| Clinical Status Improvement | |||
| Events | 329 (73.1) | 298 (65.8) | 212 (46.8) |
| Person-days | 6420 | 7497 | 9363 |
| Event ratec | 5.12 (4.59–5.70) | 3.97 (3.54–4.45) | 2.26 (1.98–2.59) |
| Unadjusted sHR | 1 (ref) | 0.78 (0.66–0.90) | 0.46 (0.39–0.54) |
| Adjusted sHR | 1 (ref) | 0.80 (0.68–0.94) | 0.47 (0.39–0.56) |
| Improvement in Oxygenation | |||
| Events | 292 (64.9) | 283 (62.5) | 227 (50.1) |
| Person-days | 6835 | 7287 | 8645 |
| Event ratec | 4.27 (3.80–4.78) | 3.88 (3.45–4.36) | 2.63 (2.30–2.99) |
| Unadjusted sHR | 1 (ref) | 0.90 (0.77–1.05) | 0.64 (0.54–0.75) |
| Adjusted sHR | 1 (ref) | 0.92 (0.78–1.10) | 0.65 (0.54–0.79) |
| Hospital Discharge | |||
| Events | 296 (65.8) | 262 (57.8) | 171 (37.7) |
| Person-days | 7433 | 8652 | 10 562 |
| Event ratec | 3.98 (3.55–4.46) | 3.03 (2.68–3.41) | 1.62 (1.39–1.88) |
| Unadjusted sHR | 1 (ref) | 0.76 (0.64–0.89) | 0.41 (0.34–0.50) |
| Adjusted sHR | 1 (ref) | 0.76 (0.64–0.91) | 0.40 (0.33–0.50) |
Abbreviations: HR, hazard ratio; ref, reference; sHR, subdistribution HR.
NOTES: Data are presented as n (%), event rates (95% confidence interval [CI]), or HRs (95% CI). The sample was split into equal tertiles using baseline viral copies/mL. Survival analysis was performed for longitudinal outcomes in middle and high viral load groups relative to the low viral load group. Since all-cause mortality is a competing risk with the other 3 outcomes, sHRs were calculated. The variables in the covariate-adjusted models include age, sex, race, ethnicity, steroid use, duration of pneumonia before baseline, body mass index, diabetes, hypertension, and treatment arm.
aNumber of patients in each tertile is 454; missing clinical data for low viral load (n = 4), middle viral load (n = 1), and high viral load (n = 1).
bPer 1000 person-days.
cPer 100 person-days.
All-Cause Mortality
Four hundred and one patients (29%) died by day 60. Patients who died had 15 times greater baseline viral load (5.04 log10 copies/mL; IQR, 3.57–6.40) n patients who survived (3.87 log10 copies/mL; IQR, 2.55–5.19; P < .001) (Supplementary Figure 3). Patients with high viral load experienced significantly greater mortality rates (HR = 2.42; 95% confidence interval [CI], 1.89–3.11; P < .001) (Figure 2A). By day 60, mortality in the low, middle, and high viral load groups were 20%, 25%, and 43%, respectively. A 10-fold (+1 log10 copies/mL) greater viral load at baseline was associated with 22% increased odds of all-cause mortality. The SI was not significantly different between patients who survived or died (P = .57), nor among the high tertile patients who survived (median 23; IQR, 3–6) versus patients who died (median 39; IQR, 5–111) (P = .52). Higher SI was consistently associated with worse clinical outcomes among patients with low viral load. In the low viral load tertile, the SI was higher in patients who died (median 150; IQR, 104–238) versus survivors (median 112; IQR, 47–172) (P = .01).
Clinical Status Improvement ≥1 Point
Since prolonged hospitalization in patients with COVID-19 could be associated with numerous factors, we explored whether baseline viral load impacted longitudinal supplemental oxygen requirements as reflected in an improvement in clinical status (Figure 2B). Patients who achieved ≥1-point clinical status improvement (3.80 log10 copies/mL; IQR, 2.55–5.11) had approximately 15-fold lower baseline viral load versus patients who did not achieve clinical status improvement (4.97 log10 copies/mL; IQR, 3.49–6.20). A 10-fold (+1 log10 copies/mL) greater viral load at baseline was associated with 22% decreased odds of 1-point clinical status improvement (P < .001) (Supplementary Figure 3). In addition, we observed an association between baseline oxygenation status and ≥1-point clinical status improvement (Supplementary Figure 4).
Improvement in Oxygenation
Patients with high viral load showed lower rates of oxygenation improvement versus low viral load patients (HR = 0.64; 95% CI, 0.54–0.75; P < .001). Patients in the middle tertile did not experience significantly lower rates (HR = .90; 95% CI, 0.77–1.05; P = .19) (Figure 2C).
Hospital Discharge
Seven hundred and twenty-nine patients (54%) were discharged, but rates were much lower in IMV (31%) versus non-IMV patients (71%) (Figure 2D). Compared with the low viral load tertile, patients in both the middle (HR = 0.76; 95% CI, 0.64–0.89; P = .002) and high tertiles (HR = 0.41; 95% CI, 0.34–0.50; P < .001) were less likely to be discharged. By day 29 in the study, just 38% of patients in the high tertile had been discharged versus 58% in the middle and 68% in the low tertiles. A 10-fold (+1 log10 copies/mL) greater viral load at baseline was associated with 23% decreased odds of hospital discharge.
Longitudinal Changes in Viral Load and Clinical Outcomes
Longitudinal SARS-CoV-2 virology data were available for a limited subset of subjects (Supplementary Figure 5, Supplementary Tables 2 and 3). No significant differences in change in viral load from baseline were observed between treatment arms on days 4 and 7. These findings are consistent with sarilumab not having a direct antiviral mechanism. Absolute levels and changes in of viral load at days 4 and 7 were predictive of all clinical outcomes assessed (Supplemental Table 4).
DISCUSSION
The relationship between SARS-CoV-2 viral load in hospitalized patients with COVID-19 and outcomes remains a key focus of clinical research. Early studies focused on the development of robust assays that could detect viral transcripts in clinical specimens to accurately diagnose infection. Additional studies characterized the time course of viral shedding in various biological specimens to understand persistence and to guide public health measures. Subsequent studies evaluated the relationship of seroconversion to symptoms and to viral persistence [1]. Viral persistence is still observed in individuals who generate anti-SARS-CoV-2 antibodies; however, serum antibody-positive individuals in the outpatient setting had lower viral load than serum antibody-negative patients [6].
In hospitalized patients with COVID-19, significant efforts have been made to understand clinical and laboratory predictors of outcomes, such as requirements for ventilation and mortality. One of the first studies to demonstrate the association of viral load and risk of intubation and mortality was in a cohort of 678 patients at 2 centers in New York City [8]. Retrospective subgrouping by viral cycle threshold (Ct) values determined upon hospital admission were similar to our study and demonstrated that patients with Ct < 25 (high viral load) had 35% mortality versus patients with medium (17.6%) or low (6.2%) viral load. Subsequent analysis corroborated the association of higher viral load and in-hospital mortality in patients with and without cancer [7]. In contrast to these studies, a study of 205 subjects concluded that viral load was not associated with requirements for oxygen or overall survival [11]. Thus, there still exists a clear need to study these same questions in data collected in a multicenter study.
Viral load in our study was determined at baseline (~1–7 days after diagnosis and 4–12 days after pneumonia). A small subset had undetectable virus at trial initiation, which could be due to levels below the lower limit of detection, false negatives, virus clearance before randomization, or virus persistence in different biological compartments that were not sampled. These patients still required hospitalization and supplemental oxygen. High baseline viral load was associated with greater disease severity at randomization and was highest in the patients on mechanical ventilation and those requiring extracorporeal life support. Patients with the highest viral loads were less likely to reduce oxygen support, less likely to be discharged, and more likely to die from COVID-19. Some of the aspects of our study include the variability in duration of illness and confirmation of SARS-CoV-2 before enrollment. In contrast to prior cohorts, our study cohort had 43%, 25%, and 20% in the high, middle, and low viral load subgroups, respectively. This suggests our cohort may have been sicker even though the timeframe of the study enrollments were similar.
Most of the patient subsets tested for serology were seropositive for anti-nucleocapsid antibodies at baseline. The SI at baseline was positively correlated with duration of pneumonia before baseline, which ranged from 0 to 41 days (median 9 days). In patients enrolled closer to symptom onset, SI and seroconversion rates were significantly lower in patients with high viral load. This study did not evaluate neutralizing antibodies, which limits the interpretation of the serology status in this cohort, but the high viral load in hospitalized patients despite a seropositivity rate of > 80% suggests these antibodies are inadequate to control viral replication in this patient population.
Limited longitudinal assessments were conducted to support conclusions about viral persistence and evaluate the contribution of anti-interleukin-6R blockade with sarilumab on viral load over time. These data confirm that sarilumab did not have a direct antiviral effect. Change in viral load at days 4 and 7 was predictive of clinical outcomes, with greater viral reductions observed in patients who survived and improved clinically.
There were several limitations in this study. Only 71% of randomized patients had available viral load for this analysis; however, baseline characteristics were similar between the overall study population and the subgroup included here. Serology testing was not available on all patients with virology; therefore, correlating serology status and viral load and clinical outcomes was challenging. However, most patients were serum antibody positive, despite having high viral loads and severe COVID-19, suggesting that serology testing may not be an ideal prognostic marker for disease progression. In addition, our study did not evaluate the viral variants with which patients were infected between March and July 2020 enrolled in this study. Despite this, our study encompasses centralized viral load measurements and standardized collection of clinical outcomes from a large multicenter trial providing a robust dataset to better understand the relationship between viral load and COVID-19 progression.
CONCLUSIONS
These analyses demonstrated that baseline viral load may be an important determinant of clinical outcomes in hospitalized patients with COVID-19. In a recent study, a phase 3 trial with REGEN-COV, a monoclonal antibody cocktail for the treatment of high-risk outpatients with COVID-19, demonstrated a significant reduction in viral load and COVID-19-related hospitalizations and death versus placebo, further supporting viral load in COVID-19 disease progression.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the patients, their families, and investigational site members. We also thank Georgia Bellingham and Lisa Boersma for operational support for virology testing.
Author contributions. A. B., M. F. W., P. J. E., and S. H. contributed to the study design, analysis plan, and implementation of the research. A. B., M. F. W., and P. J. E. authored the manuscript. A. B., E. K., and J. J. F. contributed to sample preparation and laboratory testing. A. B., S. S., D. J. L., A. W., and R. B. contributed to the primary data acquisition and data cleaning. All authors participated in data analysis and interpretation as well as manuscript review and editing. P. J. E., A. W., and S. H. had access to all data and verified the data and statistical analysis.
Data Sharing.
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the indication has been approved by a regulatory body, if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.
Financial support. This study was funded by Regeneron Pharmaceuticals, Inc. and Sanofi. Certain aspects of this project have been funded in whole or in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Other Transactions number HHSO100201700020C. Manuscript support was provided by Prime Global.
Potential conflicts of interests. All authors are employees or employees and shareholders of Regeneron Pharmaceuticals, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 2. Zheng S, Fan J, Yu F, et al. . Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020; 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fajnzylber J, Regan J, Coxen K, et al. ; Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zacharioudakis IM, Prasad PJ, Zervou FN, et al. . Association of SARS-CoV-2 genomic load with outcomes in patients with COVID-19. Ann Am Thorac Soc 2021; 18:900–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Zhang D, Du G, et al. . Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westblade LF, Brar G, Pinheiro LC, et al. . SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020; 38:661–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magleby R, Westblade LF, Trzebucki A, et al. . Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2020:ciaa851. doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sivapalasingam S, Lederer DJ, Bhore R, et al. . A randomized placebo-controlled trial of sarilumab in hospitalized patients with Covid-19 [preprint ]. medRxiv 2021:2021.05.13.21256973. doi: 10.1101/2021.05.13.21256973. [DOI] [Google Scholar]
- 10. Peterson RL, Vock DM, Powers JH, et al. ; INSIGHT FLU-IVIG Study Group. Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials 2017; 14:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Argyropoulos KV, Serrano A, Hu J, et al. . Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol 2020; 190:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.