Abstract
Objective
To describe physicians’ variation in de‐adopting concurrent statin and fibrate therapy for type 2 diabetic patients following a reversal in clinical evidence.
Data Sources
We analyzed 2007‐2015 claims data from OptumLabs® Data Warehouse, a longitudinal, real‐world data asset with de‐identified administrative claims and electronic health record data.
Study Design
We modeled fibrate use among Medicare Advantage and commercially insured type 2 diabetic statin users before and after the publication of the ACCORD lipid trial, which found statins and fibrates were no more effective than statins alone in reducing cardiovascular events among type 2 diabetic patients. We modeled fibrate use trends with physician random effects and physician characteristics such as age and specialty.
Data Extraction
We identified patient‐year‐quarters with one year of continuous insurance enrollment, type 2 diabetes diagnoses, and fibrate use. We designated the physician most responsible for patients’ diabetes care based on evaluation and management visits and prescriptions of glucose‐lowering drugs.
Principal Findings
Fibrate use increased by 0.12 percentage points per quarter among commercial patients (95% CI, 0.10 to 0.14) and 0.17 percentage points per quarter among Medicare Advantage patients (95% CI, 0.13 to 0.20) before the trial and then decreased by 0.16 percentage points per quarter among commercial patients (95% CI, −0.18 to −0.15) and 0.05 percentage points per quarter among Medicare Advantage patients (95% CI, −0.06 to −0.03) after the trial. However, 45% of physicians treating commercial patients and 48% of physicians treating Medicare Advantage patients had positive trends in prescribing following the trial. Physicians’ characteristics did not explain their variation (pseudo R 2 = 0.000).
Conclusion
On average, physicians decreased fibrate prescribing following the ACCORD lipid trial. However, many physicians increased prescribing following the trial. Observable physician characteristics did not explain variations in prescribing. Future research should examine whether physicians vary similarly in other de‐adoption settings.
Keywords: diabetes mellitus, fibric acids, hydroxymethylglutaryl‐CoA reductase inhibitors, longitudinal studies, physicians, Type 2
What is Already Known on This Topic
Physicians sometimes on average decrease their prescribing of ineffective treatments in response to new clinical evidence.
Observable physician characteristics such as age and specialty are associated with whether physicians decrease their prescribing of ineffective treatments in response to new clinical evidence.
What This Study Adds
Physicians varied in their response to new evidence showing a treatment to be ineffective, with many physicians increasing their prescribing of an ineffective treatment despite overall prescribing trends decreasing.
Observable physician characteristics did little to explain the total extent of physicians’ variation in changes in prescribing of an ineffective treatment following new evidence.
Provider organizations and policy makers evaluating interventions aimed at decreasing prescribing of ineffective treatments should be aware that average prescribing rates can mask meaningful physician‐level variation in prescribing.
1. INTRODUCTION AND BACKGROUND
While there is a substantial body of literature on the adoption of innovative treatments, the concept of “de‐adoption,” or the ending of practices as new evidence finds those practices to be ineffective or unsafe, has only recently received research attention. 1 Though a substantial proportion of medical literature focuses on “reversals,” or cases where “new studies… contradict current practice,” 2 few studies have explored how reversals are integrated into clinical practice. Furthermore, the existing literature on de‐adoption has predominantly focused on whether de‐adoption occurs on average, with less attention given to physician‐level variability and patterns of de‐adoption. 3
This study describes variation across physicians in the de‐adoption of concurrent statin and fibrate therapy for patients with type 2 diabetes following a reversal in clinical evidence after the publication of the ACCORD lipid trial, which found coprescription of statins and fibrates to be safe but ineffective in this population. Specifically, we examined two aspects of variation in the de‐adoption of concurrent statin and fibrate therapy: the magnitude of physician‐level variation in de‐adoption, and physician characteristics that explain this variation. We did so in two insured populations: Medicare Advantage and commercially insured.
1.1. Prior literature on physicians’ use of ineffective and unsafe treatments
Studies are mixed on whether physicians de‐adopt ineffective and unsafe treatments following new evidence. Several have found evidence of de‐adoption over time when clinical evidence gradually shifted from supporting to opposing a treatment. 4 , 5 , 6 , 7 , 8 However, these studies did not explicitly model changes in levels or trends in use before and after a reversal in clinical evidence. Among the limited studies that have specifically looked at treatment use before and after a reversal in clinical evidence, several found evidence of de‐adoption, 9 , 10 , 11 , 12 , 13 while another has not. 14
These studies used many data resources, including insurance claims, 4 , 8 , 10 , 12 , 13 discharge databases, 5 , 6 , 7 , 9 patient registries, 11 and electronic health records. 14 Due largely to the structure of these data resources, to our knowledge, studies of de‐adoption have not concurrently examined whether physicians exhibit the same patterns of de‐adoption when treating patients from different insured populations. One might hypothesize that physicians could differentially respond to new evidence when treating Medicare Advantage patients compared to commercially insured patients, due to differences in patient characteristics between the two populations.
Few studies of de‐adoption have measured factors associated with physicians’ variation in de‐adoption. Howard and Hockenberry 6 found physician age was associated with evidence‐concordant obstetric care, though that analysis focused on a procedure where evidence had accumulated gradually as opposed to a reversal of evidence at a specific time. Cook and colleagues 10 modeled trends in anti‐depressant prescribing before and after an FDA warning and documented variation in physicians’ response to the warning, while also finding that the racial composition of a physician's patient panel was associated with prescribing patterns. However, they did not address how much of physicians’ variation was explained by the racial composition of a physician's panel or other physician characteristics. Similarly, studies of low‐value care have found substantial variation among physicians’ use of low‐value services 15 and that time‐invariant physician characteristics such as age, gender, or specialty do not explain that variation 16 , 17 ; physicians may exhibit similar patterns in their de‐adoption decisions, but few studies have documented both whether physicians vary in de‐adoption and whether observable physician characteristics explain physicians’ variation.
1.2. Case study: concurrent statin and fibrate therapy
As a case study in the de‐adoption of a treatment identified as potentially ineffective, we examined the use of concurrent statins and fibrates among patients with type 2 diabetes. Physicians have historically used both statins and fibrates to reduce cardiovascular events among patients with type 2 diabetes. However, the evidence supporting these treatments did not address how fibrate use affected patients conditional on statin use. 18 The NIH‐sponsored ACCORD lipid trial addressed this evidence gap, assessing whether statin users with type 2 diabetes experienced declines in cardiovascular events after initiating fibrate therapy.
The ACCORD lipid trial was published in April 2010 and found using fibrates in combination with statins was on average no more effective in reducing cardiovascular events than statins alone for type 2 diabetic patients. The researchers noted that fibrates were protective for men and patients with both high triglycerides and low HDL cholesterol. However, the triglyceride‐cholesterol effect was statistically insignificant, and the sex effect observed in the overall cohort was not present in the high triglyceride‐low HDL cholesterol cohort. The study concluded that while some patients may benefit from fibrate therapy, the results ultimately “do not support the use of combination fibrate–statin therapy, rather than statin therapy alone, to reduce cardiovascular risk in the majority of patients with type 2 diabetes who are at high risk for cardiovascular disease.” 18 We assessed how physicians responded to this conclusion by examining fibrate use among a cohort of type 2 diabetic statin users before and after the publication of the ACCORD trial.
2. METHODS
2.1. Data and cohort construction
This study used de‐identified administrative claims data from the OptumLabs® Data Warehouse (OLDW), which included medical and pharmacy claims, laboratory results, and enrollment records for commercially insured and Medicare Advantage enrollees. The database contained longitudinal health information on enrollees, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States. 19
To construct our analytic sample, we identified a cohort of patients with type 2 diabetes using statins, who were therefore at risk of receiving potentially ineffective fibrate therapy. We used OLDW to identify type 2 diabetic statin users between 2007 and the third quarter of 2015 (we excluded the last quarter of 2015 to avoid artificial changes in the prevalence of diabetes and comorbidities due to the ICD 10 transition). Using diagnosis codes and place of service requirements from the diabetes specification from the Healthcare Effectiveness Data and Information Set, 20 we included patient‐quarter‐years (our unit of observation) that had two or more outpatient claims with a type 2 diabetes diagnosis (250.X0 and 250.X9), one or more inpatient claim with a type 2 diabetes diagnosis, or one or more prescription claim for glucose‐lowering drugs used to treat type 2 diabetes in the quarter‐year or preceding year. We excluded observations with type 1 diabetes diagnoses (250.X1 and 250.X3) or insulin prescriptions in the quarter‐year or preceding year. In our final analytic sample, we only included patient‐quarter‐year observations with at least one statin prescription during a given quarter‐year (ie, patients for whom fibrate therapy would be ineffective according to the ACCORD trial). We limited our sample to patients with continuous medical and prescription drug insurance enrollment in the quarter‐year and the preceding year. See Appendix S1 for details.
2.2. Attribution algorithm
After identifying our cohort, we attributed each patient‐quarter‐year observation to the physician most likely responsible for their diabetes care. We used a validated multi‐step iterative attribution algorithm 21 , 22 , 23 , 24 based on Evaluation and Management (E&M) claims with type 2 diabetes diagnosis codes and prescription claims in the year preceding the given quarter‐year. We iteratively attributed patients to physicians in four steps, moving from one step to the next if we were unable to identify a physician in a given step. We attributed patients to a physician that was (a) among the top three physicians for both their E&M services and glucose‐lowering drugs, (b) the top physician for either their E&M services or glucose‐lowering drugs, (c) the top prescriber of their statins, and (d) the most recent attributed provider from a year‐long lookback period. Patients not attributed after the fourth step were excluded from the sample. Because attribution varied by quarter, patients were not hierarchically nested within physicians. See Appendix S2 for details.
2.3. Physician characteristics
After attributing patients to physicians, we extracted linked physician data from Doximity®. Doximity aggregates physician information from sources such as provider registries and specialty societies into a comprehensive database; Doximity has been described in detail and validated in prior studies. 25 , 26 We used Doximity to acquire information about physicians’ gender, age, specialty, years since residency, and what medical school they attended. We created binary flags for whether a physician attended a top 20 medical school in the US (whether the school was in the top 20 of either the US News and World Report annual primary care or research medical school rankings in over half of the years between 2007 and 2015) 27 or whether a physician attended a non‐US medical school (based on a search of US medical school accreditation records). 28 , 29 We also created binary flags for whether a physician was a primary care physician, an endocrinologist, a cardiologist, or another specialist; whether the physician completed residency over 20 years ago; whether the physician was over 50 years old; and whether the physician was female. We calculated variance inflation factors and condition numbers for the set of physician characteristics to assess potential multicollinearity. 30 , 31 , 32
2.4. Geographic control variables
OLDW provided patient zip codes, which we used to assign patients to primary care service areas (PCSAs) according to The Dartmouth Atlas. 33 PCSAs are aggregations of census tracts reflecting geographic patterns in primary care use. We obtained PCSA‐level information from The Dartmouth Atlas on the per capita number of internists, the percent of female residents, the percent of Black residents, the percent of White residents, the percent of Hispanic residents, and the percent of residents older than 65. We also used information from The Dartmouth Atlas to calculate an SES index for each PCSA based on a formula designed by the Agency for Healthcare Research and Quality (AHRQ). The AHRQ SES index is derived from a principal component analysis that considers household crowding, property values, poverty, household income, education, and unemployment. 34
Next, to identify whether patients lived in metropolitan PCSAs, we calculated the population‐weighted proportion of census tracts classified with a USDA rural‐urban commuting area codes of 3 or lower. 35 If the weighted proportion was greater than or equal to 0.5, we considered that PCSA metropolitan. Finally, to characterize states’ malpractice environment, we incorporated Chung and colleagues’ 36 rankings of states’ malpractice environments; they ranked every US state in terms of overall legal risk and overall insurance risk (ie, low vs high premiums) based on a principal component analysis of seven common measures of malpractice environment. Using these ranks allowed us to broadly characterize malpractice environments without multicollinearity concerns.
2.5. Mixed effects with interrupted time series specification
To model de‐adoption and physician‐level variation in de‐adoption, we used a linear probability model with an interrupted time series specification and mixed effects. We chose a linear model over a logit or probit model to produce readily interpretable interaction coefficients 37 , 38 and to ease the computation associated with estimating random effects. We used two main specifications, one for describing the magnitude of variation and one for describing the factors which explain variation. The magnitude of variation specification is modeled as follows:
Equation 1: Mixed effects model, “Magnitude of variation” specification
Subscripts: i, patient; j, physician; k, PCSA; t, time period
where
Our outcome of interest was whether patient i filled a fibrate prescription in quarter‐year t. Per the standard interrupted time series specification 39 , 40 the variable Time is a linear time trend that starts at the beginning of our sample period, Post is a binary indicator variable that equals 0 prior to the publication of the ACCORD lipid trial in April 2010 and 1 after publication, and TimeSincePost is a linear time trend that equals 0 prior to the publication of the ACCORD trial and starts “counting up” each quarter afterward. The coefficient on Time is the slope of the trend in fibrate prescribing in the pre‐evidence period, the coefficient on Post is the one‐time shift in fibrate prescribing immediately following publication (ie, an intercept shift), and the coefficient on TimeSincePost is the change in slope of the trend in fibrate prescribing following publication. The sum of the coefficients from Time and TimeSincePost is the trend in fibrate prescribing in the post‐evidence period. We excluded the quarter‐year the ACCORD trial was published and the following quarter‐year as a washout period.
We modeled the intercept and the coefficients on Time, Post, and TimeSincePost with physician‐level random effects. We calculated a likelihood ratio test comparing the model in Equation 1 to a model with no random effects to test whether there was statistically significant physician‐level variation in prescribing. We also calculated several statistics to facilitate interpretation of the magnitude of physicians’ variation in prescribing. First, we calculated coefficients of variation (CVs) for each of the trend parameters:
Equation 2: Coefficient of variation (derived from Equation 1)
and
CVs are standard deviations divided by means; they are unitless and allow for comparisons of spread between distributions. A larger CV implies greater spread in a distribution. Second, we calculated a normal cumulative density function (CDF) with a mean equal to the average one‐time reduction in fibrate prescribing () and variance equal to the physician variance in the one‐time reduction in prescribing (). We used this function to estimate the proportion of physicians with an immediate increase in fibrate prescribing following the publication of the ACCORD lipid trial. Third, we used a normal CDF with the mean of the post‐evidence trend and corresponding variance ( to estimate the proportion of physicians with positive trends in the post‐ACCORD period and the proportion of physicians with post‐evidence trends indicating they increased their prescribing by over one percentage point per quarter following the publication of the trial. Fourth, to describe the combined effect of an immediate shift in prescribing and changes in trends, we estimated a normal CDF with a mean equal to the predicted prescribing rate three years after the ACCORD trial (, where , , and , physician, and geographic covariates respectively) and a corresponding variance ( ). We used this CDF to calculate the proportion of physicians, who in the third year following the ACCORD trial, had prescribing rates higher than the mean prescribing rate if the pre‐evidence trend in prescribing was projected three years into the post‐evidence period (or , where F(·) is the described normal CDF), that is, the proportion of physicians who decreased their prescribing in the three years following the ACCORD trial. We also conducted similar calculations using different amounts of follow‐up time (Appendix S3).
The model in Equation 1 was specified as a function of patient characteristics including age, gender, Elixhauser comorbidities, 41 and attribution step; physician characteristics included gender, whether the physician was a cardiologist, endocrinologist, or other specialist (with primary care physicians as the reference group), whether the physician was over 50 years old, whether the physician had practiced for under 20 years since residency, whether the physician attended a top 20 US medical school, and whether the physician attended a non‐US medical school; and PCSA characteristics included the per capita number of internists, the percent of female residents, the percent of Black residents, the percent of White residents, the percent of Hispanic residents, the percent of residents older than 65, the AHRQ SES index, metropolitan status, state malpractice insurance rank, and state malpractice legal rank.
We stratified our analyses by insurance type (commercially insured vs Medicare Advantage) to account for differences in age composition between the two populations, since statin use (a condition for being in the sample) in different age groups may indicate differences in underlying health status not captured by standard comorbidity adjustments. 42 , 43 Because the OLDW population does not necessarily reflect the US population distribution of Medicare vs. commercial insurance, models combining these groups may yield misleading estimates of overall population effects. However, we also estimated models pooling the two populations (Appendix S4).
The Equation 1 specification only described the magnitude of variation in de‐adoption. We were also interested in factors that explain physician variation in de‐adoption. Accordingly, we also estimated models using the following specification:
Equation 3: Mixed effects model, “Explanation of variance” specification
Subscripts: i, patient; j, physician; k, PCSA; t, time period
where
This specification was identical to Equation 1 with one exception: time variables were a function of both fixed physician characteristics and physician‐level random effects. This allowed us to identify characteristics associated with changes in prescribing following the ACCORD trial.
We evaluated the statistical significance of individual predictors through estimated parameters (the change in level immediately following trial publication), (the pre‐trial trend), (the post‐trial trend), and (the change in trend following trial publication) for different types of physicians, where the binary variables in vectors and for the relevant physician characteristic were set equal to one (except in the case of reference category physician types), and all other binary variables for physician characteristics were set equal to the sample mean. We also conducted Wald tests assessing whether the coefficients in vectors , , , and were jointly equal to zero.
In addition to examining the significance of individual physician characteristics, we calculated pseudo R‐squared statistics 44 to describe the proportion of physicians’ variation explained by the entire set of physician characteristics:
Equation 4: Pseudo R‐squared
where and are directly provided from the model and
If observable physician characteristics explained all the variation in prescribing, then physician variation would shrink such that 0 and . Conversely, if observable characteristics did not explain any variation, then , implying .
We also performed robustness tests in addition to our main specifications, including pooled models not stratified by insurance type (Appendix S4); models limited to observations where the attributed physician had treated at least 40 unique patients within our sample (Appendix S5); and models predicting fibrate use among new diabetic statin users as opposed to all diabetic statin users (Appendix S6).
3. RESULTS
3.1. Descriptive statistics
Our analytic sample included 3 465 582 diabetic patient‐quarters with statin use and commercial insurance and 2 707 439 diabetic patient‐quarters with statin use and Medicare Advantage. In 93% of patient‐quarter observations, the attributed provider was the same as the attributed provider from the previous quarter. Once patients entered the sample, they generally stayed in the sample, where 84% of patient‐quarter observations had an observation for the patient in the following quarter. Patients in Medicare Advantage were older and had more comorbidities compared to commercial patients. Commercially insured patients saw similar types of physicians compared to Medicare Advantage patients, with the exception that Medicare Advantage patients were more likely to see physicians that attended non‐US medical schools. Medicare Advantage patients received care in areas with more internists compared to patients with commercial insurance. Patients with Medicare Advantage also received care in states with greater legal risk for physicians committing malpractice compared to patients with commercial insurance (Table 1).
TABLE 1.
Descriptive statistics of the study samples
| Commercial | Medicare advantage | |
|---|---|---|
| # of total patient‐quarters | 3 465 582 | 2 707 439 |
| % with same attribution from previous quarter | 93.15% | 93.58% |
| % with observation in subsequent quarter | 84.62% | 84.87% |
| Patient characteristics | ||
| # of unique patients | 532,783 | 409,558 |
| Mean age | 58.61 | 72.74 |
| % Female | 40.49% | 53.67% |
| % Elixhauser category 1 (2 or fewer comorbidities) | 39.06% | 18.16% |
| % Elixhauser category 2 (3 to 5 comorbidities) | 49.38% | 51.92% |
| % Elixhauser category 3 (6 or 8 comorbidities) | 9.36% | 21.54% |
| % Elixhauser category 4 (9 or more comorbidities) | 2.20% | 8.38% |
| % attributed in step 1 | 50.19% | 40.94% |
| % attributed in step 2 | 43.38% | 48.12% |
| % attributed in step 3 | 6.28% | 10.76% |
| % attributed in step 4 | 0.16% | 0.18% |
| Physician characteristics | ||
| # of unique physicians | 107,984 | 78,188 |
| % female | 24.62% | 23.93% |
| % PCP | 80.90% | 83.92% |
| % cardiologist | 3.81% | 4.40% |
| % endocrinologist | 9.35% | 4.43% |
| % other specialist | 5.93% | 7.26% |
| % under 50 | 36.77% | 36.33% |
| % less than 20 years since residency | 40.39% | 41.31% |
| % top 20 US medical school | 11.27% | 12.41% |
| % non‐US medical school | 25.68% | 32.32% |
| % other US medical school | 63.05% | 55.27% |
| PCSA characteristics | ||
| # of unique PCSAs | 5,654 | 4,914 |
| Mean % female residents | 51.03% | 51.21% |
| Mean % black residents | 15.28% | 13.84% |
| Mean % white residents | 73.78% | 75.00% |
| Mean % Hispanic residents | 12.61% | 11.13% |
| Mean % residents older than 65 | 12.85% | 14.54% |
| Mean number of internists per 10 000 residents | 4.14 | 4.62 |
| Mean AHRQ SES index | 53.90 | 53.11 |
| % metro | 87.04% | 87.13% |
| Mean malpractice insurance rank | 29.76 | 28.37 |
| Mean malpractice legal rank | 27.78 | 30.39 |
Authors’ analysis of sample of commercially insured and Medicare Advantage patients‐quarters with a diabetes diagnosis and fibrate prescription from 2007 through the third quarter of 2015. “% with same attribution from previous quarter” excludes the first patient‐quarter observation for a patient in calculating the proportion.
In unadjusted analyses, we observed that following the publication of the ACCORD trial, the proportion of diabetic statin users that filled fibrate prescriptions decreased in both the commercial and Medicare Advantage populations (Figure 1A,B).
FIGURE 1.
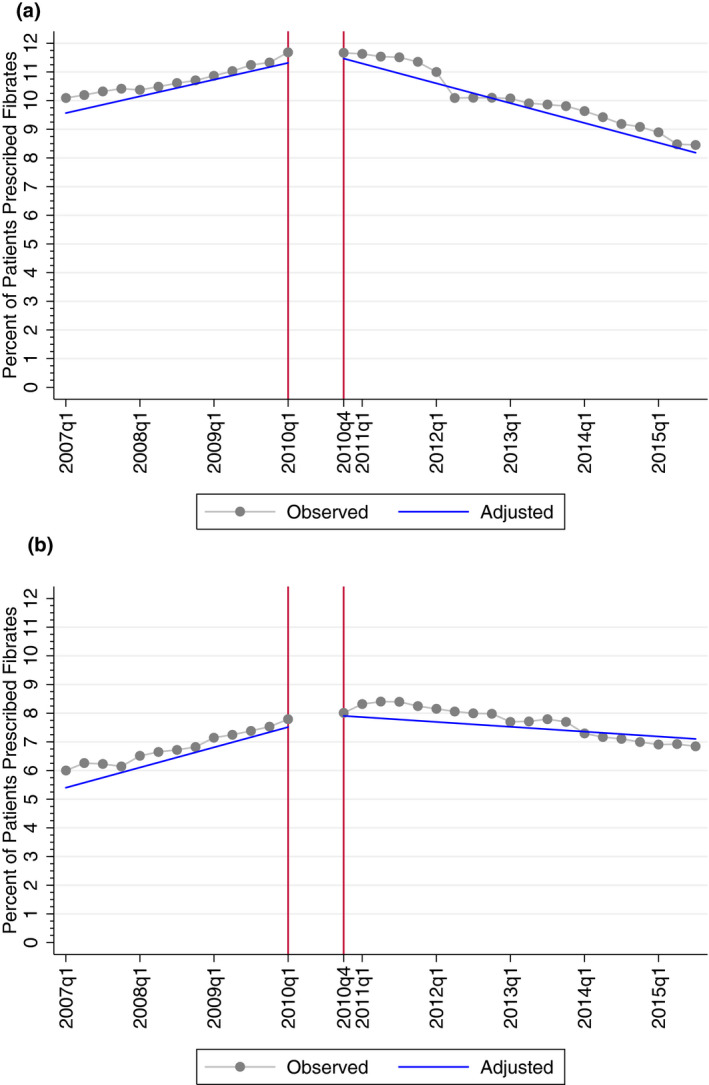
A, Unadjusted and adjusted trends in fibrate prescribing, commercial. B, Unadjusted and adjusted trends in fibrate prescribing, Medicare Advantage. Note: Trends in fibrate prescribing among patients enrolled in Medicare Advantage between 2007 and 2015; observed rates are unadjusted quarterly proportions of observations that received prescriptions, and adjusted rates are trends estimated at the means of patient, physician, and primary care service area (PCSA) covariates and at the means of best linear unbiased predictions of random effects
Collinearity statistics in Table 2 showed that the set of physician characteristics satisfied most conventional thresholds for acceptable levels of collinearity 30 , 31 , 32 ; variance inflation factors were less than 10 and condition numbers were less than 20. Accordingly, we included the entire proposed set of physician characteristics in the regression models.
TABLE 2.
Condition number and variance inflation factors for physician characteristics
| Commercial | Medicare advantage | |
|---|---|---|
| Condition number | 11.51 | 4.84 |
| Variance inflation factors | ||
| Female | 1.08 | 1.08 |
| Cardiologist | 1.02 | 1.02 |
| Endocrinologist | 1.03 | 1.01 |
| Other Specialist | 1.02 | 1.02 |
| Physician < 50 | 1.48 | 2.42 |
| Residency < 20 | 2.52 | 2.46 |
| Top 20 US Medical School | 1.05 | 1.07 |
| Non‐US Medical School | 1.10 | 1.12 |
Authors’ analysis of sample of commercially insured and Medicare Advantage patients‐quarters with a diabetes diagnosis and fibrate prescription from 2007 through the third quarter of 2015.
3.2. Physician variation in prescribing
Estimates adjusted for patient, physician, and PCSA characteristics mirrored unadjusted estimates (Table 3 and Figure 1A,B). Adjusted trends in Figure 1A,B are based on the Equation 1 specification evaluated at the means of patient, physician, and PCSA covariates and at the means of best linear unbiased predictions of random effects. Our results imply that the proportion of observations receiving fibrate therapy decreased by 3.65 percentage points (95% CI, −4.11 to −3.19) among the commercial population and by 2.74 percentage points (95% CI, −3.36 to −2.21) among the Medicare Advantage population three years after the ACCORD trial relative to if the populations had continued on their pre‐trial trends.
TABLE 3.
Trends, level changes, and physician‐level variations in fibrate prescribing before and after evidence
| Commercial | Medicare advantage | |
|---|---|---|
| N | 3 465 582 | 2 707 439 |
| Inclusion of random effects P‐value | <.001 | <.001 |
| Pre‐evidence trend, β 50 (95% CI) | 0.0012 (0.0010, 0.0014) | 0.0017 (0.0013, 0.0020) |
| Pre‐evidence trend, σ 50 | 0.0161 | 0.0111 |
| Pre‐evidence trend, CV | 13.5386 | 6.7065 |
| Pre‐evidence trend, Pseudo R 2 | 0.000 | 0.000 |
| Pre‐evidence trend, β 51 Wald P‐value | .040 | .268 |
| Post‐evidence trend, β 50 + β 60 (95% CI) | −0.0016 (−0.0018, −0.0015) | −0.0005 (−0.0006, −0.0003) |
| Post‐evidence trend, σ 50 + σ60 | 0.0123 | 0.0102 |
| Post‐evidence trend, CV | 7.5351 | 22.6538 |
| Post‐evidence trend, Pseudo R 2 | 0.000 | 0.000 |
| Post‐evidence trend, β 51 + β 61 Wald P‐value | .013 | .133 |
| Change in trend, β 60 (95% CI) | −0.0028 (−0.0031, −0.0025) | −0.0021 (−0.0025, −0.0018) |
| Change in trend, σ 60 | 0.0236 | 0.0180 |
| Change in trend, CV | 8.3563 | 8.5451 |
| Change in trend, Pseudo R 2 | 0.000 | 0.000 |
| Change in trend, β 61 Wald P‐value | .390 | .661 |
| Change in level, β 40 (95% CI) | −0.0026 (−0.0049, −0.0004) | −0.0020 (−0.0050, 0.0009) |
| Change in level, σ 40 | 0.1650 | 0.1237 |
| Change in level, CV40 | 62.6015 | 61.2152 |
| Change in level, Pseudo R 2 | 0.000 | 0.000 |
| Change in level, β 41 Wald P‐value | .480 | .476 |
The pre‐evidence trend, post‐evidence trend, change in trend, and change in level are derived from and respectively in Equation 1. Standard deviations are derived from and respectively in Equation 1. Pseudo R 2 are derived by comparing standard deviations from Equation 1 to corresponding standard deviations in Equation 3. Wald tests assessed whether physician characteristic interactions in vectors and respectively in Equation 3 were jointly equal to zero.
Abbreviations: CI, confidence interval; CV, coefficient of variation.
Likelihood ratio tests indicated significant physician‐level variation in prescribing. In the commercially insured cohort, the physician‐level standard deviation on the post‐evidence trend from Equation 1 () was 7.5 times the magnitude of the mean post‐evidence trend (). On average, physicians immediately decreased their prescribing of fibrates to commercially insured diabetic statin users by 0.26 percentage points (95% CI, −0.49 to −0.04) and continued to decrease their prescribing by 0.16 percentage points per quarter (95% CI, −0.18 to −0.15) following the publication of the ACCORD lipid trial. However, CDF calculations implied 49% of physicians had an immediate increase in their prescribing of fibrates following the ACCORD trial. Following physicians’ immediate response to the trial, 45% of physicians had positive trends in fibrate prescribing following the ACCORD trial, with 17% of physicians increasing their prescribing by more than one percentage point per quarter following the trial. Three years after the ACCORD trial, 48% of physicians treating commercially insured patients had prescribing rates higher than the mean prescribing rate projected if physicians had continued following their pre‐evidence trends.
In the Medicare Advantage cohort, the standard deviation on the post‐evidence trend from Equation 1 () was 22.7 times the magnitude of the mean post‐evidence trend (). On average, physicians’ immediate decrease in prescribing in Medicare Advantage was not significantly different from zero (95% CI, −0.50 to 0.09). However, physicians began decreasing their prescribing by 0.05 percentage points per quarter (95% CI, −0.06 to −0.03) following the publication of the ACCORD trial, a lower rate of de‐adoption compared to the commercial population. CDF calculations implied 48% of physicians had positive trends in fibrate prescribing following the ACCORD trial, with 15% of physicians increasing their prescribing by more than one percentage point per quarter following the trial. Three years after the ACCORD trial, 48% of physicians treating Medicare Advantage patients had prescribing rates higher than the mean prescribing rate projected if physicians had continued following their pre‐evidence trends; using different follow‐up periods yielded qualitatively similar percentages of physicians (Appendix S3). Prescribing patterns were also similar in our applicable robustness checks (Appendices S4‐S6).
3.3. Physician characteristics associated with variation in prescribing
Physician characteristics explained little of the total physician variation in prescribing, as evidenced by pseudo R 2 values equal to zero (Table 3). However, some individual physician characteristics were associated with post‐evidence prescribing trends.
Table 4 presents predicted pre‐evidence trends, predicted post‐evidence trends, predicted changes in trends, and predicted changes in levels associated with specific types of physicians. In the commercial cohort, cardiologists decreased their prescribing of fibrates by 0.16 percentage points per quarter (95% CI, −0.20 to −0.12) following the publication of the ACCORD trial, while primary care physicians decreased their prescribing by 0.10 percentage points per quarter (95% CI, −0.11 to −0.09), a statistically significant difference in post‐evidence trends between cardiologists and primary care physicians (P = .003). In the Medicare Advantage cohort, physicians aged 50 years or more decreased their prescribing of fibrates by 0.07 percentage points per quarter (95% CI, −0.10 to −0.04) following the publication of the trial while physicians under 50 decreased their prescribing by 0.03 percentages points per quarter (95% CI, −0.05 to −0.01), a statistically significant difference in post‐evidence trends between older and younger physicians (P = .033). However, we did not find a cardiologist effect in the Medicare Advantage population or an age effect in the commercial population (Table 4). We also did not observe differences between physician types in their changes in prescribing levels or trends following publication of the trial, as evidenced by individual tests of significance (Table 4) and Wald tests of joint significance (Table 3).
TABLE 4.
Trends and level changes in fibrate prescribing before and after evidence by physician characteristics
| Commercial | Medicare advantage | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐evidence trend | Post‐evidence trend | Change in trend | Change in level | Pre‐evidence trend | Post‐evidence trend | Change in trend | Change in level | |
| Characteristic | ||||||||
| Male Physician | 0.0013 (0.0010, 0.0015) | −0.0017 (−0.0018, −0.0015) | −0.0030 (−0.0033, −0.0026) | −0.0024 (−0.0052, 0.0003) | 0.0019 (0.0016, 0.0023) | −0.0004 (−0.0005, −0.0002) | −0.0023 (−0.0027, −0.0019) | −0.0034 (−0.0069, 0.0001) |
| Female Physician | 0.0009 (0.0005, 0.0014) | −0.0016 (−0.0019, −0.0013) | −0.0025 (−0.0031, −0.0020) | −0.0018 (−0.0064, 0.0029) | 0.0009 (0.0003, 0.0016) | −0.0006 (−0.0009, −0.0004) | −0.0015 (−0.0023, −0.0008) | 0.0029 (−0.0032, 0.0089) |
| Difference P‐value | .172 | .594 | .179 | .816 | .010 | .086 | .089 | .083 |
| Cardiologist | 0.0003 (−0.0006, 0.0012) | −0.0024 (−0.0029, −0.0018) | −0.0026 (−0.0037, −0.0015) | −0.0033 (−0.0131, 0.0065) | 0.0013 (−0.0001, 0.0028) | −0.0007 (−0.0012, −0.0002) | −0.0020 (−0.0036, −0.0004) | −0.0078 (−0.0213, 0.0058) |
| PCP | 0.0013 (0.0010, 0.0015) | −0.0016 (−0.0018, −0.0014) | −0.0029 (−0.0032, −0.0026) | −0.0019 (−0.0044, 0.0007) | 0.0016 (0.0013, 0.0020) | −0.0004 (−0.0006, −0.0003) | −0.0020 (−0.0025, −0.0016) | −0.0017 (−0.0049, 0.0016) |
| Difference P‐value | .031 | .007 | .661 | .787 | .683 | .280 | .972 | .391 |
| Endocrinologist | 0.0007 (−0.0002, 0.0017) | −0.0021 (−0.0027, −0.0016) | −0.0029 (−0.0041, −0.0017) | −0.0018 (−0.0114, 0.0078) | 0.0019 (0.0002, 0.0036) | −0.0007 (−0.0014, 0.0000) | −0.0026 (−0.0046, −0.0007) | 0.0068 (−0.0088, 0.0224) |
| PCP | 0.0013 (0.0010, 0.0015) | −0.0016 (−0.0018, −0.0014) | −0.0029 (−0.0032, −0.0026) | −0.0019 (−0.0044, 0.0007) | 0.0016 (0.0013, 0.0020) | −0.0004 (−0.0006, −0.0003) | −0.0020 (−0.0025, −0.0016) | −0.0017 (−0.0049, 0.0016) |
| Difference P‐value | .263 | .080 | .987 | .985 | .755 | .427 | .583 | .298 |
| Other specialist | 0.0014 (0.0006, 0.0021) | −0.0011 (−0.0016, −0.0007) | −0.0025 (−0.0034, −0.0016) | −0.0080 (−0.0163, 0.0002) | 0.0025 (0.0013, 0.0036) | −0.0002 (−0.0006, 0.0002) | −0.0027 (−0.0039, −0.0014) | −0.0060 (−0.0167, 0.0046) |
| PCP | 0.0013 (0.0010, 0.0015) | −0.0016 (−0.0018, −0.0014) | −0.0029 (−0.0032, −0.0026) | −0.0019 (−0.0044, 0.0007) | 0.0016 (0.0013, 0.0020) | −0.0004 (−0.0006, −0.0003) | −0.0020 (−0.0025, −0.0016) | −0.0017 (−0.0049, 0.0016) |
| Difference P‐value | .866 | .071 | .445 | .163 | .163 | .279 | .366 | .441 |
| Provider < 50 | 0.0012 (0.0008, 0.0015) | −0.0018 (−0.0020, −0.0015) | −0.0029 (−0.0034, −0.0025) | −0.0013 (−0.0048, 0.0022) | 0.0017 (0.0012, 0.0022) | −0.0003 (−0.0005, −0.0001) | −0.0019 (−0.0025, −0.0014) | −0.0032 (−0.0077, 0.0013) |
| Provider ≥50 | 0.0012 (0.0007, 0.0017) | −0.0015 (−0.0018, −0.0012) | −0.0027 (−0.0033, −0.0021) | −0.0040 (−0.0093, 0.0012) | 0.0017 (0.0010, 0.0025) | −0.0007 (−0.0010, −0.0004) | −0.0024 (−0.0033, −0.0016) | 0.0004 (−0.0064, 0.0073) |
| Difference P‐value | .860 | .194 | .620 | .461 | .962 | .033 | .424 | .456 |
| Residency < 20 | 0.0013 (0.0010, 0.0017) | −0.0016 (−0.0019, −0.0014) | −0.0029 (−0.0034, −0.0025) | −0.0029 (−0.0066, 0.0008) | 0.0016 (0.0010, 0.0021) | −0.0005 (−0.0007, −0.0003) | −0.0020 (−0.0026, −0.0014) | −0.0004 (−0.0053, 0.0044) |
| Residency ≥ 20 | 0.0010 (0.0006, 0.0015) | −0.0017 (−0.0020, −0.0014) | −0.0027 (−0.0033, −0.0021) | −0.0014 (−0.0062, 0.0035) | 0.0019 (0.0012, 0.0026) | −0.0004 (−0.0006, −0.0001) | −0.0022 (−0.0030, −0.0015) | −0.0039 (−0.0102, 0.0024) |
| Difference P‐value | .434 | .867 | .597 | .676 | .536 | .604 | .719 | .462 |
| Top 20 US Medical School | 0.0004 (−0.0002, 0.0010) | −0.0018 (−0.0022, −0.0014) | −0.0022 (−0.0029, −0.0014) | 0.0041 (−0.0026, 0.0107) | 0.0017 (0.0009, 0.0025) | −0.0006 (−0.0010, −0.0003) | −0.0023 (−0.0033, −0.0014) | −0.0051 (−0.0127, 0.0024) |
| Other US Medical School | 0.0013 (0.0010, 0.0016) | −0.0017 (−0.0018, −0.0015) | −0.0029 (−0.0033, −0.0026) | −0.0023 (−0.0052, 0.0006) | 0.0016 (0.0012, 0.0020) | −0.0004 (−0.0006, −0.0003) | −0.0020 (−0.0025, −0.0016) | −0.0018 (−0.0058, 0.0022) |
| Difference P‐value | .009 | .614 | .077 | .084 | .834 | .306 | .561 | .438 |
| Non‐US Medical School | 0.0014 (0.0009, 0.0018) | −0.0016 (−0.0019, −0.0013) | −0.0030 (−0.0035, −0.0024) | −0.0050 (−0.0095, −0.0005) | 0.0018 (0.0012, 0.0024) | −0.0004 (−0.0006, −0.0001) | −0.0022 (−0.0029, −0.0015) | −0.0008 (−0.0063, 0.0047) |
| Other US Medical School | 0.0013 (0.0010, 0.0016) | −0.0017 (−0.0018, −0.0015) | −0.0029 (−0.0033, −0.0026) | −0.0023 (−0.0052, 0.0006) | 0.0016 (0.0012, 0.0020) | −0.0004 (−0.0006, −0.0003) | −0.0020 (−0.0025, −0.0016) | −0.0018 (−0.0058, 0.0022) |
| Difference P‐value | .750 | .726 | .933 | .332 | .514 | .541 | .714 | .776 |
Pre‐evidence trends, post‐evidence trends, changes in trends, and changes in levels were calculated by estimating and respectively from Equation 3 with the binary flag for the given physician type equal to 1 (or non‐reference physician types equal to 0 if the given physician type is the reference category in the model for that set of physician characteristics) and all other binary variables in vectors and respectively equal to their sample means.
Abbreviations: PCP, primary care physician; US, United States.
4. DISCUSSION
We documented significant physician‐level variation in de‐adoption and found that physicians decreased their prescribing of fibrates on average by 0.16 percentage points per quarter in the commercially insured population and by 0.05 percentage points per quarter in the Medicare Advantage population following the publication of the ACCORD lipid trial. However, 45% of physicians treating commercially insured patients and 48% of physicians treating Medicare Advantage patients had positive trends in prescribing following the ACCORD trial. Further, while physician specialty and age were associated with prescribing trends, observable physician characteristics explained little of the total extent of physician variation in de‐adoption.
Our study had several limitations. First, we only observed prescription fills billed for payment. Prescriptions paid for with cash or manufacturer coupons were missing from our data. Missed prescriptions could have led to underestimation of the denominator population (statin users) or the numerator population (fibrate users). Second, attribution of patients to physicians is a key feature of our study, and although we followed a validated attribution methodology, in some cases the attributed physician may not have been responsible for potentially prescribing an inappropriate treatment. Third, there are potentially important factors not accounted for in our analysis. For example, prior work has found physicians’ interpretations of the ACCORD trial were associated with whether they had financial interests in companies marketing fibrates. 45 Additionally, the adoption of new therapies (rather than the de‐adoption of existing therapies) has been tied to dynamic factors including physicians’ peer networks, the organization a physician operates in, and the structure of a physician's market. 46 , 47 , 48 Future studies should consider the role of marketing, peer networks, organizations, and markets in physicians’ de‐adoption decisions.
The clinical context of our case study, specifically the potential alternative treatments, 3 informs how our results should be interpreted. For concurrent statins and fibrates, the alternative is clear; the ACCORD trial suggested that physicians could reasonably take average diabetic statin users off fibrates and not expect increases in cardiovascular events. Our case study was a relatively straightforward de‐adoption decision. Despite this, outlets had mixed responses to the ACCORD trial. In an analysis of news articles and biomedical journal articles following the trial, Downing and colleagues found that, “Articles discussing the trial offered no clear consensus on the role of fenofibrate for patients with diabetes, with nearly 20% suggesting it was effective and 30% suggesting it was ineffective. Even among those that provided a mixed interpretation of the trial's findings (ie, noting the trial's strengths and weaknesses), the authors often went on to recommend fibrate use.” 45
There may never be a “perfect” case study in de‐adoption. For ineffective but safe drugs, it seems unlikely that there will ever be universal agreement that a drug truly has no benefit for any patient group. Due to the nature of randomized trials and the assessment of efficacy via average treatment effects, some physicians are bound to argue that a given treatment has some benefit for a particular subpopulation. The complexity of de‐adoption decisions only increases when physicians consider whether to stop prescribing a treatment when no obvious safe and effective alternative exists.
The growing de‐adoption literature should focus on commonalities between different case studies in de‐adoption while using differences in contextual factors to highlight nuances in the de‐adoption process. We believe our findings make three important contributions to this growing literature. First, we studied de‐adoption among commercially insured and Medicare Advantage enrollees. To our knowledge, no study has examined de‐adoption in two different insurance populations concurrently. The heterogeneity in de‐adoption responses between our studied populations suggests there are limits to the generalizability of some prior de‐adoption studies. Second, we observed substantial physician‐level variation in de‐adoption, with many physicians increasing potentially ineffective prescribing following the publication of new evidence; prior studies have rarely described this type of variation. Future research should assess whether this variation is present in the de‐adoption of other ineffective and unsafe treatments. Third, we found that while some individual physician characteristics were associated with prescribing patterns, observable physician characteristics collectively explained little of physicians’ total variation in de‐adoption, similar to prior findings on physicians’ use of low‐value care. 16 , 17 Future studies should explore the role of drug manufacturer marketing, physician peer networks, organizational characteristics, and market structure in de‐adopting ineffective and unsafe treatments.
Supporting information
Author matrix
Supplementary Material
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: Analyses were supported by funding from the National Institute on Aging (P01 AG005842), the National Heart, Lung, and Blood Institute (R56 HL130496), and the Agency for Healthcare Research and Quality (R01 HS025164). Alexander Everhart and Laura Barrie Smith also acknowledge support from the AHRQ doctoral training program at the University of Minnesota (T32 HS000036) during the conduct of this study.
Everhart A, Desai NR, Dowd B, et al. Physician variation in the de‐adoption of ineffective statin and fibrate therapy. Health Serv Res. 2021;56:919–931. 10.1111/1475-6773.13630
REFERENCES
- 1. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de‐adoption of low‐value clinical practices: a scoping review. BMC Med. 2015;13(1):255. 10.1186/s12916-015-0488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prasad V, Vandross A, Toomey C, et al. A Decade of reversal: an analysis of 146 contradicted medical practices. Mayo Clin Proc. 2013;88(8):790‐798. 10.1016/J.MAYOCP.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 3. van Bodegom‐Vos L, Davidoff F, Marang‐van de Mheen PJ. Implementation and de‐implementation: two sides of the same coin? BMJ Qual Saf. 2017;26(6):495‐501. 10.1136/bmjqs-2016-005473 [DOI] [PubMed] [Google Scholar]
- 4. Bekelis K, Skinner J, Gottlieb D, Goodney P. De‐adoption and exnovation in the use of carotid revascularization: retrospective cohort study. BMJ. 2017;359:j4695. 10.1136/bmj.j4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard DH. Trends in the use of knee arthroscopy in adults. JAMA Intern Med. 2018;178(11):1557. 10.1001/jamainternmed.2018.4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard DH, Hockenberry J. Physician age and the abandonment of episiotomy. Health Serv Res. 2019;54(3):650‐657. 10.1111/1475-6773.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohan AV, Fazel R, Huang P‐H, Shen Y‐C, Howard D. Changes in geographic variation in the use of percutaneous coronary intervention for stable ischemic heart disease after publication of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial. Circ Cardiovasc Qual Outcomes. 2014;7(1):125‐130. 10.1161/CIRCOUTCOMES.113.000282 [DOI] [PubMed] [Google Scholar]
- 8. Wallaert JB, Nolan BW, Stone DH, et al. Physician specialty and variation in carotid revascularization technique selected for Medicare patients. J Vasc Surg. 2016;63(1):89‐97. 10.1016/J.JVS.2015.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howard DH, Shen Y‐C. Trends in PCI volume after negative results from the COURAGE Trial. Health Serv Res. 2014;49(1):153‐170. 10.1111/1475-6773.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook BL, Wang Y, Sonik R, et al. Assessing provider and racial/ethnic variation in response to the FDA antidepressant box warning. Health Serv Res. 2019;54:255‐262. 10.1111/1475-6773.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howard DH, Soulos PR, Chagpar AB, Mougalian S, Killelea B, Gross CP. Contrary to conventional wisdom, physicians abandoned a breast cancer treatment after a trial concluded it was ineffective. Health Aff. 2016;35(7):1309‐1315. 10.1377/hlthaff.2015.1490 [DOI] [PubMed] [Google Scholar]
- 12. Smith LB, Desai NR, Dowd B, et al. Patient and provider‐level factors associated with changes in utilization of treatments in response to evidence on ineffectiveness or harm. Int J Heal Econ Manag. 2020;20(3):299‐317. 10.1007/s10754-020-09282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. commercially insured patients, 2012–2017. N Engl J Med. 2019;380(11):1043‐1052. 10.1056/NEJMsa1807069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801. 10.1001/jamainternmed.2015.0157 [DOI] [PubMed] [Google Scholar]
- 15. Goodwin JS, Jaramillo E, Yang L, Kuo YF, Tan A. Is anyone listening? Variation in PSA screening among providers for men 75+ before and after United States preventive services task force recommendations against it: a retrospective cohort study. PLoS One. 2014;9(9):e107352. 10.1371/journal.pone.0107352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan A, Zhou J, Kuo Y‐F, Goodwin JS. Variation among primary care physicians in the use of imaging for older patients with acute low back pain. J Gen Intern Med. 2016;31(2):156‐163. 10.1007/s11606-015-3475-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of physician variation in provision of low‐value services. JAMA Intern Med. 2019;179(1):16. 10.1001/jamainternmed.2018.5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563‐1574. 10.1056/NEJMoa1001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. OptumLabs . OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. n.p., 2019. Reproduced with permission from OptumLabs. [Google Scholar]
- 20. Centers for Medicare and Medicaid Services . Health Insurance Exchange 2019 Quality Rating System Measure Technical Specifications. 2018. https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityInitiativesGenInfo/Downloads/QRS‐2019‐Measure‐Technical‐Specifications.pdf [Google Scholar]
- 21. Higuera L, Carlin C. A comparison of retrospective attribution rules. Am J Manag Care. 2017;23(6):e180‐e185. [PubMed] [Google Scholar]
- 22. Mehrotra A, Adams JL, Thomas JW, McGlynn EA. The effect of different attribution rules on individual physician cost profiles. Ann Intern Med. 2010;152(10):649‐654. 10.7326/0003-4819-152-10-201005180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130‐1139. 10.1056/NEJMsa063979 [DOI] [PubMed] [Google Scholar]
- 24. McCoy RG, Bunkers KS, Ramar P, et al. Patient attribution: why the method matters. Am J Manag Care. 2018;24(12):596‐603. [PMC free article] [PubMed] [Google Scholar]
- 25. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149‐1158. 10.1001/jama.2015.10680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294‐1304. 10.1001/jamainternmed.2016.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. US News and World Report . Best Graduate Schools. 2007. ‐2015. [Google Scholar]
- 28. Association of American Medical Colleges . AAMC Organization Directory Search Result. https://members.aamc.org/eweb/DynamicPage.aspx?site=AAMC&webcode=AAMCOrgSearchResult&orgtype=MedicalSchool. Accessed February 16, 2020.
- 29. American Association of Osteopathic Medicine . U S. Colleges of Osteopathic Medicine ‐ AACOM. https://www.aacom.org/become‐a‐doctor/u‐s‐colleges‐of‐osteopathic‐medicine. Accessed February 16, 2020.
- 30. Belsley DA, Kuh E, Welsch R. In: Kuh E, Welsch RE, eds. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Wiley; 1980. [Google Scholar]
- 31. Greene WH. Econometric Analysis, 7th ed. Prentice Hall; 2012. [Google Scholar]
- 32. O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673‐690. 10.1007/s11135-006-9018-6 [DOI] [Google Scholar]
- 33. The Center for the Evaluative Clinical Sciences DMS . The Dartmouth Atlas of Health Care. American Hospital Publishing; 1996. [Google Scholar]
- 34. Bonito A, Bann C, Eicheldinger C, Carpenter L. Creation of New Race‐Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries. 2012. https://archive.ahrq.gov/research/findings/final‐reports/medicareindicators/index.html#contents [Google Scholar]
- 35. United States Department of Agriculture ERS . Documentation: 2010 Rural‐Urban Commuting Area (RUCA) Codes. Published 2012. https://www.ers.usda.gov/data‐products/rural‐urban‐commuting‐area‐codes/documentation/. Accessed June 8, 2019.
- 36. Chung JW, Sohn M‐W, Merkow RP, et al. Development of a composite measure of state‐level malpractice environment. Health Serv Res. 2014;49(2):751‐766. 10.1111/1475-6773.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ai C, Norton EC. Interaction terms in logit and probit models. Econ Lett. 2003;80(1):123‐129. 10.1016/S0165-1765(03)00032-6 [DOI] [Google Scholar]
- 38. Karaca‐Mandic P, Norton EC, Dowd B. Interaction terms in nonlinear models. Health Serv Res. 2012;47(1pt1):255‐274. 10.1111/j.1475-6773.2011.01314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299‐309. 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 40. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl.):S38‐S44. 10.1016/j.acap.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 41. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 42. Curfman G. Risks of statin therapy in older adults. JAMA Intern Med. 2017;177(7):966. 10.1001/jamainternmed.2017.1457 [DOI] [PubMed] [Google Scholar]
- 43. Standards of medical care in diabetes—2017: Summary of revisions. Diabetes Care. 2017;40(Suppl. 1):S4‐S5. [DOI] [PubMed] [Google Scholar]
- 44. Raudenbush SW. In: Bryk AS, ed. Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd ed. Sage Publications; 2002. [Google Scholar]
- 45. Downing NS, Cheng T, Krumholz HM, Shah ND, Ross JS. Descriptions and interpretations of the ACCORD‐lipid trial in the news and biomedical literature. JAMA Intern Med. 2014;174(7):1176. 10.1001/jamainternmed.2014.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burke MA, Fournier GM, Prasad K. The diffusion of a medical innovation: Is success in the stars? South Econ J. 2007;73:588‐603. 10.2307/20111913 [DOI] [Google Scholar]
- 47. Molitor D. The evolution of physician practice styles: evidence from cardiologist migration. Am Econ J Econ Policy. 2018;10(1):326‐356. 10.1257/pol.20160319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karaca‐Mandic P, Town RJ, Wilcock A. The effect of physician and hospital market structure on medical technology diffusion. Health Serv Res. 2017;52(2):579‐598. 10.1111/1475-6773.12506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author matrix
Supplementary Material


