Abstract
Introduction and importance
The impact of Covid-19 pandemic on the incidence and pattern of Mucormycosis (the black fungus), has increased sharply and is featured as an epidemic within a pandemic. The majority of cases were detected at late stages, which decreases the chances of survival.
Case presentation
The authors present a case of an immunocompetent male patient diagnosed with left orbital apex syndrome post covid-19 infection, which necessitated orbital exenteration. He was later hospitalized in a quarantine facility and an area of skin breakdown on his left cheek was noted and surgical debridement performed. Later, He presented to our institution with left hemifacial skin loss, exposing the underlying diseased bone. A multidisciplinary team examined the patient clinically and radiographically, reaching a primitive diagnosis of secondary cutaneous Mucormycosis due to rhino-orbital Mucormycosis, with no cavernous sinus thrombosis nor cranial extension. Radical surgical and medical treatments were given and he had an uneventful recovery. Unfortunately, he died 5 days after the reconstructive surgery with Anterolateral Thigh (ALT) flap.
Clinical discussion
The dual effect of both covid-19 and its' associated Mucormycosis, predispose patients to increased risk of pressure injuries including Medical device related pressure injuries. Survivors of Mucormycosis are high-risk patients, and planning their reconstruction by free flaps is challenging. However, delayed reconstruction is recommended.
Conclusion
Early diagnosis and management of covid-19 associated Mucormycosis should be prioritized. Moreover, surgical debridement of necrotic tissues should not be delayed due to an unavailable or negative histopathology.
Keywords: Covid-19, Mucormycosis, Cutaneous mucormycosis, Fungal rhinosinusitis (FRS), Device related pressure ulcers (DRPU), Case report
Highlights
-
•
Medical device related pressure injuries (MDRPIs) is an iatrogenic injury, and its prevention and management should be prioritized.
-
•
Mucormycosis should be investigated in COVID-19 infected and recovered patients.
-
•
Cutaneous Mucormycosis is common among the immunocompetent patients, and knowledge of its types and subtypes is essential.
-
•
The authors highlight the value of clinical and radiographic investigation in aiding early diagnosis of Mucormycosis.
-
•
Surgical debridement of necrotic tissues is a life-saving measure, and should not be delayed due to an unavailable or negative histopathology.
1. Introduction
Mucormycosis, previously known as zygomycosis and phycomycosis, and recently called the “black fungus”. It is a rare, saprophytic, opportunistic, and potentially lethal fungal infection, caused by fungi of the phylum Zygomycota, subphylum Mucormycotina, order Mucorales [1] Mucormycosis was first reported in humans in the year 1855 and first examined in autopsy in 1956 [2]. However, reports of original description by Paultauf in 1885 [3] and A. M. Marchevsky [4] exists. Mucormycosis affects a wide range of ages, from newborns to the elderly. Its global incidence and ecology show remarkable variations. Some studies report equal sex distribution, while others show male predominance [5].
Mucormycosis presents in different locations and clinical patterns. Unlike the earlier belief that it is a disease of the immunocompromised [6], [7], Mucormycosis has been increasingly reported among immunocompetent people, as in our case report. The type and pattern of Mucormycosis should be identified according to the six well-recognized clinical categories of Mucormycosis, which are: rhino-orbital cerebral Mucormycosis (ROCM), cutaneous, pulmonary, gastrointestinal, disseminated, and miscellaneous [3]. While rhino-orbital cerebral Mucormycosis (ROCM), is prevalent among immunocompromised individuals; cutaneous Mucormycosis is common among the immunocompetent patients. [8].
Cutaneous Mucormycosis is regarded as the third most common type of Mucormycosis [1], [9] and can be classified into localized, deep, or disseminated types. Others classify it into superficial or gangrenous forms. However, it is commonly classified into primary and secondary types. Primary Cutaneous Mucormycosis (PCM) occurs due to direct inoculation of infection, while Secondary Cutaneous Mucormycosis (SCM) occurs due to dissemination of infection; usually secondary to rhino-orbital cerebral Mucormycosis (ROCM) [9], [10]. Further subtypes of primary and secondary cutaneous Mucormycosis exist. The Primary Cutaneous Mucormycosis (PCM) subtypes include a gradual or a fulminant form. On the other hand, the Secondary Cutaneous Mucormycosis (SCM) can be classified into stage I, when limited to the sino-nasal region, stage II where sino-orbital involvement occurs, and stage III, with an intracranial spread. [10].
Coronavirus disease 2019 (covid-19) is an infectious, multisystem disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is an enveloped RNA beta coronavirus-2 that resulted in the current global health crisis. Covid-19 has presented with atypical cutaneous, mucocutaneous, [11], [12], [13] as well as oral complications [13]. The latter was underreported due to the associated higher risk of infectivity and pathogenicity during the examination.
Mucormycosis has emerged as the second most common invasive fungal infection [14] and featured as an epidemic within a pandemic. Covid-19 associated Mucormycosis (CAM) presents a unique challenge, given the global inability to control the newly emerging SARS-CoV-2 virus, also the limited experience in treating Mucormycosis which was until recently a rare infection. Unlike covid-19, no person-to-person mode of transmission was reported for Mucormycosis [15]. However, the respiratory route is the most common mode of infection of both diseases. Mucormycosis-causing agents are commensal in the nasal mucosa [16] and are widespread in nature. They can grow on bread, fruit, leaves, and soil. They are also found in the hospital environment and are a known nosocomial pathogen that should not be neglected. As the pandemic is placing tremendous strain on the healthcare systems worldwide, healthcare-associated Mucormycosis (HCM) is becoming a matter of concern [17].
The Covid-19 pandemic has been associated with a range of fungal co-infections attributed to a multitude of factors [18], [19]. A “diabetes-like state” is created due to the extensive and prolonged use of steroids against covid-19. The raised glucose level provides a nutritional source for Mucorales to multiply. Moreover, the extensive use of antimicrobials, in the era of global antimicrobial resistance (AMR), without including antifungals, as part of the armamentarium against covid-19 needs reevaluation [20]. The combination of virus- and drug-induced immunosuppression [21], [22] exposes patients to increased risks of development and exacerbation of fungal infections.
2. Case report
The authors present a case of a 44-year-old, medically free, male patient who presented to our tertiary care center with left hemifacial skin loss, exposing the underlying diseased bone of the facial skeleton (Fig. 1, Fig. 2). He had a history of a positive reverse-transcriptase polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was home treated. After which, he complained of proptosis, visual disturbances, and ocular immobility and was diagnosed with orbital apex syndrome, which necessitated exenteration of the left orbit.
Fig. 1.
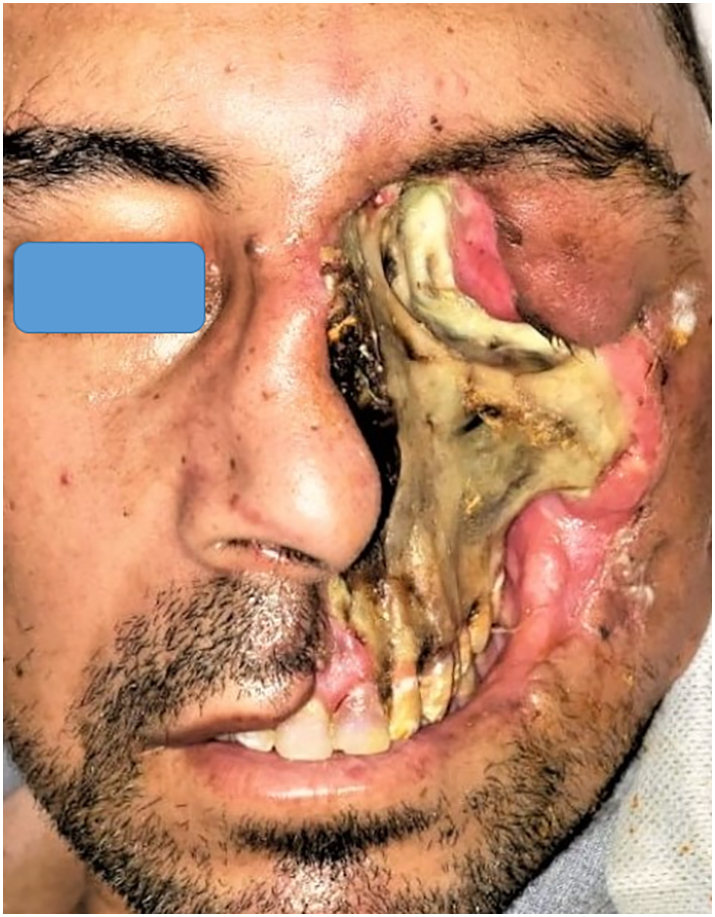
Showing the frontal view of the patient at his first clinical presentation.
Fig. 2.
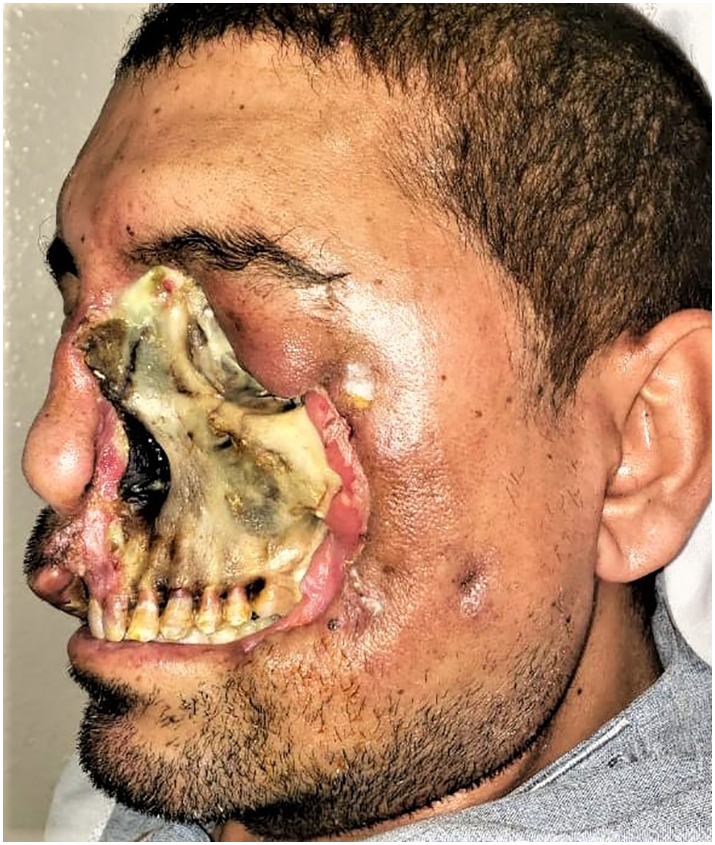
Showing the profile view of the patient at his first clinical presentation.
His Covid-19 infection did not resolve (positive nasopharyngeal swab, RT-PCR confirmed) and he was hospitalized and later intubated in an intensive care unit for treatment. During which, an area of skin breakdown on his left cheek related to the applied noninvasive ventilation (NIV) mask was noted. Although he had no history of trauma to that region; he experienced progressive necrosis of skin & soft tissues of the left cheek. This necessitated surgical debridement, which was performed in the quarantine facility where he was treated.
After his discharge, he was referred to our institution for reevaluation of the condition, with the possibility of further surgical intervention or hyperbaric oxygen therapy (HBOT). Once the patient presented to our department, an initial clinical diagnosis of cutaneous Mucormycosis was made (Fig. 1, Fig. 2). However, computed tomography (CT) scan of facial bones revealed pansinusitis with bony involvement, suggestive of rhino-orbital Mucormycosis as the infection was not limited to soft tissues. A multidisciplinary team of neurosurgeons, ophthalmologists, maxillofacial surgeons, maxillofacial prosthetists, plastic surgeons, and otolaryngologists examined the patient. Cavernous sinus thrombosis was excluded by Magnetic resonance imaging (MRI) and Magnetic Resonance Venography (MRV).
The patient was scheduled for radical surgical debridement of the diseased tissues (Fig. 3), which was continued until healthy bleeding tissues were encountered. The field was almost bloodless, although the region of the head and neck is highly vascular. Postoperative feeding was done through a percutaneous gastrostomy tube and regular dietary counseling was scheduled to assess the nutritional status. The extent of surgical debridement was beyond expected (Fig. 4, Fig. 5), and an immediate obturator could be applied neither intraoperative nor during the postoperative healing phase. Rapid crust formation was noted in the exposed surgical defect, which required manual removal, wound irrigation, and local dressing.
Fig. 3.
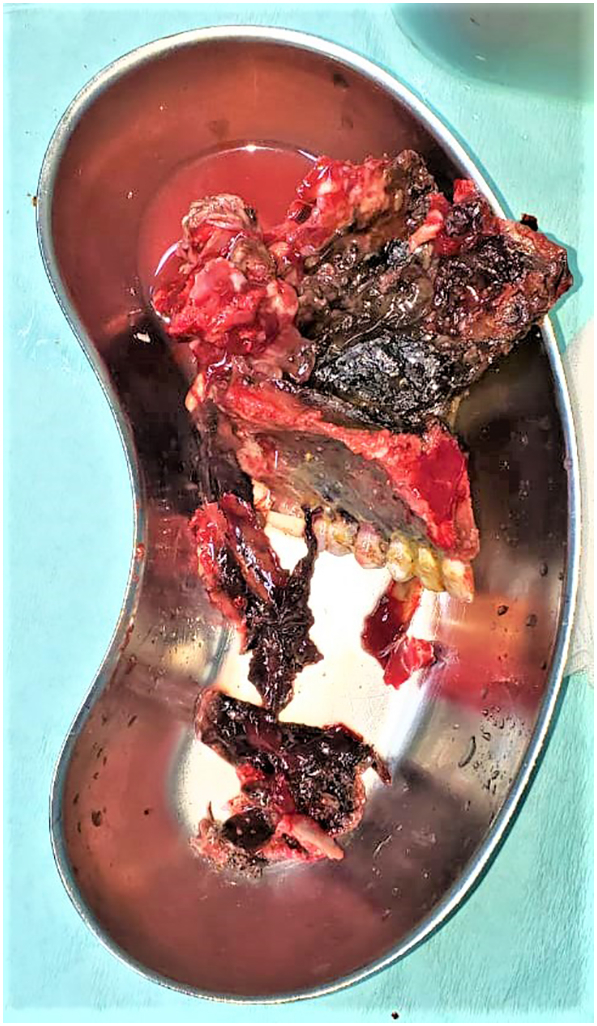
Showing the removed diseased facial bone sent as biopsy.
Fig. 4.
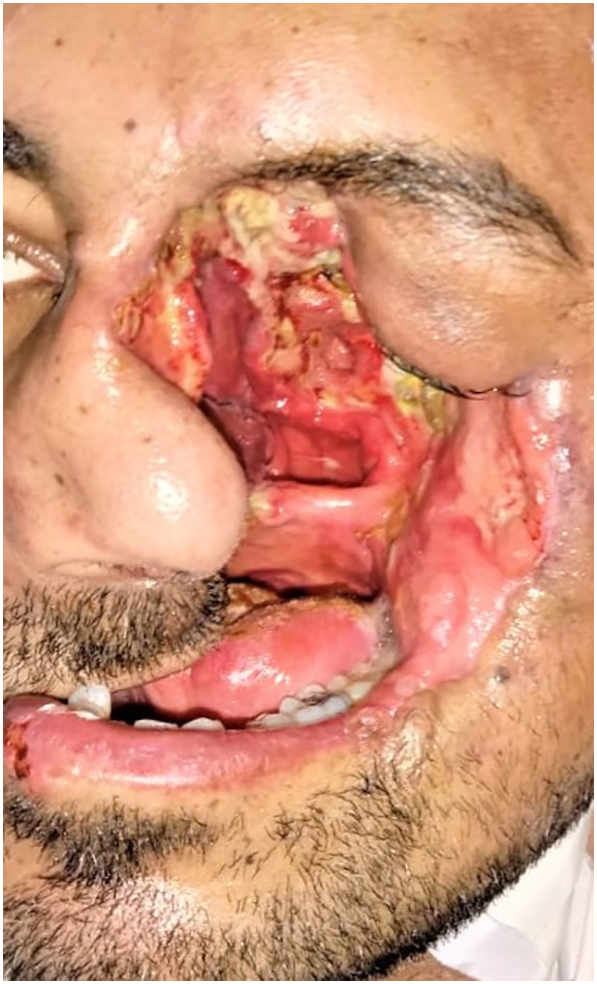
Showing the frontal view of the patient postoperative.
Fig. 5.
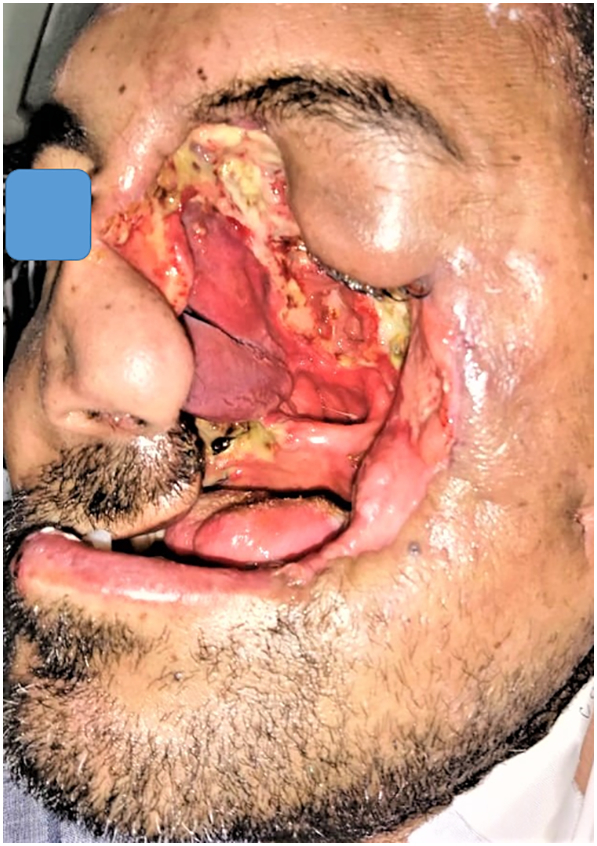
Showing the profile view of the patient postoperative.
A definitive histological study using hematoxylin and eosin stain revealed broad, branching, non-septate hyphae confirming the initial clinical diagnosis of Mucormycosis. The treatment regimen of systemic amphotericin B in conjunction with local wound irrigation with amphotericin B was performed daily. The patient's daily serum electrolytes and kidney function tests were monitored, and the amphotericin B dose was titrated accordingly.
The patient was closely monitored for the first postoperative month, by combining clinical examination (well-granulated cavities free of necrosis), and radiological investigation (resolution or stabilization on serial CT scans) [15]. Tissue diagnosis (free margins of the previously infected areas) was also used to confirm adequate disease control. Reconstructive surgery was then planned with Anterolateral Thigh (ALT) flap to reconstruct the complex orofacial surgical defect. Unfortunately, the patient died 5 days after the reconstructive surgery in the intensive care unit (ICU). This case report is compliant with the SCARE 2020 guidelines and criteria [23].
3. Discussion
The dual effect of both coronavirus disease 2019 (covid-19) and its' associated Mucormycosis, predispose patients to increased risk of pressure injuries including Medical device-related pressure injuries (MDRPIs). The covid-19 disease itself inflicts direct tissue complications, which include cytokine release storm [21], endothelial dysfunction, ischemia, hypoxemia, microvascular injury, and thrombosis [24], [25]. However, the indirect disease complications include covid-19 associated Mucormycosis (CAM) which in turn, results in added tissue angioinvasion and thromboembolism. All of which, render the tissue viability compromised and vulnerable. The incidence of MDRPIs has sharply increased in both the prevention and therapeutic phases of the treatment since the Covid-19 pandemic [24], [26]. The non-invasive ventilation (NIV) mask is known to cause facial skin damage [27] and trials to develop a personalized, fitting 3-dimensional (3D) scanning device for each patient were reported [28]. Prevention and management of this iatrogenic injury present an increasing healthcare concern, affecting the efficacy of intervention and patients' quality of life. Significant skin deformation may occur due to the unrelieved pressure, shear, and friction of non-retractable, life-preserving medical devices. Most of which, (30–70%) are respiratory equipment [29] that need to be securely fastened to the face. In contrast, pressure injuries that occur when there is continuous pressure over bony prominences can be alleviated by repositioning the patient [30].
The impact of the Covid-19 pandemic on the incidence and pattern of Mucormycosis has increased sharply and the majority of cases were detected at late stages. Barriers to accessing health care services in the lockdown period of covid-19 contributed to this diagnostic delay. Despite radical intervention, the mortality rate remains high and some cases were only diagnosed postmortem (autopsy proven Mucormycosis) [31], Moreover, SARS-CoV-2 decreased the number of autopsies performed [32]. Early diagnosis of Mucormycosis is of paramount importance as a delay of even 6 days is associated with doubling mortality from 35% to 66% [33]. However, although suspecting Mucormycosis, is just a presumptive diagnosis that is less reliable; it may improve the chances of early detection and survival. This requires a high level of clinical experience and suspicion in patients with predisposing factors, either systemic (e.g. medically compromised, or post covid-19 infection) or environmental (e.g. trauma or nosocomial). Imaging studies, despite their limitations, are useful diagnostic tools to assess the extent of infection and determine the required level of surgical intervention.
The importance of correlating radiological investigation with clinical examination cannot be overstated. Each diagnostic tool carries its strengths and weakness, therefore, combining different diagnostic modalities offer added benefit for an accurate diagnosis. Knowledge of the radiographic features of Mucormycosis such as the black turbinate sign and lack of contrast enhancement due to mucosal necrosis is important. The criteria of clinical diagnosis of Mucormycosis published in 1950, by Smith et al. [34], is still considered the gold standard [16]. Moreover, in 2021 the Indian Council of Medical Research (ICMR), published guidelines for the screening, diagnosis, and management of Mucormycosis at the time of Covid-19 [35].
Cutaneous Mucormycosis should not be confused with necrotizing soft tissue infections, as the causative agents and the treatment protocols are different. The wide range of possible differential diagnosis of early Mucormycosis and its nonspecific clinical presentation poses a diagnostic challenge and make diagnosis on clinical grounds difficult. Our patient was initially clinically diagnosed with Primary Cutaneous Mucormycosis (PCM) as it was hypothesized that the skin breach predisposed him to subsequent direct inoculation and implantation of nosocomial fungal elements. However, after the initial surgical debridement was performed; it exposed the underlying diseased bone. The black necrotic eschar seen (Fig. 1, Fig. 2), has been regarded as a hallmark of Rhino-orbital Mucormycosis but rather, a late sign of the disease [15], [36]. The anterior maxillary wall was not eroded (Fig. 1, Fig. 2), in accordance with previous reports [37]. All of which changed the primitive clinical diagnosis to secondary cutaneous Mucormycosis (SCM) due to rhino-orbital Mucormycosis.
Biopsy-proven Mucormycosis is the gold standard for a precise definitive diagnosis. However, to avoid false-negative histopathological results, the harvested tissue biopsy should be deep enough and include subcutaneous fat to facilitate recovering the characteristic hyphae [1]. These are broad, branching, aseptate hyphae that are the cause of thrombosis and tissue necrosis. Fungal Rhinosinusitis (FRS) can be divided into invasive and non-invasive types according to histopathological evidence of fungal tissue invasion [38].
However, surgical debridement of progressive disease or necrotic tissues should not be delayed due to an unavailable or negative histopathological result, especially at the time of covid-19 [2]. Surgical eradication of diseased tissues provides a life-saving measure, initially at the cost of esthetics and function. However, advances in free flaps allow surgical reconstruction of defects once considered inoperable, and bring vascularized tissue into a previously compromised area. Anterolateral thigh (ALT) flap was chosen as it offers a highly versatile reconstructive option when different coating surfaces are needed. It provides sufficient volume to ablate the previously excised sinus cavities, adequate bulk to cover the complex orofacial defect with reasonable projection to the malar region. Survivors of Mucormycosis are high-risk patients, and planning their reconstruction by free flaps is an added challenge. Many authors [39], like ourselves, prefer delayed reconstruction after surgical debridement of Mucormycosis.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Funding
The authors had no source of funding and no sponsors to this work.
Ethical approval
The data presented in the current case report is reviewed and approved by the Ethical Committee at our hospital. The patient signed a “release form” to give the authors the permission needed for publication.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
This case study was written in accordance to Helsinki guidelines.
Guarantor
Reem Hassan Saad BDS, MFD RCSI, MSc
Fahmy A. Mobarak BDS, MSc, PHD
CRediT authorship contribution statement
-
•
Reem Hassan Saad BDS, MFDRCSI, MSc
(Corresponding author, data collection, manuscript writing, patient's follow up)
-
•
Fahmy A. Mobarak BDS, MSc, PHD
(Lead clinician, data analysis, patient's surgery and follow up).
Declaration of competing interest
The authors have no conflict of interest of any aspect.
References
- 1.Ingram P.R. Cutaneous mucormycosis and motor vehicle accidents: findings from an Australian case series. Med. Mycol. 2014;52(8):819–825. doi: 10.1093/mmy/myu054. [DOI] [PubMed] [Google Scholar]
- 2.Mahalaxmi I. Mucormycosis: an opportunistic pathogen during COVID-19. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapidis A.D. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin. Microbiol. Infect. 2009;15(Suppl 5):98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 4.Dave S.P., Vivero R.J., Roy S. Facial cutaneous mucormycosis in a full-term infant. Arch. Otolaryngol. Head Neck Surg. 2008;134(2):206–209. doi: 10.1001/archoto.2007.48. [DOI] [PubMed] [Google Scholar]
- 5.Elzein F. Mucormycosis: an 8-year experience of a tertiary care centre in Saudi Arabia. J. Infect. Public Health. 2020;13(11):1774–1779. doi: 10.1016/j.jiph.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Mohindra S. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. 2007;50(4):290–296. doi: 10.1111/j.1439-0507.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- 7.Mignogna M.D. Mucormycosis in immunocompetent patients: a case-series of patients with maxillary sinus involvement and a critical review of the literature. Int. J. Infect. Dis. 2011;15(8):e533–e540. doi: 10.1016/j.ijid.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Skiada A. Global epidemiology of cutaneous zygomycosis. Clin. Dermatol. 2012;30(6):628–632. doi: 10.1016/j.clindermatol.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Bonifaz A. Cutaneous mucormycosis: mycological, clinical, and therapeutic aspects. Curr. Fungal Infect. Rep. 2015;9(4):229–237. [Google Scholar]
- 10.Castrejón-Pérez A.D. Cutaneous mucormycosis. An. Bras. Dermatol. 2017;92(3):304–311. doi: 10.1590/abd1806-4841.20176614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuno-Gonzalez A. Prevalence of mucocutaneous manifestations in 666 patients with COVID-19 in a field hospital in Spain: oral and palmoplantar findings. Br. J. Dermatol. 2021;184(1):184–185. doi: 10.1111/bjd.19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang K., Wang Y., Zhang H. Cutaneous manifestations of the coronavirus disease 2019 (COVID-19): a brief review. Dermatol. Ther. 2020;33(4) doi: 10.1111/dth.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halboub E., Al-Maweri S.A. Orofacial manifestations of COVID-19: a brief review of the published literature. Braz. Oral. Res. 2020;34 doi: 10.1590/1807-3107bor-2020.vol34.0124. [DOI] [PubMed] [Google Scholar]
- 14.Katragkou A., Walsh T.J., Roilides E. Why is mucormycosis more difficult to cure than more common mycoses? Clin. Microbiol. Infect. 2014;20(Suppl 6):74–81. doi: 10.1111/1469-0691.12466. [DOI] [PubMed] [Google Scholar]
- 15.González Ballester D. Mucormycosis of the head and neck: report of five cases with different presentations. J. Craniomaxillofac. Surg. 2012;40(7):584–591. doi: 10.1016/j.jcms.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Kumari A. Management outcomes of mucormycosis in COVID-19 patients: a preliminary report from a tertiary care hospital. Med, J, Armed Forces India. 2021;77:S289–s295. doi: 10.1016/j.mjafi.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattah S.Y. Tongue necrosis secondary to mucormycosis in a diabetic patient: a first case report in Malaysia. J. Mycol. Med. 2018;28(3):519–522. doi: 10.1016/j.mycmed.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9) doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cataldo M.A. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming "collateral effect". J, Glob, Antimicrob, Resist, 2020;23:290–291. doi: 10.1016/j.jgar.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lansbury L. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ripa M. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin. Microbiol. Infect. 2021;27(3):451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajendra Santosh A.B., Muddana K., Bakki S.R. Fungal infections of Oral cavity: diagnosis, management, and association with COVID-19. SN Compr.Clin. Med. 2021:1–12. doi: 10.1007/s42399-021-00873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agha R.A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Martel T., Orgill D.P. Medical device-related pressure injuries during the COVID-19 pandemic. J. Wound Ostomy Continence Nurs. 2020;47(5):430–434. doi: 10.1097/WON.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrillat A. Facial pressure ulcers in COVID-19 patients undergoing prone positioning: how to prevent an underestimated epidemic? J. Stomatol. Oral Maxillofac. Surg. 2020;121(4):442–444. doi: 10.1016/j.jormas.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gefen A., Ousey K. Update to device-related pressure ulcers: SECURE prevention. COVID-19, face masks and skin damage. J. Wound Care. 2020;29(5):245–259. doi: 10.12968/jowc.2020.29.5.245. [DOI] [PubMed] [Google Scholar]
- 27.Alqahtani J.S., Worsley P., Voegeli D. Effect of humidified noninvasive ventilation on the development of facial skin breakdown. Respir. Care. 2018;63(9):1102–1110. doi: 10.4187/respcare.06087. [DOI] [PubMed] [Google Scholar]
- 28.Shikama M. Development of personalized fitting device with 3-dimensional solution for prevention of NIV oronasal mask-related pressure ulcers. Respir. Care. 2018;63(8):1024–1032. doi: 10.4187/respcare.05691. [DOI] [PubMed] [Google Scholar]
- 29.Padula C.A. Prevention of medical device-related pressure injuries associated with respiratory equipment use in a critical care unit: a quality improvement project. J. Wound Ostomy Continence Nurs. 2017;44(2):138–141. doi: 10.1097/WON.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 30.Hsu M.Y., Lin J.P., Lyu J.Y. Medical device related pressure injury prevention strategies. Hu Li Za Zhi. 2019;66(3):106–111. doi: 10.6224/JN.201906_66(3).13. [DOI] [PubMed] [Google Scholar]
- 31.Zurl C., Hoenigl M. Autopsy proven pulmonary mucormycosis due to rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J. Fungi (Basel) 2021;7(2) doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nestler M. Fungal superinfection in patients with COVID-19: role of antifungal stewardship? Am. J. Infect. Control. 2021;49(2):279–280. doi: 10.1016/j.ajic.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith H.W., Kirchner J.A. Cerebral mucormycosis; a report of three cases. AMA Arch. Otolaryngol. 1958;68(6):715–726. doi: 10.1001/archotol.1958.00730020739010. [DOI] [PubMed] [Google Scholar]
- 35.Nambiar M., Varma S.R., Damdoum M. Post-covid alliance-mucormycosis, a fatal sequel to the pandemic in India. SaudiJ. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnáiz-García M.E. Cutaneous mucormycosis: report of five cases and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2009;62(11):e434–e441. doi: 10.1016/j.bjps.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 37.Hosseini S.M., Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur. Arch. Otorhinolaryngol. 2005;262(11):932–938. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 38.Ni Mhurchu E. Fungal rhinosinusitis: a radiological review with intraoperative correlation. Can. Assoc. Radiol. J. 2017;68(2):178–186. doi: 10.1016/j.carj.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Parvati R. Is single-stage microvascular reconstruction for facial mucormycosis safe? Indian J. Plast. Surg. 2021;54(2):130–137. doi: 10.1055/s-0041-1731961. [DOI] [PMC free article] [PubMed] [Google Scholar]


