Abstract
Opossums in the clade Didelphini are well known to be resistant to snake venom due to endogenous circulating inhibitors which target metalloproteinases and phospholipases. However, the mechanisms through which these opossums cope with a variety of other damaging venom proteins are unknown. A protein involved in blood clotting (von Willebrand Factor) has been found to have undergone rapid adaptive evolution in venom-resistant opossums. This protein is a known target for a subset of snake venom C-type lectins (CTLs), which bind it and then induce it to bind platelets, causing hemostatic disruption. Several amino acid changes in vWF unique to these opossums could explain their resistance; however, experimental evidence that these changes disrupt venom CTL binding was lacking. We used platelet aggregation assays to quantify resistance to a venom-induced platelet response in two species of venom-resistant opossums (Didelphis virginiana, Didelphis aurita), and one venom-sensitive opossum (Monodelphis domestica). We found that all three species have lost nearly all their aggregation response to the venom CTLs tested. Using washed platelet assays we showed that this loss of aggregation response is not due to inhibitors in the plasma, but rather to the failure of either vWF or platelets (or both) to respond to venom CTLs. These results demonstrate the potential adaptive function of a trait previously shown to be evolving under positive selection. Surprisingly, these findings also expand the list of potentially venom tolerant species to include Monodelphis domestica and suggest that an ecological relationship between opossums and vipers may be a broader driver of adaptive evolution across South American marsupials than previously thought.
Keywords: Venom resistance, Opossums, vWF, Platelet aggregation, C-type lectins
1. Introduction
Naturalists have long known that certain opossum species (in Tribe Didelphini of the marsupial family Didelphidae, Jansa and Voss, 2011), are not only resistant to snake venom but also attack and eat pitvipers with impunity (Oliveira and Santori, 1999; Jared et al., 1998; Perez et al., 1978). This tribe includes the South American opossum genera Didelphis, Philander, Lutreolina, and Chironectes (Fig. 1). Although venom resistance has not been broadly surveyed across opossum species, observational (Vellard, 1945, Wood, 1954, Fitch, 1960, Perales and Moussatche, 1986, Melo and Suarez-Kurtz, 1988, Sazima, 1992, Jared et al., 1998, Oliveira and Santori, 1999, Almeida-Santos et al., 2000) and experimental (Kilmon, 1976; Werner and Vick, 1977; Werner and Faith, 1978; Moussatche et al., 1978, 1979; Perez et al., 1979; Menchaca and Perez, 1981; Soto et al., 1988; Moussatché and Perales, 1989; Catanese and Kress, 1993; Perales et al., 1994; Lovo-Farah et al., 1996) evidence suggest that species of Didelphis, Philander, and Lutreolina can survive envenomation by pitvipers (Jansa and Voss, 2011). Whether Chironectes - the fourth genus in this clade - is venom resistant remains unknown. Metachirus nudicaudatus, the sister taxon to Didelphini, has been shown to have little if any resistance to whole venom injections (Perales et al., 1994). Consequently, this species and many other smaller-bodied opossums (the remainder of family Didelphidae) are known to be eaten by pitvipers and are thus assumed to be susceptible to venom (Voss, 2013). Evidently, snake-venom resistance has arisen at least once among South American opossums (in clade Didelphini), possibly as a dietary adaptation allowing them to exploit dangerous prey otherwise unavailable to non-resistant predators.
Fig. 1.
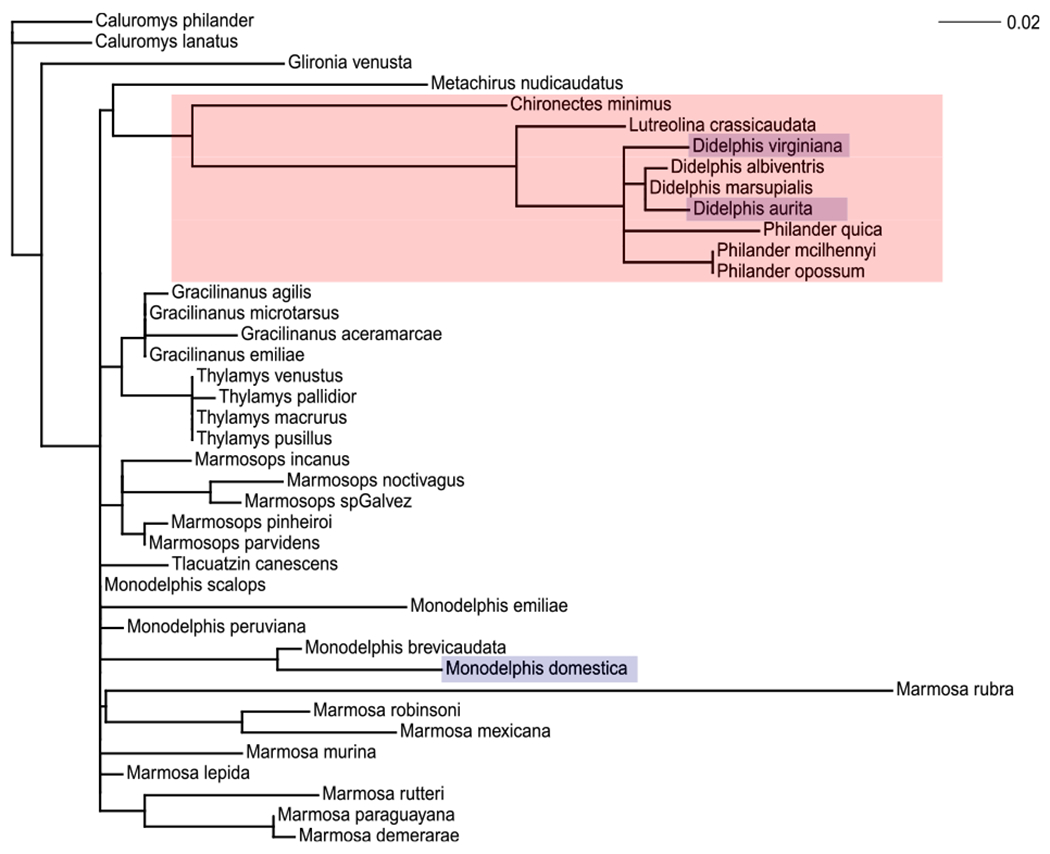
Topology of Didelphidae from Jansa and Voss (2011) with the addition of Monodelphis domestica according to Pavan et al. (2014). Branch lengths reprisent the estimated number of amino acid substitions in vWF reconstructed with the JTT model of amino acid substitution available in PAML (Yang, 2007). The clade Didelphini, known for both organismal venom resistance as well accelerated evolution of vWF, is shown in red (light grey in black and white prints). Species used for this work are highlighted in blue (dark grey in black and white prints). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
While significant work has revealed how these species cope with enzymatic-like (tissue destroying) venom components, little is known about how these species cope with non-enzymatic venom components (Kilmon, 1976; Werner and Vick, 1977; Werner and Faith, 1978; Moussatche et al., 1978, 1979; Perez et al., 1979; Menchaca and Perez, 1981; Soto et al., 1988; Moussatché and Perales, 1989; Catanese and Kress, 1993; Perales et al., 1994; Lovo-Farah et al., 1996). Non-enzymatic venom proteins are known to cause significant destructive physiological effects in susceptible species, thus adaptations which counter these effects are likely vital to a successful venom-resistant phenotype (Read et al., 1983; Qi et al., 1994; Barchan et al., 1995). An abundant and particularly destructive class of venom proteins in South American pitvipers are the C-type lectins (CTL) (Arlinghaus and Eble, 2012). A small subgroup of venom CTLs bind the mammalian blood protein von Willebrand Factor (vWF) to form a complex that binds, in turn, to the platelet-associated glycoprotein GP1bα, altering the capacity for normal blood clotting (Fukuda et al., 2005). This specific action enhances the effects of other hemorrhagic venom factors and is thought to be responsible for the devastating systemic bleeding characteristic of snake bites from several species of Bothrops vipers. A wide diversity of mammalian taxa have been shown to be susceptible to vWF-targeting CTLs, including primates, carnivores, rodents, lago-morphs, bovids, and perissodactyls, in both in vitro and in vivo assays (Sanders et al., 1995; Nichols et al., 2010). Similarly, in the presence of an altered vWF (von Willebrand’s disease; vWD) in pig and dog, the disruptive hemostatic function of vWF-binding CTLs is abolished (Sanders et al., 1995; Nichols et al., 2010). Known venom CTLs with this function include botrocetin (from Bothrops jararaca), aspercetin (from Bothrops asper), and biticetin (from Bitis arietans). Although surveys of vWF binding activity have identified several additional species of Bothrops with vWF-binding activity, no other venom CTLs have been isolated and described (Arlinghaus and Eble, 2012; Rucavado et al., 2001; Read et al., 1978).
While it is currently unknown how venom-resistant opossums cope with CTLs like botrocetin and aspercetin, recent research on the molecular evolution of vWF in marsupials has revealed that members of Didelphini show accelerated evolution at sites in the vWF A1 region that bind botrocetin (Jansa and Voss, 2011). Although signatures of positive selection suggest adaptive function and possible coevolution, explicit evidence that species with rapidly evolving vWF are physiologically resistant to vWF-targeting venom CTLs is lacking. Physiological resistance, in this case, is the failure of platelets to aggregate in the presence of venom CTLs, and can be measured by the quantity of platelets that remain suspended in solution when CTLs are introduced.
In this work, we test the hypothesis that members of Didelphini known to have a rapidly evolving vWF – especially those that are known to prey on pitvipers – possess physiological resistance to venom CTLs due to amino acid changes on vWF. We predict that opossums that eat pitvipers will show physiological resistance to CTLs in ex vivo blood assays that would otherwise (in humans) induce aggregation. To assess whether vWF-mediated resistance to venom CTLs is restricted to these large-bodied opossums, we also assay a smaller-bodied species (Monodelphis domestica) likely preyed upon by pitvipers and assumed to be susceptible to venom. We utilize a well-established ex vivo measure of vWF-mediated platelet aggregation to measure the aggregation response of opossum platelets to vWF-targeting venom CTLs. We perform these assays both in the presence and absence of plasma to exclude the influence of any unknown CTL-inhibiting plasma proteins, effectively narrowing the source of any physiological resistance seen to the three-way interaction between vWF, venom CTLs, and platelets. These assays are well correlated with in vivo aggregation response and represent a strong predictor of organismal coagulopathy (Read et al., 1983; Sanders et al., 1995; Nichols et al., 2010). Secondarily, we expect that if opossums that eat pitvipers are coevolving with their venomous prey that they will show physiological resistance to CTLs from the species they prey upon (Bothrops jararaca: botrocetin, Bothrops asper: aspercetin), but not to CTLs from viper species with which they do not share a current or historical range (e.g. the African viper Bitis arietans: bitiscetin). Utilizing these data, we provide the first evidence of physiological resistance to venom CTLs in opossums.
2. Materials and methods
2.1. Study design
We used two opossum species from the clade Didelphini hypothesized to be venom resistant (the Virginia opossum, Didelphis virginiana N = 3, and the big eared opossum Didelphis aurita N = 4), as well as one presumably non-resistant species outside Didelphini (the grey short-tailed opossum, Monodelphis domestica N = 2). Although a broader sampling of didelphid species would be welcome, these three were obtainable for whole-blood draws. Both Didelphis virginiana and Didelphis aurita were previously shown to have vWF that is evolving at an accelerated rate (Jansa and Voss, 2011). These species are also well known to exhibit organismal resistance to venom (Voss and Jansa, 2012). Monodelphis domestica is a readily available laboratory animal that belongs to a distantly related genus of opossum that is not part of the clade shown to have accelerated vWF evolution and is not expected to exhibit organismal venom resistance (Fig. 1).
We adapted a standard method of vWF-mediated platelet aggregation used extensively in biomedical studies of vWF function in humans (Rucavado et al., 2001; Hamako et al., 1996; Coller et al., 1975; Read et al., 1983). Platelet aggregation is measured by adding an agonist to Platelet Rich Plasma (PRP) and measuring the amount of light that passes through the solution over time as compared to a PPP standard. If the agonist induces platelet aggregation, PRP should become clear and transmit more light as the platelets precipitate; if the agonist is ineffective at inducing aggregation, the solution will remain cloudy. This test is performed in an aggregometer, a specialized spectrophotometer designed specifically for this measurement (Chrono-log Co.; model 560ca). Aggregometers were calibrated with ddH20 according to standard guidelines, accepting a baseline error of 5% (Chrono-log Co.).
To isolate the effect that plasma proteins might have on aggregation in PRP assays (Allain et al., 1975), we conducted an additional assay using washed platelets and purified vWF suspended in physiological buffer. This assay was performed only on Didelphis virginiana and human controls, as the platelet purification process - as well as the vWF purification process - (Allain et al., 1975, De Marco and Shapiro, 1981) both require repeated blood draws of large volumes, which were only feasible for the larger bodied D. virginiana housed in a research facility. A complete list of washed platelet tests performed can be found in Supplementary Table 8. Detailed methods for aggregation assays can be found in Supplementary Material.
2.2. Animal handling and blood collection
Three individuals of Didelphis virginiana were live-trapped in urban and suburban areas of Minneapolis, MN by licensed pest removal companies and transported in Tomahawk traps to the University of Minnesota Research Animal Resources facility (Minnesota Department of Natural Resources Permit #16312). Animal health was assessed upon arrival, and all animals were given a period of adjustment of 7–10 days before being used for blood draws. Blood draws were performed without anesthetization on the ventral tail vein (Moore, 1984). Blood was taken using sodium citrate coated syringes and stored in sodium citrate vacutainers. Multiple blood draws were conducted on each individual, with intervals of a minimum of two weeks between draws. After use in this study, animals were released near the site of capture.
Four individuals of Didelphis aurita were wild caught in baited Tomahawk traps in urban forest at the Instituto Butantan, São Paulo, Brazil (SISBIO permit #64934-1), anesthetized with 5% isoflurane by inhalation, and used for blood collection via tail vein puncture as previously described (Comitê de Etica em Experimentação Animal do Instituto Butantan protocol # 8,346,081,018) (Moore, 1984). Blood draws for D. aurita were not repeated, as all animals recovered within 30 min and were immediately released near the collection site. Two individuals of Monodelphis domestica were kindly gifted from the Oberlin College research colony by Dr. Yolanda Cruz and housed at the University of Minnesota according to protocols described in Rousmaniere et al. (2010). After a period of adjustment (3 weeks) individuals were anesthetized with 5% isoflurane and exsanguinated via cardiac puncture using a 3.2% sodium citrate coated syringe. All animals were handled in accordance with published guidelines and with supervision by veterinary staff at the University of Minnesota, or Instituto Butantan, Brazil (Sikes and Gannon, 2011; IACUC Protocol #1303–3046 A). Human samples used for controls and venom fraction isolation were obtained from healthy donors who had given informed consent. Volunteers did not consume alcohol, ibuprofen, aspirin, or any other drug which is known to disrupt coagulation within 24 h of donation. Human blood draws were performed by University of Minnesota nurses either in the Special Coagulation Laboratory or at a clinic, using standard 3.2% sodium citrate vacutainers for PRP assays or Acid Citrate Dextrose (solution A) vacutainers for vWF purification (BD Pharmaceuticals). Opossum and human vWF was purified from fresh blood and purified via size exclusion chromatography as previously described (De Marco and Shapiro, 1981). For detailed methods see Supplementary Materials.
2.3. DNA sequencing
The vWF A1 region was sequenced for all opossum individuals used in aggregation assays to assess variation among individuals within each species. DNA was extracted from whole blood using a QIAGEN DNeasy Blood and tissue kit. The vWF A1 region was amplified with PHIRE polymerase at 90 °C for 30 s, followed by 35 cycles of 98 °C for 5 s, 65 °C for 5 s, and 72 °C for 15 s using primers DvWF F1 5′-TCACTGTGATGGTGTGAACTT-3’ and DvWF R6 5′- GTCTGAGCCTTCTAGCACAAA-3′ designed from a Monodelphis domestica genome scaffold. Samples were sequenced at the University of Minnesota Genomics Center. Chromatograms were assembled and verified in GENEIOUS version 7.1.8 and MUSCLE version 3.5 (Edgar, 2004) was used to create alignments. Sequences have been deposited in GenBank (MN384757–59, MN18655-60).
2.4. Platelet agonists
Though each known venom CTL maintains similar function, their biochemical profiles differ substantially, thus different purification protocols for each known venom CTL have been previously optimized and were used for this study (Rucavado et al., 2001; Hamako et al., 1996, Sekiya et al., 1993). Aspercetin was gifted by Dr. Alexandra Rucavado, Universidad de Costa Rica, having been purified as previously described (Rucavado et al., 2001). Crude Bitis arietans venom was donated by Kristen Wiley at the Kentucky Reptile Zoo. Biticetin was purified first with salt precipitation as described previously (Hamako et al., 1996), then eluted off both strong (SP-FF), and weak (DEAE-Sepharose-FF) FPLC cation exchange columns (GE Life Sciences). Fraction isolation was performed as previously described (Hamako et al., 1996).
To obtain the volume needed to test multiple replicates in several species, we used two sources of botrocetin. The first source (here designated “botrocetin A″) was provided by Dr. Miguel Cruz (Baylor College of Medicine) and Dr. Robert Andrews (Monash University). This sample was among several previously used to assess botrocetin activity and function (e.g. Dong et al., 2001). We also purified botrocetin from pooled Bothrops jararaca venom following the protocol described by Sekiya et al. (1993), with several modifications (see Supplementary Material for detailed methods).
Ristocetin (Chronolog Co.), an antibiotic derived from bacteria, is known to induce platelet aggregation via vWF in humans and is often used as a control for vWF and platelet activity. We attempted to use ristocetin as a positive control for both human and opossum assays, however opossum platelets in plasma exposed to ristocetin produced a chalky precipitate without displaying an aggregation response. Though previously unknown in opossums, a similar response to ristocetin is known for dogs and pigs (Nichols et al., 2010; Read et al., 1983). Consequently, ristocetin could not be used as a positive control for vWF-mediated aggregation in opossums. Instead, adenosine diphosphate (ADP) was used as an alternative control. Though ADP aggregates platelets, it does not do so via vWF, and so serves as a control of platelet activity only. Ristocetin and ADP were purchased (Chronolog Co.) and reconstituted as recommended by the manufacturer.
2.5. Analyses
To test for differences in PRP aggregation response as a function of agonist concentration, assays were grouped into low (botrocetin 2 μg/ml, aspercetin <50 μg/ml), and high (botrocetin >2 μg/ml, aspercetin >51 μg/ml) concentrations. Differences in PRP aggregation response between high and low concentrations for each venom protein were tested using Wilcoxon rank-sum tests. No significant differences in percent PRP aggregation were observed between low and high concentrations for any venom protein. Therefore, subsequent analyses did not distinguish between tests run at different concentrations (e.g. all D. virginiana botrocetin PRP tests were grouped and compared to all human botrocetin PRP test regardless of concentration; Supplementary Table 2).
To compare opossum PRP aggregation with aggregation for the same agonist in human PRP, we used the non-parametric Dunn joint-ranking method to test each species-agonist treatment against the same treatment in human PRP, incorporating a Bonferroni correction for each group by agonist, as well as globally (Table 1). Virtually no within-species variation in PRP response to any agonist was observed, except for Didelphis virginiana response to aspercetin. Because individuals of Didelphis virginiana were observed to vary in their PRP response to aspercetin, an additional test was done to determine if this variation in PRP aggregation response among individuals was statistically significant, using a standard Kruskal-Wallis rank sums test.
Table 1.
One-way analysis of percent aggregation (% transmittance) in PRP. Analysis is a non-parametric comparison of PRP mean percent aggregation using Dunn method for joint ranking with Human as the control for each group. Dunn methods use a Bonferroni adjustment within each test group. Between test groups (10 tests) Asterisk indicate significance at the Bonferroni adjusted ɑ-value 0.006.
| Aggregation in Platelet Rich Plasma | |||||
|---|---|---|---|---|---|
| Species | Control | Score Mean Difference | Std Err Dif | Z-score | P-Value |
| ADP | |||||
| Didelphis virginiana n = 7 | Human n = 10 | 6.8769 | 3.453393 | 1.99135 | 0.1393 |
| Didelphis aurita n = 4 | 3.425 | 4.857221 | 0.70514 | 1 | |
| Monodelphis domestica n = 1 | −16.3 | 8.610932 | −1.89294 | 0.1751 | |
| botrocetin | |||||
| Didelphis virginiana n = 13 | Human n = 14 | 19.772 | 4.126265 | 4.79174 | <.0001* |
| Didelphis aurita n = 12 | 21.9226 | 4.21447 | 5.20175 | <.0001* | |
| Monodelphis domestica n = 3 | −21.7976 | 6.815705 | −3.19815 | 0.0041* | |
| aspercetin | |||||
| Didelphis virginiana n = 11 | Human n = 11 | 15.39583 | 4.32599 | 3.558915 | 0.0007* |
| Didelphis aurita n = 3 | 24.25 | 6.701795 | 3.618433 | 0.0006* | |
| bitiscetin | |||||
| Didelphis virginiana n = 12 | Human n = 2 | 12.35119 | 3.553028 | 3.476244 | 0.0005* |
| ristocetin | |||||
| Didelphis virginiana n = 4 | Human n = 5 | 7.866667 | 2.453116 | 3.206806 | 0.0013* |
Monodelphis domestica PRP agglutination response to aspercetin from a sample which failed ADP control was compared against human PRP and Didelphis virginiana PRP exposed to the same venom protein (aspercetin). A Tukey-Kramer HSD test for pairwise comparisons was used to evaluate pairwise differences between human, Monodelphis domestica, and Didelphis virginiana PRP aggregation response.
Differences in aggregation between vWF source (PPP vs purified vWF) in washed platelet (WP) assays were assessed using a one-way Wilcoxon test, and were grouped where no significant differences were found. The same test for dose-dependence used in PRP assays was also used to test for dose-dependent response in WP assays. No significant differences in percent aggregation were observed between low and high concentrations for any venom protein in WP assays; consequently, subsequent analyses did not distinguish between tests run at different concentrations (e.g. all D. virginiana botrocetin WP tests were grouped and compared to all D. virginiana PRP tests regardless of concentration) (Supplementary Table 5).
Average values of percent aggregation for all WP assays were compared to the same treatment (ADP, or venom protein) in PRP, using one-way Wilcoxon tests, to assess whether the removal of plasma significantly altered aggregation results.
3. Results
3.1. Platelet rich plasma assays
Comparison of human and opossum platelet aggregation tests were consistent with elevated physiological resistance to snake venom CTL activity in opossums. Results for human platelet aggregation (by percent aggregation) in response to different agonists are consistent with previous work (Fig. 2A) (Rucavado et al., 2001; Hamako et al., 1996; Sekiya et al., 1993) for all treatments. By contrast, all three opossum species showed a significant reduction or loss in aggregation response for all venom proteins tested (Table 1, Fig. 2A), but aggregated normally in the presence of ADP. Didelphis virginiana PRP did not aggregate in response to botrocetin or biticetin. Aspercetin caused Didelphis virginiana platelets to aggregate partially, although significantly less than observed in human samples (Fig. 2A, Table 1); there was no significant variation in response to aspercetin among individuals of Didelphis virginiana, and all individuals showed at least partial aggregation at the highest concentration of aspercetin (100 μg/ml) (Supplementary Tables 1 and 2). Didelphis aurita showed no aggregation response to either botrocetin or aspercetin but was not tested for bitiscetin due to limited blood volume available (Fig. 2A). Two individuals of Monodelphis domestica were sampled; however, one of the samples failed to aggregate in the presence of ADP and was thus excluded from analysis. The remaining Monodelphis domestica sample showed no aggregation response to botrocetin. Monodelphis domestica platelets from the sample that failed to aggregate with ADP did ultimately aggregate at an aspercetin concentration of 100 μg/ml (Supplementary Figure 1).
Fig. 2.
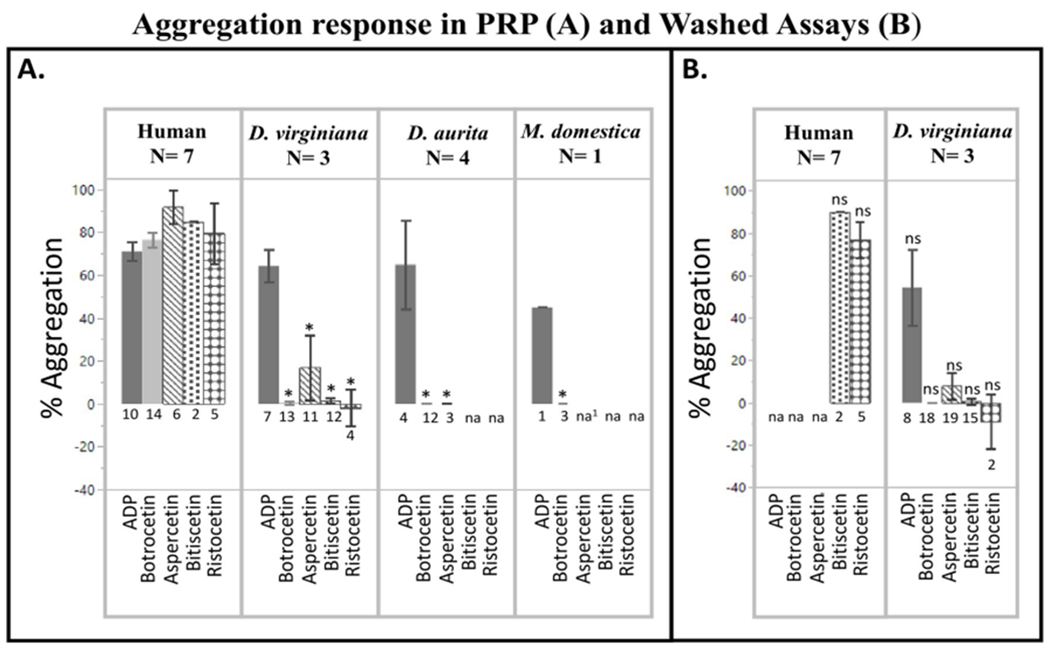
Mean percent platelet aggregation calculated as percent light transmittance (error bars 95% confidence intervals). N below species names in top panel indicates number of individual animals, numbers below each bar graph indicate total number of individual tests done for each agonist. NA indicates this test was not performed. na1 this test was performed with a sample which failed ADP control and is reported in supplementary material (Supplementary Fig. 1) Panel A are tests performed in platelet rich plasma (PRP); asterisks indicate values that are significantly different from the same PRP test in human samples. Panel B shows results from tests using washed platelets and purified vWF or 10% v/v PPP as a vWF source; ns indicates no significant difference from the same test performed with PRP (see in Panel A for comparison). ADP serves as a platelet activity control for all tests. Bitiscetin and ristocetin serve as vWF activity controls for washed assays.
3.2. Washed platelet assays
Removing plasma proteins from assays did not alter aggregation responses significantly. Hence, venom CTL resistance is not due to inhibitory plasma proteins, but rather to changes in vWF, the platelet, or both. Washed platelet assays showed similar results to those using PRP: Didelphis virginiana showed a significant reduction in aggregation response compared to human for to all venom proteins tested, but platelets aggregated normally in the presence of ADP (Fig. 2B). There were no significant differences between aggregation values from the washed platelet assay and those from the PRP assay for either opossum or human tests (Fig. 2B, Supplementary Table 4). Bitiscetin and ristocetin treatments in human assays demonstrated that platelets and vWF remained active after being purified; similarly, ADP controls showed no reduction in the ability of opossum washed platelets to aggregate (Fig. 2B, Supplementary Table 4). Opossum samples treated with ristocetin showed increases in opaqueness due to formation of a chalky precipitate but did not show platelet aggregation. As with the PRP assays, no significant differences were seen among tests performed at increasing agonist concentration (Supplementary Table 5). The source of vWF (PPP vs pure vWF) yielded no difference in response for botrocetin or bitiscetin but did for aspercetin (p = 0.021) (Supplementary Table 6). When platelet poor plasma (as opposed to purified vWF) was used as a source of vWF, aspercetin aggregation increased by an average of 7.5%, suggesting some degradation of vWF during purification, or that a low concentration impurity such as a venom serine proteinase is enhancing aggregation via a target in the plasma (e.g. fibrinogen). However, an increase of 7.5% is small (only 2.5% above machine calibration error of 5%), and thus may not reflect a biologically relevant increase.
3.3. DNA sequencing
All DNA sequences were invariant within species at the vWF A1 region known to be responsible for venom CTL binding (residues 475–710 NCBI accession AAB39987.1). Didelphis virginiana sequences from this region were identical to Genbank accession JN415020, and Monodelphis domestica sequences were identical to Genbank sample accession NM_001246274. Didelphis aurita vWF was not previously available on Genbank.
4. Discussion
We provide the first physiological evidence that the damaging hemostatic effects of snake venom CTLs are disrupted in opossums, which may contribute to whole-organism resistance to pitviper envenomation. These results remain unaltered in the absence of plasma proteins and therefore isolate the source of altered function to CTL-vWF-platelet interactions. We challenged platelets from three species of opossums using vWF-binding venom CTLs from snakes known to currently or historically co-occur with those species (botrocetin and aspercetin from species of the South American pitvpers Bothrops jararaca and Bothrops asper, respectively), and one which is found on a different continent (bitiscetin from the African viper Bitis arietans). We confirm that human platelets aggregate as expected in the presence of all three venom CTLs and show that platelets from all examined opossum species (D. virginiana, D. aurita, and M. domestica) fail to show the same response (Fig. 2A). Thus, contrary to our prediction that only opossums in Didelphini (with rapidly evolving vWF) will exhibit resistance to venom CTLs, all opossums tested to date appear to enjoy protection from CTL-mediated platelet aggregation. Furthermore, this protection appears to hold regardless of the geographic origin of the toxins tested.
4.1. Physiological function within Didelphini
Didelphis aurita inhabits a geographic range which overlaps with that of Bothrops jararaca, as well as several other species of Bothrops which have been shown to have vWF-mediated platelet aggregating activity (Read et al., 1978; McManus, 1974, Cáceres and Monteiro-Filho, 2001). Though Didelphis virginiana does not overlap geographically with Bothrops jararaca, it does share the southern portion of its range with Bothrops asper and likely shared a historical range with ancestral Bothrops species before its migration to North America ∼3 mya (McManus, 1974; Voss and Jansa, 2012). These observations, along with behavioral and experimental data which show venom resistance, predation on venomous snakes, and venom targets (vWF) evolving under positive selection, lend credence to the assertion that these species are coevolving with venomous snakes (Wood, 1954; Fitch, 1960; Menchaca and Perez, 1981; Moussatché and Perales, 1989; Jared et al., 1998; Jansa and Voss, 2011). Specifically, Jansa and Voss (2011) suggested that the detection of positive selection in didelphine vWF may be evidence of molecular changes that protect these opossums from the effects of vWF-targeting CTLs such as botrocetin. At first glance, the reduced aggregation response in these species to botrocetin and aspercetin shown in this study adds support to this assertion.
While lack of aggregation response to botrocetin and aspercetin was expected, the same result for bitiscetin was not. This is likely due to a large degree of overlap in botrocetin and bitiscetin binding sites. Of 15 bitiscetin binding sites, 13 are within the botrocetin binding pocket, 5 directly overlap with botrocetin binding sites, and another 5 are directly adjacent to a botrocetin binding site (Maita et al., 2003). Given this overlap, it is not surprising that we observed similar responses to these two agonists. Because well-resolved gene trees for venom CTLs are currently lacking, we cannot accurately interpret this as evidence against coevolution within South American species, as loss of response to Bitiscetin may be simply a result reflecting shared ancestry (and therefore shared function) between venom CTLs, or parallel evolution of Bitiscetin function for mammalian antagonists within its range. As such, this result fails to inform us regarding the potential for coevolution between South American marsupials and South American vipers but does not exclude it as a possibility.
Didelphis virginiana showed partial, though greatly reduced, aggregation for aspercetin, while all other tests involving opossums showed a nearly complete lack of a platelet aggregation response with aspercetin. Partial resistance to aspercetin in D. virginiana suggests that this protective function may not be effective against all venom CTLs in all didelphines. Given that apparent resistance to botrocetin confers resistance to bitiscetin (a distantly related African CTL) it is thus surprising that a more closely related CTL (aspercetin) would not also fail to function in the same species. This result also suggests that aspercetin may be quite divergent from botrocetin particularly at vWF binding sites—and may be part of an adaptive radiation of venom proteins evolving to target rapidly evolving vWF in mammals. Complete resolution of the aspercetin sequence and structure is not available at this time, but further work elucidating this would help decipher its divergence in form and function from botrocetin.
The observation of inter-individual variation of D. virginiana aggregation for aspercetin (though not statistically significant), suggests some variability in ability to resist this venom protein. Because no polymorphism in the vWF A1 region was present between these individuals, this result suggests that any variation in resistance to aspercetin between individuals of Didelphis virginiana is not due to changes in vWF. As vWF mediated aggregation requires that a venom protein first binds to vWF and subsequently binds to a platelet binding site (GP1bα), the variation in resistance observed may be due to aspercetin’s ability to bind to GP1bα (variation in opossum GP1bα). Additional work examining the tertiary complex of vWF-aspercetin-and GP1bα, as well as variation in GP1bα among individual opossums are needed to assess the source of reduced aggregation response in this species.
Washed platelet assays excluded the possibility that opossum plasma inhibitors are responsible for the reduction in aggregation observed (Fig. 2B). For aspercetin, a small increase in aggregation (7.5%) was seen when adding plasma to washed platelets - a pattern opposite to that expected if plasma proteins inhibited vWF CTLs. This result suggests either a small amount of a contaminating venom protein is causing aggregation via a plasma protein target, or some functional degradation of vWF is caused by the purification process. Overall, these data are consistent with the assertion that amino acid changes in opossum vWF confer physiological resistance to vWF-binding venom CTLs, but do not rule out the role of the platelet site GB1Bα in contributing to this resistance.
4.2. Physiological function outside Didelphini
Surprisingly, Monodelphis domestica, which is not a member of the clade known to be venom resistant, also showed a loss of aggregation response to botrocetin. This result is inconsistent with the hypothesis that vWF resistance to venom CTLs is restricted to Didelphini and deserves further consideration.
In their study of adaptive evolution of vWF in opossums, Jansa and Voss (2011) used phylogenetically informed tests of positive selection that require branches thought to be evolving under selection to be selected a priori to test a specific hypothesis (the branch-sites test of Yang, 2007). Though this method is powerful tool for hypothesis testing, the a priori selection of branches make it susceptible to missing possible instances of positive selection and rapid evolution. When changes in the vWF gene are mapped on the didelphid phylogeny (Fig. 1), several conspicuously long branches are apparent, including those subtending the venom-resistant Didelphini, as well as those leading to Marmosa rubra, Metachirus nudicaudatus, and several species of Monodelphis including M. domestica. Moreover, Monodelphis domestica shares several changes at botrocetin binding sites that are also seen in Didelphini. Of the 13 botrocetin binding sites, six have changes in M. domestica, three of which are identical to amino acid changes found in Didelphini. Although species of Monodelphis have been reported to occasionally eat snakes (Streilein, 1982), it is unknown whether they prey on venomous species. However, Monodelphis domestica shares a geographic range with Bothrops jararaca and is likely to be a prey item of several South American vipers (Macrini, 2004; Voss, 2013). It is therefore possible that Monodelphis domestica represents an independent instance of rapid evolution of vWF among opossum species.
Alternatively, possessing a CTL-resistant vWF may be the ancestral condition for all opossums and may have facilitated a dietary adaptation (snake eating) in Didelphini. Though rapid diversifying selection on several branches make this history less likely, it does not rule out initial resistance which may have subsequently experienced diversifying selection via coevolving venoms. This initial disabling of venom CTL function may have disrupted coagulopathy enough to allow differential survival, which subsequently may have allowed several species to evolve additional mechanisms of venom resistance such as metalloproteinase and phospholipase inhibitors that are well known for this group (Voss and Jansa, 2012). Whether this type of pre-existing resistance to CTLs would be effective for all opossums regardless of body size is unknown. Given that most members of Didelphini are large bodied compared to the remainder of Didelphidae, and that ancestral opossums were small bodied, venom CTL resistance may have been an exaptation that was maintained for snake predator defense preceding a trophic switch from prey to predators in Didelphini. This in place, the new emergence of large body size which would serve to dilute venom dosage in addition to a pre-adapted vWF may have combined to facilitate the evolution of whole venom resistance (and thus snake predation) via metalloproteinase and phospholipase inhibitors in this group.
Though vWF resistance on its own likely does not confer organismal venom resistance, it may be indicative of unexamined partial or complete organismal venom resistance. Our results suggest that the longheld assumption that M. domestica and potentially other related smaller bodied opossums are venom sensitive needs re-evaluation. Though analyses of positive selection which motivated this work are robust in detecting signals of persistent diversifying selection, they are poor at detecting selection in which a few advantageous non-synonymous mutations are either quickly reaching fixation or toggling back and forth between states (Yang and Dos Reis, 2011; Nuismer and Thompson, 2006). Therefore, while tests of positive selection are a useful tool in directing attention to potential adaptive function, they should be followed up through examination of the functional effects of observed genotypes (Yokoyama et al., 2008). These results demonstrate that ecological (predator/prey) interactions between opossums and vipers may be driving adaptive evolution at the vWF A1 region not just in Didelphini, but perhaps in a broader swath of opossums spanning family Didelphidae. This result also lends evidence to the possibility that opossums and vipers are coevolving and that venom may be beholden to selection pressure from resistant mammals as both predators and prey.
5. Conclusions
The complexities of venom have long been the focus of biochemical and evolutionary research (Markland, 1998). Comparatively little is known about the complexity of venom resistance, though it has been predicted to be equally complex (Holding et al., 2016). This work demonstrates that both target-based and inhibitor-based mechanisms are important functional components of venom resistance among several species of opossums and is the first to demonstrate that resistance in opossums is a multifaceted trait. Given that venom resistance mechanisms in this group have been shown for at least 3 classes of venom proteins (metalloproteinases, phospholipases, and now CTLs), it is reasonable to expect that resistant species have additional target-based or inhibitor-based resistance mechanisms in place to cope with other major venom toxin classes. This work highlights that opossum/viper venom-venom resistance may be an ideal system for examining the coevolution of complex traits, and lays the groundwork for future work examining the evolution of this important complex trait and its potentially coevolving counterparts.
Supplementary Material
Acknowledgements
Funding for this work was provided by the Rosemary Grant Award from the Society for the Study of Evolution, and the UMN Ecology, Evolution, and Behavior Department Travel Grant. Funding was also provided by the American Society of Mammalogists Grant in Aid, and the American Society for Ichthyologists and Herpetologists Gaige Fund Award. EHZ was financed by Biota FAPESP grant #2016/50127-5. Permission for opossum trapping was granted by the State of Minnesota Department of Natural Resources (permit No 16312). This research was conducted in accordance with University of Minnesota’s Institutional Animal Care and Use Committee (IACUC # 1303–30464 A), and Instituto Butantan’s Comitê de Ética em Experimentação Animal (CEUAIBu) (protocol #8346081018). Special thanks to Miguel Cruz for reagents and invaluable lab mentorship. Thanks to Kristen Wiley at the Kentucky Reptile Zoo, Nicole Zantec and Dan Keyler at UMN for generously sharing reagents and advice. Thanks to the undergraduate and graduate researchers in the Hingst-Zaher Lab at Instituto Butantan with special thanks to Fernanda Pricolli for help in trapping and testing in Brazil. We would like to especially thank Kalena Barros and Silvia Travaglia for their help. We are grateful for the revisions, editing, and advise from Dr. Keith barker and Dr. Georgiana May that were invaluable to this work. We would also like to thank two anonymous reviewers for their careful reading of this manuscript, and for their suggestions which have substantially improved the clarity and presentation of this work.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://doi.org/10.1016/j.toxicon.2020.02.024.
Ethical statement
All authors of this work declare that this work is original and has not been published elsewhere. All authors have participated in and contributed to this work. All aspects of this work are in compliance with ethical guidelines. Danielle Drabeck testified to the accuracy of these statements on behalf of all of the authors.
References
- Allain JP, Cooper HA, Wagner RH, Brinkhous KM, 1975. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand Factor and platelet aggregating factor. J. Lab. Clin. Med 85, 328–335. [PubMed] [Google Scholar]
- Almeida-Santos SM, Antoniazzi MM, Sant’Anna OA, 2000. Predation by the opossumDidelphis marsupialis on the rattlesnake Crotalus durissus. Curr. Herpetol 19, 1–9. [Google Scholar]
- Arlinghaus F, Eble J, 2012. C-type lectin-like proteins from snake venoms. Toxicon 60, 512–519. [DOI] [PubMed] [Google Scholar]
- Barchan D, Ovadia M, Kochva E, Fuchs S, 1995. The binding site of the nicotinic acetylcholine recepter in animal species resistant to alpha-bungarotoxin. Biochemistry 34, 9172–9176. [DOI] [PubMed] [Google Scholar]
- Cáceres NC, Monteiro-Filho ELA, 2001. Food habits, home range and activity of Didelphis aurita (Mammalia, Marsupialia) in a forest fragment of southern Brazil. Stud. Neotrop. Fauna Environ 36 (1), 85–92. [Google Scholar]
- Catanese JJ, Kress LF, 1993. Opossum α1-proteinase inhibitor: purification, linear sequence, and resistance to inactivation by rattlesnake venom metalloproteinases. Biochemistry 32, 509–515. [DOI] [PubMed] [Google Scholar]
- Coller BS, Hirschman RI, Gralnick HR, 1975. Studies on the factor VIII/von Willebrand factor antigen on human platelets. Thromb. Res 6, 469–480. [DOI] [PubMed] [Google Scholar]
- De Marco L, Shapiro S, 1981. Properties of human asialo-factor VIII. A ristocetin-independent platelet-aggregating agent. J. Clin. Invest 68 (2), 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JF, Berndt MC, Schade A, Mclntire LV, Andrews RK, López JA, 2001. ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood 97 (1), 162–168. [DOI] [PubMed] [Google Scholar]
- Edgar RC, 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch H, 1960. Autoecology of the Copperhead, vol. 13. University of Kansas Publication Museum of Natural History, pp. 85–288. [Google Scholar]
- Fukuda K, Doggett T, Laurenzi IJ, Liddington RC, Diacovo TG, 2005. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat. Struct. Mol. Biol 12, 152–159. [DOI] [PubMed] [Google Scholar]
- Hamako J, Matsui T, Suzuki M, Ito M, Makita K, Fujimura Y, Titani K, 1996. Purification and characterization of bitiscetin, a novel von Willebrand factor modulator protein from Bitis arietans snake. Venom 226, 273–279. [DOI] [PubMed] [Google Scholar]
- Holding ML, Drabeck DH, Jansa SA, Gibbs LH, 2016. Venom resistance as a model for understanding the molecular basis of complex coevolutionary adaptations. Inregr. Comp. Biol 56 (5), 1032–1043. [DOI] [PubMed] [Google Scholar]
- Jansa SA, Voss RS, 2011. Adaptive evolution of the venom-targeted vWF protein in opossums that eat pitvipers. PloS One 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jared C, Antoniazzi MM, Almeida-Santos SM, 1998. Predation of snakes by the young of opossum Didelphis marsupialis in captivity. Snake 28, 68–70. [Google Scholar]
- Kilmon J, 1976. High tolerance to snake-venom by Virginia opossum, didelphis-virginiana. Toxicon 14 (4), 337–340 (Translated From English), (In English). [DOI] [PubMed] [Google Scholar]
- Lovo-Farah MF, One M, Novello JC, Toyama MH, Perales J, Moussatche H, Domont GB, Oliveira B, Marangoni S, 1996. Isolation of protein factors from opossum (Didelphis albiventris) serum which protect against Bothrops jararaca venom. Toxicon 34, 1067–1071. [DOI] [PubMed] [Google Scholar]
- Macrini T, 2004. Monodelphis domestica. Mamm. Species 760, 1–8. [Google Scholar]
- Maita N, Nishio K, Nishimoto E, Matsui T, Shikamoto Y, Morita T, Sadler SE, Mizuno H, 2003. Crystal structure of von Willebrand factor A1 domain complexed with snake venom, bitiscetin: insight into glycoprotein Ibalpha binding mechanism induced by snake venom proteins. J. Biol. Chem 278 (39), 37777–37781. [DOI] [PubMed] [Google Scholar]
- Markland FS, 1998. Snake venoms and the hemostatic system. Toxicon 36, 1749–1800. [DOI] [PubMed] [Google Scholar]
- McManus J, 1974. Didelphis virginiana. Mamm. Species 40, 1–6. [Google Scholar]
- Melo PA, Suarez-Kurtz G, 1988. Release of sarcoplasmic enzymes from skeletal muscle by Bothrops jararacussu venom: antagonism by heparin and by the serum of South American marsupials. Toxicon 26, 87–95. [DOI] [PubMed] [Google Scholar]
- Menchaca JM, Perez JC, 1981. The purification and characterization of an antihemorrhagic factor in opossum (Didelphis virginiana) serum. Toxicon 19, 623. [DOI] [PubMed] [Google Scholar]
- Moore DM, 1984. A simple technique for blood collection in the opossum (Didelphis virginiana). Lab. Anim 1, 52–54. [DOI] [PubMed] [Google Scholar]
- Moussatche H, Leonardi F, Yates A, Borche L, 1978. Experimentos sobre la resistencia del Didelphis venezolano (rabi-pelado) a los venenos de serpientes. Acta Científica Venezolana 55 (2). [Google Scholar]
- Moussatché H, Perales J, 1989. Factors underlying the natural resistance of animals against snake venoms. Mem. Inst. Oswaldo Cruz 84 (Suppl. IV), 391–394. [Google Scholar]
- Moussatche H, Yates A, Leonardi F, Borche L, 1979. Mechanisms of resistance of the opossum to some snake venoms. Toxicon 130. [Google Scholar]
- Nichols T, Bellinger D, Merricks E, Raymer R, Kloos M, DeFriess N, Ragni MV, Griggs TR, 2010. Porcine and canine von Willebrand factor and von Willebrand disease: hemostasis, thrombosis, and atherosclerosis studies. Thrombosis 2010, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuismer SL, Thompson JN, 2006. Coevolutionary alteration in antagonistic interactions. Evolution 60 (11), 2207–2217. [PubMed] [Google Scholar]
- Oliveira M, Santori R, 1999. Predatory behavior of the opossum Didelphis albiventris on the pitviper Bothrops jararaca (translated). Stud. Neotrop. Fauna Environ 34, 72–75. [Google Scholar]
- Perales J, Moussatche H, Marangoni S, Oliveira B, Domont GB, 1994. Isolation and partial characterization of an anti-bothropic complex from the serum of South American Didelphidae. Toxicon 32, 1237–1249. [DOI] [PubMed] [Google Scholar]
- Pavan SE, Jansa SA, Voss RS, 2014. Molecular phylogeny of short-tailed opossums (Didelphidae: Monodelphis): Taxonomic implications and tests of evolutionary hypotheses. Molecular Phylogenetics and Evolution 79, 199–214. [DOI] [PubMed] [Google Scholar]
- Perales JRM, Moussatche H, 1986. Isolation and partial characterization of a protein fraction from the opossum (Didelphis marsupialis) serum, with protecting property against the Bothrops jararaca venom. An Acad. Bras Ciências 58, 155–162. [PubMed] [Google Scholar]
- Perez JC, Haws WC, Garcia VE III Jennings BM, 1978. Resistance of warmblooded animals to snake venoms. Toxicon 16, 375–383. [DOI] [PubMed] [Google Scholar]
- Perez JC, Pichyangkul P, Garcia VE, 1979. The resistance of three species of warmblooded animals to Western diamondback rattlesnake (Crotalus atrox) venom. Toxicon 17, 601–607. [DOI] [PubMed] [Google Scholar]
- Qi Z-Q, Yonaha K, Tomihara Y, Toyama S, 1994. Characterization of the antihemorrhagic factors of mongoose (Herpestes edwardsii). Toxicon 32, 1459–1469. [DOI] [PubMed] [Google Scholar]
- Read M, Shermer R, Brinkhous K, 1978. Venom coagglutinin: an activator of platelet aggregation dependent on von Willebrand factor. Proc. Natl. Acad. Sci. Unit. States Am 75 (9), 4514–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read MS, Potter JY, Brinkhous KM, 1983. Venom coagglutinin for detection of von Willebrand factor activity in animal plasmas. J. Lab. Clin. Med 101 (1), 74–82. [PubMed] [Google Scholar]
- Rousmaniere H, Silverman R, White R, Sasaki M, Wilson S, Morrison J, Cruz Y, 2010. Husbandry of Monodelphis domestica in the study of mammalian embryogenesis. Lab. Anim 39 (7), 219–226. [DOI] [PubMed] [Google Scholar]
- Rucavado A, Soto M, Kamiguti A, Theakston D, Fox J, Escalante T, Gutiérrez J, 2001. Characterization of aspercetin, a platelet aggregating component from the venom of the snake Bothrops asper which induces thrombocytopenia and potentiates metalloproteinase-induced hemorrhage. Thromb. Haemostasis 85, 710–715. [PubMed] [Google Scholar]
- Sanders WE Jr., Reddick RL, Nichols TC, Brinkhous KM, Read MS, 1995. Thrombotic thrombocytopenia induced in dogs and pigs. The role of plasma and platelet vWF in animal models of thrombotic thrombocytopenic purpura. Arterioscler Thromb Vasc Biol. June 15 (6), 793–800. [DOI] [PubMed] [Google Scholar]
- Sazima I, 1992. Natural history of the jararaca pitviper, B. jararaca in southeastern Brazil. In: Campbell JA, Brodie ED (Eds.), Biology of the Pitvipers. Selva Publ., Tyler, Texas, USA. Jr. [Google Scholar]
- Sekiya F, Atoda H, Morita T, 1993. Isolation and characterization of an anticoagulant protein homologous to botrocetin from the. Venom of Bothrops jararaca Biochem. (32), 6892–6897. [DOI] [PubMed] [Google Scholar]
- Sikes R, Gannon W, 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal 92 (1), 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto JG, Perez JC, Minton SA, 1988. Proteolytic, hemorrhagic and hemolytic activities of snake venoms. Toxicon 26, 875–882. [DOI] [PubMed] [Google Scholar]
- Streilein KE, 1982. Ecology of small mammals in the semiarid Brazilian Caatinga. I. Climate and faunal composition. Ann. Carnegie Mus 51, 79–107. [Google Scholar]
- Vellard J, 1945. Resistencia de los “Didelphis” (zarigueya) a los venenos ofidicos (Nota prévia). Rev. Bras. Biol 5, 463–467. [PubMed] [Google Scholar]
- Voss RS, 2013. Opossums (mammalia: Didelphidae) in the diets of neotropical pitvipers (serpentes: crotalinae): evidence for alternative coevolutionary outcomes? Toxicon 66, 1–6. [DOI] [PubMed] [Google Scholar]
- Voss RS, Jansa SA, 2012. Snake-venom resistance as a mammalian trophic adaptation: lessons from didelphid marsupials. Biol. Rev 87, 822–837. [DOI] [PubMed] [Google Scholar]
- Werner R, Vick J, 1977. Resistance of opossum (Didelphis-Virginiana) to envenomation by snakes of family crotalidae. Toxicon 15 (1), 29–& (Translated From English), (In English). [DOI] [PubMed] [Google Scholar]
- Werner RM, Faith RE, 1978. Decrease in the lethal effect of snake venom by serum of the opossum, Didelphis marsupialis. Lab. Anim. Sci 28, 710–713. [PubMed] [Google Scholar]
- Wood JE, 1954. Food habits of furbearers of the upland post oak region in Texas. J. Mammol 35, 406–415. [Google Scholar]
- Yang Z, 2007. Paml 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol 24, 1586–1591. [DOI] [PubMed] [Google Scholar]
- Yang Z, Dos Reis M, 2011. Statistical properties of the branch-site test of positive selection. Mol. Biol. Evol 28 (3), 1217–1228. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Tada T, Zhang H, Britt L, 2008. Elucidation of phenotypic adaptations: molecular analyses of dim-light vision proteins in vertebrates. Proc. Natl. Acad. Sci. U.S.A 105, 13480–13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


