Abstract
Active forgetting is an essential component of the brain’s memory management system1. Forgetting can be permanent, in which prior memory is lost completely; or transient, in which memory exists in a temporary state of impaired retrieval. Such temporary blocks on memory seem universal, and can disrupt an individual’s plans, social interactions, and ability to make rapid, flexible and appropriate choices. However, the neurobiological mechanisms that cause transient forgetting are unknown. Here we identify a single dopamine neuron in Drosophila that mediates memory suppression resulting in transient forgetting. Artificially activating this neuron failed to abolish the expression of long-term memory. Rather, it briefly suppressed memory retrieval, with memory becoming accessible with time. The dopamine neuron modulates memory retrieval by stimulating a unique dopamine receptor expressed in a restricted physical compartment of the axons of mushroom body neurons. This mechanism for transient forgetting is triggered by interfering stimuli presented just prior to retrieval.
Memory formation, consolidation and retrieval are well-known functions supporting memory expression; however, the processes that limit these functions, including forgetting, are less understood. Forgetting has been characterised as either passive or active, and is crucial for memory removal, flexibility, and updating1-3. Memory may be removed completely, resulting in permanent forgetting; or temporarily irretrievable, resulting in transient forgetting.
One form of active forgetting, termed intrinsic forgetting, involves ~two dopamine neurons (DAn) that innervate the γ2α’1 compartment of the mushroom body neuron (MBn) axons and the dendrites of the downstream, compartment-specific, MB output neurons (MBOn)4,5,6. These DAn reside in a cluster of 12 DAn in each brain hemisphere known as the protocerebral posterior lateral 1 (PPL1) cluster4. Current evidence indicates that the ongoing activity of these DAn following aversive olfactory conditioning slowly and chronically erodes labile, nonconsolidated behavioural memory5, as well as a corresponding cellular memory trace that forms in the MBOn6. This intrinsic forgetting mechanism is shaped by external sensory stimulation and sleep/rest7; and is mediated by a signaling cascade in the MBn initated by the activation of a DA receptor, DAMB, which leads to the downstream activation of the actin-binding protein, cofilin, and the postulated reorganization of the synaptic cytoskeleton1,8,9.
In contrast, there is little understanding of the mechanisms that arbitrate transient forgetting. Neuropsychological studies of failures or delays in retrieval in humans have primarily focused on lexical access. Phonological blockers, or interfering stimuli, produce a tip-of-the-tongue state (TOTs)10 – the failure to recall the appropriate word or phrase. TOTs are resolved when the distracting signals dissipate10. Several brain regions have been implicated in TOTs from fMRI studies11, but the neurobiological mechanisms that produce a temporary state of impaired retrieval are unknown. Our study offers an entrée into this unexplored area of brain function.
External stimuli briefly block retrieval
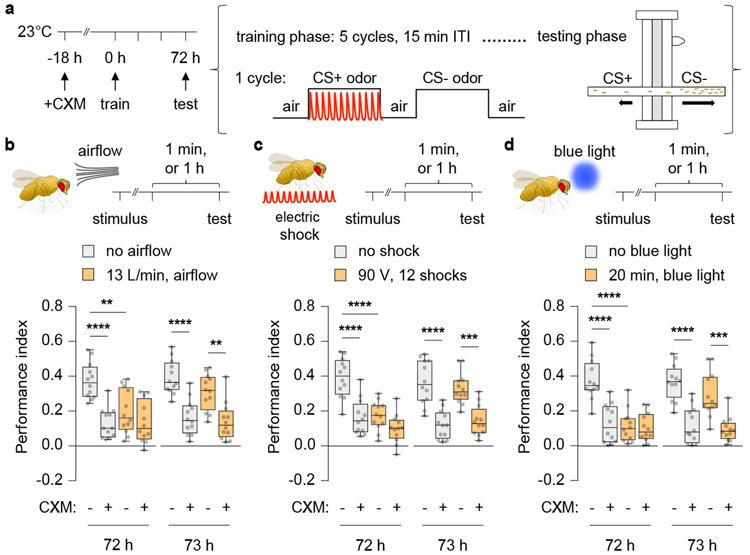
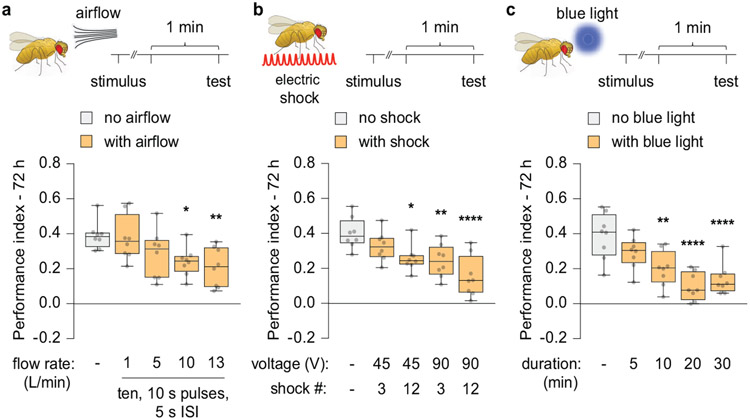
Wild-type flies subjected to aversive olfactory (odour-shock) conditioning using multiple, spaced training cycles displayed robust long-term memory (LTM) 72 h after training (Fig. 1a, b). To determine how exposure to an interfering stimulus might impact LTM expression, flies were briefly stimulated with airflow, electric shock or blue light prior to the memory retrieval test. Memory expression was weakened with increasing stimulus strength after experiencing these distractors (Extended Fig. 1a-c). These effects were observed only on cycloheximide (CXM)-sensitive memory (Fig. 1b-c). Since CXM blocks protein-synthesis dependent memory (PSD-LTM), the difference in performance index between untreated and CXM-treated flies represents the magnitude of PSD-LTM. Surprisingly, the expression of PSD-LTM resurfaced to significant levels by 1 h after airflow, shock or blue light presentation (Fig. 1b-c; Supplementary Information), indicating that the memory impairments were temporary and produced by transient forgetting. Furthermore, the flies exhibited shock and odour avoidance that was indistinguishable from controls, indicating that the observed effects were not due to anomalous sensorimotor behaviour (Supplementary Information).
Fig 1. External stimuli transiently disrupt retrieval of PSD-LTM.
a, Aversive olfactory conditioning paradigm employed to generate PSD-LTM. b-d, Cycloheximide (CXM)-treated wild-type (Canton-S) flies were exposed to interfering stimuli: airflow (b), electric shock (c), or blue light (d), and tested for 72 h or 73 h memory. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 12 (b-d), two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
Transient forgetting through dopamine
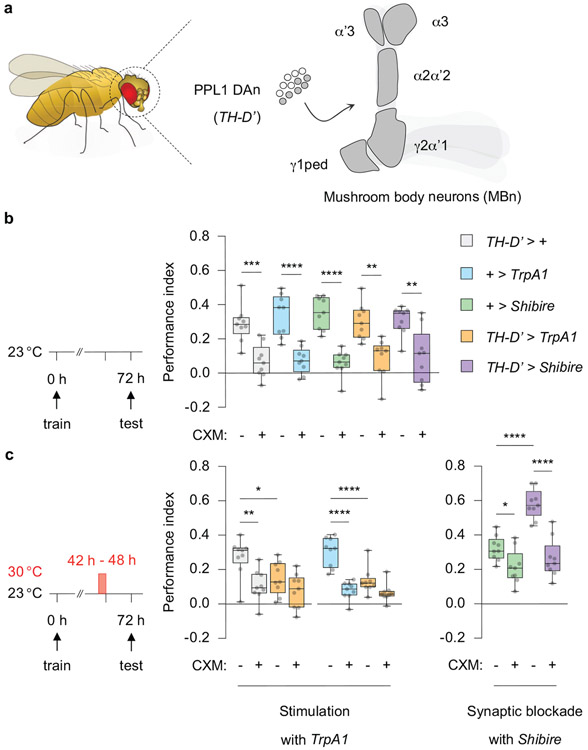
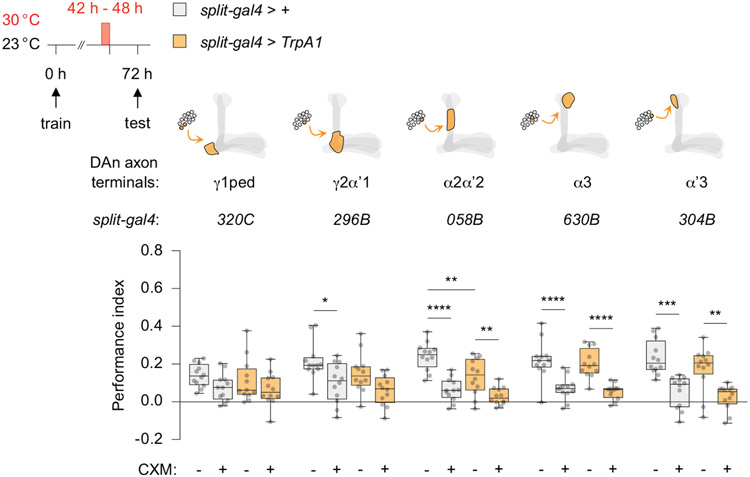
Since PPL1 DAn are involved in intrinsic forgetting, we asked whether they might also be involved in processes underlying transient forgetting. Pilot experiments demonstrated that strong, prolonged thermogenetic stimulation of all 12 PPL1 DAn per hemisphere (TH-D’ > TrpA1) significantly reduced PSD-LTM expression (Extended Fig. 2a-c) even 24 h prior to retrieval. We observed the opposite effect upon blocking synaptic output from PPL1 DAn (TH-D’ > Shibire), suggesting the existence of a memory reserve that remains hidden unless synaptic output from the DAn is suppressed (Extended Fig. 2c). We used these assays and a collection of split-gal4 lines to spatially restrict TrpA1 expression to PPL1 subpopulations (Extended Fig. 3). The phenotype observed from stimulating the entire cluster was recapitulated after manipulating only a single DAn, one per hemisphere (058B-split-gal4), whereas other PPL1 DAn subgroups had no signficant effect (Extended Fig. 3). Notably, this single DAn (058B > TrpA1) innervates the α2α’2 compartment of the MBn axons which is distinct from the compartment involved in intrinsic forgetting.
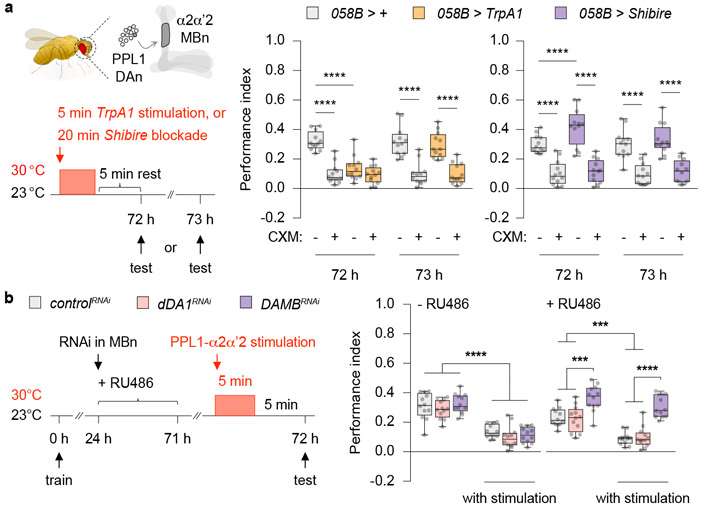
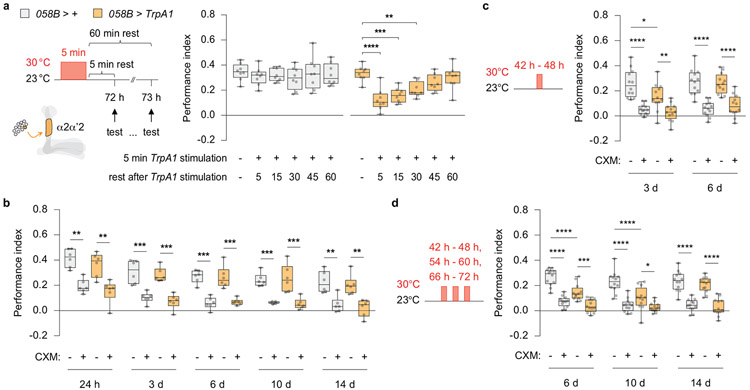
We considered the possibility that the long-lasting decrement (24 h) in performance due to extended stimulation of PPL1-α2α’2 might be an extreme, experimentally produced variant of short-lived, transient forgetting caused by distractors. So we tested whether milder stimulation presented just prior to retrieval mimicked the effects observed with external stimuli (Fig. 1). Indeed, PSD-LTM was temporarily suppressed by 5 min of TrpA1 stimulation 5 min prior to retrieval (Fig. 2a, left), while PSD-LTM was temporarily enhanced by briefly blocking synaptic output (058B > Shibire) (Fig. 2a, right). Memory performance spontaneously recovered within 1 h after a brief bout of TrpA1 stimulation (Extended Fig. 4a). Thus, the behavioural effects observed with brief artifical stimulation of PPL1-α2α’2 mimic the transient forgetting produced by presenting interfering stimuli just before retrieval, consistent with the model that PPL1-α2α’2 stimulation mediates these effects.
Fig 2. Transient memory suppression engages a single pair of PPL1 DAn and the DA receptor DAMB.
a, Schematic depicting the right hemisphere of a fly brain. One DAn (dark grey circle) in the protocerebral posterior lateral 1 (PPL1) cluster synapses onto the α2α’2-MBn compartment (dark grey). Brief stimulation of PPL1-α2α’2 (058B > TrpA1) just before retrieval transiently suppressed, while blocking synaptic output (058B > Shibire) temporarily enhanced PSD-LTM expression. b, Conditioned flies were fed with RU486 to induce RNAi expression in the MBn. Knocking down DAMB, but not dDA1, blocked the PPL1-α2α’2-induced memory suppression. PPL1-α2α’2-lexA, MB-GeneSwitch > uas-RNAi, lexAop-TrpA1. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 12, two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
Since 6 h stimulation inhibited memory expression 24 h later, we wondered whether this stimulation, or more extreme variants, could convert transient forgetting to a permanent memory loss. To test this, we delivered multiple epochs of TrpA1 stimulation. Remarkably, the PSD-LTM expression deficit was prolonged to more than one week after several spaced TrpA1 stimulation cycles (Extended Fig. 4b-d), but returned to normal levels by day 14.
Transient forgetting recruits DAMB
The PPL1-α2α’2-induced disruption of memory retrieval predicts the existence of a MB-expressed DA receptor to propagate the transient forgetting signal. One candidate was DAMB, a Gq-coupled receptor12,13 which transduces the DAn γ2α’1-MB signal involved in intrinsic forgetting5,6. Thus, we hypothesised that DAMB is the major receptor mediating the DAn-induced transient forgetting.
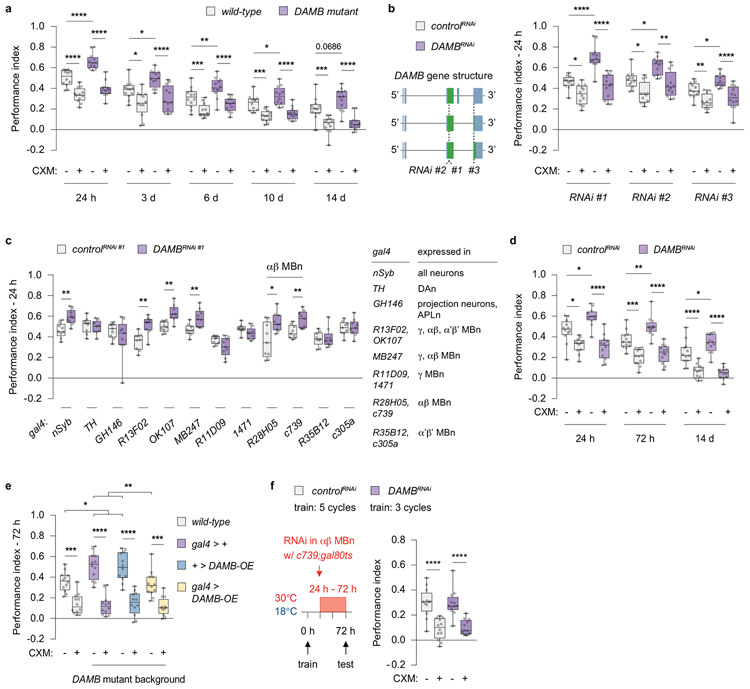
The expression of PSD-LTM in flies mutant for DAMB was dramatically elevated at all time points tested up to day 14 (Extended Fig. 5a). To identify the key neural site responsible for increased PSD-LTM, we drove DAMB RNAi expression pan-neuronally and in various types of neurons implicated in olfactory memory14 (Extended Fig. 5b, c). Since the PPL1-α2α’2 DAn forms synaptic contact with the α2-MBn and α’2-MBn compartments, we predicted that we would see differences in performance for the gal4 lines that drive RNAi expression in either of the two MBn sub-types. The enhanced memory phenotype was recapitulated only when using gal4 lines with restricted expression in the αβ MBn, and not with the α’β’ MBn lines (Extended Fig. 5c, d). We also reinstated DAMB expression only in αβ MBn, which led to a full rescue of the phenotype (Extended Fig. 5e), consistent with studies that have implicated the αβ MBn for storage of PSD-LTM15,16. These findings are consistent with the model that transient forgetting is mediated by the receptor, DAMB, located in the α2α’2 compartment of αβ MBn axons.
We next determined whether DAMB function is epistatic (downstream) to PPL1-α2α’2 stimulation using an intersectional genetics approach. We used the lexA > lexAop binary system to stimulate PPL1-α2α’2 and the gal4 > uas binary system to knock down DAMB in the αβγ MBn (Fig. 2b). The Gal4 derivative, GeneSwitch allows for spatiotemporal control of gene expression, with its activity regulated by the presence or absence of the progesterone analouge mifepristone (RU486)17. Without RU486, all groups displayed comparable performance; as expected, PPL1-α2α’2 stimulation diminished LTM (Fig. 2b). However, upon RU486 treatment after training (to express DAMBRNAi) the flies with reduced DAMB showed increased performance compared to the control, while flies with reduced dDA1 did not. Moreover, the effects of PPL1-α2α’2 stimulation were blocked in the DAMBRNAi-expressing flies, consistent with the conclusion that DAMB is epistatic to PPL1-α2α’2 stimulation for PSD-LTM. Memory performance of dDA1RNAi flies paralleled the controls, suggesting that dDA1 has no role for this behavioural plasticity. These experiments identify DAMB as the DA receptor transducing the forgetting signal in the α2-MBn to inhibit the retrieval of PSD-LTM.
Persistence of PSD-LTM cellular trace
Olfactory memory is established through the generation of cellular memory traces, defined as the biochemical and cellular changes instilled by memory formation14,18. Such cellular memory traces have often been detected by functional imaging of transgenically-supplied reporters such as GCaMP, with memory traces reported as increased responses to the CS+19-21, decreased responses to the CS+6,22, or a combined CS+ response increase and a CS− (odour without shock)23 response decrease. The latter memory traces are best represented as the differential response to the CS+/CS. Since activation of PPL1-α2α’2 causes transient forgetting, we hypothesised that cellular memory traces downstream of this signal would persist, with behavioural forgetting being due to a block on retrieval, rather than a transient suppression of the cellular memory trace.
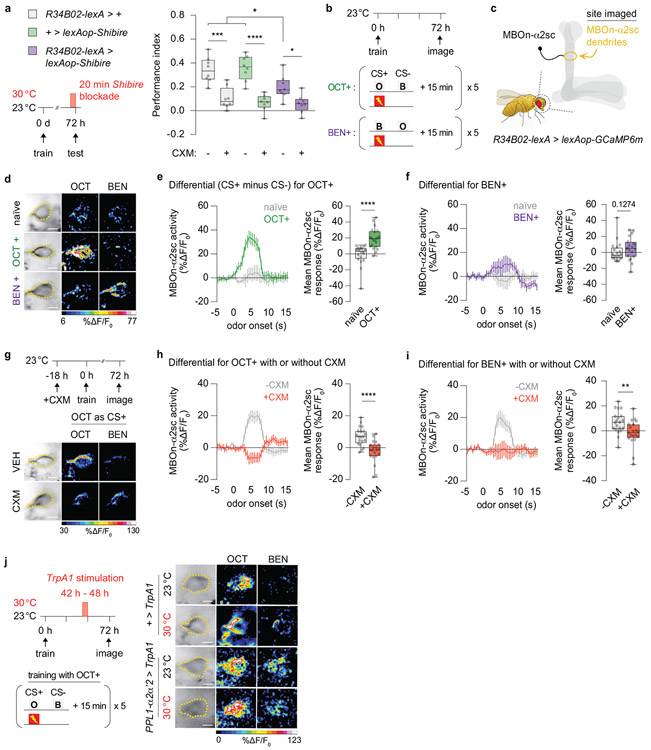
We focused on the MBOn-α2sc because the synaptic output of the α2α’2-MB compartment is funneled to the dendrites of this neuron24. We first confirmed the importance of MBOn-α2sc for PSD-LTM retrieval by blocking synaptic release before and during retrieval. This block reduced PSD-LTM expression (Extended Fig. 6a). Thus, we chose MBOn-α2sc to search for a 72 h cellular trace and then to assay its persistence in response to transient forgetting.
Flies expressing GCaMP6m in MBOn-α2sc were spaced trained and the MBOn-α2sc dendrites were imaged 72 h later (Extended Fig. 6b, c). For each trained fly, the responses to the conditioned odours, octanol (OCT) and benzaldehyde (BEN) were measured; the naïve odour responses served as controls. In naïve flies, responses to the two odours were relatively similar indicating no detectable differential response. However, there was a significantly increased enhancement in the GCaMP6m differential fluorescence signal in conditioned versus naïve animals (Extended Fig. 6d-f), which was more robust with OCT as the CS+ compared to BEN as the CS+. To confirm that this trace is dependent on protein synthesis, flies were fed with CXM prior to conditioning. Notably, the training-induced increase in the CS+/CS− differential was blunted for the CXM-treated animals (Extended Fig. 6g-i), thus identifying a previously unknown, PSD-LTM cellular trace at 72 h after conditioning.
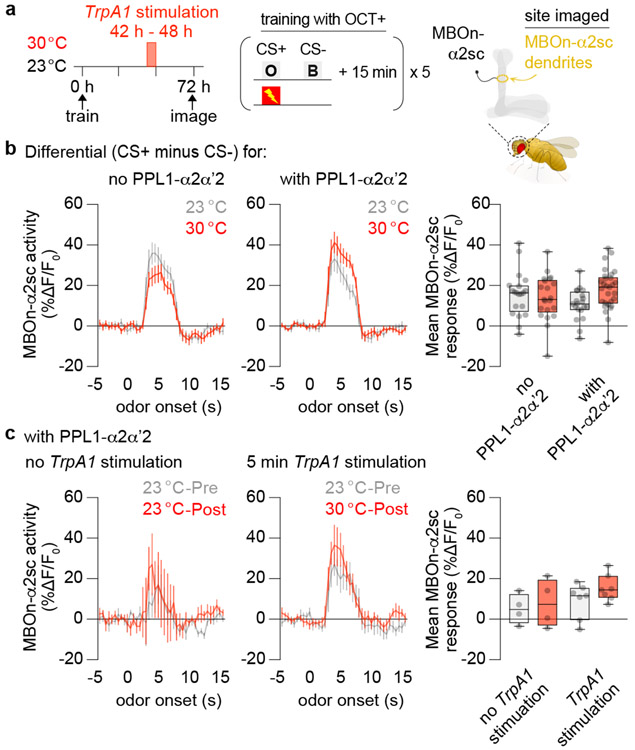
We focused on OCT-training (CS+ = OCT, CS− = BEN) for the subsequent analysis since it produced the strongest responses. Most importantly, ectopic activation of PPL1-α2α’2 did not suppress the PSD-LTM trace (Fig. 3a-c, Extended Fig. 6j). There were no obvious changes in GCaMP6m differential signal between genotypes and across temperature conditions after a 6 h TrpA1 stimulation of PPL1-α2α’2 (Fig. 3b). Furthermore, the calcium traces in MBOn-α2sc were not affected immediately after a shorter bout (5 min) of TrpA1 stimulation (Fig. 3c). Thus, our results support the first hypothesis: activation of the transient forgetting signal blocks retrieval rather than temporarily suppressing a PSD-LTM cellular trace (see Discussion).
Fig 3. Stimulating PPL1-α2α’2 failed to erase the 72 h PSD-LTM trace in MBOn-α2sc.
a, Flies were spaced-trained using octanol (OCT) as the CS+ and benzaldehyde (BEN) as the CS−. MBOn-α2sc dendrites were imaged with or without a 6 h TrpA1 stimulation of PPL1-α2α’2 (R82C10-gal4). b, Group differential traces and quantification of the odour responses to OCT+ and BEN− in flies kept at 23 °C or treated at 30 °C between 42-48 h. The left and middle panels (line graphs) display the activity as a function of time with odour stimulation. The right panel (bar graph) displays the average response magnitude within the first 5 s of odour onset (odour delivery duration). See methods for calculations. The Differential reflects the difference in odour response between CS+ minus CS−. No PPL1-α2α’2: R34B02-lexA > lexAop-GCaMP6f, uas-TrpA1. With PPL1-α2α’2: R82C10-gal4, R34B02-lexA > uas-TrpA1, lexAop-GCaMP6f. Ectopic activation of PPL1-α2α’2 did not alter the training-induced calcium transient differential. c, MBOn-α2sc dendrites in the flies with PPL1-α2α’2 were imaged before (Pre) and after (Post) a 5 min TrpA1 stimulation. Brief stimulation of PPL1-α2α’2 did not change the training-induced calcium transient differential. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. P > 0.05; n = 12 (b), n = 4 (c, no TrpA1 stimulation), n = 7 (c, TrpA1 stimulation), two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
External stimuli employ PPL1-α2α’2 and DAMB to suppress memory
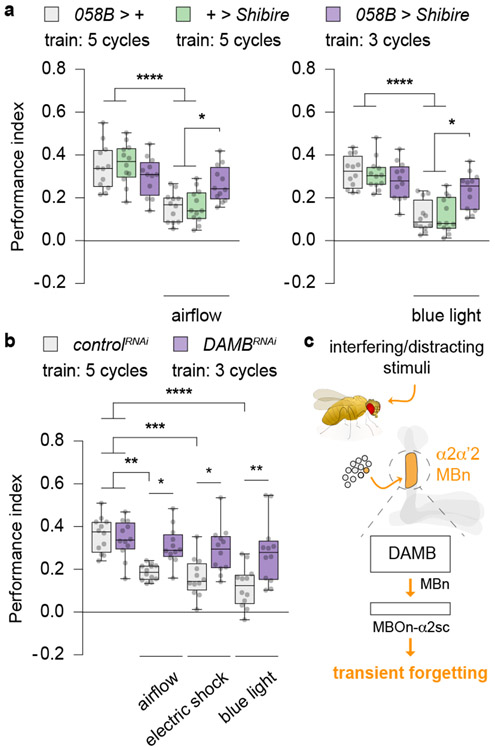
We hypothesised that transient forgetting caused by distracting stimuli prior to retrieval occurred via the PPL1-α2α’2/DAMB pathway. To test this possibility, we first blocked synaptic output from PPL1-α2α’2 while simultaneously delivering the external stimuli. Since inhibiting PPL1-α2α’2 before a memory retrieval test enhanced PSD-LTM (Fig. 2a), which may be represented by cellular memory traces of greater strength or number, we undertrained the 058B > Shibire flies using only three cycles of training so that their LTM performance was similar to the control flies (Fig. 4a). This allowed us to balance the strength of the behavioural response across all groups so that we could then subject them to interfering stimuli of constant strength – obtaining a balanced measure of PSD-LTM performance with and without the blockade of PPL1-α2α’2 activity. Remarkably, the 058B > Shibire flies with the presynaptic block and exposed to airflow or blue light exhibited significantly increased performance relative to control flies exposed to the same external stimuli, and performance that was indistinguishable from control flies unexposed to the external stimuli (Fig. 4a). This result indicates that blocking the single pair of DAn repressed the transient forgetting effects of the stimuli.
Fig 4. Airflow, electric shock or blue light require PPL1-α2α’2/DAMB function to cause transient forgetting.
a, Blocking synaptic release from PPL1-α2α’2 and normalising LTM expression by differential spaced-conditioning. Flies were exposed to airflow (Fig. 1b) or blue light (Fig. 1d), but at 30 °C (20 min) to concurrently block PPL1-α2α’2 output. Stimuli exposure and heat treatment termininated 5 min prior to a memory retrieval test at 23 °C. Inhibiting synaptic release from PPL1-α2α’2 blocked the transient forgetting induced by either airflow (left) or blue light (right). b, Flies were differentially spaced-trained at 18 °C and shifted to 30 °C for two days, 24 h after training to induce DAMBRNAi in the αβ MBn (c739-gal4, gal80ts > uas-RNAi, uas-dicer2). Flies were then exposed to airflow, electric shock or blue light just before retrieval. DAMB knockdown fully blocked the transient forgetting from exposure to external stimuli. c, Working model for transient forgetting. The expanded version contrasting permanent versus transient forgetting is shown in Extended Fig. 7. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 12 (a, b), two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
We further tested the hypothesis by obstructing the PPL1-α2α’2/DAMB pathway post-synaptically by expressing DAMBRNAi in the αβ MBn (Fig. 4b). Since DAMBRNAi expression in the αβ MBn also enhanced PSD-LTM (Fig. 2b), we similarly undertrained the DAMBRNAi expressing flies which yielded PSD-LTM performance comparable to those of the control flies (Extended Fig. 5c). Strikingly, the DAMBRNAi flies exposed to airflow, shock or blue light displayed significantly enhanced performance relative to control flies exposed to the same three external stimuli (Fig. 4b), and performance indistinguishable from control flies unexposed to the stimuli. The data obtained from this post-synaptic insult parallels precisely our observations from disrupting the proposed neural circuit at the pre-synaptic level. Taken together, these findings confirm that the interfering stimuli trigger the PPL1-α2α’2/DAMB pathway to cause transient forgetting (Fig. 4c).
Discussion
Memory retrieval is proposed to consist of an interplay between internal/external cues and memory engrams25, with cue-induced reactivation of engrams across multiple brain regions facilitating memory expression18,26. But a central question about this process is how interfering stimuli temporarily block memory retrieval, resulting in transient forgetting. Here, we offer insights into a mechanism. Our behavioural and functional imaging data reveal that the DAn, PPL1-α2α’2, working through the DAMB receptor expressed in the α2α’2-MBn axonal compartment, mediates the transient forgetting of PSD-LTM. This effect occurs without altering a cellular memory trace in the post-synaptic, MBOn-α2sc. This process can be triggered by distracting stimuli, illustrating a neural-genetic-environmental interplay to modify memory expression (Extended Fig. 7).
Why does the cellular memory trace remain unaffected by DAn stimulation despite the occurance of behavioural forgetting? Since blocking synaptic output from MBOn-α2sc reduces PSD-LTM expression (Extended Fig. 6a), the simplest hypothesis posits that cellular memory traces form with conditioning in the MBOn in addition to the cytoplasmic, Ca2+-based memory trace detected here. This is expected. Neurons undergo broad changes in physiology as they adopt new states so it is plausible that such plastic mechanisms, especially ones that gate synaptic release, are inactivated by DAn activity while leaving the Ca2+-based memory trace intact.
The discovery that DAMB loss of function leads to enhanced PSD-LTM was surprising, because of a prior study claiming that this insult attenuates PSD-LTM27. Our multiple experiments argue strongly that DAMB functions normally to suppress PSD-LTM expression. But why would a receptor involved in transient forgetting lead to enhanced PSD-LTM when inactivated? Prior experiments have shown that PPL1-α2α’2, like PPL1-γ2α’1, exhibits ongoing activity28, leading to a slow release of DA onto MBn. This activity should slowly degrade or suppress existing memory so that when the receptor is inactivated, memory expression would be enhanced.
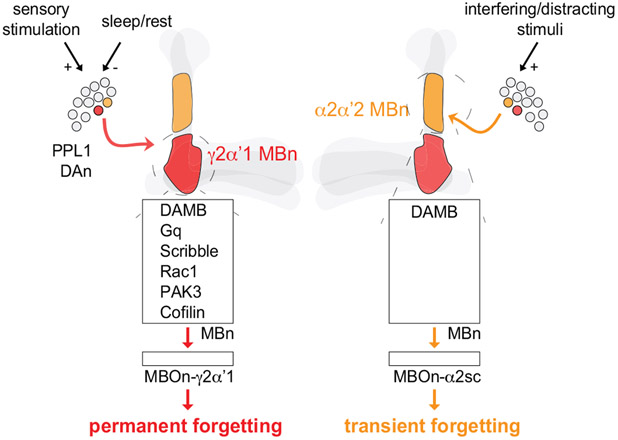
Interestingly, PPL1-α2α’2 has no significant role in the forgetting of labile, nonconsolidated memory5. Rather, our prior studies identified a different DAn, PPL1-γ2α’1, for this process and the apparent erasure of the downstream cellular memory trace5,6; perhaps an indication of “permanent forgetting”. This process is modulated by internal and external factors7, and is mediated by key molecules expressed in the MBn5,8,9,13 that receive PPL1-γ2α’1 input (Extended Fig. 7). We found no robust decrement in PSD-LTM expression after PPL1-γ2α’1 stimulation (Extended Fig. 2), pointing to the existence of two separate DA-based circuits for permanent and transient forgetting. This functional separation may indicate a fundamental principle in the organisation of circuits that mediate multiple forms of forgetting.
However, the DAMB receptor is used for both permanent and transient forgetting. DAMB is widely expressed across the MBn axons12 but alters synaptic plasticity differently across MBn compartments29. It is possible that DAMB signaling may be distinct for the two forms of forgetting. DAMB preferentially couples with Gq whose knockdown inhibits the potent erasure of memory13; but its potential role in transient forgetting is unknown. The scaffolding protein, Scribble, orchestrates the activities of Rac1, PAK3 and Cofilin8; all important for the permanent forgetting pathway (Extended Fig. 7). But importantly, Scribble knockdown or inhibition of Rac1 does not enhance the PSD-LTM8,9 like DAMB knockdown flies, providing speculation that this scaffolding signalosome does not play a large role in transient forgetting. In summary, the two distinct forms of forgetting – transient and permanent – share a dopaminergic mechanism and a common DA receptor, but differ in upstream and downstream neural circuits, and in downstream signaling pathways within MBn.
Methods
Drosophila husbandry
Fly stocks were raised on regular Drosophila medium at room temperature. Experimental fly crosses were kept at 23 °C, unless otherwise stated, and 70 % relative humidity on a 12 h-light: 12 h-dark cycle. A full list of fly strains is described in the Supplementary Information. Unless specifically noted in the figures, legends or text, the fly lines used were from the gal4 > uas binary system collection.
Behavioural experiments
The 1-4 d old flies, of mixed sex, were used for standard olfactory conditioning experiments as previously described30,31. Blotting filter paper was cut to fit the bottom of an empty vial, and 450 μl of vehicle (5 % glucose, 3 % ethanol solution) or 35 mM cycloheximide (CXM) dissolved in vehicle were pipetted onto the paper; flies were kept on the vehicle or CXM for 18 h overnight. The flies were transferred into regular food vials 1 h before conditioning, and were acclimated under dim red light, in an environmentally controlled chamber set at 23 °C and 70 % humidity. Odour-laced air was produced by bubbling fresh air through odourant dissoved in mineral oil. Groups of ~65 flies were gently tapped into a training tube where they received 30 s of fresh air, 1 min of odour A paired with electric shock (12 shocks at 90 V lasting 1.25 s each, at 5 s intervals; CS+), 30 s of fresh air, 1 min of odour B without electric shock (CS−), and 30 s of fresh air. This constitutes as one cycle. The trained flies were then transferred back onto their respective food vials for a 15 min rest interval, and this protocol was repeated for a total of five spaced cycles. After spaced training, flies were housed in a covered box inside a 23 °C incubator at 70 % humidity. For memory tests beyond 24 h, flies were flipped into fresh food vials every two days. After the specified time (24 h – 14 d), flies were loaded into a T-maze and given 1 min to acclimate, and subsequently allowed to choose between arms containing either the CS+ or CS− odour for 2 min. The number of flies were counted and used to calculate a performance index (PI) = [(CS−) – (CS+)]/[(CS−) + (CS+)]. The final PI shown in the figures denotes the averaged PI between the two CS+ odours (half PIs). Odours were counterbalanced and included benzaldehyde (BEN; 0.05-0.10 %) and 3-octanol (OCT; 0.15 %) diluted in mineral oil. The concentrations were slightly adjusted according to experimental condition, memory timepoint assayed, and genotype, so that flies displayed comparable half PIs when tested in the T-maze.
The flies’ naïve avoidance to the odours and electric shock were assessed by allowing flies to choose between the odour or fresh air (odour avoidance), or between the copper-grid arms with or without electric shock (shock avoidance), in the T-maze for 2 min. Exposing wild-type flies to blue light for 20 min, or manipulating PPL1-α2α’2 activity prior to a memory retrieval test did not affect the flies’ innate capacity to avoid BEN, OCT or electric shock. The full list of behavioural control experiments with detailed experimental conditions are in the Supplementary Information.
In the external stimulus presentation experiments for Fig. 1 and Fig. 4, spaced-trained flies were transferred into fresh food vials for 30 min and the following protocols were employed. Airflow: flies were tapped into clean and empty cylindrical tubes. Pressurised, filtered airflow was manually passed through the tubes spanning 10 cycles of 10 s airflow with 5 s inter-stimulus rest intervals; flow meters were used monitor the airflow delivery. Flies were allowed to rest for 1 min before being transferred to the T-maze for a memory retrieval test; this timing was also adopted for the electric shock and blue light experiments. Electric shock: conditioned flies were briefly exposed to mild electric shock using clean and fresh training tubes. Blue light: a 34 x 13 x 19 cm box was lined with reflective mirror boards and a computer fan for internal cooling32. Two rows of three 448 nm emitting LED modules (Luxeon SP-01-V4) were attached to the top inside the box, with a projected light intensity of 0.15 mW/mm2. Flies were housed in this box for the time indicated in the figures. Exposure to the blue light for 20 min did not compromise the flies’ capacity to form odour-shock memory (Supplementary Information).
For the temperature shift experiments involving TrpA1 stimulation or Shibire blockade, flies were tapped into fresh food vials before placing them inside an environmentally controlled chamber at at 30 °C and 70 % humidity for the length of time indicated in the figures. Subsequent memory tests were performed in a separate chamber at 23 °C and 70 % humidity, after some rest denoted in the figures. Experiments employing longer heat treatments such as those in Extended Fig. 2 and 3, required use of an incubator set at 30 °C with 70 % humidity.
For the intersectional genetics experiments employing MB-GeneSwitch in Fig. 2d, fly food vials were freshly prepared containing a final concentration of 500 μM RU486 dissolved in 1 % ethanol solution (+RU486), or food vials with the ethanol alone (−RU486). The drug treatment allowed us to pharmacogenetically induce uas-dDA1RNAi or uas-DAMBRNAi expression. Spaced-trained flies were reared on RU486 food 24 h after conditioning, until 1 h before the retrieval test measuring 72 h memory. Stimulation of PPL1-α2α’2 (R82C10-lexA) was achieved by using the lexA > lexAop binary system, which enabled us to confirm the memory suppression phenotype with an independent fly line with restricted expression in the single DAn. The lexAop-TrpA1 stimulation was performed as with TrpA1 (uas-line version) above.
For the experiments done in Fig. 4 and Extended Fig. 5c, we used the TARGET33 system. Fly crosses were reared in an incubator set at 18 °C and in 70 % humidity. Flies were then trained in an 18°C, 70 % humidity chamber to minimise leaky RNAi expression. To thermogenetically induce uas-DAMBRNAi expression in the αβ MBn (c739;gal80ts), flies were shifted to a 30 °C, 70 % humidity incubator 24 h after spaced-training until the time of testing.
In vivo GCaMP imaging
The fly mounting preparation, cuticle dissection, hemolymph saline solution (124 mM NaCl, 3 mM KCl, 20 mM MOPS, 1.5 mM CaCl2, 4 mM MgCl2.6H2O, 5 mM NaHCO3, 1 mM NaH2PO4.H2O, 10 mM trehalose, 7 mM sucrose, 10 mM glucose, pH 7.2) and microscope conditions for in vivo calcium imaging were adopted from the protocol previously detailed6, with slight modifications. To determine whether the LTM cellular trace in MBOn-α2sc was dependent on protein synthesis, female R34B02-lexA > lexAop-GCaMP6m flies were first fed with either 35 mM CXM solution mixed with 1 % blue dye, or vehicle alone with the dye. After 18 h, the flies with the most robust blue-coloured abdomen were aspirated (without CO2) and spaced-trained as described above.
A Leica TCS SP5 II confocal microscope with single photon excitation was utilised for GCaMP6m fluorescence, incorporating a 488 nm Argon laser at a resolution of 512 x 512 pixels using a 20 X objective (HCX APO L 20.0 X/1.0 NA, Leica). Emitted light was collected using a PMT (510-550 nm) for GCaMP emission at a frame rate of 2 Hz with the pinhole fully open. To deliver odours to flies under the microscope, a small stream of air (100 ml/min) was diverted (via solenoids) from flowing through a clean 20 ml glass vial to flow through a 20 ml glass vial containing a 1 ml of a 1:1000 odour/mineral oil solution. The air stream was then diluted into a faster air stream (1000 ml/min) before traveling 95 cm through Teflon tubing (~2.5 mm diameter) to reach the fly mounted beneath the microscope objective. Flies were exposed to 5 s of one odour, then 45 s of fresh air, and then 5 s of a second odour with half of the animals for a given group receiving OCT then BEN, and the other half BEN then OCT. The final responses for a particular odour were averaged across these two odour sequences.
MBOn-α2sc GCaMP activity was quantified using ImageJ and MATLAB. An ROI was first drawn around the MBOn-α2sc dendrites which wrapped around the compartment of the α2 MB axons as depicted in Extended Fig. 6c, d. The MBOn-α2sc neural “activity” was calculated across time (t), by normalising the GCaMP fluorescence (F) signal to the mean signal (F0) across the 5 s time window prior to the start of an odour pulse as follows: Activity (%ΔF/F0) (t) = 100 * ((F(t) – F0)/ F0). The line graphs represent the MBOn-α2sc activity across 20 s (−5 s to 15 s odour onset), while the bar graphs denote the mean MBOn-α2sc response within the first 5 s of odour onset. The calculated Differential reflects the change between CS+ versus CS− odour responses within animal. Representative images of the odour responses for the naïve, BEN+ or OCT+ treated flies are shown both in greyscale and in pseudo-colour (16 colours LUT, ImageJ); scaling with respect to the %ΔF/F0 range.
For the PPL1-α2α’2 stimulation (R82C10-gal4) experiments in Fig. 3b and Extended Fig. 6j, the flies were shifted to an incubator set at 30 °C with 70 % humidity for 6 h (from hour 42 to hour 48), mimicking the behavioural experiments for which we observed strong behavioural memory suppression. The flies were flipped into fresh food vials and remained in an incubator set at 23 °C with 70 % humidity until they were imaged for changes in the cellular memory trace detected 72 h after conditioning. For the shorter TrpA1 stimulation experiments in Fig. 3c, we first recorded the odour responses (Pre) which represents the 72 h cellular memory trace without PPL1-α2α’2 stimulation. To activate PPL1-α2α’2 acutely, we then perfused saline at 30 °C for 5 min, allowed for a 5 min rest period before recording the odour responses (Post), thus mimicking our short stimulation behavioural paradigm. The “Post” response represents the cellular memory trace after the PPL1-α2α’2 stimulation.
Statistics and reproducibility
All data were compiled and analysed using Excel version 16.43 (20110804) and GraphPad Prism 7, respectively. Different groups of flies, along with appropriate controls per experimental condition, were distributed evenly across each set of experiments. Each experiment was performed three to four independent times. All replication attempts were successful. The graphs displayed in all figures are box-and-whisker plots that show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. The sample sizes per group, P-values, and statistical tests used for each experiment are detailed in the Supplementary Information.
Extended Data
Extended Data Fig 1. Dose-dependent suppression of LTM.
a-c, Conditioned wild-type (Canton-S) flies were exposed to distracting stimuli of increasing potency: airflow (a), electric shock (b), or blue light (c) terminating 1 min before a 72 h memory retrieval test. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 8 (b-d), one-way ANOVA with Dunnett’s test. Exact P-values and comparisons are shown in Supplementary Information.
Extended Data Fig 2. PPL1 DAn bidirectionally modulate PSD-LTM expressed at 72 h.
a, Schematic diagram illustrating the PPL1 DAn cluster (TH-D’-gal4) innervating five subcompartments of the MB neuropil. b, c, 72 h PSD-LTM without (b) or with (c) a manipulation of PPL1 DAn activity. Stimulation (TH-D’ > TrpA1) decreased, while synaptic blockade (TH-D’ > Shibire) enhanced the expression of PSD-LTM. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 9 (b, c), two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
Extended Data Fig 3. Mapping the impaired PSD-LTM expression phenotype to a single DAn.
PPL1 DAn screen using split-gal4 lines and uas-TrpA1 to stimulate discrete DAn subpopulations. Stimulating PPL1-α2α’2 significantly decreased the expression of PSD-LTM when tested at 72 h. Other neurons from the PPL1 cluster did not impair PSD-LTM expression. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 12, two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
Extended Data Fig 4. Multiple epochs of TrpA1 stimulation extend the suppression of memory expression.
a, Memory rapidly recovered after a brief bout of TrpA1 stimulation. b, PSD-LTM retention across 14 d after spaced conditioning without PPL1-α2α’2 stimulation. c, PSD-LTM expression was significantly dampened at 3 d after a single 6 h bout of TrpA1 stimulation but resurfaced at 6 d. d, Three 6 h spaced TrpA1 stimulations prolonged the memory expression deficit to 10 d, but PSD-LTM expression resurfaced to normal levels at 14 d. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 8 (a), one-way ANOVA with Dunnett’s post-hoc test; n = 6 (b), n = 12 (c, d), two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
Extended Data Fig 5. DAMB’s enhanced PSD-LTM maps to αβ MBn.
a, Loss of DAMB (loss of function allele) elevated PSD-LTM expression up to 14 d. Wild-type: Canton-S. b, Pan-neuronal knockdown of DAMB increased 24 h PSD-LTM. The RNAi lines target non-overlapping sites of DAMB affecting all transcript variants (coding exons, green; non-coding exons, blue; introns, black line). NSyb-gal4 > uas-RNAi, uas-dicer2. RNAi #1: KK-line. RNAi #2: GD-line. RNAi #3: TRiP-line. c, DAMB knockdown in αβ MBn enhanced 24 h LTM. Gal4 > uas-RNAi(KK), uas-dicer2. d, DAMB knockdown in the αβ MBn elevated PSD-LTM up to 14 d after spaced conditioning. C739-gal4 > uas-RNAi(KK), uas-dicer2. e, Functional reinstatement of DAMB restored PSD-LTM to normal levels. Wildtype: Canton-S. f, DAMB RNAi knockdown and normalising PSD-LTM expression by differential spaced conditioning. C739-gal4, gal80ts > uas-RNAi, uas-dicer2. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 9 (c), unpaired two-tailed Welch’s t-test; n = 12 (a, b, d-f), two-way ANOVA with Tukey’s test. Exact P-values and comparisons are shown in Supplementary Information.
Extended Data Fig 6. Robust 72 h old PSD-LTM plasticity is formed in MBOn-α2sc.
a, Blocking synaptic output (R34B02-lexA > lexAop-Shibire) from MBOn-α2sc impaired retrieval of PSD-LTM. b, Schedule for training and imaging. c, MBOn-α2sc dendrites imaged as the region of interest. d, Representative pseudocoloured images (scale bar = 10 μm) showing responses to octanol (OCT) or benzaldehyde (BEN) for naïve, OCT+ (OCT as CS+), or BEN+ (BEN as CS+) flies. e, f, Response traces of group data and quantification for OCT+ (e) and BEN+ (f) conditioned animals relative to naïve. The left panel (line graph) displays the activity as a function of time with odour stimulation. The right panel (bar graph) displays the average response magnitude within the first 5 s of odour onset (duration of odour delivery). See methods for calculations of the activity versus mean responses. The Differential reflects the difference in odour response between CS+ minus CS−. Multiple spaced cycles generated increased calcium transients compared to naïve flies, with OCT+ generating a more potent differential than BEN+. g, Feeding schedule for CXM before spaced conditioning and representative pseudocoloured images (scale bar = 10 μm) showing the effects of CXM on odour responses. h, i, CXM+ blunted the OCT+ (h) or BEN+ (i) training-induced calcium transients indicating that the differential represents a PSD-LTM trace. j, Training schedule and representative pseudocoloured images (scale bar = 10 μm) without (23 °C) or with (30 °C) a 6 h TrpA1 stimuation. Response traces and quantificaiton are in Fig. 3b. Box-and-whisker plots show the range of individual data points, the interquartile spread as the box, and the median as the line bisecting each box. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; n = 8 (a), two-way ANOVA with Tukey’s test; n = 20 (e, naïve), n = 21 (e, OCT+), n = 20 (f, naïve), n = 20 (e, BEN+), n = 20 (h, i), unpaired two-tailed Mann-Whitney test (e, f, h, i). Imaging experiments (d-j) were performed three independent times with proper controls present within each set. All activity traces, mean responses and representative images shown were reproducible. Exact P-values and comparisons are shown in Supplementary Information.
Extended Data Fig 7. Working model comparing permanent versus transient forgetting.
Two forms of forgetting include permanent (red) and transient (orange) forgetting. Left, Permanent forgetting involves a PPL1 DAn that synapses onto the γ2α’1-MBn compartment (red). The slow, ongoing DAn activity after learning is transduced by the Gq-coupled, DAMB receptor. This forgetting signal mobilises the Scribble scaffolding complex and recruits Rac1, PAK3 and Cofilin to erode labile, nonconsolidated memory. The cellular memory traces formed and stored in the following neuron, γ2α’1-MBOn, are also eroded. This process can be exacerbated by enhanced sensory stimulation (+), or repressed by sleep/rest (−). Right, Transient forgetting incorporates a different PPL1 DAn that synapses onto the α2α’2-MBn compartment (orange). This forgetting signal, transduced by DAMB, temporarily impairs the expression of consolidated, PSD-LTM. The cellular memory traces stored in α2sc-MBOn are not abolished after activating the forgetting pathway. This process can be triggered by interfering or distracting stimuli (+) to transiently block the retrieval of PSD-LTM.
Supplementary Material
Supplementary Information. Reagents, behavioural control experiments and statistical analyses. Sheet number one lists the fly strains, reagents and equipment used in the study. Sheet number two contains the odour and shock avoidance across various genotypes and conditions. Sheets number three and four list the statistical comparisons for each experiment in the main and extended figures, respectively. Sheet number five details additional statistical comparisons (two-way ANOVA with repeated measures) to determine spontaneous recovery of memory.
Acknowledgements
This work was supported by NIH grant 5R35NS098224 to R.L.D. and F31MH123022 to J.M.S. We would like to thank Janelia Research for providing split-gal4 lines and all other colleagues who have supplied Drosophila stocks. We want to also thank past and current members of the Davis Lab for their constructive conversations and criticisms.
Footnotes
Competing interests The authors declare no competing interests.
Data availability
Data are available upon request.
Main references:
- 1).Davis RL, & Zhong Y (2017). The biology of forgetting—a perspective. Neuron, 95(3), 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Kitazono T, Hara-Kuge S, Matsuda O, Inoue A, Fujiwara M, & Ishihara T (2017). Multiple signaling pathways coordinately regulate forgetting of olfactory adaptation through control of sensory responses in Caenorhabditis elegans. Journal of Neuroscience, 37(42), 10240–10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Patel U, Perez L, Farrell S, Steck D, Jacob A, Rosiles T, … & Calin-Jageman IE (2018). Transcriptional changes before and after forgetting of a long-term sensitization memory in Aplysia californica. Neurobiology of learning and memory, 155, 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Mao Z, & Davis RL (2009). Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in neural circuits, 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Berry JA, Cervantes-Sandoval I, Nicholas EP, & Davis RL (2012). Dopamine is required for learning and forgetting in Drosophila. Neuron, 74(3), 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Berry JA, Phan A, & Davis RL (2018). Dopamine neurons mediate learning and forgetting through bidirectional modulation of a memory trace. Cell reports, 25(3), 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Berry JA, Cervantes-Sandoval I, Chakraborty M, & Davis RL (2015). Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell, 161(7), 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Cervantes-Sandoval I, Chakraborty M, MacMullen C, & Davis RL (2016). Scribble scaffolds a signalosome for active forgetting. Neuron, 90(6), 1230–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Shuai Y, Lu B, Hu Y, Wang L, Sun K, & Zhong Y (2010). Forgetting is regulated through Rac activity in Drosophila. Cell, 140(4), 579–589. [DOI] [PubMed] [Google Scholar]
- 10).Schwartz BL, & Metcalfe J (2011). Tip-of-the-tongue (TOT) states: retrieval, behaviour, and experience. Memory & cognition, 39(5), 737–749. [DOI] [PubMed] [Google Scholar]
- 11).Maril A, Simons JS, Weaver JJ, & Schacter DL (2005). Graded recall success: an event-related fMRI comparison of tip of the tongue and feeling of knowing. Neuroimage, 24(4), 1130–1138. [DOI] [PubMed] [Google Scholar]
- 12).Han KA, Millar NS, Grotewiel MS, & Davis RL (1996). DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron, 16(6), 1127–1135. [DOI] [PubMed] [Google Scholar]
- 13).Himmelreich S, Masuho I, Berry JA, MacMullen C, Skamangas NK, Martemyanov KA, & Davis RL (2017). Dopamine receptor DAMB signals via Gq to mediate forgetting in Drosophila. Cell reports, 21(8), 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Davis RL (2011). Traces of Drosophila memory. Neuron, 70(1), 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Pascual A, & Préat T (2001). Localization of long-term memory within the Drosophila mushroom body. Science, 294(5544), 1115–1117. [DOI] [PubMed] [Google Scholar]
- 16).Cervantes-Sandoval I, Martin-Peña A, Berry JA, & Davis RL (2013). System-like consolidation of olfactory memories in Drosophila. Journal of Neuroscience, 33(23), 9846–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Roman G, Endo K, Zong L, & Davis RL (2001). P {Switch}, a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proceedings of the National Academy of Sciences, 98(22), 12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Tonegawa S, Liu X, Ramirez S, & Redondo R (2015). Memory engram cells have come of age. Neuron, 87(5), 918–931. [DOI] [PubMed] [Google Scholar]
- 19).Yu D, Ponomarev A, & Davis RL (2004). Altered representation of the spatial code for odors after olfactory classical conditioning: memory trace formation by synaptic recruitment. Neuron, 42(3), 437–449. [DOI] [PubMed] [Google Scholar]
- 20).Yu D, Akalal DBG, & Davis RL (2006). Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron, 52(5), 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Akalal DBG, Yu D, & Davis RL (2011). The long-term memory trace formed in the Drosophila α/β mushroom body neurons is abolished in long-term memory mutants. Journal of Neuroscience, 31(15), 5643–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Liu X, & Davis RL (2009). The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nature neuroscience, 12(1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Wang Y, Mamiya A, Chiang AS, & Zhong Y (2008). Imaging of an early memory trace in the Drosophila mushroom body. Journal of Neuroscience, 28(17), 4368–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, … & Rubin GM (2014). The neuronal architecture of the mushroom body provides a logic for associative learning. Elife, 3, e04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Frankland PW, Josselyn SA, & Köhler S (2019). The neurobiological foundation of memory retrieval. Nature neuroscience, 22(10), 1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, & Tonegawa S (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature, 484(7394), 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Placais PY, de Tredern É, Scheunemann L, Trannoy S, Goguel V, Han KA, … & Preat T (2017). Upregulated energy metabolism in the Drosophila mushroom body is the trigger for long-term memory. Nature communications, 8(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Cervantes-Sandoval I, Phan A, Chakraborty M, & Davis RL (2017). Reciprocal synapses between mushroom body and dopamine neurons form a positive feedback loop required for learning. Elife, 6, e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Cohn R, Morantte I, & Ruta V (2015). Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell, 163(7), 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods references:
- 30).Beck CDO, Schroeder B, & Davis RL (2000). Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. Journal of Neuroscience, 20(8), 2944–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Walkinshaw E, Gai Y, Farkas C, Richter D, Nicholas E, Keleman K, & Davis RL (2015). Identification of genes that promote or inhibit olfactory memory formation in Drosophila. Genetics, 199(4), 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Noyes NC, Walkinshaw E, & Davis RL (2020). Ras acts as a molecular switch between two forms of consolidated memory in Drosophila. Proceedings of the National Academy of Sciences, 117(4), 2133–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).McGuire SE, Mao Z, & Davis RL (2004). Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE, 2004(220), pl6–pl6. [DOI] [PubMed] [Google Scholar]
- 34).Pfeiffer BD, Truman JW, & Rubin GM (2012). Using translational enhancers to increase transgene expression in Drosophila. Proceedings of the National Academy of Sciences, 109(17), 6626–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Liu Q, Liu S, Kodama L, Driscoll MR, & Wu MN (2012). Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Current Biology, 22(22), 2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, … & Waddell, S. (2012). Layered reward signalling through octopamine and dopamine in Drosophila. Nature, 492(7429), 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Fisher YE, Leong JC, Sporar K, Ketkar MD, Gohl DM, Clandinin TR, & Silies M (2015). A class of visual neurons with wide-field properties is required for local motion detection. Current Biology, 25(24), 3178–3189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information. Reagents, behavioural control experiments and statistical analyses. Sheet number one lists the fly strains, reagents and equipment used in the study. Sheet number two contains the odour and shock avoidance across various genotypes and conditions. Sheets number three and four list the statistical comparisons for each experiment in the main and extended figures, respectively. Sheet number five details additional statistical comparisons (two-way ANOVA with repeated measures) to determine spontaneous recovery of memory.
Data Availability Statement
Data are available upon request.