Abstract
Background and Aims
Age is a major prognostic factor for COVID-19 outcomes. The effect of inflammatory bowel disease [IBD] activity on COVID-19 is unclear. We examined the relationship between IBD activity and COVID-19 severity according to age.
Methods
We included IBD patients diagnosed with COVID-19, reported to SECURE-IBD between March 13, 2020 and August 3, 2021. Clinical IBD activity was measured by physician global assessment [PGA]. COVID-19-related outcomes were [1] intensive care unit [ICU] admission, ventilation or death, and [2] hospitalization. Using generalized estimating equations, we determined adjusted odds ratios [aOR, 95% confidence interval] for moderate and severe PGA vs clinical remission/mild PGA, controlling for demographics, medications and COVID-19 diagnosis period. We performed stratified analyses by age [≤50 vs >50 years].
Results
Among 6078 patients, adverse COVID-19 outcomes were more common with active IBD: ICU/ventilation/death in 3.6% [175/4898] of remission/mild, 4.9% [45/920] of moderate and 8.8% [23/260] of severe [p < 0.001]; and hospitalization in 13% [649/4898] of remission/mild, 19% [178/920] of moderate and 38% [100/260] of severe [p < 0.001]. Stratified by decade, effect sizes were larger for younger patients. In patients ≤50 years, severe PGA was independently associated with ICU/ventilation/death (aOR 3.27 [1.15–9.30]) and hospitalization (aOR 4.62 [2.83–7.55]). In contrast, severe PGA was not independently associated with COVID-19 outcomes in those older than 50 years.
Conclusions
Clinically active IBD may be a risk factor for severe COVID-19, particularly in younger patients. IBD disease control, including through medication compliance, and strategies to mitigate the risk of COVID-19 infection amongst patients with active IBD [e.g. distancing, immunization] are key to limit adverse COVID-19 outcomes.
Keywords: COVID-19, disease activity, inflammatory bowel disease
1. Introduction
The coronavirus disease of 2019 [COVID-19] pandemic, caused by severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], represents a global health crisis. Increasing age1 and comorbid disease2 are well-recognized risk factors for severe infection and poor outcomes. Inflammatory bowel disease [IBD], including Crohn’s disease [CD] and ulcerative colitis [UC], is a chronic inflammatory disorder of the gastrointestinal [GI] tract that affects millions of individuals worldwide.3–5 IBD often requires treatment with immune-modifying medications, such as corticosteroids, thiopurines and biologics.
Outcomes for COVID-19 are worse for the elderly in both the general population and those with IBD. However, IBD patients infected with SARS-CoV-2, particularly those on corticosteroids, may have worse outcomes regardless of their age. Risk factors for poor outcomes may also vary across the age spectrum. The Surveillance Epidemiology Under Research Exclusion for IBD [SECURE-IBD] registry is a large, international registry of IBD patients with COVID-19 derived from active surveillance and reporting by treating physicians. In previous analyses of SECURE-IBD, corticosteroids were associated with an increased risk of severe COVID-19, defined as intensive care unit [ICU] admission, invasive ventilation or death.6,7 In addition, compared to anti-tumour necrosis factor-α [anti-TNF] monotherapy, thiopurine monotherapy, thiopurine plus anti-TNF, and 5-aminosalicylates [5ASA]/sulfasalazine were independently associated with worse outcomes.6
In addition to medication-related effects, IBD patients may have worse COVID-19 outcomes as a result of gastrointestinal inflammation, potentially related to the mucosal expression of angiotensin-converting enzyme 2 [ACE2] in the gastrointestinal tract.8 This hypothesis invokes the potential role of gut inflammation and IBD activity as a mediator of COVID-19 severity. In an earlier SECURE-IBD analysis including 525 patients, clinically active disease was significantly associated with an increased risk of the composite outcome of hospitalization or death, and there was a trend toward an increased risk of ICU admission, ventilation or death.7 A more recent study from the UK, however, found no increased risk of poor COVID-19 outcomes in patients with acute severe UC.9
As the relationship between IBD activity and COVID-19 severity remains unclear, we evaluated the impact of IBD clinical disease activity on COVID-19 outcomes, while accounting for the confounding effects of medications and other factors. We specifically explored the modifying effect of age on the association between IBD activity and COVID-19 severity.
2. Materials and Methods
2.1. Study design, setting and participants
This is a retrospective cohort study utilizing data from an active surveillance registry [SECURE-IBD]. Individuals of any age with any type of IBD who developed COVID-19 and were reported to the SECURE-IBD registry between March 13, 2020 and August 3, 2021 were eligible for inclusion. Patients missing IBD activity (Physician Global Assessment [PGA]) or age were excluded.
2.2. Data source
The SECURE-IBD database [www.covidibd.org] was developed to monitor COVID-19 outcomes in paediatric and adult IBD patients. SECURE-IBD is an international collaboration supported by several regional and national organizations, as previously described.7 Healthcare providers voluntarily reported polymerase chain reaction [PCR]- or antibody-confirmed COVID-19 cases in IBD patients. Healthcare providers were instructed to report cases regardless of severity, after a minimum disease duration of 7 days and after sufficient time had elapsed to determine the final illness outcome [resolution or death]. Research Electronic Data Capture [REDCap],10 a secure, web-based electronic data capture tool hosted at the University of North Carolina at Chapel Hill, was used for data collection and management. Quality control measures were performed as previously described.6
2.2.1. Variables
The list of variables included in SECURE-IBD has been outlined elsewhere.6 We examined two adverse COVID-19 outcomes: ‘severe’ COVID-19 [primary outcome], a composite of ICU admission, mechanical ventilation or death, as previously defined,6 and COVID-19-related hospitalization. The exposure of interest was clinical IBD activity, measured by PGA. PGA was analysed as a three-level variable, combining clinical remission and mild PGA into a single reference category, against which moderate PGA and severe PGA were compared. We examined the following covariates: age, sex, comorbidities [defined as none, one or two or more], IBD type [defined as UC/IBD-unclassified or CD], systemic corticosteroids [defined as intravenous or oral corticosteroids, excluding local release formulations such as ileal-release budesonide], anti-TNF monotherapy [defined as anti-TNF without a thiopurine or methotrexate], anti-TNF combination therapy (defined as anti-TNF plus an immunomodulator [thiopurine or methotrexate]), immunomodulator monotherapy [defined as a thiopurine or methotrexate without anti-TNF], 5ASA/mesalamine, race/ethnicity [defined as Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian and other/mixed], body mass index [BMI], country gross domestic product [GDP] and other medications [anti-integrins, ustekinumab]. Recognizing that, over time, changes have occurred in COVID-19 management and variants [both with implications on outcomes], we also adjusted for period of COVID-19 diagnosis. For analyses, we dichotomized COVID-19 diagnosis period, using Joinpoint regression [https://surveillance.cancer.gov/joinpoint/, v.4.8.0.1] to identify the inflection month at which outcome rates changed significantly. This month was found to be November 2020 [Supplementary Figure 1]. We therefore defined two COVID-19 diagnosis periods, March 2020 to October 2020, and November 2020 to July 2021 [including those diagnosed August 1–3, 2021]. Variables with >10% missing data were excluded from primary analyses, but we explored their effects in sensitivity analyses.
2.3. Statistical analysis
Continuous variables were summarized using medians (interquartile range [IQR]) and categorical variables using frequencies [%]. We compared patient and disease characteristics across PGA categories using the Kruskal–Wallis and Chi-squared tests for continuous and categorical variables, respectively.
Given the clustering of participants by country, we used generalized estimating equations [GEEs] to examine the association between PGA and outcomes, in univariate and multivariable fashion. For multivariable models, we included the following covariates a priori based on clinical relevance and demonstration of significance in previous studies: age, sex, comorbidities, IBD type, corticosteroids, anti-TNF monotherapy, anti-TNF combination therapy, immunomodulator monotherapy, 5ASA/sulfasalazine and period of COVID-19 diagnosis. We included additional confounders in multivariable GEE models only if they changed the univariate estimate for PGA by ≥10% [‘change in estimate approach’].11 This change in estimation approach was repeated for each model; as a result, not all of the adjusted models presented in the results include the same combinations of covariates.
To explore for potential effect modification by age, we determined [using GEEs] the unadjusted odds ratios [ORs] of ICU admission/ventilation/death and hospitalization for severe PGA [vs remission/mild PGA] by decade and plotted them using forest plots. We also repeated the above GEE analyses stratified by age. As for COVID-19 diagnosis period, we used Joinpoint regression to guide the choice of age cut-off [found to be 50 years].
Results are reported as unadjusted ORs and adjusted ORs [aOR] with 95% confidence intervals [95% CI]. Two-sided p-values <0.05 were considered statistically significant. We performed all analyses using SAS University Edition v.3.8 [SAS Institute].
2.4. Ethical considerations
Each SECURE-IBD survey item met criteria for deidentified data, in accordance with the Health Insurance Portability and Accountability Act [HIPAA] Safe Harbor De-Identification standards. The UNC-Chapel Hill Office for Human Research Ethics has determined that the storage and analysis of de-identified data for this project does not constitute human subjects research as defined under US federal regulations [45 CFR 46.102 and 21 CFR 56.102] and does not require institutional review board approval.
2.5. Role of the funding source
The funding source had no role in the study design, collection, analysis or interpretation of the data, writing of the report, or the decision to submit the paper for publication.
3. Results
3.1. Patient characteristics
A total of 6078 cases from over 60 countries were included after excluding 342 [5.3%] cases with missing PGA and/or age data. Thirty-four per cent of included cases were from the USA, 8.1% Russia, 7.8% Spain, 5.5% Netherlands, 3.6% Germany, 3.6% UK, 3.0% Italy, 2.9% Belgium, 2.4% Canada, 2.2% Brazil, 2.1% Austria and 2.1% France, with the remainder of countries each accounting for <2% of the cohort. Patient and disease characteristics are summarized in Table 1 for the overall cohort, and by PGA category. Comorbidity details are provided in Supplementary Table 1. Forty-nine per cent of patients were male and the median age at diagnosis of SARS-CoV-2 was 38 [IQR 26–52] years. The distribution of IBD activity by PGA was as follows: 80.6% clinical remission or mild activity, 15.1% moderate and 4.3% severe. BMI was the only variable with >10% missing data and was therefore excluded from primary analyses [examined in sensitivity analyses].
Table 1.
Demographic and disease characteristics, overall and by clinical IBD activity [PGA] category
| Variable | Overall [N = 6078] | PGA remission/mild [N = 4898] | PGA moderate [N = 920] | PGA severe [N = 260] | p-value |
|---|---|---|---|---|---|
| N [%] or median [IQR] | |||||
| COVID-19 period | |||||
| Mar 20 to Oct 20 | 2729 [45%] | 2150 [44%] | 431 [47%] | 148 [57%] | <0.001 |
| Nov 21 to Jul 21 | 3349 [55%] | 2748 [56%] | 489 [53%] | 112 [43%] | |
| Country GDP [trillion USD] | 1.84 [0.91–21.4] | 1.84 [0.91–21.4] | 1.74 [0.91–21.4] | 1.70 [0.76–21.4] | 0.25 |
| Male | 2916/5990 [49%] | 2334/4830 [48%] | 445/908 [49%] | 137/252 [54%] | 0.17 |
| Age [years] | 38 [26–52] | 38 [26–52] | 38 [27–52] | 37 [26–48] | |
| ≤50 years | 4419 [73%] | 3552 [73%] | 664 [72%] | 203 [78%] | 0.68 |
| >50 years | 1659 [27%] | 1346 [27%] | 256 [28%] | 57 [22%] | 0.14 |
| Ethnicity | |||||
| Hispanic | 772/5733 [13%] | 612/4629 [13%] | 129/866 [15%] | 21/238 [13%] | 0.19 |
| White | 4315/5733 [75%] | 3495/4629 [76%] | 648/866 [75%] | 172/238 [72%] | |
| Black | 260/5733 [5%] | 205/4629 [4%] | 38/866 [4%] | 17/238 [7%] | |
| Asian | 216/5563 [4%] | 180/4629 [4%] | 23/866 [3%] | 13/238 [5%] | |
| Other/mixed | 170/5733 [3%] | 137/4629 [3%] | 28/866 [3%] | 5/238 [2%] | |
| IBD type | |||||
| CD | 3472/6052 [57%] | 2854/4876 [59%] | 499/916 [54%] | 119 [45%] | <0.001 |
| UC/IBD-U | 2580/6052 [43%] | 2022/4876 [41%] | 417/916 [46%] | 141 [54%] | |
| BMI ≥301 | 896/5175 [17%] | 732/4148 [18%] | 134/800 [17%] | 30/227 [13%] | 0.21 |
| BMI1 | 24.3 [21.6–28.0] | 24.5 [21.8–28.0] | 24.1 [21.2–27.8] | 22.5 [19.6–26.1] | <0.001 |
| Comorbidities | |||||
| None | 4178 [69%] | 3384 [69%] | 613 [67%] | 181 [70%] | 0.29 |
| 1 | 1314 [22%] | 1051 [21%] | 215 [23%] | 48 [18%] | |
| ≥2 | 586 [10%] | 463 [9%] | 92 [10%] | 31 [12%] | |
| Systemic corticosteroids | 400 [7%] | 118 [2%] | 169 [18%] | 113 [43%] | <0.001 |
| Anti-TNF mono [no IMM] | 1954 [32%] | 1666 [34%] | 236 [26%] | 52 [20%] | <0.001 |
| Anti-TNF combo [with IMM] | 602 [10%] | 488 [10%] | 88 [10%] | 26 [10%] | 0.93 |
| With thiopurine | 468 [8%] | 372 [8%] | 73 [8%] | 23 [9%] | 0.73 |
| With MTX | 135 [2%] | 117 [2%] | 15 [2%] | 3 [1%] | 0.18 |
| IMM mono [no anti-TNF] | 702 [12%] | 571 [12%] | 104 [11%] | 27 [10%] | 0.80 |
| Thiopurine [without TNF] | 611 [10%] | 499 [10%] | 89 [10%] | 23 [9%] | 0.72 |
| MTX [without TNF] | 92 [2%] | 73 [1%] | 15 [2%] | 4 [2%] | 0.95 |
| Anti-integrin | 665 [11%] | 516 [11%] | 118 [13%] | 31 [12%] | 0.11 |
| Ustekinumab | 556 [9%] | 405 [8%] | 122 [13%] | 29 [11%] | <0.001 |
| Tofacitinib | 101 [2%] | 69 [1%] | 27 [3%] | 5 [2%] | 0.004 |
| Mesalamine/sulfasalazine | 1847 [30%] | 1431 [29%] | 320 [35%] | 96 [37%] | <0.001 |
BMI, body mass index; CD, Crohn’s disease; GDP, gross domestic product; IBD-U, IBD-unclassified; IMM, immunomodulator; MTX, methotrexate; OR, odds ratio; PGA, physician global assessment; TNF, tumour necrosis factor; UC, ulcerative colitis; USD, US dollars.
If denominator not shown, no missing data.
115% missing data.
3.2. Outcomes
Fifteen per cent of patients [N = 927] required hospitalization and 4.0% [N = 243] experienced the composite outcome of ICU, ventilation or death. Supplementary Figure 1 depicts the results of Joinpoint regression applied to the proportion of patients experiencing adverse outcomes by month of COVID-19 diagnosis. An initial downward trend is appreciable for both outcomes, with a change in slope from November 2020 onwards. There was no interaction between COVID-19 diagnosis period and PGA for either outcome.
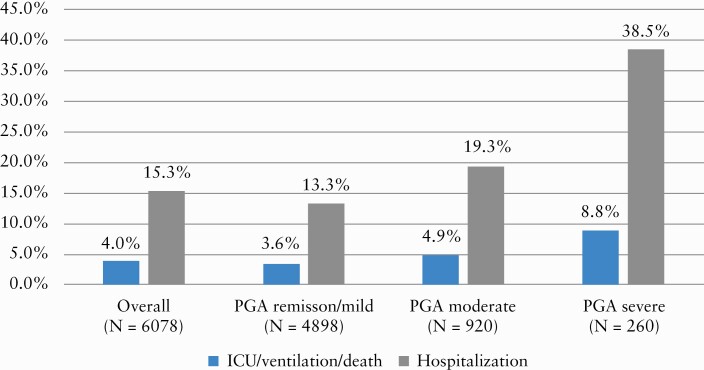
Figure 1 contrasts outcomes by IBD activity category. ICU/ventilation/death was significantly more frequent in patients with more active IBD [4.9% of moderate PGA and 8.8% of severe PGA, compared to 3.6% of remission/mild PGA, p < 0.001]. The same was true for hospitalization [19% of moderate PGA and 38% of severe PGA, compared to 13% of remission/mild PGA, p < 0.001].
Figure 1.
Proportion of patients experiencing adverse COVID-19 outcomes by PGA category [p < 0.001 for both outcomes].
3.3. Association between disease activity and outcomes
Table 2 summarizes the unadjusted associations between PGA and COVID-19 outcomes and unadjusted associations for covariates. Compared to remission/mild activity, patients with moderate PGA [OR 1.46, 95% CI 1.17–1.84] and severe PGA [OR 2.56, 95% CI 1.76–3.70] were significantly more likely to experience the composite outcome of ICU/ventilation/death. Similarly, patients with moderate PGA [OR 1.59, 95% CI 1.37–1.84] and severe PGA [OR 3.74, 95% CI 2.86–4.90] were significantly more likely to be hospitalized for COVID-19. In stratified analyses by IBD type, the effect sizes for moderate and severe PGA were larger for both outcomes in CD compared to UC/IBD-U [Supplementary Table 2]. Age over 50 years was an important risk factor for both ICU/ventilation/death [OR 8.72, 95% CI 5.96–12.77] and hospitalization [OR 3.23, 95% CI 2.82–3.70].
Table 2.
Unadjusted associations of demographic and disease characteristics with COVID-19 outcomes [using univariate GEE models]
| Variable | ICU/ventilation/death | p-value | Hospitalization | p-value |
|---|---|---|---|---|
| OR [95% CI] | OR [95% CI] | |||
| PGA | ||||
| Remission/mild | Reference | Reference | ||
| Moderate | 1.46 [1.17–1.84] | 0.0011 | 1.59 [1.37–1.84] | <0.001 |
| Severe | 2.56 [1.76–3.70] | <0.001 | 3.74 [2.86–4.90] | <0.001 |
| COVID-19 period | ||||
| Nov 21 to Jul 21 | Reference | Reference | ||
| Mar 20 to Oct 20 | 2.35 [1.67–3.30] | <0.001 | 2.44 [1.76–3.39] | <0.001 |
| Country GDP [trillions USD] | 0.97 [0.95–0.997] | 0.029 | 1.01 [0.93–1.09] | 0.87 |
| ≤50 years | Reference | Reference | ||
| >50 years | 8.72 [5.96–12.77] | <0.001 | 3.23 [2.82–3.70] | <0.001 |
| Age [years] | 1.06 [1.06–1.07] | <0.001 | 1.04 [1.03–1.05] | <0.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.31 [0.94–1.82] | 0.12 | 1.33 [1.12–1.59] | 0.012 |
| Comorbidities | ||||
| None | Reference | Reference | ||
| 1 | 3.07 [2.30–4.11] | <0.001 | 2.14 [1.83–2.51] | <0.001 |
| ≥2 | 14.39 [10.73–19.31] | <0.001 | 5.98 [4.56–7.85] | <0.001 |
| Ethnicity | ||||
| White | Reference | Reference | ||
| Hispanic | 1.25 [0.77–2.02] | 0.37 | 1.36 [1.06–1.73] | 0.014 |
| Asian | 1.52 [0.84–2.76] | 0.17 | 1.82 [1.24–2.67] | 0.0021 |
| Black | 2.74 [1.96–3.84] | <0.001 | 2.29 [1.96–2.67] | <0.001 |
| Other/mixed | 1.03 [0.53–2.01] | 0.93 | 1.56 [0.98–2.47] | 0.061 |
| BMI | 1.01 [1.01–1.02] | <0.001 | 1.01 [1.004–1.02] | 0.0038 |
| IBD type | ||||
| CD | Reference | Reference | ||
| UC/IBD-U | 1.67 [1.37–2.05] | <0.001 | 1.38 [1.21–1.58] | <0.001 |
| Systemic corticosteroids | 3.92 [2.92–5.27] | <0.001 | 3.00 [2.31–3.90] | <0.001 |
| Anti-TNF mono [no IMM] | 0.34 [0.24–0.49] | <0.001 | 0.55 [0.48–0.62] | <0.001 |
| Anti-TNF combo [with IMM] | 0.82 [0.51–1.32] | 0.41 | 0.85 [0.66–1.10] | 0.22 |
| IMM mono [without anti-TNF] | 1.16 [0.80–1.69] | 0.44 | 1.13 [0.95–1.34] | 0.18 |
| Mesalamine/sulfasalazine | 1.96 [1.48–2.60] | <0.001 | 1.55 [1.30–1.83] | <0.001 |
| Anti-integrin mono [no IMM] | 1.21 [0.68–2.17] | 0.51 | 1.03 [0.88–1.21] | 0.67 |
| Ustekinumab mono [no IMM] | 0.50 [0.31–0.84] | 0.0079 | 0.67 [0.53–0.85] | 0.0010 |
BMI, body mass index; CD, Crohn’s disease; GDP, gross domestic product; GEE, generalized estimating equation; IBD-U, IBD-unclassified; ICU, intensive care unit; IMM, immunomodulator; OR, odds ratio; PGA, physician global assessment; TNF, tumour necrosis factor; UC, ulcerative colitis; USD, US dollars.
Table 3 summarizes the results of multivariable GEE analyses. IBD activity remained independently associated with hospitalization [moderate PGA: aOR 1.48, 95% CI 1.23–1.79; severe PGA: aOR 3.24, 95% CI 2.34–4.50]. However, the association between IBD activity and ICU/ventilation/death was lost after adjusting for the covariates shown. Covariates associated with increased risk of both adverse outcomes included diagnosis earlier in the pandemic [March to October 2020], older age, comorbidities and systemic corticosteroids, while anti-TNF monotherapy was protective. Male sex was associated with an increased risk of hospitalization.
Table 3.
Adjusted associations of demographic and disease characteristics with COVID-19 outcomes [using multivariable GEE models]
| Variable1 | ICU/ventilation/death2,3 | p-value | Hospitalization4 | p-value |
|---|---|---|---|---|
| aOR [95% CI] | aOR [95% CI] | |||
| PGA | ||||
| Remission/mild | Reference | Reference | ||
| Moderate | 1.18 [0.89–1.57] | 0.26 | 1.48 [1.23–1.79] | <0.001 |
| Severe | 1.56 [0.85–2.85] | 0.15 | 3.24 [2.34–4.50] | <0.001 |
| COVID-19 period | ||||
| Nov 21 to Jul 21 | Reference | Reference | ||
| Mar 20 to Oct 20 | 1.86 [1.42–2.44] | <0.001 | 2.28 [1.71–3.03] | <0.001 |
| Age [years] | 1.05 [1.04–1.06] | <0.001 | 1.03 [1.02–1.04] | <0.001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.15 [0.81–1.62] | 0.43 | 1.30 [1.09–1.55] | 0.0030 |
| Comorbidities | ||||
| None | Reference | Reference | ||
| 1 | 2.05 [1.59–2.65] | <0.001 | 1.72 [1.49–1.99] | <0.001 |
| ≥2 | 5.57 [4.40–7.04] | <0.001 | 3.42 [2.69–4.35] | <0.001 |
| IBD type | ||||
| CD | Reference | Reference | ||
| UC/IBD-U | 1.23 [0.93–1.64] | 0.15 | 1.10 [0.91–1.32] | 0.32 |
| Systemic corticosteroids | 2.99 [2.09–4.28] | <0.001 | 1.87 [1.37–2.53] | <0.001 |
| Anti-TNF mono [no IMM] | 0.54 [0.34–0.86] | 0.0098 | 0.71 [0.60–0.83] | <0.001 |
| Anti-TNF combo [with IMM] | 1.14 [0.59–2.21] | 0.69 | 0.97 [0.76–1.25] | 0.84 |
| IMM mono [without anti-TNF] | 1.16 [0.72–1.88] | 0.54 | 1.06 [0.84–1.34] | 0.62 |
| Mesalamine/sulfasalazine | 1.18 [0.84–1.65] | 0.33 | 1.12 [0.86–1.45] | 0.42 |
aOR, adjusted odds ratio; CD, Crohn’s disease; GEE, generalized estimating equation; IBD-U, IBD-unclassified; IMM, immunomodulator; PGA, physician global assessment; TNF, tumor necrosis factor; UC, ulcerative colitis
1Age, sex, comorbidities, IBD type, corticosteroids, anti-TNF monotherapy, anti-TNF combination therapy, immunomodulator monotherapy and 5ASA included a priori based on clinical relevance; only body mass index [BMI] changed estimate for PGA by ≥10% [for outcome of ICU/ventilation/death only] – included in sensitivity analysis only due to large percentage of missing data.
2In total, 114 observations missing, 240 events.
3Sensitivity analysis including BMI: 998 observations missing, 175 events, aOR for moderate PGA – 1.20 [95% CI 0.84–1.73], aOR for severe PGA – 1.46 [95% CI 0.73–2.92].
4In total, 114 observations missing, 912 events.
A sensitivity analysis including BMI for the outcome of ICU/ventilation/death yielded almost identical PGA effect estimates [moderate PGA: aOR 1.20, 95% CI 0.84–1.73; severe PGA: aOR 1.46, 95% CI 0.73–2.92].
3.4. Effect modification by age
Supplementary Figure 2 plots unadjusted ORs for severe PGA [vs remission/mild activity] according to age by decade for ICU/ventilation/death and hospitalization. These plots illustrate that the magnitude of effect varies with age; the strength of the association between IBD activity and adverse outcomes is greatest in younger patients, especially those <50 years.
3.5. Analyses stratified by age
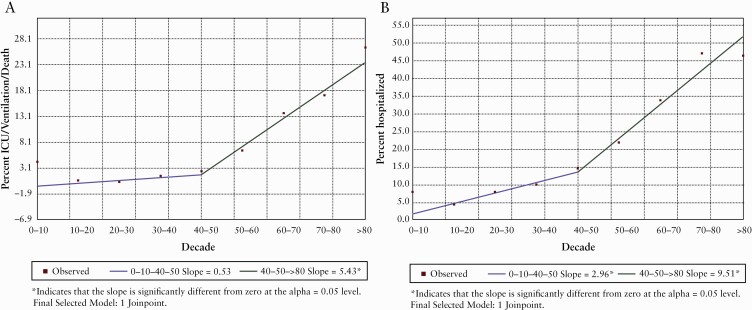
Figure 2 depicts the results of Joinpoint regression applied to the proportion of patients experiencing adverse COVID-19 outcomes by decade. A statistically significant change in slope is observed at 50 years for both ICU/ventilation/death and hospitalization. We therefore used a cut-off of 50 years [≤50 vs >50 years] for age-stratified analyses. In total, 9.6% [423/4419] and 1.2% [54/4419] of patients ≤50 years experienced hospitalization and ICU/ventilation/death, respectively, compared to 30% [504/1659] and 11% [189/1659], respectively, of patients >50 years, highlighting the major influence of older age on COVID-19 severity.
Figure 2.
Joinpoint regression. Proportion of patients experiencing ICU/ventilation/death [A] and hospitalization [B] by decade.
Supplementary Tables 3 and 4 summarize age-stratified unadjusted and adjusted analyses, respectively. In adjusted analyses, with the exception of the association between moderate PGA and hospitalization [which had a similar effect estimate for both age groups], younger patients displayed a stronger association between IBD activity and adverse outcomes. Specifically, in the ≤50 group, severe PGA was associated with an increased risk of ICU/ventilation/death [aOR 3.27, 95% CI 1.15–9.30, compared to aOR 0.89, 95% CI 0.37–2.15, in the >50 group] and hospitalization [aOR 4.62, 95% CI 2.83–7.55, compared to aOR 0.90, 95% CI 0.39–2.09, in the >50 group]. Sensitivity analyses including BMI were associated with similar results [Table 4 footnote]. In adjusted analyses, the negative effect of comorbidities, corticosteroids and earlier COVID-19 diagnosis period, and the protective effect of anti-TNF monotherapy on the outcome of ICU/ventilation death were greater in patients ≤50 years.
Table 4.
Adjusted associations of demographic and disease characteristics with COVID-19 outcomes stratified by age [using multivariable GEE models]
| Variable1 | ≤50 years | >50 years | ||||||
|---|---|---|---|---|---|---|---|---|
| ICU/vent/death2,3 | p-value | Hospitalization4,5 | p-value | ICU/vent/death6,7 | p-value | Hospitalization8,9 | p-value | |
| aOR [95% CI] | aOR [95% CI] | aOR [95% CI] | aOR [95% CI] | |||||
| PGA | ||||||||
| Remission/mild | Reference | Reference | Reference | Reference | 0.0044 | |||
| Moderate | 1.62 [0.74–3.59] | 0.23 | 1.44 [1.02–2.03] | 0.039 | 1.10 [0.94–1.29] | 0.22 | 1.53 [1.14–2.06] | |
| Severe | 3.27 [1.15–9.30] | 0.026 | 4.62 [2.83–7.55] | <0.001 | 0.89 [0.37–2.15] | 0.80 | 0.90 [0.39–2.09] | 0.81 |
| COVID-19 period | ||||||||
| Nov 21 to Jul 21 | Reference | Reference | Reference | Reference | ||||
| Mar 20 to Oct 20 | 2.46 [1.49–4.09] | <0.001 | 2.24 [1.69–2.97] | <0.001 | 1.65 [1.14–2.37] | 0.0073 | 2.23 [1.53–3.24] | <0.001 |
| Country GDP [trillions USD] | — | 0.99 [0.97–1.02] | 0.49 | — | — | |||
| Age [years] | 1.04 [1.003–1.09] | 0.034 | 1.03 [1.01–1.04] | <0.001 | 1.04 [1.02–1.05] | <0.001 | 1.03 [1.02–1.05] | <0.001 |
| Sex | ||||||||
| Female | Reference | Reference | Reference | |||||
| Male | 1.18 [0.98–1.42] | 0.076 | 1.25 [0.81–1.93] | 0.31 | 1.45 [1.07–1.95] | 0.015 | ||
| Comorbidities | ||||||||
| None | Reference | Reference | Reference | Reference | ||||
| 1 | 2.44 [1.77–3.36] | <0.001 | 1.74 [1.49–2.04] | <0.001 | 1.65 [1.04–2.60] | 0.032 | 1.73 [1.31–2.29] | <0.001 |
| ≥2 | 5.64 [2.17–14.64] | <0.001 | 2.81 [1.83–4.31] | <0.001 | 4.53 [3.58–5.73] | <0.001 | 3.54 [2.70–4.65] | <0.001 |
| Ethnicity | ||||||||
| White | Reference | Reference | ||||||
| Hispanic | 1.22 [0.81–1.84] | 0.34 | 1.20 [0.91–1.57] | 0.19 | ||||
| Asian | 1.48 [0.81–2.68] | 0.20 | 1.93 [0.82–4.58] | 0.13 | ||||
| Black | 2.22 [1.33–3.68] | 0.0022 | 2.74 [1.99–3.76] | <0.001 | ||||
| Other/mixed | 1.64 [0.76–3.50] | 0.20 | 1.79 [0.73–4.39] | 0.21 | ||||
| IBD type | ||||||||
| CD | Reference | Reference | Reference | |||||
| UC/IBD-U | — | 1.04 [0.86–1.27] | 0.66 | 1.14 [0.80–1.63] | 0.48 | 1.21 [0.93–1.58] | 0.16 | |
| Systemic corticosteroids | 3.68 [1.63–8.31] | 0.0018 | 1.83 [1.25–2.67] | 0.0018 | 2.89 [1.77–4.72] | <0.001 | 2.20 [1.39–3.49] | <0.001 |
| Anti-TNF mono [no IMM] | 0.21 [0.08–0.56] | 0.0018 | 0.70 [0.56–0.88] | 0.0022 | 0.66 [0.37–1.16] | 0.15 | 0.68 [0.51–0.90] | 0.0073 |
| Anti-TNF combo [with IMM] | — | 1.16 [0.87–1.55] | 0.31 | 1.04 [0.42–2.59] | 0.93 | 0.73 [0.44–1.22] | 0.23 | |
| IMM mono [without anti-TNF] | — | 1.06 [0.82–1.38] | 0.65 | 1.20 [0.66–2.21] | 0.55 | 1.17 [0.86–1.58] | 0.32 | |
| Mesalamine/sulfasalazine | — | 0.98 [0.81–1.19] | 0.82 | 1.16 [0.85–1.58] | 0.36 | 1.18 [0.76–1.84] | 0.47 |
aOR, adjusted odds ratio; CD, Crohn’s disease; GEE, GEE, generalized estimating equation; IBD-U, IBD-unclassified; IMM, immunomodulator; PGA, physician global assessment; TNF, tumour necrosis factor; UC, ulcerative colitis.
1Age, sex, comorbidities, IBD type, corticosteroids, anti-TNF monotherapy, anti-TNF combination therapy, immunomodulator monotherapy and 5ASA included in all models a priori based on clinical relevance [except for ICU/ventilation/death in ≤50 year group due to small number of events – see below]; other variables included if they changed estimate for PGA by ≥10% (body mass index [BMI] included in sensitivity analysis only due to large percentage of missing data).
2In total, 54 events only so covariates restricted to COVID-19 diagnosis period, age, comorbidities, corticosteroids and anti-TNF monotherapy based on clinical/statistical significance.
3Sensivity analysis including BMI: 590 observations missing, 45 events, aOR for moderate PGA – 2.09 [95% CI 0.82–5.03], aOR for severe PGA – 3.46 [95% CI 1.04–11.52].
4In total, 149 observations missing, 411 events.
5Sensitivity analysis including BMI: 716 observations missing, 326 events, aOR for moderate PGA – 1.52 [95% CI 1.05–2.20], aOR for severe PGA – 4.56 [95% CI 2.64–7.90].
6In total, 128 observations missing, 174 events.
7Sensitivity analysis including BMI: 414 observations missing, 121 events, aOR for moderate PGA 0.97 [95% CI 0.71–1.32], aOR for severe PGA – 0.75 [95% CI 0.23–2.49].
8In total, 128 observations missing, 468 events.
9Sensitivity analysis including BMI: 414 observations missing, 360 events, aOR for moderate PGA – 1.42 [95% CI 1.02–1.98], aOR for severe PGA – 0.98 [95% CI 0.38–2.53].
Recognizing that a small number of patients >50 years had severe PGA [as shown in Table 1], we repeated the above age-stratified analyses combining moderate and severe PGA [i.e. remission/mild PGA vs moderate/severe PGA]. In this analysis, there were 313 patients >50 years with moderate/severe PGA. Results are shown in Supplementary Table 4, again illustrating the greater magnitude of effect for active IBD in younger vs older patients.
4. Discussion
In this analysis of 6078 patients reported to the SECURE-IBD registry, we found that active IBD, reflected by moderate or severe PGA vs clinical remission or mild PGA, was associated with a significantly increased risk of severe COVID-19, defined as ICU admission, ventilation or death, and COVID-19-related hospitalization. The strength of the association between active IBD and adverse outcomes varied with age and was greatest in younger patients. In multivariable analyses adjusting for confounding factors, such as medication exposure, the association between severe clinical disease activity and COVID-19 outcomes was seen only in patients ≤50 years.
A few studies have previously explored the effect of IBD activity on COVID-19 outcomes. In an analysis of 209 paediatric IBD patients from the SECURE-IBD registry, moderate/severe clinical activity was associated with an increased risk of hospitalization,12 but the small sample size precluded adjustment for confounding variables, and outcomes such as ICU admission and death were too few to examine. In a prospective Italian study of 79 IBD patients with SARS-CoV-2, clinical IBD activity was identified as a risk factor for COVID-19 pneumonia and death.13 Again, multivariable analyses could not be performed due to small sample size. In a Danish nationwide IBD cohort study, amongst 76 patients with COVID-19, no independent association was observed between clinical or biochemical IBD activity and the outcomes of hospitalization, ICU admission, ventilation or death.14 Important differences with our study include the much smaller sample size and definition of active IBD as any degree of activity [including mild]. A longitudinal cohort study of 119 IBD patients from New York found that those with active IBD [clinically, endoscopically or biochemically] and those receiving corticosteroids were more likely to be infected with SARS-CoV-2, though without explicit comment on the link between IBD activity and severity of the infection.15 Beyond IBD, there is a robust body of literature supporting that poor control of other chronic diseases, such as diabetes mellitus, confers an increased risk of severe COVID-19.16
Our findings suggest that active IBD may be a particularly important modifiable risk factor for severe COVID-19 in younger patients. This may be because, in older patients, factors such as age, comorbidities and medications are primary drivers of risk, whereas in younger patients, disease activity plays a more prominent role. Differential practices by age for COVID-19 testing [possibly less testing of younger patients with mild disease] and shielding practices [possibly greater protective practices in older individuals] may also be relevant.
Several mechanisms may explain the independent association between IBD activity and COVID-19 severity. First, ACE2, the primary SARS-CoV-2 receptor, is widely expressed at the luminal surface of the GI tract.8,17 Transcriptomic analyses of intestinal biopsies demonstrate that inflammation influences ACE2 gene expression in a location-specific manner.18–20 The role of ACE2 in modulating COVID-19 progression is not entirely clear; although higher expression increases viral uptake by the host, ACE2 has important anti-inflammatory properties, which may help prevent the secondary ‘cytokine storm’ in severe COVID-19.20 The effect of gut inflammation on ACE2 expression represents an important putative mechanism by which IBD activity may influence COVID-19 outcomes. Network analyses show that IBD and COVID-19 pathways intersect molecularly and share several ‘key driver genes’.18 In addition, in severe COVID-19, thromboembolic complications are a major driver of significant morbidity and mortality.21–24 Active IBD, especially moderate–severe exacerbations requiring hospitalization, is an established risk factor for venous thromboembolism,25,26 and could potentially confer an incremental risk of thromboembolic complications in the setting of COVID-19, although this has yet to be demonstrated. Lastly, active IBD may be associated with poorer general health, which may place individuals at an increased risk of adverse COVID-19 outcomes. Notably, the association between IBD activity and more severe viral infection is not unique to COVID-19; IBD disease activity is an established risk factor for viral replication and worse outcomes with other viruses such as EBV and CMV,27,28 though it is difficult to disentangle the effects of chronic inflammation from immunosuppressive treatment.
Our finding of an association between active IBD and COVID-19 severity has several practical implications. First, it supports the position endorsed by North American and European societies that patients should continue their IBD therapies during the SARS-CoV-2 pandemic,29,30 to not precipitate a disease flare. Second, patients with moderately to severely active IBD should be counselled to adhere to practices that minimize their risk of contracting COVID-19 [such as staying home from school and/or work], given their risk of worse outcomes if they become infected. This is particularly applicable to younger patients, based on our findings. Our observations may also have implications for vaccine prioritization. Recent evidence suggests that IBD patients on anti-TNF have attenuated serological responses to SARS-CoV2 vaccines and that, accordingly, delayed second dosing should be avoided in such patients.31 Our results suggest that younger patients with severe IBD may warrant vaccine prioritization as well, including adherence to the recommended interval between vaccine doses. Along the same lines, our findings may also have implications for vaccine booster doses in the IBD population.
The strengths of our study include its very large size and international nature, the multivariable analyses controlling for multiple confounders, as well as our exploration of the effect of disease activity across the age spectrum.
We do, however, acknowledge several important limitations intrinsic to the study design and available data. First, reporting bias and selection bias are significant possible limitations given the use of a registry based on voluntary reporting. That said, the registry spans multiple countries, and the full spectrum of IBD activity and therapies [a surrogate for disease severity] was captured in our dataset. Nevertheless, our findings may not be generalizable to the broader IBD population. Given the observational nature of the data, our findings are associational, and causality cannot be inferred. An additional important limitation relates to the use of PGA, a subjective rating, as the indicator of disease activity, with no data available on biomarkers or endoscopic activity. In addition, the timing of PGA assessment may not have been precisely contemporaneous with COVID-19 diagnosis. Lastly, COVID-19-related GI symptoms may have influenced PGA assessment. While these are important limitations, there are several elements that appear to lend face validity to our findings. First, although there is probably variability in how PGA was applied, we explored the effect of using a different method to categorize PGA [remission or mild vs moderate or severe] and found similar results. Second, we generally observed a gradient effect, whereby severe PGA was associated with greater magnitudes of effect than moderate PGA. Although the PGA remains a subjective clinical assessment, it has been the reference standard in developing numerous indices,32,33 in both CD and UC, and its use is commonplace in clinical practice. Lastly, while physicians were instructed to report COVID-19-related complications, we cannot rule out the possibility that some outcomes [particularly hospitalization] were IBD-related.
In conclusion, we have demonstrated an association between clinically active IBD and adverse COVID-19 outcomes. Importantly, this association appears to vary with age, with a stronger relative effect in younger patients. These findings reinforce the importance of maintaining IBD disease control during the current pandemic and may support the implementation of strategies to mitigate the risk of SARS-CoV-2 infection [distancing, immunization] in IBD patients with severe disease to prevent poor COVID-19 outcomes.
Supplementary Material
Acknowledgments
We would like to thank all of the SECURE-IBD contributors.
Contributor Information
Amanda Ricciuto, SickKids IBD Centre, Division of Gastroenterology, Hepatology & Nutrition, The Hospital for Sick Children; Child Health Evaluative Sciences, SickKids Research Institute, Toronto, ON, Canada; Department of Paediatrics, University of Toronto, Toronto, ON, Canada.
Christopher A Lamb, Translational & Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK; Department of Gastroenterology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Eric I Benchimol, SickKids IBD Centre, Division of Gastroenterology, Hepatology & Nutrition, The Hospital for Sick Children; Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada; ICES, Toronto, ON, Canada.
Gareth J Walker, Department of Gastroenterology, Torbay and South Devon NHS Foundation Trust, Torquay, UK.
Nicholas A Kennedy, Department of Gastroenterology, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK; Exeter IBD Research Group, University of Exeter, Exeter, UK.
M Ellen Kuenzig, Child Health Evaluative Sciences, SickKids Research Institute, Toronto, ON, Canada.
Gilaad G Kaplan, Department of Medicine, University of Calgary, Calgary, AB, Canada.
Michael D Kappelman, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Ryan C Ungaro, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jean-Frederic Colombel, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Erica J Brenner, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Manasi Agrawal, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Walter Reinisch, Medical University of Vienna, Vienna, Austria.
Anne M Griffiths, SickKids IBD Centre, Division of Gastroenterology, Hepatology & Nutrition, The Hospital for Sick Children; Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada.
Shaji Sebastian, Department of Gastroenterology, Hull University Teaching Hospitals NHS Trust, Hull, UK; Faculty of Health Sciences, University of Hull, Hull, UK.
Funding
This work was supported by the Helmsley Charitable Trust [2003–04445], National Center for Advancing Translational Sciences [UL1TR002489], National Institutes of Health [T32DK007634 to E.B., K23DK129762-01 to M.A. and K23KD111995-01A1 to R.C.U.], Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion and Arenapharm. The funding agencies had no role in the design, analysis or interpretation of this work.
Conflict of Interest
A.R. has no disclosures to report. C.A.L. reports grants from Genentech, grants and personal fees from Janssen, grants and personal fees from Takeda, grants from AbbVie, personal fees from Ferring, grants from Eli Lilly, grants from Pfizer, grants from Roche, grants from UCB Biopharma, grants from Sanofi Aventis, grants from Biogen IDEC, grants from Orion OYJ, personal fees from Dr Falk Pharma, grants from AstraZeneca, all outside the submitted work. E.I.B. has acted as a legal consultant for Hoffman La-Roche Limited and Peabody & Arnold LLP for matters unrelated to a medication used to treat IBD. He has served as a consultant for McKesson Canada. G.J.W. has served as a speaker and/or advisory board member for AbbVie, Falk and Janssen. He has had support to attend meetings from AbbVie, Falk, Janssen and Norgine. His department has received research funding from Tillotts, outside the submitted work. N.A.K. reports personal fees from Falk, personal fees from Janssen, grants and personal fees from Pharmacosmos, personal fees from Takeda, personal fees from Tillotts, all outside the submitted work. M.E.K. has no disclosures to report. G.G.K. has received honoraria for speaking or consultancy from AbbVie, Janssen, Pfizer, Amgen and Takeda; has received research support from Ferring, Janssen, AbbVie, GlaxoSmith Kline, Merck and Shire; he has been a consultant for Gilead; he shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 Sept. 2018. M.D.K. has consulted for AbbVie, Janssen, Pfizer and Takeda, is a shareholder in Johnson & Johnson, and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm, all outside the submitted work. R.C.U. has served as an advisory board member or consultant for AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer and Takeda; research support from AbbVie, Boehringer Ingelheim, Pfizer, and an NIH K23 Career Development Award [K23KD111995-01A1], all outside the submitted work. J.F.C. has received research grants from AbbVie, Janssen Pharmaceuticals and Takeda; has received payment for lectures from AbbVie, Amgen, Allergan, Bristol-Myers Squibb Company, Ferring Pharmaceuticals, Shire, Takeda and Tillots; has received consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb Company, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Gilead, Iterative Scopes, Ipsen, Immunic, lmtbio, Inotrem, Janssen Pharmaceuticals, Landos, LimmaTech Biologics AG, Medimmune, Merck, Novartis, O Mass, Otsuka, Pfizer, Shire, Takeda, Tigenix, Viela bio; and holds stock options in Intestinal Biotech Development. E.J.B. receives funding from the following NIH grant: T32DK007634. She has no conflicts of interest to disclose. M.A. receives research support from the Dickler Family Fund, New York Community Trust and the Helmsley Charitable Trust Fund for SECURE-IBD. W.R. has served as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, Yakult; as a consultant for Abbott Laboratories, AbbVie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Gatehouse Bio Inc., Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia, and 4SC; as an advisory board member for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, and 4SC; and has received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, Janssen, MSD, Sandoz, Takeda. A.M.G. has received consultant fees from AbbVie, Amgen, Janssen, Merck and Pfizer, speaker fees from AbbVie and Janssen, and research support from AbbVie. S.S. holds research grants from Biogen, Takeda, AbbVie, Tillotts Pharma, Ferring and Biohit; served on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Celgene and Tillots Pharma; and has received speaker fees from AbbVie, Biogen, AbbVie, Janssen, Merck, Warner Chilcott and Falk Pharma, all outside the submitted work.
Author Contributions
A.R.: study concept and design; analysis and interpretation of data; drafting of the manuscript; statistical analysis; data verification. C.L.: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. E.I.B.: study concept and design; critical revision of the manuscript for important intellectual content; study supervision. G.J.W.: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. N.A.K.: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. M.E.K.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. G.G.K.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. M.D.K.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; data verification. R.C.U.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. J.F.C.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. E.J.B.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. M.A.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. W.R.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. A.M.G.: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision. S.S.: study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; study supervision. All authors had full access to all the data in the study and accept responsibility to submit for publication. Writing Assistance: None.
Conference Presentation
DDW 2021 [virtual], ECCO 2021 [virtual]
Data Sharing
The data dictionary is available at https://covidibd.org/. Study data for SECURE-IBD are available upon request by contacting the Steering Committee at COVID.IBD@unc.edu. Data will be shared after approval of a study proposal, with a signed data access agreement.
References
- 1. Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc 2020;21:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaughan L, Veruttipong D, Shaw JG, Levy N, Edwards L, Winget M. Relationship of socio-demographics, comorbidities, symptoms and healthcare access with early COVID-19 presentation and disease severity. BMC Infect Dis 2021;21:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananthakrishnan AN, Kaplan GG, Ng SC. Changing global epidemiology of inflammatory bowel diseases: sustaining health care delivery into the 21st Century. Clin Gastroenterol Hepatol 2020;18:1252–60. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 6. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021;70:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020;159:481–91.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg M, Royce SG, Tikellis C, et al. Imbalance of the renin–angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut 2020;69:841–51. [DOI] [PubMed] [Google Scholar]
- 9. Sebastian S, Walker GJ, Kennedy NA, et al. ; PROTECT-ASUC Study Group. Assessment, endoscopy, and treatment in patients with acute severe ulcerative colitis during the COVID-19 pandemic (PROTECT-ASUC): a multicentre, observational, case-control study. Lancet Gastroenterol Hepatol 2021;6:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee PH. Is a cutoff of 10% appropriate for the change-in-estimate criterion of confounder identification? J Epidemiol 2014;24:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brenner EJ, Pigneur B, Focht G, et al. Benign evolution of SARS-Cov2 infections in children with inflammatory bowel disease: results from two International Databases. Clin Gastroenterol Hepatol 2021;19:394–6.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bezzio C, Saibeni S, Variola A, et al. ; Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020;69:1213–7. [DOI] [PubMed] [Google Scholar]
- 14. Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID-19 among patients with inflammatory bowel disease—A Danish prospective population-based cohort study. Journal of Crohn’s and Colitis 2021;15:540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS; Jill Roberts Center Study Group Study Group; Weill Cornell Medicine-Gastrointestinal Study Group . Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology 2020;159:1541–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alyammahi SK, Abdin SM, Alhamad DW, Elgendy SM, Altell AT, Omar HA. The dynamic association between COVID-19 and chronic disorders: an updated insight into prevalence, mechanisms and therapeutic modalities. Infect Genet Evol 2021;87:104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin–angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suárez-Fariñas M, Tokuyama M, Wei G, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology 2021;160:287–301.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nowak JK, Lindstrøm JC, Kalla R, Ricanek P, Halfvarson J, Satsangi J. Age, inflammation, and disease location are critical determinants of intestinal expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology 2020;159:1151–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potdar AA, Dube S, Naito T, et al. Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease. Gastroenterology 2021;160:809–22.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58:1116–20. [DOI] [PubMed] [Google Scholar]
- 23. Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute pulmonary embolism in patients with Covid-19 at CT angiography and relationship to d-dimer levels. Radiology 2020;296:E189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology 2020;296:E186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol 2011;106:713–8. [DOI] [PubMed] [Google Scholar]
- 26. McCurdy JD, Kuenzig ME, Smith G, et al. Risk of venous thromboembolism after hospital discharge in patients with inflammatory bowel disease: a population-based study. Inflamm Bowel Dis 2020;26:1761–8. [DOI] [PubMed] [Google Scholar]
- 27. Reijasse D, Le Pendeven C, Cosnes J, et al. Epstein-Barr virus viral load in Crohn’s disease: effect of immunosuppressive therapy. Inflamm Bowel Dis 2004;10:85–90. [DOI] [PubMed] [Google Scholar]
- 28. Wisniewski A, Kirchgesner J, Seksik P, et al. ; the Saint-Antoine IBD network. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United European Gastroenterol J 2020;8:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kennedy NA, Jones GR, Lamb CA, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020;69:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology 2020;159:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy NA, Lin S, Goodhand JR, et al. ; Contributors to the CLARITY IBD study. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021;70:1884–93. [DOI] [PubMed] [Google Scholar]
- 32. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 33. Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.