Abstract
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease 2019, has caused millions of deaths worldwide. The virus is transmitted by inhalation of infectious particles suspended in the air, direct deposition on mucous membranes and indirect contact via contaminated surfaces. Disinfection methods that can halt such transmission are important in this pandemic and in future viral infections.
Aim
To highlight the efficacy of several disinfection methods against SARS-CoV-2 based on up-to-date evidence found in the literature.
Methods
Two databases were searched to identify studies that assessed disinfection methods used against SARS-CoV-2. In total, 1229 studies were identified and 60 of these were included in this review. Quality assessment was evaluated by the Office of Health Assessment and Translation's risk-of-bias tool.
Findings
Twenty-eight studies investigated disinfection methods on environmental surfaces, 16 studies investigated disinfection methods on biological surfaces, four studies investigated disinfection methods for airborne coronavirus, and 16 studies investigated methods used to recondition personal protective equipment (PPE).
Conclusions
Several household and hospital disinfection agents and ultraviolet-C (UV-C) irradiation were effective for inactivation of SARS-CoV-2 on environmental surfaces. Formulations containing povidone-iodine can provide virucidal action on the skin and mucous membranes. In the case of hand hygiene, typical soap bars and alcohols can inactivate SARS-CoV-2. Air filtration systems incorporated with materials that possess catalytic properties, UV-C devices and heating systems can reduce airborne viral particles effectively. The decontamination of PPE can be conducted safely by heat and ozone treatment.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Disinfection methods, Systematic review
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become an ongoing global health crisis responsible for causing millions of deaths and has devastated the world's economy [1,2]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel betacoronavirus, is known to be transmitted through exposure to infectious particles in respiratory droplets of infected individuals [3]. This can take place by inhalation of viral particles suspended in the air, deposition of exhaled infectious droplets directly on mucous membranes, or indirect contact with contaminated secondary surfaces, such as hands or fomites [4]. It is believed that airborne transmission may be the dominant form of transmission that best explains the occurrence of superspreading events, the higher risk of transmission in indoor settings, and the fact that more than half of transmission events are observed in asymptomatic or pre-symptomatic patients [[5], [6], [7], [8]]. Particles emitted from infected individuals can be deposited on environmental surfaces and can remain viable for hours to days; as such, it is possible that transmission occurs due to indirect contact with contaminated surfaces [[9], [10], [11]].
The process of decontaminating surfaces normally uses chemical agents such as alcohol or quaternary ammonium compounds (QACs). There is evidence that these agents are active against viruses including SARS-CoV-2 [12]. The World Health Organization (WHO) recommends alcohol-based formulations to disinfect hands; such formulations have been shown to inactivate SARS-CoV-2 efficiently [13]. Many other accessible formulations with a broad range of application, such as hydrogen peroxide or povidone-iodine (PVP-I), possess antiviral properties, potentially serving as effective alternatives for the disinfection of biological surfaces [14,15].
As recent findings suggest that the airborne route is the most plausible and dominant form of transmission, this matter should be highlighted, and methods that can inactivate viruses suspended in the air may contribute substantially to lower the number of cases. Besides natural and mechanical ventilation, only two methods are available commercially: air cleaners fitted with filters or ultraviolet light; and upper room fixtures of ultraviolet germicidal irradiation (UVGI) [16]. UVGI uses short-wavelength ultraviolet C (UV-C) light which, in turn, has been tested against SARS-CoV-2 and proven to be effective [17].
Personal protective equipment (PPE) is essential to protect healthcare workers (HCWs) from contracting infections. Frontline HCWs are at higher risk of contracting SARS-CoV-2 infection compared with the general public [18]. While it is recommended that PPE should be disposable, in times of crisis, a shortage of PPE can cause more harm than benefit, as observed in many countries during the COVID-19 pandemic [19]. In cases when PPE is scarce, methods that provide proper sterilization, while preserving functionality, can be highly beneficial.
The first vaccines, distributed by the end of 2020, have reduced the number of hospitalizations, deaths and incidence of infection, proving to be the most effective tool to combat the COVID-19 pandemic [[20], [21], [22]]. However, disinfection methods will continue to play a major role and must still be put into practice to control local transmissions, whether from human to human, fomites or airborne. Halting the chain of transmission through the implementation of disinfection methods is not only useful in this present pandemic but also in any future similar pandemic. Therefore, the goal of this systematic review is to highlight the best disinfection methods to eliminate SARS-CoV-2 from environmental surfaces, biological surfaces and the air, and to determine the best methods to recondition PPE adequately.
Methods
Eligibility criteria
This review included original articles and experimental studies. Guidelines, protocols, recommendations and non-experimental studies, such as case reports, case series, cross-sectional, prospective case–control studies, opinions and review articles, were excluded. No limitations were considered regarding language, date or status of publication.
Participants
Studies that mentioned SARS-CoV-2 as the main target of any type of disinfection method tested were included. If the study did not mention SARS-CoV-2 specifically, inclusion of the family of coronaviruses that shares genetic or morphological similarities with SARS-CoV-2, preferentially the betacoronaviruses responsible for previous outbreaks of respiratory diseases such as severe acute respiratory syndrome (SARS-CoV) and/or Middle Eastern respiratory syndrome (MERS-CoV), was mandatory. During an outbreak of a highly contagious viral disease such as COVID-19, the availability of the virus under investigation can be limited. Therefore, surrogate viruses (i.e. enveloped virus references) used to study the efficacy of disinfection methods were also included. Studies that did not meet the participant criteria were excluded.
Interventions
Trials that compared the virucidal effects of disinfection methods with the potential to halt transmission of SARS-CoV-2 on environmental surfaces, biological surfaces, air and PPE were assessed.
Information sources, search and study selection
A search was conducted by two reviewers in two separate databases from January to June 2021. PubMed and Web of Science were searched using the following terms: (‘SARS-CoV-2’ OR ‘Coronavirus’ OR ‘COVID-19’) AND (‘Disinfection Methods’ OR’ Surface Disinfection’ OR ‘Hand Disinfection’ OR ‘Air disinfection’ OR ‘Environmental disinfection’ OR ‘Inactivation’). Thirteen articles were identified from other sources and included in the screening process. Two reviewers screened (by title and abstract) the initial 1229 articles found, and the information collected was registered on a shared EndNote Vx9 (Clarivate Analytics, Philadelphia, PA, USA) library and a shared online Microsoft Excel V16.42/2020 (Microsoft Corp., Redmond, WA, USA) document. Eligibility assessment was performed independently in an unblinded standardized manner by two reviewers and disagreements between reviewers were solved by consensus. In total, 60 articles were found to meet the inclusion criteria and were included in this systematic review.
Risk of bias
To determine the risk of bias in the individual studies selected, the Office of Health Assessment and Translation Risk-of-Bias Rating Tool for Human and Animal Studies was used. This tool includes a questionnaire aimed to study risk of bias in several domains:
-
•
selection bias;
-
•
performance bias;
-
•
attrition/exclusion bias;
-
•
detection bias;
-
•
selective reporting bias; and
-
•
other bias.
Potential source of bias was graded as low risk (++), probable low risk (+), probable high risk or not reported (-), and high risk (--).
Results
Study selection
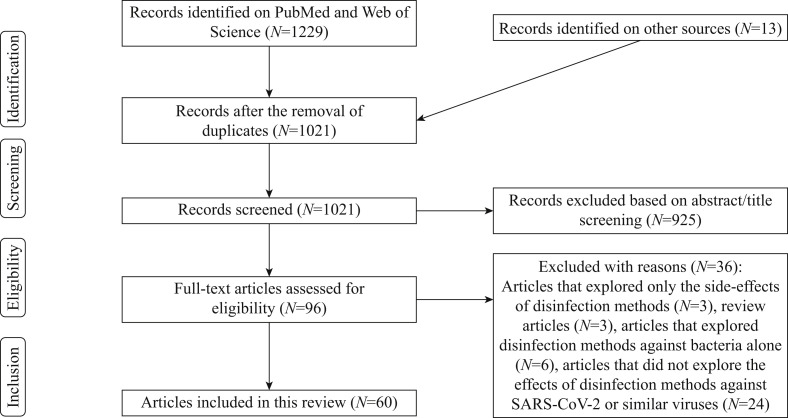
In total, 1229 articles were identified through a search of two databases, PubMed and Web of Science, from January 2021; 13 of these articles were included from other sources. After eliminating duplicate articles, 1021 articles remained. All articles were screened based on the title and abstract, leaving 83 articles eligible for this review. A further 36 articles were eliminated as they did not meet the inclusion criteria. In total, 60 articles were included in this systematic review. The details of this process are represented in Figure 1 . The main characteristics of each individual study included in the systematic review are summarized in Table I .
Figure 1.
PRISMA flow diagram of included articles. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Table I.
Characteristics of included studies (N=60)
| Study | Country | Year | Study design | Environmental surfaces | Personal protective equipment (masks/respirators etc.) | Biological surfaces (hands, skin, oral cavity, respiratory tract) | Air |
|---|---|---|---|---|---|---|---|
| Anderson et al. | UK | 2020 | In vitro | X | |||
| Bedell et al. | USA | 2016 | In vitro | X | |||
| Behzadinasab et al. | Hong Kong | 2020 | In vitro | X | |||
| Biryukov et al. | USA | 2020 | In vitro | X | x | ||
| Casanova et al. | USA | 2010 | In vitro | X | |||
| Colnago et al. | Brasil | 2020 | In vitro | X | x | ||
| Criscuolo et al. | Italy | 2020 | In vitro | X | |||
| Gamble et al. | USA | 2020 | In vitro | X | |||
| Gerchman | Israel | 2020 | In vitro | X | |||
| He et al. | China | 2004 | In vitro | x | |||
| Heilingloh et al. | Germany | 2020 | In vitro | X | |||
| Hulkower et al. | USA | 2011 | In vitro | X | x | ||
| Khaiboullina et al. | USA | 2020 | In vitro | X | |||
| Liu et al. | China | 2020 | In vitro | X | |||
| Malenovská | Czech Republic | 2020 | In vitro | X | |||
| Martins et al. | Brasil | 2020 | In vitro | X | |||
| Meyers et al. | USA | 2021 | In vitro | X | |||
| Monge et al. | USA | 2020 | In vitro | X | |||
| Rabenau et al. | Germany | 2005 | In vitro | X | x | ||
| Ratnesar-Shumate et al. | USA | 2020 | In vitro | X | |||
| Wood and Payne | UK | 1998 | In vitro | X | |||
| Blanchard et al. | USA | 2020 | In vitro | X | |||
| Campos et al. | USA | 2020 | In vitro | X | |||
| Buonanno et al. | USA | 2020 | In vitro | X | |||
| Daeschler et al. | Canada | 2020 | In vitro | X | |||
| Gopal et al. | USA | 2020 | In vitro | X | |||
| Ibanez-Cervantes et al. | Mexico | 2020 | In vitro | X | |||
| Ludwig-Begall et al. | Belgium | 2020 | In vitro | X | |||
| Ma et al. | China | 2020 | In vitro | X | |||
| Mantlo et al. | USA | 2020 | In vitro | X | |||
| Ozog et al. | USA | 2020 | In vitro | X | |||
| Perkins et al. | USA | 2020 | In vitro | X | |||
| Rathnasinghe et al. | USA | 2020 | In vitro | X | |||
| Rockey et al. | USA | 2020 | In vitro | X | |||
| Bidra et al. | USA | 2020 | In vitro | X | |||
| Bidra et al. | USA | 2020 | In vitro | X | |||
| Eggers et al. | Germany | 2015 | In vitro | X | |||
| Frank et al. | USA | 2020 | In vitro | X | |||
| Gudmundsdottir et al. | Iceland | 2020 | In vitro | X | |||
| Kratzel et al. | Germany | 2020 | In vitro | X | |||
| Leslie et al. | USA | 2020 | In vitro | X | |||
| Liang et al. | China | 2020 | In vivo and in vitro | X | |||
| Meister et al. | Germany | 2020 | In vitro | X | |||
| Mukherjee et al. | India | 2020 | In vitro | X | |||
| Buonanno et al. | USA | 2020 | In vitro | x | |||
| Qiao et al. | USA | 2020 | In vitro | x | |||
| Yu et al. | USA | 2020 | In vitro | x | |||
| Franke et al. | Germany | 2021 | In vitro | x | |||
| Gidari et al. | Italy | 2021 | In vitro | x | |||
| Glasbrenner et al. | USA | 2021 | In vitro | x | |||
| Hirose et al. | Japan | 2020 | In vitro | x | |||
| Hu et al. | China | 2021 | In vitro | x | |||
| Huang et al. | USA | 2020 | Prospective cohort | x | |||
| Ijaz et al. | USA | 2021 | In vitro | x | x | ||
| Messina et al. | Italy | 2021 | In vitro | x | |||
| Steinhauer et al. | Germany | 2020 | In vitro | x | x | ||
| Steinhauer et al. | Germany | 2020 | In vitro | x | |||
| Trivellin et al. | Italy | 2021 | In vitro | x | |||
| Uppal et al. | USA | 2021 | In vitro | x | |||
| Valdez-Salas et al. | Mexico | 2021 | In vitro | x |
Study characteristics
The studies included were from 15 countries. Thirty-eight studies used SARS-CoV-2 in their experiments, and the other studies depended on surrogate viruses to represent virucidal activities of some disinfection methods. Six of the articles included were pre-prints.
Stability and survival of SARS-CoV-2 exposed to heat and high humidity
SARS-CoV-2 can remain viable on glass, stainless steel and plastic for more than 3.5 h at ambient temperature and humidity [23]. Increasing relative humidity alone at a constant temperature of 25°C can reduce the survival of SARS-CoV-2 on non-porous surfaces from approximately 15 h–8 h. When temperature and relative humidity are increased simultaneously, the half-life can be reduced remarkably to approximately 1 h [24]. The findings of another study conducted on other coronaviruses [mouse hepatitis virus (MHV) and transmissible gastroenteritis virus (TGEV)] revealed similar results. However, at low temperatures of 4°C and relative humidity of 20%, viruses can persist for up to 28 days [25]. SARS-CoV-2 can be deactivated at different rates when exposed to distinct heating procedures; one study showed that conditions that block evaporation can speed up virus inactivation rates substantially [26].
Disinfection methods on environmental surfaces
Amongst all the reviewed and included studies, 28 articles were categorized as disinfection methods with potential activity on environmental surfaces [12,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]]. A summary of this category is provided in Table II .
Table II.
Results of disinfection methods used on environmental surfaces
| Study | Virus | Disinfectant | Disinfection phase | Exposure time | Reduction of viral infectivity (log10) or (%) | Reduction of viral half-life (t1/2) | |
|---|---|---|---|---|---|---|---|
| 1 | Anderson et al. (2020) | SARS-CoV-2 | Virusend (TX-10) detergent | Suspension test and stainless steel disc surface | 1 min | 4.0 log10 | (-) |
| 10 min | 4.0 log10 | ||||||
| 2 | Bedell et al. (2016) | MHV-A59 | Multiple-emitter, automated, continuous, whole-room UV-C disinfection system | Glass coverslip surface | 5 min | 2.71 log10 | (-) |
| 10 min | 6.11 log10 | ||||||
| MERS-CoV | 5 min | 5.91 log10 | |||||
| 10 min | (-) | ||||||
| 3 | Behzadinasab et al. (2020) | SARS-CoV-2 | Cu2O/PU coating film | Glass surface | 1 h | 3.64 log10 | (-) |
| Stainless steel surface | 2.97 log10 | ||||||
| 4 | Biryukov et al. (2020) | SARS-CoV-2 | 24°C + 20% relative humidity | Stainless steel, ABS plastic and nitrile rubber surfaces | (-) | (-) | 15.33 h ± 2.75 |
| 24°C + 40% relative humidity | 11.52 h ± 1.72 | ||||||
| 24°C + 60% relative humidity | 9.15 h ± 3.39 | ||||||
| 24°C + 80% relative humidity | 8.33 h ± 1.80 | ||||||
| 35°C + 20% relative humidity | 7.33 h ± 1.33 | ||||||
| 35°C + 40% relative humidity | 7.52 h ± 1.22 | ||||||
| 35°C + 60% relative humidity | 2.26 h ± 1.42 | ||||||
| 5 | Casanova et al. (2010) | TGEV and MHV | 4°C + 20% relative humidity | Stainless steel surface | 28 days | 0.5 log10 | (-) |
| 4°C + 50% relative humidity | 21 days | 3.5 log10 | |||||
| 4°C + 80% relative humidity | 28 days | 3.2 log10 (TGEV) and 2.5 log10 (MHV) | |||||
| 20°C + 20% relative humidity | 28 days | 2 log10 | |||||
| 20°C + 50% relative humidity | 3 days (TGEV) and 5 days (MHV) | 2 log10 (TGEV) and 3 log10 (MHV) | |||||
| 20°C + 80% relative humidity | 14 days (TGEV) 11 days (MHV) | 3 log10 (TGEV) and 5 log10 (MHV) | |||||
| 40°C + 20% relative humidity | 5 days | 3.5 log10 (TGEV) and 4.7 log10 (MHV) | |||||
| 40°C + 50% relative humidity | (-) | (-) | |||||
| 40°C + 80% relative humidity | 3 h | 2.8 log10 (TGEV) and 4.1 log10 (MHV) | |||||
| 6 | Colnago et al. (2020) | ACoV | Household dishwashing detergent (2% sodium dodecyl sulfate and 6% linear alkylbene sulfonates) | Suspension test | 10 min | >4 log10 | (-) |
| 7 | Criscuolo et al. (2021) | SARS-CoV-2 | UV-C | Glass | 15 min | >99.9% | (-) |
| Plastic | >99.9% | ||||||
| Gauze | >99.9% | ||||||
| Wood | 0.0% | ||||||
| Fleece | 90.0% | ||||||
| Wool | 94.4% | ||||||
| Ozone (0.2 ppm) | Glass | 2 h | 90.0% | ||||
| Plastic | 82.2% | ||||||
| Gauze | 96.8% | ||||||
| Wood | 93.3% | ||||||
| Fleece | >99.9% | ||||||
| Ozone (4 ppm) | Glass | 2 h | 94.4% | ||||
| Plastic | 90.0% | ||||||
| Gauze | 99.8% | ||||||
| Wood | (-) | ||||||
| Fleece | 99.7% | ||||||
| 8 | Gamble et al. (2020) | SARS-CoV-2 | Uncovered plate oven (70°C) | Suspension test | (-) | (-) | ∼37 min |
| Covered plate oven (70°C) | ∼3 min and 56 s | ||||||
| Closed vial oven (70°C) | ∼51.6 s | ||||||
| Closed vial heat block (70°C) | ∼1 min and 55 s | ||||||
| 9 | Gerchman et al. (2020) | HCoV-OC43 | UV-LED (267 nm wavelength, 6–7 mJ/cm2) | Suspension test | 60 s | >3 log10 | (-) |
| UV-LED (279 nm wavelength, 6–7 mJ/cm2) | >3 log10 | ||||||
| UV-LED (286 nm wavelength, 13 mJ/cm2) | 90 s | >3 log10 | |||||
| UV-LED (297 nm wavelength, 32 mJ/cm2) | >3 log10 | ||||||
| 10 | Heilingloh et al. (2020) | SARS-CoV-2 | UV-C 1.94 mJ/cm2/s | Suspension test | 9 min | Total inactivation | (-) |
| UV-A 0.54 mJ/cm2/s | 1 log reduction | ||||||
| combined (UV-C and UV-A) | Total inactivation | ||||||
| 11 | Hulkower et al. (2011) | TGEV | 9.09% O-phenylphenol, 7.66% P-tertiary amylphenol | Stainless steel surface | 1 min | 2.03 log10 | (-) |
| 6% sodium hypochlorite | 0.35 log10 | ||||||
| 0.55% ortho-phthalaldehyde | 2.27 log10 | ||||||
| 70% ethanol | 3.19 log10 | ||||||
| 62% ethanol | 4.04 log10 | ||||||
| 71% ethanol | 3.51 log10 | ||||||
| MHV | 9.09% O-phenylphenol, 7.66% P-tertiary amylphenol | 1.33 log10 | |||||
| 6% sodium hypochlorite | 0.62 log10 | ||||||
| 0.55% ortho-phthalaldehyde | 1.71 log10 | ||||||
| 70% ethanol | 3.92 log10 | ||||||
| 62% ethanol | 2.66 log10 | ||||||
| 71% ethanol | 1.98 log10 | ||||||
| 12 | Khaiboullina et al. (2020) | HCoV | TNP coating + UV-C (254 nm wavelength) | Glass coverslip surface | TNP (20 min to dry or left wet) o and UV-C (30 s and 1 minute) | Reduction in viral copies on both wet and dry surfaces potentiated by the addition of TNP | (-) |
| 13 | Liu et al. (2020) | SARS-CoV-2 | Ultra-high power UV-C | Suspension test | 1 s | 100% | (-) |
| 14 | Malenovská (2020) | SARS-CoV-2 | 99% water, caprylyl/capryl glucoside, citric acid, sodium citrate, sodium benzoate | Plastic (4°C) | 24 h | ∼1.9 log10 | (-) |
| 48 h | ∼2.6 log10 | ||||||
| 72 h | ∼2.2 log10 | ||||||
| 96 h | >1 log10 | ||||||
| 120 | >0.3 log10 | ||||||
| Water, ethanol (0.6 g/wipe), glycerine, Aloe barbadensis leaf extract, chlorhexidine digluconate | 24 h | 2.4 log10 | |||||
| 48 h | 2.2 log10 | ||||||
| 72 h | >1.8 log10 | ||||||
| 96 h | >1 log10 | ||||||
| 120 h | >0.3 log10 | ||||||
| 0.75% didecyl-dimethyl-ammonium chloride, 0.5% hydrogen peroxide, less than 5% non-ionic surface active agent, cationic surface active agent, bleaching agent based on oxygen, perfume, limonene, iodopropynyl butylcarbamate | 24 h | >3.3 log10 | |||||
| 48 h | >3.1 log10 | ||||||
| 72 h | >2.3 log10 | ||||||
| 96 h | Not performed | ||||||
| 120 h | Not performed | ||||||
| 15 | Martins et al. (2020) | SARS-CoV-2 | Ozonated water [0.2–0.8 ppm (mg/L)] | Suspension test | 1 min | 2 log10 | (-) |
| 16 | Meyers et al. (2021) | HCoV | 62% ethanol | Porcelain surface | 15 s, 30 s, 1 min | >4 log10, >4 log10, >4 log10 | (-) |
| 70% ethanol | >4 log10, >4 log10,>4 log10 | ||||||
| 75% ethanol | >4 log10, >4 log10, >4 log10 | ||||||
| 80% ethanol | >4 log10, ≥4 log10, >4 log10 | ||||||
| 95% ethanol | >2 log10, 2–3 log10, 1–2 log10 | ||||||
| 70% isopropanol | >4 log10, >4 log10, >4 log10 | ||||||
| 75% isopropanol | >4 log10, >4 log10, >4 log10 | ||||||
| 80% isopropanol | >4 log10, >4 log10,>4 log10 | ||||||
| 95% isopropanol | >4 log10, 3–4 log10, 3–4 log10 | ||||||
| 0.0525% sodium hypochlorite | 1–2 log10, 2–3 log10, 2–3 log10 | ||||||
| 0.525% sodium hypochlorite | >4 log10, >4 log10, >4 log10 | ||||||
| 0.1% sodium hypochlorite | Not performed | ||||||
| Glutaraldehyde | >4 log10, >4 log10, >4 log10 | ||||||
| 62% ethanol | Ceramic surface | >4 log10, >4 log10, >4 log10 | |||||
| 70% ethanol | >4 log10, >4 log10, >4 log10 | ||||||
| 75% ethanol | 3–4 log10, >4 log10, >4 log10 | ||||||
| 80% ethanol | >4 log10, >4 log10, >4 log10 | ||||||
| 95% ethanol | 1–2 log10, 1–2 log10, 1–2 log10 | ||||||
| 70% isopropanol | >4 log10, 3–4 log10, >4 log10 | ||||||
| 75% isopropanol | >4 log10, >4 log10, >4 log10 | ||||||
| 80% isopropanol | >4 log10, >4 log10, >4 log10 | ||||||
| 95% isopropanol | 3–4 log10, 1–2 log10, 1–2 log10 | ||||||
| 0.0525% sodium hypochlorite | 1–3 log10, 1–2 log10, 1–2 log10 | ||||||
| 0.525% sodium hypochlorite | >4 log10, >4 log10, >4 log10 | ||||||
| 0.1% sodium hypochlorite | >4 log10, >4 log10, >4 log10 | ||||||
| Glutaraldehyde | >4 log10, >4 log10, >4 log10 | ||||||
| 17 | Monge et al. (2020) | SARS-CoV-2 | Cationic phenylene ethynylene polymers (conjugated electrolytes) | Suspension test | 10 min | 1–5 log | (-) |
| Cationic phenylene ethynylene oligomers (conjugated electrolytes) | 20 min | 1.5 log | |||||
| 60 min | 5 log | ||||||
| 18 | Rabenau et al. (2005) | SARS-CoV | Mikrobac forte (0.5% benzalkonium chloride and laurylamine) | Suspension test | 30 min | ≥6.13 log10 | (-) |
| Korsolin FF (0.5% benzalkonium chloride, glutaraldehyde and didecyldimonium chloride) | ≥3.75 log10 | ||||||
| Dismozon pur (magnesium monoperphthalate) | ≥4.5 log10 | ||||||
| Korsolex basic [4% glutaraldehyde and (ethylenedioxy) dimethanol] | 15 min | ≥3.5 log10 | |||||
| Korsolex basic [3% glutaraldehyde and (ethylenedioxy) dimethanol] | 30 min | ≥3.5 log10 | |||||
| Korsolex basic [2% glutaraldehyde and (ethylenedioxy) dimethanol] | 60 min | ≥3.5 log10 | |||||
| 19 | Ratnesar-Shumate et al. (2020) | SARS-CoV-2 | 37°C + 5% CO2 | Stainless steel coupons | 20 min | 1.6 W/m2 UV-B ->∼2.5 log10 | (-) |
| 0.7 W/m2 UV-B ->∼2.2 log10 | |||||||
| 0.3 W/m2 UV-B ->∼2.5 log 10 | |||||||
| Darkness ->0.5 log10 | |||||||
| 20 | Wood and Payne (1998) | HCoV | Dettol (5% chloroxylenol) | Suspension test | 1 min | 0.0 log10 | (-) |
| Dettol for hospitals (1% benzalkonium chloride) | 0.0 log10 | ||||||
| Savlon (5% cetrimide and chlorhexidine gluconate) | 0.0 log10 | ||||||
| 21 | Franke et al. (2021) | Bacteriophage F6 (phi 6) | Ozone (80 ppm) + 90% relative humidity | Melamine-coated solid core panels | 60 min | 4.29 log10 | (-) |
| Ceramic tiles | 6.15 log10 | ||||||
| Stainless steel carriers | 5.31 log10 | ||||||
| Bovine coronavirus | Melamine-coated solid core panels | 5.03 log10 | |||||
| Ceramic tiles | 4.88 log10 | ||||||
| Stainless steel carriers | 5.31 log10 | ||||||
| 22 | Gidari et al. (2021) | SARS-CoV-2 | 23–25°C + 40–50 relative humidity | Plastic | (-) | (-) | 3.5 h |
| Stainless steel carriers | 4.4 h | ||||||
| Glass | 4.2 h | ||||||
| UV-C (254 nm) | Plastic | 20.06 mJ/cm2 (36 s) | >4.00 log10 | (-) | |||
| Stainless steel carriers | 20.06 mJ/cm2 (36 s) | >4.00 log10 | |||||
| Glass | 10.25 mJ/cm2 (21 s) | >4.00 log10 | |||||
| 23 | Hu et al. (2021) | SARS-CoV-2 | Ozonated water (36 mg/L) | Suspension test | 0 min | 0.0 log10 | (-) |
| 1 min | ∼5 log10 | ||||||
| 5 min | ∼5 log10 | ||||||
| 10 min | ∼5 log10 | ||||||
| Ozonated water (18 mg/L) | 1 min | ∼5 log10 | |||||
| 24 | Ijaz et al. (2020) | MHV-1 | 0.12% p-chloro-m-xylenol (PCMX) | Glass | 0.5 min | ≥4.2 log10 | (-) |
| HCoV-229E | 10 min | ≥4.0 log10 | |||||
| SARS-CoV | 5 min | ≥6.0 log10 | |||||
| MERS-CoV | 5 min | ≥5.0 log10 | |||||
| SARS-CoV-2 | Suspension test | 1 min | ≥5.0 log10 | ||||
| HCoV-229E (1), SARS-CoV (2), SARS-CoV-2 (3) | PCMX (0.125% w/v) | Glass + organic load | 5–10 min | (1) ≥4.0 log10, (2) ≥6.0 log10, (3) not performed | |||
| Alkyl dimethyl benzyl ammonium chloride QAC (0.19% w/w) | 1.75 min | (1) ≥6.0 log10, (2) ≥5.8 log10, (3) ≥3.5 log10 | |||||
| Citric acid (2.4% w/w) | 0.5 min | (1) ≥4.3 log10, (2) ≥3.0 log10, (3) ≥3.0 log10 | |||||
| Ethanol (50% w/w)/QAC (0.082% w/w) | 0.5–1.75 min | (1) ≥5.5 log10, (2) not performed, (3) ≥4.5 log10 | |||||
| Alkyl dimethyl benzyl ammonium chloride (0.0916%) | 5 min | (1) ≥3.5 log10, (2) ≥4.8 log10, (3) not performed | |||||
| QAC (0.092% w/w) | 2 min | (1) ≥3.3 log10, (2) ≥3.8 log10, (3) ≥4.0 log10 | |||||
| HCoV-229E (1), SARS-CoV-2 (2) | QAC (0.077% w/w) | Suspension test | 5 min | (1) Not performed (2) ≥4.1 log10 | |||
| Lactic acid (1.9% w/w) | 5 min | (1) Not performed (2) ≥5.5 log10 | |||||
| Hydrochloric acid (0.25% w/w) | 0.5 min | (1) Not performed (2) ≥4.1 log10 | |||||
| Sodium hypochlorite (0.14% w/w) | 0.5 min | (1) Not performed (2) ≥5.1 log10 | |||||
| Benzalkonium chloride (0.45% w/w) | 5 min | (1) Not performed (2) ≥4.5 log10 | |||||
| Ethanol (44% w/w) | 5 min | (1) ≥4.0 log10 (2) ≥4.1 log10 | |||||
| Sodium hypochlorite (0.32% w/w) | 5 min | (1) Not performed (2) ≥5.1 log10 | |||||
| 25 | Messina et al. (2021) | SARS-CoV-2 | UV irradiation chips (265–350 nm) box with lid - reflected light | Suspension test | 3 min | 4.70 log10 | (-) |
| UV irradiation chips (265–350 nm) box with lid | 3 min | 3.45 log10 | |||||
| UV irradiation chips (265–350 nm) box with lid | 6 min | 5.53 log10 | |||||
| UV irradiation chips (265–350 nm) box with lid | 6 min | 5.53 log10 | |||||
| UV irradiation chips (265–350 nm) box with lid | 10 min | 5.70 log10 | |||||
| UV irradiation chips (265–350 nm) box with lid | 10 min | 5.70 log10 | |||||
| UV irradiation chips (265–350 nm) box without lid - direct light | 3 min | 4.62 log10 | |||||
| UV irradiation chips (265–350 nm) box without lid | 3 min | 5.53 log10 | |||||
| UV irradiation chips (265–350 nm) box without lid | 10 min | 5.70 log10 | |||||
| UV irradiation chips (265–350 nm) box without lid | 10 min | 5.70 log10 | |||||
| 26 | Steinhauer et al. (2020) | Modified vaccinia virus Ankara | 20% surface disinfectant - propan-2-ol, ethanol | Suspension test | 15 s | ∼0 log10 | (-) |
| 90% surface disinfectant - propan-2-ol, ethanol | 15 s | ≥4.25 log10 | |||||
| Vaccinia virus Elstree | 80% surface disinfectant - QAC | 30 s | ≥4.32 log10 | ||||
| 80% surface disinfectant - QAC | 60 s | ≥4.51 log10 | |||||
| SARS-CoV-2 | 20% surface disinfectant - propan-2-ol, ethanol | 15 s | ≥4.02 log10 | ||||
| 80% surface disinfectant - propan-2-ol, ethanol | 15 s | ≥4.02 log10 | |||||
| 20% surface disinfectant - QAC | 15 s | ≥4.02 log10 | |||||
| 20% surface disinfectant - QAC | 60 s | ≥3.17 log10 | |||||
| 80% surface disinfectant - QAC | 15 s | ≥4.38 log10 | |||||
| 80% surface disinfectant - QAC | 30 s | ≥4.38 log10 | |||||
| 80% surface disinfectant - QAC | 60 s | ≥2.17 log10 | |||||
| 27 | Trivellin et al. (2020) | SARS-CoV-2 | UV-C LED (275 nm) spherical irradiation box | Football | 1 min | >3 log10 | (-) |
| 2 min | >3 log10 | ||||||
| Basketball | 1 min | >3 log10 | |||||
| 2 min | >3 log10 | ||||||
| Volleyball | 1 min | >3 log10 | |||||
| 2 min | >3 log10 | ||||||
| 28 | Uppal et al. (2021) | HCoV-OC43 | Ozone (20 ppm) | Glass | 10 min | 90.71% | (-) |
| Ozone (25 ppm) | 10 min | 92.3245% | |||||
| 15 min | 99.99% | ||||||
| 20 min | 100.00% | ||||||
| Ozone (50 ppm) | 10 min | 99.987% | |||||
| 15 min | 99.985% | ||||||
| 20 min | 100.00% |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; MHV, mouse hepatitis virus; MERS-COV, Middle East respiratory syndrome coronavirus; TGEV, transmissible gastroenteritis coronavirus; HCoV, human coronavirus; ACOV, avian coronavirus; UV-C, ultraviolet C irradiation; UV-A, ultraviolet A irradiation; UV-LED, ultraviolet light emitting diode; Cu2O/PU, cuprous oxide/polyurethane; TiO2, titanium dioxide; TNP, TiO2 nanoparticle; ABS plastic, acrylonitrile butadiene styrene plastic.
Several studies demonstrated the virucidal properties of commonly used alcohols, leading to the inactivation of viruses on environmental surfaces. One study illustrated inactivation of human coronavirus (HCoV) on porcelain and ceramic surfaces with different concentrations of ethanol and isopropanol. Ethanol with concentrations ranging from 62% to 80% can cause a 4 log10 reduction of viral titres after exposure ≥15 s. Isopropanol 60–70% exposed on surfaces for at least 15 s demonstrated similar results with a 4 log10 reduction of viral titre [40]. Hulkower et al. demonstrated the virucidal effects of three products containing different concentrations of alcohol on stainless steel surfaces. Ethanol 62%, 70% and 71% showed approximately 1.98–3.92 log10 reduction of MHV and 3.19–4.04 log10 reduction of TGEV after 1 min of exposure [29]. Hygiene wipes containing water and ethanol (0.6 g/wipe) destined to decontaminate plastic food packaging can reduce alphacoronavirus 1 to undetectable levels after 72 h of refrigeration (4°C) compared with wipes containing 99% water. However, this study showed evidence that hygiene wipes can potentially transfer viral particles to secondary surfaces [38]. Only two studies showed virucidal efficacy with at least 20% ethanol against SARS-CoV-2 in suspension [12,47].
In the case of sodium hypochlorite, one study showed that 0.525% and 0.1% sodium hypochlorite was sufficient to produce a 4 log10 reduction of HCoV after 15 s of exposure on porcelain and ceramic surfaces [40]. Sodium hypochlorite 0.06% caused <1 log10 reduction of MHV and TGEV after 1 min of exposure on stainless steel. This indicates that either a higher concentration of sodium hypochlorite is needed to cause a more significant reduction in viral titre after 1 min of exposure or a longer exposure time should be considered if 0.06% sodium hypochlorite is used [29]. When it comes to SARS-CoV-2, 0.14% sodium hypochlorite has been shown to reduce the viral titre significantly after 30 s of exposure [12].
With reference to aldehydes, one study showed that glutaraldehyde can lead to a >4 log10 reduction in HCoV with contact times as low as 15 s on porcelain and ceramic surfaces [40]. Glutaraldehyde 4% and ethylenedioxy dimethanol at different concentrations were also capable of causing more than 3.5 log10 reduction in SARS-CoV titre after 15 min in a suspension test [28]. Ortho-phthalaldehyde (OPA) 0.55% caused <2.5 log10 reduction of MHV and TGEV after 1 min of exposure, indicating that OPA may need a longer exposure time to reach its total inactivation capacity [29].
QACs are common disinfection agents with a wide range of microbicidal action. Disinfectant wipes containing 0.75% didecyl-dimethyl-ammonium chloride associated with 0.5% hydrogen peroxide can reduce the alphacoronavirus 1 titre by 3.8 log10 on plastic carriers, and can prevent transmission to secondary surfaces [38]. Combined surface disinfection solutions containing 0.5% benzalkonium chloride with laurylamine can reduce the SARS-CoV titre by 6.13 log10 after 30 min of exposure, while 0.5% benzalkonium chloride associated with glutaraldehyde and didecyldimonium chloride showed a 3.75 log10 reduction in the SARS-CoV titre in 30 min [28]. However, a study conducted in 1997 on suspended HCoV revealed that 1% of benzalkonium chloride and a combination of 5% cetrimide and chlorhexidine gluconate were both ineffective in reducing viral titre after 1 min of exposure [27]. Moreover, QACs were shown to be active against SARS-CoV-2, vaccinia virus Elstree and modified vaccinia virus Ankara with contact times ≤5 min [12,47].
Phenols are another group of disinfectants active against a variety of micro-organisms. Cleaners that consist of 9.09% O-phenylphenol and 7.66% P-tertiary amylphenol showed a moderate reduction in infectivity for MHV and TGEV, revealing approximately 0.8–3.17 log10 reduction on stainless steel surfaces [29]. Chloroxylenol 5% was ineffective for reducing the HCoV titre, but a study conducted in 2020 demonstrated that lower concentrations can efficiently inactivate a number of coronaviruses, including SARS-CoV-2, deposited on glass and in suspension after 1 min of exposure [12,27].
Ozonated water could be an alternative for environmental disinfection as it can cause a 2.0–5.0 log10 reduction in SARS-CoV-2 titre after only 1 min of exposure [39,45].
Other chemical agents, such as magnesium monoperoxyphthalate, lead to a ≥4.5 log10 reduction in SARS-CoV titre after 15 min of exposure [28]. Surface disinfectants based on citric acid, hydrochloride acid or lactic acid were shown to reduce viral titres of coronaviruses (including SARS-CoV-2) efficiently [12]. Virusend (TX-10), a detergent-based disinfectant, was able to reduce infectious SARS-CoV-2 with high titre inoculum by at least 4.0 log10 plaque-forming units (PFU)/mL, and reduce infectious SARS-CoV-2 with low titre inoculum by at least 2.3 log10 PFU/mL on hard surfaces, such as stainless steel, and in solution [31].
UV-C irradiation and ozone exposure
On glass surfaces, UV-C radiation can reduce MHV titre by an average of 2.71 log10 and 6.11 log10 with exposure times of 5 and 10 min, respectively. It is also able to reduce MERS-CoV titre by 5.9 log10 after 5 min of exposure [30]. Findings in two studies indicated that at least 3 min of exposure to UV-C irradiation is able to inactivate SARS-CoV-2 in suspension completely [35,46]. Spherical objects such as footballs, volleyballs and basketballs were completely decontaminated from SARS-CoV-2 after 1 min of exposure to a UV-C-LED device (275 nm) [48]. UV-A, characterized by a longer wavelength (315–400 nm) is less efficient in viral inactivation, revealing only 1 log10 reduction after 9 min of exposure to radiation [35]. It is suggested that peak emission of approximately 286 nm can be effective in inactivating coronaviruses [34]. An in-vitro study provided evidence that UV-B (280–315 nm) levels similar to natural sunlight can significantly reduce SARS-CoV-2 titre by 2.5 log10 on stainless steel surfaces after 20 min of exposure [42].
Exposure of glass, plastic and gauze samples infected with SARS-CoV-2 to UV-C irradiation for 15 min led to a 99.99% reduction of viral titre, while a reduction of 90–95% was obtained for fleece and wool samples. No reduction in viral titre was quantified on wood samples with this method [43]. In the same study, 2 h of exposure to ozone 0.2 ppm was able to completely disinfect (99.99% reduction) fleece samples, and to achieve a 96.8% reduction on gauze, 93.3% on wood, 90% on glass and 82.2% on plastic. Exposure of the same materials to higher concentrations of ozone was effective in reducing viral titre in a shorter period. Uppal et al. demonstrated that ozone gas of at least 25 ppm can optimally eliminate ≥99% of HCoV deposited on glass in 15 min, while another study showed that ozone 80 ppm and 90% relative humidity obtained significant viral inactivation after 60 min [44,49].
Complete inactivation of HCoV is seen on TiO2 nanoparticle (TNP)-coated glass coverslips exposed to UV-C for 30 s and 1 min. Viral inactivation was enhanced and accelerated with TNP coating, making viral titres undetectable after shorter time exposures to UV-C irradiation [36].
SARS-CoV-2 can be eliminated completely after only 1 s of exposure to a high-powered deep UV light. The UV light source is an aluminium gallium nitride-based device and can achieve an output power as high as 2 W at a current of 1.3 A allowing the ultra-rapid inactivation of SARS-CoV-2 [37].
Coatings and films
Coating surfaces with cuprous oxide/polyurethane or conjugated electrolytes such as cationic phenylene ethynylene polymers and oligomers was shown to have virucidal activity against SARS-CoV-2, and reduce viral titre significantly after 1 h of exposure on glass, stainless steel and in suspension [32,41]. Films made from an accessible household dishwashing detergent containing 8% surfactant can provide longer virucidal activity on inanimate surfaces, reducing avian coronavirus to undetectable levels after 10 min of exposure. The activity of these films can persist for up to 7 days [33].
Disinfection methods on biological surfaces
Sixteen articles addressed disinfection methods that can be used on biological surfaces (Table III ) with application on skin, hands and mucous membranes, such as the oral cavity and upper respiratory tract [12,13,28,47,[50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]].
Table III.
Results of disinfection methods used on biological surfaces
| Study | Virus | Disinfectant | Disinfection phase | Exposure time | Reduction of viral infectivity (log10) or (%) | Type | |
|---|---|---|---|---|---|---|---|
| 1 | Bidra et al. (2020) | SARS-CoV-2 | PVP-I 1.0% oral rinse | ST | 15 s and 30 s | ∼4.33 log10 | Oral |
| PVP-I 2.5% oral rinse | ∼4.33 log10 | ||||||
| PVP-I 3.0% oral rinse | ∼4.33 log10 | ||||||
| H2O2 3.0% | 1.33 log10 | ||||||
| H2O2 6.0% | 1 log10 | ||||||
| 2 | Bidra et al. (2020) | SARS-CoV-2 | PVP-I (3.0%) oral rinse antiseptic | ST | 30 s | 3.33 log10 | Oral |
| PVP-I (1.5%) oral rinse antiseptic | 3.33 log10 | ||||||
| PVP-I (1.0%) oral rinse antiseptic | 3.33 log10 | ||||||
| 3 | Eggers et al. (2015) | MERS-CoV | PVP-I surgical scrub (7.5 g/L available iodine) | ST | 15 s | 4.64 log10 | Skin and oral |
| PVP-I skin cleanser (4 g/L available iodine) | 4.97 log10 | ||||||
| PVP-I gargle and mouthwash (1 g/L available iodine) | 4.30 log10 | ||||||
| Modified vaccinia virus Ankara | PVP-I surgical scrub (7.5 g/L available iodine) | 15 s, 30 s and 60 s | ≥4.17 log10, ≥4.17 log10, ≥4.17 log10 | ||||
| PVP-I skin cleanser (4 g/L available iodine) | ≥4.00 log10, ≥4.00 log10, ≥4.00 log10 | ||||||
| PVP-I gargle and mouthwash (1 g/L available iodine) | ≥6.50 log10, ≥6.50 log10, ≥6.50 log10 | ||||||
| 4 | Frank et al. (2020) | SARS-CoV-2 | PVP-I nasal antiseptic 5.0% | Dilution test | 15 s and 30 s | 3 log10 (15 s), 3.33 log10 (30 s) | Respiratory tract |
| PVP-I nasal antiseptic 2.5% | 3 log 10 (15 s), 3.33 log10 (30 s) | ||||||
| PVP-I nasal antiseptic 1.0% | 3log10 (15 s), 3.33 log10 (30 s) | ||||||
| 5 | Gudmundsdottir et al. (2020) | SARS-CoV-2 and HCoV | Coldzyme (glycerol, water, cod trypsin, ethanol, calcium chloride, hydroxymethy, and menthol) | ST | 20 min | 1.76 log 10 (SARS-CoV-2), 2.88 log10 (HCoV) | Oral |
| 6 | Kratzel et al. (2020) | SARS-CoV-2 | Original WHO formulation I consists of 80% (vol/vol) ethanol, 1.45% (vol/vol) glycerol, and 0.125% (vol/vol) hydrogen peroxide | ST | 30 s | >3.8 log10 | Hands |
| Original WHO formulation II consists of 75% (vol/vol) 2-propanol, 1.45% (vol/vol) glycerol, and 0.125% (vol/vol) hydrogen peroxide | >3.8 log10 | ||||||
| Modified WHO formulation I consists of 80% (wt/wt) ethanol, 0.725% (vol/vol) glycerol, and 0.125% (vol/vol) hydrogen peroxide | >5.9 log10 | ||||||
| Modified isopropyl-based WHO formulation II contains 75% (wt/wt) 2-propanol, 0.725% (vol/vol) glycerol, and 0.125% (vol/vol) hydrogen peroxide | >5.9 log10 | ||||||
| 7 | Leslie et al. (2020) | SARS-CoV-2 | PURELL hand sanitizer gel, 70% ethanol (vol/vol) | ST | 30 s | ≥3.22 log10 | Hands |
| PURELL hand sanitizer foam, 70% ethanol (vol/vol) | ≥3.10 log10 | ||||||
| 8 | Liang et al. (2020) | SARS-CoV-2 | Povidone-iodine in-situ gel (polyvinylpyrrolidinone-iodine complex) (0.9%) | ST in tear fluid | 30 s, 2 min and 10 min | 3.5 log10 (30 s), 2.9 log10 (2 min), 3.3 log10 (10 min) | eyes |
| Povidone-iodine in-situ gel (polyvinylpyrrolidinone-iodine complex) (0.5%) | 3.2 log10 (30 s), 2.9 log10 (2 min), 3.3 log10 (10 min) | ||||||
| Povidone-iodine in-situ gel (polyvinylpyrrolidinone-iodine complex) (0.28%) | 2.2 log10 (30 s), 2.6 log10 (2 min), 3.3 log10 (10 min) | ||||||
| Povidone-iodine in-situ gel (polyvinylpyrrolidinone-iodine complex) (0.09%) | 1.2 log10 (30 s), 0.8 log10 (2 min), 1.0 log10 (10 min) | ||||||
| Povidone-iodine nasal spray (1-vinyl-2-pyrrolidinone polymers, iodine complex) (0.54%) | ST in nasal fluid | 3.1 log10 (30 s), 2.9 log10 (2 min), 3.3 log10 (10 min) | Respiratory tract | ||||
| Povidone-iodine nasal spray (1-vinyl-2-pyrrolidinone polymers, iodine complex) (0.3%) | 3.1 log10 (30 s), 2.9 log10 (2 min), 3.3 log10 (10 min) | ||||||
| Povidone-iodine nasal spray (1-vinyl-2-pyrrolidinone polymers, iodine complex) (0.17%) | 2.9 log10 (30 s), 2.9 log10 (2 min), 3.3 log10 (10 min) | ||||||
| Povidone-iodine nasal spray (1-vinyl-2-pyrrolidinone polymers, iodine complex) (0.05%) | 2.3 log10 (30 s), 1.9 log10 (2 min), 1.6 log10 (10 min) | ||||||
| 9 | Meister et al. (2020) | SARS-CoV-2 | Cavex oral pre rinse (hydrogen peroxide) | ST | 30 s | 0.33–0.78 log | Oral |
| Chlorhexamed Forte [chlorhexidinebis (D-gluconate)] | 0.78–1.17 log | ||||||
| Dequonal (dequalinium chloride, benzalkonium chloride) | ≥2.61–3.11 log | ||||||
| Dynexidine Forte 0.2% [chlorhexidinebis (D-gluconate)] | 0.50–0.56 log | ||||||
| Iso-betadine mouthwash 0% (polyvidone-iodine) | ≥2.61–3.11 log | ||||||
| Listerine cool mint (ethanol, essential oils) | ≥2.61–3.11 log | ||||||
| Octenident mouthwash (octenidine dihydrochloride) | 0.61–1.11 log | ||||||
| ProntOral mouthwash (polyaminopropyl biguanide (polyhexanide) | 0.61–≥1.78 log | ||||||
| 10 | Mukherjee et al. (2020) | SARS-CoV-2 | Soap bar with 67 total fatty matter | ST | 20 s | ≥3.14 log10 | Hands |
| Soap bar with 68 total fatty matter | 20 s | ≥3.06 log10 | |||||
| Soap bar with 72 total fatty matter | 20 s | ≥4.06 log10 | |||||
| Liquid cleansers with 10% surfactant w/w | 20 s | ≥3.10 log10 | |||||
| Liquid cleansers with 12% surfactant w/w | 10 s | ≥3.01 log10 | |||||
| Liquid cleansers with 19% surfactant w/w | 10 s | ≥3.42 log10 | |||||
| Alcohol-based sanitizers (60.5% alcohol w/w) | 10 s | ≥3.25 log10 | |||||
| Alcohol-based sanitizers (65% alcohol w/w) | 10 s | ≥4.01 log10 | |||||
| Alcohol-based sanitizers (95% alcohol w/w) | 15 s | ≥4.01 log10 | |||||
| 11 | Rabenau et al. (2005) | SARS-CoV | Sterillium (45% iso-propanol, 30% n-propanol and 0.2% mecetronium etilsulphate) | ST | 30 s | ≥4.25 log10 | Hands |
| Sterillium rub (80% ethanol) | ≥4.25 log10 | ||||||
| {Gopal, 2020 #204} | ≥5.5 log10 | ||||||
| Sterillium Virugard (95% ethanol) | ≥5.5 log10 | ||||||
| 12 | Hirose et al. (2020) | IAV | 80% EA (ethanol) | ST and HS | ST (5 s, 15 s and 60 s)/HS (5 s, 15 s and 60 s) | ST (>4.10, >4.11, >4.07 log)/HS (>4.12, >4.16, >4.16 log) | Skin |
| 60% EA | ST (>4.10, >4.11, >4.07 log)/HS (>4.12, >4.16, >4.16 log) | ||||||
| 40% EA | ST (>4.10, >4.11, >4.07 log)/HS (>4.12, >4.16, >4.16 log) | ||||||
| 20% EA | ST (∼0.09, ∼0.07, ∼0.06 log)/HS (∼0.73, ∼0.85, ∼0.88 log) | ||||||
| 70% IPA (isopropanol) | ST (>4.10, >4.11, >4.07 log)/HS (>4.12, >4.16, >4.16 log) | ||||||
| 0.2% CHG (chlorhexidine gluconate) | ST (∼0.08, ∼0.17, ∼0.19 log)/HS (∼0.74, ∼0.95, ∼1.02 log) | ||||||
| 1.0% CHG | ST (∼0.23, ∼0.24, ∼0.40 log)/HS (∼2.85, ∼3.25, ∼3.39 log) | ||||||
| 0.05% BAC (benzalkonium chloride) | ST (∼0.69, ∼1.78, ∼2.71 log)/HS (∼0.78, ∼1.04, ∼1.23 log) | ||||||
| 0.2% BAC | ST (∼2–43, ∼2.34, >4.07 log)/HS (∼1.64, ∼2.85, ∼3.24 log) | ||||||
| SARS-CoV-2 | 80% EA | ST (>4.50, >4.50, >4.50 log)/HS (>4.19, >4.17, >4.14 log) | |||||
| 60% EA | ST (>4.50, >4.50, >4.50 log)/HS (>4.19, >4.17, >4.14 log) | ||||||
| 40% EA | ST (>4.50, >4.50, >4.50 log)/HS (>4.19, >4.17, >4.14 log) | ||||||
| 20% EA | ST (∼0.08, ∼0.25, ∼0.33 log)/HS (∼0.53, ∼0.61, ∼0.81 log) | ||||||
| 70% IPA | ST (>4.50, >4.50, >4.50 log)/HS (>4.19, >4.17, >4.14 log) | ||||||
| 0.2% CHG | ST (∼0.33, ∼0.42, ∼0.58 log)/HS (∼2.19, ∼2.31, ∼2.42 log) | ||||||
| 1.0% CHG | ST (∼1.00, ∼1.42, ∼1.83 log)/HS (∼2.62, ∼2.94, ∼3.17 log) | ||||||
| 0.05% BAC | ST (∼1.33, ∼1.75, ∼2.17 log)/HS (∼2.03, ∼2.19, ∼2.36 log) | ||||||
| 0.2% BAC | ST (∼1.83, ∼2.42, ∼3.00 log)/HS (∼2.72, ∼2.97, ∼3.19 log) | ||||||
| 13 | Huang et al. (2020) | Patients with SARS-CoV-2 infection | Chlorhexidine oral rinse (15 mL) | Oral and oropharyngeal cavity | 30 s twice a day for 4 days | 37.9% positive SARS-CoV-2 test, 62.1% negative test | Oral and oropharyngeal cavity |
| without exposure | (-) | 94.5% positive SARS-CoV-2 test, 5.5% negative test | |||||
| Chlorhexidine oral rinse (15 mL) + oropharyngeal spray (1.5 mL) | 30 s oral rinse + spray, twice a day for 4 days | 14.0% positive SARS-CoV-2 test, 80% negative test | |||||
| without exposure | (-) | 93.8% positive SARS-CoV-2 test, 6.2% negative test | |||||
| 14 | Ijaz et al. (2020) | HCoV-229E (1), SARS-CoV-2 (2) | Bar soap PCMX - (0.090% w/w) | ST | 0.5–1 min | (1) ≥3.3 log10, (2) ≥4.1 log10 | Hands |
| Liquid gel handwash - salicylic acid (0.025% w/w) | 0.5–1 min | (1) ≥3.6 log10, (2) ≥3.6 log10 | |||||
| Foaming handwash - benzalkonium chloride (0.025% w/w) | 1 min | (1) ≥3.3 log10, (2) ≥3.4 log10 | |||||
| Foaming handwash - salicylic acid (0.023% w/w) | 0.5–1 min | (1) ≥3.5 log10, (2) ≥5.0 log10 | |||||
| Antiseptic liquid - PCMX (0.021% w/v) | 5 min | (1) ≥5.2 log10, (2) ≥4.7 log10 | |||||
| Hand sanitizer gel - ethanol (53% w/w) | 1 min | (1) ≥5.4 log10, (2) ≥4.2 log10 | |||||
| Hand sanitizer gel - citric acid (1.5% w/w), lactic acid (0.41% w/w) | 0.5–1 min | (1) ≥5.2log10, (2) ≥4.7 log10 | |||||
| 15 | Steinhauer et al. (2020) | Modified vaccinia virus Ankara | 20% hand disinfectant - propan-2-ol | ST | 15 s | ∼0.17 log10 | Hands |
| 80% hand disinfectant - propan-2-ol | 15 s | ≥4.19 log10 | |||||
| SARS-CoV-2 | 20% hand disinfectant - propan-2-ol | 15 s | ≥4.02 log10 | ||||
| 20% hand disinfectant - propan-2-ol | 30 s | ≥3.02 log10 | |||||
| 80% hand disinfectant - propan-2-ol | 15 s | ≥2.02 log10 | |||||
| 80% hand disinfectant - propan-2-ol | 30 s | ≥4.38 log10 | |||||
| 16 | Steinhauer et al. (2020) | SARS-CoV-2 | 80% chlorhexidine bis-(D-gluconate) 0.1 g | ST | 5–10 min | <1.00 log10 | Oral |
| 80% chlorhexidine bis-(D-gluconate) 0.2 g | 1–5 min | <1.00 log10 | |||||
| 80% 0.1 g octenidine dihydrochloride, 2 g phenoxyethanol | 15 s | ≥4.00 log10 | |||||
| 80% 0.1 g octenidine dihydrochloride, 2 g phenoxyethanol | 30 s | ≥4.00 log10 | |||||
| 80% 0.1 g octenidine dihydrochloride, 2 g phenoxyethanol | 1 min | ≥4.00 log10 | |||||
| 20% 0.1 g octenidine dihydrochloride, 2 g phenoxyethanol | 15 s | ≥4.00 log10 |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; MERS-CoV, Middle East respiratory syndrome coronavirus; HCoV, human coronavirus; PVP-I, povidone-iodine; WHO, World Health Organization; ST, suspension test; HS, human skin.
Alcohols were mainly evaluated via suspension tests showing optimal virucidal activity (including SARS-CoV-2) at concentrations >65% and with exposure times of 15–60 s specifically for application to hands and the oral cavity [12,13,28,47,54,55,57,58,61]. One study evaluated the efficacy of ethanol and propanol directly on human skin against SARS-CoV-2 and found that 40% concentrations of these alcohols can cause >4 log10 reduction in viral titre after just 5 s of exposure [59]. Interestingly, WHO-recommended hand rub formulations containing 80% ethanol or propanol showed inferior efficacy compared with modified formulations (with 75% ethanol or propanol and half of the concentration of glycerol from the original formulation) when tested with SARS-CoV-2 [13]. Soap bars evaluated in two studies were shown to reduce the quantity of SARS-CoV-2 significantly, with optimal results seen with a contact time of 20 s to 1 min [12,58]. QACs, specifically benzalkonium chloride 0.2%, can produce maximum virucidal activity after 60 s of exposure, verified in suspension tests and on human skin [12,59]. Liquids containing chloroxylenol, citric acid, lactic acid or salicylic acid were also effective in reducing coronavirus titres, including SARS-CoV-2 [12].
Oral rinses containing PVP-I 1–3% lead to >4.33 log10 reduction of SARS-CoV-2, MERS-CoV and modified vaccinia virus Ankara titres after 15–30 s of contact time [[50], [51], [52],57]. The action of hydrogen peroxide oral rinses, on the other hand, is inferior to PVP-I [51]. Chlorhexidine gluconate (oral and skin formulations) seems to provide suboptimal virucidal activity compared with other agents in in-vitro suspension test experiments. However, a prospective cohort study on patients who were initially admitted to hospital with a positive SARS-CoV-2 test indicated that the application of chlorhexidine gluconate mouthwash and nasopharyngeal spray of the same agent can accelerate the clearance of SARS-CoV-2 in these areas, resulting in a negative reverse transcriptase polymerase chain reaction test after 4 days [60]. Other antiseptic oral rinses containing chloride and benzalkonium or ethanol have also been shown to deactivate SARS-CoV-2 [54,57].
PVP-I can also be applied topically on eyes as an additional pre-procedure disinfection as concentrations of 0.9% can reduce SARS-CoV-2 titre significantly after 30 s of exposure [56]. On the other hand, a toxicity study carried out in rabbits revealed that groups exposed to ocular PVP-I 0.6% and 1.0% every day for 7 days showed signs of mild and transient ocular irritation [56]. Nasal cavity formulations consisting of PVP-I 0.54–5% are able to cause >3 log10 reduction in SARS-CoV-2 titre after 15 s of exposure [62].
Disinfection methods against airborne viruses
Regarding disinfection methods against airborne coronaviruses, four articles were identified (Table IV ) [[63], [64], [65], [66]]. Wafers containing silver and copper combined with aluminium oxide display catalytic properties and can be incorporated in air conditioning systems to trap and kill viruses. These wafers are active against coronaviruses and can cause complete viral inactivation after 5 min of exposure [63].
Table IV.
Results of disinfection methods against airborne viruses
| Study | Virus | Disinfectant | Disinfection phase | Exposure time | Reduction of viral infectivity (log10) or (%) | |
|---|---|---|---|---|---|---|
| 1 | Buonanno et al. (2020) | Alphacoronavirus HCoV-229E | Far-UV-C light at 222 nm (0.5, 1 and 2 mJ/cm2) | Dynamic aerosol/virus irradiation chamber | ∼20 s | 1.7 mJ/cm2 produce 99.9% inactivation (3-log reduction) of aerosolized alpha HCoV-229E |
| Betacoronavirus HCoV-OC43 | 1.2 mJ/cm2 produce 99.9% inactivation (3-log reduction) of aerosolized beta HCoV-OC43 | |||||
| 2 | Qiao et al. (2020) | PRCV | UV-C light 200–850 nm (13.9 mJ/cm2) | Wind tunnel (high flow rate of 2439 L/min) | 1.3 s | 2.2 log10 (99.4% removal efficiency) |
| UV-C light 253±1 nm (49.6 mJ/cm2) | Wind tunnel (low flow rate of 684 L/min) | 5.1 s | 3.7 log10 (99.98% removal efficiency) | |||
| 3 | Yu et al. (2020) | SARS-CoV-2 | Novel Ni-foam-based filter (up to 200°C) | Aerosolized SARS-CoV-2 | Single pass | 99.8% reduction |
| 4 | He et al. (2004) | SARS coronavirus | Ag/Al2O3 (Ag 5 wt%) catalytic oxidation | Ag/Al2O3 and Cu/Al2O3 wafers | 5 min and 20 min | Virus undetectable |
| Cu/Al2O3 (Cu 10 wt%) catalytic oxidation | Virus undetectable |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; PRCV, porcine respiratory coronavirus; HCoV, human coronavirus; UV-C, ultraviolet C irradiation; Ni, nickel; Ag, silver; Cu, copper.
UV-C can efficiently inactivate up to 99.9% of aerosolized coronaviruses [64]. Ventilation systems fitted with a UV-C light source that can control its flow rate, control the exposure time of air passage indirectly, as lower flow rates translate into longer exposure times which results in superior viral removal efficacy [65]. As all human coronaviruses have similar genomic size, a key determinant of radiation sensitivity, it is likely that UV-C irradiation will show comparable inactivation efficiency against other human coronaviruses, including SARS-CoV-2 [64].
Methods to decontaminate and recondition personal protective equipment
Methods with potential use to decontaminate and recondition PPE were examined by 16 studies (Table V ) [49,[67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]]. Most of these studies investigated filtering facepiece respirators (FFRs), especially 3M N95 masks. Heat (70–95ºC) combined with different levels of relative humidity is capable of inactivating enveloped viruses, including SARS-CoV-2, inoculated on N95 level melt-blown polypropylene fabric after at least 20 min of exposure [68,74,79]. Filtration efficacy was maintained after several cycles. However, cycles should be limited to avoid compromising mask function. A limit of 20 disinfection cycles is suggested for treatments under high relative humidity (100%) and temperatures ≤85°C. Treatment should also be limited to five cycles under high relative humidity (100%) and temperatures ≤95°C [[68], [69], [70],73]. Caution must be taken when a dry oven is utilized to generate dry heat (0% relative humidity) as samples placed on parchment paper prior to heating can result in lower efficacy of viral inactivation [77].
Table V.
Results of methods to recondition personal protective equipment
| Study | Virus | Disinfectant | Disinfection phase | Exposure time | Reduction of viral infectivity (log10) or (%) | Effect on material properties and functionality | |
|---|---|---|---|---|---|---|---|
| 1 | Blanchard et al. (2020) | IAV and RSV | Ozone (20 ppm) + 50–70% RH | Surgical facemasks (1 cm x 1 cm sample swatches) | 40 min | Equal to 70% ethanol inactivation | Material properties were preserved and filtration capacity of masks was maintained. |
| Tyvek (disposable gown) 1 cm x 1 cm | |||||||
| N95 respirators 1 cm x 1 cm | |||||||
| Bunny suits 1 cm x 1 cm | |||||||
| PAPR hoods 1 cm x 1 cm | |||||||
| 2 | Campos et al. (2020) | SARS-CoV-2 | Ambient humidity (60%) without BSA | Meltblown fabric from N95-grade FFRs | 60°C for 30 min | 2.16 ± 0.23 log10 | Temperatures of 75–85 °C are able to efficiently inactivate the virus in 20–30 min under 100% RH, without lowering filtration efficiency. Filtration efficacy started to decrease significantly after 10 cycles with temperature of 95°C probably due to the absorption of water or other mechanisms that can decay the electrostatic charge. |
| Ambient humidity (60%) without BSA | 75°C for 30 min | 3.69 ± 0.32 log10 | |||||
| Ambient humidity (60%) without BSA | 85°C for 20 min | >4.77 log10 | |||||
| Ambient humidity (60%) without BSA | 95°C for 5 min | >4.77 log10 | |||||
| Ambient humidity (60%) with BSA | 60°C for 30 min | 1.07 ± 0.06 log10 | |||||
| Ambient humidity (60%) with BSA | 75°C for 30 min | 2.89 ± 0.31 log10 | |||||
| Ambient humidity (60%) with BSA | 85°C for 20 min | 4.3 ± 0.55 log10 | |||||
| Ambient humidity (60%) with BSA | 95°C for 5 min | 4.8 ± 0.44 log10 | |||||
| 100% humidity without BSA | 60°C for 30 min | 2.82 ± 0.09 log10 | |||||
| 100% humidity without BSA | 75°C for 30 min | >4.97 log10 | |||||
| 100% humidity without BSA | 85°C for 20 min | >4.97 log10 | |||||
| 100% humidity without BSA | 95°C for 5 min | >4.97 log10 | |||||
| 100% humidity with BSA | 60°C for 30 min | 2.27 ± 0.09 log10 | |||||
| 100% humidity with BSA | 75°C for 30 min | 4.92 ± 0.12 log10 | |||||
| 100% humidity with BSA | 85°C for 20 min | >5.02 log10 | |||||
| 100% humidity with BSA | 95°C for 5 min | >5.02 log10 | |||||
| 3 | Choi et al. (2020) | SARS-CoV-2 | Moist heat generated by multi-cooker | FFRs 3M Model 1860 in simulated saliva | 65°C for 30 min | ∼1.5 log10 | All FFRs absorbed <1 g of water when in a paper bag. Collection efficacy exceeded 95% and inhalation resistance was preserved. After five cycles of moist heat treatment, 3M 8210 and NS 721 had no change in strap elasticity, while 3M 1860 and 3M 8511 showed a small change (<10%). |
| FFRs 3M Model 1860 in simulated lung fluid | ∼3.2 log10 | ||||||
| FFRs 3M Model 8511 in simulated saliva | ∼2.5 log10 | ||||||
| FFRs 3M Model 8511 in simulated lung fluid | ∼3.2 log10 | ||||||
| FFRs 3M Model 8210 in simulated saliva | ∼2.2 log10 | ||||||
| FFRs NS Model 7210 in simulated saliva | ∼2.2 log10 | ||||||
| 4 | Daeschler et al. (2020) | SARS-Cov-2 | 70°C + 50% RH | N95 respirators | 2–18 min | Reduced to undetectable levels | Masks maintained fibre diameters similar to untreated masks and continued to meet standards for fit, filtration efficiency and breathing resistance. |
| 5 | Gopal et al. (2020) | SARS-Cov-2 | Zinc oxide embedded into fabrics (only tested on PA66) | Cotton, polypropylene (PPP) fabrics and polyamide (PA66) | 60 min | 2 log | Cotton and polyamide 66 (PA66) can strongly trap viruses as only 56% of SARS-CoV-2 can be recovered from cotton samples and 92% from PA66 after viral inoculation. PPP is poor at trapping viruses. |
| 6 | Ibanez-Cervantes et al. (2020) | SARS-CoV-2 | Hydrogen peroxide plasma | N95 3M Model 8210 | 47 min | Undetectable by RT-PCR | Not tested |
| 7 | Ludwig-Begall et al. (2020) | PRCV | UV irradiation | Surgical mask coupons | 2 min | ∼5 log10 | Not tested |
| Vaporized H2O2 (59% liquid H2O2) 750 ppm | 28 min | ∼5 log10 | |||||
| Dry heat (102°C) | 60 min | ∼5.5 log10 | |||||
| UV irradiation | Surgical mask straps | 2 min | ∼2.9 log10 | ||||
| Vaporized H2O2 (59% liquid H2O2) 750 ppm | 28 min | Non-significant | |||||
| Dry heat (102°C) | 60 min | ∼1.2 log10 | |||||
| UV irradiation | FFR coupons | 4 min | ∼3.2 log10 | ||||
| Vaporized H2O2 (59% liquid H2O2) 750 ppm | 28 min | ∼4 log10 | |||||
| Dry heat (102°C) | 60 min | ∼2.5 log10 | |||||
| UV irradiation | FFR straps | 4 min | (-) | ||||
| Vaporized H2O2 (59% liquid H2O2) 750 ppm | 28 min | ∼1.2 log10 | |||||
| Dry heat (102°C) | 60 min | (-) | |||||
| 8 | Ma et al. (2020) | Avian infectious bronchitis virus | Steam | N95 FFR masks | 5 min | Undetectable by RT-PCR | Blocking efficacy of 99% verified in all masks except for one model that seemed to have thinner layers compared with other models. Therefore, masks with thinner layers can have reduced blocking efficacy. |
| 9 | Mantlo et al. (2020) | SARS-CoV-2 | Clyraguard copper iodine complex undiluted | Suspension test | 10 min | 2 log | Not tested |
| 30 min | Below limit of detection (<75 TCID50 per mL) | ||||||
| 60 min | Below limit of detection (<75 TCID50 per mL) | ||||||
| 10 | Ozog et al. (2020) | SARS-CoV-2 | UV-C irradiation (1.5 J/cm2 to each side) | N95 FFR models (3M 1860, 8210, 8511, 9211; Moldex 1511) | 60–70 s (for each side) | Below limit of detection (101.3 TCID50/4 mm punch) | Not tested |
| 11 | Perkins et al. (2020) | SARS-CoV-2 | Dry heat (60°C) | N95 respirator coupons + parchment paper | 60 min | All samples were positive analysed by microscopy for cytopathic effect | Not tested |
| Dry heat (70°C) | 60 min | All samples were positive analysed by microscopy for cytopathic effect | |||||
| Dry heat (75°C) | 60 min | All samples were positive analysed by microscopy for cytopathic effect | |||||
| Dry heat (60°C) | N95 respirator coupons + tissue culture | (-) | (-) | ||||
| Dry heat (70°C) | 60 min | All samples were positive analysed by microscopy for cytopathic effect | |||||
| Dry heat (75°C) | 60 min | All samples were positive analysed by microscopy for cytopathic effect | |||||
| Dry heat (60°C) | Intact N95 respirators | (-) | (-) | ||||
| Dry heat (70°C) | 60 min | All samples were positive analysed by microscopy for cytopathic effect | |||||
| Dry heat (75°C) | 60 min | Most samples were positive analysed by microscopy for cytopathic effect | |||||
| Ambient temperature | 5 days | 5/9 samples were positive analysed by microscopy for cytopathic effect | |||||
| 12 | Rathnasinghe et al. (2020) | SARS-CoV-2 | UV-C irradiation (5.43 mW/cm2) | N95 mask squares | 120 s per side | 3.5 log | Not tested |
| 13 | Rockey et al. (2020) | Bacteriophage MS2 | Temperature (72°C and 82°C) + PBS | N95 respirator coupons | 30 min | 0.24 log 10 (72°C + 1% RH), 0.19 log10 (82°C + 1% RH) | Not tested |
| 6.87 log 10 (72°C + 89% RH), 6,90 log10 (82°C + 89% RH) | |||||||
| Temperature (72°C and 82°C) + DMEM-A | 1.44 log 10 (72°C + 1% RH), 2.77 log10 (82°C + 1% RH) | ||||||
| 6.56 log 10 (72°C + 89% RH), 7.16 log10 (82°C + 89% RH) | |||||||
| Temperature (72°C and 82°C) + saliva | 0.99 log 10 (72°C + 13% RH), 0.88 log10 (82°C + 1% RH) | ||||||
| 1.45 log 10 (72°C + 25% RH), 1.74 log10 (82°C + 13% RH) | |||||||
| Temperature (72°C and 82°C) + (PBS + BSA) | 1.5 log 10 (72°C + 13% RH), 0.77 log10 (82°C + 1% RH) | ||||||
| 2.72 log 10 (72°C + 25% RH), 3.56 log10 (82°C + 13% RH) | |||||||
| Bacteriophage phi6 | Temperature (72°C and 82°C) + PBS | 0.99 log 10 (72°C + 1% RH), 1.48 log10 (82°C + 1% RH) | |||||
| 6.79 log 10 (72°C + 89% RH), 6,70 log10 (82°C + 89% RH) | |||||||
| Temperature (72°C and 82°C) + DMEM-A | 2.58 log 10 (72°C + 1% RH), 3.87 log10 (82°C + 1% RH) | ||||||
| 6.81 log 10 (72°C + 89% RH), 7.63 log10 (82°C + 89% RH) | |||||||
| Temperature (72°C and 82°C) + saliva | 0.95 log 10 (72°C + 13% RH), 1.09 log10 (82°C + 1% RH) | ||||||
| 1.69 log 10 (72°C + 25% RH), 2.62 log10 (82°C + 13% RH) | |||||||
| Temperature (72°C and 82°C) + (PBS + BSA) | 1.33 log 10 (72°C + 13% RH), 0.76 log10 (82°C + 1% RH) | ||||||
| 1.34 log 10 (72°C + 25% RH), 1.98 log10 (82°C + 13% RH) | |||||||
| MHV | Temperature (72°C and 82°C) + DMEM-A | 2.51 log 10 (72°C + 1% RH), 3.30 log10 (82°C + 1% RH) | |||||
| 4.19 log 10 (72°C + 89% RH), 4.38 log10 (82°C + 89% RH) | |||||||
| IAV | Temperature (72°C and 82°C) + DMEM-A | 1.25 log 10 (72°C + 1% RH), 2.71 log10 (82°C + 1% RH) | |||||
| 3.71 log 10 (72°C + 89% RH), 3.37 log10 (82°C + 89% RH) | |||||||
| 14 | Glasbrenner et al. (2021) | TGEV | UV (300–400 nm) simulated sunlight | FFR 3M 1860 | (-) | (-) | All FFRs maintained collection efficacy and breathing resistance after one and five cycles ((Model 3M 8210 not tested for five cycles). Reduced strap elasticity from NS 7210 model with 19% change in stress). |
| FFR 3M 8210 | (-) | (-) | |||||
| FFR 3M 8511 | (-) | Inactivation less efficient | |||||
| FFR NS 7210 | (-) | Inactivation below level of detection | |||||
| SARS-CoV-2 | FFR 3M 1860 + SS and LF | 20 min (13.3 J cm2) SS/40 min (26.5 J cm2) FL | Inactivation below level of detection | ||||
| FFR 3M 8210 + SS and LF | (-) | (-) | |||||
| FFR 3M 8511 + SS and LF | 60 min (37.8 J cm2) for SS and FL | Complete inactivation | |||||
| FFR NS 7210 + SS and LF | 20 min (13.3 J cm2) for SS and LF | Inactivation below level of detection | |||||
| 15 | Uppal et al. (2021) | HCoV-OC43 | Ozone (20 ppm) | N95 FFRs | 10 min | 98.1411% | Not tested |
| Ozone (25 ppm) | 10 min | 97.4138% | |||||
| 15 min | 99.9947% | ||||||
| 20 min | 99.9966% | ||||||
| Ozone (50 ppm) | 10 min | 99.9860% | |||||
| 15 min | 99.9956% | ||||||
| 20 min | 99.9925% | ||||||
| 16 | Valdez-Salas et al. (2021) | Enveloped H5N1 avian influenza virus | Formulated disinfectant - 0.2% benzalkonium chloride, 85% ethanol-water, 0.03% triclosan, 10% silver nanoparticles, 0.3% lauryl alcohol ethoxylate, 0.2% Triton X-100, 2% citric acid, microdacyn | Suspension test | 15 min | No presence of haemagglutinine - complete inactivation | Not tested |
IAV, avian influenza virus; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; PRCV, porcine respiratory coronavirus; IBV, avian infectious bronchitis virus; MHV, mouse hepatitis coronavirus; PAPR, powered air purifying respirator; FFR, filtering facepiece respirator; RH, relative humidity; UV-C, ultraviolet C irradiation; PSB, phosphate-buffered saline; BSA, bovine serum albumin; DMEM, Dulbecco's modified Eagle medium; SS, simulated saliva; FF, lung fluid.
Other than heat treatment, face masks made with cloth fabric, disposable gowns and powered air purifying respirator hoods can all be decontaminated successfully with doses of at least 20 ppm of ozone [67]. N95 respirators inoculated with HCoV were also adequately decontaminated after 10–20 min of exposure to 20–50 ppm ozone gas [49].
Metals such as copper and zinc possess antiviral activity. Zinc ions incorporated in fabrics, such as cotton and polyamide 66 (PA66), can inactivate SARS-CoV-2 while maintaining virucidal activity after 50 washes, supporting the possibility of long-lasting virucidal protection [71]. It is worth noting that cotton and PA66 can trap viruses, as only 56% and 92% of SARS-CoV-2 can be recovered from cotton samples and PA66, respectively, after viral inoculation. This information is relevant as cotton- and PA66-based masks can trap large amounts of SARS-CoV-2, making cross-contamination more probable when masks are reused without decontamination [71]. Copper iodine complex has the potential to be used on non-critical PPE as it has been shown to completely deactivate SARS-CoV-2 in suspension after 30 min of exposure [75]. An innovative formulation that consists of silver and antimicrobial substances (ethanol and QACs) has also been shown to possess antiviral activity when impregnated in the matrix of surgical masks [81].
Hydrogen peroxide vapour can also inactivate SARS-CoV-2 deposited on N95 masks and FFRs. This last process can be conducted in a STERRAD 100NX sterilization system or a V-PRO Max Sterilizer providing exposure cycles of ≤47 min [72,73].
UV irradiation was able to inactivate coronaviruses deposited on surgical masks and FFRs [73,76,78]. Exposure times needed to decontaminate these materials completely ranged from 60 s to 4 min when the models tested were N95 FFRs. It is worth noting that the efficacy of UV-C irradiation is model-dependent, and straps that contain hydrophilic properties seem to cause a lower reduction in viral titre [76]. Exposure to simulated sunlight for 20 min, characterized by UV irradiation with wavelengths ranging between 300 and 400 nm, can reduce SARS-CoV-2 titre significantly on specific models of N95 masks [80].
Risk of bias
It was only possible to evaluate selection bias in two studies as the majority of experiments took place in in-vitro settings. Only one study blinded the personnel, so the other studies may contain performance bias. Thirteen studies were considered to have a probable risk of attrition or exclusion bias, eight studies had probable risk of detection bias, two studies had probable risk of selective reporting bias, and three studies had probable risk of potential threat to internal validity. A summary of the evaluation is provided in Table VI .
Table VI.
Risk of bias assessment using the Office of Health Assessment and Translation (OHAT) Risk of Bias Rating Tool for Human and Animal Studies Potential source of bias was graded as low risk (++), probable low risk (+), probable high risk or not reported (−) and high risk (−−)
| Study | Study design | Was administered dose or exposure level adequately randomized? | Was allocation to study groups adequately concealed? | Were experimental conditions identical across study groups? | Were research personnel blinded to the study group during the study? | Were outcome data complete without attrition or exclusion from analysis? | Can we be confident in the exposure characterization? | Can we be confident in the outcome assessment (including blinding of assessors)? | Were all measured outcomes reported? | Were there no other potential threats to internal validity? |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (NR) | (NR) | (NR) | (+) | (++) | (+) |
| Bedell et al. (2016) | In vitro | Not applicable | Not applicable | (++) | (-) | (NR) | (NR) | (-) | (++) | (-) |
| Behzadinasab et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Bidra et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (NR) | (++) | (++) | (++) | (+) |
| Bidra et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (NR) | (++) | (++) | (++) | (+) |
| Biryukov et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (+) | (++) |
| Blanchard et al. (2020) | In vitro | Not applicable | Not applicable | (-) | (-) | (NR) | (++) | (++) | (+) | (+) |
| Buonanno et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (NR) | (++) | (++) | (++) | (++) |
| Campos et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Casanova et al. (2010) | In vitro | Not applicable | Not applicable | (+) | (-) | (++) | (+) | (++) | (++) | (++) |
| Choi. et al. (2020) | In vitro | Not applicable | Not applicable | (+) | (-) | (++) | (+) | (++) | (++) | (+) |
| Colnago et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (-) | (-) | (++) | (++) | (+) |
| Criscuolo et al. (2021) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (+) | (++) | (+) |
| Daeschler et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (+) | (++) | (++) | (+) | (++) | (++) |
| Eggers et al. (2015) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (++) | (+) |
| Frank et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (+) | (++) | (++) | (+) |
| Gamble et al. (2020) | In vitro | Not applicable | Not applicable | (+) | (-) | (+) | (++) | (++) | (++) | (+) |
| Gerchman et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (-) | (++) | (+) | (++) | (+) |
| Gopal et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (++) | (+) |
| Gudmundsdottir et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (-) | (++) | (++) | (+) |
| He et al. (2004) | In vitro | Not applicable | Not applicable | (-) | (-) | (+) | (++) | (++) | (-) | (+) |
| Heilingloh et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (+) | (++) |
| Hulkower et al. (2011) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Ibanez-Cervantes et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (+) | (++) | (++) | (++) |
| Khaiboullina et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (+) | (++) |
| Kratzel et al. (2020) | In vitro | Not applicable | Not applicable | (NR) | (-) | (++) | (++) | (++) | (++) | (++) |
| Leslie et al. (2020) | In vitro | Not applicable | Not applicable | (NR) | (-) | (+) | (+) | (++) | (+) | (-) |
| Liang et al. (2020) | In vivo and in vitro | (+) | (NR) | (++) | (NR) | (++) | (++) | (+) | (++) | (+) |
| Liu et al. (2020) | In vitro | Not applicable | Not applicable | (+) | (-) | (+) | (++) | (+) | (++) | (+) |
| Ludwig-Begall et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Ma et al. (2020) | In vitro | Not applicable | Not applicable | (-) | (-) | (NR) | (+) | (+) | (++) | (+) |
| Malenovská (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (++) | (++) |
| Mantlo et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (++) | (++) |
| Martins et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (+) | (++) |
| Meister et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (-) | (++) | (+) | (+) |
| Meyers et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (-) | (++) | (++) | (+) | (-) |
| Monge et al. (2020) | In vitro | Not applicable | Not applicable | (NR) | (-) | (+) | (+) | (+) | (++) | (+) |
| Mukherjee et al. (2020) | In vitro | Not applicable | Not applicable | (+) | (-) | (+) | (-) | (+) | (++) | (+) |
| Ozog et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Perkins et la (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (-) | (+) | (++) | (+) |
| Qiao et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (+) |
| Rabenau et al. (2005) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (+) | (+) | (++) |
| Rathnasinghe et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (+) | (++) | (++) |
| Ratnesar-Shumate et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (+) | (++) | (++) | (++) | (++) | (++) |
| Rockey et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Wood and Payne (1998) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (+) | (+) | (+) |
| Yu et al. (2020) | In vitro | Not applicable | Not applicable | (NR) | (-) | (NR) | (++) | (+) | (NR) | (+) |
| Franke et al. (2021) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Gidari et al. (2021) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Glasbrenner et al. (2021) | In vitro | Not applicable | Not applicable | (+) | (-) | (-) | (-) | (++) | (+) | (+) |
| Hirose et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Hu et al. (2021) | In vitro | Not applicable | Not applicable | (++) | (-) | (+) | (++) | (++) | (+) | (+) |
| Huang et al. (2020) | Prospective cohort | (++) | (-) | (-) | (-) | (++) | (++) | (++) | (++) | (+) |
| Ijaz et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Messina et al. (2021) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Steinhauer et al. (2020) | In vitro | Not applicable | Not applicable | (+) | (-) | (+) | (++) | (++) | (+) | (+) |
| Steinhauer et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Trivellin et al. (2020) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Uppal et al. (2021) | In vitro | Not applicable | Not applicable | (++) | (-) | (++) | (++) | (++) | (++) | (++) |
| Valdez-Salas et al. (2021) | In vitro | Not applicable | Not applicable | (-) | (-) | (-) | (++) | (+) | (+) | (+) |
Discussion
Stability and survival of SARS-CoV-2 exposed to heat and high humidity
Under ambient conditions (temperatures of 21–23°C and relative humidity of 40%), SARS-CoV-2 can remain viable on surfaces for hours to days [7,40]. The findings of this review support the evidence that coronaviruses are less viable when exposed to higher temperatures and higher relative humidity. It is not always possible to change the room temperature or humidity in indoor settings. However, rooms with the possibility to set these parameters between a determined range, such as intensive care units, operating rooms or hospital wards, can benefit as the survival of viruses is reduced markedly in warmer and higher humidity conditions.
Disinfection methods on environmental surfaces
Although most chemical agents have demonstrated virucidal activity against the coronavirus family, alcohols with concentrations of at least 60% showed a more constant and significant reduction in viral titres, promoting viral inactivation with shorter time exposures. This suggests that alcohols may be a better option when it comes to choosing a fast-acting and effective agent. Sodium hypochlorite, if preferred, should be used as a 0.1% solution, at least. If using QACs, a minimum exposure time of 30 min is recommended.
As household dishwashing detergent is more accessible compared with the other coatings discussed, it can be an effective alternative in providing long-lasting virucidal protection on surfaces in household settings or in countries that have difficulty in accessing other products, such as alcohols. However, further investigation is still needed to determine the efficacy and practicality of these coatings.
Ozone has virucidal activity targeting proteins on the viral envelope, inhibiting its entry to host cells. Higher concentrations of ozone must be used with caution due to the potential toxicity to humans; therefore, an ozone concentration of 20 ppm and an exposure time of 15 min is considered to be sufficient for optimal disinfection of surfaces [43,82,83].
For surface disinfection, UV-C irradiation seems to be the best alternative, as it is widely available and exceptionally convenient. It may be preferred over ozone as it is safer and less toxic to humans. However, when used with the purpose of whole-room disinfection, other methods, such as surface antimicrobial agents, could complement the strategy, as some surfaces may not be fully decontaminated due to shadowing or the composition of absorbable materials, such as fleece and wood.
Disinfection methods on biological surfaces
Adequate disinfection of hands is an important way to prevent indirect transmission of respiratory infections, especially during the era of SARS-CoV-2. Based on the review findings and evidence in the literature, the original formulations of WHO-recommended hand rubs seem to be less active against SARS-CoV-2 compared with modified formulations [13,84]. This is significant as many companies seek standard recommendations from WHO to produce disinfectants with the adequate proportion of ethanol/isopropanol and glycerol. These formulations could be updated to ensure optimal disinfection efficacy of formulations against SARS-CoV-2. Commercially available personal care products, such as soap bars, liquid cleansers (containing surfactant) and alcohol-based hand sanitizers (at least 30% ethanol or propanol), were all able to reduce SARS-CoV-2 titre after 10–20 s of exposure [13,55,58,85]. This suggests that the current procedure for handwashing is effective against SARS-CoV-2 at the established concentrations and duration.
At present, no methods are in place regarding eye or respiratory tract disinfection in order to stop the transmission of SARS-CoV-2, and this deserves further investigation due to potential toxicity. However, there are viable options in specific settings, such as during ophthalmologic procedures or interventions where aerosols may be generated. While very low concentrations of PVP-I showed in-vitro viral inactivation, in-vivo conditions must be taken into account due to the fact that biological debris such as physiological buffers in nasal secretions can lower the effective concentration of PVP-I. Therefore, a concentration of at least 1.25% PVP-I is recommended for in-vivo application [53].
In summary, for oral rinses and skin cleansers, products containing PVP-I should be preferred, as its action is rapid and efficient. Soap bars, surfactant and alcohol-based hand sanitizers are all excellent alternatives for hand hygiene.
Disinfection methods against airborne viruses
Recent evidence indicates that airborne transfer is the main route of transmission of SARS-CoV-2, being more evident in indoor spaces with poor ventilation. Considering that coronaviruses cannot tolerate high temperatures, filtration or ventilation systems coupled with heatable metal filters may be an effective option. It is also evident that SARS-CoV-2 is susceptible to UV-C irradiation. As the latter is the only commercially available option at present, the installation of an upper room germicidal UV-C irradiation device, for example, in healthcare facilities, indoor spaces that accommodate a large number of people, or even in household settings, can be beneficial. Other than UV irradiation, the remaining methods in this section provide preliminary evidence of effective ways to decontaminate the air, indicating the future of more sophisticated and efficient air conditioning systems.
Methods to decontaminate and recondition personal protective equipment
The COVID-19 pandemic has had a significant impact on the environment and mass production of PPE to meet the world's rapid and urgent demand, creating major challenges in waste management on a global scale [[86], [87], [88]]. Surgical masks, for instance, are composed of plastic that is not biodegradable and may end up in waterbeds, causing harm to the environment and the fauna of these areas. Methods that aim to decontaminate and recondition PPE for reuse can be beneficial not only for the environment but also in cases of shortages of PPE, as experienced by many countries during the COVID-19 pandemic.
Based on these studies, there is still insufficient evidence to support the virucidal efficacy of metal-embedded fabrics. Moreover, as it is important to preserve the functionality of PPE after decontamination, the only methods that provided evidence of effective sterilization without compromising the integrity of PPE (with a limited number of cycles) were heat and ozone treatment, making these methods better and safer options at the present time.
Deposition solutions
Three of the studies included in this review addressed how different deposition solutions can change the viral inactivation rate. It was found that the viral load of SARS-CoV-2 and bacteriophages MS2 and Phi6 deposited in DMEM-A (cell culture medium formulations) showed, under different temperature and humidity exposures, a more significant reduction in viral titre compared with the viral load deposited in phosphate-buffered saline (PBS) [40]. Interestingly, the viral load deposited in freshly collected human saliva demonstrated a log10 reduction trend more similar to PBS compared with DMEM-A. Bovine serum albumin containing higher concentrations of protein can be used to mimic body fluids, particularly sputum [31,42]. This may suggest that laboratory-made solutions may not fully represent the behaviour of biological fluids.
Limitations of this review
One major limitation of this systematic review is that all the studies included are based on in-vitro findings, with some extensive experiments trying to mimic in-vivo conditions. However, the real efficacy in in-vivo settings needs further investigation.
Nineteen of the studies included in this review used surrogate viruses to mimic the behaviour of SARS-CoV-2. Surrogate viruses were included due to the biosafety level of SARS-CoV-2 that may hinder the use of this virus in some experiments. It may also have been unavailable in some laboratories, especially at the beginning of the pandemic when little was known about SARS-CoV-2. To evaluate the efficacy of disinfectants, vaccinia virus, in particular, is a reference virus used in Europe as a surrogate for enveloped viruses (EN 14476) [89]. As SARS-CoV-2 is an enveloped virus easily susceptible to disinfection, as verified in the review findings, methods that can effectively target more resilient surrogate enveloped viruses translate into efficacy against SARS-CoV-2.
In conclusion, the results demonstrate that several household and hospital disinfection agents, UV-C irradiation, ozone and surface coatings are effective for inactivation of the coronavirus family, including SARS-CoV-2, on environmental surfaces. While SARS-CoV-2 can survive for hours to days depending on the surface, high temperature and humidity are key factors in viral decay. Decontamination of PPE can be performed effectively using heat treatment, UV-C irradiation and hydrogen peroxide vapour. Zinc ions can potentially provide prolonged disinfection when embedded into fabrics. Formulations containing PVP-I at different concentrations can provide virucidal action in the form of oral rinses, topical eye disinfection and skin cleansers. In the case of hand hygiene, typical soap bars, ethanol and propanol can inactivate SARS-CoV-2. Regarding disinfection methods against airborne particles, air filtration systems with materials that possess catalytic properties, UV-C devices and heating systems can reduce viral particles effectively. This review supports improved selection of the most effective disinfection method for each specific setting, potentially resulting in better outcomes during the present pandemic, and also the prevention of viral healthcare-associated infections.
Acknowledgements
The first author wishes to thank Dr. Luís Cobrado for his expertise and guidance throughout this work, and Carolina Xavier for support, effort and assistance regarding the writing and language of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.07.014.
Conflicts of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mofijur M., Fattah I.M.R., Alam M.A., Islam A.B.M.S., Ong H.C., Rahman S.M.A., et al. Impact of COVID-19 on the social, economic, environmental and energy domains: lessons learnt from a global pandemic. Sustain Prod Consum. 2021;26:343–359. doi: 10.1016/j.spc.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2021. COVID-19 weekly epidemiological update. [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2021. Scientific brief: SARS-CoV-2 transmission. [PubMed] [Google Scholar]
- 5.Bulfone T.C., Malekinejad M., Rutherford G.W., Razani N. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: a systematic review. J Infect Dis. 2021;223:550–561. doi: 10.1093/infdis/jiaa742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson M.A., Quandelacy T.M., Kada S., Prasad P.V., Steele M., Brooks J.T., et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Network Open. 2021;4:e2035057. doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis D. Superspreading drives the COVID pandemic – and could help to tame it. Nature. 2021;590:544–546. doi: 10.1038/d41586-021-00460-x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO; Geneva: 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. [Google Scholar]
- 10.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mondelli M.U., Colaneri M., Seminari E.M., Baldanti F. Bruno R. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect Dis. 2021;21:e112. doi: 10.1016/S1473-3099(20)30678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ijaz M.K., Nims R.W., Zhou S.S., Whitehead K., Srinivasan V., Kapes T., et al. Microbicidal actives with virucidal efficacy against SARS-CoV-2 and other beta- and alpha-coronaviruses and implications for future emerging coronaviruses and other enveloped viruses. Sci Rep. 2021;11:5626. doi: 10.1038/s41598-021-84842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratzel A., Todt D., V’Kovski P., Steiner S., Gultom M., Thao T.T.N., et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mentel R., Shirrmakher R., Kevich A., Dreĭzin R.S., Shmidt I. Virus inactivation by hydrogen peroxide. Vopr Virusol. 1977:731–733. [PubMed] [Google Scholar]
- 15.Sriwilaijaroen N., Wilairat P., Hiramatsu H., Takahashi T., Suzuki T., Ito M., et al. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol J. 2009;6:124. doi: 10.1186/1743-422X-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardell E.A., Nathavitharana R.R. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. JAMA. 2020;324:141–142. doi: 10.1001/jama.2020.7603. [DOI] [PubMed] [Google Scholar]
- 17.Storm N., McKay L.G.A., Downs S.N., Johnson R.I., Birru D., de Samber M., al at. Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation. Sci Rep. 2020;10:22421. doi: 10.1038/s41598-020-79600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C., Ma W., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Publ Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burki T. Global shortage of personal protective equipment. Lancet Infect Dis. 2020;20:785–786. doi: 10.1016/S1473-3099(20)30501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moghadas S.M., Vilches T.N., Zhang K., Wells C.R., Shoukat A., Singer B.H., et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenforde M.W., Olson S.M., Self W.H., Talbot H.K., Lindsell C.J., Steingrub J.S., et al. MMWR Morb Mortal Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gidari A., Sabbatini S., Bastianelli S., Pierucci S., Busti C., Bartolini D., et al. SARS-CoV-2 survival on surfaces and the effect of UV-C light. Viruses. 2021;13 doi: 10.3390/v13030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Reese A.L., et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere. 2020;5 doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamble A., Fischer R.J., Morris D.H., Yinda K.C., Munster V.J., Lloyd-Smith J.O. Heat-treated virus inactivation rate depends strongly on treatment procedure. bioRxiv. 2020 doi: 10.1101/2020.08.10.242206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood A., Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J Hosp Infect. 1998;38:283–295. doi: 10.1016/S0195-6701(98)90077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabenau H.F., Kampf G., Cinatl J., Doerr H.W. Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulkower R.L., Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Am J Infect Control. 2011;39:401–407. doi: 10.1016/j.ajic.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedell K., Buchaklian A.H., Perlman S. Efficacy of an automated multiple emitter whole-room ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infect Control Hosp Epidemiol. 2016;37:598–599. doi: 10.1017/ice.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson E.R., Hughes G.L., Patterson E.I. Inactivation of SARS-CoV-2 on surfaces and in solution with Virusend (TX-10), a novel disinfectant. bioRxiv. 2020 doi: 10.1101/2020.11.25.394288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behzadinasab S., Chin A., Hosseini M., Poon L., Ducker W.A. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl Mater Interfaces. 2020;12:34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colnago L.A., Trevisol I.M., Voss Rech D., Forato L.A., Igreja do Nascimento Mitre C., Gagliardi L.J.P., et al. Simple, low-cost and long-lasting film for virus inactivation using avian coronavirus model as Challenge. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17186456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerchman Y., Mamane H., Friedman N., Mandelboim M. UV-LED disinfection of coronavirus: wavelength effect. J Photochem Photobiol B-Biol. 2020;212:7. doi: 10.1016/j.jphotobiol.2020.112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D., et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am J Infect Control. 2020;48:1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaiboullina S., Uppal T., Dhabarde N., Subramanian V.R., Verma S.C. Inactivation of human coronavirus by titania nanoparticle coatings and UVC radiation: throwing light on SARS-CoV-2. Viruses. 2020;13 doi: 10.3390/v13010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S.F., Luo W., Li D., Yuan Y., Tong W., Kang J.J., et al. Sec-eliminating the SARS-CoV-2 by AlGaN based high power deep ultraviolet light source. Adv Funct Mater. 2020 doi: 10.1002/adfm.202008452. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malenovská H. Coronavirus persistence on a plastic carrier under refrigeration conditions and its reduction using wet wiping technique, with respect to food safety. Food Environ Virol. 2020;12:361–366. doi: 10.1007/s12560-020-09447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins R.B., Castro I.A., Pontelli M., Souza J.P., Lima T.M., Melo S.R., et al. SARS-CoV-2 inactivation by ozonated water: a preliminary alternative for environmental disinfection. Ozone Sci Eng. 2021;43:108–111. [Google Scholar]
- 40.Meyers C., Kass R., Goldenberg D., Milici J., Alam S., Robison R. Ethanol and isopropanol inactivation of human coronavirus on hard surfaces. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monge F.A., Jagadesan P., Bondu V., Donabedian P.L., Ista L., Chi E.Y., et al. Highly effective inactivation of SARS-CoV-2 by conjugated polymers and oligomers. ACS Appl Mater Interfaces. 2020;12:55688–55695. doi: 10.1021/acsami.0c17445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., et al. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J Infect Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Criscuolo E., Diotti R.A., Ferrarese R., Alippi C., Viscardi G., Signorelli C., et al. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg Microbes Infect. 2021 doi: 10.1080/22221751.2021.1872354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franke G., Knobling B., Brill F.H., Becker B., Klupp E.M., Belmar Campos C., et al. An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. J Hosp Infect. 2021;112:108–113. doi: 10.1016/j.jhin.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X., Chen Z., Su Z., Deng F., Chen X., Yang Q., et al. Ozone water is an effective disinfectant for SARS-CoV-2. Virol Sin. 2021 doi: 10.1007/s12250-021-00379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messina G., Della Camera A., Ferraro P., Amodeo D., Corazza A., Nante N., et al. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinhauer K., Meister T.L., Todt D., Krawczyk A., Paßvogel L., Becker B., et al. Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J Hosp Infect. 2021;111:180–183. doi: 10.1016/j.jhin.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trivellin N., Buffolo M., Onelia F., Pizzolato A., Barbato M., Orlandi V.T., et al. Inactivating SARS-CoV-2 using 275 nm UV-C LEDs through a spherical irradiation box: design, characterization and validation. Materials (Basel) 2021;14 doi: 10.3390/ma14092315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uppal T., Khazaieli A., Snijders A.M., Verma S.C. Inactivation of human coronavirus by FATHHOME's dry sanitizer device: rapid and eco-friendly ozone-based disinfection of SARS-CoV-2. Pathogens. 2021;10 doi: 10.3390/pathogens10030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA) Infect Dis Ther. 2015;4:491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont. 2020;29:599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. 2020;29:529–533. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank S., Brown S.M., Capriotti J.A., Westover J.B., Pelletier J.S., Tessema B. In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2. JAMA Otolaryngol Head Neck Surg. 2020;146:1–5. doi: 10.1001/jamaoto.2020.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gudmundsdottir Á., Scheving R., Lindberg F., Stefansson B. Inactivation of SARS-CoV-2 and HCoV-229E in vitro by ColdZyme® a medical device mouth spray against the common cold. J Med Virol. 2020 doi: 10.1002/jmv.26554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leslie R.A., Zhou S.S., Macinga D.R. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang B., Yuan X., Wei G., Wang W., Zhang M., Peng H., et al. In-vivo toxicity studies and in-vitro inactivation of SARS-CoV-2 by povidone-iodine in-situ gel forming formulations. bioRxiv. 2020 doi: 10.1101/2020.05.18.103184. [DOI] [Google Scholar]
- 57.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee S., Vincent C.K., Jayasekera H.W., Yekhe A.S. Antiviral efficacy of personal care formulations against severe acute respiratory syndrome coronavirus 2. Infect Dis Health. 2020;26:63–66. doi: 10.1016/j.idh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirose R., Bandou R., Ikegaya H., Watanabe N., Yoshida T., Daidoji T., et al. Disinfectant effectiveness against SARS-CoV-2 and influenza viruses present on human skin: model-based evaluation. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y.H., Huang J.T. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J Med Virol. 2021;93:4370–4373. doi: 10.1002/jmv.26954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinhauer K., Meister T.L., Todt D., Krawczyk A., Paßvogel L., Becker B., et al. Virucidal efficacy of different formulations for hand and surface disinfection targeting SARS CoV-2. J Hosp Infect. 2021;112:27–30. doi: 10.1016/j.jhin.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frank S., Capriotti J., Brown S.M., Tessema B. Povidone-iodine use in Sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J. 2020;99:586–593. doi: 10.1177/0145561320932318. [DOI] [PubMed] [Google Scholar]
- 63.He H., Dong X., Yang M., Yang Q., Duan S., Yu Y., et al. Catalytic inactivation of SARS coronavirus, Escherichia coli and yeast on solid surface. Catal Commun. 2004;5:170–172. doi: 10.1016/j.catcom.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buonanno M., Welch D., Shuryak I., Brenner D.J. Far-UVC light (222nm)efficiently and safely inactivates airborne human coronaviruses. Sci Rep. 2020;10:10285. doi: 10.1038/s41598-020-67211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiao Y., Yang M., Marabella I.A., McGee D.A.J., Aboubakr H., Goyal S., et al. Greater than 3-log reduction in viable coronavirus aerosol concentration in ducted ultraviolet-C (UV-C) systems. Environ Sci Technol. 2020 doi: 10.1021/acs.est.0c05763. [DOI] [PubMed] [Google Scholar]
- 66.Yu L., Peel G.K., Cheema F.H., Lawrence W.S., Bukreyeva N., Jinks C.W., et al. Catching and killing of airborne SARS-CoV-2 to control spread of COVID-19 by a heated air disinfection system. Mater Today Phys. 2020;15:5. doi: 10.1016/j.mtphys.2020.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanchard E.L., Lawrence J.D., Noble J.A., Xu M., Joo T., Ng N.L., et al. Enveloped virus inactivation on personal protective equipment by exposure to ozone. medRxiv. 2020 doi: 10.1101/2020.05.23.20111435. [DOI] [Google Scholar]
- 68.Campos R.K., Jin J., Rafael G.H., Zhao M., Liao L., Simmons G., et al. Decontamination of SARS-CoV-2 and other RNA viruses from N95 level meltblown polypropylene fabric using heat under different humidities. Acs Nano. 2020;14:14017–14025. doi: 10.1021/acsnano.0c06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi Y.W., Richardson A.W., Sunderman M., Mladineo M.J., Keyes P.H., Hofacre K.C., et al. Decontamination of SARS-CoV-2 contaminated N95 filtering facepiece respirators (FFRs) with moist heat generated by a multicooker. Lett Appl Microbiol. 2020 doi: 10.1111/lam.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daeschler S.C., Manson N., Joachim K., Chin A.W.H., Chan K., Chen P.Z., et al. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ. 2020;192:E1189–E1197. doi: 10.1503/cmaj.201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gopal V., Nilsson-Payant B.E., French H., Siegers J.Y., Yung W.S., Hardwick M., et al. Zinc-embedded fabrics inactivate SARS-CoV-2 and influenza A virus. bioRxiv. 2020 doi: 10.1101/2020.11.02.365833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibanez-Cervantes G., Bravata-Alcantara J.C., Najera-Cortes A.S., Meneses-Cruz S., Delgado-Balbuena L., Cruz-Cruz C., et al. Disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria using hydrogen peroxide plasma: impact on the reutilization of disposable devices. Am J Infect Control. 2020;48:1037–1041. doi: 10.1016/j.ajic.2020.06.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ludwig-Begall L.F., Wielick C., Dams L., Nauwynck H., Demeuldre P.F., Napp A., et al. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J Hosp Infect. 2020;106:577–584. doi: 10.1016/j.jhin.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma Q.X., Shan H., Zhang C.M., Zhang H.L., Li G.M., Yang R.M., et al. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: experimental supports. J Med Virol. 2020;92:1971–1974. doi: 10.1002/jmv.25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantlo E., Rhodes T., Boutros J., Patterson-Fortin L., Evans A., Paessler S. In vitro efficacy of a copper iodine complex PPE disinfectant for SARS-CoV-2 inactivation. F1000Res. 2020;9:674. doi: 10.12688/f1000research.24651.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozog D.M., Sexton J.Z., Narla S., Pretto-Kernahan C.D., Mirabelli C., Lim H.W., et al. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int J Infect Dis. 2020;100:224–229. doi: 10.1016/j.ijid.2020.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perkins D.J., Nofchissey R.A., Ye C., Donart N., Kell A., Foo-Hurwitz I., et al. COVID-19 global pandemic planning: dry heat incubation and ambient temperature fail to consistently inactivate SARS-CoV-2 on N95 respirators. Exp Biol Med. 2020 doi: 10.1177/1535370220977819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rathnasinghe R., Karlicek R.F., Schotsaert M., Koffas M.A., Arduini B., Jangra S., et al. Scalable, effective, and rapid decontamination of SARS-CoV-2 contaminated N95 respirators using germicidal ultra-violet C (UVC) irradiation device. medRxiv. 2020 doi: 10.1101/2020.10.05.20206953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rockey N., Arts P.J., Li L., Harrison K.R., Langenfeld K., Fitzsimmons W.J., et al. Humidity and deposition solution play a critical role in virus inactivation by heat treatment of N95 respirators. mSphere. 2020;5 doi: 10.1128/mSphere.00588-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glasbrenner D.C., Choi Y.W., Richardson A.W., Edwards E.W., Mladineo M.J., Sunderman M., et al. Decontamination of SARS-CoV-2 contaminated N95 filtering facepiece respirators using artificial sun lamps. J Appl Microbiol. 2021 doi: 10.1111/jam.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valdez-Salas B., Beltran-Partida E., Cheng N., Salvador-Carlos J., Valdez-Salas E.A., Curiel-Alvarez M., et al. Promotion of surgical masks antimicrobial activity by disinfection and impregnation with disinfectant silver nanoparticles. Int J Nanomed. 2021;16:2689–2702. doi: 10.2147/IJN.S301212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rojas-Valencia M.N. In: Science against microbial pathogens: communicating current research and technological advances. Méndez-Vilas A., editor. National Autonomous University of Mexico, Institute of Engineering, Coordination of Environmental Engineering; Mexico: 2011. Research on ozone application as disinfectant and action mechanisms on wastewater microorganisms. [Google Scholar]
- 83.Nuvolone D., Petri D., Voller F. The effects of ozone on human health. Environ Sci Pollut Res Int. 2018;25:8074–8088. doi: 10.1007/s11356-017-9239-3. [DOI] [PubMed] [Google Scholar]
- 84.Suchomel M., Steinmann J., Kampf G. Efficacies of the original and modified World Health Organization-recommended hand-rub formulations. J Hosp Infect. 2020;106:264–270. doi: 10.1016/j.jhin.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Binda G., Bellasi A., Spanu D., Pozzi A., Cavallo D., Bettinetti R. Evaluating the environmental impacts of personal protective equipment use by the general population during the COVID-19 pandemic: a case study of Lombardy (Northern Italy) Environments. 2021;8:33. [Google Scholar]
- 87.Rizan C., Reed M., Bhutta M.F. Environmental impact of personal protective equipment distributed for use by health and social care services in England in the first six months of the COVID-19 pandemic. J R Soc Med. 2021;114:250–263. doi: 10.1177/01410768211001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang E.J., Aitchison L.P., Phillips N., Shaban R.Z., Kam A.W. Protecting the environment from plastic PPE. BMJ. 2021;372:n109. doi: 10.1136/bmj.n109. [DOI] [PubMed] [Google Scholar]
- 89.Eggers M., Schwebke I., Suchomel M., Fotheringham V., Gebel J., Meyer B., et al. The European tiered approach for virucidal efficacy testing – rationale for rapidly selecting disinfectants against emerging and re-emerging viral diseases. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.3.2000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.