Abstract
Background
Previous studies have demonstrated an association between gait speed and cognitive function. However, the relationship between balance and cognition remains less well explored. This study examined the cross-sectional and longitudinal relationship of balance and cognitive decline in older adults.
Methods
A cohort of 4,811 adults, aged ≥65 years, participating in the Cardiovascular Health Study was followed for 6 years. Modified Mini-Mental State Examination (3MSE) and Digit Symbol Substitution Test (DSST) were used to measure cognition. Tandem balance measures were used to evaluate balance. Regression models were adjusted for demographics, behavioural and disease factors.
Results
Worse balance was independently associated with worse cognition in cross-sectional analysis. Longitudinally, participants aged ≥76 years with poorer balance had a faster rate of decline after adjustment for co-variates: −0.97 points faster decline in 3MSE per year (95% confidence interval (CI): −1.32, −0.63) compared to the participants with good balance. There was no association of balance and change in 3MSE among adults aged <76 years (P value for balance and age interaction < 0.0001). DSST scores reflected −0.21 (95% CI: −0.37, −0.05) points greater decline when adjusted for co-variates. In Cox proportional hazard models, participants with worse balance had a higher risk of being cognitively impaired over the 6 years of follow-up visits (adjusted HR:1.72, 95% CI: 1.30, 2.29).
Conclusions
Future studies should evaluate standing balance as a potential screening technique to identify individuals at risk of cognitive decline. Furthermore, a better understanding of the pathophysiological link between balance and cognition may inform strategies to prevent cognitive decline.
Keywords: physical performance, cognition, ageing, older people
Key points
Standing balance and cognitive function were examined in older adults.
Older adults with worse balance scores had lower cognitive function.
Older adults with worse balance had a larger decrease in cognitive function over 6 years of follow-up.
Adults aged 76 and older saw a larger difference between worse balance scores and cognitive decline.
Older adults with worse balance had a higher risk of being cognitively impaired over the 6 years of follow-up.
Introduction
In 2011, the Centers for Disease Control and Prevention (CDC) estimated 16 million people with cognitive impairment in the USA based on the prevalence of adult brain disorders [1]. Early detection of cognitive impairment and dementia can reduce the burden of the disease by allowing timely action from health care providers,patients and family members [2]. Because of their strong association with cognitive function, measures of physical performance have been suggested as possible screening tools for cognitive decline [3–6]. A better understanding of factors that precede cognitive decline can aid clinicians and members of the health care community in identifying at-risk individuals. Moreover, a better understanding of the shared pathophysiology of physical performance and cognitive impairment could help inform interventions aimed at preventing cognitive decline.
Previous literature has shown a cross-sectional link between physical performance, including balance, and cognitive decline, although few have examined the relationship over time [3,5,7–9]. One study, examining physical performance and the risk of dementia, found higher risk of dementia with balance measures than with other physical measures [10]. Balance relies on signals and feedback from the vestibular system and brain, providing a physiological link between the two [11–13]. Evaluating the relationship over time may establish temporality between cognitive function and balance that has not been thoroughly demonstrated. Determining the potential of balance as a risk factor for cognitive impairment has the possibility to inform interventions that prevent cognitive decline in an older population in the future.
The objective of this study was to evaluate the cross-sectional and the longitudinal relationship between standing balance and cognitive function in a cohort of older adults participating in the Cardiovascular Health Study (CHS). We hypothesised that worse balance would be associated with lower cognitive function scores and greater decline over time in a large cohort of older adults. Previous research has shown that cognitive function can vary by age, race and gender [14–16], so we also evaluated effect modification by these factors.
Methods
Study population
The population used in this study consisted of adults aged 65 years and older in the CHS. The study was comprised of 5,201 participants in 1989–90, with an additional 687 African Americans recruited in 1992–93 [17]. Participants were seen annually in the clinic over 10 years (1989–99) and took part in biannual phone interviews [17, 18]. We excluded participants diagnosed with Parkinson’s disease (n = 47) for all analyses. For the cross-sectional analytical sample, we used three assessments at the seventh follow-up year (1996–97) since this year had more detailed balance assessments. Participants who did not have balance measures (n = 1,627) for that year were excluded, resulting in a final sample size of 3,184. The longitudinal analytical sample was based on the third yearly follow-up visit (1992–93) and excluded participants who did not have balance measures (n = 1,044) based on one assessment resulting in a total of 4,811 participants. Additionally, participants with Modified Mini-Mental State Examination (3MSE) scores below 80 at the third follow-up year were removed for the Cox proportional hazard regression model, as they were considered cognitively impaired, for a sample size of 4,290. Participants were followed for up to 6 years in longitudinal follow-up.
Primary exposure
Balance measures were assessed using validated tandem stance measures at the third and seventh follow-up visits (see timeline in Supplemental Figure S1) (Supplementary data are available in Age and Ageing online) [19]. Only full-tandem stance was assessed as a binary variable at the third follow-up visit, which required participants to stand with one foot in front of the other, for 5 s. Semi-tandem stance and side-by-side stance were also included in the balance measures for the seventh follow-up visit. The measures involved holding a full-tandem stance for 10 s; holding a semi-tandem stance, one foot slightly in front of the other, for 10 s and side-by-side stance, both feet next to each other, for 10 s. For these measurements, time was recorded in seconds if the 10 s were not reached [18].
In the seventh follow-up visit, we followed the previous methods of Houston et al., used with CHS data, to categorized balance as: (i) participants who failed to hold a tandem stance for more than 2 s and included participants who could complete a side-by-side stance and semi-tandem stance for 10 s; (ii) participants who held a full-tandem stance for 3–9 s and (iii) participants who held a full-tandem stance for a completion of 10 s [20]. Balance at the third follow-up visit was defined as a dichotomous variable for the baseline of the longitudinal analysis based on the ability to complete the 5 s tandem stance hold.
Outcome
Cognitive function was measured annually using the 3MSE and the Digit Symbol Substitution Test (DSST). The 3MSE scores range from 0 to 100 and were collected during yearly clinical visits that evaluated domains of memory, registration, mental reversal, recall, temporal and special orientation, writing and more [17]. The 3MSE is a reliable and valid measure of global cognitive function often used for dementia screening [21,22]. Study participants were considered cognitively impaired if they had 3MSE score below 80 for 2 consecutive years or a score below 80 with a consecutive missing score [23]. DSST is a well-known valid and reliable measure that tests psychomotor performance that requires matching symbols and numbers with scores ranging from 0 to 90 [17,24,25].
Over the 6 years of follow-up, the 3MSE measure had 7,128 (21.43%) missing out of 33,677 possible observations and 141 (4.4%) missing out of the 3,184 participants at the seventh follow-up visit. Due to the missing values of 3MSE, telephone interview responses were used to address the missing values using the method discussed in Arnold et al. [26], reducing the missing values to 5,945 (17.65%) of the 33,677. The seventh year follow-up visit had two missing values out of 3,184 participants with balance measures. DSST measure had 8,724 (25.9%) missing observations out of the 33,677 observations throughout the 6 years.
Co-variates
We considered the following variables as potential continuous co-variates: age, weekly alcohol consumption, depression score, body mass index (BMI), number of medications, kidney function and physical activity. Categorical co-variates considered as potential confounders were sex, race, education, previous physical activity level, smoking status, diabetes, stroke, coronary heart disease, heart failure, hypertension, apolipoprotein E-4 (APOE-4) allele and MRI brain infarcts. All values were taken from the third follow-up visit, except gender, age, race and education, which were recorded at baseline. Age was dichotomized at the median (≥76 versus <76 years). Education was categorized as less than high school, high school or general education development test (GED), some college or vocational school, graduate or professional school. Race was recoded as white and non-white; <1% of non-white participants identified as a race other than African American. Physical activity was measured as weekly calories burned (kcal). Previous physical activity level compared activity level before 65 years of age to baseline activity level and was categorized as less active, about as active, a little more active and a lot more active. Diabetes was defined by fasting glucose levels of ≥126 (mg/dl) or use of diabetes medication. Borderline diabetes was defined as fasting glucose levels of 100–126 (mg/dl). Smoking was categorized into current smoker, former smoker and never smoker. Hypertension was measured as systolic blood pressure of ≥140 mg/dl or diastolic blood pressure of ≥90 mg/dl or use of hypertension medication. Pre-hypertension was defined as the systolic blood pressure between 120 and 140 mg/dl or diastolic blood pressure between 80 and 90 mg/dl. BMI was a continuous variable calculated as weight (kg)/height (m)2. Kidney function was measured by the estimated glomerular filtration rate (eGFR) using cystatin-C (ml/min/1.73m2). Depressive symptoms were classified based on the Center of Epidemiologic Studies Depression Scale [18].
Analysis
We compared the baseline descriptive characteristics using a chi-square or t-test for comparisons between the balance groups at the third follow-up visit. We used linear regression for the cross-sectional analysis of standing balance (tandem stance measure) and cognitive function outcome; this cross-sectional analysis used data from the seventh follow-up visit to incorporate the more extensive balance measures. The longitudinal analyses used the third follow-up visit as the baseline for the standing balance measures and evaluated 3MSE and DSST through the ninth follow-up visit based on linear mixed models (6 years of follow-up). This model was specified as:
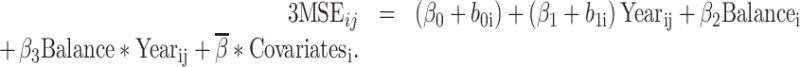 |
Finally, a Cox proportional hazard regression model was performed to examine the hazard of standing balance exposure measure and incident cognitive impairment over 6 years of follow-up. While the linear mixed model evaluates the average change across all participants, the Cox model estimates the likelihood of new cognitive impairment among those who were not cognitively impaired at baseline. For all analyses, interactions with gender, race and age were evaluated to assess effect modification, and a P value < 0.05 was considered significant for the interaction terms. Model 1 adjusted for education, gender, age and race; Model 2 further adjusted for smoking, drinking, BMI, number of medication and physical activity level; Model 3 was Model 2 plus APOE-4, diabetes, hypertension, eGFR, heart failure, heart disease, stroke, depression and MRI infarcts. Participants who did not agree to the use of genetic data were excluded from the analysis involving APOE. In addition, we performed sensitivity analyses that included walking speed as a co-variate and excluded participants who used assisted devices. Finally, we performed a sensitivity analysis by evaluating alternative age cut points of 75 and 80 years. All analyses were conducted using SAS/STAT™ software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
At study baseline, 919 (19%) of the 4,811 participants failed to hold a tandem stance for 5 s. Participants who failed the balance test were slightly older and more likely to be female, have less education, be diabetic and hypertensive, suffer from small and large MRI brain infarcts, have lower eGFR and have lower physical activity levels (Table 1). Participants who failed the balance test also had lower 3MSE and DSST scores, were less likely to suffer from heart failure, more likely to suffer from stroke and depressive symptoms and have fewer alcoholic drinks in a week. There were similar patterns across balance groups among participants at the seventh follow-up visit (Supplemental Table S1) (Supplementary data are available in Age and Ageing online).
Table 1.
Characteristics of participants stratified by balance scores at the third follow-up visit (1992–93) in the CHS
| Characteristic | Subjects with failed tandem stanced (n = 919) | Subjects with completed tandem stanced (n = 3,892) | ||
|---|---|---|---|---|
| n | %/Mean (SD) | n | %/Mean (SD) | |
| Age (years)* | 919 | 79 (6.3) | 3,892 | 76 (5.0) |
| Female sex* | 610 | 66 | 2,208 | 57 |
| Non-white race | 171 | 19 | 702 | 18 |
| Education* | ||||
| Less than high school | 182 | 20 | 472 | 12 |
| High school or GED | 367 | 40 | 1,621 | 42 |
| Some college or vocational school | 289 | 32 | 1,353 | 35 |
| Graduate or professional school | 76 | 8 | 441 | 11 |
| Smoking | ||||
| Never smoked | 422 | 47 | 1,726 | 45 |
| Previous smoked | 385 | 43 | 1,725 | 45 |
| Current smoker | 91 | 10 | 374 | 10 |
| Weekly alcoholic drinks* | 916 | 1.4 (4.4) | 3,887 | 2.2 (6.5) |
| Depression score* | 916 | 6 (5.2) | 3,890 | 5 (4.7) |
| Diabetes*,a | ||||
| Normal | 430 | 51 | 2,016 | 54 |
| Pre-diabetic | 238 | 28 | 1,199 | 32 |
| Diabetic | 179 | 21 | 525 | 14 |
| Hypertension*,b | ||||
| Normal | 73 | 8 | 504 | 13 |
| Pre-hypertensive | 163 | 17 | 819 | 21 |
| Hypertensive | 683 | 74 | 2,568 | 66 |
| BMI (kg/m2) | 872 | 27 (5.3) | 3,833 | 27 (4.6) |
| MRI brain small infarct* | 109 | 18 | 360 | 13 |
| MRI brain large infarct* | 261 | 43 | 806 | 28 |
| MRI brain cortical infarcts* | 261 | 43 | 806 | 28 |
| Number of medication* | 918 | 3 (2.5) | 3,890 | 2 (2.2) |
| eGFR (ml/min/1.73 m2)*,c | 833 | 64 (18.0) | 3,707 | 70 (16.7) |
| Calories burned during exercise a week (kcal)* | 913 | 1,033 (1,468.2) | 3,878 | 1,506 (1,791.9) |
| APOE-4 allele | 204 | 25 | 1,103 | 25 |
| Activity level before age 65 years compared to now | ||||
| Less active | 68 | 7 | 311 | 8 |
| About as active | 247 | 27 | 1,053 | 27 |
| A little more active | 283 | 31 | 1,246 | 32 |
| A lot more active | 318 | 35 | 1,263 | 33 |
| Heart failure* | 97 | 2 | 187 | 5 |
| Coronary heart disease | 220 | 24 | 818 | 21 |
| Stroke* | 90 | 10 | 155 | 4 |
| 3MSE* | 916 | 85 (13.2) | 3,889 | 91 (8.2) |
| DSST* | 843 | 32 (13.8) | 3,813 | 40 (13.4) |
Note. Abbreviation: SD, standard deviation.
aDiabetes: Normal: fasting glucose < 100, pre-diabetic: ≥ 100 and <26 and diabetic: fasting glucose ≥ 126 or taking hypoglycemic oral medications.
bHypertension: Normal: systolic blood pressure < 120 and diastolic blood pressure < 80, pre-hypertensive: systolic blood pressure ≥ 120 and <140 or diastolic blood pressure ≥ 80 and <90; hypertensive: systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 or usage of antihypertensive medication.
ceGFR calculated using the cystatin-C based CKD-Epi equation.
dIndividuals who could hold a tandem stance for 5 s were considered to have completed the tandem stance, and individuals who were unable to hold the stance for 5 s were considered to have failed.
*Differed by balance scores with P value <0.05.
In the cross-sectional analyses (Table 2), worse balance was associated with lower cognitive function, and this association was present after the adjustment for potential confounders. Participants who did not hold a full-tandem stance for more than 2 s had a 4.57 (95% confidence interval (CI): −5.73, −3.40) point lower 3MSE, and those who held the stance for 3–9 s had a 0.89 (95% CI: −1.68, −0.10) lower 3MSE than those who held it for the full 10 s. The difference was larger in adults ≥76 years compared to adults <76 years (P value for the interaction of balance and age = 0.025 in the demographic-adjusted model). In the fully adjusted model, participants 76 years and older with the worst balance scored had an average 3MSE of −6.20 (95% CI: −7.93, −4.47) lower than those with the best balance compared to −2.18 (95% CI: −3.77, −0.59) for participants <76 years of age. The adjusted models using DSST as an outcome had similar overall findings.
Table 2.
Cross-sectional association of standing balance and cognitive function at the seventh follow-up visit (1996–97) in a population over 65 in the CHS
| Cognitive function testa | Difference in cognitive function scores | ||
|---|---|---|---|
| Model 1c (95% CI) | Model 2d (95% CI) | Model 3e (95% CI) | |
| 3MSEb | |||
| Balance 0 | −5.19*** (−6.19, −4.19) | −4.82*** (−5.84, −3.80) | −4.57*** (−5.73, −3.40) |
| Balance 1 | −1.47*** (−2.19, −0.76) | −1.53*** (−2.25, −0.81) | −0.89* (−1.68, −0.10) |
| Balance 2 (reference) | 0.00 | 0.00 | 0.00 |
| <76 years old | |||
| Balance 0 | −6.25*** (−7.80, −4.69) | −3.33*** (−4.64, −2.01) | −2.18* (−3.77, −0.59) |
| Balance 1 | −0.87 (−2.04, 0.29) | −2.33*** (−3.23, −1.44) | −1.57* (−2.59, −0.55) |
| Balance 2 (reference) | 0.00 | 0.00 | 0.00 |
| ≥76 years old | |||
| Balance 0 | −5.31*** (−6.77, −3.86) | −5.93*** (−7.52, −4.33) | −6.20*** (−7.93, −4.47) |
| Balance 1 | −1.21* (−2.25, −0.17) | −1.46* (−2.82, −0.09) | −0.48 (−1.70, 0.75) |
| Balance 2 (reference) | 0.00 | 0.00 | 0.00 |
| DSSTb | |||
| Balance 0 | −5.43*** (−6.82, −4.05) | −4.91*** (−6.33, −3.49) | −4.23*** (−5.96, −2.51) |
| Balance 1 | −2.94*** (−3.89, −1.99) | −2.69*** (−3.66, −1.72) | −1.79* (−2.91, −0.67) |
| Balance 2 (reference) | 0.00 | 0.00 | 0.00 |
Notes. The seventh follow-up visit was used for the cross-sectional analyses since additional balance measures were included at that year. The beta co-efficient is the difference in cognitive function scores in people who failed tandem stance balance test compared to completed tandem stance test. Stratified due to interaction P value < 0.05.
aBalance scores: Zero participant who could not hold a full-tandem stance for >2 s, one participant held the tandem stance for 2–9 s and two participants held the tandem stance for a completion of 10 s.
b3MSE which is a cognitive assessment test, DSST which is also a cognitive assessment test.
c3MSE and DSST Model 1 were adjusted for age, race, gender and education.
d3MSE and DSST Model 2 was additionally adjusted for active calories, smoking status, BMI, weekly alcohol consumption, number of medication and previous activity level.
e3MSE and DSST Model 3 was further adjusted by APOE-4, diabetes, hypertension, eGFR, heart failure, heart disease, stroke, depression score and small and cortical MRI infarcts.
*P value < 0.05.
**P value < 0.01.
***P value < 0.001.
The longitudinal analyses (Table 3) demonstrated worse cognitive decline over 6 years of follow-up among participants who failed the tandem stance. Participants who failed to hold a tandem stance had an average −0.88 (95% CI: −1.06, −0.69) greater decline in 3MSE score per year. This association was attenuated to −0.74 (95% CI: −0.97, −0.51) when adjusted for all co-variates. There was an interaction between balance and age in the demographic-adjusted longitudinal models (P value for interaction < 0.0001). Interactions between balance and gender or race were not significant (P value > 0.39). When adjusted for all co-variates, adults ≥76 years had a decrease of 0.97 3MSE points (95% CI: −1.32, −0.63). No significant differences were found among adults <76 years. Similar results were found for DSST. The interaction terms were not significant, with a 0.45 P value for balance and age interaction.
Table 3.
Longitudinal association of failed tandem stance test with annual change in cognitive function over 6 years of follow-up (1992/93–98/99) in the CHS
| Cognitive function test | Annual decline in cognitive function | |
|---|---|---|
| Beta co-efficient (per year) | 95% CI | |
| 3MSEa | ||
| Model 1b | -0.88*** | (−1.06, −0.69) |
| Model 2c | -0.88*** | (−1.07, −0.69) |
| Model 3d | -0.74*** | (−0.97, −0.51) |
| <76 years old | ||
| Model 1b | -0.25 | (−0.50, 0.00) |
| Model 2c | -0.24 | (−0.50, 0.02) |
| Model 3d | -0.06 | (−0.36, 0.23) |
| ≥76 years old | ||
| Model 1b | -0.98** | (−1.24, −0.71) |
| Model 2c | -1.01*** | (−1.28, −0.73) |
| Model 3d | -0.97*** | (−1.32,-0.63) |
| DSSTa | ||
| Model 1b | -0.32*** | (−0.46, −0.19) |
| Model 2c | -0.30*** | (−0.43, −0.16) |
| Model 3d | -0.21* | (−0.37, −0.05) |
Note. Stratified due to interaction P value < 0.05.
a3MSE, cognitive assessment test; DSST, cognitive assessment test.
b3MSE and DSST Model 1 were adjusted for age, race, gender and education.
c3MSE and DSST Model 2 was additionally adjusted for active calories, smoking status, BMI, weekly alcohol consumption, number of medication and previous activity level.
d3MSE and DSST Model 3 were further adjusted by APOE-4, diabetes, hypertension, eGFR, heart failure, heart disease, stroke, depression score and small and cortical MRI infarcts.
eBalance was based on completion or failure of holding a 5-s tandem stance at Year 5.
*P value < 0.05.
**P value < 0.01.
***P value < 0.001.
Participants who had worse balance at baseline were at higher risk of becoming cognitively impaired, and this association was independent of potential demographic confounders (HR: 1.55, 95% CI: 1.24, 1.92) (Supplemental Figure S2 (Supplementary data are available in Age and Ageing online) and Table 4). The model additionally adjusted for disease and behaviour showed a stronger association. There was no significant interaction with age (P value = 0.42).
Table 4.
Association between failed tandem stance test and incident cognitive impairment (3MSE score < 80) over 6 years of follow-up (1992/93–98/99) in the CHS
| Balancea | HR for cognitive impairmentb | 95% CI |
|---|---|---|
| Model 1c | 1.55*** | (1.24, 1.92) |
| Model 2d | 1.64*** | (1.31, 2.05) |
| Model 3e | 1.72*** | (1.30, 2.29) |
Note. HR, hazard ratio.
aBalance is based on completion or failure of holding a 5-s tandem stance at Year 5. In this model, completing the tandem stance was the reference, so the HR values above represent failed tandem stance cognitive function scores compared to completed tandem stance.
bCognitive Impairment considered individuals with 3MSE scores less than 80 for two consecutive years or a score below 80 with a consecutive missing score from Year 5 to Year 11 of the study.
cModel 1 was adjusted for age, race, gender and education.
dModel 2 was additionally adjusted for active calories, smoking status, BMI, weekly alcohol consumption, number of medication and previous activity level.
eModel 3 was further adjusted by APOE-4, diabetes, hypertension, eGFR, heart failure, heart disease, stroke, depression score and small and cortical MRI infarcts.
*P value < 0.05.
**P value < 0.01.
***P value < 0.001.
Sensitivity analyses that included walking speed as a co-variate demonstrated similar results. After adjustment for timed walk in the fully adjusted models, participants with balance scores of 0 had an average of 4.34 (95% CI: −5.51, −3.34) 3MSE points lower than participants with balance scores of 2, and the annual decline was −0.72 (95% CI: −0.92, −0.49) 3MSE scores. The second sensitivity analysis removed 181 (4%) participants who used assisted devices from the original study population. The cross-sectional results were similar; participants with worse balance had lower 3MSE scores (−4.68; 95% CI: −5.87, −3.50). However, the longitudinal association was not significant after this exclusion. Finally, results using age cut points of 75 and 80 years were similar to the results with the 76 years of age cut point (data not shown).
Discussion
In this study of adults aged 65 and older participating in the CHS, those with worse balance had lower cognitive function scores and had a faster rate of decline than others with better balance scores. For example, participants who failed a tandem balance test had an estimated 4.4-point greater decline in 3MSE scores over 6 years and had 1.7 times the risk of becoming cognitively impaired compared with participants who passed the tandem stance assessment. These results were stronger in adults ≥76 years. As previous research has described a five-point change in the 3MSE as a clinically important decline, our results have high potential clinical importance [27]. These findings highlight the importance of physical performance as an early and robust risk factor for cognitive decline, especially among adults aged ≥76 years.
Results from this study are supported by previous literatures that have found an association with balance and cognitive function [7–9]. A study by Bullain et al. followed 578 dementia-free participants from the 90+ study for a mean of 2.6 years of follow-up. This study, which examined the physical performance and the risk of dementia in the 90 years and above population, found higher risk of dementia-associated balance measures than with handgrips and chair stands [10]. Finkel et al. examined the bivariate relationship between motor skills, which included balance and fine motor movements. Within a subset population of 813 twins aged 50–85 years who were followed for 19 years, results suggested that the decline in some domains of cognitive function is associated with the decline in physical function, including balance [28]. Our study builds upon this work by looking specifically at balance both cross-sectionally and longitudinally in a large sample of older adults.
The vestibular system provides a physiological link between standing balance and cognitive function. Balance relies on signals and feedbacks from parts of the body, including the brain through the vestibular system [11–13]. Studies have examined association between cognitive impairment and vestibular decline as well as vestibular decline and balance. These studies have shown that vestibular decline and dysfunction is associated with worse balance and cognitive decline [29–31]. A better understanding of common pathophysiology between balance and cognitive function could inform interventions to preserve cognitive function in old age.
Strengths of this study are the large sample size and the established clinical measures which were used. The limitations of the study include the moderate length of follow-up time and the potential residual confounding unaccounted for in the analysis. Another limitation includes the moderate amount of missing values connected with the outcome variables and co-variates; however, we used maximum likelihood to fit our multilevel models which can account for the data that are missing at random. Furthermore, while results for our age cut point at the median and at 75 and 80 years were similar, the relationship of age and cognitive decline based on balance performance may be more nuanced than our results indicate.
In addition to enhancing our understanding of the shared mechanism between balance and cognition, balance could be a useful screening tool to predict future cognitive decline in older individuals. Tandem stance is an easy low-cost test that can be used to identify at-risk individuals, which could allow for the early detection of cognitive decline. Future research should continue to explore the relationship between balance and sensory health with cognitive function in an older population and should evaluate the predictive performance of balance and a variety of physical performance measures as early markers of cognitive decline.
Supplementary Material
Contributor Information
Claire C Meunier, School of Biological and Population Health Sciences, Oregon State University, Corvallis, OR, USA.
Ellen Smit, School of Biological and Population Health Sciences, Oregon State University, Corvallis, OR, USA.
Annette L Fitzpatrick, Departments of Family Medicine, Epidemiology, and Global Health, University of Washington, Seattle, WA, USA.
Michelle C Odden, School of Biological and Population Health Sciences, Oregon State University, Corvallis, OR, USA; Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA.
Declaration of Conflicts of Interest
Dr. Odden is a paid consultant for Cricket Health Inc., a kidney care company.
Declaration of Sources of Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC15103 and R01AG15928 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Financial sponsors played no role in the design, execution, analysis and interpretation of the data.
References
- 1.Cognitive Impairment: A Call for Action, Now!: Available at https://www.cdc.gov/aging/pdf/cognitive_impairment/cogimp_poilicy_final.pdf (accessed 31 October 2018).
- 2.Morley JE, Morris JC, Berg-Weger M et al. Brain health: the importance of recognizing cognitive impairment: an IAGG consensus conference. J Am Med Dir Assoc 2015; 16: 731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolandzadeh N, Kording K, Salowitz N et al. Predicting cognitive function from clinical measures of physical function and health status in older adults. PLoS One 2015; 10: e0119075 doi: 10.1371/journal.pone.0119075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu C-L, Liang C-K, Liao M-C, Chou MY, Lin YT. Slow gait speed as a predictor of 1-year cognitive decline in a veterans’ retirement community in southern Taiwan. Geriatr Gerontol Int 2017; 17: 14–9. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Pinillos F, Cozar-Barba M, Munoz-Jimenez M, Soto-Hermoso V, Latorre-Roman P. Gait speed in older people: an easy test for detecting cognitive impairment, functional independence, and health state. Psychogeriatrics 2016; 16: 165–71. [DOI] [PubMed] [Google Scholar]
- 6.Otobe Y, Hiraki K, Hotta C et al. Mild cognitive impairment in older adults with pre-dialysis patients with chronic kidney disease: prevalence and association with physical function. Nephrol Ther 2019; 24: 50–5. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins CH, Roe CM, Morris JC, Galvin JE. Mild physical impairment predicts future diagnosis of dementia of the Alzheimer type. J Am Geriatr Soc 2013; 61: 1055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto S, Sasaki A, Takahashi I, Mitsuhashi Y, Nakaji S, Matsubara A. Relationship between cognitive function and balance in a community-dwelling population in Japan. Acta Otolaryngol (Stockh) 2018; 138: 471–4. [DOI] [PubMed] [Google Scholar]
- 9.Bahureksa L, Najafi B, Saleh A et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology 2017; 63: 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullain SS, Corrada MM, Perry SM, Kawas CH. Sound body sound mind? Physical performance and the risk of dementia in the oldest-old: the 90+ study. J Am Geriatr Soc 2016; 64: 1408–15. [DOI] [PubMed] [Google Scholar]
- 11.Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev 1998; 22: 465–72. [DOI] [PubMed] [Google Scholar]
- 12.van der Kooij H, Jacobs R, Koopman B, Grootenboer H. A multisensory integration model of human stance control. Biol Cybern 1999; 80: 299–308. [DOI] [PubMed] [Google Scholar]
- 13.Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 2012; 35: 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine DA, Galecki AT, Langa KM et al. Blood pressure and cognitive decline over 8 years in middle-aged and older black and white Americans. Hypertension 2019; 73: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snitz BE, O'Meara ES, Carlson MC et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA 2009; 302: 2663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benn EKT, Fox A, Fei K, Roberts E, Boden-Albala B. Moving towards a more comprehensive investigation of racial/ethnic differences in cognitive disability among US adults. J Immigr Minor Health 2015; 17: 1105–13. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P et al. The cardiovascular health study: design and rationale. Ann Epidemiol 1991; 1: 263–76. [DOI] [PubMed] [Google Scholar]
- 18.CHS-NHLBI | chs-nhlbi , ed. chs-nhlbi. In: Available at https://chs-nhlbi.org/ (accessed 3 December 2018).
- 19.Franchignoni F, Tesio L, Martino MT et al. Reliability of four simple, quantitative tests of balance and mobility in healthy elderly females. Aging Milan Italy 1998; 10: 26–31. [DOI] [PubMed] [Google Scholar]
- 20.Houston DK, Tooze JA, Davis CC et al. Serum 25-hydroxyvitamin D and physical function in older adults: the Cardiovascular Health Study All Stars. J Am Geriatr Soc 2011; 59: 1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry 1987; 48: 314–8. [PubMed] [Google Scholar]
- 22.McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: the mini mental state exam (MMSE) and modified mini-mental state exam (3MS) compared. J Clin Epidemiol 1997; 50: 377–83. [DOI] [PubMed] [Google Scholar]
- 23.Darsie B, Shlipak MG, Sarnak MJ et al. Kidney Function and Cognitive Health in Older Adults: The Cardiovascular Health Study. Am J Epidemiol 2014; 180: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeger J. Digit symbol substitution test. J Clin Psychopharmacol 2018; 38: 513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S. Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing 2016; 45: 688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold AM, Newman AB, Dermond N, Haan M, Fitzpatrick A. Using telephone and informant assessments to estimate missing modified mini-mental state exam scores and rates of cognitive decline. The Cardiovascular Health Study. Neuroepidemiology 2009; 33: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winovich DT, Longstreth WT, Arnold AM et al. Factors associated with ischemic stroke survival and recovery in older adults. Stroke 2017; 48: 1818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkel D, Ernsth-Bravell M, Pedersen NL. Temporal dynamics of motor functioning and cognitive aging. J Gerontol A Biol Sci Med Sci 2016; 71: 109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bigelow RT, Agrawal Y. Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J Vestib Res Equilib Orientat 2015; 25: 73–89. [DOI] [PubMed] [Google Scholar]
- 30.Smith PF, Zheng Y. From ear to uncertainty: vestibular contributions to cognitive function. Front Integr Neurosci 2013; 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Silva LJ, Lin J, Staecker H, Whitney SL, Kluding PM. Impact of diabetic complications on balance and falls: contribution of the vestibular system. Phys Ther 2016; 96: 400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


