Abstract
Patient: Female, 33-year-old
Final Diagnosis: Cervix cancer • squamous cell carcinoma
Symptoms: Cervical mass • lymphadenopathy • vaginal bleeding
Medication: —
Clinical Procedure: Magnetic resonance imaging (MRI)
Specialty: Obstetrics and Gynecology
Objective:
Unusual clinical course
Background:
Cervical cancer is the most common gynecologic malignancy diagnosed in pregnancy. When cervical cancer is diagnosed late in pregnancy, pelvic lymphadenectomy is avoided. Advanced imaging adds an alternative way to assess nodal involvement. The aim of this case report is to demonstrate how magnetic resonance imaging (MRI) can contribute to the clinical staging of cervical cancer and inform treatment decisions when diagnosis is made late in pregnancy. We report the case of a woman in the third trimester who was diagnosed with advanced-stage squamous cell cervical carcinoma by MRI and biopsy.
Case Report:
A 33-year-old woman at 33 weeks of gestation was admitted to our hospital for recurrent vaginal bleeding. Examination revealed a large cervical mass. A gynecologic oncologist was consulted, an examination under anesthesia was performed, and cervical biopsy samples were obtained. Pathology results revealed squamous cell carcinoma of the cervix. A clinical stage of IB3 was assumed. MRI revealed a 5.2×5.8-cm cervical mass and an enlarged left pelvic lymph node. These findings upstaged the patient to IIIC1. Instead of undergoing a radical cesarean hysterectomy, the patient had a cesarean delivery and pelvic lymph node dissection. Four weeks later, she began chemotherapy and pelvic radiation.
Conclusions:
There is significant value in advanced imaging for cervical cancer staging. This is especially relevant in pregnancy, where the under-staging of disease is a concern. This case report demonstrates the value of MRI in cervical cancer staging, particularly in pregnant women, in whom treatment and the timing of treatment should be individualized.
Keywords: Pregnancy, Case Reports, Uterine Cervical Neoplasms, Magnetic Resonance Imaging
Background
Cervical cancer is the most common gynecologic malignancy diagnosed during pregnancy [1–3]. The incidence of cervical cancer in pregnancy is approximately 1.4 to 4.6 cases per 100 000 pregnancies [3,4]. The diagnosis of cervical cancer in pregnancy is thought to occur with similar frequency in each trimester of pregnancy [5]. Patients diagnosed in the first trimester or early in the second trimester who elect to continue their pregnancy have the option to undergo clinical staging and surgical evaluation of the pelvic lymph nodes via laparoscopy and/or laparotomy to aid treatment decisions [6]. Nodal resection is not recommended after 22 weeks of gestation [6]. Thus, a diagnosis of cervical cancer later in pregnancy can pose a challenge for optimal staging and potentially lead to under-staging.
Prior to the introduction of the 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging guidelines, cervical cancer was primarily staged clinically [6,7]. This included a pelvic examination, colposcopy, hysteroscopy, cervical conization, cystoscopy, and proctoscopy. The primary imaging studies recommended were an intravenous pyelo-gram, chest X-ray, and barium enema [6,7]. The release of the new guidelines, which redefined the cervical cancer staging system, encouraged the incorporation of pathology results and advanced imaging [6,8]. In these guidelines, there is no consensus on the use of lymph node biopsy, surgical assessment of the extent of the tumor, or a specific imaging technique [6,8], but it is recognized that the use of advanced imaging is an accurate way to assess tumor size and spread and lymph node involvement when surgical exploration is not feasible [3,9,10].
In this case report, we describe a 33-year-old woman in the third trimester of pregnancy who was diagnosed with advanced-stage squamous cell cervical carcinoma by biopsy and magnetic resonance imaging (MRI).
Case Report
A 33-year-old woman (gravida 3, para 2) at 33 weeks and 4 days of gestation was admitted to our hospital’s antenatal service with vaginal bleeding. She had an insignificant medical, family, and psychosocial history. Her 2 prior pregnancies were uncomplicated and resulted in spontaneous vaginal deliveries. The patient’s gynecologic history was otherwise unremarkable. Her most recent Papanicolaou test was performed in 2018, revealing normal cytology. A Papanicolaou test was not performed during this pregnancy, and no visual abnormalities of the cervix were noted during her first trimester prenatal visits. Her only medication was a prenatal vitamin. A complete blood count, basic metabolic panel, and coagulation panel were obtained and found to be within normal limits.
The patient had 2 prior admissions for the same symptom at 27 and 30 weeks of gestation. During those admissions, common causes of vaginal bleeding were ruled out, including placental pathology, abruption, and subchorionic hemorrhage. Notably, during her admission at 30 weeks of gestation, she was found to have a cervical lesion that was nodular and originally described as smooth and non-friable. It was thought to be a benign finding, possibly a Nabothian cyst, cervical polyp, or cervical ectopy. Despite its characteristics not raising great concern for malignancy, a referral was made to the Gynecology Oncology Department for an expert examination. The patient had yet to be seen by the Gynecologic Oncology team at the time of her admission at 33 weeks and 4 days of gestation.
On hospital day 2, at 33 weeks and 6 days of gestation, the antenatal team consulted a gynecologic oncologist who, on examination of the patient, noted a firm, cylindrical, downward-facing cervix, with a friable mass felt at the 12 o’clock position. A complete blood count, basic metabolic panel, and coagulation panel were obtained and found to be within normal limits. A pelvic sonogram revealed a cervical mass, a fetus in a vertex presentation, and a posterior placenta. Given the suspicion of cervical malignancy, the gynecologic oncology service took the patient to the operating room for an examination under anesthesia. Intraoperatively, the mass was visualized to be at least 7 cm in size. Biopsy samples of the lesion were sent for frozen section pathology analysis and revealed squamous cell carcinoma. Final pathology results from all biopsy locations revealed moderately differentiated, non-keratinizing squamous cell carcinoma (Figures 1, 2). Clinical staging in the absence of imaging was determined to be stage IB3.
Figure 1.
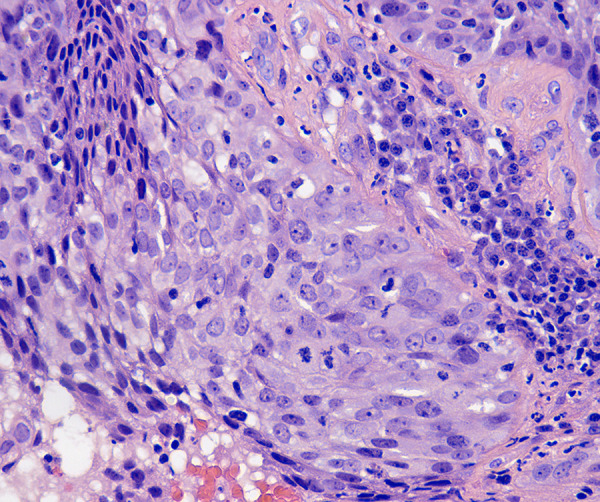
Squamous cell carcinoma of the cervix, nonkeratinizing carcinoma. Hematoxylin and eosin staining (original magnification ×400) high-power microscopic view showing malignant cells with irregular, large nuclei containing multiple nucleoli and cells with abundant eosinophilic cytoplasm.
Figure 2.
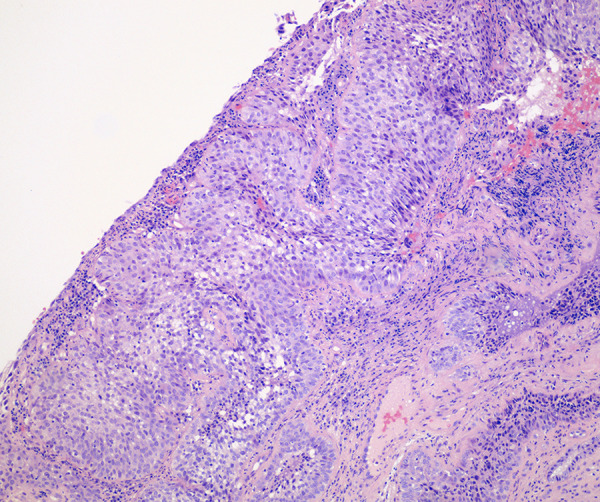
Squamous cell carcinoma of the cervix, nonkeratinizing carcinoma with invasion. Hematoxylin and eosin staining (original magnification ×40) low-power microscopic view showing malignant cells infiltrating as nests. The cytoplasm is moderate and eosinophilic. Large nuclei with multiple nucleoli are prominent.
Given the size and bulk of this lesion, there was a concern for metastatic disease. Therefore, a pelvic MRI examination was conducted, revealing a 5.2×5.8-cm mass within the anterior cervix and an enlarged left-sided pelvic lymph node with diffusion restriction, suggestive of metastasis (Figure 3). Incorporating the MRI results according to the 2018 FIGO staging guidelines changed the patient’s stage to IIIC1.
Figure 3.
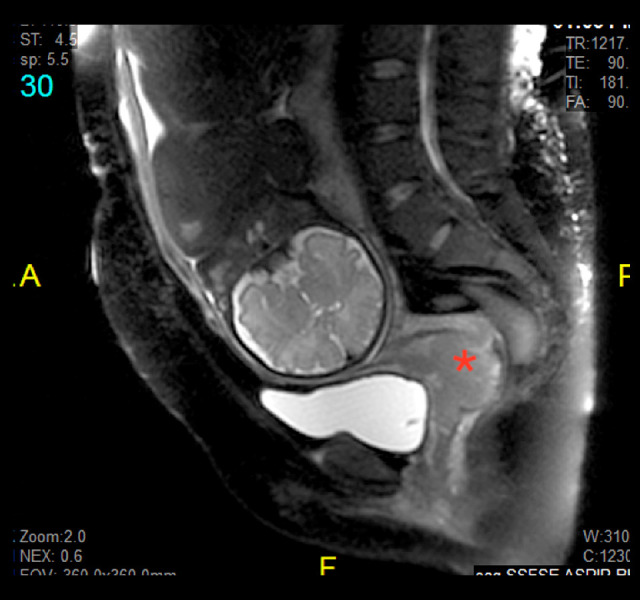
A sagittal magnetic resonance imaging of the abdomen and pelvis. This sagittal view magnetic resonance image of the patient’s abdomen and pelvis shows a fetus in a cephalic presentation with a clear and measurable tumor (red asterisk) involving the anterior lip of the cervix. The mass measured 5.2×5.8 cm; biopsy revealed a moderately differentiated squamous cell carcinoma of the cervix.
To develop a delivery and treatment plan for this patient, a multidisciplinary team was assembled, including the patient’s obstetrician along with the gynecologic oncology, neonatology, and maternal fetal medicine teams. Prior to obtaining the MRI, there was consideration of performing a cesarean hysterectomy at the time of delivery. Given the change in clinical stage (from IB3 to IIIC1), the delivery plan was changed, and a plan was made to proceed with a cesarean delivery during week 34 of gestation and to treat the patient with chemotherapy and radiation following delivery.
Forty-eight hours after the administration of corticosteroids, a liveborn female infant was delivered via a primary cesarean delivery, weighing 2720 g and having an Apgar score of 9 at 1 min and 9 at 5 min. The newborn was admitted to the Neonatal Intensive Care Unit because of prematurity. Following closure of the hysterotomy, the patient underwent a bilateral salpingectomy and bilateral pelvic and para-aortic lymphadenectomy. In anticipation of pelvic radiation, she underwent bilateral ovarian transposition, where both ovaries were secured to the peritoneum above the pelvic brim on their respective sides with 0 silk suture. The procedure was uncomplicated.
Final pathology from the procedure revealed that 2 of 5 left pelvic lymph nodes were positive for metastatic squamous cell carcinoma, with the largest lymph node measuring 3.5 cm in its greatest dimension. The patient’s postoperative course was uncomplicated, and she was discharged home on postoperative day 5. Prior to discharge, the patient was seen by the Radiation Oncology Department, and a radiation therapy plan was made with the patient for outpatient follow-up.
Following hospital discharge, the patient underwent a positron emission tomography scan, which demonstrated no evidence of disseminated disease. Four weeks after her delivery, she began a course of treatment that included 5 cycles of cisplatin chemotherapy and external beam pelvic radiation with 45 gray (Gy) to the pelvis and a 9-Gy parametrial boost to the left parametrium. In addition, a 29-Gy dose of radiation was delivered to the pelvis in 5 fractions using tandem and ovoid brachytherapy. Further, the patient was scheduled to undergo adjuvant chemotherapy with 6 cycles of cisplatin, paclitaxel, and bevacizumab.
Discussion
This case highlights the impact of advanced imaging on the staging and management of cervical cancer diagnosed during the third trimester of pregnancy. Clinical staging was used with advanced imaging to gain greater insight into the extent of this patient’s disease state, thereby allowing for a more appropriate treatment plan to be devised. Painless bleeding, which our patient had, is the most commonly cited symptom associated with the diagnosis of cervical cancer in pregnancy [11]. Additionally, abnormal discharge that is watery, malodorous, or purulent should warrant investigation for cervical pathology via a thorough examination [12]. Although bleeding, pelvic pressure, back pain, and/or bowel and urinary symptoms can be nonspecific in pregnancy, they can also be a sign of more advanced disease [11].
Previous case reports have found a similar incidence of initial diagnosis of cervical cancer during the first, second, and third trimesters [13,14]. Clinical stage is the most important prognostic factor in cervical cancer [15,16]. Best practice guidelines are created to determine treatment planning once a patient is diagnosed with cervical cancer in pregnancy, and these are primarily based on small case series [3,8]. Delayed treatment of cervical cancer in pregnancy should be discouraged because under-diagnosis is more common in pregnant than in non-pregnant patients [17]. Therefore, decisions such as delivery timing and when to initiate treatment should be individualized, based on stage of disease and gestational age.
The type of advanced imaging used in pregnancy has been studied, and evidence-based recommendations exist to help guide what is acceptable. Imaging that utilizes ionizing radiation is commonly used in non-pregnant patients. Doses less than 0.05 Gy (5 rads) are usually sufficient for pretreatment imaging to investigate cervical cancer, and this level of radiation exposure has not been associated with fetal complications [18]. The American College of Obstetrics and Gynecology (ACOG) recommends that ultrasound and non-contrast MRI be used as first-line imaging studies for the pregnant patient [10,18,19]. Gadolinium use with MRI during pregnancy puts the fetus at increased risk for rheumatologic, inflammatory, or infiltrative skin conditions as well as stillbirth and neonatal death [10,20]. Based on this evidence, it is recommended that gadolinium not be used in pregnancy [10,20]. Another modality to consider would be a diffusion-weighted MRI, as there are no recognized adverse effects to the growing fetus [3,9]. A recent meta-analysis study showed that whole-body diffusion-weighted MRI, when compared with positron emission tomography/computed tomography, had similar detection rates of nodal metastasis [21]. Therefore, whole-body diffusion-weighted MRI should also be considered [3,9].
Prior case reports have looked at the delay in treatment of cervical cancer diagnoses during the late second trimester and early third trimester [8]. In these cases, providers, including a multidisciplinary team of obstetrician, oncologist, radiation oncologist, maternal fetal medicine specialist, and pathologist, can use shared decision-making for the optimal treatment and
timing of delivery. Prior case reports have also shown promise that the use of neoadjuvant chemotherapy can occur during the peri-viable period [22]. Our patient presented late in the third trimester. Thus, expedited delivery and prompt post-partum treatment was initiated. Treatment is best initiated within 4 to 6 weeks of diagnosis [3,23].
In our case, preoperative imaging helped determine a management strategy that eliminated the plan for a radical hysterectomy. This is notable in that others have observed that when cervical cancer is diagnosed in pregnancy at stage II or higher, the disease can be under-staged (as deemed by postoperative pathology) [3,24]. The decision to treat this patient with a cesarean delivery followed by chemo-radiation, including therapy with whole-pelvic radiation and brachytherapy, after her delivery was made to give her the best chance for progression-free survival and overall survival [3,6,25].
In addition, the decision to give cisplatin and paclitaxel chemotherapy following her initial course of therapy was based on the findings of the Gynecologic Oncologic Group (GOG) 169 trial. In GOG 169, patients receiving the combination of cisplatin and paclitaxel had a survival advantage over those receiving cisplatin alone for the treatment of advanced-stage cervical cancer [6,8,25]. In addition, we added bevacizumab for our patient based upon findings from the GOG 240 trial, which showed a significantly longer overall survival (in months) in patients with metastatic cervical cancer who received the medication compared with those who did not [6,8,25].
Conclusions
The clinical staging of cervical cancer can be aided by advanced imaging techniques, which provide greater insight into disease stage when compared with clinical staging without imaging. It is considered unsafe to accurately perform lymph node staging surgically during the third trimester. Therefore, accurate advanced imaging with MRI allows for confidence when staging a patient in the third trimester. Choosing an advanced imaging technique can be based on the FIGO recommendations for staging cervical cancer combined with the ACOG recommendations for the imaging techniques most accepted in pregnancy. The information obtained by advanced imaging can result in the implementation of a management plan that could meaningfully alter treatment and therefore a patient’s prognosis. The present case report has shown the value of MRI in staging cervical cancer, particularly in cases of pregnant women, when treatment and the timing of treatment should be individualized.
In pregnancy, advanced imaging can be employed safely and adverse fetal effects avoided while optimal imaging is achieved.
In this report, we discussed a patient who presented at 33 weeks and 4 days of gestation with vaginal bleeding resulting from a cervical mass that was determined to be squamous cell carcinoma of the cervix, with a change in clinical stage (from IB3 to IIIC) following MRI of the abdomen and pelvis, which altered her treatment plan. Advanced imaging is an important tool to aid the evaluation and management of patients diagnosed with cervical cancer in pregnancy.
Acknowledgments
We would like to acknowledge Dr. Pankaj Singhal, Department Chair and Division Director Gynecology Oncology, Good Samaritan Hospital, Dr. Claudeus Murphy, Obstetrician and Gynecologist, Good Samaritan Hospital, and Dr. Wei Lu, Pathologist, Good Samaritan Hospital.
Footnotes
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Ji YI, Kim KT. Gynecologic malignancy in pregnancy. Obstet Gynecol Sci. 2013;56(5):289–300. doi: 10.5468/ogs.2013.56.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith LH, Dalrymple JL, Leiserowitz GS, et al. Obstetrical deliveries associated with maternal malignancy in California, 1992 through 1997. Am J Obstet Gynecol. 2001;184(7):1504–12. doi: 10.1067/mob.2001.114867. ; discussion 1512–13. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Berveiller P, Boere IA, et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann Oncol. 2019;30(10):1601–12. doi: 10.1093/annonc/mdz228. [DOI] [PubMed] [Google Scholar]
- 4.Eibye S, Kjær SK, Mellemkjær L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet Gynecol. 2013;122(3):608–17. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- 5.Jones WB, Shingleton HM, Russell A, et al. Cervical carcinoma and pregnancy. A national patterns of care study of the American College of Surgeons. Cancer. 1996;77(8):1479–88. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1479::AID-CNCR9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129–35. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo K, Machida H, Mandelbaum RS, et al. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152(1):87–93. doi: 10.1016/j.ygyno.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han J, Hu X, He X, et al. Cervical cancer in pregnancy: One case report and a review of current treatment recommendations. Gynecol Pelvic Med. 2019;2 gpm.2019.0701. [Google Scholar]
- 9.Halaska MJ, Uzan C, Han SN, et al. Characteristics of patients with cervical cancer during pregnancy: a multicenter matched cohort study. An initiative from the International Network on Cancer, Infertility and Pregnancy. Int J Gynecol Cancer. 2019;29(4):676–82. doi: 10.1136/ijgc-2018-000103. [DOI] [PubMed] [Google Scholar]
- 10.Balleyguier C, Fournet C, Ben Hassen W, et al. Management of cervical cancer detected during pregnancy: role of magnetic resonance imaging. Clin Imaging. 2013;37(1):70–76. doi: 10.1016/j.clinimag.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Han SN, Mhallem Gziri M, Van Calsteren K, Amant F. Cervical cancer in pregnant women: Treat, wait or interrupt? Assessment of current clinical guidelines, innovations and controversies. Ther Adv Med Oncol. 2013;5(4):211–19. doi: 10.1177/1758834013494988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen C, Montz FJ, Bristow RE. Management of stage I cervical cancer in pregnancy. Obstet Gynecol Surv. 2000;55(10):633–43. doi: 10.1097/00006254-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Shan Y, Yang J, et al. [Management of invasive cervical cancer in pregnancy: Clinical analysis of 13 cases] Zhonghua Fu Chan Ke Za Zhi. 2012;47(12):893–97. [in Chinese] [PubMed] [Google Scholar]
- 14.McDonald SD, Faught W, Gruslin A. Cervical cancer during pregnancy. J Obstet Gynaecol Can. 2002;24(6):491–98. doi: 10.1016/s1701-2163(16)31097-0. [DOI] [PubMed] [Google Scholar]
- 15.Creasman WT, Rutledge FN, Fletcher GH. Carcinoma of the cervix associated with pregnancy. Obstet Gynecol. 1970;36(4):495–501. [PubMed] [Google Scholar]
- 16.Allen HH, Nisker JA, Anderson RJ. Primary surgical treatment in one hundred ninety-five cases of stage IB carcinoma of the cervix. Am J Obstet Gynecol. 1982;143(5):581–84. doi: 10.1016/0002-9378(82)90551-8. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima K, Ogawa S, Tsukimori K, et al. Can we diagnose invasive cervical cancer during pregnancy as precise as in nonpregnant women?: Maternal and perinatal outcome in pregnancies complicated with cervical cancers. Int J Gynecol Cancer. 2009;19(8):1439–45. doi: 10.1111/IGC.0b013e3181a83ebf. [DOI] [PubMed] [Google Scholar]
- 18.Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Accessed 23 August 2021. Available from: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/10/guidelines-for-diagnostic-imaging-during-pregnancy-and-lactation.
- 19.Streffer C, Shore R, Konermann G, et al. Biological effects after prenatal irradiation (embryo and fetus). A report of the International Commission on Radiological Protection. Ann ICRP. 2003;33(1–2):5–206. [PubMed] [Google Scholar]
- 20.Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952–61. doi: 10.1001/jama.2016.12126. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Gao S, Li S. A Comprehensive Comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical cancer: A Meta-Analysis Based on 67 Studies. Gynecol Obstet Invest. 2017;82(3):209–22. doi: 10.1159/000456006. [DOI] [PubMed] [Google Scholar]
- 22.Vincenzo RD, Tortorella L, Ricci C, et al. Locally advanced cervical cancer complicating pregnancy: A case of competing risks from the Catholic University of the Sacred Heart in Rome. Gynecol Oncol. 2018;150(3):398–405. doi: 10.1016/j.ygyno.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Hunter MI, Tewari K, Monk BJ. Cervical neoplasia in pregnancy. Part 2: Current treatment of invasive disease. Am J Obstet Gynecol. 2008;199(1):10–18. doi: 10.1016/j.ajog.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Leath CA, Monk BJ. Twenty-first century cervical cancer management: A historical perspective of the gynecologic oncology group/NRG oncology over the past twenty years. Gynecol Oncol. 2018;150(3):391–97. doi: 10.1016/j.ygyno.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beharee N, Shi Z, Wu D, Wang J. Diagnosis and treatment of cervical cancer in pregnant women. Cancer Med. 2019;8(12):5425–30. doi: 10.1002/cam4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]


