Abstract
Asthma is a disease of reversible airflow obstruction characterised clinically by wheezing, shortness of breath, and coughing. Increases in airway type 2 cytokine activity, including interleukin-4 (IL-4), IL-5, and IL-13, are now established biological mechanisms in asthma. Inhaled corticosteroids have been the foundation for asthma treatment, in a large part because they decrease airway type 2 inflammation. However, inhaled or systemic corticosteroids are ineffective treatments in many patients with asthma and few treatment options exist for patients with steroid resistant asthma. Although mechanisms for corticosteroid refractory asthma are likely to be numerous, the development of a new class of biologic agents that target airway type 2 inflammation has provided a new model for treating some patients with corticosteroid refractory asthma. The objective of this Therapeutic paper is to summarise the new type 2 therapeutics, with an emphasis on the biological rationale and clinical efficacy of this new class of asthma therapeutics.
Introduction
Asthma is a chronic airway disease that inflicts between 300 million and 400 million people worldwide.1 A diagnosis of asthma requires verifying the presence of reversible airflow obstruction,2 which is accomplished by showing either airflow limitation that improves following bronchodilator administration or worsening airflow obstruction in the setting of airway provocation.3 The disease is characterised by coughing, shortness of breath, chest tightness, and wheezing.4 These symptoms result from impaired airway inflammatory responses that cause mucus hypersecretion, bronchial hyperresponsiveness, and activation of airway granulocytes.5 Mouse models of asthma were used to identify pivotal roles for the cytokines interleukin-4 (IL-4), IL-5, and IL-13 in driving the pathophysiological features of allergic asthma.6,7-9 Because T-helper-2 (Th2) cells were believed to be the principle source of these signalling molecules they were originally named Th2 cytokines,10 but other cells, including innate lymphocytes, can also produce these proteins, and the research community has since migrated to the broader term of type 2 (or T2) cytokines. Confirming the pathological role of these factors in human asthma would take nearly 25 years as initial human trials of targeted therapies returned negative results.11-13 In fact, establishing the efficacy of these cytokines for asthma in humans required a convergence of two concepts. First, that asthma was a complex heterogeneous disease, and second, that biologic therapies needed to target the population of asthma patients with elevated type 2 cytokine activity in their airways.14-16
Type 2-high asthma
Inspired by observations that allergic inflammation in mice was driven by Th2 cytokine activity8,9 and that these cytokines were measured at high concentrations in the lungs of patients with asthma,17-20 multiple monoclonal antibodies were developed to inhibit type 2 inflammation. Unfortunately, the first clinical trials testing the inhibition of IL-5 (and IL-4) with these antibodies were profoundly disappointing.11-13 Proving the efficacy of type 2 cytokine inhibition would have to wait until a new insight emerged, namely that Th2 inflammation was not a causal disease mechanism in all patients with asthma. Furthermore, multiple immune cells other than Th2 cells have been increasingly recognised as able to produce IL-4, IL-5 and IL-13, including several innate immune cells such as basophils, mast cells, and type 2 innate lymphoid cells,21-24 with potentially differing regulatory mechanisms than those observed for adaptive immune Th2 cells (figure 1). This concept prompted the community to refer to these factors as type 2 cytokines and their downstream effects (or signatures) as type 2 inflammation. Additionally, measuring the protein concentrations of type 2 cytokines proved difficult, thus necessitating the need for downstream or associated biomarkers to identify the subgroup of patients with type 2-high asthma. Eosinophil cell counts in the blood and sputum, fraction exhaled nitric oxide (FeNO), periostin concentrations, and measurements of airway type 2 cytokine gene expression have now all been used successfully as surrogate biomarkers for airway type 2 inflammation.25-28 Through the use of these biomarkers, only a subset (40–70%) of asthma patients clearly show increases in airway type 2 inflammation (type 2-high), with the remaining subgroup demonstrating low to normal type 2 inflammatory measures (type 2-low).14,16,25,29-32
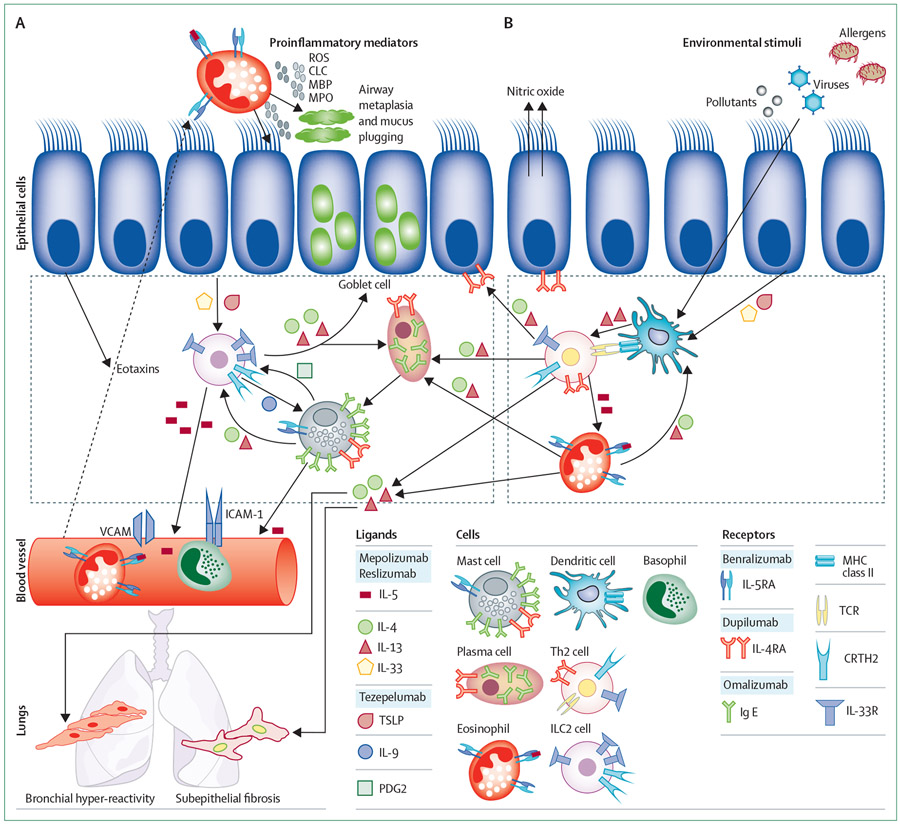
Figure 1: ILC2s and Th2 cells are key activators of airway type-2 inflammation.
The type 2 cytokines are responsible for the key pathological features of asthma, including goblet cell metaplasia, mucus plugging, bronchial hyper-reactivity, and airway eosinophilia. The type 2 immune cascade is initiated by epithelial cell exposure to environmental stimuli (ie, allergens, viruses, and pollutants). Epithelial cells secrete eotaxins that promote chemotaxis of eosinophils, basophils, and T-helper-2 (Th2) cells. (A) The role of the group 2 Innate lymphoid cell (ILC2) in driving the type 2 immune response. ILC2 cells are activated through the epithelial production of IL-33 and TSLP, and in this state secrete large amounts of type 2 cytokines (IL-4, IL-5, and IL-13). ILC2 cells induce mast cell proliferation via IL-9 and assist plasma cell class switching to immunoglobulin E (IgE) through the release of IL-4 and IL-13. (B) The role of Th2 cells as propagators of the type 2 immune response. Dendritic cells process and present antigens leading to the production of type 2 cytokines by Th2 cells. ROS=reactive oxygen species. CLC=charcot-leyden crystals. MBP=myelin basic protein. MPO=myeloperoxidase.
By recognising that not all asthma is the same, studies of type 2 cytokine inhibition began to target patients with elevations in these type 2 biomarkers. For example, an anti-eosinophilic medication (anti-IL-5 monoclonal antibody) did not meet its primary or secondary endpoints in all-comers trials, but clinical efficacy became apparent when targeted to patients with increased blood and sputum eosinophil counts.33,34 This success was followed by studies of an anti-IL-13 antibody in which responses to therapy were greater in those patients with elevated serum periostin and exhaled FeNO, than in those patients without these elevations.31 With this new realisation regarding the heterogeneity of asthma, multiple type 2 biologics were tested with eosinophil counts or other type 2 biomarkers as predictors of type 2-high asthma in patients that met criteria for severe asthma. The majority of these targeted trials proved efficacious and led to the development of a growing list of type 2 biologic agents. To date, there are four approved drugs that directly inhibit type 2 cytokines. Three of these agents, mepolizumab, benralizumab, and reslizumab target the IL-5 cytokine or its receptor (IL-5RA), whereas the fourth agent, dupilumab, targets IL-4RA, which is the primary signalling receptor for IL-4 and IL-13.
Omalizumab
Although patients and asthma physicians are excited about the type 2-targeted biologics, the first approved biologic for asthma (omalizumab) was infact targeted to immunoglobulin E (IgE). Multiple reviews have discussed in detail the use of omalizumab as a treatment for asthma.35,36 Free or circulating IgE binds to high-affinity IgE receptors (FcεRI) expressed on the surface of basophils and mast cells, leading to their cellular activation. Omalizumab is a monoclonal antibody that binds to circulating IgE and inhibits the binding of IgE to FcεRI. The most consistent clinical benefit of this treatment is a reduction in asthma exacerbations. Importantly, the biomarkers initially used in the early omalizumab trials (IgE and the presence of specific IgE) have not been proven to be effective at predicting clinical response, and retrospective analysis suggests that type 2 biomarkers, including blood eosinophils and the amount of exhaled FeNO, are more effective.37 Thus, although not initially thought of as a type 2-targeted drug, there is strong overlap with the type 2-high phenotype; yet the drug has never been studied in this population. In a pooled analysis of 25 randomised controlled trials (in patients who met total and specific IgE criteria only), omalizumab reduced the number of patients with asthma exacerbation from 26% in the placebo group to 16% in the omalizumab treatment group over 48 weeks.38 Although the effect size is less than the benefit seen with anti-IL-5 or anti-IL-4RA therapies, some key differences in the trial designs need to be highlighted between the major omalizumab trials and those with the newer type 2 biologic agents.39-41 Principally, the omalizumab trials did not exclusively restrict participation to those patients with eosinophilic asthma and the inclusion criteria for these trials did not include a requirement to have had an asthma exacerbation in the previous year. In fact, many of the registered trials for omalizumab were completed in patients who would not meet more recent definitions for severe asthma.42,43 Therefore, directly comparing the effect sizes between omalizumab versus the newer type 2 biologic agents is challenging.44
Eosinophils and IL-5 inhibition
Eosinophils are granulocytes that release a variety of proinflammatory mediators, including proinflammatory cytokines following activation, major basic protein, eosinophil peroxidase, eosinophil cationic protein, eosinophil-derived neurotoxin, and galactin-10 or Charcot-Leyden crystals (figure 1).7 Some patients with asthma show increases in airway eosinophilia, which has been appreciated for over 100 years.45,46 This observation prompted a considerable amount of research focused on understanding the role of eosinophils as mediators of asthmatic disease. There is now an understanding that these granulocytes instigate airway dysfunction through degranulation and the release of reactive oxygen species that promote airway epithelial-barrier dysfunction (figure 1).47 Although traditionally characterised as innate cells, new findings also suggest that eosinophils are directly involved as pivotal orchestrators of the type 2 immune response.48 Together these studies support a crucial role for eosinophils as drivers of type 2 immune-inflammatory responses in asthma.
IL-5 is required for eosinophil maturation, survival, and the translocation of these cells from the bone marrow into the systemic circulation. Therefore, inhibiting IL-5 signalling was an obvious therapeutic target in asthma. IL-5 signals via an IL-5 specific receptor, IL5RA, and a signal-transducing β receptor that is shared with the cytokines IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF).49 When used in patients with evidence of eosinophilic inflammation, medications that inhibit binding of the ligand to its IL5RA receptor reduce systemic eosinophil counts, decrease basement membrane thickening, reduced airway tissue remodelling,50 and might promote airway mucus plug formation.51
The first of these agents to be tested was the IL-5 ligand-directed IgG1 antibody, mepolizumab. As previously noted, the initial mepolizumab trials were disappointing, questioning the role of eosinophils as active mediators of asthmatic disease.11,12 However, the results were statistically significant when mepolizumab was directed to biomarker-defined eosinophilic asthma.30,33,34,52,53 The initial trial targeted patients with eosinophilic asthma using sputum eosinophil cell counts greater than 3%,33 and the pivotal DREAM study30 used a combination of sputum eosinophil percentages (>3%) or cell count in the blood (≥300 cells per μL) to define eosinophilic asthma. However, counting sputum cells is technically challenging and subsequent phase 3 trials used more convenient blood eosinophil cell measurements. Specifically, in the first phase 3 clinical trial, mepolizumab (Subcutaneous, 100 mg every 4 weeks) met its primary outcome by decreasing asthma exacerbation by 53% when compared with placebo in patients with blood eosinophil counts of more than 150 cells per μL at screening or 300 cells per μL or higher during the previous year (figure 2A). Small but significant improvements in forced expiratory volume in 1 s (FEV1) of 98 mL (figure 2B) and an asthma control questionnaire-5 result of 0·42 (which did not reach a clinically significant difference) were also noted.53 Using similar eosinophilic inclusion criteria, in a study of patients who were dependent on systemic corticosteroids, mepolizumab treatment also improved the likelihood of decreasing systemic-oral-prednisone dosing, with patients given mepolizumab showing a 2·39 greater increase in reducing oral prednisone treatment compared with patients given placebo.52 Impressively, despite this corticosteroid dose reduction, patients given mepolizumab also achieved better lung function and improved asthma control questionnaire scores when compared with placebo.52
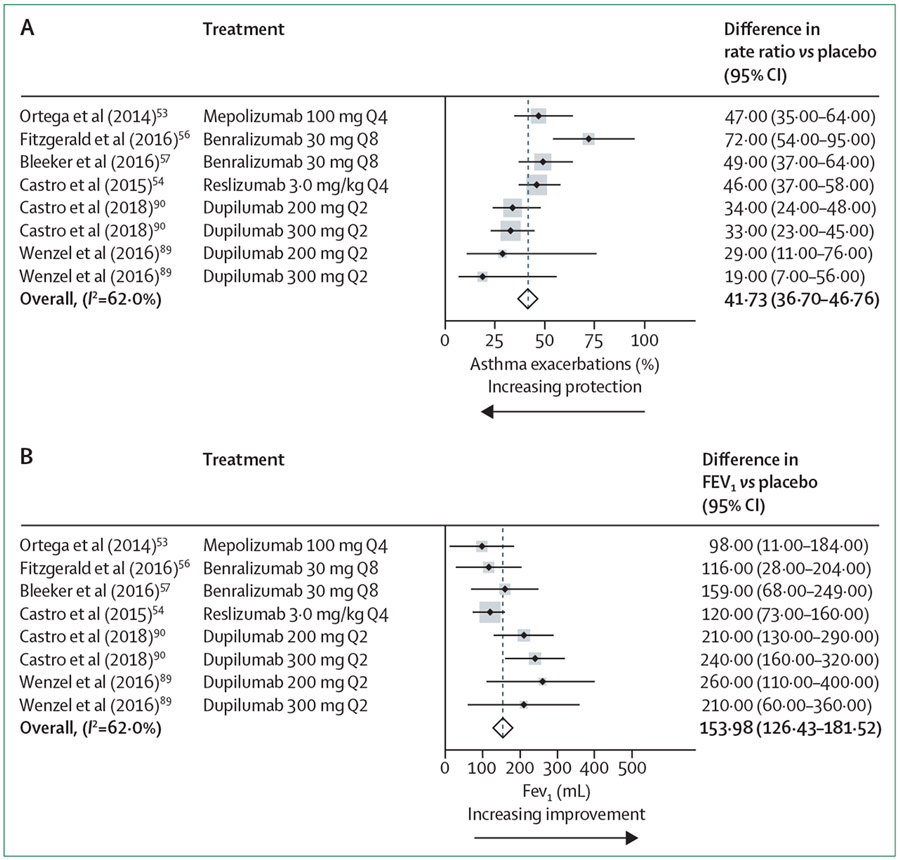
Figure 2: Forest plots showing the effect size of type 2 biologic agents in patients with eosinophilic asthma.
(A) Effect of biologic agents on asthma exacerbation. (B) Effect of biologic agents on forced expiratory volumes in 1 s (FEV1). The standardised mean difference (dashed line) and 95% CI for the combined treatment effects are shown. Q2=dose every 2 weeks. Q4=dose every 4 weeks. Q8=dose every 8 weeks.
The success of mepolizumab was duplicated with reslizumab, a similar anti-IL-5 monoclonal antibody (IgG4). The inclusion criteria for the reslizumab trials differed slightly from the mepolizumab studies with the inclusion of patients on a medium-to-high dose of inhaled corticosteroids and a slightly higher blood-eosinophil threshold of 400 cells per μL or more. Despite these differences, reslizumab decreased asthma exacerbation by 54% when given intravenously (3 mg/kg every 4 weeks),54 with similar improvements in FEV1 (120 mL) and symptom scores (asthma control questionnaire-7 score of 0·25) when compared with placebo (figure 2A, B).54
The third IL-5 pathway inhibitor to show clinical efficacy was benralizumab. Unlike mepolizumab or reslizumab, benralizumab is an IgG1 monoclonal antibody targeting the α subunit of the IL-5 receptor. One unique aspect of benralizumab is that it lacks a fucose molecule in the constant segment (Fc fragment) of the monoclonal antibody. This afucosylation results in the enhanced affinity of benralizumab for the human Fcγ receptor that is expressed on cytotoxic cells, such as natural killer cells, macrophages, and neutrophils.55 As such, benralizumab has a unique capacity to induce antibody-mediated cellular toxicity resulting in prolonged eosinophil depletion when compared with monoclonal antibodies that directly bind to a ligand. Pharmacodynamically, this characteristic means that benralizumab can be dosed at 30 mg every 4 weeks for the first 3 months and then every 8 weeks thereafter. However, despite these theoretical benefits, the clinical efficacy of benralizumab was similar to that observed in the ligand-antibody trials (figure 2A, B). Specifically, in patients with eosinophilic asthma (defined by ≥300 cells per μL), benralizumab decreased asthma exacerbation by 28% in one trial and 51% in the other when compared with placebo.56,57 Small improvements in FEV1 (159 mL, 116 mL) (figure 2B) and symptoms were also shown. In addition, in an oral corticosteroid reduction trial in which patients were only required to have blood eosinophil counts of 150 cells per μL or more, patients treated with benralizumab were able to decrease oral-prednisone doses by 75% compared with 25% in the placebo group.58 The percentage of patients able to decrease oral prednisone dose by more than half after starting benralizumab (48%) was greater than the fraction of patients on mepolizumab (37%).52,58 However, the clinical or statistical meaning of this difference is difficult to compare.
Overall, these findings support the clinical efficacy of all three IL-5 pathway antagonists as they showed similar effect sizes in the primary outcome for reducing asthma exacerbation (table 1). Small improvements in FEV1, asthma symptoms, and quality of life were also seen, as was a reduction in systemic corticosteroid dependency.52,56-58 However, reslizumab in subcutaneous form did not show a reduction in systemic corticosteroid dependency (NCT02501629). Although the improvements in FEV1 were statistically significant when compared with placebo, the overall improvement of between 98 mL and 159 mL was relatively modest. Conversely, these medications show consistent and relatively robust effects on asthma exacerbations, with overall decreases of 35–55% when compared with the placebo group (figure 2A, B; table 1). Importantly, the effect of anti-IL-5 therapy on asthma exacerbation was sustained even after multiple years of treatment.59,60
Table 1:
Type 2 biologic medications for severe asthma
| Target | Dose | Primary treatment group | Primary benefits | Stage of development | |
|---|---|---|---|---|---|
| Mepolizumab (GlaxoSmithKline, Brentford, UK) | IL-5 | Subcutaneous, 100 mg, Q4 weeks | Severe eosinophilic asthma (≥150 cells per μL at screening or ≥300 cells per μL in past year) | Considerable improvement in asthma exacerbations and symptoms; mild improvement in FEV1 and steroid sparing | FDA approved for severe eosinophilic asthma |
| Reslizumab (Teva Pharmaceuticals, Petah Tikva, Israel) | IL-5 | Intravenous, 3·0 mg/kg, Q4 weeks | Moderate to severe eosinophilic asthma (≥400 cells per μL) | Considerable improvement in asthma exacerbations; mild improvement in FEV1 and symptoms | FDA approved for severe eosinophilic asthma |
| Benralizumab (MedImmune, Gaithersburgh, USA; and AstraZeneca, Cambridge, UK) | IL-5RA | Subcutaneous, 30 mg, Q8 weeks | Severe eosinophilic asthma (≥300 cells per μL) | Considerable improvement in asthma exacerbations; mild improvement in FEV1 and steroid sparing | FDA approved for severe eosinophilic asthma |
| Lebrikizumab (Genentech, San Francisco, USA; and Roche, Basel, Switzerland) | IL-13 | Subcutaneous, 38–125 mg, Q4 weeks | Severe asthma with periostin concentrations ≥50 ng/mL or blood eosinophils ≥300 cells per μL | Mild improvement in asthma exacerbations | No longer in development for asthma |
| Pitrakinra (Amgen, Thousand Oaks, USA) | IL-4RA | Subcutaneous, 25 mg once a day or 60 mg nebulised twice a day | Atopic asthma | Modest efficacy in allergen challenge model | No longer in development for asthma |
| Dupilumab (Regeneron, Tarrytown, USA; and Sanofi, Paris, France) | IL-4RA | Subcutaneous, 200 or 300 mg, Q4 weeks | Moderate to severe eosinophilic asthma (>300 cells per μL) | Considerable improvement in asthma exacerbations, FEV1, and symptoms; mild improvement in steroid sparing | FDA approved for moderate to severe eosinophil asthma or oral corticosteroid-dependent asthma |
| Tezepelumab (Amgen; and MedImmune) | TSLP | Subcutaneous, 70 mg Q4 weeks, 210 mg Q4 weeks, or 280 mg Q2 weeks | Moderate to severe asthma | Considerable improvement in asthma exacerbations; mild improvement in FEV1 and symptoms | Ongoing phase 3 trial |
| REGN3500 (Regeneron) | IL-33 | Subcutaneous, dose to be determined | Moderate to severe eosinophilic asthma (≥300 cells per μL) | Mild improvement in loss of asthma control and FEV1 | Recently completed phase 2B trial |
FEV1=forced expiratory volumes in 1 s. FDA=US Food and Drug Administration. Q2=every 2 weeks. Q4=every 4 weeks. Q8=every 8 weeks.
Adverse effects of IL-5 inhibition
The most severe adverse reaction observed with the IL-5 inhibitors was anaphylaxis. This reaction was more common with the intravenously administered reslizumab than the other IL-5 antagonists and occurred in 0·3% of patients61—the US Food and Drug Administration (FDA) has given the drug a black box warning in this regard (table 2). This frequency is similar to that seen with the subcutaneous anti-IgE monoclonal antibody (omalizumab).62 Hypersensitivity reactions were slightly less frequent with the subcutaneous medications of mepolizumab and benralizumab, but such events did occur, and prescribers should be able to manage anaphylaxis and hypersensitivity while administering these medications.60,63
Table 2:
Risk for type 2 therapeutics
| Observed risks | Hypothetical risks | |
|---|---|---|
| Mepolizumab | Herpes zoster | Parasitic infections, malignancy, obesity or metabolic dysfunction |
| Reslizumab | Anaphylaxis | Parasitic infections, malignancy, obesity or metabolic dysfunction |
| Benralizumab | Prolonged decrease in eosinophil counts | Parasitic infections, malignancy, obesity or metabolic dysfunction |
| Dupilumab | Eosinophilia, conjunctivitis | Parasitic infections, obesity or metabolic dysfunction, eosinophilic granulomatosis with polyangiitis |
All medications report low and similar frequencies for injection site reactions (2–10%) and hypersensitivity reactions (<1–3%).
An unexpected observation was that two serious herpes zoster infections occurred in patients given mepolizumab, but none in the participants given placebo (table 2). The association between herpes zoster infections and mepolizumab treatment has been observed in subsequent observational studies, but uncertainty remains regarding the clinical impact of IL-5 inhibition on the rates of these infections.60 Due to this uncertainty, herpes zoster vaccination might be considered in patients with a high risk of infection, but uniform vaccinations before initiating anti-IL-5 medications is not yet standard practice.
Eosinophils are commonly elevated in helminth infections64 and although the essential role of eosinophils in the elimination of different types of parasites remains controversial, one concern of IL-5 inhibition is the potential to increase the risk of these infections. These infections, however, rarely occur in the high-income countries in which these therapies are tested, and in initial studies patients were screened to exclude participants with a parasitic infection.53 As such, the potential risk has not been confirmed. However, the risk remains, and caution is advisable in countries where parasites are endemic.
Two additional hypothetical concerns arise when treating patients with IL-5 inhibitors. First, an inverse relationship exists between blood and mucosal eosinophil cell counts and colon cancer risk.65,66 These findings suggest that decreasing eosinophil numbers might increase the risk of certain mucosal cancers. Second, eosinophils have a crucial role in the maintenance of adipose tissue metabolism,23,67 and decreasing eosinophil numbers in this tissue leads to obesity and metabolic dysfunction.67,68 Therefore, prolonged inhibition of eosinophils could lead to obesity and metabolic dysfunction, including insulin resistance. These complications could potentially be of greater concern with benralizumab as this treatment has a prolonged effect on eosinophil depletion; however, this therapy does not appear to completely eliminate tissue eosinophilia (table 2).69,70 Furthermore, using fewer systemic corticosteroids in patients treated with these biologics could be speculated to also limit further weight gain. Certainly, long-term followup studies are needed in patients treated with anti-IL-5 therapies to address these potential concerns.
IL-4RA inhibition
The cytokines, IL-4 and IL-13, are complementary both in their biologic roles and in their signalling machinery. Namely, the primary receptor for IL-4 is IL-4RA, which upon binding with IL-4, complexes with the common γ-chain (γc) to signal via intracellular JAK1 or JAK3 pathways (type 1 receptor).71 IL-13 also uses the IL-4RA receptor through a heterodimerisation with IL-13RA1 that signals via JAK1, JAK2, and TYK2 (type 2 receptor).71,72 Thus, blocking IL-4RA inhibits the primary signalling pathways of both IL-4 and IL-13.72 Both cytokines promote B lymphocyte class switching from IgM antibodies to IgE antibodies,73 induce airway smooth-muscle hyper-reactivity,8,73 and promote eosinophilic chemotaxis through expression of vascular cell adhesion molecule-1 (VCAM-1)74 and numerous eosinophilic chemokines (figure 1). However, IL-4 is essential for promoting the differentiation of Th2 cells from T0 lymphocytes,75 and IL-13 is a prominent driver of the airway epithelial transformations that occur in asthma.8 Specifically, both IL-4 and IL-13 can promote goblet cell metaplasia, mucus production, subepithelial fibrosis, and basement membrane thickening in conjunction with, or independent of, IL-4 (figure 1).6,76-78
Because of the strong animal data supporting the role of IL-13 in driving asthma pathogenesis and the success of the anti-IL-5 medications, IL-13 inhibition was reasonably assumed to prove efficacious in asthma. Unfortunately, clinical trials that selectively targeted IL-13 did not show consistent efficacy, supporting the broader importance of both IL-4 and IL-13 in asthma.31,79-85 Subsequently, IL-4RA became a target as it is a dual receptor for IL-4 and IL-13.
Efforts to block the IL-4 and IL-13 signalling axis with the IL-4RA inhibitors, pitrakinra and AMG 317, were initially done in all-comers trials and this unstratified approach did not show clinical efficacy (table 1).13,86,87 However, 3 years later with the added insight of asthma heterogeneity, and secondary analysis showing the efficacy of pitrakinra in patients with eosinophilic asthma,86 a new IL-4RA antibody was tested in an initial proof-of-concept phase 2A trial.88 In this study, 104 patients on medium-to-high dose combination therapy (inhaled corticosteroids and long-acting β agonists) with blood eosinophil counts of 300 cells per μL or higher, or sputum eosinophils of 2% or more, were randomly assigned to drug versus placebo groups. Following a 4-week stable treatment phase, background medication was successively withdrawn, with the primary endpoint being loss of asthma control. Dupilumab treatment led to an 87% reduction in loss of asthma control compared with placebo, and improved FEV1 and asthma symptoms despite withdrawal of background medications. This proof-of-concept study was followed by a phase 2B study of dupilumab at 200 mg or 300 mg doses given subcutaneously at 2-week or 4-week intervals. The prespecified analysis plan in this trial subdivided patients into eosinophilic (blood eosinophils ≥300 cells per μL) and non-eosinophilic (<300 cells per μL) subgroups,89 and the primary endpoint of improvement in lung function was measured at 12 weeks in the eosinophil-high subgroup. The trial met its primary endpoint, improved FEV1 at 12 weeks in both eosinophil-low and eosinophil-high patients, and maintained this improvement at 6 months while decreasing severe exacerbations in both subgroups at 6 months.89 However, the treatment effect size for asthma exacerbations and FEV1 was larger in eosinophil high patients compared with eosinophil low patients.89 A phase 3 follow-up trial confirmed these findings, with large effect sizes seen for reducing asthma exacerbations and improving FEV1 measurements in patients with blood eosinophil counts of 300 cells per μL or higher, and gradually diminishing responses over the follow-up period (52 weeks) in patients with lower blood eosinophil cell counts.90 Specifically, asthma exacerbations decreased by 48% in all patients treated with dupilumab at 200 mg and 46% in those treated with 300 mg subcutaneously, and FEV1 increased by 140 mL (200 mg dose) and 130 mL (300 mg dose).90 In patients with blood eosinophil counts of 300 cells per μL or higher, the overall effect size was larger, with an exacerbation reduction of 66% and 67% (figure 2A) and FEV1 improvement of 210 mL and 240 mL at the 200 mg and 300 mg doses (figure 2B; table 1). Conversely, no reduction in asthma exacerbation or improvement in FEV1 was seen in patients with blood eosinophil counts of less than 150 cells per μL.90 As with mepolizumab and benralizumab, dupilumab has also been shown to enable patients on systemic glucocorticoids to decrease their corticosteroid dose, with a 70% reduction of oral corticosteroids in patients treated with dupilumab compared with a 42% decrease in patients treated with placebo.91 Unlike the steroid-sparing trials for mepolizumab and benralizumab,52,58 dupilumab did not require blood eosinophilia as an inclusion criteria. However, patients with blood eosinophil counts of 300 cells per μL or higher at baseline were over two times as likely to reduce their corticosteroid dose by 50% than those participants with lower eosinophil numbers. Although the differences in trial design and inclusion criteria (primarily related to eosinophil counts) make directly comparing the effects on corticosteroid reduction difficult, the overall decrease in oral prednisone by 70% for patients on dupilumab was similar to the decrease observed with benralizumab (75%).52,58,91
Adverse effects of IL-4RA inhibition
Dupilumab is relatively well tolerated and the most common adverse events are injection site reactions. In addition, treatment with dupilumab increased the frequency of a poorly characterised conjunctivitis (about 10%) in the atopic dermatitis studies, an effect not yet seen in the asthma trials.92 As with the anti-IL-5 monoclonal antibodies, there was a reported increase in herpes-related events, and IL-4RA inhibition also increased blood eosinophil counts after treatment, peaking at 1–2 months and typically falling back to baseline values by 3 months. The biological mechanism and clinical relevance of this increase remains unknown, but a few patients did develop eosinophil counts higher than 5000 cells per μL, and several cases of eosinophilic granulomatosis with polyangiitis have also been reported (table 2).93 Current recommendations are to exclude patients with blood eosinophil counts greater than 1500 cells per μL at baseline. Finally, similar to the anti-IL-5 medications, theoretical concerns exist regarding increased risk of parasitic infections and potential increases in obesity and metabolic dysfunction. There is a dose effect with dupilumab, and the higher 300 mg dose is associated with a higher frequency of adverse events. Thus, the lower 200 mg dupilumab dose is recommended for the majority of patients with moderate-to-severe asthma, and 300 mg is reserved for patients with systemic corticosteroid-dependent asthma or with comorbid conditions responsive to dupilumab, such as atopic dermatitis or nasal polyposis.
Comparing clinical efficacy and differing trial designs of phase 3 trials
Comparing clinical efficacy between medications requires a randomised blinded trial design directly testing the medications in a head-to-head analysis. To our knowledge, no such trial has been done and would probably require large numbers of patients. Furthermore, because study populations and analysis plans differed greatly between the type 2 biologic clinical trials, directly comparing the treatment effect sizes for each drug is difficult.94 For example, the threshold to discriminate eosinophilic from non-eosinophilic asthma differed substantially between the trials. The phase 3 mepolizumab asthma exacerbation trial required patients to have a blood eosinophil count of more than 150 cells per μL at the time of enrolment or 300 cells per μL in the past year,53 whereas the reslizumab trials required patients to show one blood eosinophil count of 400 cells per μL or higher over a 2–4 week screening period.54 Alternatively, the phase 3 benralizumab and dupilumab studies enrolled patients with eosinophilic and non-eosinophilic asthma, and these studies used a cutoff of 300 cells per μL or higher in the blood to discriminate eosinophilic from non-eosinophilic subgroups.57 The dupilumab trials did not exclude patients with eosinophil blood counts of less than 150 cells per μL, even though efficacy was only seen at concentrations of greater than 150 cells per μL. Furthermore, although all the trials used a similar primary outcome that measured clinical asthma exacerbations (defined as a treating physician electing to administer systemic corticosteroids for at least 3 days, or an emergency department visit, or hospitalisation for asthma), each of the trials included slight modifications to the inclusion criteria. Specifically, mepolizumab and benralizumab required a history of at least two asthma exacerbations requiring systemic corticosteroid treatment in the past year,53 whereas reslizumab and dupilumab required at least one exacerbation treated with systemic corticosteroids in the past year. The anti-IL-5 trials primarily enrolled patients with severe asthma on a high dose of inhaled corticosteroids, whereas dupilumab was tested in a slightly less severe population in patients on both medium and high doses of this treatment.90 These minor differences are important because restricting inclusion criteria to patients with more severe asthma improves study power to detect differences in clinical outcomes between the drug and placebo. The phase 2B and phase 3 dupilumab trials also excluded patients who were taking systemic corticosteroids before study enrolment.90 Not surprisingly, these protocol variations resulted in robust differences in the exacerbation rates in the placebo group. The highest placebo exacerbation rate occurred in the reslizumab trials at 1·8 clinical asthma exacerbations per year, followed by the mepolizumab studies at 1·7 clinical asthma exacerbations per year, the benralizumab studies at 1·3 clinical asthma exacerbations per year (in the eosinophil-high subgroup), and the lowest rate in the dupilumab studies at 1·1 clinical asthma exacerbations per year (in the eosinophil-high subgroup).53,54,57,90 These relatively large differences in placebo exacerbation rates amplify the complexity in comparing clinical efficacy across the type 2 biologic agents.
Acknowledging these limitations, a reasonable interpretation of the data is that any clinical differences between these biologic agents are likely to be small. For example, the effect sizes for exacerbation reduction and improvements in FEV1 are related to starting eosinophil numbers, with a greater reduction in asthma exacerbations in the dupilumab trials (approximately 60%) than reductions of 40–50% in anti-IL-5 and anti-IL-5RA studies in patients with eosinophil counts of 300 cells per μL or more (figure 2A). Despite these differences, the confidence interval for the reduction in asthma exacerbations overlaps in all phase 3 type 2 biologic trials. Improvements in FEV1 are comparably higher in the dupilumab studies in patients with eosinophilic asthma, with a similar overlap in confidence intervals. Specifically, in patients with eosinophil counts of 300 cells per μL or higher, dupilumab improved FEV1 by 210–260 mL compared with 98–159 mL in the IL-5 inhibitor trials, which is an overall difference of about 100 mL (figure 2B).53,54,56,57,90
Fevipiprant
Although it is not a monoclonal antibody, fevipiprant is an oral medication that blocks the binding of prostaglandin D2 to its receptor, the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2 or PTGDR2). As the name implies, this receptor is commonly expressed on Th2 cells, and would therefore be expected to work in patients with type 2-high asthma. However, clinical trials of fevipiprant have shown mixed results, with the most consistent finding showing a small improvement in FEV1 measurements when compared with placebo.95,96 This response was similar to the effect size seen with montelukast, a leukotriene receptor antagonist.95 As an oral medication fevipiprant is relatively easy to administer, but additional data are needed to assess the added benefit of fevipiprant over other available asthma medications.
Biomarkers of treatment response
Blood eosinophil counts are a predictor of response for each of the type 2 biologic agents.54,90,97,98 Patients with blood eosinophil counts of 300 cells per μL or higher have approximately a 50% reduction in asthma exacerbation when treated with either anti-IL-5 or anti-IL-4RA therapies, whereas the clinical benefit in patients with blood eosinophil counts of less than 300 cells per μL is considerably reduced.90,98 However, blood eosinophilia does not uniformly predict treatment response, and not all patients with elevations in type 2 biomarkers respond to these biologic agents. For example, the benralizumab and dupilumab trials enrolled patients with eosinophilic asthma and non-eosinophilic asthma, and both studies showed decreased asthma exacerbation in patients with blood eosinophil counts between 0 cells per μL and 300 cells per μL.56,57,90 In the benralizumab studies, inconsistent improvements were seen in those patients with blood eosinophil counts of less than 300 cells per μL across the two pivotal trials, and the patients with blood eosinophil counts below this threshold were not subdivided further. By contrast, clinically significant responses to IL-4RA antibodies were consistently observed in patients with blood eosinophil counts between 150 cells per μL and 300 cells per μL (but not with <150 cells per μL). These responses were greater in patients who also had elevated concentrations of FeNO (>24 parts per billion; ppb), although dupilumab did not benefit patients with FeNO measurements of less than 25 ppb and eosinophil counts of less than 150 cells per μL.90 Thus, considering both FeNO values and blood eosinophil cell counts could conceivably improve the ability to predict response patterns.
Improved targeting of type 2 biologics will probably require additional biomarkers beyond those currently available. For example, eosinophils are key regulators of glucose homoeostasis23 and changes in nutrition or intermittent fasting can change blood eosinophil counts.99-101 Blood eosinophil counts are poor biomarkers for type 2 airway inflammation in patients with obesity, suggesting that these patients might benefit from IL-5 inhibition even when blood eosinophil counts remain low.25,102 Finally, although blood eosinophilia is an effective predictive biomarker for treatment response (before starting treatment), it is inadequate as a monitoring biomarker to distinguish medication responders from non-responders after starting treatment. As such, there are no biomarker-based rules to identify and stop these medications in patients with a low likelihood of benefitting after treatment has been started.
Indications for treatment
The American Thoracic Society and the European Respiratory Society’s definition of severe asthma is the presence of poor asthma control despite maximal treatment with high doses of inhaled corticosteroids and one additional controller medication.42,103 The relatively small percentage of patients with severe asthma (5–10%) contributes to the majority of asthma-associated healthcare costs.104 The cost of health care for each patient helps to justify the high expense of these new biologic treatments. Furthermore, use of blood eosinophil cell counts and FeNO values as biomarkers has initiated a more precision medicine-based approach to asthma treatment. Namely, asthma control and asthma exacerbations are likely to improve in patients with frequent asthma exacerbations and high blood eosinophil cell counts after starting on a type 2 biologic therapy. Despite this biomarker driven approach, the reduced cost to health care by preventing a single asthma exacerbation needs to be weighed against the current market value of the type 2 biologic.105 Thus, more precise biomarkers are needed before more cost-effective therapeutics become available.
New therapies for severe non-eosinophilic or type 2-low asthma
Treatment options for patients with type 2-low severe asthma remain limited, and aggressive efforts to identify non-type 2 treatment options remain scarce. Multiple cytokines with roles that overlap with the prototypical type 2 cytokines have been tested in asthma with mixed results. An early phase 2B trial with an inhibitor of the epithelial cell-derived cytokine thymic stromal lymphopoietin (TSLP) have been positive, with tezepelumab decreasing asthma exacerbation by 60–70%.106 By contrast, inhibitors of IL-33, a member of the IL-1 cytokine family that has a role in promoting type 2 innate lymphoid cell activation, showed some clinical efficacy but was inferior in a head-to-head analysis with dupilumab.107 Thus, tezepelumab and IL-33 inhibitors might prove efficacious in broader patient populations that include people with type 2-high and type 2-low asthma.
The prominence of old age (>50 years old)25 and obesity are among the phenotypic features of severe asthma108,109 and raise the possibility that the systemic inflammation associated with ageing, obesity, and metabolic dysfunction could have effects on the airway to worsen asthma. Recent work supports this hypothesis and patients with asthma with elevated IL-6 concentrations in the plasma (IL-6-high asthma) show lower lung function and increased asthma exacerbation than patients with low amounts of IL-6 (IL-6-low asthma).110 Targeting this systemic IL-6 inflammation has shown efficacy in the treatment of cardiovascular diseases111 and a similar benefit might plausibly exist in patients with IL-6-high asthma.
Another interesting molecular endotype is the observation that many patients with asthma have impairments in the resolution of inflammation. Traditionally, asthma has been described as a disease of chronic airway inflammation,2 but little attention has been dedicated to understanding how different types of inflammation (type 2 and others) are restored back to homoeostatic concentrations. Recent work has shown that in severe asthma these mechanisms of inflammation resolution might be impaired112-114 and treatments that restore inflammation resolution pathways to homoeostatic concentrations might be beneficial.
Finally, microbial imbalances (dysbiosis) of the asthmatic airway have been implicated as a possible mechanism of disease in some patients. Initial trials testing the use of antibiotics (specifically macrolide antibiotics) for the treatment of asthma were ineffective,115,116 but the AMAZES trial117 showed that azithromycin reduced asthma exacerbations in adult patients with both eosinophilic and non-eosinophilic asthma. These findings raise the possibility that some patients could benefit from antibiotic treatment.117,118 Unfortunately, no biomarker exists to identify responders from non-responders in terms of antibiotics treatment and alterations in microbial dysbiosis is unlikely to be specific for the type 2 pathway. Therefore, considerable debate remains regarding the appropriate approach for the use of antibiotics in asthma.119
Future directions and remaining controversies
Although the results of the clinical trials do not provide evidence that inhibition of IL-5 is superior or inferior to inhibiting IL-4RA, there are clues that heterogeneity in type 2 inflammatory-immune processes might eventually define pathobiological subgroups of patients with type 2 asthma who respond better to inhibition of one pathway versus the other. For example, airway eosinophilia can be induced by activation of Th2 cells or type 2 innate lymphoid cells. Type 2 innate lymphoid cells generate considerably more IL-5 and IL-13 than IL-4, whereas Th2-driven processes are likely to have elevations in IL-4, IL-5, and IL-13 (figure 1). Thus, if the type 2 subtype is related to type 2 innate lymphoid cells, then inhibiting IL-5 alone could be sufficient to improve disease outcomes. Conversely, in Th2-driven processes (as in allergic asthma phenotypes), inhibiting IL-4 and IL-13 could be more important for improving disease outcomes.120 These differences might explain some of the observed differences in response patterns to IL-4RA versus IL-5 pathway-targeted therapies. For example, IL-4RA targeted therapies (and IL-13) inhibit the late asthmatic response (bronchoconstriction that recurs 3–4 h after the initial allergen challenge), whereas mepolizumab, an anti-IL-5 monoclonal antibody, was found to be ineffective against this response despite a large reduction in blood eosinophils.11,87,121 By contrast, post-hoc analyses of anti-IL-5 trials have suggested that despite similar starting eosinophil counts, both reslizumab and benralizumab are more effective in patients whose asthma developed in adulthood (>40 years for reslizumab and >18 years for benralizumab) or in those individuals with nasal polyps, which are subgroups of asthma that show lower blood IgE concentrations.97,122-124 In addition, although traditionally viewed as a granulocyte with minimal immunological activity, eosinophils and eosinophil-derived proteins, such as Charcot-Leyden crystals, could possibly initiate or amplify an airway type 2 immune response and have a pivotal role in airway mucus formation in some patients.48,51 Thus, in patients with asthma where eosinophils are the key orchestrators of the type 2 immune response, or function as key propagators of airway mucus plugging, IL-5 inhibition might be superior to IL-4RA inhibition.51 Targeted and mechanistic comparison studies could help to distinguish these potential pathobiological differences.
Intriguingly, anti-IL-5 agents have been disappointing as treatments for eosinophilic oesophagitis and atopic dermatitis (NCT03055195),125 and two small studies have shown some efficacy for nasal polyposis.126,127 Conversely, the blockade of IL-4RA signalling is a highly effective therapy for atopic dermatitis,128 is FDA-approved for treating nasal polyposis (dupilumab),129,130 and IL-4RA has also shown promise as a therapeutic target for eosinophilic oesophagitis.131 These studies all support immunological differences among these diseases (or subgroups), all of which are considered to have type 2 inflammatory processes that might explain the differences in clinical response patterns. Thus, although the overall efficacy is similar, the different biological or clinical characteristics of these diseases might eventually be used to better identify the most appropriate treatment for patients from the two drug classes (IL-5 and IL-4RA inhibitors). However, better biomarkers are required to match patients to the most effective treatment.
The fundamental goal of asthma research is to find a cure. Multiple biological defects are likely to contribute to the initiation and maintenance of abnormal increases in airway type 2 inflammation. Therefore, developing an asthma cure will require a deeper understanding of how airway type 2 inflammation develops and persists in airway tissue. For example, work investigating airway sputum gene expression has shown that categorising patients into type 2-high and type 2-low asthma is too simplistic.22 Some patients show uniform and robust elevations in multiple airway type 2 gene expression networks compared with other asthmatics. These increases occur despite treatment with systemic or inhaled corticosteroids and these so-called type 2 ultra-high patients show unique clinical features such as older age (>50 years old at time of study), reduced lung function, and elevations in airway genes specific for CD11b and IRF4 double-positive type 2 dendritic cells.22,25,132,133 These immunological findings suggest that the immune senescence that occurs during ageing could explain the increase in asthma severity seen in older patients.108,134 Multiple other biological pathways probably have similar roles and a better understanding is needed for how type 2 inflammation develops in lung tissue.4
Although these new type 2 biologic agents have fundamentally changed the lives of many patients with severe asthma, questions remain regarding key clinical issues for patient care. What is the long-term safety of these medications? Are these medications disease modifying so that patients could eventually be taken off these medications? Do certain subgroups of patients respond preferentially to the different type 2 biologic agents? Will similar efficacy be observed in children? Will guidelines for their use evolve? Answers to these questions require a continued focus on identifying and understanding the molecular mechanisms that contribute to the pathogenesis of human asthma. Furthermore, the growing list of type 2 therapies will require the development of new and improved biomarkers to direct patients to medications with the highest likelihood of success.4
Conclusion
The emergence of type 2 biologics for the treatment of severe asthma is a welcomed and much needed advance in the management of patients with asthma. Although a cure for asthma remains elusive, many patients with severe asthma show a robust and sustained response to this new class of medication. Critical needs remain regarding better biomarkers to identify patients that are most likely to respond to these drugs and a deeper understanding for how airway type 2 inflammation develops in airway tissue. Few treatment options exist for patients with type 2-low asthma and developing new medications for this patient subgroup is essential.
Search strategy and selection criteria.
We evaluated the biological target and clinical efficacy of type 2 monoclonal antibodies in asthma. References for this Review were identified through searches of PubMed for articles published between Jan 1, 1950, and Oct 31, 2019 (last searched Nov 7, 2019). The search terms “Asthma/drug therapy” [MeSH], “Antibodies, monoclonal/therapeutic use” [MeSH], “Clinical Trial” [publication type], “Eosinophilia/drug therapy” [MeSH], “Asthma/immunology” [MeSH], “Th2 Cells/immunology” [MeSH], and “asthma and type-2 inflammation” [MeSH] were used and applied no language restrictions. A total of 577 items were found.
Acknowledgments
This study was funded by the National Institutes of Health Grants: (K23 HL138303, P30 DK098722, U10 HL109152, AI106684-01A1, HL109152-05, 5UG1HL139098-02, GM114311-01A1).
Footnotes
Declaration of interests
MCP reports personal fees from Merck, and grants from AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline (GSK), Sanofi Genzyme-Regeneron, and TEVA Pharmaceuticals Industries, outside the submitted work. SEW reports grants and personal fees from Sanofi, AstraZeneca, GSK, grants from Novartis, and personal fees from Pieris Pharmaceuticals. She also reports grants from AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi Genzyme-Regeneron, and Teva Pharmaceuticals Industries, outside the submitted work.
Contributor Information
Michael C Peters, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of California San Francisco, San Francisco, CA, USA.
Sally E Wenzel, Department of Environmental and Occupational Health, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012; 12: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther 2013; 26: 710–15. [DOI] [PubMed] [Google Scholar]
- 3.Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–79. [DOI] [PubMed] [Google Scholar]
- 4.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008; 372: 1107–19. [DOI] [PubMed] [Google Scholar]
- 6.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan SP, Koskinen A, Foster PS. Interleukin-5 and eosinophils induce airway damage and bronchial hyperreactivity during allergic airway inflammation in BALB/c mice. Immunol Cell Biol 1997; 75: 284–88. [DOI] [PubMed] [Google Scholar]
- 8.Grünig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998; 282: 2261–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol 1995; 12: 25–59. [DOI] [PubMed] [Google Scholar]
- 10.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992; 326: 298–304. [DOI] [PubMed] [Google Scholar]
- 11.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness, and the late asthmatic response. Lancet 2000; 356: 2144–48. [DOI] [PubMed] [Google Scholar]
- 12.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 2007; 176: 1062–71. [DOI] [PubMed] [Google Scholar]
- 13.Corren J, Busse W, Meltzer EO, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med 2010; 181: 788–96. [DOI] [PubMed] [Google Scholar]
- 14.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007; 62: 1043–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999; 160: 1001–08. [DOI] [PubMed] [Google Scholar]
- 17.Kotsimbos TC, Ernst P, Hamid QA. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc Assoc Am Physicians 1996; 108: 368–73. [PubMed] [Google Scholar]
- 18.Bodey KJ, Semper AE, Redington AE, et al. Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy 1999; 54: 1083–93. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007; 104: 15858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert M, Durham SR, Ying S, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med 1996; 154: 1497–504. [DOI] [PubMed] [Google Scholar]
- 21.Gordon ED, Simpson LJ, Rios CL, et al. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci USA 2016; 113: 8765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters MC, Ringel L, Dyjack N, et al. A transcriptomic method to determine airway immune dysfunction in T2-high and T2-low asthma. Am J Respir Crit Care Med 2019; 199: 465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502: 245–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang H-E, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol 2016; 9: 275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters MC, Kerr S, Dunican EM, et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol 2019; 143: 104–13.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol 2014; 133: 388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhakta NR, Solberg OD, Nguyen CP, et al. A qPCR-based metric of Th2 airway inflammation in asthma. Clin Transl Allergy 2013; 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med 2019; 380: 2009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath KW, Icitovic N, Boushey HA, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med 2012; 185: 612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–59. [DOI] [PubMed] [Google Scholar]
- 31.Corren J, Lemanske RF Jr, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011; 365: 1088–98. [DOI] [PubMed] [Google Scholar]
- 32.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet 1999; 353: 2213–14. [DOI] [PubMed] [Google Scholar]
- 33.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009; 360: 973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009; 360: 985–93. [DOI] [PubMed] [Google Scholar]
- 35.Thomson NC, Chaudhuri R. Omalizumab: clinical use for the management of asthma. Clin Med Insights Circ Respir Pulm Med 2012; 6: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C-H, Cheng S-L. A review of omalizumab for the management of severe asthma. Drug Des Devel Ther 2016; 10: 2369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–11. [DOI] [PubMed] [Google Scholar]
- 38.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014; 1: CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154: 573–82. [DOI] [PubMed] [Google Scholar]
- 40.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011; 364: 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18: 254–61. [DOI] [PubMed] [Google Scholar]
- 42.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–73. [DOI] [PubMed] [Google Scholar]
- 43.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society Guideline. Eur Respir J 2019; published online September 26. DOI: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 44.Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy 2018; 73: 490–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet 1958; 2: 1245–47. [DOI] [PubMed] [Google Scholar]
- 46.McFadden ER Jr. A century of asthma. Am J Respir Crit Care Med 2004; 170: 215–21. [DOI] [PubMed] [Google Scholar]
- 47.Frigas E, Motojima S, Gleich GJ. The eosinophilic injury to the mucosa of the airways in the pathogenesis of bronchial asthma. Eur Respir J 1991; 13: 123–35. [PubMed] [Google Scholar]
- 48.Persson EK, Verstraete K, Heyndrickx I, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 2019; 364: eaaw4295. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol 2003; 112: 653–65. [DOI] [PubMed] [Google Scholar]
- 50.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 2003; 112: 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest 2018; 128: 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–97. [DOI] [PubMed] [Google Scholar]
- 53.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–207. [DOI] [PubMed] [Google Scholar]
- 54.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–66. [DOI] [PubMed] [Google Scholar]
- 55.Ghazi A, Trikha A, Calhoun WJ. Benralizumab--a humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity–a novel approach for the treatment of asthma. Expert Opin Biol Ther 2012; 12: 113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016: 388: 2128–41. [DOI] [PubMed] [Google Scholar]
- 57.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–27. [DOI] [PubMed] [Google Scholar]
- 58.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376: 2448–58. [DOI] [PubMed] [Google Scholar]
- 59.Busse WW, Bleecker ER, FitzGerald JM, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med 2019; 7: 46–59. [DOI] [PubMed] [Google Scholar]
- 60.Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2019; 143: 1742–51.e7. [DOI] [PubMed] [Google Scholar]
- 61.Hom S, Pisano M. Reslizumab (Cinqair): an interleukin-5 antagonist for severe asthma of the eosinophilic phenotype. P T 2017; 42: 564–68. [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HL, Leigh R, Becker A. Omalizumab: practical considerations regarding the risk of anaphylaxis. Allergy Asthma Clin Immunol 2010; 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.No authors listed. Benralizumab (Fasenra) for severe eosinophilic asthma. JAMA 2018; 319: 1501–02. [DOI] [PubMed] [Google Scholar]
- 64.Huang L, Appleton JA. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol 2016; 32: 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Inverse association of eosinophil count with colorectal cancer incidence: atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev 2011; 20: 1861–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol 2015; 28: 403–13. [DOI] [PubMed] [Google Scholar]
- 67.Molofsky AB, Nussbaum JC, Liang H-E, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013; 210: 535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu D, Molofsky AB, Liang H-E, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332: 243–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol 2018; 141: 1529–32.e8. [DOI] [PubMed] [Google Scholar]
- 70.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132: 1086–96.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev 2010; 19: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vatrella A, Fabozzi I, Calabrese C, Maselli R, Pelaia G. Dupilumab: a novel treatment for asthma. J Asthma Allergy 2014; 7: 123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol 1998; 161: 3813–16. [PubMed] [Google Scholar]
- 74.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med 1994; 179: 1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 1996; 380: 630–33. [DOI] [PubMed] [Google Scholar]
- 76.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 2019; 50: 975–91. [DOI] [PubMed] [Google Scholar]
- 77.Zuyderduyn S, Ninaber DK, Schrumpf JA, et al. IL-4 and IL-13 exposure during mucociliary differentiation of bronchial epithelial cells increases antimicrobial activity and expression of antimicrobial peptides. Respir Res 2011; 12: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 1999; 162: 6233–37. [PubMed] [Google Scholar]
- 79.Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016; 4: 781–96. [DOI] [PubMed] [Google Scholar]
- 80.Korenblat P, Kerwin E, Leshchenko I, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med 2018; 134: 143–49. [DOI] [PubMed] [Google Scholar]
- 81.De Boever EH, Ashman C, Cahn AP, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol 2014; 133: 989–96. [DOI] [PubMed] [Google Scholar]
- 82.Brightling CE, Chanez P, Leigh R, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 2015; 3: 692–701. [DOI] [PubMed] [Google Scholar]
- 83.Busse WW, Brusselle GG, Korn S, et al. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur Respir J 2019; 53: 1800948. [DOI] [PubMed] [Google Scholar]
- 84.Panettieri RA Jr, Sjöbring U, Péterffy A, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med 2018; 6: 511–25. [DOI] [PubMed] [Google Scholar]
- 85.Russell RJ, Chachi L, FitzGerald JM, et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med 2018; 6: 499–510. [DOI] [PubMed] [Google Scholar]
- 86.Otulana BA, Wenzel SE, Ind PW, et al. A phase 2b study of inhaled pitrakinra, an IL-4/IL-13 antagonist, successfully identified responder subpopulations of patients with uncontrolled asthma. Am J Respir Crit Care Med 2019; 183: A6179 (abstr). [Google Scholar]
- 87.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 2007; 370: 1422–31. [DOI] [PubMed] [Google Scholar]
- 88.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368: 2455–66. [DOI] [PubMed] [Google Scholar]
- 89.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388: 31–44. [DOI] [PubMed] [Google Scholar]
- 90.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–96. [DOI] [PubMed] [Google Scholar]
- 91.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–85. [DOI] [PubMed] [Google Scholar]
- 92.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol 2019; 181: 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.No authors listed. Dupilumab (Dupixent) for asthma. JAMA 2019; 321: 1000–01. [DOI] [PubMed] [Google Scholar]
- 94.Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol 2019; 143: 190–200.e20. [DOI] [PubMed] [Google Scholar]
- 95.Bateman ED, Guerreros AG, Brockhaus F, et al. Fevipiprant, an oral prostaglandin DP2 receptor (CRTh2) antagonist, in allergic asthma uncontrolled on low-dose inhaled corticosteroids. Eur Respir J 2017; 50: 1700670. [DOI] [PubMed] [Google Scholar]
- 96.Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2016; 4: 699–707. [DOI] [PubMed] [Google Scholar]
- 97.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J 2018; 52: 1800936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016; 4: 549–56. [DOI] [PubMed] [Google Scholar]
- 99.Acland JD, Gould AH. Normal variation in the count of circulating eosinophils in man. J Physiol 1956; 133: 456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh RK, Chandra R, Narang RK, et al. Circadian variations of the absolute eosinophil count and serum histaminase activity in tropical pulmonary eosinophilia. Trop Geogr Med 1987; 39: 49–52. [PubMed] [Google Scholar]
- 101.Halberg F, Cornélissen G, Katinas G, et al. Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms 2003; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lugogo N, Green CL, Agada N, et al. Obesity’s effect on asthma extends to diagnostic criteria. J Allergy Clin Immunol 2018; 141: 1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Global Initiative for Asthma. Difficult-to-treat and severe asthma guide. 2019. https://ginasthma.org/difficult-to-treat-and-severe-asthma-guide/ (accessed Jan 3, 2020).
- 104.Lang DM. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc 2015; 36: 418–24. [DOI] [PubMed] [Google Scholar]
- 105.ICER. Biologic therapies for treatment of asthma associated with type 2 inflammation: effectiveness, value, and value-based price benchmarks. 2018. https://icer-review.org/wp-content/uploads/2018/04/Asthma-Final-Report_FOR-PUBLICATION.pdf (accessed Jan 3, 2020).
- 106.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–46. [DOI] [PubMed] [Google Scholar]
- 107.Market Watch. Sanofi and Regeneron announce positive topline phase 2 results for IL-33 antibody in asthma. 2019. https://www.marketwatch.com/press-release/sanofi-and-regeneron-announce-positive-topline-phase-2-results-for-il-33-antibody-in-asthma-2019-06-21 (accessed July 31, 2019).
- 108.Zein JG, Dweik RA, Comhair SA, et al. Asthma is more severe in older adults. PLoS One 2015; 10: e0133490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181: 315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peters MC, McGrath KW, Hawkins GA, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 2016; 4: 574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–31. [DOI] [PubMed] [Google Scholar]
- 112.Duvall MG, Barnig C, Cernadas M, et al. Natural killer cell-mediated inflammation resolution is disabled in severe asthma. Sci Immunol 2017; 2: eaam5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krishnamoorthy N, Douda DN, Brüggemann TR, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol 2018; 3: eaao4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ricklefs I, Barkas I, Duvall MG, et al. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight 2017; 2: 93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sutherland ER, King TS, Icitovic N, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol 2010; 126: 747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013; 68: 322–29. [DOI] [PubMed] [Google Scholar]
- 117.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659–68. [DOI] [PubMed] [Google Scholar]
- 118.Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA 2015; 314: 2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A. Antibiotics for exacerbations of asthma. Cochrane Database Syst Rev 2018; 6: CD002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mindt BC, Fritz JH, Duerr CU. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Front Immunol 2018; 9: 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gauvreau GM, Boulet L-P, Cockcroft DW, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med 2011; 183: 1007–14. [DOI] [PubMed] [Google Scholar]
- 122.Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther 2017; 43: 39–45. [DOI] [PubMed] [Google Scholar]
- 123.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119: 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004; 113: 101–08. [DOI] [PubMed] [Google Scholar]
- 125.Roufosse F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne) 2018; 5: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol 2017; 140: 1024–31.e14. [DOI] [PubMed] [Google Scholar]
- 127.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol 2011; 128: 989–95.e1–8. [DOI] [PubMed] [Google Scholar]
- 128.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–48. [DOI] [PubMed] [Google Scholar]
- 129.Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016; 315: 469–79. [DOI] [PubMed] [Google Scholar]
- 130.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394: 1638–50. [DOI] [PubMed] [Google Scholar]
- 131.Wechsler JB, Hirano I. Biological therapies for eosinophilic gastrointestinal diseases. J Allergy Clin Immunol 2018; 142: 24–31.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plantinga M, Guilliams M, Vanheerswynghels M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013; 38: 322–35. [DOI] [PubMed] [Google Scholar]
- 133.Williams JW, Tjota MY, Clay BS, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun 2013; 4: 2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teague WG, Phillips BR, Fahy JV, et al. Baseline Features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract 2018; 6: 545–54.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]