Abstract
Studies on the Hsp70 chaperone machine in eukaryotes have shown that Hsp70 and Hsp40/Hdj1 family proteins are sufficient to prevent protein misfolding and aggregation and to promote refolding of denatured polypeptides. Additional protein cofactors include Hip and Bag1, identified in protein interaction assays, which bind to and modulate Hsp70 chaperone activity in vitro. Bag1, originally identified as an antiapoptotic protein, forms a stoichiometric complex with Hsp70 and inhibits completely Hsp70-dependent in vitro protein refolding of an unfolded polypeptide. Given its proposed involvement in multiple cell signaling events as a regulator of Raf1, Bcl2, or androgen receptor, we wondered whether Bag1 functions in vivo as a negative regulator of Hsp70. In this study, we demonstrate that Bag1, expressed in mammalian tissue culture cells, has pronounced effects on one of the principal activities of Hsp70, as a molecular chaperone essential for stabilization and refolding of a thermally inactivated protein. The levels of Hsp70 and Bag1 were modulated either by transient transfection or conditional expression in stably transfected lines to achieve levels within the range detected in different mammalian tissue culture cell lines. For example, a twofold increase in the concentration of Bag1 reduced Hsp70-dependent refolding of denatured luciferase by a factor of 2. This effect was titratable, and higher levels of wild-type but not a mutant form of Bag1 further inhibited Hsp70 refolding by up to a factor of 5. The negative effects of Bag1 were also observed in a biochemical analysis of Bag1- or Hsp70-overexpressing cells. The ability of Hsp70 to maintain thermally denatured firefly luciferase in a soluble state was reversed by Bag1, thus providing an explanation for the in vivo chaperone-inhibitory effects of Bag1. Similar effects on Hsp70 were observed with other cytoplasmic isoforms of Bag1 which have in common the carboxyl-terminal Hsp70-binding domain and differ by variable-length amino-terminal extensions. These results provide the first formal evidence that Bag1 functions in vivo as a regulator of Hsp70 and suggest an intriguing complexity for Hsp70-regulatory events.
The challenge for protein folding, within the densely packed environment of the cell, is to ensure that polypeptides acquire and maintain their native state. Under normal conditions of cell growth, protein homeostasis is balanced. However, during cell stress, an imbalance in protein synthesis, folding, translocation, and degradation occurs, resulting in the elevated expression of heat shock proteins, which ensure that misfolded proteins do not accumulate and that nonnative intermediates are captured, subsequently refolded, or degraded. In eukaryotes, these events are monitored by Hsp104, Hsp90, Hsp70, Hsp60, and Hsp27, which function in concert with cochaperones and ATP (3, 10, 12, 17). The Hsp70 chaperones have specialized domains which recognize stretches of hydrophobic residues in polypeptide chains that are transiently exposed in early folding intermediates and are typically confined to the hydrophobic core in the native state (28). The consequence of chaperone interactions, therefore, is to shift the equilibrium of protein folding reactions towards productive on-pathway events and to prevent the appearance of nonproductive intermediates which otherwise would misfold.
Affinity of the Hsp70 peptide-binding domain for unfolded substrates is strongly influenced by its nucleotide state and cochaperones, or accessory proteins which modulate Hsp70 function. These cochaperones have been studied extensively for Escherichia coli, where the Hsp70 homologue, DnaK, was originally identified in a genetic screen for host proteins required for replication of bacteriophage lambda (9). DnaK is regulated by DnaJ, which stimulates the DnaK ATPase, yielding the high-affinity ADP state for substrate binding, and GrpE, which functions as a nucleotide exchange factor for release of substrate and allows the cycle to begin anew (15, 16, 21).
In mammalian cells, the regulation of chaperone activities appears more complex, with the identification of increasing numbers of proposed accessory proteins which could confer substrate specificity to Hsp70, influence the assembly of Hsp70 into chaperone complexes, or alter Hsp70 chaperone activity (13, 14, 27, 29, 35). In addition to the DnaJ homologue Hdj-1/Hsp40 which can stimulate the activity of the cytosolic Hsp70 chaperones, two new classes of proteins which modulate the Hsp70 and Hdj-1/Hsp40 reaction cycle have been identified (6, 24). Hip, initially identified in a protein interaction assay with the Hsp70 ATPase domain, forms a complex and enhances Hsp70 chaperone activity (14). Independently, Hip was identified as a component of steroid aporeceptor complexes and shown to stimulate the assembly of Hsp70-Hsp90 heteromeric complexes (27). Bag1 associates with and stimulates the ATPase domain of Hsp70, but with opposite effects, and inhibits Hsp70-dependent in vitro chaperone activity. Independently, Bag1 was identified as a regulator of cell signaling molecules, including Raf1, Bcl2, Siah1A, and a subset of growth factor and steroid receptors. Overexpression of Bag1 and its isoforms stimulates the activities of these cell signaling molecules with positive or negative effects on cell growth (1, 2, 8, 13, 20, 29, 31–33, 35).
The objective of this study was to examine whether Bag1 functions in vivo as a modulator of Hsp70. We demonstrate that overexpression of Bag1 or its human isoforms inhibits the ability of Hsp70 to reactivate heat-inactivated luciferase and interferes with the ability of Hsp70 to maintain denatured luciferase in a soluble state.
MATERIALS AND METHODS
Plasmids and constructs.
pCytluc (pRSVLL/V) encodes cytoplasmic luciferase under the control of a Rous sarcoma virus long terminal repeat promoter (provided by S. Subramani, University of California, San Diego). pCDNA/Bag1 (corresponding to the murine 30-kDa Bag1 protein), pCDNA/Bag1ΔC, pCDNA/HA-Bag1, and pCDNA/HA-Bag1ΔC were created by cloning an EcoRI-XhoI fragment from pGEX-4T-1/Bag1 and pGEX-4T-1/Bag1Δ (29) behind a cytomegalovirus promoter in pCDNA-3 (Invitrogen) or pCDNA-3/HA (provided by S. Ness, Northwestern University, Evanston, Ill.). Insertion of pCDNA-3/HA resulted in an in-frame fusion of a triple hemagglutinin (HA) tag. pUHD/Bag1 was created by insertion of an EcoRI-XbaI fragment of pCDNA/Bag1 downstream of the tetracycline-responsive TA (tTA)-dependent promoter of pUHD10-3 (provided by H. Bujard, University of Heidelberg) (11). pHGR272 carries a hygromycin resistance gene under the control of a thymidine kinase minimal promoter from herpes simplex virus. Construction of pCMV70 has previously been described (22). p29K, p33K, p46K, and p50K encode the 29,000-molecular-weight (29K), 33K, 46K, and 50K isoforms of human Bag1 under the control of a cytomegalovirus promoter (provided by S.-C. Tang, Memorial University of Newfoundland, St. John's, Newfoundland) (34).
Cell culture and transfections.
OT70 cells are hamster lung fibroblasts (O23) in which Hsp70 expression can be controlled by the tetracycline-responsive tTA expression system (25). Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Sigma), 1 mg of G418 (Gibco/BRL, Inc.) per ml, 1 mg of hygromycin (Boehringer Mannheim) per ml, and 3 μg of tetracycline (Sigma) per ml. G418 and hygromycin were absent during all experiments. Transient transfections were performed using Lipofectamine according to the procedure of the manufacturer (Gibco/BRL, Inc.). OTBag1 cells are O23 cells in which the expression of Bag1 is under the control of the tTA expression system. The cell line was created by CaPO4 transfection of pUHD/Bag1 and pHGR272 at a ratio of 80:1 into O23 cells that constitutively express the tTA protein (25). Hygromycin-resistant clones were selected in medium containing 1 mg of G418 (Gibco/BRL, Inc.) per ml, 1 mg of hygromycin (Boehringer Mannheim) per ml, and 3 μg of tetracycline (Sigma) per ml. A clone that showed a good induction of Bag1 expression after tetracycline withdrawal, as judged by Western blot analysis, was used for further experiments.
Luciferase reactivation assay, insolubilization, and Western blot analysis.
Cells were transiently transfected with pCytluc and cochaperone- or Hsp70-encoding plasmids or pCDNA as a control. Twenty-four hours after transfection, cells were transferred into tissue culture tubes in medium with or without 3 μg of tetracycline per ml for induction of Hsp70 or Bag1 expression. Another 24 h later, the medium was replaced with medium containing 20 μg of cycloheximide per ml and 20 mM morpholinepropanesulfonic acid (MOPS; pH 7.0). After 30 min at 37°C, luciferase was inactivated by heating the cells at 45°C for 30 min. During a subsequent recovery period at 37°C, triplicate samples were taken and cells were lysed and assayed for luciferase activity as previously described (23). For Western blot analysis the cells were trypsinized, resuspended in phosphate-buffered saline, lysed by addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and sonicated prior to SDS-PAGE and Western blot analysis. Solubility of luciferase was determined by cell trypsinization and lyses in 100 μl of BLUC (25 mM Tris-H3PO4 [pH 7.8], 10 mM MgCl2, 1% Triton X-100, 15% glycerol, and 1 mM EDTA per 1.5 × 106 cells). Supernatant and pellet fractions were separated by centrifugation at 21,000 × g for 15 min and analyzed by SDS-PAGE and Western blot analysis.
Bag1 and luciferase were detected by polyclonal antibodies to Bag1ΔC (amino acids 1 to 172) and luciferase (Cortex), respectively. Hsp70 was detected by C92, a monoclonal antibody specific for the heat-inducible form of Hsp70 (Stressgen). Hsc70 expression was detected by N27, a monoclonal antibody specific for Hsc70 and Hsp70 (Stressgen). Human Bag1 isoforms were detected by a monoclonal antibody to human Bag1 (provided by S.-C. Tang, Memorial University of Newfoundland) (34). Recombinant Hsp70 and Bag1 were purified as described previously (5, 29).
Immunofluorescence analysis.
Indirect immunofluorescence was performed as described previously (22). OT70 cells transiently transfected with pCDNA or pCDNA/HA-Bag1 were immunostained with a rabbit polyclonal antibody to the heat-inducible Hsp70 (Stressgen) and a mouse monoclonal anti-HA tag antibody (provided by R. Lamb, Northwestern University) simultaneously. A mixture of tetramethyl rhodamine isocyanate-labeled anti-rabbit and fluorescein isothiocyanate-labeled anti-mouse secondary antibodies (Sigma) was used to visualize binding of the primary antibodies. The images were made with a confocal laser scanning microscope (Zeiss LSM 410).
RESULTS
Bag1 functions in vivo as an inhibitor of Hsp70-dependent refolding.
The absolute levels of chaperones and cochaperones vary considerably among different human tissues and mammalian tissue culture cell lines. Among multiple human tissue culture cells (primary fibroblasts and HeLa, K562, U937, Jurkat, and MCF7 cells), the levels of Hsc70 and Hsp70 range from 5 to 50 μM, a level which is 10- to 50-fold greater than the levels of the cochaperones Hdj1, Hdj2, Hip, and Bag1; furthermore, the level of Hdj1 can range from 0.5 to 2 μM and the level of Bag1 can range from 0.1 to 2 μM (2, 30, 32, 34; C. Schmidt, D. Winchester, and R. Morimoto, unpublished observations). The relative concentration of Bag1 to Hsp70, which in vitro influences directly Hsp70 protein refolding activity, may also modulate Hsp70 activity in vivo.
To examine whether Bag1 affects Hsp70, we transiently expressed Bag1 in OT70 cells that have the Hsp70 gene under the control of the tetracycline-responsive promoter. Under physiological conditions Hsp70 is expressed at very low levels in these cells (25). We coexpressed firefly luciferase, whose enzymatic activity during recovery from thermal inactivation is highly dependent on chaperone activity. Overexpression of Hsp70 alone is sufficient to enhance reactivation of luciferase activity during recovery at 37°C (22).
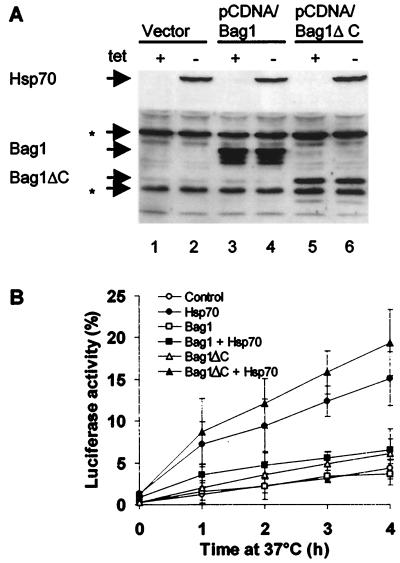
OT70 cells were cotransfected with expression vectors for wild-type Bag1 or a deletion mutant (Bag1ΔC) lacking the carboxyl-terminal domain required for Hsp70 interaction (29). The expression of Bag1, Bag1ΔC, and Hsp70 was monitored by Western blot analysis (Fig. 1A), and complex formation between Bag1 and Hsp70 was detected by coimmunoprecipitation (data not shown) as previously described (29). The levels of Hsp70 were not influenced by coexpression of either wild-type or mutant Bag1 (Fig. 1A, lanes, 2, 4, and 6). Overexpression of Hsp70 alone resulted in reactivation of heat-denatured cytoplasmic luciferase to 15% of the initial enzyme activity after 4 h at 37°C (Fig. 1B), whereas in the absence of exogenous Hsp70, luciferase activity was reactivated to 4% (Fig. 1B). In cells overexpressing both Bag1 and Hsp70, reactivation of luciferase was diminished to 6%. Thus, we conclude that relative to the basal level (4%) of enzyme reactivation in control cells, Bag1 reduced Hsp70-mediated reactivation of luciferase by a factor of approximately 5 (Fig. 1B). Expression of Bag1ΔC did not inhibit Hsp70 chaperone activity, consistent with in vitro results, which had indicated an essential role for the C-terminal Hsp70-binding domain of Bag1 (Fig. 1B). The inhibitory effect of Bag1 on Hsp70-mediated reactivation of luciferase ranged from two- to fivefold and was proportional to the level of coexpressed Bag1 (data not shown).
FIG. 1.
Overexpression of Bag1 inhibits Hsp70-mediated reactivation of heat-denatured firefly luciferase. (A) Western blot analysis of Hsp70, Bag1, and Bag1ΔC expression. OT70 cells were transiently transfected with pCytluc (encoding luciferase) together with pCDNA (vector), pCDNA/Bag1, or pCDNA/Bag1ΔC and grown in medium with or without tetracycline (tet) for induction of Hsp70 expression. Asterisks indicate endogenous hamster proteins that are recognized by the polyclonal anti-Bag1ΔC antibody. (B) OT70 cells were transfected as described above. After pretreatment with cycloheximide (20 μg/ml), luciferase was inactivated by heating the cells at 45°C for 30 min. During a subsequent recovery period at 37°C, samples were taken at the indicated time points and assayed for luciferase activity. The data points represent averages of three independent experiments. Error bars indicate the standard errors of the means.
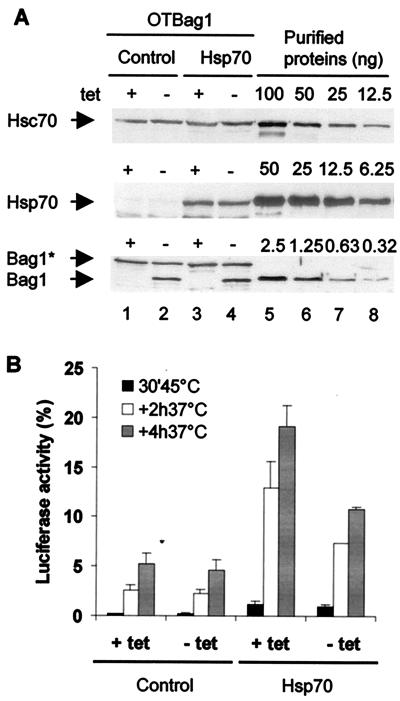
While these results demonstrate that overexpression of Bag1 inhibited the in vivo chaperone activity of Hsp70, it is difficult to assess the biological relevance directly, given the high levels of Bag1 expression attained in transient-transfection assays. To regulate the levels of Bag1, we created a stable cell line for the tetracycline-regulated conditional expression of Bag1. The concentrations of Bag1 from either endogenous or exogenous genes relative to endogenous Hsc70 or transiently expressed Hsp70 were determined by quantitative Western blot analysis using known concentrations of the respective purified recombinant proteins as standards. The cellular concentrations of Hsc70 and endogenous Bag1 were 11 and 2 μM, respectively. Following induction, the total concentration of Bag1 increased to 3.6 μM (Fig. 2A) relative to the concentration of Hsp70 (17 μM) in the subpopulation of transfected cells. Therefore, the ratio of Hsc70 or Hsp70 to Bag1 was 28:4, which corresponds to a sevenfold excess of Hsc70 or Hsp70.
FIG. 2.
Analysis of Bag1 activity on Hsp70 in a regulated cell line. (A) Western blot analysis of Hsc70, Hsp70, and Bag1 expression levels. OTBag1 cells were transiently transfected with a plasmid encoding cytoplasmic luciferase and pCDNA (control) or pCMV70 (Hsp70). At 24 h after transfection, the cells were grown in medium with or without tetracycline (tet) for induction of Bag1 expression. Protein levels in cell lysates (lanes 1 to 4) were compared to levels of purified Hsp70 and Bag1 (lanes 5 to 8). Levels of Hsc70, Hsp70, endogenous Bag1 (Bag1*; also recognized by polyclonal anti-BagΔC antibody), and mouse Bag-1 were 11.5 ± 2.7, 17.3 ± 6.1, 2.0 ± 0.7, and 1.6 ± 0.4 μM, respectively (averages ± standard errors of the means of three independent measurements). Hsp70 levels were corrected for transfection efficiencies that were determined by indirect immunofluorescence. (B) OTBag1 cells were transfected with pCytluc and pCDNA or pCMV70 as described above. After pretreatment with cycloheximide (20 μg/ml), the cells were heated at 45°C for 30 min. During a subsequent recovery period at 37°C, samples were taken at 2 and 4 h after heat shock and assayed for luciferase activity. The data points represent averages of three independent experiments. Error bars indicate the standard errors of the means.
Reactivation of heat-inactivated luciferase was examined in OTBag1 cells. Under normal physiological conditions, Hsp70 is undetected (Fig. 2A, lanes 1 and 2) and the level of luciferase reactivation (5%) is not affected by higher levels of Bag1 (Fig. 2B). Transient overexpression of Hsp70 to a level of 17 μM resulted in a 3.5-fold enhancement (18%) in recovery of luciferase activity. Elevating the levels of Bag1 twofold resulted in a twofold reduction in the level of luciferase reactivation (Fig. 2B). This reveals that the inhibitory effects of Bag1 occur even when the molar ratio of Hsp70 or Hsc70 to Bag1 is 7:1 at concentrations found in mammalian tissue culture cells.
Influence of Bag1 on the biochemical properties of the unfolded substrate in cells expressing Hsp70.
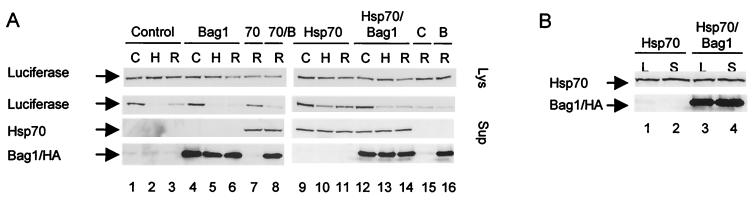
Having established that the recovery of enzymatic activity of thermally inactivated luciferase is enhanced by Hsp70 and inhibited by Bag1, we next addressed whether this is also reflected by changes in the overall levels or the folded state of luciferase. To examine this, we transfected O23 cells with luciferase alone or together with HA-Bag1, Hsp70, or HA-Bag1 and Hsp70. HA-tagged Bag1 inhibits Hsp70-mediated refolding similarly to Bag1 (data not shown) and was used to facilitate detection. In this assay, overexpression of Hsp70 increased the recovery of luciferase enzymatic activity. The overall levels of luciferase protein detected by Western blot analysis in the total lysate were the same regardless of the levels of Hsp70 or Bag1 or whether the cells were exposed to heat shock (Fig. 3A, upper panel). In control cells, luciferase is soluble and can be quantitatively recovered from the soluble fraction of cell extracts (Fig. 3A, lane 1). However, following heat shock at 45°C, all of the luciferase was retained in the insoluble fraction, and even after recovery at 37°C for 3 h, less than 25% of the luciferase was in the soluble fraction (Fig. 3A, lanes 2 and 3). Expression of Hsp70 (Fig. 3A, lanes 9 to 11) increased significantly the amount of luciferase recovered in the soluble fraction; this was clearly observed immediately after heat shock and yielded a higher level of recovery after 3 h at 37°C (Fig. 3A, lanes 9 to 11). Coexpression of Bag1 reversed this effect (lanes 12 to 14), and the level of soluble luciferase was reduced to background levels as in control cells. As expression of Bag1 did not affect the levels of Hsp70 in the soluble fraction, we conclude that Bag1 interferes with the ability of Hsp70 to protect denatured luciferase and its subsequent reactivation to the native state.
FIG. 3.
Bag1 inhibits the Hsp70-mediated increase in substrate solubility. (A) O23 cells were transfected to express luciferase either alone (control or C) or in combination with HA-Bag1 (Bag1 or B), Hsp70 (Hsp70 or 70), or HA-Bag1 and Hsp70 (70/B or Hsp70/Bag1). After pretreatment with cycloheximide (20 μg/ml), the cells were heated at 45°C for 30 min. Before heat shock (C), after heat shock (H), and after recovery at 37°C for 3 h (R), cells were lysed in BLUC containing 1% Triton X-100. The supernatant fraction after centrifugation at 21,000 × g for 15 min was taken as the soluble fraction. The presence of luciferase, Hsp70, and HA-Bag1 in the lysates (Lys) and supernatant fractions (Sup) was analyzed by SDS-PAGE and Western blot analysis. (B) Western blot analysis of Hsp70 and HA-Bag1 in the lysates (L) and supernatant fractions (S) of unheated cells.
Subcellular localization of Bag1 and Hsp70 under physiological and heat shock conditions.
How could Bag1 interfere with the ability of Hsp70 to interact with the denatured substrate? To examine whether Bag1 alters the subcellular localization of Hsp70, we used indirect immunofluorescence to monitor Hsp70 and Bag1 in cells transfected with vector alone or a plasmid encoding HA-tagged Bag1 under conditions where the levels of Hsp70 were modulated by the tetracycline-inducible promoter. Using a collection of antibodies specific to the HA tag or Hsp70, we analyzed the subcellular distribution of HA-Bag1 and Hsp70 in the same cells. Bag1 was detected in the cytosol and nucleus and was enriched in the nucleus (Fig. 4D) in control cells transfected with HA-Bag1 (Fig. 4D to I). Only background levels of staining were detected in untransfected cells (data not shown) or cells transfected with vector alone (Fig. 4A to C). To examine whether elevated levels of Bag1 altered the subcellular distribution of Hsp70, cells expressing both HA-Bag1 and Hsp70 were examined. The primarily cytosolic distribution of Hsp70 typically observed in control cells persisted when the levels of Bag1 were elevated (Fig. 4A and G). Even upon heat shock or during recovery, when Hsp70 relocalizes to the nucleus, expression of Bag1 did not influence compartmentalization of Hsp70 (Fig. 4B, C, H, and I). Taken together, these results show that Bag1 and Hsp70 coexist in the cytosol of control cells together with luciferase and that increased levels of Bag1 did not alter the overall subcellular distribution of Hsp70, even under conditions of heat shock.
FIG. 4.
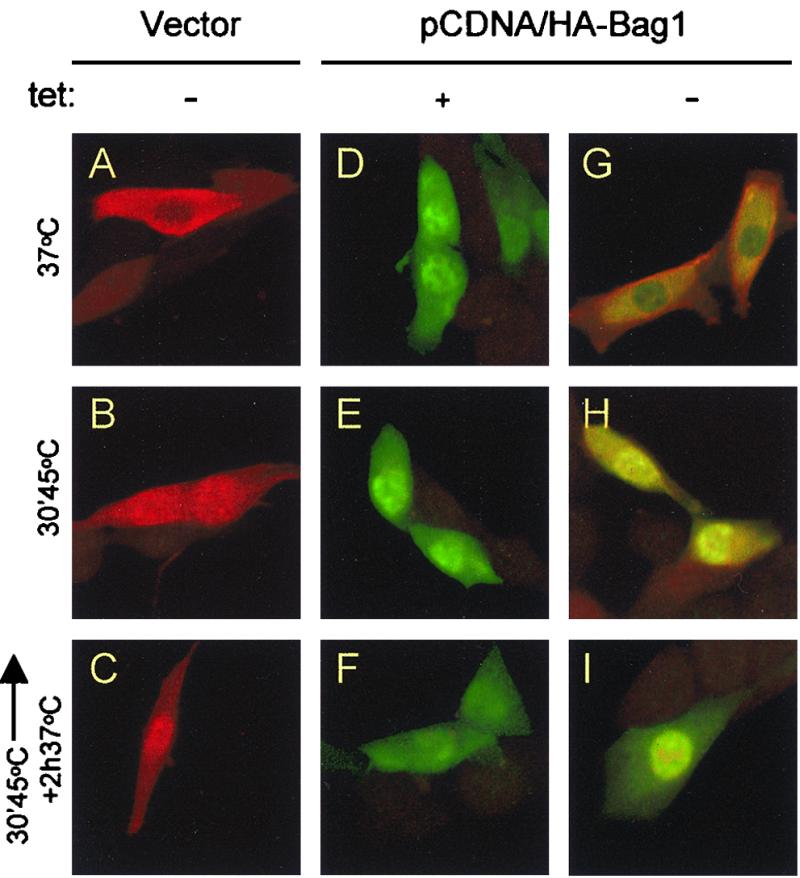
Immunofluorescence analysis of Hsp70 and Bag1 localization. OT70 cells were transiently transfected with pCDNA (vector) or pCDNA/HA-Bag1. Twenty-four hours after transfection the cells were grown in medium with or without tetracycline (tet) for another 24 h. Cells were immunostained after cycloheximide treatment for 30 min (A, D, and G), after heat treatment at 45°C for 30 min (B, E, and H), or after a recovery period for 2 h at 37°C (C, F, and I). All cells were stained with a polyclonal antibody to the heat-inducible Hsp70 and a monoclonal anti-HA tag antibody simultaneously, followed by an incubation with a mixture of tetramethyl rhodamine isocyanate-labeled (red) (Hsp70) and fluorescein isothiocyanate-labeled (green) (HA-Bag1) secondary antibodies. The images were made by confocal microscopy.
Hsp70 chaperone activity is affected by multiple cytosolic isoforms of Bag1.
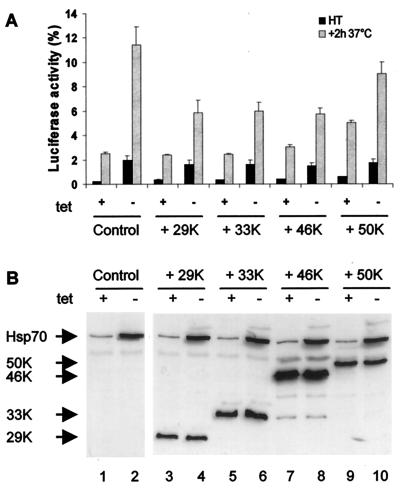
Human Bag1 exists as multiple isoforms with molecular weights of 29, 33, 46, and 50K which originate from different start codons, resulting in proteins that vary by amino-terminal extensions but have a common carboxyl-terminal Hsp70-binding domain (34). We addressed whether the in vivo effect of Bag1 on Hsp70 was exclusive to the 29K isoform or common to all of the cytosolic and longer Bag1 isoforms. To address this, we expressed the 29, 33, 46, and 50K isoforms of Bag1 in OT70 cells and assayed their effects on Hsp70 chaperone activity (Fig. 5). The 29K isoform of Bag1 reduced the Hsp70-mediated reactivation of heat-inactivated luciferase more than twofold (compare Fig. 5A and 1B). Nearly identical inhibitory effects were observed for the 33 and 46K cytoplasmic isoforms of Bag1 (Fig. 5A), while in contrast the nucleus-localized 50K isoform did not affect Hsp70 chaperone activity (Fig. 5A and data not shown) (34). These results reveal that the inhibitory effects of Bag1 on the cytoplasmic chaperone activity of Hsp70 are shared among multiple cytoplasmic forms of Bag1.
FIG. 5.
Human Bag1 and its longer isoforms similarly inhibit Hsp70 chaperone activity. (A) pCytluc together with an empty vector (control) or p29K, p33K, p46K, or p50K (encoding Bag1 isoforms) was transfected into OT70 cells. After induction of Hsp70 expression, cells were treated as described for Fig. 1B. Luciferase activity was measured directly after heat shock at 45°C for 30 min (HT) or after a subsequent incubation at 37°C for 2 h. All data are the averages of three independent experiments; error bars indicate the standard errors of the means. (B) Cells transfected as described above were analyzed for Bag1 and Hsp70 expression by SDS-PAGE and Western blot analysis with monoclonal antibodies to Hsp70 and human Bag1.
DISCUSSION
This study provides the first in vivo evidence to support a role for Bag1 as a negative regulator of Hsp70 molecular chaperone activity. In contrast to Hip or the human DnaJ proteins (Hdj1/Hsp40, Hdj2, and Hsj) which enhance the refolding activity of mammalian Hsp70, or GrpE, which in prokaryotes and mitochondria enhances the DnaK and DnaJ machinery, Bag1 has the distinctive property of inhibiting the ability of Hsp70 to maintain heat-inactivated luciferase in a soluble state and, associated with this, reactivating inert luciferase to a native enzymatically active state. Many of the experiments presented here were accomplished in living cells in which the in vivo levels of either Hsp70 or Bag1 were altered, and while recognizing concerns regarding results obtained in such overexpression studies, every attempt was made to stay within the range of levels of either protein found in human tissue culture cells. Rather than altering the levels of Bag1 to nonphysiological levels, our experiments show that just a twofold increase in the cellular levels of Bag1 inhibits Hsp70-dependent refolding of denatured luciferase by a factor of 2 and that this relationship is proportional to the levels of Bag1. Extending these observations to recent in vitro studies on Bag1 and Hsp70, the inhibitory effect of Bag1 requires the formation of stable Bag1-Hsp70 complexes at a molar ratio of 1:1 and that such complexes lack Hsp70 chaperone activity. The in vivo level at which Bag1 affects Hsp70 is approximately 2 μM (above a basal 2 μM endogenous level), yet the concentration of Hsp70 is approximately 10-fold higher. One interpretation of these results is that only a relatively small fraction (approximately 10 to 15%) of the total cellular level of Hsp70 is available to protect and reactivate thermally inactivated luciferase and that relatively modest changes in the cellular concentrations of Bag1 may have profound effects on Hsp70 function.
In vitro studies on Bag1 interaction with Hsp70 provide some insights on the underlying mechanism by which Bag1 inhibits Hsp70 chaperone activity. In the presence of Hsp70, an unfolded polypeptide is maintained in a soluble intermediate folded state and conversion to the native state is enhanced strongly by Hdj1/Hsp40 and ATP. However, if Bag1 is added to this reaction, refolding to the native state is completely inhibited, even in the presence of cochaperones and ATP (2). These results provide an explanation for the in vivo data presented here showing that Bag1 interferes with the ability of Hsp70 to maintain luciferase in a soluble state, leading to refolding of luciferase to an enzymatically active state. However, the in vitro studies on Hsp70 and Bag1 show that the unfolded substrate remains bound to Hsp70 in a soluble state, whereas the in vivo observations show that the denatured luciferase is not bound to Hsp70 in a soluble inert state. Perhaps this is because Hsp70 is also engaged in interactions with other substrates which in the presence of Bag1 are not dissociated, with the consequence that this population of Hsp70 is inaccessible to denatured luciferase and for subsequent Hsp70-dependent refolding reactions.
What firm conclusions can be drawn from these studies on the role of Bag1 as a cochaperone which unlike Hip and Hdj1/Hsp40 has negative regulatory effects? Bag1 is not a classical heat shock protein, as its levels are not induced in stressed cells, in contrast to Hsp70 and Hdj1/Hsp40, which are strongly induced and accumulate to high levels following heat shock (26). Therefore, the relative ratio of Hsp70 and the cochaperones required for the stress-induced chaperone activity to cope with the rapid flux of stress-induced unfolded and misfolded proteins is enhanced significantly during stress relative to Bag1. The levels of Bag1 and cochaperones also vary significantly under normal conditions of cell growth and differentiation in a cell-type-specific manner among different human tumor cell lines (30; C. Schmidt, personal communication).
Mammalian cells express multiple Bag1-related proteins (Bag1 to Bag5) which have in common the Hsp70-binding domain and inhibit Hsp70 chaperone-dependent, in vitro refolding reactions (32). Additionally, Bag1 is evolutionarily conserved from humans to Caenorhabditis elegans and is differentially expressed as multiple alternatively spliced isoforms which have in common the C-terminal Hsp70-binding domain and differ by N-terminal extensions (32; E. Nollen, J. Morley, and S. Satyal, personal communication). All of the cytoplasmic Bag1 isoforms inhibit, to a similar extent, Hsp70 chaperone activity in vivo, suggesting that this effect on chaperone activity may be a universal feature of Bag1 proteins (32). How do we reconcile these results with the independent identification of Bag1 as a partner protein for a number of key cell signaling proteins, including Bcl-2, Raf-1, hepatocyte growth factor receptor, platelet-derived growth factor receptor, and retinoic acid receptor (1, 7, 8, 18, 31, 33)? Although Bag1 and its isoforms associate with these cell signaling proteins and Hsp70, there is no evidence of whether the function of Bag1 is to select these regulatory proteins for subsequent association with Hsp70. Located at the amino terminus of Bag1 is a region which has a ubiquitin-like motif; this could provide a mechanism for Bag1 or Bag1-associated-protein targeting to the ubiquitin-dependent proteasome (31). Other possibilities are that such heteromeric complexes could be used to deliver substrates to Hsp70 for translocation, targeting, or folding or as a means to regulate or confer a particular folded state. Alternatively, Bag1 could bring specific proteins to Hsp70 or function as a selector molecule which alternatively regulates Hsp70 or proteins involved in cell signaling events. Distinguishing among such alternatives, however, may prove challenging, as interactions between chaperones and their substrates have been elusive in vivo. Nevertheless, Bag1 represents a new family of conserved proteins which brings together by direct biochemical interactions key proteins associated with cell stress and cell death.
ACKNOWLEDGMENTS
E.A.A.N. was supported by grants from the Dutch Cancer Society, Groningen Utrecht Institute for Drug Exploration (GUIDE), and The Dutch Science Foundation (NWO). These studies were supported by grants from the NIH (GM38109) to R.I.M. and from the Dutch Cancer Society (NKB-grant 95-1082) to H.H.K.
We thank Susan G. Fox and Masahiro Takeda for excellent technical assistance. We thank S.-C. Tang for generously providing us with the plasmids encoding the human Bag1 isoforms and the monoclonal antibody to human Bag1.
REFERENCES
- 1.Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio P M. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–6212. [PMC free article] [PubMed] [Google Scholar]
- 2.Bimston D, Song J H, Winchester D, Takayama S, Reed J C, Morimoto R I. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 1998;17:6871–6878. doi: 10.1093/emboj/17.23.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 4.Freeman B C, Morimoto R I. The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman B C, Myers M P, Schumacher R, Morimoto R I. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman B C, Toft D O, Morimoto R I. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- 7.Froesch B A, Takayama S, Reed J C. BAG-1L protein enhances androgen receptor function. J Biol Chem. 1998;273:11660–11666. doi: 10.1074/jbc.273.19.11660. [DOI] [PubMed] [Google Scholar]
- 8.Gebauer M, Zeiner M, Gehring U. Proteins interacting with the molecular chaperone hsp70/hsc70: physical associations and effects on refolding activity. FEBS Lett. 1997;417:109–113. doi: 10.1016/s0014-5793(97)01267-2. [DOI] [PubMed] [Google Scholar]
- 9.Georgopoulos C. A new bacterial gene (groPC) which affects lambda DNA replication. Mol Gen Genet. 1977;151:35–39. doi: 10.1007/BF00446910. [DOI] [PubMed] [Google Scholar]
- 10.Gething M-J. The difference with prokaryotes. Nature. 1997;388:329–331. doi: 10.1038/40979. [DOI] [PubMed] [Google Scholar]
- 11.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 13.Hohfeld J, Jentsch S. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohfeld J, Minami Y, Hartl F U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 15.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberek K, Skowyra D, Zylicz M, Johnson C, Georgopoulos C. The Escherichia coli DnaK chaperone, the 70-kDa heat shock protein eukaryotic equivalent, changes conformation upon ATP hydrolysis, thus triggering its dissociation from a bound target protein. J Biol Chem. 1991;266:14491–14496. [PubMed] [Google Scholar]
- 17.Lindquist S. Mad cows meet psi-chotic yeast: the expansion of the prion hypothesis. Cell. 1997;89:495–498. doi: 10.1016/s0092-8674(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Takayama S, Zheng Y, Froesch B, Chen G Q, Zhang X, Reed J C, Zhang X K. Interaction of BAG-1 with retinoic acid receptor and its inhibition of retinoic acid-induced apoptosis in cancer cells. J Biol Chem. 1998;273:16985–16992. doi: 10.1074/jbc.273.27.16985. [DOI] [PubMed] [Google Scholar]
- 19.Luders J, Demand J, Schonfelder S, Frien M, Zimmermann R, Hohfeld J. Cofactor-induced modulation of the functional specificity of the molecular chaperone Hsc70ss. Biol Chem. 1998;379:1217–1226. doi: 10.1515/bchm.1998.379.10.1217. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa S, Takayama S, Froesch B A, Zapata J M, Reed J C. p53-inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. EMBO J. 1998;17:2736–2747. doi: 10.1093/emboj/17.10.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mccarty J S, Buchberger A, Reinstein J, Bukau B. The role of ATP in the functional cycle of the DnaK chaperone system. J Mol Biol. 1995;249:126–137. doi: 10.1006/jmbi.1995.0284. [DOI] [PubMed] [Google Scholar]
- 22.Michels A A, Kanon B, Konings A W T, Ohtsuka K, Bensaude O, Kampinga H H. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J Biol Chem. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- 23.Michels A A, Nguyen V T, Konings A W, Kampinga H H, Bensaude O. Thermostability of a nuclear-targeted luciferase expressed in mammalian cells—destabilizing influence of the intranuclear microenvironment. Eur J Biochem. 1995;234:382–389. doi: 10.1111/j.1432-1033.1995.382_b.x. [DOI] [PubMed] [Google Scholar]
- 24.Minami Y, Hohfeld J, Ohtsuka K, Hartl F U. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 25.Nollen E A, Brunsting J F, Roelofsen H, Weber L A, Kampinga H H. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsell D A, Lindquist S. Heat shock proteins and stress tolerance. In: Morimoto R I, et al., editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1994. pp. 457–494. [Google Scholar]
- 27.Prapapanich V, Chen S, Nair S C, Rimerman R A, Smith D F. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996;10:420–431. doi: 10.1210/mend.10.4.8721986. [DOI] [PubMed] [Google Scholar]
- 28.Rudiger S, Buchberger A, Bukau B. Interaction of Hsp70 chaperones with substrates. Nat Struct Biol. 1997;4:342–349. doi: 10.1038/nsb0597-342. [DOI] [PubMed] [Google Scholar]
- 29.Takayama S, Bimston D N, Matsuzawa S, Freeman B C, Aimesempe C, Xie Z H, Morimoto R I, Reed J C. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takayama S, Krajewski S, Krajewska M, Kitada S, Zapata J M, Kochel K, Knee D, Scudiero D, Tudor G, Miller G J, Miyashita T, Yamada M, Reed J C. Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res. 1998;58:3116–3131. [PubMed] [Google Scholar]
- 31.Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan J A, Reed J C. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- 32.Takayama S, Xie Z H, Reed J C. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 33.Wang H G, Takayama S, Rapp U R, Reed J C. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci USA. 1996;93:7063–7068. doi: 10.1073/pnas.93.14.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Chernenko G, Hao Y, Ding Z, Pater M M, Pater A, Tang S C. Human BAG-1/RAP46 protein is generated as four isoforms by alternative translation initiation and overexpressed in cancer cells. Oncogene. 1998;17:981–989. doi: 10.1038/sj.onc.1202032. [DOI] [PubMed] [Google Scholar]
- 35.Zeiner M, Gebauer M, Gehring U. Mammalian protein RAP46: an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J. 1997;16:5483–5490. doi: 10.1093/emboj/16.18.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeiner M, Gehring U. A protein that interacts with members of the nuclear hormone receptor family: identification and cDNA cloning. Proc Natl Acad Sci USA. 1995;92:11465–11469. doi: 10.1073/pnas.92.25.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]