Abstract
The relationship between the genetic loci that influence mean corpuscular volume (MCV) and those associated with excess alcohol drinking is unknown. We used white British participants from the UK Biobank (n = 362 595) to assess the association between alcohol consumption and MCV, and whether this was modulated by genetic factors. Multivariable regression was applied to identify predictors of MCV. GWAS, with and without stratification for alcohol consumption, determined how genetic variants influence MCV. SNPs in ADH1B, ADH1C and ALDH1B were used to construct a genetic score to test the assumption that acetaldehyde formation is an important determinant of MCV. Additional investigations using Mendelian randomization and phenomewide association analysis were conducted. Increasing alcohol consumption by 40 g/week resulted in a 0.30% [95% confidence interval CI: 0.30–0.31%] increase in MCV (P < 1.0 × 10−320). Unstratified (irrespective of alcohol intake) GWAS identified 212 loci associated with MCV, of which 108 were novel. There was no heterogeneity of allelic effects by drinking status. No association was found between MCV and the genetic score generated from alcohol metabolizing genes. Mendelian randomization demonstrated a causal effect for alcohol on MCV. Seventy-one SNP-outcome pairs reached statistical significance in phenomewide association analysis, with evidence of shared genetic architecture for MCV and thyroid dysfunction, and mineral metabolism disorders. MCV increases linearly with alcohol intake in a causal manner. Many genetic loci influence MCV, with new loci identified in this analysis that provide novel biological insights. However, there was no interaction between alcohol consumption and the allelic variants associated with MCV.
Introduction
Alcohol misuse and abuse is a leading cause of morbidity and mortality (1). In 2016, global statistics suggested that 5.1% (~3 million) of deaths and 5.3% (~133 million) of disability-adjusted life years were caused by the harmful use of alcohol (2). Early identification of individuals who are misusing alcohol is critical for interventions to stop progression towards alcohol dependence and alcohol-related end-organ damage. Unfortunately, a history from a patient is not always reliable, and laboratory tests vary in their diagnostic accuracy, availability and usage. In clinical practice, therefore, it is usual to use a combination of history (including alcohol intake and symptoms consistent with organ damage), physical examination (to look for features of organ damage) and laboratory markers that support alcohol misuse as the underlying aetiology. The most widely used laboratory tests are (a) liver function tests (in particular, gamma-glutamyl transferase), which indicates liver damage, and (b) the mean corpuscular volume (MCV), a measure of the mean size and volume of erythrocytes, which is a non-specific marker of alcohol misuse (3).
The molecular basis for the increase in MCV that occurs with alcohol misuse is incompletely understood. A study of 105 alcohol-dependent individuals, 62 moderate drinkers and 24 abstainers was able to show that the increase in MCV was dose-dependent (4). Alcohol may have direct haematotoxic effects by interfering with cell structure and erythrocyte stability (5). Interestingly, the levels of acetaldehyde, a metabolite of alcohol, show a significant increase inside erythrocytes of alcohol-dependent individuals (6). Since acetaldehyde is a toxic metabolite, and can bind to proteins, this may lead to erythrocyte damage directly or through an immune-mediated mechanism via the development of anti-acetaldehyde adduct antibodies (4). Folate deficiency also occurs with alcohol misuse, particularly in those patients with liver disease (7), and therefore may be implicated in increasing the MCV (8).
It is important to note, however, that MCV is a non-specific biomarker in that other factors such as age, smoking, malnutrition and underlying diseases, including hypothyroidism, liver disease and pernicious anaemia, are also known to affect MCV (7,9,10). There is also a genetic contribution to the MCV, in addition to the genetic factors that have been identified for other haematological indices (11,12). Genetic factors have also been implicated in high alcohol consumption—our recent study showed genome-wide significant effects across six loci following meta-analysis in two large independent cohorts (13). The relationship between the genetic loci that determine the MCV and those associated with excess alcohol drinking is, however, unknown.
In this study, we have used genome-wide association study (GWAS) data from the UK Biobank (UKB) to understand if genetic variants and level of alcohol consumption interact to influence MCV. The specific aims were as follows: (1) determine how genetic loci influence MCV, with and without stratification for alcohol consumption; (2) confirm the causal effect of alcohol consumption on MCV using Mendelian randomization and (3) explore the association between acetaldehyde accumulation and MCV using genotype data from alcohol metabolizing genes.
Results
Demographics
A total of 139 921 individuals were excluded from the UKB cohort: 1502 declined to provide information on their drinking status, 1984 were drinking at levels at least 4 SDs above sex-specific means, 7060 had liver disease, 23 385 had missing MCV data, 104 788 failed GWAS quality control (including ethnic inclusion) and 1202 had missing covariate information. This study therefore included 362 595 participants, of whom 194 706 (53.7%) were females and the average age was 56.9 (SD = 7.9) years. There were 82 235 (24.6%) zero, 146 436 (40.1%) light, 114 946 (30.2%) moderate and 18 978 (5.0%) heavy drinkers, with the median units/week being 6.0 [interquartile (IQR) = 13.0] in females and 15.6 (IQR = 23.9) in males. There was evidence of hypothyroidism and vitamin B12 deficiency in 14 781 and 777 participants, respectively.
Alcohol consumption and MCV
Alcohol consumption was associated with higher MCV (P < 1.0 × 10−320). Increasing alcohol consumption by 5 units (40 g) per week resulted in a 0.3% increase in MCV. Variation by drinking status was evident; compared with light drinkers (reference group), zero drinkers had 0.9% lower mean values, while moderate and heavy drinkers had 1.1 and 2.8% higher mean values, respectively (all P < 1.0 × 10−320; Table 1). Results from multivariate analysis for MCV were consistent in terms of direction and magnitude when those classified as teetotal were removed (Supplementary Material Table S1).
Table 1.
Summary of linear and logistic regression models with all participants (n = 362 595)
| Risk factor | Change in MCV (%) | 95% CI | P | |
|---|---|---|---|---|
| Alcohol: continuous variable | ||||
| Alcohol (5 units) | 0.30 | 0.30 to 0.31 | <1.0 × 10−320 | |
| Sex (Ref: female) | −0.21 | −0.25 to −0.18 | 5.2 × 10−38 | |
| Age at recruitment | 0.06 | 0.06 to 0.06 | <1.0 × 10−320 | |
| Never smoker (Ref: current) | −2.00 | −2.05 to −1.95 | <1.0 × 10−320 | |
| Previous smoker (Ref: current) | −1.87 | −1.93 to −1.82 | <1.0 × 10−320 | |
| Hypothyroidism | −0.20 | −0.28 to −0.13 | 2.4 × 10−7 | |
| B12 deficiency | 0.20 | −0.13 to 0.53 | 0.23 | |
| Alcohol: categorical variable | ||||
| Drinking status (Ref: light) Non-drinker Moderate Heavy |
−0.89 1.14 2.80 |
−0.93 to −0.85 1.10 to 1.17 2.72 to 2.87 |
< 1.0 × 10−320 <1.0 × 10−320 <1.0 × 10−320 |
|
| Sex (Ref: female) | −0.08 | −0.11 to −0.05 | 1.9 × 10−6 | |
| Age at recruitment | 0.06 | 0.06 to 0.07 | <1.0 × 10−320 | |
| Never smoker (Ref: current) | −2.11 | −2.16 to −2.06 | <1.0 × 10−320 | |
| Previous smoker (Ref: current) | −1.97 | −2.02 to −1.92 | <1.0 × 10−320 | |
| Hypothyroidism | −0.17 | −0.25 to −0.09 | 1.4 × 10−5 | |
| B12 deficiency | 0.26 | −0.06 to 0.59 | 0.12 |
GWAS of MCV
An unstratified (i.e. irrespective of alcohol intake) GWAS in white British individuals identified 212 loci associated with MCV at P < 5 × 10−8 (Fig. 1). Presented P-values are corrected based on a LD score regression intercept = 1.20. The large sample size (n = 362 595) resulted in identification of variants with small effect sizes, equivalent to a change in MCV of 0.057% (Table 2). There was evidence to suggest that lower minor allele frequency was associated with larger effect sizes (Fig. 2). The largest effect size was observed with rs144861591 [effect allele frequency (EAF) 0.076; P = 3.4 × 10−640], where the minor allele (T) was associated with an increase in MCV by 1.11%. This variant is located ~13.5 bp downstream of LOC108783645, an HFE antisense RNA. HFE itself is involved with iron regulation and has been associated with haemochromatosis (14). Strong associations were also reported in loci mapping to HBS1L-MYB, TMPRSS6, CCND3, CARMIL1, ODF3B and CCDC162P (11,12,15–18). We compared (using SNP ID and reported gene symbol) our findings to those of other equivalently sized genome-wide studies of MCV in UKB (11,19) and found that 58.0% (n = 123) of our loci were unique, likely due to targeted study of MCV and trait-specific covariate control whereas the cited studies explored multiple traits. Investigation in the GWAS catalog (https://www.ebi.ac.uk/gwas/) found replication with a further 15 mapped genes, leaving 108 new findings (Table 2). The SNP-based heritability of MCV was estimated to be 24.2% through LD score regression.
Figure 2 .
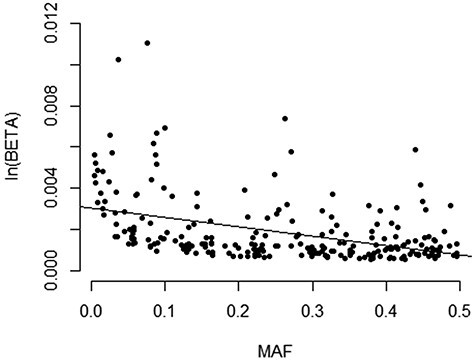
Relationship between minor allele frequency and effect size.
Table 2.
Summary of genome-wide significant SNPs following distance-based clumping
| SNP | CHR | BP | Locus (overlapping/nearest) | Effect allele | EAF | % change MCV | 95%LCI | %UCI | P (GC corrected) | Uniquea |
|---|---|---|---|---|---|---|---|---|---|---|
| rs7775698 | 6 | 135 418 635 | HBS1L | C | 0.738 | --0.73 | --0.76 | --0.71 | 2.8e−790 | N |
| rs144861591 | 6 | 26 072 992 | HFE | C | 0.924 | --1.10 | --1.14 | --1.06 | 3.4e−640 | N |
| rs855791 | 22 | 37 462 936 | TMPRSS6 | A | 0.440 | --0.59 | --0.61 | --0.56 | 3.0e−610 | N |
| rs9471708 | 6 | 41 956 353 | CCND3 | C | 0.728 | 0.58 | 0.56 | 0.60 | 1.3e−493 | N |
| rs592423 | 6 | 139 840 693 | RP11-12A2.3 | A | 0.447 | 0.42 | 0.40 | 0.44 | 2.2e−327 | N |
| rs149359690 | 6 | 25 526 319 | LRRC16A | C | 0.900 | --0.69 | --0.72 | --0.65 | 3.6e−325 | N |
| rs218264 | 4 | 55 408 875 | AC006552.1 | A | 0.752 | --0.46 | --0.49 | --0.44 | 8.7E−287 | Y |
| rs13191659 | 6 | 27 001 055 | VN1R12P | C | 0.911 | --0.66 | --0.70 | --0.63 | 1.7E−264 | Y |
| rs140522 | 22 | 50 971 266 | ODF3B | T | 0.327 | --0.37 | --0.39 | --0.35 | 1.4E−221 | N |
| rs9487023 | 6 | 109 590 004 | C6orf183 | A | 0.551 | --0.34 | --0.36 | --0.31 | 3.6E−212 | N |
| rs71559031 | 6 | 27 519 947 | XXbac-BPG34I8.3 | G | 0.916 | --0.62 | --0.66 | --0.58 | 2.7E−203 | Y |
| rs117747069 | 16 | 170 076 | NPRL3 | G | 0.963 | 1.03 | 0.96 | 1.10 | 9.1E−188 | N |
| rs10758657 | 9 | 4 853 751 | RCL1 | A | 0.791 | 0.39 | 0.37 | 0.42 | 1.8E−182 | N |
| rs6014993 | 20 | 55 991 637 | RBM38 | A | 0.513 | --0.32 | --0.34 | --0.30 | 1.2E−180 | N |
| rs147493146 | 6 | 28 054 465 | ZNF165 | C | 0.913 | --0.56 | --0.60 | --0.52 | 5.2E−179 | Y |
| rs9801017 | 7 | 100 236 202 | TFR2 | G | 0.376 | --0.31 | --0.34 | --0.29 | 5.7E−168 | N |
| rs7428496 | 3 | 142 320 532 | PLS1 | A | 0.414 | 0.30 | 0.28 | 0.33 | 7.4E−164 | N |
| rs6592965 | 7 | 50 427 982 | IKZF1 | G | 0.546 | --0.30 | --0.32 | --0.28 | 1.3E−158 | N |
| rs8110787 | 19 | 12 999 458 | KLF1 | C | 0.609 | --0.29 | --0.31 | --0.27 | 3.2E−149 | N |
| rs114179634 | 6 | 28 626 101 | LINC00533 | C | 0.911 | --0.51 | --0.55 | --0.47 | 2.3E−146 | Y |
| rs13194984 | 6 | 26 500 563 | BTN1A1 | G | 0.856 | --0.38 | --0.41 | --0.35 | 1.7E−134 | N |
| rs41298087 | 3 | 195 779 736 | TFRC | C | 0.687 | 0.29 | 0.27 | 0.32 | 4.0E−134 | N |
| rs1045267 | 2 | 112 187 041 | NA | A | 0.266 | 0.32 | 0.29 | 0.35 | 2.8E−122 | N |
| rs9952469 | 18 | 43 812 010 | C18orf25 | T | 0.254 | 0.30 | 0.27 | 0.32 | 9.1E−121 | N |
| rs362538 | 6 | 29 510 630 | GPR53P | G | 0.902 | --0.40 | --0.44 | --0.36 | 2.4E−109 | Y |
| rs113700287 | 3 | 24 334 511 | THRB | T | 0.326 | --0.26 | --0.28 | --0.24 | 1.1E−107 | N |
| rs78744187 | 19 | 33 754 548 | CTD-2540B15.12 | C | 0.918 | 0.44 | 0.40 | 0.48 | 7.7E−107 | N |
| rs113968785 | 15 | 65 734 015 | DPP8 | A | 0.748 | 0.28 | 0.25 | 0.30 | 4.3E−101 | Y |
| rs243076 | 2 | 60 617 563 | AC007381.2 | G | 0.594 | --0.23 | --0.25 | --0.21 | 1.9E−94 | Y |
| rs17476364 | 10 | 71 094 504 | HK1 | T | 0.890 | --0.36 | --0.39 | --0.32 | 1.5E−92 | N |
| rs2057726 | 6 | 30 335 350 | UBQLN1P1 | C | 0.143 | 0.31 | 0.28 | 0.34 | 1.5E−91 | Y |
| rs56273049 | 12 | 121 156 041 | UNC119B | A | 0.612 | 0.23 | 0.21 | 0.25 | 4.3E−90 | N |
| rs7089063 | 10 | 45 946 389 | RP11-67C2.2 | C | 0.761 | --0.26 | --0.28 | --0.23 | 8.0E−88 | Y |
| rs738264 | 22 | 32 874 258 | FBXO7 | G | 0.728 | 0.24 | 0.22 | 0.27 | 1.7E−85 | N |
| rs536350318 | 12 | 4 331 068 | CCND2-AS1 | C | 0.788 | --0.26 | --0.28 | --0.23 | 1.1E−81 | N |
| rs3811444 | 1 | 248 039 451 | TRIM58 | C | 0.667 | 0.22 | 0.20 | 0.24 | 1.1E−81 | N |
| rs2026428 | 10 | 45 389 452 | TMEM72-AS1 | T | 0.416 | --0.21 | --0.23 | --0.19 | 3.3E−75 | N |
| rs16843346 | 1 | 198 543 027 | RP11-553K8.2 | C | 0.972 | --0.57 | --0.64 | --0.51 | 1.7E−66 | Y |
| rs1042391 | 6 | 16 290 761 | GMPR | T | 0.619 | 0.19 | 0.17 | 0.21 | 9.5E−66 | N |
| rs56142708 | 17 | 19 934 963 | SPECC1 | A | 0.478 | --0.19 | --0.21 | --0.17 | 2.8E−65 | N |
| rs12193223 | 6 | 24 978 511 | FAM65B | C | 0.938 | --0.37 | --0.42 | --0.33 | 1.3E−62 | Y |
| rs17296501 | 8 | 21 849 566 | XPO7 | C | 0.838 | --0.24 | --0.27 | --0.21 | 8.1E−59 | N |
| rs7705526 | 5 | 1 285 974 | TERT | C | 0.674 | 0.19 | 0.17 | 0.21 | 1.6E−57 | N |
| rs10901252 | 9 | 136 128 000 | ABO | G | 0.940 | 0.36 | 0.32 | 0.41 | 2.5E−55 | N |
| rs74401481 | 19 | 1 855 583 | KLF16 | C | 0.975 | 0.66 | 0.57 | 0.74 | 3.5E−55 | N |
| rs13207150 | 6 | 110 092 900 | FIG4 | C | 0.313 | --0.18 | --0.20 | --0.15 | 1.2E−52 | N |
| rs4134058 | 1 | 47 670 911 | TAL1 | T | 0.457 | --0.17 | --0.19 | --0.15 | 5.4E−52 | N |
| rs2834256 | 21 | 35 125 373 | AP000304.12 | A | 0.655 | 0.18 | 0.15 | 0.20 | 1.4E−51 | N |
| rs381500 | 6 | 164 478 388 | RP1-155D22.2 | C | 0.549 | 0.16 | 0.14 | 0.18 | 2.5E−50 | N |
| rs1172113 | 1 | 205 226 288 | TMCC2 | T | 0.587 | --0.16 | --0.19 | --0.14 | 3.0E−49 | N |
| rs12128171 | 1 | 158 580 477 | SPTA1 | A | 0.719 | 0.18 | 0.15 | 0.20 | 3.3E−48 | N |
| rs6730558 | 2 | 8 756 183 | AC011747.6 | C | 0.620 | 0.16 | 0.14 | 0.18 | 9.6E−46 | N |
| rs111918234 | 5 | 154 027 482 | MIR1303 | GT | 0.860 | --0.23 | --0.26 | --0.19 | 3.1E−45 | N |
| rs558567978 | 17 | 76 124 810 | TMC6 | A | 0.777 | 0.19 | 0.16 | 0.21 | 6.8E−44 | N |
| rs67775544 | 4 | 122 796 917 | RP11-63B13.1 | G | 0.603 | --0.16 | --0.18 | --0.13 | 8.4E−44 | Y |
| rs17534202 | 1 | 203 281 175 | BTG2 | G | 0.464 | 0.15 | 0.13 | 0.17 | 5.9E−42 | N |
| rs111721712 | 6 | 31 315 407 | HLA-B | C | 0.534 | 0.15 | 0.13 | 0.17 | 8.5E−42 | N |
| rs10766533 | 11 | 19 224 677 | CSRP3 | T | 0.278 | 0.16 | 0.14 | 0.19 | 1.7E−40 | N |
| rs11227793 | 11 | 67 194 593 | RPS6KB2 | C | 0.561 | 0.15 | 0.12 | 0.17 | 3.4E−39 | Y |
| rs67971539 | 16 | 87 886 726 | SLC7A5 | T | 0.764 | --0.17 | --0.19 | --0.14 | 8.6E−39 | N |
| rs4134898 | 7 | 99 711 614 | TAF6 | C | 0.868 | 0.21 | 0.18 | 0.24 | 1.7E−37 | Y |
| rs17776547 | 10 | 51 581 143 | NCOA4 | A | 0.965 | --0.38 | --0.44 | --0.32 | 4.3E−36 | N |
| rs1567668 | 8 | 23 418 122 | SLC25A37 | A | 0.429 | --0.14 | --0.16 | --0.12 | 1.6E−35 | N |
| rs144894688 | 9 | 100 746 855 | ANP32B | G | 0.780 | 0.16 | 0.13 | 0.19 | 1.6E−33 | N |
| rs35979828 | 12 | 54 685 880 | RP11-968A15.8 | C | 0.931 | --0.26 | --0.30 | --0.22 | 1.8E−32 | N |
| rs114165892 | 14 | 74 223 968 | ELMSAN1 | C | 0.976 | --0.43 | --0.50 | --0.36 | 6.1E−32 | Y |
| rs7789162 | 7 | 44 872 900 | H2AFV | T | 0.495 | --0.13 | --0.15 | --0.11 | 1.5E−31 | N |
| rs145946844 | 2 | 111 612 050 | ACOXL | T | 0.662 | --0.13 | --0.16 | --0.11 | 7.1E−31 | N |
| rs59027521 | 8 | 128 966 573 | PVT1 | A | 0.657 | 0.13 | 0.11 | 0.16 | 2.4E−30 | N |
| rs6440006 | 3 | 141 142 691 | ZBTB38 | G | 0.552 | 0.13 | 0.11 | 0.15 | 3.0E−30 | Y |
| rs6788010 | 3 | 16 929 109 | PLCL2 | T | 0.920 | 0.23 | 0.19 | 0.27 | 9.1E−30 | N |
| rs496321 | 11 | 94 886 632 | RP11-712B9.2 | T | 0.386 | 0.13 | 0.10 | 0.15 | 1.3E−28 | N |
| rs1991866 | 8 | 130 624 105 | CCDC26 | G | 0.424 | 0.12 | 0.10 | 0.15 | 1.7E−28 | N |
| rs592229 | 6 | 31 930 441 | SKIV2L | G | 0.486 | 0.12 | 0.10 | 0.14 | 1.9E−28 | Y |
| rs10883359 | 10 | 101 274 033 | RP11-129J12.2 | A | 0.716 | --0.13 | --0.16 | --0.11 | 5.0E−27 | Y |
| rs10923397 | 1 | 118 251 143 | RP11-134N8.2 | C | 0.837 | --0.16 | --0.19 | --0.13 | 1.1E−26 | N |
| rs78147199 | 15 | 66 245 962 | MEGF11 | G | 0.955 | 0.29 | 0.23 | 0.34 | 1.2E−26 | Y |
| rs12582170 | 12 | 53 757 831 | SP1 | A | 0.156 | --0.16 | --0.19 | --0.13 | 1.4E−26 | N |
| rs2713936 | 15 | 56 545 985 | TEX9 | A | 0.577 | 0.12 | 0.10 | 0.14 | 3.8E−26 | N |
| rs11723371 | 4 | 145 025 810 | RP11-673E1.4 | T | 0.527 | --0.12 | --0.14 | --0.10 | 4.3E−26 | N |
| rs151305716 | 20 | 52 222 106 | ZNF217 | C | 0.984 | --0.48 | --0.57 | --0.39 | 4.9E−26 | N |
| rs111836360 | 16 | 89 786 649 | VPS9D1 | A | 0.594 | --0.12 | --0.14 | --0.09 | 4.7E−25 | N |
| rs2277339 | 12 | 57 146 069 | PRIM1 | T | 0.897 | --0.19 | --0.22 | --0.15 | 6.6E−25 | N |
| rs4900538 | 14 | 102 994 065 | MIR4309 | T | 0.355 | --0.12 | --0.14 | --0.10 | 9.0E−25 | N |
| rs1134634 | 4 | 15 603 069 | CC2D2A | G | 0.414 | --0.11 | --0.14 | --0.09 | 1.9E−24 | N |
| rs136211 | 22 | 36 758 547 | MYH9 | A | 0.313 | 0.12 | 0.10 | 0.14 | 3.9E−24 | N |
| rs371182872 | 11 | 108 300 854 | C11orf65 | CT | 0.588 | 0.11 | 0.09 | 0.14 | 7.7E−24 | N |
| rs322918 | 1 | 199 068 614 | RP11-16L9.4 | A | 0.539 | 0.11 | 0.09 | 0.13 | 8.0E−23 | N |
| rs1569419 | 1 | 2 996 602 | PRDM16 | T | 0.233 | 0.13 | 0.10 | 0.15 | 4.2E−22 | N |
| rs9532563 | 13 | 41 160 270 | FOXO1 | T | 0.782 | 0.13 | 0.10 | 0.16 | 4.8E−22 | N |
| rs200234036 | 1 | 39 869 512 | MACF1 | GC | 0.728 | 0.12 | 0.10 | 0.15 | 7.1E−22 | Y |
| rs875742 | 5 | 173 287 763 | CPEB4 | G | 0.594 | 0.11 | 0.09 | 0.13 | 7.3E−22 | N |
| rs9370792 | 6 | 15 099 585 | RP1-190J20.2 | A | 0.601 | --0.11 | --0.13 | --0.08 | 1.1E−21 | Y |
| rs138191091 | 7 | 99 105 676 | ZKSCAN5 | A | 0.877 | 0.17 | 0.13 | 0.20 | 1.3E−21 | Y |
| rs4955426 | 3 | 49 143 438 | QARS | C | 0.222 | 0.12 | 0.10 | 0.15 | 4.2E−21 | Y |
| rs2143583 | 1 | 114 989 211 | TRIM33 | T | 0.747 | --0.12 | --0.14 | --0.09 | 4.2E−21 | N |
| rs10883710 | 10 | 103 885 557 | LDB1 | T | 0.545 | --0.10 | --0.13 | --0.08 | 5.9E−21 | Y |
| rs7487314 | 12 | 88 836 215 | Y_RNA | G | 0.299 | --0.11 | --0.14 | --0.09 | 6.0E−21 | N |
| rs35362007 | 14 | 96 003 198 | GLRX5 | G | 0.749 | --0.12 | --0.15 | --0.10 | 7.8E−21 | N |
| rs632959 | 1 | 68 197 671 | GNG12 | A | 0.295 | --0.11 | --0.14 | --0.09 | 9.2E−21 | N |
| rs1047891 | 2 | 211 540 507 | CPS1 | C | 0.684 | --0.11 | --0.13 | --0.09 | 1.1E−20 | N |
| rs2923403 | 8 | 42 447 748 | RP11-503E24.3 | G | 0.407 | 0.10 | 0.08 | 0.13 | 2.2E−20 | Y |
| rs2224539 | 20 | 38 552 107 | HSPE1P1 | A | 0.584 | --0.10 | --0.13 | --0.08 | 3.5E−20 | N |
| rs149472343 | 10 | 64 905 218 | NRBF2 | C | 0.967 | 0.28 | 0.22 | 0.34 | 1.3E−19 | N |
| rs2237572 | 7 | 92 260 260 | CDK6 | T | 0.804 | --0.12 | --0.15 | --0.10 | 2.9E−19 | N |
| rs545709142 | 1 | 11 881 141 | CLCN6 | C | 0.837 | 0.13 | 0.10 | 0.16 | 3.3E−19 | N |
| rs12779263 | 10 | 104 886 533 | NT5C2 | G | 0.706 | 0.11 | 0.08 | 0.13 | 3.6E−19 | Y |
| rs322351 | 5 | 172 194 873 | RP11-779O18.3 | C | 0.532 | --0.10 | --0.12 | --0.08 | 4.5E−19 | Y |
| rs72766638 | 9 | 136 931 778 | BRD3 | C | 0.836 | --0.13 | --0.16 | --0.10 | 6.1E−19 | Y |
| rs174567 | 11 | 61 593 005 | FADS2 | A | 0.649 | 0.10 | 0.08 | 0.12 | 8.3E−19 | Y |
| rs4663199 | 2 | 236 368 039 | AGAP1 | T | 0.601 | --0.10 | --0.12 | --0.08 | 1.7E−18 | N |
| rs74929147 | 19 | 18 413 061 | LSM4 | G | 0.941 | 0.21 | 0.16 | 0.26 | 2.2E−18 | N |
| rs201581170 | 10 | 105 682 344 | OBFC1 | T | 0.845 | 0.13 | 0.10 | 0.16 | 3.2E−18 | Y |
| rs9866749 | 3 | 49 650 935 | BSN | A | 0.296 | 0.11 | 0.08 | 0.13 | 4.7E−18 | Y |
| rs2492301 | 1 | 37 939 173 | LINC01137 | T | 0.471 | 0.10 | 0.07 | 0.12 | 6.0E−18 | N |
| rs80226431 | 8 | 48 267 917 | SPIDR | A | 0.905 | 0.16 | 0.12 | 0.20 | 2.0E−17 | Y |
| rs36225153 | 4 | 146 081 852 | OTUD4 | C | 0.886 | 0.15 | 0.11 | 0.18 | 2.8E−17 | Y |
| rs7641761 | 3 | 178 740 422 | ZMAT3 | T | 0.302 | --0.10 | --0.12 | --0.08 | 3.1E−17 | N |
| rs6116019 | 20 | 3 742 066 | C20orf27 | T | 0.901 | --0.16 | --0.19 | --0.12 | 3.7E−17 | N |
| rs4936291 | 11 | 114 009 982 | ZBTB16 | A | 0.611 | 0.10 | 0.07 | 0.12 | 4.0E−17 | Y |
| rs6531706 | 4 | 39 296 167 | RFC1 | T | 0.565 | --0.09 | --0.12 | --0.07 | 5.8E−17 | N |
| rs73079476 | 12 | 21 343 833 | SLCO1B1 | A | 0.850 | 0.13 | 0.10 | 0.16 | 6.1E−17 | Y |
| rs79953286 | 3 | 132 226 100 | DNAJC13 | A | 0.942 | 0.20 | 0.15 | 0.24 | 8.3E−17 | Y |
| rs1867146 | 15 | 75 354 971 | PPCDC | C | 0.818 | 0.12 | 0.09 | 0.15 | 9.0E−17 | Y |
| rs2723513 | 7 | 17 814 888 | SNX13 | A | 0.461 | --0.09 | --0.11 | --0.07 | 1.2E−16 | Y |
| rs1119279 | 8 | 47 071 977 | AC113134.1 | C | 0.903 | 0.15 | 0.12 | 0.19 | 1.9E−16 | Y |
| rs145498761 | 4 | 128 456 129 | RP11-18O11.2 | T | 0.991 | 0.49 | 0.37 | 0.61 | 3.7E−16 | Y |
| rs920112 | 2 | 174 219 135 | AC092573.2 | G | 0.947 | --0.20 | --0.25 | --0.15 | 4.7E−16 | N |
| rs2134814 | 6 | 90 987 512 | BACH2 | C | 0.646 | --0.09 | --0.11 | --0.07 | 5.4E−16 | N |
| rs12196049 | 6 | 121 786 091 | RNU4-35P | A | 0.801 | 0.11 | 0.08 | 0.14 | 6.7E−16 | Y |
| rs964184 | 11 | 116 648 917 | ZNF259 | G | 0.132 | --0.13 | --0.16 | --0.10 | 6.7E−16 | Y |
| rs13209786 | 6 | 131 421 040 | AKAP7 | A | 0.769 | 0.10 | 0.08 | 0.13 | 8.0E−16 | Y |
| rs145185045 | 17 | 60 091 881 | MED13 | T | 0.770 | 0.11 | 0.08 | 0.13 | 1.4E−15 | Y |
| rs3892355 | 19 | 5 696 962 | LONP1 | G | 0.674 | --0.09 | --0.12 | --0.07 | 1.9E−15 | Y |
| rs61952071 | 12 | 133 076 439 | FBRSL1 | C | 0.687 | --0.09 | --0.12 | --0.07 | 2.0E−15 | Y |
| rs6711700 | 2 | 86 987 987 | RMND5A | G | 0.329 | --0.09 | --0.12 | --0.07 | 2.2E−15 | N |
| rs147707926 | 9 | 115 914 583 | SLC31A2 | C | 0.981 | 0.34 | 0.25 | 0.42 | 2.6E−15 | Y |
| rs4672497 | 2 | 62 523 565 | snoU13 | C | 0.779 | 0.10 | 0.08 | 0.13 | 5.0E−15 | N |
| rs657036 | 6 | 35 901 151 | SLC26A8 | G | 0.703 | 0.09 | 0.07 | 0.12 | 5.0E−15 | Y |
| rs7137095 | 12 | 6 739 907 | LPAR5 | C | 0.452 | --0.09 | --0.11 | --0.07 | 8.3E−15 | Y |
| rs1330826 | 9 | 85 129 970 | RP11-15B24.5 | G | 0.772 | --0.10 | --0.13 | --0.08 | 1.2E−14 | N |
| rs139012450 | 2 | 160 684 717 | LY75 | T | 0.504 | --0.09 | --0.11 | --0.06 | 1.3E−14 | Y |
| rs75497126 | 3 | 141 655 542 | RP11-271K21.11 | A | 0.993 | 0.52 | 0.39 | 0.66 | 2.8E−14 | Y |
| rs200401106 | 2 | 197 024 922 | STK17B | A | 0.871 | 0.12 | 0.09 | 0.16 | 4.2E−14 | Y |
| rs75581061 | 6 | 134 858 499 | RP11-557H15.4 | A | 0.877 | --0.12 | --0.16 | --0.09 | 7.9E−14 | Y |
| rs62472014 | 7 | 98 517 117 | TRRAP | C | 0.966 | 0.23 | 0.17 | 0.29 | 8.8E−14 | N |
| rs184837332 | 17 | 44 359 783 | ARL17B | G | 0.775 | 0.10 | 0.07 | 0.13 | 9.4E-14 | Y |
| rs78378222 | 17 | 7 571 752 | TP53 | T | 0.987 | 0.38 | 0.28 | 0.48 | 9.9E−14 | Y |
| rs6747952 | 2 | 239 069 926 | FAM132B | C | 0.556 | --0.08 | --0.10 | --0.06 | 1.3E−13 | Y |
| rs10865309 | 2 | 58 984 870 | LINC01122 | C | 0.863 | 0.12 | 0.09 | 0.15 | 1.3E−13 | N |
| rs117325033 | 6 | 140 511 519 | MIR3668 | T | 0.995 | --0.56 | --0.71 | --0.41 | 1.4E−13 | Y |
| rs115447786 | 6 | 34 354 073 | NUDT3 | C | 0.955 | 0.19 | 0.14 | 0.24 | 1.6E−13 | Y |
| rs4285804 | 10 | 104 386 309 | SUFU | T | 0.441 | 0.08 | 0.06 | 0.10 | 2.2E−13 | Y |
| rs1958078 | 14 | 70 354 858 | SMOC1 | A | 0.155 | --0.11 | --0.14 | --0.08 | 2.5E−13 | N |
| rs35158985 | 16 | 68 796 746 | CDH1 | A | 0.693 | 0.09 | 0.06 | 0.11 | 4.1E−13 | N |
| rs2023335 | 19 | 10 695 959 | AP1M2 | A | 0.056 | --0.18 | --0.23 | --0.13 | 4.2E−13 | Y |
| rs10893817 | 11 | 127 951 980 | RP11-702B10.2 | A | 0.351 | --0.08 | --0.11 | --0.06 | 4.9E−13 | Y |
| rs766009815 | 3 | 176 878 261 | TBL1XR1 | CA | 0.432 | --0.08 | --0.10 | --0.06 | 5.3E−13 | Y |
| rs34406510 | 1 | 209 936 964 | TRAF3IP3 | T | 0.766 | 0.09 | 0.07 | 0.12 | 7.4E−13 | N |
| rs6538413 | 12 | 93 749 125 | RP11-486A14.2 | G | 0.692 | --0.09 | --0.11 | --0.06 | 1.0E−12 | N |
| rs557536055 | 17 | 40 535 384 | STAT3 | C | 0.701 | --0.09 | --0.11 | --0.06 | 1.3E−12 | N |
| rs60152331 | 1 | 63 177 539 | RP11-230B22.1 | G | 0.623 | --0.08 | --0.10 | --0.06 | 2.6E−12 | Y |
| rs73369896 | 17 | 80 478 877 | FOXK2 | G | 0.922 | 0.15 | 0.11 | 0.19 | 3.4E−12 | Y |
| rs10811408 | 9 | 20 805 270 | FOCAD | T | 0.777 | 0.09 | 0.07 | 0.12 | 4.1E−12 | Y |
| rs34817 | 5 | 102 435 260 | GIN1 | G | 0.695 | --0.08 | --0.11 | --0.06 | 5.1E−12 | Y |
| rs71446622 | 13 | 113 365 480 | ATP11A | G | 0.910 | 0.13 | 0.10 | 0.17 | 5.8E−12 | N |
| rs8138197 | 22 | 43 114 551 | A4GALT | G | 0.529 | 0.08 | 0.05 | 0.10 | 5.9E−12 | N |
| rs72755040 | 15 | 64 342 757 | DAPK2 | G | 0.945 | 0.17 | 0.12 | 0.21 | 7.1E−12 | Y |
| rs12146644 | 11 | 95 492 878 | FAM76B | A | 0.618 | 0.08 | 0.06 | 0.10 | 9.6E−12 | Y |
| rs117107603 | 7 | 149 261 825 | ZNF767 | C | 0.984 | 0.30 | 0.21 | 0.39 | 9.6E−12 | Y |
| rs10910476 | 1 | 234 734 956 | IRF2BP2 | C | 0.444 | 0.08 | 0.05 | 0.10 | 1.2E−11 | Y |
| rs1520195 | 3 | 183 736 882 | ABCC5 | G | 0.509 | --0.07 | --0.10 | --0.05 | 1.3E−11 | Y |
| rs55693403 | 9 | 84 320 639 | RP11-154D17.1 | T | 0.942 | --0.16 | --0.20 | --0.11 | 2.4E−11 | Y |
| rs140073759 | 2 | 152 356 937 | RIF1 | T | 0.378 | 0.08 | 0.05 | 0.10 | 2.5E−11 | Y |
| rs61871633 | 11 | 2 366 260 | CD81-AS1 | C | 0.913 | --0.13 | --0.17 | --0.09 | 2.7E−11 | Y |
| rs762418439 | 16 | 691 325 | AL022341.1 | A | 0.616 | 0.09 | 0.06 | 0.11 | 2.7E−11 | Y |
| rs62553882 | 9 | 91 497 782 | PCNPP2 | C | 0.945 | 0.16 | 0.11 | 0.21 | 3.9E−11 | Y |
| rs10770059 | 11 | 9 770 910 | SWAP70 | T | 0.351 | 0.08 | 0.05 | 0.10 | 5.3E−11 | N |
| rs12650679 | 4 | 69 771 836 | RP11-468N14.13 | A | 0.871 | 0.11 | 0.08 | 0.14 | 5.4E−11 | Y |
| rs4541821 | 7 | 148 446 377 | CUL1 | T | 0.788 | 0.09 | 0.06 | 0.12 | 5.7E−11 | Y |
| rs117111916 | 19 | 14 529 082 | DDX39A | C | 0.945 | --0.16 | --0.20 | --0.11 | 7.1E−11 | Y |
| rs10916527 | 1 | 229 746 970 | TAF5L | T | 0.495 | --0.07 | --0.09 | --0.05 | 8.1E−11 | Y |
| rs1844428 | 4 | 145 574 196 | HHIP-AS1 | A | 0.867 | 0.11 | 0.07 | 0.14 | 8.2E−11 | Y |
| rs13255193 | 8 | 11 309 192 | FAM167A | T | 0.457 | --0.07 | --0.09 | --0.05 | 1.2E−10 | Y |
| rs759544745 | 7 | 150 759 219 | SLC4A2 | CGTGTGTGAGT | 0.424 | --0.07 | --0.09 | --0.05 | 1.6E−10 | Y |
| rs12478953 | 2 | 71 618 599 | ZNF638 | T | 0.339 | --0.07 | --0.10 | --0.05 | 1.8E−10 | Y |
| rs758263745 | 3 | 12 395 972 | PPARG | CA | 0.683 | 0.08 | 0.05 | 0.10 | 1.9E−10 | Y |
| rs62054589 | 16 | 81 068 748 | RP11-303E16.3 | T | 0.875 | 0.11 | 0.07 | 0.14 | 2.3E−10 | Y |
| rs118153075 | 12 | 112 825 973 | HECTD4 | C | 0.982 | 0.27 | 0.19 | 0.35 | 3.5E−10 | N |
| rs2011082 | 6 | 110 734 999 | DDO | G | 0.222 | 0.08 | 0.06 | 0.11 | 4.1E−10 | Y |
| rs2337113 | 18 | 46 452 327 | SMAD7 | A | 0.536 | --0.07 | --0.09 | --0.05 | 5.7E−10 | Y |
| rs11380525 | 7 | 124 427 517 | GPR37 | T | 0.715 | --0.08 | --0.10 | --0.05 | 7.9E−10 | Y |
| rs78587207 | 11 | 57 654 991 | RP11-734C14.2 | T | 0.681 | 0.07 | 0.05 | 0.10 | 8.2E−10 | Y |
| rs2140875 | 7 | 129 602 879 | RP11-306G20.1 | A | 0.186 | 0.09 | 0.06 | 0.11 | 1.2E−09 | N |
| rs9783086 | 1 | 225 588 376 | LBR | T | 0.835 | --0.09 | --0.12 | --0.06 | 1.2E−09 | Y |
| rs67795055 | 3 | 169 529 895 | LRRC34 | C | 0.776 | --0.08 | --0.11 | --0.05 | 1.6E−09 | Y |
| rs139372052 | 16 | 67 695 483 | PARD6A | T | 0.995 | --0.46 | --0.61 | --0.31 | 1.7E−09 | Y |
| rs2535922 | 14 | 73 447 240 | NA | A | 0.609 | --0.07 | --0.09 | --0.05 | 2.4E−09 | Y |
| rs200127094 | 7 | 123 430 826 | RNU6-11P | A | 0.920 | 0.12 | 0.08 | 0.16 | 2.6E−09 | Y |
| rs11072763 | 15 | 78 724 256 | IREB2 | A | 0.222 | --0.08 | --0.10 | --0.05 | 3.4E−09 | Y |
| rs7649045 | 3 | 196 519 878 | PAK2 | T | 0.406 | 0.07 | 0.04 | 0.09 | 7.1E−09 | Y |
| rs2672092 | 15 | 81 870 620 | CTD-2034I4.1 | T | 0.772 | 0.08 | 0.05 | 0.10 | 8.9E−09 | Y |
| rs6433891 | 2 | 181 969 709 | AC068196.1 | G | 0.299 | 0.07 | 0.05 | 0.09 | 9.7E−09 | Y |
| rs574063085 | 1 | 118 769 284 | RNA5SP56 | C | 0.993 | --0.43 | --0.57 | --0.28 | 1.0E−08 | Y |
| rs12514956 | 5 | 177 635 181 | HNRNPAB | G | 0.919 | --0.12 | --0.16 | --0.08 | 1.4E−08 | Y |
| rs111362998 | 15 | 74 760 541 | UBL7-AS1 | A | 0.940 | --0.14 | --0.18 | --0.09 | 1.5E−08 | Y |
| rs552000109 | 11 | 85 682 778 | PICALM | C | 0.841 | 0.09 | 0.06 | 0.12 | 2.3E−08 | Y |
| rs74346567 | 15 | 86 249 722 | AKAP13 | G | 0.944 | --0.13 | --0.18 | --0.09 | 3.0E−08 | N |
| rs4446237 | 3 | 171 472 296 | PLD1 | C | 0.534 | --0.06 | --0.08 | --0.04 | 3.5E−08 | Y |
| rs562038 | 1 | 120 254 545 | PHGDH | G | 0.320 | 0.06 | 0.04 | 0.09 | 4.0E−08 | Y |
| rs117629721 | 10 | 101 786 635 | snoU13 | C | 0.965 | 0.17 | 0.11 | 0.23 | 4.6E−08 | Y |
| rs190629717 | 8 | 144 310 590 | GPIHBP1 | G | 0.759 | --0.07 | --0.10 | --0.05 | 4.8E−08 | Y |
aNot reported in [12, 37] or in the GWAS catalog (https://www.ebi.ac.uk/gwas/) to be associated with MCV.
Figure 1 .
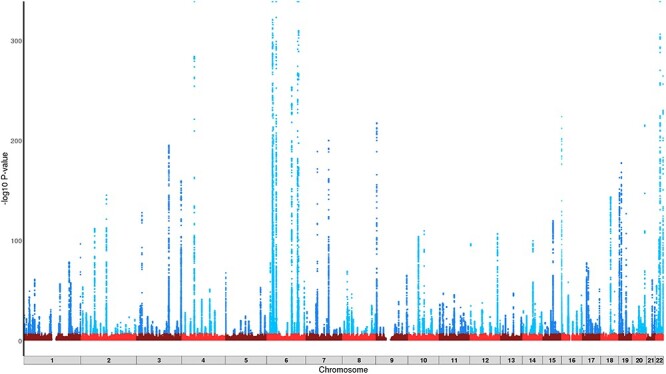
Manhattan plot of the unstratified GWAS outcomes for MCV (n = 362 595). Y axis truncated at 1 × 10−320.
GWAS of MCV stratified by alcohol intake
Analysis of the heterogenous effects between individuals with different alcohol intakes found that no variants reached the threshold for statistical significance (P < 2.4 × 10−4). SNP rs218264 was the closest to this threshold at P = 5.2 × 10−4, although both the low and heavy drinking groups showed significant associations with this variant (Table 3). No variants reached genome-wide significance (P < 5 × 10−8) when exploring the heterogeneity of allelic effects between the different drinking groups. Specific assessment of the alcohol metabolizing pathway found no evidence of an alcohol-related association between MCV and either the ADH or ALDH SNPs (Supplementary Material, Table S2).
Table 3.
Summary of variants reaching nominal significance for heterogeneity of allelic effects (significance threshold: P < 2.1 × 10−4)
| Low drinkers (n = 228 671) | Heavy drinkers (n = 133 924) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | % change MCV | 95%LCI | 95%UCI | P | % change MCV | 95%LCI | 95%UCI | P | Heterogeneity P |
| rs218264 | −0.50 | −0.53 | −0.47 | 4.5 × 10−232 | −0.42 | −0.45 | −0.38 | 5.1 × 10−104 | 5.2 × 10−4 |
| rs855791 | −0.61 | −0.64 | −0.59 | 2.3 × 10−462 | −0.54 | −0.57 | −0.51 | 2.3 × 10−230 | 5.9 × 10−4 |
| rs144861591 | −1.15 | −1.20 | −1.11 | 5.0 × 10−487 | −1.03 | −1.09 | −0.97 | 3.8 × 10−246 | 2.0 × 10−3 |
| rs4936291 | 0.12 | 0.10 | 0.15 | 7.9 × 10−19 | 0.06 | 0.02 | 0.09 | 7.4 × 10−4 | 4.0 × 10−3 |
| rs10758657 | 0.41 | 0.38 | 0.44 | 1.4 × 10−139 | 0.34 | 0.30 | 0.38 | 2.9 × 10−63 | 0.01 |
| rs243076 | −0.25 | −0.28 | −0.23 | 9.4 × 10−79 | −0.20 | −0.23 | −0.17 | 2.3 × 10−32 | 0.01 |
| rs56142708 | −0.17 | −0.20 | −0.15 | 6.5 × 10−39 | −0.22 | −0.26 | −0.19 | 1.8 × 10−41 | 0.02 |
| rs67971539 | −0.20 | −0.23 | −0.17 | 8.4 × 10−37 | −0.14 | −0.18 | −0.10 | 2.4 × 10−12 | 0.02 |
| rs6592965 | −0.32 | −0.34 | −0.29 | 1.7 × 10−125 | −0.27 | −0.30 | −0.23 | 1.6 × 10−57 | 0.02 |
| rs12650679 | 0.09 | 0.05 | 0.13 | 1.2 × 10−5 | 0.16 | 0.11 | 0.21 | 7.3 × 10−11 | 0.02 |
| rs9471708 | 0.60 | 0.58 | 0.63 | 5.0 × 10−369 | 0.55 | 0.51 | 0.59 | 3.6 × 10−192 | 0.02 |
| rs12582170 | −0.19 | −0.22 | −0.15 | 4.7 × 10−25 | −0.12 | −0.17 | −0.08 | 1.3 × 10−7 | 0.02 |
| rs17296501 | −0.26 | −0.30 | −0.23 | 1.3 × 10−47 | −0.20 | −0.24 | −0.15 | 4.4 × 10−18 | 0.03 |
| rs9801017 | −0.33 | −0.35 | −0.30 | 4.1 × 10−126 | −0.28 | −0.31 | −0.25 | 1.5 × 10−60 | 0.04 |
| rs74401481 | 0.60 | 0.50 | 0.70 | 1.5 × 10−32 | 0.76 | 0.64 | 0.89 | 5.6 × 10−34 | 0.05 |
Allele score for alcohol metabolism pathway
The genetic score used as a proxy for acetaldehyde accumulation rate/speed of clearance in drinkers only was independent of confounding factors (i.e. covariates included in multivariate model). The frequencies of the effect alleles contributing to the allele score were as follows: rs1229984_T = 0.021; rs698_T = 0.588 and rs2228093_T = 0.121. We found no evidence for an association between MCV and the allele score (P = 0.53). There was however evidence that the allele score was associated with alcohol intake (P < 2 × 10−16). Categorization of the allele score demonstrated that this relationship with alcohol consumption was dose-dependent (negative direction), and thus, the score can be considered valid given current knowledge of alcohol metabolism and its relationship with intake (Supplementary Material, Table S3).
Phenome-wide analysis
We performed Phenomewide association analysis (PheWAS) to detect whether the variants implicated in MCV might impact other diseases or clinically relevant phenotypes. This showed that the SNPs contribute to a range of different diseases, with 71 SNP-outcome pairs reaching P < 4.8 ×10−7 (Supplementary Material, Table S4). The most consistent outcomes were observed for ICD-10 chapter IV codes, including disorders of mineral metabolism and disorders of lipoprotein metabolism and other lipidaemias. There was also strong evidence from three SNPs for a shared risk with neoplasms of the skin. Thyroid-related disorders were also found in two SNPs (rs2134814, rs592229), with evidence for both under- and overactive thyroid diagnoses. The G allele in rs2134814 was associated with increased MCV and hypothyroidism, while the T allele in rs592229 was associated with decreased MCV and hyperthyroidism. Other outcomes included diabetes, multiple sclerosis, hypertension, varicose veins and rheumatoid arthritis.
Mendelian randomization
Mendelian randomization analysis demonstrated a significant causal effect of alcohol consumption on MCV. Each copy of the effect allele at rs1229984 in ADH1B was associated with a 0.19 decrease in drinks per week in the work by Jorgenson et al. (20) and was also found to reduce MCV by 0.18 femtoliters (fL) (SE = 0.002; P = 0.002). However, the addition of rs7686419 (KLB) returned a null outcome with evidence of effect heterogeneity, although the effect size of rs7686419 for drinks per week was approximately 6-fold smaller than rs1229984 (20).
Discussion
In the largest study undertaken to date, we have shown, as would be expected, that alcohol was clearly associated with an increase in MCV in a dose-dependent manner. However, the effects of alcohol on MCV were largely independent of genetic architecture, despite the association of MCV with genetic variation at 212 autosomal loci. Our analysis using Mendelian randomization provides evidence of a causal relationship between alcohol intake and MCV. However, we demonstrated a lack of association between alcohol metabolizing genes and MCV using a genetic score approach. Taken together, these findings support MCV as a marker of alcohol use disorder, although lack of specificity remains a substantial barrier in predictive accuracy and therefore clinical utility (3).
The strengths of this study are as follows: (1) the large sample size for GWAS analyses, (2) post-GWAS analysis including fixed effect inverse-variance weighted meta-analysis to generate heterogeneity statistics, (3) the use of a mixed-model approach in GWAS to account for relatedness and maximize sample size and (4) use of allele scores to explore the functional consequences of alcohol metabolizing gene variants as a proxy for acetaldehyde accumulation. There are, however, several limitations. First, the alcohol measures were based on self-report. The accuracy of self-report alcohol consumption has been questioned due to under-coverage compared with sales data (21). Second, we restricted our analysis to those of white British ancestry to limit population structure variability on the outcomes. This limits generalizability of our findings to other ethnic groups. Third, we did not undertake formal replication of findings, but our top GWAS outcomes are consistent with those reported elsewhere (11,12,15–18). Finally, we considered including folate in our models. However, folate levels were not measured in the UKB and the prevalence of folate deficiency anaemia was low (<0.002%).
The large sample size of the UKB enabled the detection of genetic variants with small effect sizes. The replication of findings in loci such as HBS1L-MYB, TMPRSS6 and CCND3, which have been identified in previous GWAS for MCV (11,12,15–18), supports the validity of our outcomes. Indeed, many of the low-frequency variants with smaller effect sizes were reported in an analysis of 36 blood cell traits (11). However, we also identified 108 new loci associated with MCV providing new biological insights. We observed associations between MCV and several loci involved in DNA modification through binding and/or processing alterations (e.g. ZNF165, TAF6, ZBTB38, ZKSCAN5, SPIDR). It is known that impaired DNA synthesis delays cell division resulting in macrocytosis (22). Of the new loci identified, rs13191659 (VN1R12P/LINC00240) has been associated with total iron binding capacity in Hispanics (23); DPP8 has been suggested as a candidate gene for mean corpuscular haemoglobin (MCH) in Europeans (24) and was identified as part of an LD block at 15q22.3 containing IGDCC4-DPP8-PTPLAD1-C15orf44-SLC24A1-DENND4A for MCH in Japanese (25); OBFC1 and MEGF11 have been associated with MCH but not MCV (26,27); PAK2 has been reported to have a role in eryptosis of erythrocytes, and therefore the effect of PAK2 on red blood cell indices might be greater than previously recognized (28); LDB1 influences erythrocyte development by the protein product acting as a cofactor for transcription factor complexes with, for example, Gata1, Tal1, E2A and Lmo2 (29). Indeed, the critical requirement for LDB1 during early-stage erythropoiesis has been demonstrated in rodent models (30). Furthermore, several of our lead SNPs were missense variants, including rs1047891 (EAF 0.684; P = 1.1 × 10−20) (CPS1) alongside more well-described MCV-associated SNPs such as rs855791 (EAF 0.440; P = 3.0 × 10−610) (TMPRSS6) and rs3811444 (EAF 0.667; P = 1.1 × 10−81) (TRIM58). rs1047891 is in the 3′ untranslated section of CPS1, a region reported to play a key role in glycine and serum homocysteine metabolism. Allelic variation in rs1047891 has been associated with various cardiometabolic traits (31,32) and lower platelet count (33). The substitution at this SNP (T-->N; p.Thr1412Asn) increases enzymatic activity and influences nitric oxide production (34), an important mediator of vascular function. MCV has been reported to be an independent predictor for cardiovascular events (35) and rs1047891 variation is therefore a potential pathway for this relationship.
Stratification of participants by drinking status did not identify any loci that determined the effect of alcohol intake on MCV. This suggests that the pathways through which alcohol influences MCV are not mediated by genetic variation. This was supported by the causal inference for alcohol on MCV levels when using rs1229984 as a proxy for alcohol consumption in the Mendelian randomization analysis. However, the discriminatory power of MCV in identifying heavy alcohol use is modest given that alcohol accounts for only ~65% of MCV values above 100 fL (36). In addition, the turnover of erythrocytes is around 120 days meaning that recently abstinent individuals will present with evidence of alcohol consumption for several months.
Using a genetic score to define alcohol metabolism, we did not find evidence to support that acetaldehyde accumulation is important in determining MCV levels. This is contrary to the findings in Asians for MCV (37) and other alcohol-related liver function in Europeans (38). The lack of association with MCV is likely to be due to the fact that rs1229984 (ADH1B) is rare in Europeans and the ubiquitous presence of active ALDH2, the enzyme primarily involved in the rapid metabolism of acetaldehyde to acetate (39). Similar results to our own for ALDH gene polymorphisms were reported in a study of 510 white alcohol-dependent patients (40).
The PheWAS analysis showed SNP level pleiotropy for variants involved in MCV suggesting a shared genetic risk with a number of conditions. Many of these combinations have strong physiological connections with one another (e.g. mineral metabolism disorders and liver disease). The association between MCV and thyroid dysfunction is well described, with thyroid hormones being essential for erythropoiesis (41). Indeed, we found evidence to support the relationship between hypothyroidism and increased MCV (42) alongside hyperthyroidism and decreased MCV (43). Our findings suggest that some pathways, as mediated by rs2134814 (BACH2) and rs592229 (SKIV2L), convey shared genetic architecture for MCV and thyroid dysfunction. Other findings offer additional insight in areas of ongoing investigation such as the association between psoriasis and red blood cell deformability (44).
In summary, we have demonstrated that the impact of alcohol consumption on MCV is independent of allelic variation and provided new biological insights into the genetic loci determining MCV itself. The role of acetaldehyde, although likely important in determining MCV, is difficult to measure in Europeans due to rare variation in alcohol metabolizing genes. Interindividual variability in MCV in the setting of moderate to heavy alcohol consumption is likely to be due to a complex (and at present incompletely understood) interaction between genetic factors, underlying medical conditions and lifestyle factors.
Materials and Methods
A complete description of the methods can be found in the Supplementary Material.
UKB
The UKB is a large population cohort of ~502 000 individuals from the United Kingdom aged 40–69 years at recruitment. Only white British participants were included in this study. Ethical approval for the UKB was gained from the Research Ethics Service (reference: 17/NW/0274), and written informed consent was obtained from all participants. Analyses were conducted under approved application 15110.
Alcohol consumption
Questions from the UKB baseline assessment were used to estimate alcohol consumption. We applied a standardized number of UK alcohol units to each drink to enable estimation of the number of units per week, as described previously (13).
MCV measurement
Components of full blood counts were measured in UKB participants using clinical haematology analysers at the centralized processing laboratory of the UK Biocenter (Stockport, UK). Full information on the protocol can be found elsewhere (45).
Multivariable analyses for predictors of MCV
MCV was natural log-transformed to normalize the distribution of residuals. Multivariable linear regression was applied to identify predictors of MCV. Analyses examined alcohol consumption as both a continuous and categorical predictor of MCV. All multivariable analyses were adjusted for age, sex, smoking status, history of hypothyroidism and vitamin B12 deficiency, and individuals with liver disease were removed due to the interaction between alcoholic liver disease risk and macrocytosis (7). Models were rerun with those reporting zero alcohol consumption removed.
Genetic analyses
In July 2017, UKB released genetic information (directly typed and imputed genotypes) for 487 406 individuals to approved collaborators. Genotyping, quality control and imputation were performed centrally by UKB and have been described previously (46).
GWAS analysis
Autosomal genetic association analysis was conducted for ln(MCV) using a linear mixed model in BOLT-LMM v2.3.4 (47), adjusted for genotyping array and covariates outlined in multivariable analyses plus alcohol consumption in units/week as a continuous variable. Distance-based clumping was used for defining loci. Genomic control adjustments were applied for standard errors and P-values.
Heterogeneity of allelic effects by drinking group
Variants reaching P < 5 × 10−8 and surviving distance-based clumping (i.e. lead SNPs) were explored for heterogeneous outcomes based on drinking category. GWAMA was used to run a fixed effect inverse-variance weighted meta-analysis on outcomes and generate heterogeneity statistics for allelic effects between groups, which is equivalent to fitting an interaction term (48). Any variant reaching the Bonferroni-corrected threshold (P < 0.05/‘number of lead SNPs from unstratified GWAS’) was considered statistically significant.
MCV heritability
To characterize the heritability of MCV, we applied single-trait LDscore regression through LD Hub v1.9.3 (http://ldsc.broadinstitute.org/ldhub/) (49).
Phenomewide association analysis
Gene ATLAS (http://geneatlas.roslin.ed.ac.uk/) was used as a lookup for outcomes from PheWAS analysis performed on UKB traits (50).
Impact of genetic score for acetaldehyde on MCV
To test the assumption that acetaldehyde is important in MCV, we used genotype data for SNPs in ADH1B, ADH1C and ALDH1B to construct a genetic score. The SNPs rs1229984 (ADH1B), rs698 (ADH1C) and rs2228093 (ALDH1B) were used to generate an unweighted allele score based on number of ADH alleles increasing the metabolism of ethanol to acetaldehyde and the number of ALDH alleles slowing the metabolism of acetaldehyde to acetate. This score (0–6) was used as a continuous predictor alongside covariates previously outlined in multivariable analyses. The selected variants were independent (r2 < 0.01 for all SNP pairs).
Mendelian randomization
MR-Base v0.4.21 was used for performing Mendelian randomization to explore the causal relationship between alcohol consumption and MCV (51). The causal estimates between exposure and outcome were obtained using the two-sample Mendelian randomization inverse variance-weighted method.
Results are reported using STROBE guidelines. A checklist can be found in the Supplementary Material.
Supplementary Material
Contributor Information
Andrew Thompson, Wolfson Centre for Personalised Medicine, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, UK; MRC Centre for Drug Safety Science, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, UK; Liverpool Centre for Alcohol Research, University of Liverpool, Liverpool L69 3BX, UK.
Katharine King, Wolfson Centre for Personalised Medicine, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, UK; MRC Centre for Drug Safety Science, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, UK; Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9NT, UK.
Andrew P Morris, Division of Musculoskeletal and Dermatological Sciences, Centre for Genetics and Genomics Versus Arthritis, Centre for Musculoskeletal Research, The University of Manchester, Manchester M13 9PL, UK; Department of Biostatistics, University of Liverpool, Liverpool L69 3GL, UK.
Munir Pirmohamed, Wolfson Centre for Personalised Medicine, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, UK; MRC Centre for Drug Safety Science, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, UK; Liverpool Centre for Alcohol Research, University of Liverpool, Liverpool L69 3BX, UK; Liverpool University Hospital, Liverpool L9 7AL, UK; Liverpool Health Partners, Liverpool L3 5TF, UK.
Funding
Medical Research Council [grant number: MR/S000607/1]. The funders did not engage in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review and approval of the manuscript.
Ethics approval and consent to participate
This study is based on data from the UK Biobank. However, the interpretation and conclusions contained in this paper are those of the authors’ alone. The study protocol was approved by the UK Biobank under application number 15110 and all participants provide written, informed consent prior to any data collection.
Data sharing statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The data from UKB were provided under license by UKB, who is the owner of the data. Requests for access to the data should be directed to UKB as per the material transfer agreement. Additional data related to this paper may be requested from the authors.
Authors’ Contributions
A.T. and M.P. conceived the project. A.T., A.P.M and M.P developed the analysis plan. A.T. and K.K. performed the analysis. A.T. produced data visualization. A.T. and K.K. performed searches for previous GWAS in MCV. A.P.M. and M.P. supervised the project. A.T. wrote the original draft. All authors critically reviewed the drafts of the manuscript and approved the final version.
Conflicts of Interest statement. M.P. receives research funding from various organizations including the MRC and NIHR. He has also received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (co-funded by MRC and Roche, UCB, Eli Lilly and Novartis); a PhD studentship jointly funded by EPSRC and Astra Zeneca; and grant funding from Vistagen Therapeutics. He has also received unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb and UCB. He has developed an HLA genotyping panel with MC Diagnostics but does not benefit financially from this. He is part of the IMI Consortium ARDAT (www.ardat.org).
References
- 1. Abat, C., Roussel, Y., Chaudet, H. and Raoult, D. (2019) Alcohol and the global burden of disease. Lancet, 393, 2390–2391. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (2019) Global Status Report on Alcohol and Health 2018. World Health Organization, Geneva. [Google Scholar]
- 3. Bornhorst, J.A. and Mbughuni, M.M. (2019) Critical Issues in Alcohol and Drugs of Abuse Testing. Elsevier, Amsterdam, in press., pp. 25–42. [Google Scholar]
- 4. Koivisto, H., Hietala, J., Anttila, P., Parkkila, S. and Niemelä, O. (2006) Long-term ethanol consumption and macrocytosis: diagnostic and pathogenic implications. J. Lab. Clin. Med., 147, 191–196. [DOI] [PubMed] [Google Scholar]
- 5. Beaug, F., Gallay, J., Stibler, H. and Borg, S. (1988) Alcohol abuse increases the lipid structural order in human erythrocyte membranes: a steady-state and time-resolved anisotropy study. Biochem. Pharmacol., 37, 3823–3828. [DOI] [PubMed] [Google Scholar]
- 6. Hernández-Muñoz, R., Baraona, E., Blacksberg, I. and Lieber, C.S. (1989) Characterization of the increased binding of acetaldehyde to red blood cells in alcoholics. Alcohol. Clin. Exp. Res., 13, 654–659. [DOI] [PubMed] [Google Scholar]
- 7. Maruyama, S., Hirayama, C., Yamamoto, S., Koda, M., Udagawa, A., Kadowaki, Y., Inoue, M., Sagayama, A. and Umeki, K. (2001) Red blood cell status in alcoholic and non-alcoholic liver disease. J. Lab. Clin. Med., 138, 332–337. [DOI] [PubMed] [Google Scholar]
- 8. Aslinia, F., Mazza, J.J. and Yale, S.H. (2006) Megaloblastic anemia and other causes of macrocytosis. Clin. Med. Res., 4, 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Reilly, M.A., Millar, S.R., Buckley, C.M., Harrington, J.M., Perry, I.J. and Cahill, M.R. (2015) Smoking as an independent risk factor for macrocytosis in middle-aged adults: a population-based observational study. Am. J. Hematol., 90, E196–E197. [DOI] [PubMed] [Google Scholar]
- 10. Kaferle, J. and Strzoda, C.E. (2009) Evaluation of macrocytosis. Am. Fam. Physician, 79, 203–208. [PubMed] [Google Scholar]
- 11. Astle, W.J., Elding, H., Jiang, T., Allen, D., Ruklisa, D., Mann, A.L., Mead, D., Bouman, H., Riveros-Mckay, F. and Kostadima, M.A. (2016) The allelic landscape of human blood cell trait variation and links to common complex disease. Cell, 167, 1415–1429.e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganesh, S.K., Zakai, N.A., Van Rooij, F.J., Soranzo, N., Smith, A.V., Nalls, M.A., Chen, M.-H., Kottgen, A., Glazer, N.L. and Dehghan, A. (2009) Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat. Genet., 41, 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson, A., Cook, J., Choquet, H., Jorgenson, E., Yin, J., Kinnunen, T., Barclay, J., Morris, A.P. and Pirmohamed, M. (2020) Functional validity, role, and implications of heavy alcohol consumption genetic loci. Sci. Adv., 6, eaay5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen, K.J., Gurrin, L.C., Constantine, C.C., Osborne, N.J., Delatycki, M.B., Nicoll, A.J., McLaren, C.E., Bahlo, M., Nisselle, A.E. and Vulpe, C.D. (2008) Iron-overload-related disease in HFE hereditary hemochromatosis. N. Engl. J. Med., 358, 221–230. [DOI] [PubMed] [Google Scholar]
- 15. McLachlan, S., Giambartolomei, C., White, J., Charoen, P., Wong, A., Finan, C., Engmann, J., Shah, T., Hersch, M. and Podmore, C. (2016) Replication and characterization of association between ABO SNPs and red blood cell traits by meta-analysis in Europeans. PLoS One, 11, e0156914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li, J., Glessner, J.T., Zhang, H., Hou, C., Wei, Z., Bradfield, J.P., Mentch, F.D., Guo, Y., Kim, C. and Xia, Q. (2013) GWAS of blood cell traits identifies novel associated loci and epistatic interactions in Caucasian and African-American children. Hum. Mol. Genet., 22, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kullo, I.J., Ding, K., Jouni, H., Smith, C.Y. and Chute, C.G. (2010) A genome-wide association study of red blood cell traits using the electronic medical record. PLoS One, 5, e13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Read, R.W., Schlauch, K.A., Elhanan, G., Metcalf, W.J., Slonim, A.D., Aweti, R., Borkowski, R. and Grzymski, J.J.J.P.o. (2019) GWAS and PheWAS of red blood cell components in a Northern Nevadan cohort. PLoS One, 14, e0218078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng, W., Ramachandran, S. and Crawford, L. (2020) Estimation of non-null SNP effect size distributions enables the detection of enriched genes underlying complex traits. BioRxiv, in press, 597484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorgenson, E., Thai, K.K., Hoffmann, T.J., Sakoda, L.C., Kvale, M.N., Banda, Y., Schaefer, C., Risch, N., Mertens, J. and Weisner, C. (2017) Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol. Psychiatry, 22, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gmel, G. and Rehm, J. (2004) Measuring alcohol consumption. Contemp. Drug Probl., 31, 467–540. [Google Scholar]
- 22. Green, R. and Dwyre, D.M. (2015) Seminars in Hematology. Elsevier, Amsterdam, Vol. 52, pp. 279–286. [DOI] [PubMed] [Google Scholar]
- 23. Raffield, L.M., Louie, T., Sofer, T., Jain, D., Ipp, E., Taylor, K.D., Papanicolaou, G.J., Avilés-Santa, L., Lange, L.A. and Laurie, C.C. (2017) Genome-wide association study of iron traits and relation to diabetes in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL): potential genomic intersection of iron and glucose regulation? Hum. Mol. Genet., 26, 1966–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding, K., Shameer, K., Jouni, H., Masys, D.R., Jarvik, G.P., Kho, A.N., Ritchie, M.D., McCarty, C.A., Chute, C.G. and Manolio, T.A. (2012) Mayo Clinic Proceedings. Elsevier, Amsterdam, Vol. 87, pp. 461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamatani, Y., Matsuda, K., Okada, Y., Kubo, M., Hosono, N., Daigo, Y., Nakamura, Y. and Kamatani, N. (2010) Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet., 42, 210–216. [DOI] [PubMed] [Google Scholar]
- 26. Chami, N., Chen, M.-H., Slater, A.J., Eicher, J.D., Evangelou, E., Tajuddin, S.M., Love-Gregory, L., Kacprowski, T., Schick, U.M. and Nomura, A. (2016) Exome genotyping identifies pleiotropic variants associated with red blood cell traits. Am. J. Hum. Genet., 99, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kichaev, G., Bhatia, G., Loh, P.-R., Gazal, S., Burch, K., Freund, M.K., Schoech, A., Pasaniuc, B. and Price, A.L. (2019) Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet., 104, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zelenak, C., Föller, M., Velic, A., Krug, K., Qadri, S.M., Viollet, B., Lang, F. and Macek, B. (2011) Proteome analysis of erythrocytes lacking AMP-activated protein kinase reveals a role of PAK2 kinase in eryptosis. J. Proteome Res., 10, 1690–1697. [DOI] [PubMed] [Google Scholar]
- 29. Meier, N., Krpic, S., Rodriguez, P., Strouboulis, J., Monti, M., Krijgsveld, J., Gering, M., Patient, R., Hostert, A. and Grosveld, F. (2006) Novel binding partners of Ldb1 are required for haematopoietic development. Development, 133, 4913–4923. [DOI] [PubMed] [Google Scholar]
- 30. Li, L., Lee, J.Y., Gross, J., Song, S.-H., Dean, A. and Love, P.E. (2010) A requirement for Lim domain binding protein 1 in erythropoiesis. J. Exp. Med., 207, 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willer, C.J., Schmidt, E.M., Sengupta, S., Peloso, G.M., Gustafsson, S., Kanoni, S., Ganna, A., Chen, J., Buchkovich, M.L. and Mora, S. (2013) Discovery and refinement of loci associated with lipid levels. Nat. Genet., 45, 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabater-Lleal, M., Huang, J., Chasman, D., Naitza, S., Dehghan, A., Johnson, A.D., Teumer, A., Reiner, A.P., Folkersen, L. and Basu, S. (2013) Multiethnic meta-analysis of genome-wide association studies in >100 000 subjects identifies 23 fibrinogen-associated loci but no strong evidence of a causal association between circulating fibrinogen and cardiovascular disease. Circulation, 128, 1310–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Polfus, L.M., Khajuria, R.K., Schick, U.M., Pankratz, N., Pazoki, R., Brody, J.A., Chen, M.-H., Auer, P.L., Floyd, J.S. and Huang, J. (2016) Whole-exome sequencing identifies loci associated with blood cell traits and reveals a role for alternative GFI1B splice variants in human hematopoiesis. Am. J. Hum. Genet., 99, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Summar, M.L., Gainer, J.V., Pretorius, M., Malave, H., Harris, S., Hall, L.D., Weisberg, A., Vaughan, D.E., Christman, B.W. and Brown, N.J. (2004) Relationship between carbamoyl-phosphate synthetase genotype and systemic vascular function. Hypertension, 43, 186–191. [DOI] [PubMed] [Google Scholar]
- 35. Solak, Y., Yilmaz, M.I., Saglam, M., Demirbas, S., Verim, S., Unal, H.U., Gaipov, A., Oguz, Y., Kayrak, M. and Caglar, K. (2013) Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrol. Ther., 18, 728–735. [DOI] [PubMed] [Google Scholar]
- 36. Savage, D.G., Ogundipe, A., Lindenbaum, J., Stabler, S.P. and Hallen, R. (2000) Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci, 319, 343–352. [DOI] [PubMed] [Google Scholar]
- 37. Yokoyama, A., Yokoyama, T., Brooks, P.J., Mizukami, T., Matsui, T., Kimura, M., Matsushita, S., Higuchi, S. and Maruyama, K. (2014) Macrocytosis, macrocytic anemia, and genetic polymorphisms of alcohol dehydrogenase-1 B and aldehyde dehydrogenase-2 in Japanese alcoholic men. Alcohol. Clin. Exp. Res., 38, 1237–1246. [DOI] [PubMed] [Google Scholar]
- 38. Lawlor, D.A., Benn, M., Zuccolo, L., De Silva, N.M.G., Tybjaerg-Hansen, A., Smith, G.D. and Nordestgaard, B.G. (2014) ADH1B and ADH1C genotype, alcohol consumption and biomarkers of liver function: findings from a Mendelian randomization study in 58,313 European origin Danes. PLoS One, 9, e114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edenberg, H.J. and McClintick, J.N. (2018) Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: a critical review. Alcohol. Clin. Exp. Res., 42, 2281–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun, L., König, I.R., Jacobs, A., Seitz, H.K., Junghanns, K., Wagner, T., Ludwig, D., Jacrobs, A. and Homann, N. (2005) Mean corpuscular volume and ADH1C genotype in White patients with alcohol-associated diseases. Alcohol. Clin. Exp. Res., 29, 788–793. [DOI] [PubMed] [Google Scholar]
- 41. Kendrick, T.S., Payne, C.J., Epis, M.R., Schneider, J.R., Leedman, P.J., Klinken, S.P. and Ingley, E. (2008) Erythroid defects in TRα−/− mice. Blood, 111, 3245–3248. [DOI] [PubMed] [Google Scholar]
- 42. Horton, L., Coburn, R., England, J. and Himsworth, R. (1976) The haematology of hypothyroidism. Q. J. Med., 45, 101–123. [PubMed] [Google Scholar]
- 43. Ford, H. and Carter, J. (1988) The haematology of hyperthyroidism: abnormalities of erythrocytes, leucocytes, thrombocytes and haemostasis. Postgrad. Med. J., 64, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Biran, R., Hadayer, N., Ramot, Y., Zlotogorski, A., Yedgar, S. and Barshtein, G. (2019) Phototherapy decreases red blood cell deformability in patients with psoriasis. Clin. Hemorheol. Microcirc., 73, 489–496. [DOI] [PubMed] [Google Scholar]
- 45. Sheard, S., Nicholls, R. and Froggat, J. (2017) UK Biobank Haematology Data Companion Document V1.9. UK Biobank, Oxford, UK. [accessed 19 Sep 2019]. in press.
- 46. Bycroft, C., Freeman, C., Petkova, D., Band, G., Elliott, L.T., Sharp, K., Motyer, A., Vukcevic, D., Delaneau, O. and O'Connell, J. (2017) Genome-wide genetic data on ~500,000 UK Biobank participants. BioRxiv, in press, 166298. [Google Scholar]
- 47. Loh, P.-R., Tucker, G., Bulik-Sullivan, B.K., Vilhjalmsson, B.J., Finucane, H.K., Salem, R.M., Chasman, D.I., Ridker, P.M., Neale, B.M. and Berger, B. (2015) Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet., 47, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mägi, R. and Morris, A.P. (2010) GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics, 11, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng, J., Erzurumluoglu, A.M., Elsworth, B.L., Kemp, J.P., Howe, L., Haycock, P.C., Hemani, G., Tansey, K., Laurin, C. and Pourcain, B.S. (2017) LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics, 33, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Canela-Xandri, O., Rawlik, K. and Tenesa, A. (2018) An atlas of genetic associations in UK Biobank. Nat. Genet., 50, 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hemani, G., Zheng, J., Elsworth, B., Wade, K.H., Haberland, V., Baird, D., Laurin, C., Burgess, S., Bowden, J. and Langdon, R. (2018) The MR-Base platform supports systematic causal inference across the human phenome. elife, 7, e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


