Abstract
Background
Comedication with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) during treatment with tumour necrosis factor inhibitors (TNFi) is extensively used in psoriatic arthritis (PsA), although the additive benefit remains unclear. We aimed to compare treatment outcomes in patients with PsA treated with TNFi and csDMARD comedication versus TNFi monotherapy.
Methods
Patients with PsA from 13 European countries who initiated a first TNFi in 2006–2017 were included. Country-specific comparisons of 1 year TNFi retention were performed by csDMARD comedication status, together with HRs for TNFi discontinuation (comedication vs monotherapy), adjusted for age, sex, calendar year, disease duration and Disease Activity Score with 28 joints (DAS28). Adjusted ORs of clinical remission (based on DAS28) at 12 months were calculated. Between-country heterogeneity was assessed using random-effect meta-analyses, combined results were presented when heterogeneity was not significant. Secondary analyses stratified according to TNFi subtype (adalimumab/infliximab/etanercept) and restricted to methotrexate as comedication were performed.
Results
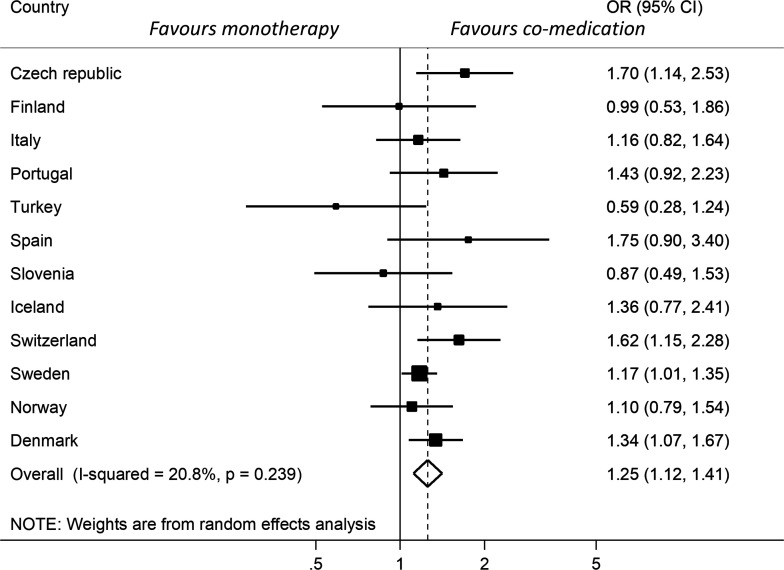
In total, 15 332 patients were included (62% comedication, 38% monotherapy). TNFi retention varied across countries, with significant heterogeneity precluding a combined estimate. Comedication was associated with better remission rates, pooled OR 1.25 (1.12–1.41). Methotrexate comedication was associated with improved remission for adalimumab (OR 1.45 (1.23–1.72)) and infliximab (OR 1.55 (1.21–1.98)) and improved retention for infliximab. No effect of comedication was demonstrated for etanercept.
Conclusion
This large observational study suggests that, as used in clinical practice, csDMARD and TNFi comedication are associated with improved remission rates, and specifically, comedication with methotrexate increases remission rates for both adalimumab and infliximab.
Keywords: arthritis, psoriatic, tumour necrosis factor inhibitors, methotrexate
Key messages.
What is already known about this subject?
Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) are often used in combination with tumour necrosis factor (TNF)-inhibitors in psoriatic arthritis, although the added benefit of such comedication, over TNF-inhibitor monotherapy, has been disputed.
What does this study add?
Treatment retention of TNF-inhibitors varied significantly across countries, as did the utilisation of a concomitant csDMARD, and overall, there was no additional improvement in TNF-inhibitor retention when used together with a csDMARD.
Comedication with methotrexate in patients treated with adalimumab or infliximab was associated with a 50% increase in the probability of achieving DiseaseActivity Score with 28 joints (DAS28) remission at 1 year, compared with TNF-inhibitor monotherapy.
Comedication with csDMARDs in patients treated with etanercept provided no additional advantage over TNF-inhibitor monotherapy, in terms of either retention or DAS28 remission rates.
How might this impact on clinical practice or future developments?
Our findings support the prevailing clinical strategy of combining monoclonal TNF-inhibitors with methotrexate in psoriatic arthritis.
Introduction
Tumour necrosis factor inhibitors (TNFi) have become a cornerstone in the treatment of psoriatic arthritis (PsA). Despite this, no international consensus has been reached regarding the optimal use of TNFi in PsA. Thus, the current American College of Rheumatology guidelines recommend using TNFi as a first-line disease-modifying antirheumatic drug (DMARD), and, in patients previously failing a conventional synthetic conventional synthetic DMARD (csDMARD), to switch to rather than to add a TNFi.1 In contrast, the European Alliance of Associations for Rheumatology (EULAR) recommendations suggest using csDMARDs (methotrexate in particular) as the first-line DMARD and to then step up the treatment by adding, rather than switching to, a biological DMARD, such as a TNFi.2
The discrepancy in international guidelines stems from the limited and conflicting data comparing the different treatment strategies. While the effect of TNFi has been compellingly demonstrated in all domains of PsA,3 the effect of methotrexate is derived from expert opinion,2 one large randomised clinical trial (RCT) (which failed to show an effect of methotrexate),4 randomised trials not specifically designed to assess this effect5 6 and a few smaller randomised trails.7 8 The recent Study of Etanercept and Methotrexate in Subjects with Psoriatic Arthritis (SEAM)-trial, comparing etanercept and methotrexate monotherapy with combination therapy, demonstrated a superior response for etanercept compared with methotrexate, but additionally, a good response to methotrexate monotherapy.9 Importantly, no additional effect of combination therapy over etanercept monotherapy was demonstrated.9
Available data so far suggest that csDMARD (especially methotrexate) and TNFi comedication therapy, compared with TNFi monotherapy, is not superior in terms of treatment response but may be beneficial for TNFi treatment retention.10–14 Nevertheless, a recent study from a collaboration across European treatment registries (the European Spondyloarthritis Research collaboration Network: EuroSpA) indicated that 60% of patients with PsA starting a TNFi used a concomitant csDMARD, and that 81% had previously used a csDMARD, suggesting extensive use of comedication in routine care.15 The 2019 EULAR recommendations for the management of PsA stipulate that more data are needed on this subject.2
The objective of this observational study of patients with PsA in routine care was, therefore, to compare the 1-year TNFi treatment retention and treatment response on joint manifestations, in patients starting a first TNFi as monotherapy compared with those starting a TNFi as combination therapy, that is, together with a csDMARD.
Methods
This is an observational study based on prospectively collected data from 13 rheumatology registers in Europe, aggregated through the EuroSpA collaboration (as previously described15).
Data sources
Patients with PsA, aged 18 years or older, starting a first TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab) in 2006–2017 were identified from the following countries (registers): Czech Republic (ATTRA), Denmark (DANBIO), Finland (ROB-FIN), Iceland (ICEBIO), Italy (GISEA), Norway (NOR-DMARD), Portugal (Reuma.pt), Romania (RRBR), Slovenia (biorx.si), Spain (BIOBADASER), Sweden (ARTIS), Switzerland (SCQM) and Turkey (TURKBIO).
Time-point definitions and treatment groups
All participating registers recorded start dates (and stop dates in case of withdrawal) for the TNFi. The start date of the patients’ first TNFi was set as the baseline date, and the baseline visit was defined as the visit closest to the registered start date, within −100 to +30 days, giving priority to dates before the start date. The 3-month, 6-month and 12-month follow-up visits during treatment were defined as the date of visit closest to these time points, within the ranges: day 60–150, day 151–270 and day 271–545, respectively, counting from the baseline date.
Registration of csDMARD use varied across the registers, with some recording start and stop dates, while others record treatment status (use/no use) at registered visits. Thus, comedication use was either based on the start and stop dates (where available) for the csDMARD or on data on treatment status at registered visits.
The following two treatment exposure groups were defined: (1) The TNFi monotherapy group (=monotherapy group) including all patients starting a first TNFi without concurrent use of a csDMARD in a period from 100 days before to 30 days after baseline and (2) The TNFi and csDMARD comedication group (=comedication group) including all patients either starting a first TNFi together with a csDMARD (within 30 days) or starting a TNFi added to an already ongoing (and continued) csDMARD treatment. Changes in csDMARD treatment (withdrawal or switch to other csDMARD) during follow-up beyond 30 days from the baseline visit were not considered.
In the main analyses, the following csDMARDs were included: methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, chloroquine, cyclosporine, azathioprine, mycophenolate and cyclophosphamide.
Patients were followed up from the baseline date until first of: TNFi stop date, last visit date +12 months, end of patient’s participation in the register or end of the study period (31 December 2017).
Treatment retention
The 1-year treatment retention of the TNFi in the two treatment groups was compared through crude survival curves (Kaplan-Meier curves), and as HRs for TNFi discontinuation, with monotherapy as the reference.
Treatment response
Clinical remission at 12 months of treatment was defined following a hierarchical approach (online supplemental figure S1): for patients remaining on TNFi treatment beyond 12 months, clinical remission was defined as 28-joint Disease Activity Score with 28 joints and CRP (DAS28-CRP) <2.6 at the 12- month visit.16 For patients with follow-up and treatment longer than 12 months, but with DAS28 missing at the 12-month visit, a DAS28 recorded at 6 months and up to 12 months was carried forward. Patients discontinuing the TNFi before 12 months due to adverse events or lack of effect were considered not having achieved remission. Patients discontinuing the TNFi due to remission and not starting another TNFi before 12 months (27 patients) were considered as remaining in remission. Patients discontinuing the TNFi before 12 months for other reasons (eg, pregnancy) were considered as missing data. The reason for TNFi discontinuation was missing in only 11% of the patients with monotherapy and 3% with comedication.
annrheumdis-2021-220097supp001.pdf (686.8KB, pdf)
Statistical analyses
Baseline characteristics of the patients are presented country specific and pooled for all countries, as means and SD (continuous variables) or percentages (categorical variables).
Significant heterogeneity between countries was anticipated for both TNFi retention and treatment response. Therefore, all analyses were first performed individually per country, and only after disproving heterogeneity, combined results were presented (see below).
Country-specific HR of TNFi discontinuation was estimated using Cox regression adjusted for age, sex, calendar year, DAS28-CRP and disease duration at baseline.
Country-specific response rates of the two treatment groups were compared, based on the proportions and ORs of achieving clinical remission at 12 months and visualised through the average across individual patients’ delta-(∆)-DAS28-CRP (baseline DAS28-CRP minus 12 month’s DAS28-CRP). ORs of achieving remission at 12 months (yes/no) were estimated using logistic regression adjusted for the same variables as mentioned above.
Overall results from both the Cox regression and the logistic regression analyses, per country, were combined using a random-effect meta-analysis. Statistical heterogeneity among countries was evaluated with the Cochran Q-test and the I2 statistic in order to assess the proportion of total variation that was due to between-country variation, based on the included cases with nonmissing data on the respective outcome.17
All data are reported as observed. No imputation of missing data was performed. In the regression models, baseline disease duration and DAS28 were categorised into quartiles to enable the use of a fifth category for missing data. The effectiveness analyses were modelled only on patients with remission outcome data, and DAS28 at 6 months was carried forward if missing at 12 months.
Retention and effectiveness analyses were performed using R, V.3.6.3 (R Core Team (2020)). Meta-analyses were performed using Stata, V.14.2 (StataCorp, College Station, Texas, USA). Throughout all analyses, the level of statistical significance was set to 0.05. The proportional hazards assumption (for retention analyses) was assessed using the cox.zph function of the R survival package.
Secondary analyses
All initial analyses were performed combining data for the different TNFi. Biosimilars were not distinguished from originators, and switches between originators and corresponding biosimilars were disregarded.
Due to the potential differential effect of csDMARD comedication according to the type of TNFi and comedication, secondary analyses were performed separately for the subgroup of patients treated with the most common TNFi (infliximab, adalimumab and etanercept: constituting 82% of the TNFi in the comedication group and 86% in the monotherapy group) and exploring the most common csDMARD, that is, methotrexate. In these subset analyses, country-specific comparisons were only performed if at least 30 patients were included in each of the treatment groups.
Patient and public involvement
Patients were not involved in the study.
Results
In total, 15 332 patients starting a TNFi were included, of whom 9440 (62%) were included in the comedication group (table 1) and 5892 (38%) in the TNFi monotherapy group.
Table 1.
Baseline characteristics (mean (SD) or percentages) of patients with PsA starting their first TNFi as monotherapy or in combination with a csDMARD, pooled across all countries
| TNFi and csDMARD co-medication | TNFi monotherapy | |||
| Proportion missing data | Proportion missing data | |||
| N (%) | 9440 (62%) | 5892 (38%) | ||
| Age, years, mean (SD) | 48.9 (12.1) | 0% | 49.0 (12.6) | 0% |
| Sex (male), % | 50% | 0% | 48% | 0% |
| Disease duration, years mean (SD) | 6.3 (7.1) | 28% | 6.2 (7.3) | 32% |
| CRP, mg/L, mean (SD) | 13.6 (21.3) | 18% | 9.1 (16.9) | 25% |
| Tender joints 28, mean (SD) | 6.2 (5.9) | 21% | 5.6 (6.1) | 32% |
| Swollen joints 28, mean (SD) | 3.9 (4.2) | 21% | 2.9 (4.0) | 32% |
| VAS global health, mm, mean (SD) | 59.2 (24.0) | 21% | 58.1 (25.6) | 28% |
| VAS pain, mm, mean (SD) | 57.4 (23.6) | 30% | 56.3 (25.7) | 34% |
| DAPSA28, mean (SD) | 28.8 (16.0) | 38% | 26.0 (16.9) | 46% |
| DAS28-CRP, mean (SD) | 4.2 (1.2) | 29% | 3.8 (1.3) | 41% |
| Type of TNFi | ||||
| Adalimumab, % | 32% | – | 33% | – |
| Certolizumab pegol, % | 6% | – | 4% | – |
| Etanercept, % | 30% | – | 40% | – |
| Golimumab, % | 11% | – | 10% | – |
| Infliximab, % | 20% | – | 13% | – |
| Type of csDMARD used as co-medication | ||||
| Methotrexate, % | 79% | – | – | – |
| Sulfasalazine, % | 15% | – | – | – |
| Leflunomide, % | 11% | – | – | – |
| Other*, % | 6% | – | – | – |
*See the list of all csDMARDs in Methods section.
CRP, C reactive protein; csDMARD, conventional synthetic disease modifying anti-rheumatic drugs; DAPSA28, disease activity index for psoriatic arthritis with 28 joints; DAS28-CRP, disease activity score with 28 joints and CRP; PsA, psoriatic arthritis; TNFi, tumour necrosis factor inhibitors; VAS, visual analogue scale.
Methotrexate was the most frequently used csDMARD (79%). There was a large variation in the proportion of monotherapy versus comedication across registers (monotherapy 1%–79%, figure 1 and online supplemental table S2). Overall, baseline characteristics were similar between the two treatment groups, with a slightly higher DAS28-CRP in the comedication group compared with the monotherapy group (4.2 vs 3.8, p value <0.001), a higher proportion of patients treated with infliximab in the comedication group and a higher proportion with etanercept in the monotherapy group. The mean number of swollen and tender joints was also higher in the comedication group. Baseline characteristics per country are presented in online supplemental table S2 and the proportions of missing data in online supplemental table S3. Data from one register (Romania) were excluded from the stratified analyses due to a large imbalance between the treatment groups (<5 patients included in the monotherapy group).
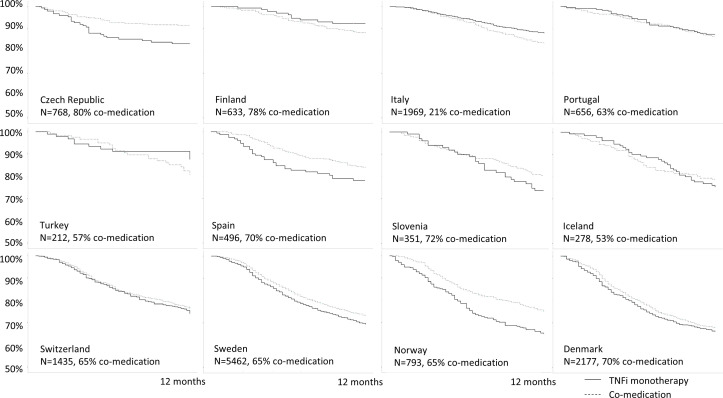
Figure 1.
Country-specific 1 year treatment retention of co-medication and TNFi monotherapy (Kaplan-Meier retention curves), ordered by overall TNFi retention of the countries, from highest to lowest. Romania is not included in the figure due to <5 patients in the monotherapy group. TNFi, tumour necrosis factor inhibitors.
Treatment retention
The overall treatment retention of the TNFi differed between the countries, and the heterogeneity was statistically significant (I2=62.7%; p=0.002). It was, therefore, determined inappropriate to proceed with presentation of the combined result in a meta-analysis. The country-specific crude retention curves (figure 1) showed not only modest differences between the two treatment groups but also different directions of the effect of comedication versus TNFi monotherapy across countries. The retention curves of the individual countries by csDMARD use, demonstrated in figure 1, are ordered by the overall retention rates (from highest to lowest) of the TNFi in the registers. The overall TNFi retention regardless of comedication is shown in online supplemental figure S2.
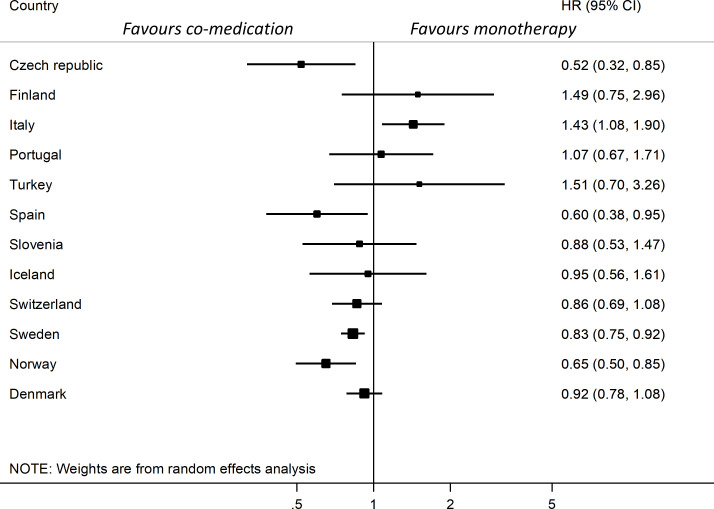
The country-specific HR for discontinuation, adjusted for age, sex, calendar year, disease duration and DAS28, also showed different directions for the association between drug retention and use of comedication (figure 2). The HRs in eight of the countries were below 1 (range 0.52–0.95), indicating better retention for comedication, and above 1 in four countries (range 1.07–1.51) favouring monotherapy, but only in five of all 12 countries were these findings statistically significant, figure 2. The assumption of proportional hazards was not rejected for all countries but Spain. Due to the clear direction of the results in Spain, this was not analysed further.
Figure 2.
Forest plot of country-specific hazard ratios for TNFi discontinuation at 12 months comparing TNFi and csDMARD co-medication with TNFi monotherapy, ordered by overall TNFi retention rate per country. Adjusted for baseline age, sex, calendar year, DAS28 and disease duration. Combined results are not presented due to significant heterogeneity. Data from Romania are not presented due to <5 patients with monotherapy. csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; DAS28, Disease Activity Score with 28 joints; TNFi, tumour necrosis factor inhibitors.
Treatment response
In contrast to the retention rates, the variation in response rates across the included countries was less pronounced, and there was no significant heterogeneity (I2=20.8%; p=0.239). The overall OR for achieving clinical remission at 12 months in the comedication group versus the monotherapy group was 1.25 (1.12–1.41) favouring comedication (figure 3). Excluding the two countries considered to be outliers in terms of the proportion of patients on comedication treatment (21% and 99%) resulted in a very similar pooled OR of 1.27 (1.11–1.44) for remission. The ∆DAS28-CRP and the crude proportions achieving remission across the different registers (figure 4A, B) also suggested a tendency towards better outcomes in the co-medication groups. In total, 72% of the patients had complete outcome data and were included in the response analyses. Regarding patients in the csDMARD group who remained on the TNFi at 6 months, 82% also remained on csDMARD treatment.
Figure 3.
Forest-plot of country-specific ORs and overall OR for clinical remission at 12 months in TNFi and csDMARD co-medication compared with TNFi monotherapy. Adjusted for baseline age, sex, calendar year, DAS28 and disease duration. Data from Romania are not presented separately due to <5 patients with monotherapy. csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; DAS28, Disease Activity Score with 28 joints; TNFi, tumour necrosis factor inhibitors.
Figure 4.
DAS28-CRP response at 12 months per country.(A) Delta DAS28-CRP between baseline and 12 months. (B) Proportions achieving remission at 12 months. Data from Romania are not presented due to <5 patients with monotherapy. csDMARDs, conventional synthetic disease-modifying ant-irheumatic drugs; DAS28, Disease Activity Score with 28 joints; TNFi, tumour necrosis factor inhibitors.
Secondary analyses
The results of the secondary analyses, assessing the effect of methotrexate comedication separately for infliximab, adalimumab and etanercept, are presented in table 2.
Table 2.
Crude TNFi retention and adjusted HR for TNFi discontinuation (upper part of table), and crude proportion achieving remission and adjusted OR for clinical remission (lower part of table) for infliximab, adalimumab and etanercept, in co-medication with methotrexate compared with monotherapy
| Infliximab | Adalimumab | Etanercept | ||
| One-year TNFi retention (%) and adjusted* HR for TNFi discontinuation (ref=monotherapy) | ||||
| Czech republic | co-med/mono | NA | 92%/79% | 89%/89% |
| HR (95% CI) | NA | 0.36 (0.17 to 0.77) | 1.06 (0.31 to 3.63) | |
| Finland | co-med/mono | NA | 87%/90% | 95%/96% |
| HR (95% CI) | NA | 1.18 (0.41 to 3.35) | 2.08 (0.31 to 13.94) | |
| Italy | co-med/mono | 80%/87% | 82%/88% | 85%/89% |
| HR (95% CI) | 1.45 (0.63 to 3.35) | 1.63 (0.98 to 2.72) | 1.45 (0.84 to 2.51) | |
| Portugal | co-med/mono | NA | 89%/97% | 89%/84% |
| HR (95% CI) | NA | 7.39 (1.46 to 37.54) | 0.60 (0.28 to 1.30) | |
| Spain | co-med/mono | NA | 82%/82% | 77%/78% |
| HR (95% CI) | NA | 0.70 (0.26 to 1.90) | 0.76 (0.30 to 1.90) | |
| Slovenia | co-med/mono | NA | 80%/67% | NA |
| HR (95% CI) | NA | 0.90 (0.41 to 1.96) | NA | |
| Iceland | co-med/mono | 81%/78% | NA | NA |
| HR (95% CI) | 0.81 (0.39 to 1.70) | NA | NA | |
| Switzerland | co-med/mono | 77%/73% | 79%/72% | 80%/78% |
| HR (95% CI) | 0.78 (0.39 to 1.58) | 0.67 (0.45 to 1.00) | 0.81 (0.49 to 1.35) | |
| Sweden | co-med/mono | 71%/63% | 78%/66% | 76%/74% |
| HR (95% CI) | 0.65 (0.50 to 0.85) | 0.58 (0.47 to 0.72) | 0.94 (0.77 to 1.14) | |
| Norway | co-med/mono | NA | 83%/68% | 81%/70% |
| HR (95% CI) | NA | 0.59 (0.24 to 1.48) | 0.59 (0.35 to 1.01) | |
| Denmark | co-med/mono | 64%/45% | 71%/70% | 70%/72% |
| HR (95% CI) | 0.56 (0.41 to 0.78) | 0.93 (0.70 to 1.24) | 1.12 (0.77 to 1.62) | |
| Crude proportion (%) reaching remission at 12 months and adjusted* OR for clinical remission (ref=monotherapy) | ||||
| Pooled | co-med/mono | 38%/32% | 47%/38% | 44%/42% |
| OR (95% CI) | 1.55 (1.21 to 1.98) | 1.45 (1.23 to 1.72) | 1.12 (0.95 to 1.31) | |
| Czech republic | co-med/mono | NA | 57%/38% | 68%/57% |
| OR (95% CI) | NA | 2.25 (1.23 to 4.20) | 1.69 (0.66 to 4.33) | |
| Finland | co-med/mono | NA | NA | NA |
| OR (95% CI) | NA | NA | NA | |
| Italy | co-med/mono | 29%/42% | 43%/45% | 46%/47% |
| OR (95% CI) | 0.59 (0.12 to 2.58) | 1.07 (0.55 to 2.07) | 1.09 (0.59 to 2.02) | |
| Portugal | co-med/mono | NA | 54%/49% | 51%/54% |
| OR (95% CI) | NA | 1.26 (0.49 to 3.27) | 1.40 (0.64 to 3.15) | |
| Spain | co-med/mono | NA | NA | NA |
| OR (95% CI) | NA | NA | NA | |
| Slovenia | co-med/mono | NA | 42%/47% | NA |
| OR (95% CI) | NA | 0.69 (0.28 to 1.68) | NA | |
| Iceland | co-med/mono | NA* | NA | NA |
| OR (95% CI) | NA* | NA | NA | |
| Switzerland | co-med/mono | NA | 40%/33% | 37%/21% |
| OR (95% CI) | NA | 1.68 (0.94 to 3.06) | 2.94 (1.28 to 7.18) | |
| Sweden | co-med/mono | 37%/27% | 43%/33% | 41%/38% |
| OR (95% CI) | 1.73 (1.17 to 2.60) | 1.59 (1.2 to 2.11) | 1.03 (0.82 to 1.31) | |
| Norway | co-med/mono | NA | 54%/47% | 49%/50% |
| OR (95% CI) | NA | 1.58 (0.54 to 4.66) | 0.95 (0.52 to 1.74) | |
| Denmark | co-med/mono | 34%/21% | 45%/37% | 42%/37% |
| OR (95% CI) | 2.01 (1.13 to 3.72) | 1.42 (0.99 to 2.03) | 1.15 (0.72 to 1.85) | |
HR and OR are adjusted for age, sex, calendar year, disease duration and DAS28-CRP.
co-med/mono indicates the crude 1 year TNFi retention rate and the proportion in the co-medication/monotherapy groups reaching remission, respectively.
*NA=not available due to <30 patients in at least one of the exposure groups. Data from Romania and Turkey are not included in the table because they provided no strata in the analysis with ≥30 patients in both groups. Adjusted for baseline age, sex, calendar year, DAS28-CRP and disease duration.
CRP, C reactive protein; DAS28, Disease Activity Score with 28 joints; TNFi, tumour necrosis factor inhibitors.
Only three countries provided enough patients for all different strata of the secondary analyses. The number of patients included in the secondary analyses is presented in online supplemental table S4. In general, the differences in crude retention rates and the proportion reaching clinical remission were modest across the different TNFi, particularly between etanercept and adalimumab. The retention rate of etanercept was in line with that of the adalimumab comedication groups and somewhat higher than the infliximab groups (table 2). In four out of five contributing countries, the HR for infliximab discontinuation was in favour of methotrexate comedication, but this was only statistically significant in two of the countries. For adalimumab, the HR of TNFi discontinuation was in favour of methotrexate comedication in 7 out of 10 contributing countries (with statistically significant values below 1 in two countries and above 1 in one country). For etanercept, the HR of discontinuation was in favour of methotrexate comedication in five countries and in favour of monotherapy in four contributing countries (all results were nonsignificant).
The combined OR for remission indicated better outcomes for methotrexate comedication compared with monotherapy for infliximab (1.55 (1.21–1.98)) and adalimumab (1.45 (1.23–1.72)), whereas no such association was observed for etanercept (1.12 (0.95–1.31)).
The distribution of DAS28 and the number of tender and swollen joints at baseline in the secondary analyses are presented in online supplemental figure S3. The comedication treatment groups were associated with higher baseline values of both DAS28 and number of swollen joints, indicating more active peripheral joint disease.
Discussion
In this study of 15 332 patients with PsA initiating a first ever TNFi, based on data from 13 European countries, we found that csDMARD comedication was associated with a 25% improved rate of clinical remission at 12 months, compared with TNFi monotherapy. Regarding TNFi treatment retention, the results varied across the countries and no combined estimate could be assessed due to the significant heterogeneity. In the secondary analyses, significantly improved remission rates were observed for adalimumab and infliximab when used together with methotrexate, and a trend towards better treatment retention for infliximab, while no advantage was found for combining etanercept with methotrexate.
The initial RCTs for etanercept,18 infliximab19 and adalimumab20 did not indicate a difference in response between patients on monotherapy versus comedication with methotrexate, although these trials were generally restricted to patients with inadequate response to csDMARD. Similarly, the recent SEAM-trial9 and previous observational studies have in general not indicated any additional treatment response for methotrexate when used in combination with a TNFi,10 although several studies have demonstrated improved TNFi retention.10–13 21 Based on these previous studies, our finding of an increased proportion achieving clinical remission in the csDMARD comedication group was unexpected.
Another recent register-based study (including data from three of the same registers, as the present study: Italy, Czech Republic and Switzerland) found no effect of comedication on treatment response.22 However, in that study, all TNFi were analysed together. By contrast, the results of our stratified secondary analysis suggest that clear differences between the TNFi can explain some of this discrepancy and that combining the drug-specific effects may have diluted the overall effect observed in that study. In line with previous studies, we found a trend for better TNFi treatment retention for infliximab, when used together with methotrexate, but not for etanercept, which corroborates the results from the SEAM trial.9 Further and similar to the SEAM trial, we found no additive effect on treatment response of methotrexate when added to etanercept.9 Our findings of a 55% and 45% higher odds for reaching remission at 12 months in the methotrexate comedication groups of infliximab and adalimumab, respectively, are novel findings. In particular, for adalimumab, the validity of these results is supported by the fact that all except one of the countries, included in that analysis, presented ORs in the same direction.
This study has some limitations. First, in contrast to the relatively uniform results for the response rates, the pronounced intercountry differences in TNFi retention suggests that factors other than biological/pharmaceutical may have an influence on observed retention rates. Since the understanding of how such factors (eg, availability of drugs, prescription regulations and insurance policies) may influence the retention rates is incomplete, it was deemed beyond the scope of this paper to explore this further. However, these findings suggest that treatment retention should be analysed in a way that accounts for such factors, and that pooling of retention data across countries should be performed with caution. Furthermore, use of csDMARD comedication in TNFi-treated patients varied from 21% to 99% across the registers, suggesting large differences in treatment strategies in the participating countries. However, we do not believe that this biases the results, since excluding the outliers barley changed the pooled response outcome, and since there were no clear correlations between the proportions treated with comedication, and the direction and magnitude of remission and retention rates.
Second, use of 28-joint counts to define remission in PsA is inferior to the recommended 66/68-joint counts. However, this should not introduce a bias across the exposure groups, particularly not with regards to differentiation between axial and peripheral disease.
Third, misclassification may be an issue, and since PsA classification criteria are not uniformly registered across the registers, case ascertainment was based on the clinical diagnosis entered by the treating rheumatologist. When aggregating data from a large number of different registers, further difficulties arise regarding the operational definitions of exposures and outcomes and in assessment of nonrandom missing data. Fourth, of the 13 countries initially included, only three contributed enough patients to be included in all analyses (including secondary analyses). The large differences in the number of patients from each register will also inevitably lead to an unequal impact on the combined results.
Fifth, confounding by indication is likely to affect the results, since both the choice of TNFi and the decision to use csDMARD comedication may be influenced by factors such as axial versus peripheral disease, the extent of cutaneous psoriasis and other comorbidities. In this study, we could not precisely identify axial disease or other concomitant comorbidities. In the secondary analyses, the baseline distributions of swollen joint count and DAS28 (online supplemental figure 3) suggested that patients with methotrexate comedication had a higher peripheral joint disease activity, despite having higher OR of reaching clinical remission, thus supporting an effect of comedication.
Finally, changes in csDMARD treatment over the 1-year follow-up period were not taken into account, since it was not within the aims of the present study to assess csDMARD retention and since csDMARD data (eg, doses and start/stop dates) are poorly captured in the majority of the registers.
Conversely, our study has several strengths. First, it includes a large number of patients with PsA with prospectively collected data. Second, the possibility of comparing retention and response rates across several different registers and to assess heterogeneity, adds considerable robustness to the results and their interpretation. Third, the large number of patients enabled stratification of the analysis according to the type of TNFi and methotrexate comedication.
In conclusion, we found improved clinical response rates when combining TNFi with a csDMARD. More specifically, the rate of clinical remission for infliximab and adalimumab increased when combined with methotrexate, and the retention of infliximab was improved. For etanercept, the remission and retention rates did not differ between comedication and monotherapy—and were in line with the rates observed for adalimumab comedication. Our findings support the prevailing strategy, in a situation of incomplete response, to continue methotrexate therapy when commencing treatment with infliximab or adalimumab, while for etanercept methotrexate may be discontinued.
Footnotes
Handling editor: Josef S Smolen
Twitter: @hteraG_senoJ
Contributors: All coauthors have contributed significantly in accordance with contributorship guidelines.
Funding: This work was supported by Novartis. Novartis had no influence on the data collection, statistical analyses, manuscript preparation or decision to submit. The work was also supported by NordForsk.
Competing interests: BeG reports grants from BMS, grants from Pfizer, grants from AbbVie, outside the submitted work. AC reports consultancy and speaker fees from Abbvie, Eli Lilly, Merck Sharp & Dohme, Novartis and Pfizer. MP-S reports speaker and research fees from Gilead, Janssen, MSD and Sanofi. KE reports consulting fees from Lilly, Biogene, Sobi, Pfizer and Gilead. HR reports personal fees from AbbVie, personal fees from Roche, personal fees from Pfizer, outside the submitted work. BjG reports other from Novartis, other from Amgen, outside the submitted work. GTJ reports grants from AbbVie, grants from Pfizer, grants from UCB, grants from Celgene / Amgen, grants from GSK, outside the submitted work. CC reports speaker fees and grants from AbbVie, Amgen, Egis, Novartis, Pfizer and UCB. RI reports speaker fee from Abbvie, Amgen, Novartis, Pfizer, Lilly and UCB. JZ reports speaker and consulting fees from Elli-Lilly, Abbvie, Novartis and UCB. SY reports speaker fees from Abbvie, MSD, Novartis, UCB, Sanofi and Pfizer. BM reports grants and personal fees from Novartis, outside the submitted work. ZR reports speaker fees, consultancy fees and support to biorx.si registry by, AbbVie, Amgen, Biogen, Eli Lilly, Janssen, Medias, Medis, MSD, Novartis, OPH Oktal Pharma, Sandoz and Pfizer. MTreports speaker fees, consultancy fees and support to biorx.si registry by, AbbVie, Amgen, Biogen, Eli Lilly, Janssen, Medias, Medis, MSD, Novartis, OPH Oktal Pharma, Sandoz and Pfizer. FI reports consultancy fees and/or speaker honoraria from Pfizer, AbbVie, MSD, BMS, Lilly, Novartis, Sanofi, Celgene and UCB, outside this work. MJS reports personal fees from Abbvie, Novartis and Pfizer, outside the submitted work. PA-R reports research grant (paid to academic research institute) from Novartis. LMØ reports grants from Novartis, during the conduct of the study. MØ reports grants, personal fees and non-financial support from AbbVie, grants, personal fees and non-financial support from BMS, personal fees from Boehringer-Ingelheim, personal fees from Eli Lilly, personal fees and non-financial support from Janssen, grants, personal fees and non-financial support from Merck, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Roche, grants, personal fees and non-financial support from UCB, grants and personal fees from Celgene, personal fees from Sanofi, personal fees from Regeneron, grants, personal fees and non-financial support from Novartis, personal fees from Gilead, outside the submitted work. LJ reports lecture and consulting fees from Pfizer, Abbvie, Novartis, Eli-Lily and Janssen. JA reports grants from Abbvie, Astra-Zeneca, BMS, Eli Lilly, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB, outside the submitted work. MN reports research and consulting fees from Abbvie, Celgene, Eli-Lilly, Janssen, Novartis and Pfizer.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the respective national data protection agencies and research ethical committees according to legal regulatory requirements in the participating countries (online supplemental table S1).
References
- 1. Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. 10.1002/art.40726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700.1–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- 4. Kingsley GH, Kowalczyk A, Taylor H, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology 2012;51:1368–77. 10.1093/rheumatology/kes001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baranauskaite A, Raffayová H, Kungurov NV, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the respond study. Ann Rheum Dis 2012;71:541–8. 10.1136/ard.2011.152223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. 10.1016/S0140-6736(15)00347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willkens RF, Williams HJ, Ward JR, et al. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum 1984;27:376–81. 10.1002/art.1780270403 [DOI] [PubMed] [Google Scholar]
- 8. Scarpa R, Peluso R, Atteno M, et al. The effectiveness of a traditional therapeutical approach in early psoriatic arthritis: results of a pilot randomised 6-month trial with methotrexate. Clin Rheumatol 2008;27:823–6. 10.1007/s10067-007-0787-7 [DOI] [PubMed] [Google Scholar]
- 9. Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24. 10.1002/art.40851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Behrens F, Cañete JD, Olivieri I, et al. Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PsA: a systematic review of the literature. Rheumatology 2015;54:915–26. 10.1093/rheumatology/keu415 [DOI] [PubMed] [Google Scholar]
- 11. George MD, Baker JF, Ogdie A. Comparative persistence of methotrexate and tumor necrosis factor inhibitors in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2020;47:826–34. 10.3899/jrheum.190299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kristensen LE, Gülfe A, Saxne T, et al. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish arthritis treatment group register. Ann Rheum Dis 2008;67:364–9. 10.1136/ard.2007.073544 [DOI] [PubMed] [Google Scholar]
- 13. Fagerli KM, Lie E, van der Heijde D, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis 2014;73:132–7. 10.1136/annrheumdis-2012-202347 [DOI] [PubMed] [Google Scholar]
- 14. Maneiro JR, Souto A, Salgado E, et al. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 2015;1:e000017. 10.1136/rmdopen-2014-000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brahe CH, Ørnbjerg LM, Jacobsson L, et al. Retention and response rates in 14 261 PsA patients starting TNF inhibitor treatment-results from 12 countries in EuroSpA. Rheumatology 2020;59:1640–50. 10.1093/rheumatology/kez427 [DOI] [PubMed] [Google Scholar]
- 16. Michelsen B, Sexton J, Smolen JS, et al. Can disease activity in patients with psoriatic arthritis be adequately assessed by a modified disease activity index for psoriatic arthritis (DAPSA) based on 28 joints? Ann Rheum Dis 2018;77:1736–41. 10.1136/annrheumdis-2018-213463 [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18. Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. 10.1002/art.20335 [DOI] [PubMed] [Google Scholar]
- 19. Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36. 10.1002/art.20967 [DOI] [PubMed] [Google Scholar]
- 20. Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. 10.1002/art.21306 [DOI] [PubMed] [Google Scholar]
- 21. Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90. 10.1002/art.30117 [DOI] [PubMed] [Google Scholar]
- 22. Thomas ML, Shaddick G, Charlton R, et al. Tumor necrosis factor inhibitor monotherapy versus combination therapy for the treatment of psoriatic arthritis: combined analysis of European biologics databases. J Rheumatol 2021;48:48–57. 10.3899/jrheum.190815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-220097supp001.pdf (686.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.