Supplemental Digital Content is available in the text.
Keywords: 3-D echocardiography, left ventricular dysfunction, outcomes research, right ventricle, right ventricular dysfunction
Abstract
Background:
The functional adaptation of the right ventricle (RV) to the different degrees of left ventricular (LV) dysfunction remains to be clarified. We sought to (1) assess the changes in RV contraction pattern associated with the reduction of LV ejection fraction (EF) and (2) analyze whether the assessment of RV longitudinal, radial, and anteroposterior motion components of total RVEF adds prognostic value.
Methods:
Consecutive patients with left-sided heart disease who underwent clinically indicated transthoracic echocardiography were enrolled in a single-center prospective observational study. Adverse outcome was defined as heart failure hospitalization or cardiac death. Cross-sectional analysis using the baseline 3-dimensional echocardiography studies was performed to quantify the relative contribution of the longitudinal, radial, and anteroposterior motion components to total RVEF.
Results:
We studied 292 patients and followed them for 6.7±2.2 years. In patients with mildly and moderately reduced LVEF, the longitudinal and the anteroposterior components of RVEF decreased significantly, while the radial component increased resulting in preserved total RVEF (RVEF: 50% [46%–54%] versus 47% [44%–52%] versus 46% [42%–49%] in patients with no, mild, or moderate LV dysfunction, respectively; data presented as median and interquartile range). In patients with severe LV systolic dysfunction (n=34), a reduction in all 3 RV motion components led to a significant drop in RVEF (30% [25%-39%], P<0.001). In patients with normal RVEF (>45%), the anteroposterior component of total RVEF was a significant and independent predictor of outcome (hazard ratio, 0.960 [CI, 0.925–0.997], P<0.001).
Conclusions:
In patients with left-sided heart disease, there is a significant remodeling of the RV associated with preservation of the RVEF in patients with mild or moderate LV dysfunction. In patients with normal RVEF, the measurement of the anteroposterior component of RV motion provided independent prognostic value.
Clinical Perspective.
Three main mechanisms contribute to the total right ventricular (RV) pump function: shortening of the longitudinal axis with the traction of the tricuspid valve towards the apex (longitudinal shortening); inward motion of the RV free wall (radial shortening); and contraction of the interventricular septum and its bulging into the RV (causing shortening in the anteroposterior direction). The functional adaptation of the RV to different degrees of left ventricular dysfunction remains to be clarified. We investigated 292 patients with left heart disease by 3-dimensional echocardiography and followed them for 6.7±2.2 years. The RV longitudinal and anteroposterior components of RV contraction decreased significantly from early stages of left ventricular dysfunction, while the radial component increased to preserve the RV ejection fraction. In patients with normal RV ejection fraction, the anteroposterior component of the total RV ejection fraction was a significant and independent predictor of adverse outcome. Echocardiographic parameters that refer exclusively to the longitudinal shortening of the RV (ie, tricuspid annular plane systolic excursion, S’ by tissue Doppler imaging) can underestimate total RV function as radial shortening provides compensation in patients with left-sided heart disease. Three-dimensional imaging allows accurate assessment of RV functional adaptation to left ventricular dysfunction with important diagnostic and prognostic consequences. Further research is warranted to investigate whether the prognostic value of different mechanical components in the face of normal RV ejection fraction persists in other disease states.
Development of the right ventricular (RV) failure in patients with left-sided heart disease is a well-known adverse clinical and prognostic factor.1 Left ventricular (LV) systolic dysfunction and remodeling influences RV function via the mechanical interdependence between the 2 ventricles and, at later stages, also by hemodynamic overload.2 Although RV ejection fraction (EF) remains preserved for a long time during the disease course, early adaptive (or even maladaptive) changes may develop in the RV contraction pattern.
Total RVEF is the cumulative result of the complex interplay among distinct mechanical components (ie, shortening along the longitudinal, radial, and anteroposterior directions) and, therefore, may not capture subtle changes that occur at the early stages of RV dysfunction.3 Moreover, the functional adaptations of the RV at different degrees of LV dysfunction and their prognostic relevance remain to be clarified. Three-dimensional echocardiography (3DE) offers the opportunity to separately analyze the above-mentioned 3 main components of total RVEF.3 Thus, it can provide insights into both the functional adaptation of the RV to LV dysfunction and the prognostic significance of the various components of total RVEF.
Accordingly, the aims of this study were to use 3DE: (1) to assess the changes in the RV contraction pattern in relation to the reduction of LVEF in patients with left-sided heart disease and (2) to analyze whether the longitudinal, radial, and anteroposterior motion components of total RVEF may predict outcomes in this patient population.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
Consecutive patients with sinus rhythm and left-sided heart disease who underwent clinically indicated transthoracic echocardiography between October 2010 and December 2012 at the University of Padua (Italy) were enrolled in a single-center prospective observational study. The following inclusion criteria were used: 18 years of age or older; left-sided structural heart disease with any value of LVEF; recordings of both LV and RV 3DE full-volume data sets; sufficient image quality and volume rate to measure LV and RV volumes; availability of follow-up data. Exclusion criteria were primary RV disease (eg, arrhythmogenic RV cardiomyopathy); primary tricuspid or pulmonary valve disease; severe tricuspid regurgitation (even if functional or pacemaker-related); congenital heart diseases affecting the right heart; type I, III, IV, or V pulmonary hypertension; pericardial diseases; and acute myocarditis. Demographic and clinical data (age, weight, height, body surface area, body mass index, cardiovascular risk factors, and comorbidities) were retrieved from the electronic clinical records of the hospital database. The study was approved by the local Ethics Committee and performed according to the principles of the Declaration of Helsinki. Participants provided their written informed consent.
Echocardiographic Data Acquisition and Analysis
Patients underwent complete echocardiographic examination at baseline, including both conventional and 3DE data acquisition using the Vivid E9 system (GE Vingmed Ultrasound, Horten, Norway) equipped with M5S and 4V-D probes. Digitally stored data sets in raw-data format were analyzed offline using commercially available software package (EchoPAC BT12, GE Vingmed Ultrasound).
LV and RV volumes, EF and LV mass were measured by 3DE. In particular, 4- or 6-beat full-volume 3D data sets of both the LV and the RV (volume rate 31±5 vol/s) were obtained during breath-holding from the LV focused standard apical 4-chamber view and the RV focused apical 4-chamber view, respectively. Pyramidal data sets were optimized for width and depth. During both acquisitions, the 12-slice display was used to ensure a complete inclusion of either the LV or the RV in the data set, respectively. Digitally stored data sets in raw-data format were analyzed offline by 2 independent and experienced investigators (LV by D. Muraru and RV by E. Surkova) who were blinded to both clinical information and outcomes, using commercially available software packages (4D AutoLVQ [EchoPAC BT12] and 4D RV-Function 2.0 [TomTec Imaging Systems GmbH, Unterschleissheim, Germany]) previously validated against cardiac magnetic resonance (CMR).4–6 A detailed description of the analysis technique was published elsewhere.7 LV and RV volumes and LV mass were indexed to body surface area.
LVEF was classified as normal (≥50%), mildly reduced (50%>EF≥40%), moderately reduced (40%>EF≥30%), or severely reduced (<30%).8,9 RVEF was classified as normal (>45%) or reduced (≤45%).10
Conventional echocardiographic parameters of RV size and function (eg, tricuspid annular plane systolic excursion [TAPSE], RV diameters, areas, and fractional area change [FAC]) were measured from M-mode and 2-dimensional echocardiographic data sets, respectively, according to the current American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines.9
Detailed Quantification of 3D RV Mechanics
Three main mechanisms contribute to the total RV pump function: (1) shortening of the longitudinal axis with the traction of the tricuspid valve towards the apex (longitudinal shortening); (2) inward motion of the RV free wall (radial shortening); and (3) contraction of the interventricular septum and its bulging into the RV (causing shortening in the anteroposterior direction). To separately quantify these 3 major functional components contributing to total RVEF, we used the ReVISION software package (Argus Cognitive, Inc, Lebanon, NH). First, the 3D mesh model’s orientation obtained from 4D RV-Function software package was adjusted by a standard, automated method to identify the axes of the longitudinal, radial, and anteroposterior directions. The mesh consists of 2 structures: a set of vertices, each denoting a 3D position on the RV endocardial surface, and a set of edges that define the connections between the vertices (example meshes are shown in Figure 1). Each vertex corresponds to the same anatomic position on the RV endocardium throughout the cardiac cycle. After standardizing the orientation of the mesh, motion decomposition was performed along the aforementioned axes in a vertex-based manner by considering the movement of each vertex along only a single given direction and ignoring the other 2 directions. We applied the edges of the original meshes on the transformed vertices, thus generating 3 new mesh series (ie, one for each axis where movement was allowed) over the cardiac cycle. We computed the EFs of these newly generated mesh series to separately quantify partial RVEFs (ie, longitudinal EF [LEF=stroke volume generated by the longitudinal shortening divided by RV end-diastolic volume], radial EF [REF=stroke volume generated by the radial shortening divided by RV end-diastolic volume], and anteroposterior EF [AEF=stroke volume generated by the anteroposterior shortening divided by RV end-diastolic volume]).11 Of note, the absolute RV volume change is generated by the aggregated contribution of the 3 motion components, but due to the nonlinear deformations of the RV, the values of decomposed EFs are not additive (LEF+AEF+REF≠RVEF). To facilitate interpretation, one can apply rescaling, that is, LEF′=LEF/(LEF+AEF+REF)×RVEF and then LEF′+AEF′+REF′=RVEF will hold. In our analysis, we used the native values and not the rescaled ones to allow comparisons with our previous studies (clinical results and the correlations with the CMR-derived 3D models). The relative contribution of each component to the total RV pump function was defined as the ratio between LEF, REF, and AEF and total RVEF (LEFi=LEF/RVEF, REFi=REF/RVEF, and AEFi=AEF/RVEF). The comparison of these metrics using 3DE versus CMR-derived mesh models showed a robust agreement between the 2 modalities.12
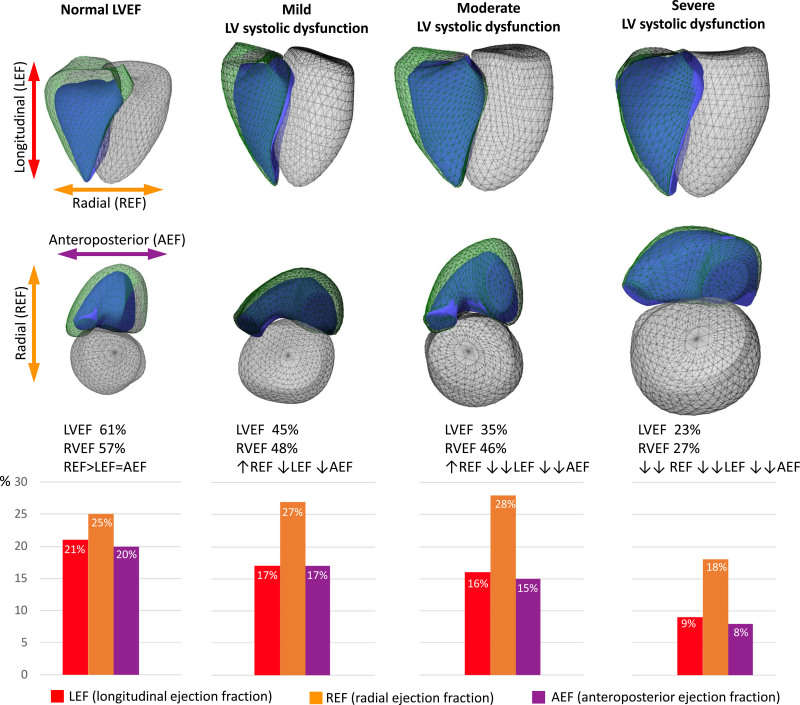
Figure 1.
Functional adaptation of the right ventricle (RV) to the different degrees of left ventricular (LV) systolic dysfunction in patients with left-sided heart disease: representative cases. Three-dimensional schematic representation of the 3 major components contributing to total RV pump function: (i) longitudinal shortening along the long-axis (red) contributing to RV longitudinal ejection fraction component (LEF), (ii) inward (radial) motion of the RV free wall (orange) contributing to radial ejection fraction component (REF), and (iii) short-axis shortening in the anteroposterior direction (purple) contributing to anteroposterior ejection fraction component (AEF). Green mesh represents RV end-diastolic and the blue surface the RV end-systolic volume. In the case of preserved LV ejection fraction (LVEF), the 3 components show a balanced relative contribution. RV shortening along the longitudinal and anteroposterior directions continuously decreases with LVEF. However, shortening in the radial direction shows a compensatory increase in mild and moderate LV dysfunction, maintaining RVEF. In severe LV dysfunction, all motion components drop significantly, resulting in severe RV dysfunction.
Follow-Up
The primary end point of our study was the composite of the first heart failure hospitalization (defined as hospital admission due to worsening signs and symptoms of heart failure, requiring intravenous treatment aimed predominantly at managing fluid overload and hemodynamic compromise) or cardiac death (defined as death resulting from an acute myocardial infarction, heart failure, cardiovascular procedures, and sudden cardiac death), whichever occurred first. Follow-up data were collected by an investigator (A. Ruocco) who was not involved in the echocardiographic measurements through the analysis of clinical records, telephone contacts to patients, physicians, or the next of kin when the patient was not available. The last update of follow-up was performed on July 10, 2019, 13 patients (4.5%) were lost to follow-up.
Statistical Analysis
Continuous variables were expressed as mean±SD or median (interquartile range), whereas categorical variables were reported as frequencies and percentages. After verifying the normal distribution of each variable using the Shapiro-Wilk test, the clinical and echocardiographic characteristics of patient subsets were compared with unpaired Student t test or Mann-Whitney U test for continuous variables, and χ2 or Fisher exact test for categorical variables, as appropriate. Multiple group comparisons (>2 groups) were performed using ANOVA (with Tukey post hoc test) or Kruskal-Wallis test (with Dunn post hoc test) and χ2 or Fisher exact test, as appropriate. Spearman rank correlation coefficients were computed to assess the correlation between continuous variables. Cox proportional hazards models were used to compute hazard ratios with 95% confidence intervals (CIs). Including significant variables identified at the univariable Cox regression analysis, multivariable Cox regression models were built to identify independent predictors of outcomes. Collinearity of variables was tested at each multivariable model by variance inflation factor (excessive if variance inflation factor >3). Hazard ratios of LEFi, REFi, and AEFi refer to the effect of 0.01 unit change. Variables with >10% missing values were not included in multivariable analyses. Receiver operating characteristic curves were generated to assess the discriminatory power of RV systolic functional parameters with regards to the composite end point. Youden index was used to identify the optimal cutoff points of each parameter, then these values were used to dichotomize the study population. Outcomes of the dichotomized groups were visualized on Kaplan-Meier curves and compared by log-rank test. To assess intraobserver and interobserver variability and reliability, the operator of the first measurements repeated the analysis in a randomly chosen subset of patients (n=20, 5 from each LVEF subgroup) blinded to previous results. A second experienced operator (A. Kovács) also analyzed these patients in a blinded fashion. Intraclass correlation coefficient and coefficient of variation values suggested acceptable intraobserver and interobserver variability and reliability for both global and component RV metrics (Table I in the Data Supplement).
A 2-sided P<0.05 was considered as statistically significant. Statistical analysis was performed in R (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Of a total of 462 patients screened, 71 patients (15%) had echocardiographic images unsuitable for 3DE analysis: 42 patients due to unavailability of either the RV or the LV 3DE data sets; 21 patients because of poor acoustic window; and 8 patients because of irregular rhythm and stitching artifacts. From the remaining 391, 292 patients had primary left-sided heart disease, and they formed the study cohort. The median follow-up time was 7.6 (interquartile range 6.6-8.0) years.
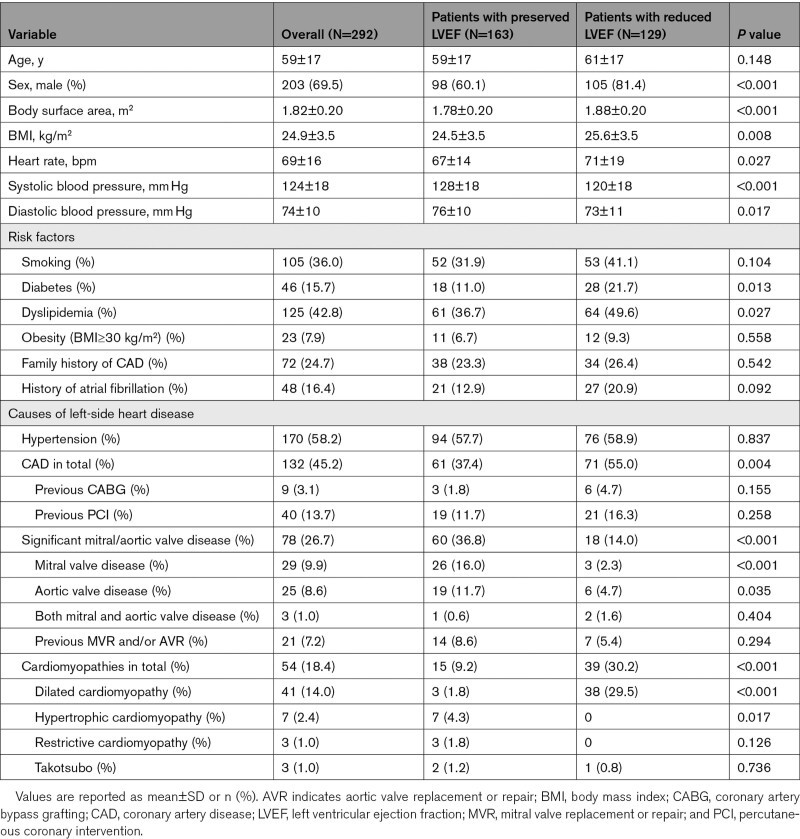
Baseline demographic, clinical, and echocardiographic characteristics were summarized in Tables 1 and 2. The main causes of left-sided heart disease in our study cohort were arterial hypertension (diagnosed in 170 patients, 58.2%), coronary artery disease (132, 45.2%), mitral or aortic valve disease (≥moderate stenosis or regurgitation; 78, 26.7%), and cardiomyopathies (54, 18.4%).
Table 1.
Demographic and Clinical Characteristics of Study Samples
Table 2.
Echocardiographic Characteristics of Study Samples
One hundred sixty-three patients (55.8%) had preserved LVEF (59.9±5.6%, range 50%–72%), and 129 patients (44.2%) had reduced LVEF (36.4±10.9%, range 5%–49%). Patients from the latter subgroup were more likely to be diagnosed with coronary artery disease, dilated cardiomyopathy, diabetes, or dyslipidemia. Significant mitral or aortic valve diseases were more frequent in patients with preserved LVEF (Table 1).
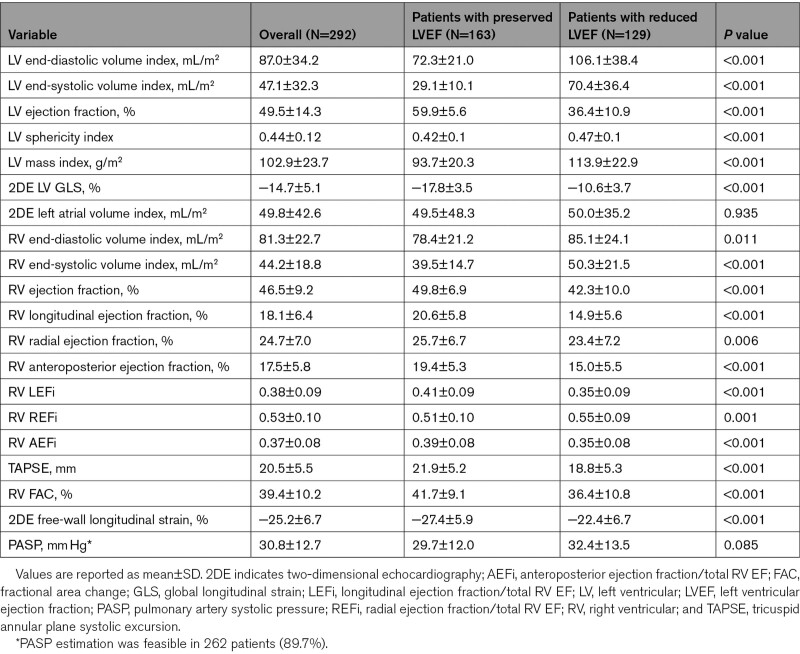
Patients with reduced LVEF also had poorer RV systolic function compared to the patients with preserved LVEF (RVEF 42.3±10.0% versus 49.8±6.9%, P<0.001; FAC 36.4±10.8% versus 41.7±9.1%, P<0.001; and TAPSE 18.8±5.3 mm versus 21.9±5.2 mm, P<0.001, respectively; Table 2). Accordingly, the prevalence of reduced RVEF was significantly higher in patients with reduced LVEF compared with those with preserved LVEF (45% versus 19%, P<0.001).
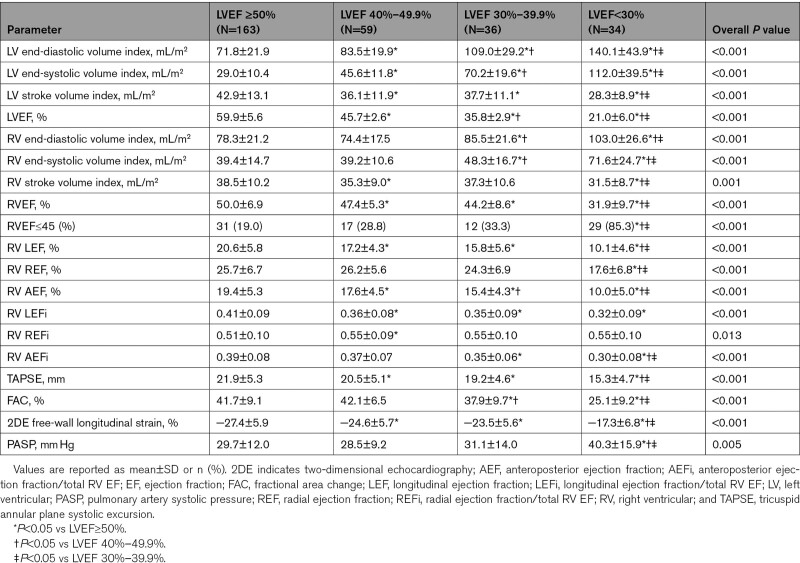
LV end-diastolic and end-systolic volumes increased significantly starting from the early stages of LV systolic dysfunction while stroke volume dropped (Table 3). However, RV volumes started increasing significantly only in patients with moderate LV dysfunction, and the RV stroke volume dropped significantly in those with severely reduced LVEF (Table 3 and Figure I in the Data Supplement).
Table 3.
Echocardiographic Characteristics of Patients Stratified According to LVEF
Different Components of the RVEF in Patients With Left-Sided Heart Disease
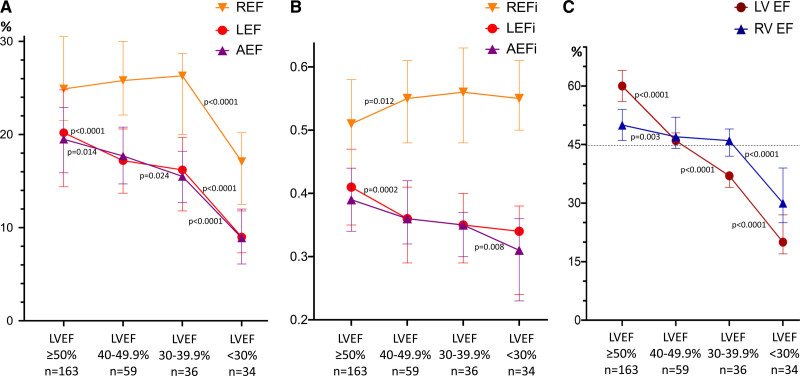
The relative contribution of the radial component to the total RVEF was significantly higher than that of longitudinal and anteroposterior components both in patients with preserved LVEF (REFi: 0.51±0.10 versus LEFi: 0.41±0.09 versus AEFi: 0.39±0.08; P<0.001) and reduced LVEF (REFi: 0.55±0.09 versus LEFi: 0.35±0.09 versus AEFi: 0.35±0.08; P<0.001; Figure II in the Data Supplement). The relative contribution of the longitudinal and anteroposterior components decreased, while radial component contribution further increased in patients with reduced LVEF compared with those with preserved LVEF (Table 2).
RV LEF and AEF showed moderate correlations with LVEF (Spearman ρ: 0.51 and 0.48, respectively, P<0.001) and with LV global longitudinal strain (−0.60 and −0.51, respectively, P<0.001), while the correlations between RV REF and the same parameters were weak (0.23, P<0.001 and −0.14, P=0.032, respectively). RV REF correlated weakly with the 3DE-derived LV sphericity index (Spearman ρ: −0.24, P<0.001), while other components did not. All 3 components demonstrated weak correlations with pulmonary arterial systolic pressure (Spearman ρ: LEF −0.27, P<0.001, REF −0.18, P=0.003, and AEF −0.33, P<0.001).
Changes in RV Contraction Pattern at Different Degrees of LV Systolic Dysfunction
When patients were further stratified according to the degree of LVEF impairment (Table 3), there was a significant drop in both RV LEF and AEF, and in their relative contribution to the total RVEF, starting from the earlier stages of LV dysfunction (Figure 2A and 2B). However, in patients with mildly and moderately reduced LVEF, there was a significant increase in the radial RV component: REFi increased from 0.51 (0.44–0.58) in patients with normal LVEF to 0.55 (0.48–0.61; P=0.012) in those with mild LV dysfunction, and to 0.56 (0.48–0.63; P=0.031) in those with moderate LV dysfunction (Figure 2A and 2B). As a result, total RVEF remained normal in patients with normal, mildly, and moderately reduced LVEF (RVEF 50% [46%–54%], 47% [44%–52%], and 46% [42%–49%], respectively; Figure 2C). Conversely, in patients with severely reduced LVEF, a significant reduction in all RVEF components was observed, which ultimately led to a decrease of the total RVEF (30% [25%–39%]; P<0.001; Figure 2C and Table 3). Similar trends could be observed when the subgroups of patients with coronary artery disease or dilated and hypertrophic cardiomyopathy were assessed separately (Tables II and III in the Data Supplement).
Figure 2.
Comparison of right ventricular (RV) longitudinal, radial, and anteroposterior ejection fractions (LEF, REF, and AEF, respectively) and their relative contributions at different stages of left ventricular (LV) dysfunction. A, Longitudinal (red line) and anteroposterior (purple line) ejection fraction (EF) values are decreasing continuously and parallel with the decrease of LVEF. However, REF (orange line) is even increasing in mild and moderately decreased stages of LV dysfunction but drops significantly below LVEF of 30%. B, The relative contributions show similar phenomena, with REF being the dominant contributor to RVEF even in severe LV dysfunction. C, In our cohort, total RVEF remained preserved (>45%) in patients with mild and moderate LV dysfunction but decreased significantly below LVEF of 30%, which is clearly attributable to the drop in REF values at this stage. Dotted line demonstrates a cutoff value of RVEF 45%. Data are presented as median and interquartile range. AEFi indicates anteroposterior ejection fraction indexed to total right ventricular ejection fraction; LEFi, longitudinal ejection fraction indexed to total right ventricular ejection fraction; and REFi radial ejection fraction indexed to total right ventricular ejection fraction.
Prognostic Value of the Different Components of RV Mechanics
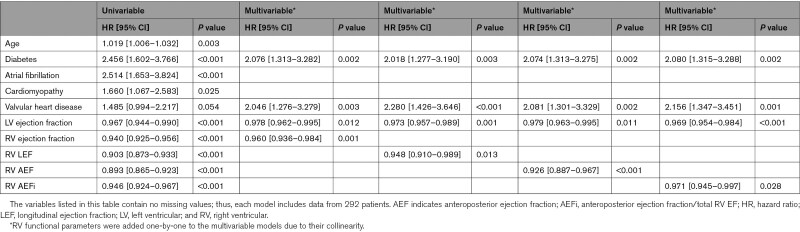
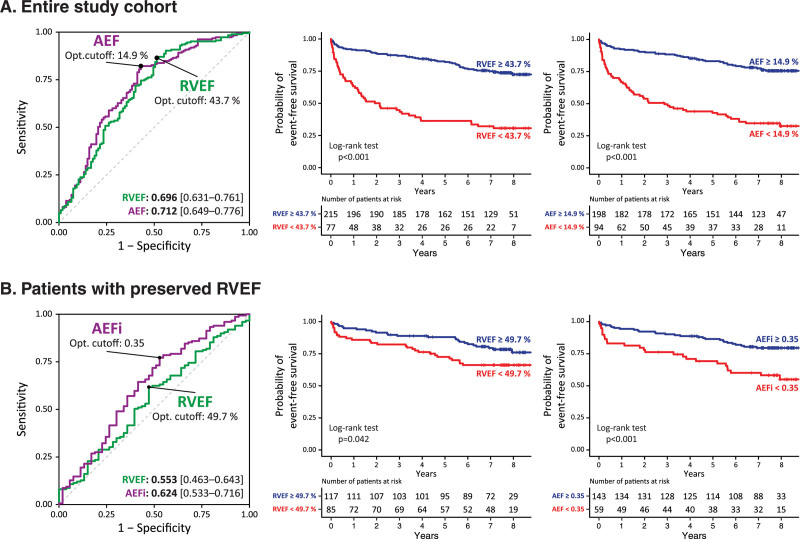
In our cohort, 107 patients (37%) met the composite end point: 76 patients were hospitalized due to heart failure, and 31 patients died due to cardiac cause (these patients were not hospitalized for heart failure during the follow-up period). Out of the 76 patients who were hospitalized for heart failure, 29 died due to cardiac cause during the follow-up period. Patients with adverse outcomes were older and more likely diagnosed with diabetes or atrial fibrillation compared to patients who remained free of events. RV functional parameters were all significantly decreased except for the relative contribution of radial shortening (REFi; Table IV in the Data Supplement). Results of the univariable Cox proportional hazards regression analysis are summarized in Table V in the Data Supplement. Multivariable Cox regression models were built, including significant demographic and medical history parameters, LVEF, and RV measures (RV parameters were added one-by-one to the models to avoid collinearity). In multivariable models including LVEF, age, diabetic status, history of atrial fibrillation, cardiomyopathies, and valvular heart diseases as etiological factors, several RV morphological, and functional measurements (including RVEF, LEF, AEF, and AEFi) were found to be independently associated with adverse outcomes (Table 4 and Tables VI through X in the Data Supplement). In the receiver operating characteristic analysis, RV AEF exhibited the highest area under the curve value compared with the other RV functional measures (Figure 3 and Table XI in the Data Supplement).
Table 4.
Factors Associated With Cardiac Death and Heart Failure Hospitalization in the Entire Study Cohort
Figure 3.
Comparison of the discriminatory power of right ventricular ejection fraction (RVEF) vs parameters of anteroposterior shortening with regards to the composite end point. A, RVEF vs anteroposterior ejection fraction (AEF) in the entire population; (B) RVEF vs AEFi (AEF/RVEF) in patients with preserved (>45%) RVEF. Based on the optimal cutoff values of each parameter assessed with the receiver operating characteristic (ROC) analysis, patient cohorts were dichotomized and their outcomes were visualized on Kaplan-Meier curves. Area under the ROC curve values (with 95% CIs) are displayed in the right bottom corner of the ROC plots.
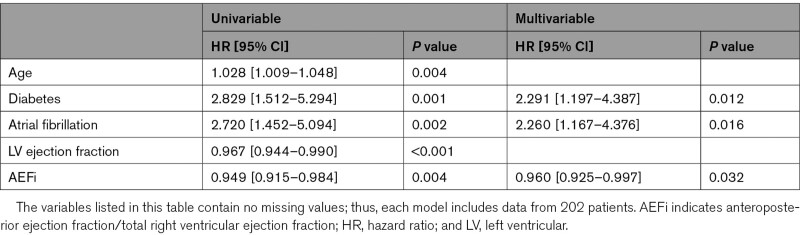
We further investigated patients with normal RV function (RVEF>45%, N=202). Fifty-three patients met the composite end point: 37 patients were hospitalized due to heart failure, and 16 patients died due to cardiac cause (these patients were not hospitalized for heart failure during the follow-up period). Out of the 37 patients who were hospitalized for heart failure, 7 died due to cardiac cause during the follow-up period. Patients who experienced adverse outcomes were older, more likely to have diabetes or history of atrial fibrillation, had larger LV and left atrial volumes, and lower LVEF than patients who did not (Table XII in the Data Supplement). RV end-diastolic volume, RVEF, FAC, and RV free-wall longitudinal strain did not differ between the 2 groups. Conversely, AEF and AEFi were significantly lower, whereas REFi was significantly higher among patients who met the end point. The latter 3 RV mechanical component measures were also significantly associated with adverse outcomes by univariable Cox analysis, while the RVEF was not (Table XIII in the Data Supplement). In a multivariable Cox regression model including age, diabetic status, atrial fibrillation, LVEF and AEFi, AEFi (hazard ratio, 0.960 [95% CI, 0.925–997], P=0.032) was found to be independently associated with adverse outcomes in patients with preserved RVEF besides diabetes and atrial fibrillation (Table 5). No other RV morphological or functional parameter was found to be independently associated with outcome in this model (Tables XIV–XVIII in the Data Supplement). In the receiver operating characteristic analysis, AEFi exhibited the highest area under the curve value (Figure 3 and Table XIX in the Data Supplement).
Table 5.
Factors Associated With Cardiac Death and Heart Failure Hospitalization in Patients With Preserved Right Ventricular Ejection Fraction
Discussion
To the best of our knowledge, this is the first study that used 3DE to investigate the changes of RVEF and RV contraction patterns at the different grades of LV systolic dysfunction in patients with left-sided heart disease.
Our main findings can be summarized as follows (Figure 1): (1) in patients with left-sided heart disease, the longitudinal and anteroposterior components of the RV pump function were directly related to the LV systolic function, and decreased significantly even in patients with mild LV dysfunction; (2) the relative increase in the radial component of the RV pump function compensated the decrease of the RV longitudinal and anteroposterior components to maintain the total RVEF in patients with mild and moderate LV dysfunction; (3) in patients with severe LV dysfunction all the 3 components of the RV pump function were impaired resulting in a significant reduction of the total RVEF; (4) 3DE-derived RVEF was significantly, and independently of LV systolic function, associated with a composite outcome of cardiac death or heart failure hospitalization; and (5) anteroposterior RV motion component held additional prognostic value in patients with normal RVEF.
Pathophysiology of RV Dysfunction in Patients With Left-Sided Heart Disease
Despite significant embryological, morphological, and physiological differences, a close relationship exists between the RV and the LV.13 The 2 cardiac chambers are interdependent as they are nested within the pericardium and share common helical myocardial fibers both in the septum and across the interventricular groove.13,14 Approximately 20% to 40% of RV systolic performance can be attributed to LV contraction.2
Three main mechanisms contribute to the total RV pump function: shortening of the longitudinal axis with the traction of the tricuspid valve towards the apex; inward motion of the RV free wall; and contraction of the interventricular septum and its bulging into the RV.2 The RV fiber orientation determines these mechanisms: free wall has predominantly circumferential fibers which narrow the cavity during systole, while the septal helical fibers twist and shorten the longitudinal axis of the RV.15
Reduction of LV contractility and stretching of the septum in patients with left-sided heart disease alter the helical fiber orientation to >60 degrees, making the helix fibers more transverse, thus impairing the efficiency of the longitudinal contraction.15 At the compensatory stage, the RV increases its transverse function in relation to the decrease of the longitudinal shortening.16,17 If the injury persists, the RV transitions from a compensated to a decompensated phenotype characterized by myocyte loss and replacement fibrosis,18 limiting the free-wall inward motion alongside the longitudinal function. As the LV becomes progressively more spherical, the septal fibers become less oblique, dramatically reducing their mechanical advantage and further impairing RV contractile function. These mechanisms ultimately lead to clinical RV failure.19
Prevalence of the RV Systolic Dysfunction and Changes in RV Contraction Pattern in Left-Sided Heart Disease
The prevalence of RV dysfunction in patients with left-sided heart disease varies widely in different populations, but its presence is universally associated with poor prognosis and increased mortality.1 Thus, in a recent retrospective study involving nearly 1300 patients with RV dysfunction, the left-sided heart disease was the most common cause of severe RV dysfunction (46%) and led to increased mortality: 1- and 5-year survival rates were 61% (95% CI, 57%–65%) and 33% (95% CI, 28%–37%), respectively.1
According to a large meta-analysis, the prevalence of RV dysfunction in patients with heart failure with reduced LVEF reached 47%, and it was significantly associated with overall mortality and admissions for heart failure during follow-up.20 In patients with preserved LVEF (HFpEF), RV systolic dysfunction was also associated with increased morbidity and mortality.16 The reported 2-year mortality in a cohort of patients with HFpEF reached 45% in those with RV dysfunction compared with 7% in those without.21 The prevalence of RV dysfunction in patients with HFpEF can exceed 30%, depending on the methods and cutoff values used for its assessment.16 A CMR study of patients with HFpEF reported that RVEF <45% was present in 19.3% of patients.22 This was in agreement with our results: the prevalence of RV systolic dysfunction assessed by 3DE was 19% in those with preserved LVEF, but it reached 45% in those with reduced LVEF (increasing gradually from 28% in patients with mild LV dysfunction to 85% for those with LVEF <30%).
Importantly, the prevalence of RV dysfunction differs depending on the methodology and the imaging modalities used to assess it. A meta-analysis of patients with HFpEF reported RV systolic dysfunction in 31% assessed by TAPSE, 26% by RV S’, and 13% by FAC.16 A more recent study of a similar patients cohort confirmed that the prevalence of RV systolic dysfunction was the highest if defined by TAPSE <17 mm (37%), lower if defined by FAC <35% (26%), and the lowest if defined by CMR-derived RVEF <45% (10%).23 Observed differences in the rates of RV dysfunction in patients with both reduced and preserved LVEF may reflect not only the well-known limitations of TAPSE and S’ but also indicate that the reduction of longitudinal RV shortening (reflected by TAPSE and S’) is an earlier process than the reduction of radial function (partially reflected by FAC and RVEF), and it is present in a larger proportion of patients with left-sided heart diseases.
In the normal RV, the longitudinal component has long been considered as the dominant contributor to >50% of the RV pump function.2 Recent studies using more advanced imaging techniques demonstrated that under normal conditions, contribution of radial and anteroposterior shortening of the RV to the total RVEF appears equal to the longitudinal component.24 The postprocessing of 3DE-derived RV models using a vertex-based motion decomposition enabled the separate quantification and head-to-head comparison of the different mechanical components along 3, anatomically relevant orthogonal axes.11 Using this methodology, both the inward (radial) motion of the free wall (the so-called bellows effect) and the routinely neglected anteroposterior component can be evaluated. Contraction of the subepicardial circumferential fibers contribute to the radial and also the anteroposterior shortening of the RV, and because of the large surface of the RV free wall, a relatively limited inward movement in these directions can result in a significant stroke volume. Moreover, the anteroposterior shortening implies the contribution of the interventricular septum and the LV, as the circumferential shortening of the LV mid-layer myofibers stretches the RV free-wall insertion lines towards each other. Accordingly, the LV function was found to be a significant and independent predictor of RV anteroposterior shortening in healthy volunteers.24
Significant correlations of LEF and AEF with LV systolic function parameters (ie, LVEF and global longitudinal strain) shown in our study, confirm this finding. RV radial component, on the contrary, may be partially explained by the alterations in LV shape, as demonstrated by the correlation between REF and LV sphericity index. Because all RV components correlated rather weakly with pulmonary artery systolic pressure, one can speculate that the mechanisms behind the RV functional adaptation and remodeling are more complex than just postcapillary pulmonary hypertension but may be a combination of LV contractility, LV shape, and RV afterload. Future research is needed to identify the pathophysiological determinants of the various RVEF components.
There are no data specifically addressing the changes in RV contraction pattern at different grades of LV systolic dysfunction. Our results suggest that the LV remodeling occurring in left-sided heart disease is associated with an early and steady reduction in both the RV longitudinal and anteroposterior components of RV pump function, compensated by an increase in the RV radial component, which leads to the maintenance of the total RVEF in patients with mild and moderate LV systolic dysfunction. Consequently, in these patients, the RV systolic function may be underestimated if assessed by parameters that reflect the longitudinal shortening only (ie, TAPSE, tricuspid annular systolic velocity). In patients with severe LV systolic dysfunction, this compensatory mechanism is lost due to a decrease of the radial RV shortening resulting in both the reduction of the total RVEF and the development of severe RV systolic dysfunction.
Prognostic Value of RVEF and the Different Components of RV Pump Function
Our study confirmed the findings derived from similar cohorts that RVEF is significantly associated with adverse outcomes, independently from LVEF.7,25 Importantly, in patients with normal RVEF, only the anteroposterior component’s relative contribution to total RV function was significantly and independently associated with the composite end point of cardiac death and hospitalization for heart failure, whereas neither RVEF nor LVEF was.
As mentioned above, the systolic anteroposterior shortening of the RV is mainly the result of LV circumferential contraction by stretching the RV free-wall insertion lines.26 Accordingly, the parameters referring to the anteroposterior component of total RVEF may be perceived as surrogates of the function of the interventricular septum and also of the LV contribution to RV performance. We hypothesize that, in patients with normal RVEF, this additional prognostic value of AEF is still related to LV dysfunction and deteriorated ventricular interdependence that could not be sensitively captured by LVEF either. Relative predictive power of the different RV components may vary according to the underlying pathophysiology. Therefore, further research is warranted either in cardiac diseases affecting the function of the interventricular septum (left bundle branch block, septal myocardial ischemia/infarction, etc) or in conditions with different RV pressure- and volume-overload profile. Of note, no conventional 2-dimensional echocardiographic parameter represents anteroposterior RV motion. Therefore, our results strongly emphasize the usefulness of 3D imaging in the thorough characterization of RV function.
Limitations
Several limitations in the current study warrant consideration. First, the study design was cross-sectional, and the study cohort was referral-based. Our results need to be confirmed in longitudinal studies designed to assess the adaptations of the RV function to the changes of LV function over time and also in specific subgroups (ie, patients with coronary artery disease, cardiomyopathies, etc) with a larger number of cases. Second, since the study protocol included the acquisition of 3DE data sets of the cardiac chambers, only patients with sinus rhythm, relatively good image quality, and able to tolerate breath-holding for at least 4 to 6 cardiac cycles have been selected. This may have caused a selection bias. The extent to which the results can be extrapolated to other patient groups (eg, patients with atrial fibrillation) is not known. However, latest generation 3DE scanners have implemented single-beat full-volume acquisitions at a temporal resolution high enough to allow the quantification of both the LV and the RV. We did not collect data about the LV diastolic function, however, our work was not aimed to assess relationships between diastolic function parameters and different components of RVEF but to study the functional adaptation of the RV to different degrees of the LV systolic dysfunction.
Finally, we did not assess 3DE volumetric analysis accuracy with a reference modality (CMR). However, this study was not designed to validate 3DE volumetric analysis but assess its prognostic value in the clinical routine. Good correlations of 3DE-derived RV and LV volumes and EF with CMR data have been demonstrated previously, including studies performed by our group.5,6
Conclusions
In our cohort of patients with left-sided structural heart disease, the performance of the longitudinal and the anteroposterior components of the RV systolic function were rather related to the LV systolic function, and they decreased significantly according to the degree of LV dysfunction. The relative increase in the radial component of the RV systolic function compensated the loss of longitudinal and anteroposterior RV shortening in patients with mild and moderate LV dysfunction to maintain total RVEF. Conversely, the drop in all the 3 components of RV systolic function resulted in a significant reduction of total RVEF in patients with severe LV dysfunction.
3DE-derived measurements of RV systolic function were associated with outcomes in patients with left-sided heart disease independently of LV function. The separate quantification of RV mechanical components can hold additional prognostic value, particularly in patients with normal RVEF.
Sources of Funding
The research was partly funded by the Thematic Excellence Programme (2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapeutic Development and Bioimaging thematic programmes of the Semmelweis University. Dr Surkova has received a research grant from European Society of Cardiology (2016) and a training grant from European Association of Cardiovascular Imaging (2016). Dr Kovács was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the ÚNKP 21-5 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund.
Disclosures
None.
Supplemental Materials
Supplemental Figures I–II
Supplemental Tables I–XIX
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 3DE
- three-dimensional echocardiograph
- AEF
- anteroposterior ejection fraction
- AEFi
- anteroposterior ejection fraction indexed to total right ventricular ejection fraction
- CMR
- cardiac magnetic resonance imaging
- FAC
- fractional area change
- HFpEF
- heart failure with preserved left ventricular ejection fraction
- LEF
- longitudinal ejection fraction
- LEFi
- longitudinal ejection fraction indexed to total right ventricular ejection fraction
- LV
- left ventricular
- LVEF
- left ventricular ejection fraction
- REF
- radial ejection fraction
- REFi
- radial ejection fraction indexed to total right ventricular ejection fraction
- RV
- right ventricular
- RVEF
- right ventricular ejection fraction
- TAPSE
- tricuspid annular plane systolic excursion
E. Surkova and A. Kovács contributed equally.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.121.012774.
For Sources of Funding and Disclosures, see page 993.
Contributor Information
Márton Tokodi, Email: tokmarton@gmail.com.
Bálint Károly Lakatos, Email: lakatosbalintka@gmail.com.
Béla Merkely, Email: merkely.study@gmail.com.
Denisa Muraru, Email: denisa.muraru@gmail.com.
Alessandro Ruocco, Email: alessandroruocco89@hotmail.com.
Gianfranco Parati, Email: gianfranco.parati@unimib.it.
Luigi P. Badano, Email: lpbadano@gmail.com.
References
- 1.Padang R, Chandrashekar N, Indrabhinduwat M, Scott CG, Luis SA, Chandrasekaran K, Michelena HI, Nkomo VT, Pislaru SV, Pellikka PA, et al. Aetiology and outcomes of severe right ventricular dysfunction. Eur Heart J 2020411273–1282.doi: 10.1093/eurheartj/ehaa037 [DOI] [PubMed] [Google Scholar]
- 2.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 20081171436–1448.doi: 10.1161/CIRCULATIONAHA.107.653576 [DOI] [PubMed] [Google Scholar]
- 3.Kovács A, Lakatos B, Tokodi M, Merkely B. Right ventricular mechanical pattern in health and disease: beyond longitudinal shortening. Heart Fail Rev 201924511–520.doi: 10.1007/s10741-019-09778-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leibundgut G, Rohner A, Grize L, Bernheim A, Kessel-Schaefer A, Bremerich J, Zellweger M, Buser P, Handke M. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr 201023116–126.doi: 10.1016/j.echo.2009.11.016 [DOI] [PubMed] [Google Scholar]
- 5.Muraru D, Badano LP, Piccoli G, Gianfagna P, Del Mestre L, Ermacora D, Proclemer A. Validation of a novel automated border-detection algorithm for rapid and accurate quantitation of left ventricular volumes based on three-dimensional echocardiography. Eur J Echocardiogr 201011359–368.doi: 10.1093/ejechocard/jep217 [DOI] [PubMed] [Google Scholar]
- 6.Muraru D, Spadotto V, Cecchetto A, Romeo G, Aruta P, Ermacora D, Jenei C, Cucchini U, Iliceto S, Badano LP. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging 2016171279–1289.doi: 10.1093/ehjci/jev309 [DOI] [PubMed] [Google Scholar]
- 7.Surkova E, Muraru D, Genovese D, Aruta P, Palermo C, Badano LP. Relative prognostic importance of left and right ventricular ejection fraction in patients with cardiac diseases. J Am Soc Echocardiogr 2019321407, e3–1415.doi: 10.1016/j.echo.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015281, e14–39.doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Muraru D, Badano LP, Nagata Y, Surkova E, Nabeshima Y, Genovese D, Otsuji Y, Guida V, Azzolina D, Palermo C, et al. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging 20202110–21.doi: 10.1093/ehjci/jez233 [DOI] [PubMed] [Google Scholar]
- 11.Lakatos B, Tősér Z, Tokodi M, Doronina A, Kosztin A, Muraru D, Badano LP, Kovács A, Merkely B. Quantification of the relative contribution of the different right ventricular wall motion components to right ventricular ejection fraction: the ReVISION method. Cardiovasc Ultrasound 2017158.doi: 10.1186/s12947-017-0100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokodi M, Staub L, Budai Á, Lakatos BK, Csákvári M, Suhai FI, Szabó L, Fábián A, Vágó H, Tősér Z, et al. Partitioning the right ventricle into 15 segments and decomposing its motion using 3D echocardiography-based models: the updated ReVISION method. Front Cardiovasc Med 20218622118.doi: 10.3389/fcvm.2021.622118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 20141291033–1044.doi: 10.1161/CIRCULATIONAHA.113.001375 [DOI] [PubMed] [Google Scholar]
- 14.Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res 20171131474–1485.doi: 10.1093/cvr/cvx160 [DOI] [PubMed] [Google Scholar]
- 15.Buckberg G, Hoffman JI. Right ventricular architecture responsible for mechanical performance: unifying role of ventricular septum. J Thorac Cardiovasc Surg 20141483166, e1–71.doi: 10.1016/j.jtcvs.2014.05.044 [DOI] [PubMed] [Google Scholar]
- 16.Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 2016181472–1487.doi: 10.1002/ejhf.630 [DOI] [PubMed] [Google Scholar]
- 17.Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo-Leiro MG, Guazzi M, Harjola VP, Heymans S, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 20182016–37.doi: 10.1002/ejhf.1029 [DOI] [PubMed] [Google Scholar]
- 18. Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, et al. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:e578–e622. doi: 10.1161/CIR.0000000000000560. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz K, Singh S, Dawson D, Frenneaux MP. Right ventricular function in left ventricular disease: pathophysiology and implications. Heart Lung Circ 201322507–511.doi: 10.1016/j.hlc.2013.03.072 [DOI] [PubMed] [Google Scholar]
- 20.Iglesias-Garriz I, Olalla-Gómez C, Garrote C, López-Benito M, Martín J, Alonso D, Rodríguez MA. Contribution of right ventricular dysfunction to heart failure mortality: a meta-analysis. Rev Cardiovasc Med 201213e62–e69. [DOI] [PubMed] [Google Scholar]
- 21.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014353452–3462.doi: 10.1093/eurheartj/ehu193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aschauer S, Kammerlander AA, Zotter-Tufaro C, Ristl R, Pfaffenberger S, Bachmann A, Duca F, Marzluf BA, Bonderman D, Mascherbauer J. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail 20161871–80.doi: 10.1002/ejhf.418 [DOI] [PubMed] [Google Scholar]
- 23.Lejeune S, Roy C, Ciocea V, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, et al. Right ventricular global longitudinal strain and outcomes in heart failure with preserved ejection fraction. J Am Soc Echocardiogr 202033973, e2–984.doi: 10.1016/j.echo.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 24.Lakatos BK, Nabeshima Y, Tokodi M, Nagata Y, Tősér Z, Otani K, Kitano T, Fábián A, Ujvári A, Boros AM, et al. Importance of nonlongitudinal motion components in right ventricular function: three-dimensional echocardiographic study in healthy volunteers. J Am Soc Echocardiogr 202033995, e1–1005.doi: 10.1016/j.echo.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Nagata Y, Wu VC, Kado Y, Otani K, Lin FC, Otsuji Y, Negishi K, Takeuchi M. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging 201710e005384.doi: 10.1161/CIRCIMAGING.116.005384 [DOI] [PubMed] [Google Scholar]
- 26.Addetia K, Muraru D, Badano LP, Lang RM. New directions in right ventricular assessment using 3-dimensional echocardiography. JAMA Cardiol 20194936–944.doi: 10.1001/jamacardio.2019.2424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.