Abstract
Human fibroblast activation protein (FAP), a member of the serine prolyl oligopeptidase family, is a type II cell surface glycoprotein selectively expressed by fibroblastic cells in areas of active tissue remodeling, such as the embryonic mesenchyme, areas of wound healing, the gravid uterus, and the reactive stroma of epithelial cancers. Homologues of FAP have been identified in the mouse and Xenopus laevis. FAP is a dual-specificity enzyme that acts as a dipeptidyl peptidase and collagenase in vitro. To explore the role of FAP in vivo, Fap−/− mice were generated by homologous recombination. RNase protection analysis and reverse transcription-PCR confirmed the absence of full-length Fap transcripts in mouse embryonic tissues. No FAP protein was detected in Fap−/− animals by immunohistochemistry, and no FAP-specific dipeptidyl peptidase activity was found. We report that Fap−/− mice are fertile, show no overt developmental defects, and have no general change in cancer susceptibility.
The study of oncogenesis and tumor progression encompasses a broad range of genetically programmed signaling events within the transformed cancer cells (4, 5) and changes affecting the cellular and biochemical tissue environment of the transformed cells (11, 15). Among the cellular and biochemical changes associated with the cancer environment, neoangiogenesis and activation of proteolyic enzymes have received much attention (2, 20).
The supporting stroma of most types of epithelial cancers contains an abundance of specialized fibroblastic cells, referred to as reactive tumor fibroblasts. A highly consistent and specific molecular trait of tumor stromal fibroblasts found in carcinomas of the breast, colorectum, lung, stomach, pancreas, and esophagus is the expression of fibroblast activation protein (FAP), a cell surface-bound type II transmembrane protein, which belongs to the family of serine prolyl oligopeptidases and which acts as a dual-specificity dipeptidyl peptidase (DPP) and collagenase in vitro (3, 12, 17). FAP-expressing fibroblasts have been detected also in the granulation tissue of healing wounds and certain chronic inflammatory lesions, whereas resting fibrocytes in normal adult tissues generally lack detectable FAP expression (3, 13, 14, 17). Consequently, a role for the FAP protease in extracellular matrix degradation or growth factor activation in tumor stroma and in other sites of tissue remodeling has been suggested.
FAP homologues in Xenopus laevis and in mice have been studied (1, 9). Murine FAP has 89% amino acid sequence identity to human FAP and exhibits DPP activity indistinguishable from that of its human counterpart (9, 10). Mouse embryonic fibroblasts grown in vitro and mouse embryonic tissues were found to express Fap, consistent with the findings for human FAP expression. In addition, the fibroblast-rich stroma of epithelial cancer xenografts grown in immunodeficient mice expresses mouse Fap, supporting the similarities between the two molecules in both species. Interestingly, the Xenopus FAP was also identified as a marker of tissue remodeling (1). A search of gene databases has identified no isoforms of FAP in any species. In humans and mice, the closest relative of FAP is DPP IV, also known as CD26, a DPP that differs markedly in its tissue expression profile and enzyme activity (7).
In this study, we generated homozygous Fap-deficient mice to address the biological function of FAP in embryonic development and tissue remodeling, notably cancer development.
(These studies were carried out in partial fulfillment of the requirements for the Ph.D. thesis of J. Niedermeyer.)
MATERIALS AND METHODS
Oligonucleotides.
Oligonucleotides were obtained from Roth (Karlsruhe, Germany). The following oligonucleotides were used: mFap7 (5′-GGAAACACAAAGAGAGCTCGTACCT-3′), mFap36 (5′-ATTCTGAAGGTCGTAGATG-3′), mFap37 (5′-GCTCTGGCGATATTCATAC-3′), mFap41 (5′-CATAAACCCAGTCTGGTATTCC-3′), ko1 (5′-AACCTCGAGGAATTCGTCGACAAGATGATATTTACAACAAAAGACCCCCTC-3′), ko2 (5′-CAGGCGGCCGCATCATTTATTCTGTGAGTTTGTAAAATG-3′), neo5′ (5′-GACGTTGTCACTGAAGCGGGAAGGG-3′), and neo3′ (5′-AATCGGGAGCGGCGATACCGTAAAGC-3′).
Fap targeting vector.
The targeting construct was based on pPNT, a vector containing a neomycin resistance and a thymidine kinase cassette under the control of the phosphoglycerate kinase-1 promoter (18) facilitating positive and negative selection. To generate a Fap targeting construct, a 7.5-kb XbaI fragment of the Fap gene spanning parts of exon 5 and exons 6 to 9 was isolated from a mouse genomic library (Stratagene, Heidelberg, Germany) and was cloned into pPNT cleaved with XbaI, yielding plasmid pJo44. Subsequently, with primer set ko1 and ko2, a 1.2-kb genomic fragment encompassing intronic sequences between exons 3 and 4 was amplified by PCR, cleaved with NotI and XhoI, and inserted into pJo44/NotI/XhoI resulting in the final targeting construct, pJo49.
Homologous recombination in ES cells and generation of Fap−/− mice.
The embryonic stem (ES) cell line R1 (8), derived from 129/Sv male mice, was cultured in the presence of leukemia inhibitory factor as described earlier (19). Trypsinized ES cells (1.2 × 107) were mixed with 30 μg of the NotI-linearized targeting vector/ml and transfected by electroporation with a single pulse at 285 V and 490 μF by using a gene pulser (Hoefer Scientific Instruments, San Francisco, Calif.). G418- and gancyclovir-resistant clones were doubly selected in medium containing G418 (300 μg/ml) and gancyclovir (2 μM). The homologous recombination event was assessed by Southern blotting and PCR analysis. Mutant clones were expanded and injected into C57BL/6 recipient blastocysts. Male founder animals exhibiting extensive coat color chimerism were crossed into C57BL/6 females and screened for agouti offspring. Germ line transmission was assessed by Southern blotting. Heterozygous Fap-deficient mice were interbred to homozygosity, and the genotypes of F2 progeny were determined by Southern blot analysis. Fap−/− mice were repeatedly backcrossed into 129/Sv and C57BL/6 wild-type animals (eight times) in order to obtain two Fap−/− mouse strains with defined genetic background.
RNase protection assay.
Total RNA was isolated from Fap+/− and Fap−/− 14.5-day embryos by the guanidine thiocyanate method. RNase protection analysis was performed with the Ambion ribonuclease protection assay kit according to the manufacturer's protocol. Briefly, a 319-bp SacI DNA fragment spanning the mouse Fap cDNA from nucleotides 122 to 441 (9) was subcloned into SacI-linearized pBS/KS II (Stratagene). To generate a radioactively labeled riboprobe, the resulting plasmid was linearized with BamHI and used as a template for in vitro transcription with T7 RNA polymerase. The 32P-labeled riboprobe was hybridized to 20 μg of total RNA in 20 μl of hybridization buffer (80% deionized formamide, 100 mM sodium citrate [pH 6.4], 300 mM sodium acetate [pH 6.4], 1 mM EDTA) for 16 h at 55°C. Nonprotected RNA was digested with RNase A (40 μg/ml) and RNase T1 (2 μg/ml) for 1 h at 37°C, followed by phenol extraction and ethanol precipitation. Protected 32P-labeled RNA fragments were separated in an 8% polyacrylamide gel and visualized by autoradiography. The sizes of the protected RNA fragments were determined by coelectrophoresis of a sequencing reaction mixture. To control for the completeness of the RNase digest, 20 μg of yeast RNA was used instead of total RNA.
RT-PCR and sequencing.
Reverse transcription-PCRs (RT-PCRs) were carried out with 25 μg of total RNA, derived from embryonic day 14.5 (E14.5) Fap+/− and Fap−/− mouse embryos, as the template. First-strand synthesis was performed at 42°C for 2 h in the presence of 1 μg of oligo(dT) primer and 400 U of Superscript II reverse transcriptase (Gibco-BRL, Eggenstein, Germany). PCRs were performed in a 50-μl mixture consisting of 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, a 0.2 mM deoxynucleoside triphosphate mixture, 1 μM concentrations of each of various primers, 1.25 U of Taq DNA polymerase (Roche Diagnostics, Mannheim, Germany), and 1.5 μl of the reverse transcription reaction mixture as a template. PCR cycles were run at 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min, for a total of 30 cycles. The following primer sets were used: mFap7 and mFap36 to amplify a 300-bp segment spanning exon 3 to exon 6, neo5′ and neo3′ to amplify a 500-bp fragment of the neo cassette, and mFap37 and mFap41 to generate a 244-bp amplicon corresponding to an exon 6-to-exon 8 segment. The resulting PCR products were cloned into pCRII (TA cloning kit; Invitrogen, San Diego, Calif.) and were sequenced by the dideoxynucleotide chain termination method with Sequenase (United States Biochemical, Renner, Dannstadt, Germany).
Immunological reagents.
Protein A-Sepharose was obtained from Pharmacia Fine Chemicals (Stockholm, Sweden). Antisera used were 3D11, a mouse monoclonal antibody (MAb; immunoglobulin G2b [IgG2b]) recognizing an extracellular epitope of mouse and human FAP (data not shown), rabbit anti-mouse IgG (Dianova, Hamburg, Germany), and H194-112, a rat IgG2a antibody recognizing mouse DPP IV (PharMingen, San Diego, Calif.).
Preparation of membrane extracts.
Two embryos (E17.5) of each genotype were homogenized in 5 ml of ice-cold lysis buffer (1% Triton X-114, 10 mM MgCl2, 1× Tris-buffered saline [TBS]). After incubation on ice for 3 min, the homogenates were cleared by centrifugation for 10 min (4,500 × g) at 4°C and phase partitioned at 37°C for 20 min, followed by a 20-min centrifugation (4,500 × g) at room temperature. The detergent phase was diluted by adding 4 volumes of dilution buffer (0.75% Empigen BB, 5 mM CaCl2, 5 mM MgCl2, 1× TBS). The resulting membrane extracts were either used for immunoprecipitation or stored frozen at −80°C.
Immunopurification and DPP assays.
Membrane extracts were precleared by incubation for 1 to 2 h with 100 μl of protein A-Sepharose/ml at 4°C. Beads were pelleted by centrifugation for 30 s (1,000 × g), and the supernatant was incubated with 20 μl of protein A-Sepharose conjugated with rabbit anti-mouse IgG and anti-FAP MAb 3D11. After incubation at 4°C for 12 h, the beads were washed four times with 1 ml of wash buffer (0.05% Triton X-100, 50 mM Tris [pH 8.4], 150 mM NaCl, 1 mM CaCl2, 1 mg of chicken albumin/ml), once with 1 ml of buffer containing 25 mM Tris, pH 8.4, and 250 mM NaCl, and four times with phosphate-buffered saline (PBS). DPP activity was assayed in the presence of 100 μl of reaction buffer (500 μM Ala-Pro 7-amino-trifluromethyl coumarin [AFC] [Bachem, Bubendorf, Switzerland], 100 mM Tris-HCl [pH 7.8], 100 mM NaCl) for 1 h at 37°C. The release of free AFC was measured in a Cytofluor fluorometer (PerSeptive Biosystems, Inc.) with the 395-nm excitation and 538-nm emission filter set.
Immunohistochemical procedures.
Tissues were obtained from Fap+/+ and Fap−/− E13.5 embryos (129/Sv-C57BL/6 chimeras). For embryo staging, noon of the day on which the copulatory plug was detected was designated E0.5. Tissues were embedded in Optimal-Cryo-Temperature compound (Miles, Kankakee, Ill.), frozen in isopentane precooled in liquid nitrogen, and stored at −70°C. The avidin-biotin complex immunoperoxidase procedure was performed as previously described (3). Briefly, 5-μm-thick frozen sections were cut, mounted on poly-l-lysine-coated slides, air dried, and fixed in acetone for 10 min at 4°C. Sections were treated with H2O2 for 3 min to block endogenous peroxidase and then incubated with biotinylated MAb 3D11 (20 μg/ml) in 2% bovine serum albumin-PBS for 1 h at room temperature. The sections then were washed in PBS and subsequently incubated with avidin-biotin horseradish peroxidase complex (ABC Elite; Vector Labs, Burlingame, Calif.). The final reaction product was visualized with 3,3′-diaminobenzidine. Sections were counterstained with Harris hematoxylin. For histological evaluation, sections were stained with hematoxylin and eosin.
RESULTS
Generation of Fap-deficient mice.
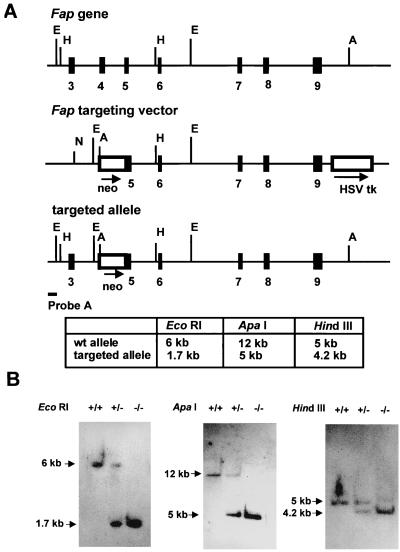
To disrupt the Fap gene, a targeting construct was generated by deleting exon 4, intron 4, and part of exon 5 sequences and replacing them with a PGK-1 neo cassette (Fig. 1A). The targeting vector was electroporated into R1 ES cells followed by positive and negative selection with G418 and gancyclovir. Two independent mutant cell clones were identified by Southern blot analysis; these were expanded in cell culture and injected into C57BL/6 recipient blastocysts. Male founder animals exhibiting extensive coat color chimerism were crossed with C57BL/6 females, offspring were screened for agouti coat color, and germ line transmission was assessed by Southern blotting of tail DNA (data not shown).
FIG. 1.
(A) Targeted disruption of the Fap gene. The targeting vector, its relationship to the wild-type allele, and the organization of the targeted allele are depicted. The relative positions of exons 3 to 9 (solid boxes) are indicated. Relevant restriction sites (A, ApaI; E, EcoRI; H, HindIII) and the location of the probe (probe A) used for Southern blot analysis are shown. The table indicates the expected fragments of the wild-type (wt) allele and targeted alleles after digestion with the respective enzyme and Southern blotting with probe A. (B) Southern blot analysis of tail DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Fap-deficient animals. DNA was digested with ApaI, EcoRI, or HindIII, and blots were hybridized to probe A. HSV tk, herpes simplex virus thymidine kinase cassette.
Fap−/− mice are viable and fertile.
Heterozygous Fap+/− F1 animals, which appeared phenotypically normal, were crossed, and the genotypes of F2 progeny were determined by Southern blot analysis, as exemplified in Fig. 1B. The ratios of numbers of homozygous, heterozygous, and wild-type offspring were found to be in close agreement with normal Mendelian ratios (data not shown).
Absence of functional Fap transcripts in Fap−/− mice.
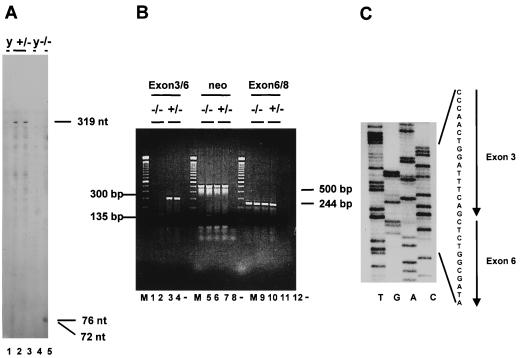
To confirm that the targeted mutation results in a null allele, Fap−/− animals were analyzed for FAP expression at the RNA, protein, and enzyme levels. Thus, RNase protection assays were performed with RNAs isolated from E14.5 Fap+/− and Fap−/− embryos. An RNA probe specific for exons 3 to 7 was used. With this probe, wild-type transcripts protect a 319-nucleotide (nt) fragment, whereas transcripts expressed from the targeted allele are expected to protect fragments 93 and 72 nt in length. As shown in Fig. 2A, a 319-nt fragment is protected by RNA from Fap+/− mice (lanes 2 and 3) but not by that from Fap−/− mice (lane 5). Instead these transcripts protected 72- and 76-nt fragments (Fig. 2A, lane 5). To analyze the nature of the unpredicted 76-nt fragment, RT-PCRs based on a primer set specific for exons 3 to 6 were performed. These primers amplified the expected 300-bp DNA fragment from RNAs isolated from wild-type (data not shown) and Fap+/− mice (Fig. 2B, lanes 3 and 4) and a 135-bp fragment from Fap−/− mice (Fig. 2B, lanes 1 and 2). Nucleotide sequence analysis of the 135-bp DNA fragment revealed a precise splicing from exon 3 to 6 (Fig. 2C). This event completely deletes the neo sequences and the remaining 17 nt of exon 5 from the primary mutant transcript, resulting in a frameshift at the exon 3-to-exon 6 border and a stop codon in exon 7 (data not shown). Consequently, due to the absence of an additional 17 nt in the mutant transcript, a 76-nt instead of a 93-nt fragment is detected by RNase protection analysis.
FIG. 2.
Molecular analysis of Fap transcripts. (A) RNase protection assay. Total RNA isolated at E14.5 from Fap+/− (lanes 2 and 3) or Fap−/− (lane 5) embryos or from yeast RNA (lanes 1 and 4) was analyzed. The lengths of the protected fragments are indicated. (B) RT-PCR assay. Total RNA from E14.5 Fap+/− and Fap−/− embryos was reverse transcribed and amplified with a primer set specific for Fap exons 3 and 6 (lanes 1 to 4), a neo-specific primer set (lanes 5 to 8), and a primer set specific for exons 6 and 8 (lanes 9 to 12). The lengths of the amplified cDNA fragments are indicated. M, 100-bp DNA ladder (Gibco-BRL). (C) Nucleotide sequence analysis. The 135-bp fragment identified by RT-PCR specific for exons 3 and 6 in Fap−/− embryos was subjected to sequence analysis. Nucleotides derived from exons 3 and 6 are indicated.
A PCR with a primer set encompassing exon 6 to exon 8 revealed the expected 244-bp amplicon in Fap+/+, Fap+/−, and Fap−/− mice, adding additional support to the hypothesis that the mutated Fap locus is transcriptionally active (Fig. 2B, lanes 9 to 12, and data not shown). A primer set specific for the neo cassette was used to amplify a 500-bp fragment from Fap+/− and Fap−/− mice by PCR, showing that the neomycin resistance gene is expressed in Fap mutant mice (Fig. 2B, lanes 5 to 8).
Absence of FAP protein and enzyme activity in Fap−/− embryos.
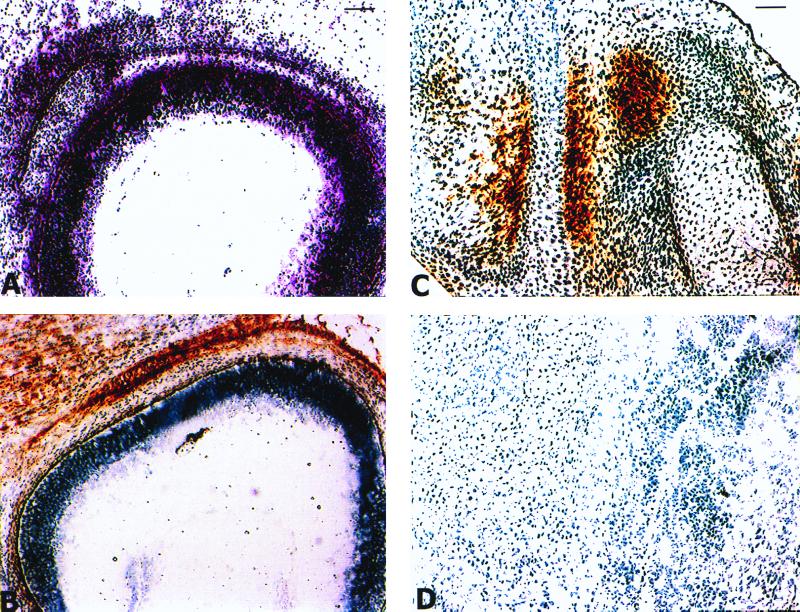
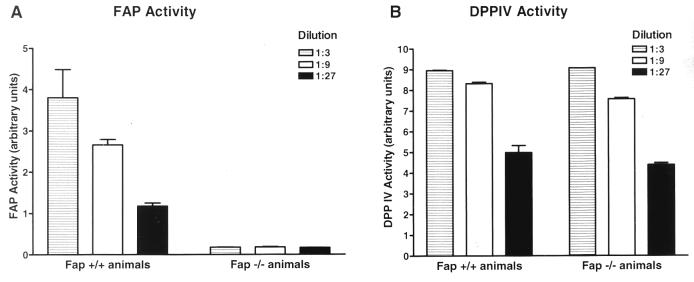
To confirm the absence of FAP at the protein and functional levels, we performed immunohistochemistry and FAP-specific activity assays. As shown in Fig. 3, in E13.5 wild-type embryos FAP is selectively expressed in the primitive mesenchymal condensation adjacent to the eye and in primitive mesenchymal cells surrounding the cartilaginous primordia of the bones (Fig. 3A to C). In contrast, mesenchymal cells in the forelimb of an E13.5 Fap−/− embryo do not show reactivity with anti-FAP MAb 3D11 (Fig. 3D). To extend these analyses to a functional level, 3D11 was used to immunoprecipitate the FAP protein present in detergent-soluble extracts isolated from E17.5 Fap+/+ and Fap−/− embryos. Subsequently, the immunopurified material was analyzed in a DPP activity assay. As shown in Fig. 4A, FAP-mediated DPP activity was present in Fap+/+ embryos but not in Fap−/− embryos.
FIG. 3.
Histological analysis of FAP expression in E13.5 mouse embryos. (A to C) Frozen sections from an E13.5 Fap+/+ embryo. (A) Hematoxylin- and eosin-stained section of the head showing the eye and the primitive mesenchymal condensation. (B) Adjacent frozen sections stained with anti-FAP MAb 3D11. (C) Section through the forelimb showing FAP expression in the primitive mesenchymal cells surrounding the cartilaginous primordia of the bones. (D) Section through the forelimb of an E13.5 Fap−/− embryo stained with 3D11. Bars: A and B, 100 μm; C and D, 50 μM.
FIG. 4.
FAP activity but not DPP IV activity is absent in Fap−/− embryos. (A) FAP-specific DPP activity assay. (B) DPP IV-specific DPP activity assay. Detergent-soluble membrane extracts obtained from two Fap+/+ and Fap−/− embryos (E17.5) were immunoprecipitated with MAb 3D11 (A) and with anti-CD26 MAb H194-112 (B), and different dilutions (indicated) of the immunoprecipitates were used in the DPP assay.
We previously showed that the genomic organization and chromosomal localization of the Fap gene is similar to those of the gene encoding DPP IV (10). To confirm that the disruption of the Fap gene did not abolish the adjacent DPP IV gene function, an anti-DPP IV MAb was used to immunoprecipitate DPP IV from E17.5 Fap+/+ and Fap−/− detergent-soluble extracts. As shown in Fig. 4B, the extracts exhibited comparable levels of DPP IV-mediated DPP activity, suggesting intact DPP IV gene function in the Fap mutant mice.
Anatomical and histopathological analysis of Fap-deficient mice.
Fap+/− and Fap−/− littermates were autopsied to investigate the presence of developmental abnormalities. Histological analysis of the internal organs of E13.5 mutant embryos did not reveal any obvious structural anomalies. During growth to adulthood, Fap−/− mice grew to sizes and body weights similar to those of the heterozygous littermates. Moreover, the weights and sizes of the major organs of Fap−/− mice were not discernibly different from those of heterozygous mice (data not shown). For more-detailed analysis, 10 homozygous mutant mice and 10 heterozygous control mice (5 of each sex) were sacrificed at 11 weeks, 25 weeks, and 18 months of age and were subjected to an extensive histopathological analysis. We noted no significant differences in any of the major organs examined, including lung, kidney, heart, brain, liver, thymus, and spleen, between Fap+/− and Fap−/− mutant animals and normal mice, both in C57BL/6 and 129/SV backgrounds. Similarly, hematological analysis, including erythrocyte counts, hemoglobin levels, and hematocrits, and clinical chemistry of blood and sera isolated from these animals showed no differences between mutant animals and controls. Based on two postmortem investigations with a total of 98 animals of the heterogenetic 129.BL6 background which were nearly 2 years old, no differences in tumor incidence and tumor types were observed.
DISCUSSION
To study the role of FAP in embryogenesis, tumorigenesis, and wound healing, processes marked by FAP expression, Fap-deficient mice were generated after homologous recombination in embryonic stem cells. Inactivation was achieved by replacing exon 4 and part of exon 5 by a neo selection marker cassette. Southern blot analysis confirmed the presence of the targeted allele in Fap+/− and Fap−/− mice. To investigate whether any message is expressed from the inactivated allele, RNase protection assays and RT-PCR assays were performed. Both techniques revealed that a nonfunctional Fap transcript is produced from the targeted allele. This truncated transcript encodes a peptide of 30 amino acids encompassing the most N-terminal intracellular and transmembrane domains of FAP. Since the entire extracellular catalytic domain is missing, these data strongly suggest that a FAP-null mutant mouse strain has been generated. These findings were extended by the use of 3D11, a MAb recognizing an extracellular epitope of mouse FAP. Employing this antibody, we detected FAP expression in primitive mesenchymal cells of E13.5 Fap+/+ embryos but not in E13.5 Fap−/− animals. Furthermore, we did not detect FAP-mediated enzymatic activity in detergent-soluble extracts isolated from Fap−/− embryos.
The major sources of FAP expression are primitive mesenchymal cells, which are likely to play an important role in the epithelium-mesenchyme interactions contributing to pattern formation during normal development, wound healing, and carcinogenesis. Due to the conservation of the expression pattern and in vitro activities of FAP in humans and mice, it was tempting to speculate that FAP fulfills a critical function in tissue remodeling. The notion that FAP may be involved in these process was reinforced by the observation that xFAP expression is upregulated in the tadpole tail resorption program of Xenopus laevis induced by the thyroid hormone (1). Nevertheless, our results show that a Fap deficiency per se does not compromise embryonic development or normal organ function in mice. The Fap−/− animals have no apparent anatomical or developmental abnormalities as judged by macroscopic examination of neonates and adults or by histological analysis of all major organs from animals 11 weeks, 25 weeks, and 18 months old. Hematopoietic and lymphoid cells appeared normal in number and distribution in the peripheral blood and lymphoid organs. Furthermore, we observed no evidence of any unusual disease in these mice.
Distinct mechanisms may be invoked to explain the apparently normal phenotype of Fap−/− mice. First, Fap-deficient mice may express residual FAP enzymatic activity. This is unlikely, however, since neither a functional transcript nor a catalytically active protein is detected in Fap−/− mice. Second, the loss of Fap expression could upregulate a compensatory pathway. The pathway could include the deregulated expression of unknown prolyl oligopeptidase family members or of other collagenolytic proteins, such as serine proteases and metalloproteases. A compensatory coordinated upregulation of matrix metalloproteinase (MMP) family members has been observed in matrilysin-deficient animals (16). Third, functional redundancy by proteins with overlapping function may mark the loss of FAP. Double or multiple knockouts generated by crossing Fap−/− animals with mice nullizygous for serine proteases or MMPs may address this issue by overcoming any built-in redundancy, thereby demonstrating the function of these proteins in embryogenesis and development. Finally, FAP may have a specialized but nonvital function which might become obvious only in elaborated murine models of human diseases in which a function of FAP has been suggested (6, 17), including mouse tumor models and models of liver cirrhosis and chronic inflammation.
ACKNOWLEDGMENTS
We thank R. Klein, (EMBL, Heidelberg, Germany) for providing pPNT. The expert technical assistance of M. Dettenmaier, C. Daiber, R. Femming, G. Koscis, G. Maier, A. Mohl, E. Mueller, G. Sauter, J. Schmidt, H. Schneider, A. Schuetze, E. Sladek-Kottisch, R. Sterner, B. Tuschen, R. Unger, and H. P. Kurray is acknowledged. We thank K. Damm for critically reading the manuscript.
REFERENCES
- 1.Brown D D, Wang Z, Furlow J D, Kanamori A, Schwartzman R A, Remo B F, Pinder A. The thyroid hormone-induced tail resorption program during Xenopus laevis metamorphosis. Proc Natl Acad Sci USA. 1996;93:1924–1929. doi: 10.1073/pnas.93.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dano K, Romer J, Nielsen B S, Bjorn S, Pyke C, Rygaard J, Lund L R. Cancer invasion and tissue remodeling—cooperation of protease systems and cell types. APMIS. 1999;107:120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 3.Garin-Chesa P, Old L J, Rettig W J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody-target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 5.Kinzler K W, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 6.Levy M T, McCaughan G W, Abbott C A, Park J E, Cunningham A M, Muller E, Rettig W J, Gorrell M D. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodeling interface in human cirrhosis. Hepatology. 1999;29:1768–1778. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto C, Schlossman S F. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niedermeyer J, Scanlan M J, Garin-Chesa P, Daiber C, Fiebig H H, Old L J, Rettig W J, Schnapp A. Mouse fibroblast activation protein: molecular cloning, alternative splicing and expression in the reactive stroma of epithelial cancers. Int J Cancer. 1997;71:383–389. doi: 10.1002/(sici)1097-0215(19970502)71:3<383::aid-ijc14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Niedermeyer J, Enenkel B, Park J E, Lenter M, Rettig W J, Damm K, Schnapp A. Mouse fibroblast activation protein: conserved fap gene organization and biochemical function as a serine protease. Eur J Biochem. 1998;254:650–654. doi: 10.1046/j.1432-1327.1998.2540650.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohtani H. Stromal reaction in cancer tissue: pathophysiologic significance of the expression of matrix-degrading enzymes in relation to matrix turnover and immune/inflammatory reactions. Pathol Int. 1998;48:1–9. doi: 10.1111/j.1440-1827.1998.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 12.Park, J. E., M. C. Lenter, R. N. Zimmermann, P. Garin-Chesa, L. J. Old, and W. J. Rettig. Fibroblast activation protein: a dual-specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem., in press. [DOI] [PubMed]
- 13.Rettig W J, Garin-Chesa P, Beresford H R, Oettgen H F, Melamed M R, Old L J. Cell surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA. 1988;85:3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rettig W J, Su S L, Forunato S R, Scanlan M J, Mohan Raj B K, Garin-Chesa P, Healey J H, Old L J. Fibroblast activation protein: purification, epitope mapping, and induction by growth factors. Int J Cancer. 1994;58:385–392. doi: 10.1002/ijc.2910580314. [DOI] [PubMed] [Google Scholar]
- 15.Ronnov-Jessen L, Petersen O W, Bissell M J. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph-Owen L A, Hulboy D L, Wislon C L, Mudgett J, Matrisian L M. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1-deficient mice. Endocrinology. 1997;138:4902–4911. doi: 10.1210/endo.138.11.5478. [DOI] [PubMed] [Google Scholar]
- 17.Scanlan M J, Mohan Raj B K, Calvo B, Garin-Chesa P, Sanz-Moncasi M P, Healey J H, Old L J, Rettig W J. Molecular cloning of fibroblast activation protein, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA. 1994;91:5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 19.Wurst W, Joyner A L. Production of targeted embryonic stem cell clones. In: Joyner A L, editor. Gene targeting, a practical approach. Oxford, United Kingdom: IRL Press, Oxford University Press; 1993. [Google Scholar]
- 20.Yancopoulos G D, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]