Abstract
Background
Seroprevalence studies are essential to understand the epidemiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Various technologies, including laboratory assays and point-of-care self-tests, are available for antibody testing. The interpretation of seroprevalence studies requires comparative data on the performance of antibody tests.
Methods
In June 2020, current and former members of the United Kingdom police forces and fire service performed a self-test lateral flow immunoassay (LFIA), had a nurse-performed LFIA, and provided a venous blood sample for enzyme-linked immunosorbent assay (ELISA). We present the prevalence of antibodies to SARS-CoV-2 and the acceptability and usability of self-test LFIAs, and we determine the sensitivity and specificity of LFIAs compared with laboratory ELISA.
Results
In this cohort of 5189 current and former members of the police service and 263 members of the fire service, 7.4% (396 of 5348; 95% confidence interval [CI], 6.7–8.1) were antibody positive. Seroprevalence was 8.9% (95% CI, 6.9–11.4) in those under 40 years, 11.5% (95% CI, 8.8–15.0) in those of nonwhite ethnicity, and 7.8% (95% CI, 7.1–8.7) in those currently working. Self-test LFIA had an acceptability of 97.7% and a usability of 90.0%. There was substantial agreement between within-participant LFIA results (kappa 0.80; 95% CI, 0.77–0.83). The LFIAs had a similar performance: compared with ELISA, sensitivity was 82.1% (95% CI, 77.7–86.0) self-test and 76.4% (95% CI, 71.9–80.5) nurse-performed with specificity of 97.8% (95% CI, 97.3–98.2) and 98.5% (95% CI, 98.1–98.8), respectively.
Conclusions
A greater proportion of this nonhealthcare key worker cohort showed evidence of previous infection with SARS-CoV-2 than the general population at 6.0% (95% CI, 5.8–6.1) after the first wave in England. The high acceptability and usability reported by participants and similar performance of self-test and nurse-performed LFIAs indicate that the self-test LFIA is fit for purpose for home testing in occupational and community prevalence studies.
Keywords: antibody testing, COVID-19 diagnostic testing, SARS-CoV-2, sensitivity and specificity
Self-test lateral flow immunoassays (LFIAs) had a high acceptability and usability and similar performance to nurse-performed LFIAs. Self-test LFIAs are suitable for home-testing in SARS-CoV-2 prevalence studies in nonhealthcare worker populations.
During the response to the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK), people who, due to the nature of their work, were unable to work from home were known as “key workers” [1]. The UK key worker population is estimated to be 10.6 million people (33% of the total workforce) with the majority (69%) working in industries other than health and social care, including education and childcare, key public services, transport industry, and food sector [1, 2]. It is well established that key workers are at increased risk from infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and subsequent COVID-19 [3, 4].
In April 2020, the UK government initiated a program of COVID-19 testing that included mass testing for the prevalence of antibodies to SARS-CoV-2 in the community, clinically indicated swab testing to diagnose infection, and population surveillance of the epidemic using swab tests [5]. To facilitate widespread community antibody testing, the REal Time Assessment of Community Transmission-2 (REACT-2) study undertook a program of development work using self-test finger prick lateral flow immunoassays (LFIAs) for the detection of antibodies to SARS-CoV-2. The initial laboratory validation and usability of the test was carried out among healthcare professionals [6], and further usability and acceptability studies were conducted among a random sample of adults in the population [7].
In this study, as part of the REACT-2 program [8], we aimed both (1) to quantify the prevalence of SARS-CoV-2 (current and past infection) among a subset of public-facing nonhealthcare key workers after the first wave of infection in England and (2) to test at scale the performance of self-test LFIAs versus nurse-administered LFIAs. This was done to determine the acceptability, usability, and performance of self-test point-of-care LFIAs in key worker occupations who do not have specialist training in the use of medical devices. Finally, we evaluated the performance of the LFIA against an established enzyme-linked immunosorbent assay (ELISA) antibody test in sera.
METHODS
Cross-Sectional Study Design
Nonhealthcare key worker participants were identified through an established occupational research cohort (Airwave Health Monitoring Study), a longitudinal occupational cohort of over 50000 people who work, or have worked, for the Police forces in the UK [9], and a register of current employees of the West Midlands (UK) Fire Service. Airwave participants (n = 23 634) with a residential address within a reasonable distance (approximately 50 kilometers) of 1 of 6 testing sites (Bournemouth, Derby, Keele, London, Manchester, and Warwick) and no known medical condition that might increase bleeding risk were sent a letter or e-mail inviting them to take part together with a participant information sheet. Those who did not respond were sent a reminder at 4 weeks. The Fire Service sent e-mail invitations to staff through established mailing lists. Recruitment was capped at 5500 participants [8].
Study Procedures
Participants were recruited between June 1 and July 10, 2020. Social distancing (except during finger-prick and venipuncture) and personal protective equipment were implemented at the clinics. Consent was obtained from participants who had been symptom-free for the previous 7 days and they were provided with instructions, a set of study identification (ID) stickers (barcode), a self-test LFIA kit, a nasopharyngeal kit, and a saliva kit.
Participants were then invited to conduct the LFIA (“self-test”) by following the written and/or visual instructions provided [10]. No direct instruction was provided from staff at the clinic facility. The LFIA (Fortress, Antrim, United Kingdom) used tests antibody against the spike (“S”) protein. It was previously evaluated as having sensitivity 84% (95% confidence interval [CI], 70.5%–93.5%) and specificity 98.6% (95% CI, 97.1%–99.4%) [6]. While participants were waiting the 10–15 minutes to read the LFIA test, they performed a self-administered saliva collection (2mL, using Oragene kit [DNA Genotek, Ottawa, Ontario, Canada] and drool technique) using the instructions provided. After reading the LFIA result, participants uploaded an image of the completed self-test LFIA. Participants then underwent a repeat finger-prick LFIA administered by a healthcare professional (“nurse-performed”) and provided a set of venous blood samples. After leaving the building, participants completed a self-administered nasopharyngeal swab in an outside shelter area or their private vehicle, following the instructions and video, and then placed the sample in the “drop box” at reception. Participants were also asked to complete a brief questionnaire, which included questions on demographic characteristics, occupation status, health status (including COVID-19), and usability and acceptability of the LFIA device [7].
Samples were transported daily via courier to laboratories for processing. The saliva sample and nasopharyngeal swab were analyzed by reverse-transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2, and participants were contacted (e-mail or phone) with the results within 3 days, and, if positive, they were provided with the latest government instructions on self-isolation and household isolation. Participants had a venous blood sample analyzed by ELISA (Abbott Laboratories, Lake Bluff, IL, USA) for SARS-CoV-2 immunoglobulin (Ig)G antibody. We applied the manufacturer-recommended diagnostic cutoff (binding ratio of 1.4) to determine a positive result from this quantitative immunoassay [11]. Participants received the results of the blood tests within 4 weeks via letter.
Outcomes
We define the acceptability of the self-test LFIA as the proportion of participants attending the test center who consented to, and used, the provided kit [7]. We define the usability of the self-test LFIA as the proportion of participants that used the kit who achieved a valid result. A positive LFIA test was defined as IgG positive, and a negative test was defined as IgG negative, regardless of IgM result [7]. For those that did not complete the test, we present the reason given.
Data Analysis
We excluded people without valid questionnaire responses from relevant analyses and retained tests with a positive or negative result. We estimated the prevalence of RT-PCR positivity from nasopharyngeal swab and saliva samples, and antibody positivity was defined as a positive Abbott ELISA test on serum.
After excluding LFIAs with an invalid result, we report the concordance of self-test to nurse-performed LFIA results using the Cohen’s kappa statistic: <0, poor agreement; 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and >0.8, almost perfect agreement [12]. For participants with valid LFIA (participant and nurse) and Abbott ELISA results, we determined the positive (sensitivity) and negative (specificity) percent agreement of the LFIA tests compared to the serum Abbott ELISA test.
We undertook an independent visual inspection of the uploaded images from the discordant LFIA test pairs (self-test and nurse-performed) and a similar-sized sample of concordant pairs, to investigate reasons for any differences. Two reviewers (B.D. and M.A.) independently reviewed the images and a third (H.W.) was consulted to adjudicate on and resolve any differences.
We used logistic regression to quantify associations between demographic and clinical characteristics and (1) detected antibody status (positive Abbott ELISA test) and (2) not having a valid self-test LFIA result. The 95% CIs were calculated using the Wilson’s method. Analyses were conducted in STATA version 13.1 or higher (StataCorp, College Station, TX).
Data Availability
Aggregate data from study participants is presented in tables and Supplementary information.
Participant Consent Statement
Written consent was obtained from all participants. This study was undertaken as part of the REACT-2 study, with ethical approval from South Central – Berkshire B Research Ethics Committee (REC reference: [20]/SC/0206; Integrated Research Application System [IRAS] 283805). Airwave study participants have given consent to be contacted for other research studies (IRAS project ID: 259978).
RESULTS
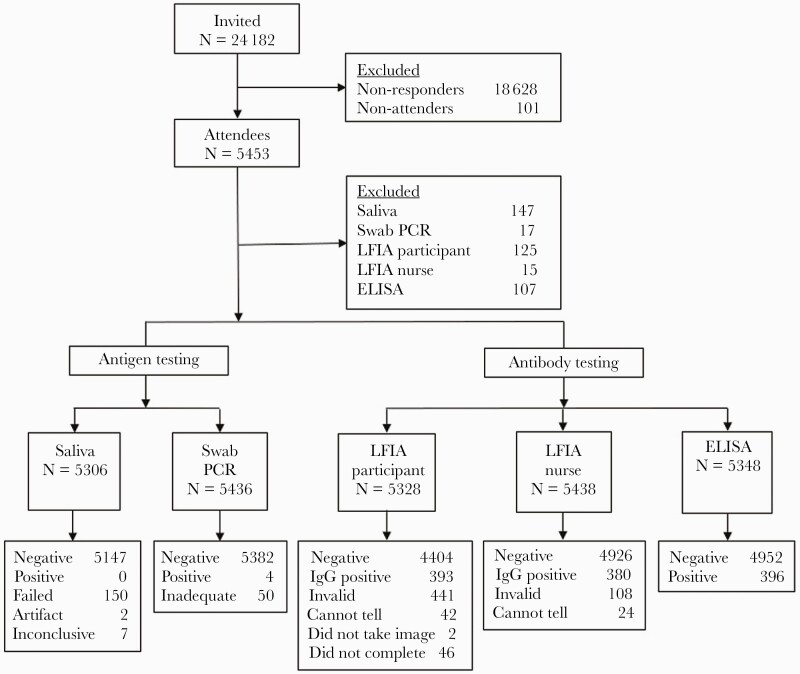
Study recruitment was capped at 5500, 5554 (23.0%) individuals booked into clinic and, of these, 5453 (98.2%) attended (Figure 1). The majority (88%, n = 4729) of participants were 40 years and older, 65% (n = 3483) were men, and 92% (n = 4882) were white British (Table 1). Overall, 5306 (97.3%) participants provided a saliva sample for RT-PCR, 5436 (99.7%) performed a nasopharyngeal swab for RT-PCR, and 5348 (98.1%) provided blood for an Abbott ELISA test.
Figure 1.
STARD flow diagram. ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; LFIA, lateral flow immunoassay; PCR, polymerase chain reaction.
Table 1.
Demographic Characteristics of Participants by Antibody Status (Abbott ELISA or LFIA) and Logistic Regression in Relation to Abbott ELISA
| Characteristic | Abbott ELISA | LFIA Self-Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants N (% of total) | No. Positive ELISA/No. Valid ELISAa | Crude Prevalence ELISA Positive % (95% CI) | P Valueb | Adjusted ORc (95% CI) | P Value | No. Positive LFIA/No. Valid LFIA | Crude Prevalence LFIA Positive % (95% CI) | P Value | ||
| Overall | 396/5348 | 7.4 (6.7–8.1) | 393/4404 | 8.1 (7.4–9.0) | ||||||
| Age (years) | <40 | 626 (11.6) | 56/626 | 8.9 (6.9–11.4) | .045 | - | 62/545 | 11.3 (8.9–14.3) | .013 | |
| 40–49 | 1686 (31.4) | 125/1684 | 7.4 (6.2–8.7) | 0.81 (0.58–1.13) | .233 | 122/1497 | 8.1 (6.8–9.6) | |||
| 50–59 | 2192 (40.9) | 170/2189 | 7.7 (6.7–8.9) | 0.85 (0.62–1.16) | .317 | 152/1935 | 7.8 (6.7–9.1) | |||
| 60 and over | 851 (15.8) | 45/849 | 5.3 (3.9–7.0) | 0.56 (0.37–0.84) | .006 | 47/734 | 6.4 (4.8–8.4) | |||
| Gender | Male | 3483 (65.0) | 266/3478 | 7.6 (6.8–8.5) | .354 | - | 244/3054 | 7.9 (7.0–9.0) | .632 | |
| Female | 1872 (34.9) | 130/1870 | 6.9 (5.8–8.1) | 0.88 (0.71–1.10) | .279 | 139/1657 | 8.3 (7.1–9.8) | |||
| Ethnicity | White | 4882 (92.0) | 336/4789 | 7.0 (6.3–7.7) | .001 | - | 337/4343 | 7.7 (7.0–8.5) | .000 | |
| Nonwhite | 424 (7.9) | 48/415 | 11.5 (8.8–15.0) | 1.66 (1.20–2.29) | .002 | 52/388 | 13.4 (10.3–17.1) | |||
| Currently Working | Yes | 4459 (84.2) | 344/4372 | 7.8 (7.1–8.7) | .003 | - | 346/3992 | 8.6 (7.8–9.5) | .012 | |
| Nod | 836 (15.7) | 40/821 | 4.8 (3.5–6.5) | 0.65 (0.45–0.94) | .024 | 43/731 | 5.8 (4.3–7.8) | |||
| Education | Degree | 1629 (30.7) | 109/1600 | 6.8 (5.6–8.1) | .299 | - | 122/1448 | 8.4 (7.0–9.9) | .733 | |
| No degree | 3678 (69.3) | 275/3605 | 7.6 (6.8–8.5) | 1.16 (0.91–1.46) | .208 | 267/3284 | 8.1 (7.2–9.1) | |||
| Region | East Midlands | 789 (14.7) | 50/789 | 6.3 (4.8–8.2) | .000 | - | 46/699 | 6.5 (4.9–8.6) | .000 | |
| London | 1068 (19.9) | 125/1066 | 11.7 (9.9–13.8) | 1.90 (1.35–2.68) | .000 | 128/956 | 13.4 (11.2–15.6) | |||
| North West | 800 (14.9) | 70/799 | 8.7 (6.9–10.9) | 1.42 (0.97–2.07) | .069 | 58/704 | 8.2 (6.4–10.5) | |||
| South West | 764 (14.2) | 23/761 | 3.0 (2.0–4.5) | 0.46 (0.27–0.76) | .003 | 28/663 | 4.2 (2.9–6.0) | |||
| West Midlands | 1934 (36.1) | 128/1933 | 6.6 (5.5–7.8) | 1.06 (0.75–1.48) | .732 | 123/1689 | 7.2 (6.1–8.6) | |||
| Self-report COVID-19e | No | 3747 (70.3) | 74/3679 | 2.0 (1.6–2.5) | .000 | - | 86/3353 | 2.5 (2.0–3.1) | .000 | |
| Yes | 1582 (29.6) | 312/1547 | 20.1 (18.2–22.2) | 12.6 (9.6–16.4) | .000 | 303/1396 | 21.7 (19.6–23.9) | |||
| Comorbidityf | No | 3081 (57.8) | 233/3021 | 7.3 (6.5–8.3) | .899 | - | 233/2748 | 8.4 (7.4–9.5) | .701 | |
| 1–2 | 2017 (37.8) | 148/1978 | 7.4 (6.4–8.7) | 1.05 (0.84–1.30) | .658 | 140/1793 | 7.8 (6.6–9.1) | |||
| ≥3 | 229 (4.3) | 15/226 | 6.6 (4.0–10.7) | 0.96 (0.56–1.66) | .906 | 16/207 | 7.7 (4.7–12.2) | |||
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; LFIA, lateral flow immunoassay; OR, odds ratio.
Participants with a valid questionnaire and valid Abbott ELISA result.
P value from χ2 test.
Adjusted for age and sex.
Includes people in government-supported training, unemployed and available for work, wholly retired from work, full-time education at school, college, or university, looking after home/family, permanently sick/disabled, and “doing something else”.
Self-reported COVID-19 infection, “yes” includes confirmed by a positive test, suspected by a doctor but not tested and my own suspicions.
Number of self-reported comorbidities from the following list: organ transplant recipient, diabetes (type I or II), heart disease or heart problems, hypertension, overweight, stroke, kidney disease, liver disease, anemia, asthma, other lung condition (such as COPD, bronchitis, or emphysema), cancer, condition affecting the brain and nerves (eg, dementia, Parkinson’s, multiple sclerosis), a weakened immune system/reduced ability to deal with infections (as a result of a disease or treatment), depression, anxiety, psychiatric disorder.
Four (0.07%; 95% CI, 0.03–0.19) participants were RT-PCR positive from nasopharyngeal swabs; none of 5306 saliva tests were positive. Due to the low numbers testing positive, no further analysis of prevalence of SARS-CoV-2 infection is presented.
Antibody Prevalence
The IgG antibodies to SARS-CoV-2 were found in 396 of 5348 participants using the Abbott ELISA, a prevalence of 7.4% (95% CI, 6.7–8.1) (Table 1). Prevalence varied by the following: (1) age, being highest in people under 40 years of age (8.9%; 95% CI, 6.9–11.4) and lowest in people over 60 years (5.3%; 95% CI, 3.9–7.0); (2) ethnicity, being higher in people of nonwhite ethnicity (11.5%; 95% CI, 8.8–15.0) compared to people of white ethnicity (7.0%; 95% CI, 6.3–7.7); (3) employment status, being higher in people currently working (7.8%; 95% CI, 7.1–8.7) compared to people not currently working (4.8%; 95% CI, 3.5–6.5); and (4) region, being higher in London (11.7%; 95% CI, 9.9–13.8) compared to other regions except North West and lowest in the South West (3.0%; 95% CI, 2.0–4.5) (Table 1). These associations persisted after controlling for age and sex, with the odds of positivity in London being almost twice as high as those in East Midlands (adjusted odds ratio [AOR] of 1.90 [95% CI, 1.35–2.68]), and AOR in people of nonwhite ethnicity of 1.66 (95% CI, 1.20–2.29) compared to people of white ethnicity (Table 1). Three hundred ninety-three (8.1%; 95% CI, 7.4–9.0) participants were antibody positive on the self-test LFIA.
Comparative Performance of Antibody Tests
For participants with a valid result from self-test LFIA, nurse-performed LFIA, and Abbott ELISA, 95.2% (4363 of 4582) were concordant (positive or negative) across all 3 tests (Supplementary Table S1). Compared with Abbott ELISA, self-test LFIAs had a sensitivity of 82.1% (95% CI, 77.7–86.0) and specificity of 97.8% (95% CI, 97.3–98.2), and nurse-performed LFIAs had a sensitivity of 76.4% (95% CI, 71.9–80.5) and a specificity of 98.5% (95% CI, 98.1–98.8) (Table 2).
Table 2.
Comparison of Results from LFIAs and Abbott ELISA
| ELISA | |||||
|---|---|---|---|---|---|
| Positive | Negative | Total | Performance (95% CI) | ||
| Self-Test LFIA | Positive | 285 | 98 | 383 | Sensitivity: 82.1% (77.7–86.0) |
| Negative | 62 | 4260 | 4322 | Specificity: 97.8% (97.3–98.2) | |
| Total | 347 | 4358 | 4705 | ||
| Nurse-Performed LFIA | Positive | 298 | 72 | 370 | Sensitivity: 76.4% (71.9–80.5) |
| Negative | 92 | 4744 | 4836 | Specificity: 98.5% (98.1–98.8) | |
| Total | 390 | 4816 | 5206 | ||
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; LFIA, lateral flow immunoassay.
There was substantial agreement between the 2 LFIA results (self-test and nurse-performed) from the same participants, with 4540 of 4670 (97.2%) having the same result, kappa 0.80 (95% CI, 0.77–0.83) (Table 3). Overall, there were discordant results between the self-test and nurse-performed tests in 130 cases (2.8%). Uploaded images were available for review from 101 of these discordant pairs, 56 (55%) of which were confirmed as discordant (Supplementary Table S2). The review showed that participants were more likely to report negative tests as positive, and nurses were more likely to miss faint positives, which may explain the lower sensitivity of nurse-tests compared with Abbott ELISA (Supplementary Table S3). Review of the uploaded images for the sample of 145 concordant self-test and nurse-performed tests found that 129 of 129 available image pairs were concordant (Supplementary Tables S4 and S5).
Table 3.
Comparison of Results from Self-Test LFIA and Nurse-Performed LFIA
| Nurse-Performed LFIA | |||||
|---|---|---|---|---|---|
| Positive | Negative | Total | %agreement (95% CI) | ||
| Self-Test LFIA | Positive | 294 | 93 | 387 | Positive: 88.8% (84.9–92.0) |
| Negative | 37 | 4246 | 4283 | Negative: 97.9% (97.4–98.3) | |
| Total | 331 | 4339 | 4670 | Kappa: 0.80 (0.77–0.83) | |
Abbreviations: CI, confidence interval; LFIA, lateral flow immunoassay.
Self-Test Lateral Flow Immunoassay Acceptability and Usability
The self-test LFIA test was attempted by 5328 participants, an acceptability of 97.7%. Of the participants who attempted the test, 4797 obtained a valid result, giving a usability of 90.0%. The most commonly cited reasons for not successfully completing the LFIA were “I could not read the result” (n = 78), “It was too fiddly for me to manage” (n = 51), and “I did not manage to get the buffer onto the test” (n = 37). An invalid self-test LFIA result was 20% less likely for women compared with men (OR, 0.78; 95% CI, 0.63–0.97) and 40% more likely for people who were not currently working compared with people currently working (OR, 1.38; 95% CI, 1.03–1.85) (Supplementary Table S6). The majority of participants (88.5%, n = 4638) were “very confident” that they had interpreted their results correctly. Of the 50 participants who provided a preference for where to perform the test, 82.0% (95% CI, 62.9–90.2) stated home.
DISCUSSION
One in thirteen people (7.4%; 95% CI, 6.7–8.1) in this nonhealthcare key worker cohort had evidence of previous infection with SARS-CoV-2 after the first wave of the epidemic in England, rising to 1 in 9 in London (11.7%; 95% CI, 9.9–13.8). This prevalence was higher than in a representative sample of the population at the time at 6.0% (95% CI, 5.8–6.1), but lower than in key workers in healthcare at 11.7% (95% CI, 10.5–13.1) [4]. The participants reported a high acceptability and usability of self-test LFIAs, with 90% of participants obtaining a valid antibody test result. There was no significant difference in the performance of self-test and nurse-performed LFIAs.
The prevalence of infection in the cohort was low at 0.07% (n = 4) on RT-PCR from nasopharyngeal swab, and none of the saliva samples tested positive. This finding is in keeping with the national REACT prevalence study (adjusted prevalence of 0.077% [95% CI, 0.065%–0.092%] in England, June 19–July 8, 2020) and reflects the declining epidemic at the time of the study, during June to July 2020 [13, 14].
The Fortress LFIA used in this study was selected after a rigorous evaluation of 5 commercially available LFIAs [6]. It has since been compared with 7 newer LFIAs and none had a superior diagnostic performance [15]. We used the Abbott ELISA as the primary reference standard. An evaluation by Public Health England (PHE) found that it met the manufacturer-reported performance for specificity, although not for sensitivity [11].
Our study provides further evidence for the association of occupation, involving key workers in a public-facing role, with risk of infection by SARS-CoV-2. Previous research suggests that in mid-June to mid-July 2020, the crude prevalence in key workers (excluding healthcare and care home workers) in England was 6.1% (95% CI, 5.7–6.4) [4], more than a percentage point lower than the 7.4% (95% CI, 6.7–8.1) reported here. However, it is possible that the prevalence after the first wave of the pandemic among participants in our study could be underestimated given the uncertainty about antibody waning and the duration of a detectable antibody response [16, 17]. We found similar demographic patterns in prevalence to those seen in the national REACT-2 study, where the highest prevalence was also found in the London region, in people of nonwhite ethnicity and younger adults [4].
The sensitivity of self-test LFIAs estimated against Abbott ELISA in this cohort of nonhealthcare key workers was 82.1% (95% CI, 77.8–85.8), similar to previously reported sensitivity of 84% (95% CI, 70.5–93.5) in a cohort of healthcare workers with PCR-confirmed COVID-19 [6]. The specificity against Abbott ELISA in this study was 97.8% (95% CI, 97.3–98.2), similar to the 98.6% (95% CI, 97.1–99.4) using prepandemic sera in the laboratory [6].
Overall, there was substantial agreement between self-test and nurse-performed LFIAs, with only 2.8% having discrepant results. There was no evidence that nurse-performed tests were better than self-tests, although there was a suggestion that participants were more likely to record a test as positive and healthcare practitioners were more likely to report as negative. This was supported by the visual reinspection of the small number of discordant pairs of LFIAs. The study suggests that there is no gain in accuracy to be had by committing extra resource to obtain healthcare practitioner performed LFIAs in large-scale community surveys and that self-test is a viable and acceptable approach.
Strengths and Limitations
We aimed to replicate the experience of completing a home self-test LFIA as a means of obtaining prevalence of previous SARS-CoV-2 infection in nonhealthcare occupational settings by providing participants with no additional assistance, beyond the test instruction materials. The idea was that, if successful, this could then be extended to obtain prevalence estimates in the wider population at low cost, without the need for supervision by a healthcare practitioner. We found little difference between the results of LFIAs administered as a self-test or by a nurse, supporting the unsupervised use of the LFIA. However, our results might not be fully generalizable. The majority of participants were recruited from the Airwave occupational cohort [9]. The cohort is not demographically (age, sex, ethnicity, geography) representative of the adult population of England, participants had to be able to travel to a test site (mostly in a private vehicle), and, being an occupational cohort, participants were healthier than the general population (“healthy cohort” effect [9]). Participants (emergency service staff) may also have been more familiar with medical procedures, which may have had an impact on the generalizability of the usability and acceptability findings to the general population. Nonetheless, parallel studies carried out in the community at around the same time provided similar results on usability and acceptability (although estimates of prevalence were lower, reflecting greater exposure of public-facing workers to risk of infection) [7].
We compared results of the self-test and nurse-performed LFIA with a quantitative ELISA test (Abbott) using an established cut-point to denote positivity. To the extent that the “gold standard” ELISA test itself does not have perfect sensitivity and specificity [11] will have introduced some error into those comparisons.
CONCLUSIONS
In June 2020, after the first wave of the COVID-19 pandemic in England, approximately 1 in 13 of this nonhealthcare key worker cohort had evidence of past infection with SARS-CoV-2. The epidemiological patterns in prevalence of previous infection were similar to the national picture, with highest prevalence observed in people who (1) were under 40 years, (2) had a nonwhite ethnicity, (3) were currently in employment and (4) were living in London. Participants reported a high acceptability and usability for the LFIAs, and there was little difference in performance of self-test compared with a nurse-performed LFIA. Overall, our study suggests that the self-test LFIA is fit for purpose for home-testing for use in occupational and—by extension—community prevalence studies of anti-SARS-CoV-2 antibodies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank key collaborators on this research: Eric Johnson and Rob Elliott (Department of Epidemiology and Biostatistics at Imperial College); Gianluca Fontana, Sutha Satkunarajah, and Lenny Naar (Institute of Global Health Innovation at Imperial College); Andy Heard, Paul Downey, and Antoinette Amuzu (Airwave study, Imperial College).
Disclaimer. The views expressed are those of the authors and not necessarily those of the Department of Health and Social Care or the National Institute for Health Research.
Financial support. This work was supported by the Department of Health and Social Care in England. We acknowledge funding from National Institute of Health Research Professorship (to G. C.); National Institute of Health Research Senior Investigator Award (to A. D. and H. W.); Medical Research Council Centre for Environment and Health (Grant Numbers MR/L01341X/1 and MR/S019669/1; to P. E.); National Institute of Health Research Imperial College NHS Trust Biomedical Research Centre (to P. E. and H. W.); National Institute of Health Research Applied Research Collaborative (to H. W.); National Institute of Health Research Health Protection Research Unit in Chemical and Radiation Threats and Hazards (Grant Number NIHR-200922; to P. E. and B. D.); National Institute of Health Research Health Protection Research Unit in Environmental Exposures and Health (Grant Number NIHR-200880; to P. E.); British Heart Foundation Centre for Research Excellence at Imperial College London (RE/18/4/34215; to P. E.); Wellcome Trust (Grant Numbers 200861/Z/16/Z and 200187/Z/15/Z; to H.W.); Health Data Research UK (to P. E.); and UK Dementia Research Institute at Imperial (Grant Number MC_PC_17114; to P. E.). We thank The Huo Family Foundation for their support of our work on COVID-19.
Potential conflicts of interest. A. D. is Chair of the Health Security initiative at Flagship Pioneering UK Ltd. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Office for National Statistics. Official Statistics: Key workers: population and characteristics, 2019. Available at: https://www.gov.uk/government/statistics/key-workers-population-and-characteristics-2019. Accessed 29 November 2020.
- 2. UK Cabinet Office and Department for Education. Guidance: Critical workers who can access schools or educational settings. Available at: https://www.gov.uk/government/publications/coronavirus-covid-19-maintaining-educational-provision/guidance-for-schools-colleges-and-local-authorities-on-maintaining-educational-provision. Accessed 21 November 2020.
- 3. Office for National Statistics. Coronavirus and key workers in the UK. Available at: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/articles/coronavirusandkeyworkersintheuk/2020-05-15. Accessed 29 November 2020.
- 4. Ward H, Atchison C, Whitaker M, et al. . SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun 2021; 12:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Department of Health and Social Care. Policy paper: Coronavirus (COVID-19): Scaling up our testing programmes. UK, 2020 04/04/2020. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/878121/coronavirus-covid-19-testing-strategy.pdf. Accessed 31 October 2021. [Google Scholar]
- 6. Flower B, Brown JC, Simmons B, et al. . Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax 2020; 75:1082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atchison C, Pristera P, Cooper E, et al. . Usability and acceptability of home-based self-testing for SARS-CoV-2 antibodies for population surveillance. Clin Infect Dis 2020; 72:e384–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riley S, Atchison C, Ashby D, et al. . REal-time Assessment of Community Transmission (REACT) of SARS-CoV-2 virus: study protocol [version 2; peer review: 2 approved]. Wellcome Open Res 2021; 5:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott P, Vergnaud AC, Singh D, et al. . The airwave health monitoring study of police officers and staff in Great Britain: rationale, design and methods. Environ Res 2014; 134:280–5. [DOI] [PubMed] [Google Scholar]
- 10. Helix Centre. Your at-home antibody test for coronavirus. Imperial College London and IPSOS MORI. 2020: Available at: https://vimeo.com/423592077/ed946fe3f6. Accessed 31 October 2021. [Google Scholar]
- 11. Public Health England. Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARS-CoV-2 antibodies. London, UK: PHE; 2020. [Google Scholar]
- 12. Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 13. Riley S, Ainslie KEC, Eales O, et al. . Community prevalence of SARS-CoV-2 virus in England during May 2020: REACT study [preprint]. medRxiv 2020: 2020.07.10.20150524. [Google Scholar]
- 14. Riley S, Ainslie KEC, Eales O, et al. . Transient dynamics of SARS-CoV-2 as England exited national lockdown [preprint]. medRxiv 2020: 2020.08.05.20169078. [Google Scholar]
- 15. Moshe M, Daunt A, Flower B, et al. ; React study team. SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (React 2): diagnostic accuracy study. BMJ 2021; 372:n423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lumley SF, Wei J, O’Donnell D, et al. . The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis 2021;73:e699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ward H, Cooke GS, Atchison C, et al. . Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: serial cross-sectional studies of 365,000 adults. Lancet Reg Health Eur 2021; 4:100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregate data from study participants is presented in tables and Supplementary information.