Abstract
Objective:
This study aimed to investigate quality of life (QOL) improvement in long-term cancer survivors using complementary therapy (CT) as mind–body practice.
Methods:
A quasi-experimental study including intervention and control groups was conducted. Participants in the intervention group engaged in CTs, including music therapy, progressive muscle relaxation, and deep-breathing exercises for 8 weeks at home. QOL was evaluated in both the groups using Short Form-8 (SF-8) questionnaire before the experiment and at 4 and 8 weeks after starting the experiment. To examine QOL, we compared SF-8 subscale scores, the physical and mental component summaries of QOL.
Results:
Cancer survivors were assigned to the intervention and control groups, comprising 69 and 59 individuals. There were no significant differences in QOL between the two groups with low scores, but there was a significant difference in the mental aspect of QOL in 4 weeks, indicating that the intervention group was lower than the control group. Meanwhile, the intervention group tended to experience increased changes in the mental aspect of QOL in 8 weeks compared to 4 weeks, although there was no significant difference.
Conclusions:
CT did not exhibit an effect on QOL among cancer survivors, especially in 4 weeks. This might have been due to sample size, participants' potential low compliance resulting in an inability to confirm whether the CTs were performed accurately and continuously, and consideration of what CT suited them. Meanwhile, CT may require a longer time to increase QOL. We recommend further studies to address these factors when conducting CT as mind–body practice.
Keywords: Cancer survivors, complementary therapy, mind–body practice, quality of life
Introduction
In Japan, cancer has been the most common cause of death since 1981. In 2021, the predicted number of new cancer diagnoses was 1,009,800, and the predicted number of cancer deaths was 378,500.[1] The survival rates of patients with cancer more than 5 years and 10 years after the completion of their initial treatment are 64.1% and 57.2%, respectively. These are higher than the 5-year survival rates from 10 years ago, which were under 60%. The 5-year survival rates are more than 90% for prostate, skin, thyroid, and breast cancers but <50% for pancreatic, gallbladder, bile duct, lung, and brain cancers.[2] Owing to an increase in the number of long-term survivors, oncology nurses are expected to consider the care of long-term cancer survivors in addition to those undergoing treatment. A previous study described how nursing care for cancer survivors in Japan was mostly provided in acute care settings at the time of diagnosis and during the subsequent treatment period, while long-term survivors were poorly cared for after their active treatments (operation, strong chemotherapy, and radiotherapy) because there were likely few opportunities for them to contact medical staff when they were engaged in active treatments.[3] This showed that there was a demand for nursing care among cancer survivors after active treatment, and this demand continues. Thus, we aimed to investigate the care of long-term cancer survivors in this study.
“Cancer survivorship” refers to how well individuals can live from the time of their diagnosis until the end of their lives.[4] This concept implies that cancer survivors require improved quality of life (QOL) in order to return to normal life after active treatment. Meanwhile, long-term cancer survivors live with psychosocial problems, such as anxiety about physical symptoms and recurrences, even after returning to their roles in society. According to some reports, for at least 2 years after diagnosis, cancer survivors had a higher prevalence of anxiety and depression than individuals without cancer,[5] and cancer survivors who received treatment have reportedly suffered health-related problems such as fatigue and sleep disturbances.[6] Thus, they need to be cared for after receiving treatment to minimize psychosocial and physical distress, thereby enhancing the QOL of long-term cancer survivors.
In nursing care, one method of self-care management promoting QOL is complementary therapy (CT), practiced by nurses,[7] among others.[8] CT is brought by the US National Center for Complementary and Integrative Health as a group of diverse medical and health-care systems, practices, and products that are not generally considered a part of conventional medicine, such as natural products and mind–body practices (e.g., acupressure, aromatherapy, hypnosis, massage, relaxation/meditation, music therapy, reflexology, tai chi, qigong, and yoga).[9,10] Mind–body practices can be defined as techniques to help modify biological, physiological, or psychosocial processes as well as improve QOL outcomes.[11] The terms “CT” or “complementary and alternative medicine” (CAM) are often used to describe a broad range of therapies and practices that are nonconventional or nontraditional. In the current study, “CT” refers to a form of mind–body practice of self-care management in nursing care.
The mechanism of CT as a mind–body practice is to reduce the stress responses such as anxiety, depression, and tension. When someone experiences a stressful event, firstly the unpleasant information of danger or threat gathered through a sense organ consisting of five senses is sent to the amygdala, an area of the brain that contributes to emotional processing, then the danger signal is sent to hypothalamus.
In the stress response,[12] the hypothalamus functions like a command center, communicating with the rest of the body through the autonomic nervous system (consisting of the sympathetic and parasympathetic nervous systems) and the hypothalamic–pituitary–adrenal axis (which secretes a corticotropic-releasing hormone, adrenocorticotropic hormone). The sympathetic nervous system, which triggers the release of the hormone epinephrine, results in a number of physical changes, such as pulse and respiratory rates, blood pressure, and blood sugar. As the initial surge of epinephrine subsides, the hypothalamus activates the second component of the stress response system known as the hypothalamic–pituitary–adrenal axis. Continuous stress contributes to health problems such as lifestyle-related diseases and also decreases QOL. Thus, CT works on the amygdala and hypothalamus by providing comforting stimulation of the five senses to reduce stress by inducing calm.
According to Onishi's study,[7,13] the CT used as a mind–body practice program for self-care included music therapy, progressive muscle relaxation (PMR), and deep-breathing exercises, which were effective for alleviating anxiety and depression among cancer survivors. Music therapy has been reported to have biological, psychological, and epidemiological effects on the central and peripheral nervous systems.[14] In music therapy, classical music is used as healing music,[15] and Mozart's music has been reported as effective for improving QOL.[16] PMR, a relaxation method developed by Edmund Jacobson, has been as effective for alleviating symptoms and improving QOL by reducing tension and anxiety among cancer patients.[17,18] Deep-breathing exercises heighten parasympathetic nerve activity and relax patients; therefore, they are psychologically effective for improving mood and quality of sleep and reducing stress. These CT types are also effective for self-care management.[19,20]
The current study involved conducting a secondary data analysis to examine improvements in QOL by using CTs as a mind–body practice program for self-care for long-term cancer survivors after active treatment.
Methods
Study design
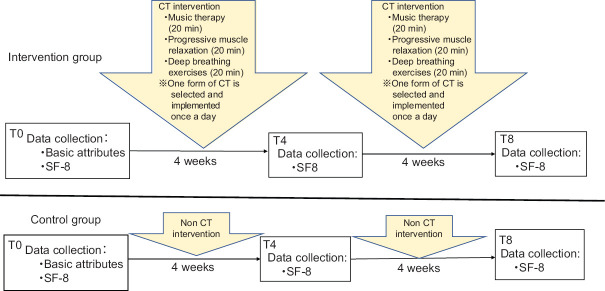
This study was a quasi-experimental design including patients who visited a cancer support center. Eligible patients, who consented to participate, were assigned to either the intervention or control group based on whether their study enrollment was during an odd or even month, respectively. Participants in the intervention group were asked to use CT for 8 weeks. In both the groups, QOL was measured before the start of the experiment (T0), at 4 weeks (T4), and at 8 weeks (T8) after the start of the study. The changes in QOL at T4 and T8 with respect to T0 were evaluated and compared between the two groups.
Participants
The participants were cancer patients in Japan. We calculated the sample size using G*Power 3.1.[21] It was estimated that a sample of 67 participants per group could provide 80% power at a 5% level of significance to detect a medium effect size of 0.5 for changes in QOL after the intervention (effect size = 0.5, α =0.05, power = 0.8). Allowing for an attrition rate of 20%, a total of 168 participants were required, 84 in each group.
The study was described to outpatients visiting the cancer support center, and verbal and written consent to participate in the study was obtained from 190 people. The inclusion criteria were as follows: (1) aged 18 years or older, (2) diagnosed with Stage I-III cancer, (3) completed active treatment, and (4) able to communicate in Japanese. The exclusion criterion included previous consultation in a psychiatric department.
Instruments
We used the Japanese version of the health-related QOL scale Short Form-8 (SF-8), which has previously been used in the cancer population.[22] The questionnaire has also been used in large-scale epidemiological studies[23,24,25] and has confirmed validity and reliability. The Cronbach's α coefficient in this study was 0.823–0.914.[22] The SF-8, excerpted from the main items of the SF-36, can be used for comparisons after calculating national standard scores in a simple test. The SF-8 measures each item in the eight health concepts of physical functioning, role limitation due to physical health problem, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. Based on the SF-8 subscale scores, the scores of the two summary measures, the physical component summary (PCS) and mental component summary (MCS), were calculated by adding the scores for each item. The PCS and MCS are generally used to evaluate QOL.[22]
These were converted to a range of 0–100, where 50 marks the national standard value. Higher scores indicated better QOL.
Procedure
We contacted patients when they visited the cancer support center and explained the purpose and procedures of this study. Participants who gave their consent were asked to visit the center after 4 and 8 weeks to respond to the questionnaire. Data were collected from 2012 to 2013. However, this set of data is still applicable in the current local context. Patients who visited the center during odd months were assigned to the intervention group, and those who visited in even months were assigned to the control group. The study participants, data collector, and interventionist were not blinded.
The participants in both the groups were asked to respond to the questions at the cancer support center, taking privacy into consideration, at the times T0, T4 and T8. In the intervention group, the CT implementation status was confirmed in the provided checklist at times T4 and T8, while the control group did not need it. All of these procedures were performed by the author.
Intervention
The intervention group was assigned a set of CTs containing three types of compact discs (CDs) that were used for 20 min a day for 8 weeks at home by the participants themselves. CT (music therapy, PMR, or deep-breathing exercises) was carried out with participants using either a commercial CD of a Mozart piano sonata and concerto, a CD of PMR created by Onishi,[26] or a commercial CD of deep-breathing exercises, each for lasting 20 min. These CTs have been validated in previous studies.[7,13,26] Participants in this group were asked to select their favorite CD from one of the three CTs to use as a mind–body practice. Participants were asked to use one of the three CTs during each session because all three were considered effective based on previous studies confirming their effectiveness and because participants could continue to practice without getting tired using the same CT every time. We provided participants with a checklist so that they could practice CT daily as part of their routine. The checklist included items to record which of the three CTs was used for practice, the time of practice, and health conditions. Participants were required to bring their completed checklist to the 4- and 8-week visits for a compliance check. The study design is illustrated in Figure 1.
Figure 1.
Study design
Ethical approval
Ethical approval for this study was obtained from the Institutional Review Board (IRB) before the start of the study. The purpose and methods of the study were explained to the participants, and written consent was obtained. We also explained that participation was voluntary and that the obtained data would be anonymized.
Statistical analysis
Participant characteristics between the intervention and control groups were compared using independent t-test and Chi-squared test. The subscale scores, PCS, and MCS of SF-8 between the two groups at T0, T4, and T8 did not all follow a normal distribution according to the Shapiro–Wilk test. Then the subscale scores, PCS, and MCS of the SF-8 between the two groups at T0, T4, and T8 were compared using the Mann–Whitney U-test. The changes in subscale scores, PCS, and MCS after 4 (T4-T0) and 8 weeks (T8-T0) were compared between the groups using the Mann–Whitney U-test. The Statistical Package for the Social Sciences version 25 (IBM Japan, Tokyo, Japan) was used for all analyses, and the significance level was set at P < 0.05.
Results
Recruitment and completion
According to the sample size estimation, among the 190 consenting participants, 69 (62.7%) participants in the intervention group and 59 (73.8%) in the control group were eligible for analysis [Figure 2]. In the intervention group, 36 participants (24 in 4 weeks and 12 in 8 weeks) dropped out and 5 had missing data, while 20 participants dropped out and 1 was untraceable in the control group. The reasons for dropout from the intervention group included the following: “busy schedule made it difficult to continue,” “could not continue owing to physical or mental problems,” and “the CTs, especially music did not match my tastes.” Participant dropout in the control group was attributed to failure to visit the center at the appointed times.
Figure 2.
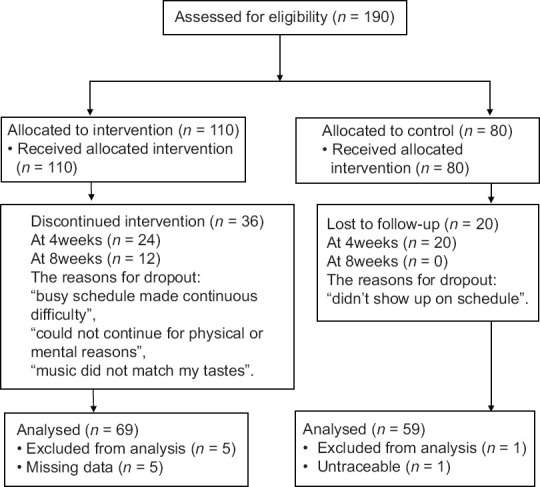
Study flowchart
Demographic and clinical characteristics
A comparison of the basic attributes of the two groups is shown in Table 1. No statistically significant differences were observed for marital status, education, employment status, and recurrence. However, there was a difference for age (P = 0.02). The average ages were 51.0 ± 10.2 in the intervention and 55.4 ± 11.5 in the control groups.
Table 1.
Demographics and clinical characteristics n=128
| Characteristics | Intervention Group n=69, n(%) | Control Group n=59, n(%) | P |
|---|---|---|---|
| Age, years | mean: 51.0 (SD, 10.2) | mean: 55.4 (SD, 11.5) | 0.02*,† |
| Gender | |||
| Male | 4 (5.8) | 5 (8.5) | 0.73‡ |
| Female | 65 (94.2) | 54 (91.5) | |
| Marital status | |||
| Married | 52 (75.4) | 47 (79.7) | 0.42‡ |
| Single, divorces, or widowed | 13 (18.8) | 10 (17.0) | |
| No answer | 4 (5.8) | 2 (3.4) | |
| Education | |||
| Junior high school | 1 (1.4) | 4 (6.8) | 0.62‡ |
| Senior high school | 28 (40.6) | 24 (40.7) | |
| Vocational school | 10 (14.5) | 8 (13.6) | |
| Junior college | 13 (18.8) | 12 (20.3) | |
| University, graduate school | 15 (21.7) | 9 (15.3) | |
| No answer | 2 (2.9) | 2 (3.4) | |
| Employment status | |||
| Employed | 23 (33.3) | 20 (33.9) | 0.26‡ |
| Unemployed/retired | 45 (65.2) | 37 (62.7) | |
| No answer | 1 (1.4) | 2 (3.4) | |
| Recurrence | |||
| Yes | 13 (18.8) | 12 (20.3) | 0.63‡ |
| No | 39 (56.5) | 26 (44.1) | |
| No answer | 17 (24.6) | 21 (35.6) | |
| Cancer type | |||
| Breast cancer | 38 (55.1) | 32 (54.2) | |
| Gynecological cancers | 14 (20.3) | 13 (22.0) | |
| Pancreatic and Gallbladder cancer | 3 (4.3) | 2 (3.4) | |
| Leukemia and Lymphoma | 3 (4.3) | 1 (1.7) | |
| Colorectal cancer | 2 (2.9) | 0 | |
| Stomach cancer | 2 (2.9) | 2 (3.4) | |
| Lung cancer | 1 (1.4) | 4 (6.8) | |
| Renal and Urinary tract cancer | 1 (1.4) | 2 (3.4) | |
| Tongue cancer | 1 (1.4) | 1 (1.7) | |
| Peritoneal cancer | 1 (1.4) | 1 (1.7) | |
| Thyroid cancer | 0 | 1 (1.7) | |
| Cancer of unknown origin | 1 (1.4) | 0 | |
| No answer | 2 (2.9) | 0 | |
| Treatments (Multiple answers) | |||
| Surgical treatment | 63 | 54 | |
| Chemotherapy | 59 | 42 | |
| Radiation therapy | 21 | 14 | |
| Hormone therapy | 23 | 13 | |
| others | 1 | 1 |
SD: Standard deviation, *P<0.05, †t-test, ‡Chi-squared test
Changes in quality of life scores after undergoing complementary therapy
The means and standard deviations for the SF-8 subscale scores, PCS, and MCS of QOL at T0, T4, and T8 along with changes in QOL scores at T4-T0 and T8-T0 are displayed in Table 2.
Table 2.
Comparison between intervention and control groups on quality of life (n=128)
| SF-8 subscales (PCS and MCS) | n | T0 score (mean±SD) | P # | T4 score (mean±SD) | P # | T8 score (mean±SD) | P # | T4-T0 change (mean±SD) | P # | T8-T0 change (mean±SD) | P # |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical functioning | |||||||||||
| Intervention group | 69 | 44.37±8.70 | 0.60 | 44.83±5.62 | 0.56 | 44.92±5.85 | 0.96 | 0.47±8.32 | 0.61 | 0.55±8.89 | 0.59 |
| Control group | 59 | 45.19±8.70 | 44.45±9.62 | 44.69±7.51 | −0.73±10.24 | −0.49±8.81 | |||||
| Role limitation due to physical health problem | |||||||||||
| Intervention group | 69 | 42.56±9.53 | 0.57 | 43.48±6.63 | 0.91 | 43.77±7.56 | 1.00 | 0.92±8.55 | 0.70 | 1.21±9.29 | 0.41 |
| Control group | 59 | 43.56±9.06 | 43.16±8.58 | 43.81±8.23 | −0.39±9.06 | 0.25±9.25 | |||||
| Bodily pain | |||||||||||
| Intervention group | 69 | 47.79±9.45 | 0.47 | 48.29±8.68 | 0.92 | 47.03±7.72 | 0.37 | 0.49±10.2 | 0.51 | −0.77±8.17 | 0.81 |
| Control group | 59 | 46.95±9.27 | 48.36±7.79 | 45.80±8.03 | 1.41±9.62 | −1.15±10.21 | |||||
| General health | |||||||||||
| Intervention group | 69 | 46.02±7.51 | 0.99 | 45.81±7.21 | 0.70 | 45.87±6.97 | 0.31 | −0.21±7.27 | 0.77 | −0.15±7.90 | 0.29 |
| Control group | 59 | 45.68±8.35 | 45.11±8.47 | 44.62±7.95 | −0.57±9.64 | −1.06±8.85 | |||||
| Vitality | |||||||||||
| Intervention group | 69 | 48.63±7.10 | 0.90 | 47.14±6.38 | 0.58 | 47.41±5.22 | 0.86 | −1.48±8.22 | 0.80 | −1.22±7.03 | 0.97 |
| Control group | 59 | 48.77±7.41 | 47.44±8.13 | 47.58±7.4 | −1.34±7.14 | −1.19±6.68 | |||||
| Social functioning | |||||||||||
| Intervention group | 69 | 42.88±9.32 | 0.73 | 41.26±9.50 | 0.09 | 43.01±9.38 | 0.64 | −1.62±9.99 | 0.04* | 0.13±10.30 | 0.95 |
| Control group | 59 | 42.12±10.45 | 43.76±9.22 | 42.47±8.92 | 1.63±9.56 | 0.35±10.27 | |||||
| Role limitations due to emotional problems | |||||||||||
| Intervention group | 69 | 44.34±10.79 | 0.14 | 45.96±8.44 | 0.39 | 46.34±7.31 | 0.30 | 1.61±10.62 | 0.46 | 2.00±9.53 | 0.47 |
| Control group | 59 | 42.19±10.55 | 44.05±9.67 | 44.63±8.60 | 1.86±10.79 | 2.44±10.79 | |||||
| Mental health | |||||||||||
| Intervention group | 69 | 45.91±7.56 | 0.57 | 45.89±6.40 | 0.11 | 46.49±6.58 | 0.18 | −0.02±7.11 | 0.01* | 0.59±7.09 | 0.91 |
| Control group | 59 | 45.38±6.88 | 47.47±8.04 | 45.24±7.07 | 2.08±7.88 | −0.14±7.71 | |||||
| PCS | |||||||||||
| Intervention group | 69 | 44.10±8.14 | 0.46 | 44.44±6.38 | 0.84 | 43.93±6.56 | 0.83 | 0.33±7.57 | 0.21 | −0.17±7.83 | 0.19 |
| Control group | 59 | 44.95±8.12 | 43.81±8.34 | 43.81±7.47 | −1.14±9.63 | −1.14±8.75 | |||||
| MCS | |||||||||||
| Intervention group | 69 | 45.16±8.50 | 0.18 | 44.83±7.57 | 0.41 | 46.00±7.62 | 0.16 | −0.33±8.96 | 0.03* | 0.84±7.65 | 0.84 |
| Control group | 59 | 43.56±8.49 | 45.78±7.74 | 44.69±6.74 | 2.22±8.83 | 1.12±8.82 |
#: Mann-Whitney U-test; *P<0.05, SF-8: Short form 8; SD: Standard deviation; T0: CTs used at baseline; T4: CTs used at 4eeks; T8: CTs used at 8eeks; PCS: Physical component summary; MCS: Mental component summary
All QOL scores from the SF-8 in this study were lower than the national reference value of 50.[22] In other words, participants had low QOL throughout the experiment since before the start of this study.
Regarding the measured values of the SF-8 subscales, PCS, and MCS, and the changes in T4-T0 and T8-T0, there were statistically significant differences in the changes for “social functioning” (P = 0.04), “mental health” (P = 0.01), and MCS (P = 0.03) in T4-T0 between the two groups. In T4-T0, “social functioning” was −1.62 ± 9.99, “mental health” was −0.02 ± 7.11, and MCS was −0.33 ± 8.96 for the intervention group, while was 1.63 ± 9.56, mental health was 2.08 ± 7.88, and MCS was 2.22 ± 8.83 for the control group. All of these scores were worse in the intervention group. MCS was affected by “social functioning” and “mental health”, which are important factors in calculating the mental QOL score.[22] There were no statistically significant differences in the change for T8-T0. This indicates that there are potential psychosocial factors at work during the 4-week period.
Figure 3 shows comparisons of changes in the PCS and MCS scores for T4-T0 and T8-T0 for each of the groups. The PCS of QOL showed that there were almost no changes in QOL for both the groups. The MCS of QOL indicated that there was a tendency for less change in QOL for T8-T0 than T4-T0 in the intervention group, while fewer changes were noted in the control group. However, there were no statistically significant differences between the two groups.
Figure 3.
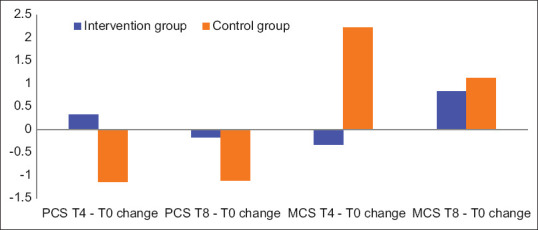
The change in physical component summary (PCS) and mental component summary (MCS) of quality of life for T4-T0 and T8-T0
Discussion
The aim of this study was to explore improvement in QOL by using CT as a mind–body practice for long-term cancer survivors after active treatment. The results of this study indicated no statistically significant difference in QOL between the intervention and control groups in 8 weeks. Furthermore, the participants' QOL scores since the start of the experiment were below the national standard value (score of 50). Meanwhile, in the study using the same CTs, anxiety and depression were alleviated using a stress rating scale.[7,13] This difference may be attributed to the following points. Measurement of health-related QOL includes several components, such as “physical functioning”, “mental health”, and social function, which considers the whole person, while measurement of the emotional anxiety–depression scale only includes mental components. Therefore, more than 1 month may be needed to measure the effect of QOL as the emotional anxiety–depression scale from the stress rating scale is measured at one point in time. We do acknowledge, however, that it may not be possible to measure QOL using the same procedures as an anxiety–depression scale. On the other hand, some studies have reported improvement in QOL after a 6-week intervention in which patients selected the CAM they preferred[27] and in a 6-month survey on CAM used by patients undergoing treatment.[28] Considering these reports, a longer period may be needed to ascertain the effect of CT as a mind–body practice for QOL, which can be a process for obtaining self-care management.
Regarding QOL in 4 weeks, the psychosocial aspect of the QOL presented significantly lower QOL for the intervention group compared to the control group. This may have been due to “a large number of participants dropping out,” or “not becoming accustomed to practicing the CTs,” although we were unable to accurately explain these. There were 36 dropouts in the CT intervention group, of which 24 dropped out at 4 weeks, which was very significant. The reasons for dropout included the inability to continue the CT every day because of a busy schedule, physical or psychosocial problems, or a dislike for the CTs, especially the music offered. Alternatively, participants were unfamiliar with using CTs at the beginning of the study. Thus, there were unknown levels of adherence to the intervention whereby patients were supposed to perform the CTs correctly. The intervention group's age (51.0 ± 10.2) was younger than the control group's age (55.4 ± 11.5), which might explain some differences due to psychosocial distress associated with a menopausal disorder in the early 50s' women, although this is not well substantiated with empirical data. Regarding busy schedules and physical or psychosocial problems that might be influenced by a menopausal disorder, it is possible that we should have contacted participants more frequently by phone or E-mail to inquire about their circumstances at home, their schedule, their psychosocial and physical distress, how the CTs were going, and so on to encourage accurate and continuous CT performance. Further, the use of information technology, such as a smartphone app instead of phone or E-mail, should be considered. This may help prevent participant dropout.
Regarding a dislike for the CTs, especially the music, we did not consider that specific CTs could be unsuitable for some patients because the three CTs were effective for anxiety and depression. However, some studies found that dislike for the music being used can cause a mental burden,[29] as musical tastes are affected by personality[30] and are interrelated with other mental aspects.[31] Based on these study findings that mental burden and music taste can be influenced by personality and mental states, it is possible that some participants suffered a mental burden related to QOL when using music therapy. Meanwhile, a Cochrane database of systematic reviews showed that music therapy interventions may have beneficial effects on anxiety, pain, fatigue, and QOL in people with cancer.[32]
In addition to PMR and deep breathing, it has been reported that the effects of PMR can be shaped by patient characteristics that influence the effectiveness of interventions, such as active involvement, guided instructions, providing a source of distraction, stimulating relaxation, individual abilities and preferences, and pain qualities.[33] In some systematic reviews, PMR reduced anxiety levels associated with other CTs, but few studies have shown improvements in QOL by PMR alone.[34] No studies have investigated the effects of deep-breathing therapy on the QOL of cancer survivors after active treatment. For patients who used either music therapy, PMR, or deep-breathing exercises, without considering what suited them, it should be noted that different effects could have been obtained from the use of a form of CT on QOL that is better suited to patients. The discussions in these reports suggest that maintenance and improvement of QOL, especially for the mental aspect of cancer survivors, might be possible when implementing favorable CTs, considering patient differences, such as personality and psychosocial state. Thus, it might have been necessary to confirm whether the CTs were performed accurately and continuously and consider what CT best suited the patients.
Moreover, the small sample size in relation to the 36 dropouts in this study may be another possible reason why the CTs did not improve QOL. The estimated sample size was 84 participants per group. The high attrition rate of the study greatly reduced the sample size, which may have affected the ability to detect a difference in the outcomes between the two groups. Thus, a larger sample size may be needed to determine the effects of CT as a mind–body practice on QOL.
For QOL in 8 weeks, the results in Figure 3 show that using CTs improved the mental aspect of QOL in 8 weeks compared to 4 weeks, although the difference was not statistically significant. The effects of the physical aspect of participants' QOL on CT were not observed during the 8 weeks. This might be because the CT used in this study was expected to have an effect on the mental aspects rather than the physical aspects. This might represent the necessary duration for the effects of CT to appear related to the mental aspect of QOL.
Limitations
The limitation of this study included unknown adherence to the intervention, which could represent a lack of confirmation regarding accurate and continuous use of CT and consideration of the best-suited CT for each participant in the intervention. It is because that CT did not exhibit an effect on QOL, especially in 4 weeks, perhaps owing to a large number of participants dropping out due to busy schedule, physical and psychosocial problem, or dislike to the CTs, especially the music offered. It might have been necessary to confirm the use of CT, investigate the physical and psychosocial problems including menopausal symptoms, and consider which CTs are best suited for individual participants. Moreover, the small available sample size was due to a high attrition rate. Therefore, these limitations need to be considered in future studies.
Conclusions
No improvement was noted for the physical and mental aspects of QOL in cancer survivors after undergoing CT for 8 weeks, especially for the mental aspects of QOL in 4 weeks. This might have been due to a short period for obtaining the effects of CT on QOL, a potentially low compliance of CT use by participants as there was no confirmation of unknown levels of performance, perhaps owing to busy schedules, physical and psychosocial problems including menopausal symptoms, and not having a CT that best suited them, which was the cause of a large number of dropout. Future studies should consider these limitations and encourage participants to perform continuously via the use of current information technology.
Although the mental aspect of QOL increased at 8 weeks for the intervention group, there was not a statistically significant difference. Considering some studies showed improvements in QOL when using CT as a mind-body practice over a longer period, a longer follow-up period may be warranted to demonstrate CT's effectiveness for enhancing QOL among cancer survivors.
Therefore, a larger sample size and a longer follow-up period are needed.
Financial support and sponsorship
This study was supported by a fund from Grand-in-Aid for Scientific Research (A) in Japan 2009-2013 (Grant No. 21249095).
Conflicts of interest
The corresponding author, Prof. Kazuko Onishi, is an editorial board member of Asia-Pacific Journal of Oncology Nursing. The article was subject to the journal's standard procedures, with peer review handled independently of Prof. Onishi and their research groups.
References
- 1.Cancer Registration and Statistics. National Cancer Research Center in Japan. [Last accessed on 2021 Jul 20]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/short_pred.html .
- 2.Cancer Registration and Statistics. National Cancer Research Center in Japan. [Last accessed on 2021 Jul 20]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/summary.html .
- 3.Miura A, Matsuda Y, Ogawa I, Takagai J, Hirai K, Hosoda Y, et al. Oncology nurses' recognition of a long cancer survivorship care in Japan. Asian Pac J Oncol Nurs. 2015;2:136–43. doi: 10.4103/2347-5625.163412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: A new challenge in delivering quality cancer care. J Clin Oncol. 2006;24:5101–4. doi: 10.1200/JCO.2006.09.2700. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013;14:721–32. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 6.Westin SN, Sun CC, Tung CS, Lacour RA, Meyer LA, Urbauer DL, et al. Survivors of gynecologic malignancies: Impact of treatment on health and well-being. J Cancer Surviv. 2016;10:261–70. doi: 10.1007/s11764-015-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onishi K, Tsujikawa M, Inoue K, Yoshida K, Goto S. The effect of complementary therapy for hospital nurses with high stress. Asia Pac J Oncol Nurs. 2016;3:272–80. doi: 10.4103/2347-5625.189810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: A randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. doi: 10.1186/1472-6882-14-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieland LS, Manheimer E, Berman BM. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane collaboration. Altern Ther Health Med. 2011;17:50–9. [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Complementary and Integrative Health. [Last accessed on 2021 Jan 04]. Available from: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-aname .
- 11.Chaoul A, Milbury K, Sood AK, Prinsloo S, Cohen L. Mind-body practices in cancer care. Curr Oncol Rep. 2014;16:417. doi: 10.1007/s11912-014-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Understanding the Stress Response. [Last accessedon 2021 May 03]. Available from: https:/ /www.health.harvard.edu/staying-healthy/understanding-the-stress-response .
- 13.Onishi K. Complementary therapy for cancer survivors: Integrative nursing care. Asia Pac J Oncol Nurs. 2016;3:41–4. doi: 10.4103/2347-5625.178170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans D. The effectiveness of music as an intervention for hospital patients: A systematic review. J Adv Nurs. 2002;37:8–18. doi: 10.1046/j.1365-2648.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 15.Scheufele PM. Effects of progressive relaxation and classical music on measurements of attention, relaxation, and stress responses. J Behav Med. 2000;23:207–28. doi: 10.1023/a:1005542121935. [DOI] [PubMed] [Google Scholar]
- 16.Trappe HJ. Music and health – What kind of music is helpful for whom? What music not. Dtsch Med Wochenschr. 2009;134:2601–6. doi: 10.1055/s-0029-1243066. [DOI] [PubMed] [Google Scholar]
- 17.Arakawa S. Relaxation to reduce nausea, vomiting, and anxiety induced by chemotherapy in Japanese patients. Cancer Nurs. 1997;20:342–9. doi: 10.1097/00002820-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Shahriari M, Dehghan M, Pahlavanzadeh S, Hazini A. Effects of progressive muscle relaxation, guided imagery and deep diaphragmatic breathing on quality of life in elderly with breast or prostate cancer. J Educ Health Promot. 2017;6:1. doi: 10.4103/jehp.jehp_147_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoi K. Trends in nursing research on complementary and alternative therapies for chronic patients in Japan; A comparison of nursing research papers on complementary and alternative therapies for chronic disease patients and cancer patients. J Hum Nurs Stud. 2010;8:25–33. [Google Scholar]
- 20.Kondo Y. Effect of repeated intervention with progressive muscle relaxation in cancer patients. J Jpn Soc Cancer Nurs. 2008;22:86–97. [Google Scholar]
- 21.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 22.Fukuhara T, Suzukamo Y. Manual of the SF-8 Japanese Version. Kyoto: IHope International Co; 2014. [Google Scholar]
- 23.Taguchi R, Yamazaki Y, Takayama T, Saito M. Life-lines of relapsed breast cancer patients: A study of post-recurrence distress and coping strategies. Jpn J Health Hum Ecol. 2008;74:217–35. [Google Scholar]
- 24.Shiozaki M, Hirai K, Dohke R, Morita T, Miyashita M, Sato K, et al. Measuring the regret of bereaved family members regarding the decision to admit cancer patients to palliative care units. Psychooncology. 2008;17:926–31. doi: 10.1002/pon.1312. [DOI] [PubMed] [Google Scholar]
- 25.Zenger M, Glaesmer H, Höckel M, Hinz A. Pessimism predicts anxiety, depression and quality of life in female cancer patients. Jpn J Clin Oncol. 2011;41:87–94. doi: 10.1093/jjco/hyq168. [DOI] [PubMed] [Google Scholar]
- 26.Onishi K. Study Report Stress Nursing. Mie, Japan: Mie University, College of Medical Science; 1998. pp. 1–210. [Google Scholar]
- 27.Bar-Sela G, Danos S, Visel B, Mashiach T, Mitnik I. The effect of complementary and alternative medicine on quality of life, depression, anxiety, and fatigue levels among cancer patients during active oncology treatment: Phase II study. Support Care Cancer. 2015;23:1979–85. doi: 10.1007/s00520-014-2560-1. [DOI] [PubMed] [Google Scholar]
- 28.Kang DH, McArdle T, Suh Y. Changes in complementary and alternative medicine use across cancer treatment and relationship to stress, mood, and quality of life. J Altern Complement Med. 2014;20:853–9. doi: 10.1089/acm.2014.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okawa A, Kato M, Miyachi Y, Ishiguro C, Kume R, Taketani H, et al. Dependency on personal attributes of complementary alternative therapies: Emotional stability and relaxation effects produced by exercise and music. Nagoya City University Academic Repository. 2006;6:7–12. [Google Scholar]
- 30.Nuki M, Nagata K, Kawakami H. The relations among EEG, mood, preference, personality and spectrum power analysis in listening to healing music. IPSJ SIG Tech Rep. 2004;57:35–40. [Google Scholar]
- 31.Otsuji M, Sato N. Relationship between listeners' mental health and music preference. Jpn J Psychosom Med. 2017;57:160–72. [Google Scholar]
- 32.Bradt J, Dileo C, Magill L, Teague A. Music interventions for improving psychological and physical outcomes in cancer patients? Cochrane Database Syst Rev. 2016;8:CD006911. doi: 10.1002/14651858.CD006911.pub3. doi: 10.1002/14651858.CD006911.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Kwekkeboom KL, Hau H, Wanta B, Bumpus M. Patients' perceptions of the effectiveness of guided imagery and progressive muscle relaxation interventions used for cancer pain. Complement Ther Clin Pract. 2008;14:185–94. doi: 10.1016/j.ctcp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charalambous A, Giannakopoulou M, Bozas E, Marcou Y, Kitsios P, Paikousis L. Guided Imagery And Progressive Muscle Relaxation as a Cluster of Symptoms Management Intervention in Patients Receiving Chemotherapy: A Randomized Control Trial. PLoS One. 2016;11:e0156911. doi: 10.1371/journal.pone.0156911. [DOI] [PMC free article] [PubMed] [Google Scholar]