ABSTRACT
Clinical efficacy of the influenza antiviral baloxavir marboxil (baloxavir) is compromised by treatment-emergent variants harboring a polymerase acidic protein I38T (isoleucine-38-threonine) substitution. However, the fitness of I38T-containing influenza B viruses (IBVs) remains inadequately defined. After the pharmacokinetics of the compound were confirmed in ferrets, animals were injected subcutaneously with 8 mg/kg of baloxavir acid (BXA) at 24 h postinoculation with recombinant BXA-sensitive (BXA-Sen, I38) or BXA-resistant (BXA-Res, I38T) B/Brisbane/60/2008 (Victoria lineage) virus. BXA treatment of donor ferrets reduced virus replication and delayed transmission of the BXA-Sen but not the BXA-Res IBV. The I38 genotype remained dominant in the BXA-Sen-infected animals, even with BXA treatment. In competitive-mixture experiments, no transmission to aerosol contacts was seen from BXA-treated donors coinfected with the BXA-Sen and BXA-Res B/Brisbane/60/2008 viruses. However, in parallel mixed infections with the B/Phuket/3073/2013 (Yamagata lineage) virus background, BXA treatment failed to block airborne transmission of the BXA-Res virus, and the I38T genotype generally predominated. Therefore, the relative fitness of BXA-Res IBVs is complex and dependent on the virus backbone and within-host virus competition. BXA treatment of single-virus-infected ferrets hampers aerosol transmission of the BXA-Sen virus and does not readily generate BXA-Res variants, whereas mixed infections may result in propagation of BXA-Res IBVs of the Yamagata lineage. Our findings confirm the antiviral potency of baloxavir against IBVs, while supporting optimization of the dosing regimen to maximize clinical benefit.
KEYWORDS: influenza B virus, baloxavir treatment, antiviral resistance, fitness, ferret model
INTRODUCTION
Influenza B viruses (IBVs) are important human respiratory pathogens, accounting for approximately 21% of global influenza cases annually (1, 2). Cocirculating alongside the A(H1N1)pdm09 and A(H3N2) subtypes of influenza A virus (IAV), IBVs exist as antigenically and genetically diverse Victoria and Yamagata lineages (3, 4). Because IBVs generally cause only mild to moderate disease in healthy individuals and are not known to cause pandemics, IBVs attract less clinical attention than do IAVs. However, they can cause severe disease burden in children and young adults, often with complications, including encephalitis and myositis (3, 5, 6).
The neuraminidase inhibitors (NAIs) oseltamivir, zanamivir, peramivir, and laninamivir (approved only in Japan) are the prevailing standard of care for treating influenza, but they are less effective against IBVs than against IAVs (7–10). Moreover, the emergence of NAI-resistant viruses is concerning because of the limited options for influenza treatment, as indicated by the global spread of NAI-resistant A(H1N1) viruses in the 2008-2009 influenza season (11–13). The 2018 approval of baloxavir marboxil (here, baloxavir) as a single-dose oral agent to treat uncomplicated influenza introduced a promising new class of influenza antivirals (14). The active metabolite, baloxavir acid (BXA), inhibits the cap-dependent endonuclease activity of the virus polymerase acidic (PA) protein, impairing viral replication (15). In clinical trials, baloxavir was superior to placebo and oseltamivir in reducing the time-to-alleviation of influenza symptoms among otherwise healthy and high-risk patients by rapidly reducing the infectious virus titer and the duration of virus shedding (14, 16). Recently, baloxavir was demonstrated (17) and has been approved for postexposure prophylaxis of influenza for individuals 12 years of age and older following contact with influenza patients (18, 19). However, as with NAIs, BXA is less active against IBVs than against IAVs in vitro (20–22). Accordingly, smaller reductions in virus titer were observed in baloxavir-treated, IBV-infected patients (23), and the median time to sustained cessation of infectious virus detection was delayed relative to that in IAV-infected patients (16).
Virus variants with reduced baloxavir susceptibility emerge frequently during monotherapy. This reduced susceptibility is primarily defined by the isoleucine-38-threonine (I38T) substitution in the virus PA protein (14, 24). The frequencies of treatment-emergent variants differ between virus types and subtypes (25). Compared to the frequencies of such variants in patients infected with A(H1N1)pdm09 or A(H3N2) virus (2.2% to 6% and 9% to 13.2%, respectively), variants were rarely detected in IBV-infected patients, being found in <3% of cases (16, 24–26). In antiviral surveillance, only two IBVs containing either the I38T or the I38V substitution were isolated from non-baloxavir-exposed patients (27, 28). Therefore, there is a need to understand the clinical relevance and impact of the PA I38T substitution on the fitness and spread of IBVs.
Previous studies have suggested that orally administered baloxavir has unfavorable pharmacokinetics (PK) in ferrets (29, 30), which constitute the principal animal model for studying influenza pathogenesis, transmission, and antiviral efficacy (31). Subcutaneously administered BXA demonstrated efficacy in IAV-infected ferrets (29, 30), but its efficacy against IBVs is insufficiently explored. Here, in single-virus inoculation experiments, we investigated the replication efficiency and transmissibility in ferrets of a BXA-sensitive (BXA-Sen) IBV with wild-type PA I38 or BXA-resistant (BXA-Res) IBV with PA I38T. In competitive-mixture experiments, we examined the interactions of BXA-Sen and BXA-Res viruses within the host by using representative viruses from both the Victoria and Yamagata lineages: B/Brisbane/60/2008 (BR/08) and B/Phuket/3073/2013 (PH/13), respectively. Both experimental approaches were conducted in the presence or absence of BXA treatment to evaluate the risks posed by treatment-emergent variants.
RESULTS
Pharmacokinetics of BXA in ferrets after subcutaneous administration.
A single BXA administration at 4 mg/kg yielded plasma concentrations sufficient to reduce influenza A(H1N1)pdm09 viral shedding and transmission in ferrets (30). Given that IBVs are generally less susceptible than IAVs to BXA in vitro (20–22), a single dose of BXA at 8 mg/kg was administered to ferrets in this study. Sampling began at 1 h postadministration, with BXA plasma concentrations gradually increasing to a peak (Cmax, 21.1 ± 4.4 ng/ml) at 24 h after administration (see Fig. S1 in the supplemental material). Thereafter, the mean plasma concentration in all dosed ferrets was 9 ng/ml through 120 h postdosing, which exceeds the 6.85 ng/ml estimated target mean for baloxavir efficacy (32). The BXA plasma concentration in ferrets was higher than the half-maximal effective concentration (EC50) values of 2.9 ± 1.1 nmol/liter (1.4 ± 0.5 ng/ml) to 3.4 ± 0.8 nmol/liter (1.6 ± 0.4 ng/ml) obtained in vitro for recombinant BXA-Sen viruses, but it is 2- to 3-fold lower than the values obtained for their recombinant BXA-Res counterparts (see Fig. S1). Therefore, the 8-mg/kg dose did not effectively elevate BXA plasma concentration in ferrets relative to a previous report (30). However, since it resulted in a plasma concentration above the target efficacy level, we adopted this BXA preparation for our subsequent in vivo experiments in ferrets.
Antiviral activity of BXA in ferrets inoculated with BXA-Sen BR/08 virus.
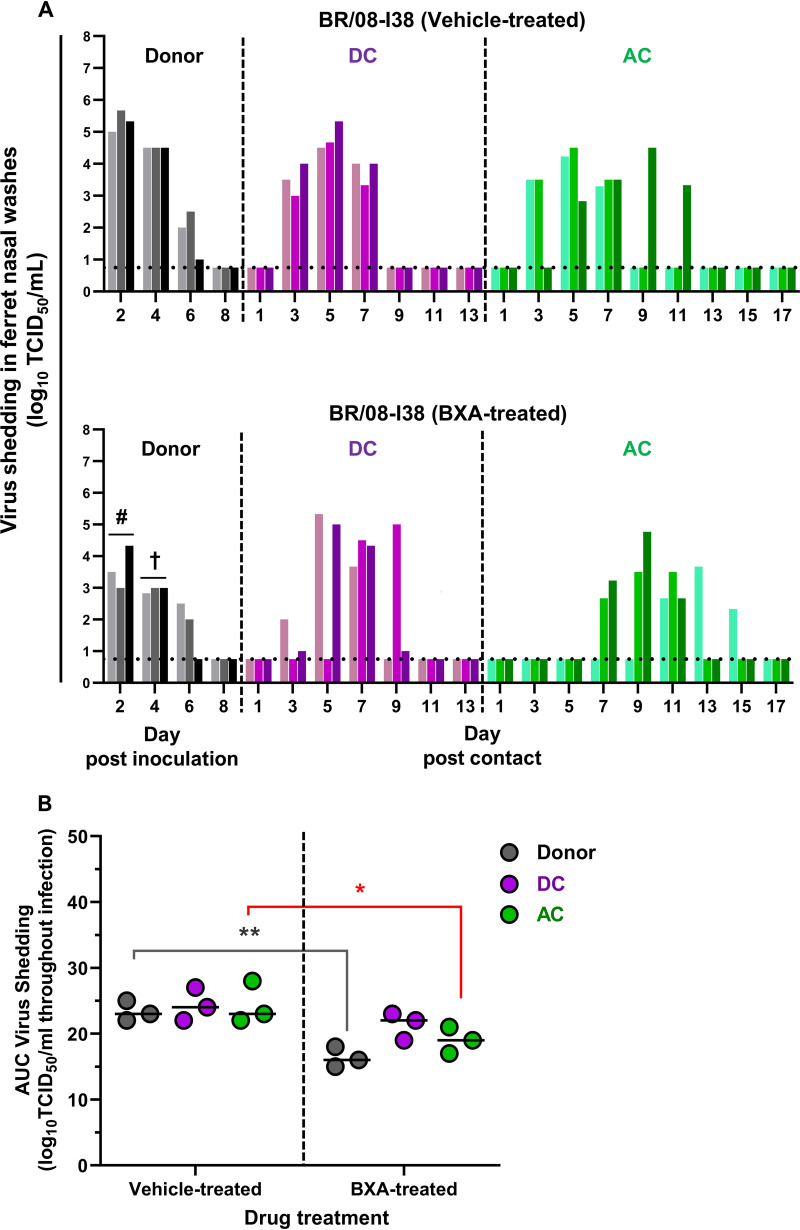
To examine the efficacy of BXA against IBV infection, ferrets inoculated with the BXA-Sen BR/08-I38 virus (in a single-virus inoculation) were treated at 1 day postinoculation (dpi) with either BXA or the suspension vehicle (0.5% methylcellulose) (Fig. 1). All inoculated donor ferrets in the vehicle-treated and BXA-treated groups were similarly active, with some animals displaying transient pyrexia (1 to 2°C above baseline) at 2 dpi (see Table S1). Infrequent sneezing was observed, and the recorded weight loss was <10%. Regardless of treatment, BR/08-I38 was shed by donor ferrets at 2 to 6 dpi (Fig. 1A; see also Table S1). The viral load in the upper respiratory tracts (URTs) of BXA-treated donors was consistently lower than that in the URTs of vehicle-treated donors on all sampling days: viral titer reductions were 1.7 (P = 0.03), 1.6 (P = 0.05), and 0.3 (P > 0.05) log10 50% tissue culture infective dose(s) (TCID50)/ml at 2, 4, and 6 dpi, respectively. The area under the curve (AUC) for viral shedding over the course of infection was significantly lower for the BXA-treated group than for the vehicle-treated group (16.3 ± 1.5 versus 23.3 ± 1.5; P = 0.007) (Fig. 1B). Therefore, our PK-derived BXA dosing significantly reduced the titers of the BR/08-I38 virus in the ferret URT, particularly at early times after dosing.
FIG 1.
Effect of BXA treatment on the replication and transmission of BXA-Sen BR/08 virus in ferrets. (A) Donor ferrets (n = 3) were inoculated with 105 TCID50/ferret of recombinant influenza BR/08-I38 virus. At 1 dpi, donors were injected subcutaneously with a single dose of BXA at 8 mg/kg or 0.5% methylcellulose, and naive DC and AC ferrets were individually paired with each donor immediately after antiviral treatment. Virus titers (log10TCID50/ml; limit of detection: 0.75 log10TCID50/ml, horizontal dotted lines) in individual ferrets were assayed using nasal washes collected at the indicated time points after donor inoculation or contact exposure. The mean viral titers in vehicle-treated and BXA-treated donors were compared at 2 dpi (#, P = 0.03) and 4 dpi (†, P = 0.05), using two-way ANOVA, followed by Šidák’s multiple-comparison post hoc test. (B) The AUCs for virus shedding from ferrets are presented as individual animal shedding curve values, which were calculated in GraphPad Prism 8.0. P values were calculated by two-way ANOVA with Šidák’s multiple-comparison post hoc test; the associated bars are color coded to indicate the groups being compared. *, P < 0.05; **, P < 0.01.
To determine the effect of BXA treatment on virus transmission, each donor ferret was paired with naive ferrets immediately after BXA administration by cohousing a direct-contact (DC) ferret in the same cage (to model the DC route of transmission) and placing an aerosol-contact (AC) ferret in a cage adjacent to the donor-DC pair (to model the AC route of transmission). In the vehicle-treated donor group, all three DC ferrets became positive for virus detection at 3 days postcontact (dpc) whereas the three AC animals started shedding virus at 3 to 5 dpc (Fig. 1A; see also Table S1). Virus transmission also occurred in the BXA-treated donor group, but with a trend toward delayed transmission starting at 3 to 5 dpc for DC ferrets and at 7 to 11 dpc for AC animals. Moreover, BR/08-I38 virus replication in ferrets exposed to the BXA-treated donors was generally attenuated, particularly in AC animals (P < 0.05) (Fig. 1B). All donors and contacts seroconverted by 21 dpi (see Table S1). Therefore, BXA treatment of donors delayed both routes of virus transmission and resulted in less-robust virus replication in contacts. However, there was no immediate effect on the number of DC and AC ferrets that became infected.
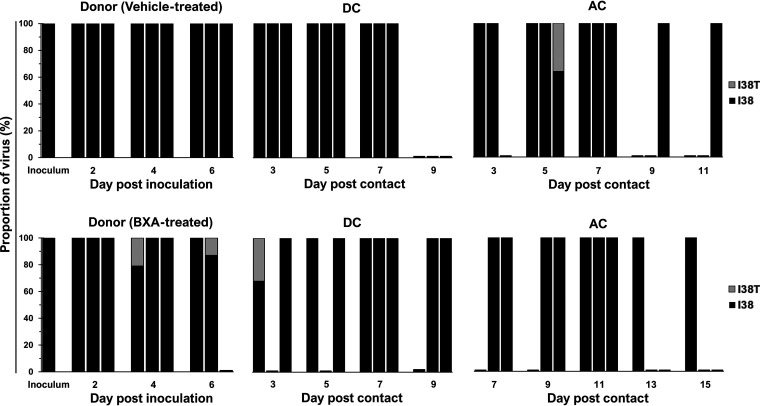
Because variants with PA substitutions may arise during baloxavir treatment, all ferret nasal washes that were virus positive were subjected to next-generation sequencing (NGS) analysis. One AC ferret in the vehicle-treated donor group shed I38T variants (36% of the total) on its initial day of shedding only (Fig. 2). Two BXA-treated donors and one DC ferret transiently shed the I38T variant at low abundance (≤20% of the total). Therefore, treatment-emergent I38T variants may arise late in infection, at which time the antiviral drug concentration is waning.
FIG 2.
Proportions of BXA-Sen and BXA-Res virus subpopulations in the URTs of ferrets inoculated with the BR/08-I38 virus. All virus-positive ferret nasal washes were processed for amplification of the full-length PA gene by RT-PCR. The proportions of the respective PA I38 and I38T subpopulations were determined by using the Illumina sequencing platform to call variants in the nucleotides encoding the amino acid at position 38 in the PA protein. High-quality reads were mapped to the consensus PA sequence of the BR/08 virus. The data shown are the virus proportions (expressed as the percentages of the total reads) in individual ferrets.
Antiviral activity of BXA in ferrets inoculated with BXA-Res BR/08 virus and within-host fitness.
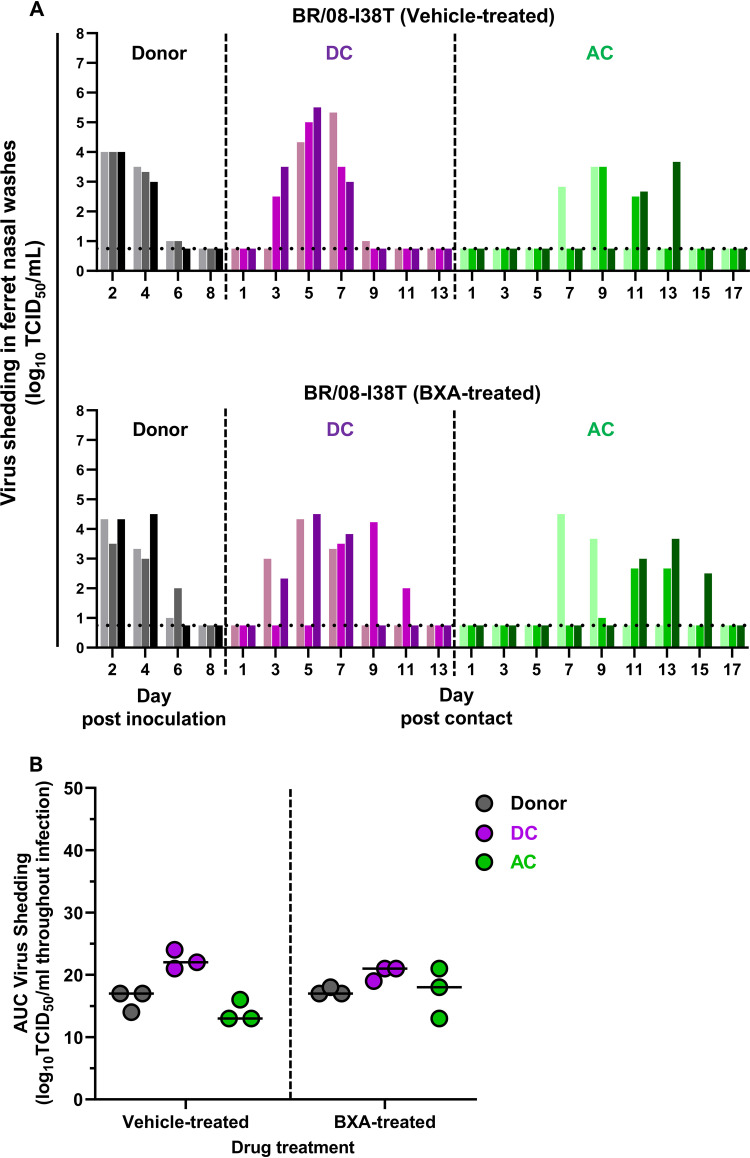
We previously evaluated IBVs harboring PA I38X substitutions in ferrets, but only in the absence of antiviral treatment (20). Consistent to results obtained in these studies, the BXA-Res BR/08 virus replicated in the URTs of donor ferrets until 6 dpi, and virus-induced morbidity remained mild (Fig. 3A; see also Table S1). Virus replication in the URT did not differ significantly between vehicle-treated and BXA-treated donors (Fig. 3B). Therefore, BXA treatment could not regulate virus replication in ferrets inoculated with BXA-Res virus, confirming that the I38T substitution confers reduced BXA susceptibility of IBVs in vivo.
FIG 3.
Effect of BXA treatment on the replication and transmission of BXA-Res BR/08 virus in ferrets. (A) Donor ferrets (n = 3) were inoculated with recombinant influenza BR/08-I38T virus at 105 TCID50/ferret. At 1 dpi, donors were injected subcutaneously with a single dose of BXA at 8 mg/kg or 0.5% methylcellulose, and naive DC ferrets (n = 3) and AC animals (n = 3) were individually paired with each donor immediately after antiviral treatment. Virus titers (log10TCID50/ml; limit of detection: 0.75 log10TCID50/ml, horizontal dotted lines) in individual ferrets were assayed using nasal washes collected at the indicated time points after donor inoculation or contact exposure. (B) The AUCs for virus shedding from ferrets are presented as individual animal shedding curve values, which were calculated in GraphPad Prism 8.0. P values were calculated using two-way ANOVA with Šidák’s multiple-comparison post hoc test; the associated bars are color coded to indicate the groups being compared.
The between-host fitness of the BXA-Res virus was also determined by exposing DC and AC ferrets to donors. In the vehicle-treated donor control group, all cohoused DC ferrets shed virus from 3 to 5 dpc, whereas each of the paired AC animals was virus positive at 7, 9, and 11 dpc (Fig. 3A). In the BXA-treated donor, antiviral treatment had no noticeable effect on the dissemination of BR/08-I38T to DC and AC ferrets. All naive contact ferrets shed virus at frequencies comparable to those observed in ferrets exposed to vehicle-treated donors. All donors and contacts seroconverted at 21 dpi. Therefore, the single dose of BXA at 8 mg/kg did not inhibit the replication or transmission of the BXA-Res virus in ferrets.
When the virus replication kinetics were compared to those in ferrets inoculated with BR/08-I38, BR/08-I38T was found to induce lower viral titers in the URTs of untreated donors (P < 0.01), consistent with its attenuated replication in vivo (Fig. 3A; see also Fig. S2A) (19). Similarly, less-robust replication was noted in the corresponding DC (P = 0.6) and AC (P < 0.01) animals despite the successful transmission. Although the results of single-virus inoculations indicated that the within-host replication fitness of the BR/08-I38T virus was attenuated relative to that of the BR/08-I38 virus, the transmission fitness appeared to be similar for the two viruses and transmission was inefficiently blocked by the BXA treatment.
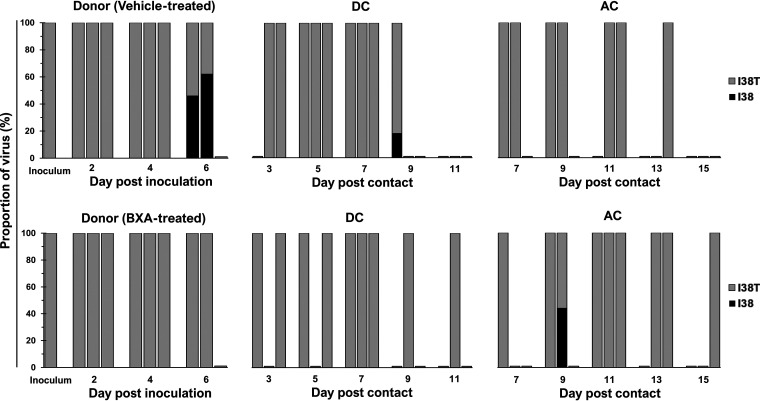
The stability of the I38T substitution was monitored in all virus-positive nasal washes, and the results showed that the BXA-Res genotype was largely maintained in the vehicle-treated group, with wild-type I38 revertant virus being detected in only two donors (at 6 dpi) and one DC ferret (at 9 dpc) (Fig. 4). In the BXA-treated group, only one of the three AC ferrets initially contained a minor BR/08-I38 virus subpopulation at 9 dpc, and this was undetectable thereafter. No other variants with substitutions at I38 or at non-I38 positions associated with reduced BXA susceptibility were observed. Therefore, the I38T substitution is generally stable in the BR/08 genetic background.
FIG 4.
Proportions of BXA-Sen and BXA-Res virus subpopulations in the URTs of ferrets inoculated with the BR/08-I38T virus. All virus-positive ferret nasal washes were processed for amplification of the full-length PA gene by RT-PCR. The proportions of the respective PA I38 and I38T subpopulations were determined by using the Illumina sequencing platform to call variants in the nucleotides encoding the amino acid at position 38 of the PA protein. High-quality reads were mapped to the consensus PA sequence of the BR/08 virus. The data shown are the virus proportions (expressed as the percentages of the total reads) in individual ferrets.
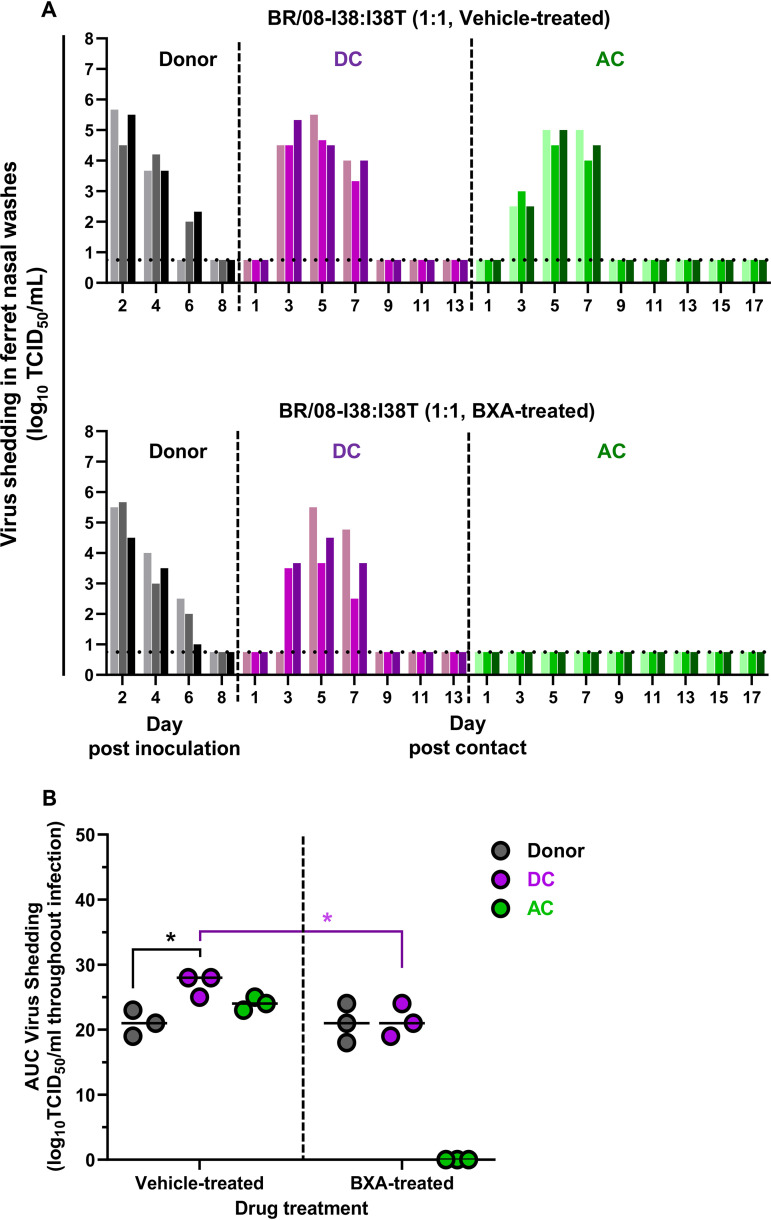
Competitive fitness of BXA-Sen and BXA-Res BR/08 viruses in the presence or absence of BXA.
To understand the dynamics of virus-virus interactions in infected individuals, we modeled the replication and transmission of the virus in ferrets coinoculated with a 1:1 mixture of the BXA-Sen and BXA-Res BR/08 viruses (Fig. 5). As with single-virus inoculations, clinical symptoms, including weight loss, temperature elevations, and lethargy, were typically mild in both vehicle-treated and BXA-treated donors (see Table S2). In vehicle-treated donors, virus shedding was detected at 2 dpi and lasted up to 6 dpi (Fig. 5A; see also Table S2). All three DC ferrets and all three AC animals shed virus from 3 to 7 dpc, with high titers being reached at 5 dpc in most animals. The AUC for virus shedding in contact ferrets was greater than that for shedding in donors, particularly among DC animals (P < 0.05) (Fig. 5B).
FIG 5.
Replication and transmission of BXA-Sen BR/08-I38 and BXA-Res BR/08-I38T viruses in a competitive fitness ferret model. (A) Donor ferrets (n = 3) were coinoculated with a mixture (at a 1:1 ratio) of the recombinant BXA-Sen BR/08-I38 virus (5 × 104 TCID50) and the BXA-Res BR/08-I38T virus (5 × 104 TCID50) in 0.5 ml of PBS. At 1 dpi, donors were injected subcutaneously with a single dose of BXA at 8 mg/kg or 0.5% methylcellulose, and naive DC and AC ferrets were individually paired with each donor immediately after antiviral treatment. Virus titers (log10TCID50/ml; limit of detection: 0.75 log10TCID50/ml, horizontal dotted lines) in individual ferrets were assayed using nasal washes collected at the indicated time points after donor inoculation or contact exposure. (B) The AUCs for virus shedding from ferrets are presented as individual animal shedding curve values, which were calculated in GraphPad Prism 8.0. P values were calculated using two-way ANOVA with Šidák’s multiple-comparison post hoc test; the associated bars are color coded to indicate the groups being compared. *, P < 0.05.
The single-dose BXA regimen did not reduce virus titers in the nasal washes of BXA-treated donors relative to those in nasal washes of vehicle-treated donors (Fig. 5). All cohoused DC ferrets shed virus from 3 to 5 dpc, with shedding lasting up to 7 dpc regardless of treatment. Although BXA-treated donor and DC ferrets exhibited comparable virus shedding overall, shedding was significantly lower (P = 0.05) compared to that in DC ferrets in the vehicle-treated donor group (Fig. 5B). In contrast to the DC ferrets, no AC animal exposed to the BXA-treated donors became virus positive during the experimental period, and this was further confirmed by the lack of seroconversion against BR/08 at 21 dpi (Fig. 5; see also Table S2). The virus replication in ferrets inoculated with the BR/08 competitive-mixture appeared to mirror better that in the BXA-Sen-inoculated group than that in the BXA-Res–inoculated group (see Fig. S2A). However, except in the AC ferrets, virus shedding did not differ significantly between these groups under BXA treatment (see Fig. S2B). Together, these data indicate that BXA inefficiently reduced the viral load in donors inoculated with the BR/08 virus-mixture but that it did dampen virus replication in cohoused DC ferrets and restrict aerosol transmission.
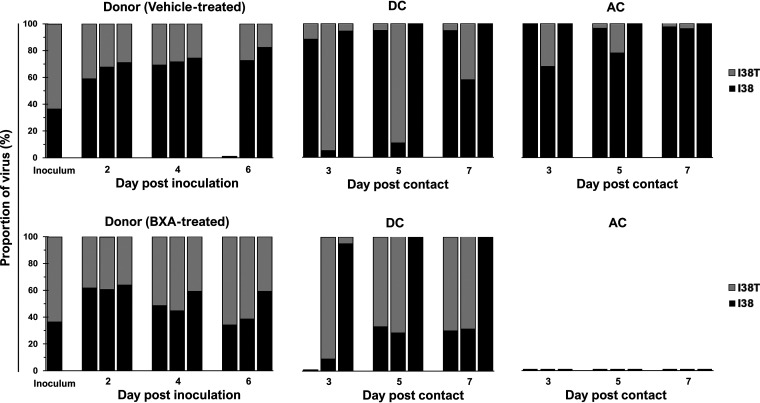
Dynamics of viral subpopulations in ferrets inoculated with the BXA-Sen and BXA-Res BR/08 mixture.
Understanding the dynamics of viral subpopulations in ferrets actively shedding influenza virus is a valuable tool for examining the predominance of drug-sensitive or drug-resistant viruses within mixed-inoculation groups (33, 34). Therefore, we evaluated virus fitness based on how the ratio (expressed in percentages) of the BR/08-I38 and BR/08-I38T viruses in ferret nasal washes changed from the first day to the last day of virus shedding. As determined by NGS, the ratio of BR/08-I38 to BR/08-I38T in the virus inoculum was 37% versus 63% (Fig. 6). Such a ratio does not reflect the intended proportions of the viruses in the mixed-virus inoculum based on the infectious virus titer; this may be attributed to the presence of nonreplicating virions (35). Moreover, given its lower infectivity in vitro (20), more BR/08-I38T virus was needed to match the infectivity of the BR/08-I38 virus. Despite this, the virus with the I38 genotype had already outgrown the I38T virus in vehicle-treated donors at 2 dpi (ratio = 64% to 36%). Mixed-virus populations persisted until the end of infection and were dominated by I38 virus (constituting >50% of the total) at all time points. Similarly, I38 maintained dominance over I38T in most contacts except in one of three DC ferrets, in which the proportions mostly oscillated, but >50% of the virus subpopulations harbored I38T on the first and last days of shedding (Fig. 6; see also Fig. S3). These results indicate that most viruses that transmitted from vehicle-treated donors to DC or AC ferrets possessed the I38 genotype, suggesting that it has both within-host and between-host fitness advantages over I38T in the absence of BXA treatment. However, this advantage might still be limited, because the I38T variants remained detectable in at least two ferrets on the last day of virus shedding in each of the donor and contact groups (Fig. 6; see also Fig. S3).
FIG 6.
Proportions of BXA-Sen and BXA-Res virus subpopulations in nasal washes collected from individual ferrets inoculated with a BR/08 competitive mixture. All virus-positive ferret nasal washes were processed for amplification of the full-length PA gene by RT-PCR. The proportions of the respective PA I38 and I38T subpopulations were determined by using the Illumina sequencing platform to call variants in the nucleotides encoding the amino acid at position 38 of the PA protein. High-quality reads were mapped to the consensus PA sequence of the BR/08 virus. The data shown are the virus proportions (expressed as the percentages of the total reads) in individual ferrets.
In the BXA-treated group, the virus subpopulations in infected donors did not differ significantly from those in vehicle-treated donors suggesting that possession of I38T PA did not provide apparent growth advantage in BXA-treated donor ferrets (Fig. 6; see also Fig. S3). The I38 virus was initially more prevalent (constituting an average of 62% of the total) at 2 dpi in all donors (see Fig. S3). Mixed-virus subpopulations in proportions almost identical to those expected were established at 4 dpi, but the average proportion of I38T virus at 6 dpi was already 56%, suggesting that replication of this variant was slightly better than that of the wild-type virus on the last day of shedding. PA I38 was detected in one of three DC ferrets, whereas the remaining two DC animals contained higher proportions (>53%) of I38T virus throughout the period examined (Fig. 6). Despite these results, neither the BR/08-I38 virus nor the I38T variant was able to transmit to any of the AC ferrets.
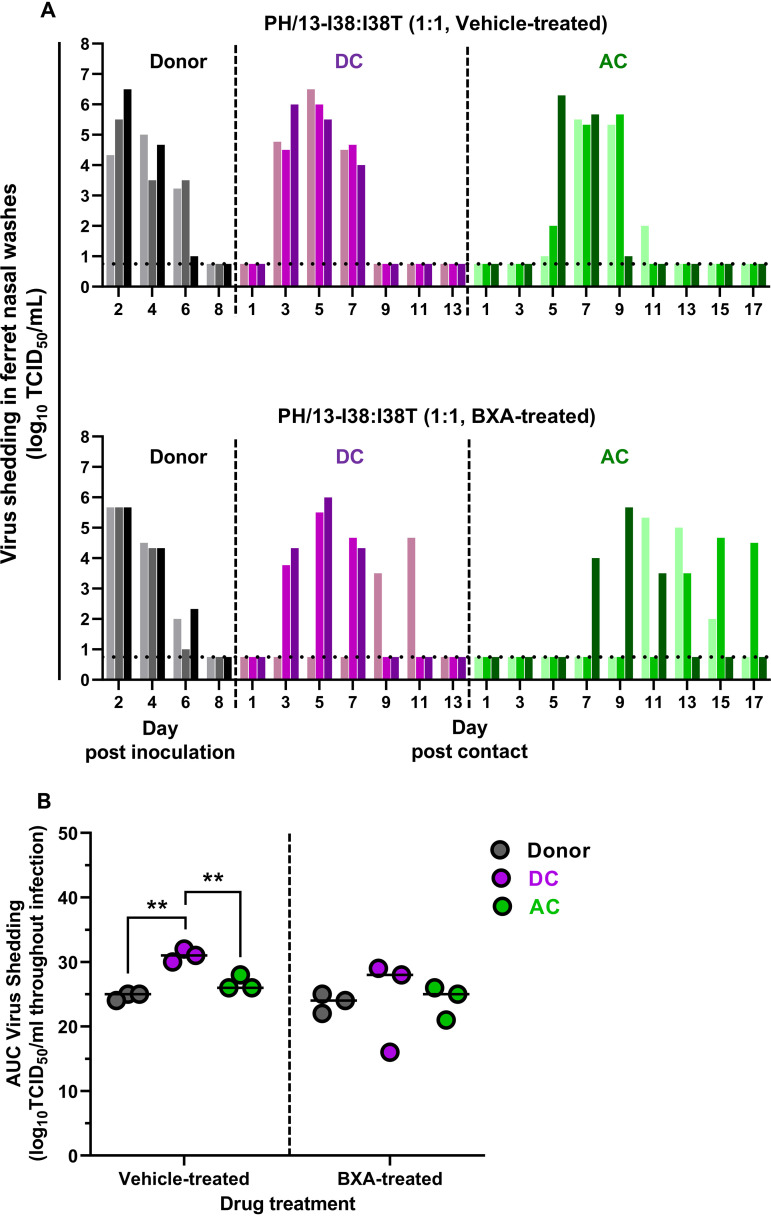
Competitive fitness of BXA-Sen and BXA-Res PH/13 viruses in the presence or absence of BXA.
We extended our competitive fitness experiments by coinoculating ferrets with BXA-Sen and BXA-Res PH/13 viruses (Yamagata lineage). In vehicle-treated donors, infectious virus was shed from 2 dpi through 6 dpi, despite the ferrets exhibiting mild morbidity (Fig. 7A; see also Table S2). The transmission frequency was comparable with that of the BR/08 Victoria-lineage virus, with all three DC ferrets and all three AC animals being successfully infected. Whereas all DC ferrets efficiently shed infectious virus at 3 dpc, AC animals were not infected until 5 to 7 dpc, indicating delayed PH/13 virus dissemination through the aerosol route (Fig. 7A). The AUC for virus shedding in DC ferrets was greater than those for shedding in the donor and AC ferrets (P < 0.01) (Fig. 7B). Such shedding was also greater than that in the corresponding DC ferrets of the BR/08 mixture group (P < 0.01) (see Fig. S4A). Notably, virus shedding in ferrets infected with the PH/13 virus-mixture was generally higher than that in animals infected with the BR/08 virus mixture, particularly in donor (P < 0.05) and DC (P < 0.01) ferrets (see Fig. S4).
FIG 7.
Replication and transmission of BXA-Sen PH/13-I38 and BXA-Res PH/13-I38T viruses in a competitive fitness ferret model. (A) Donor ferrets (n = 3) were coinoculated with a mixture (in a 1:1 ratio) of the recombinant BXA-Sen PH/13-I38 virus (5 × 104 TCID50) and the BXA-Res PH/13-I38T virus (5 × 104 TCID50) in 0.5 ml of PBS. At 1 dpi, donors were injected subcutaneously with a single dose of BXA at 8 mg/kg or 0.5% methylcellulose, and naive DC and AC ferrets were individually paired with each donor immediately after antiviral treatment. Virus titers (log10TCID50/ml; limit of detection: 0.75 log10TCID50/ml, horizontal dotted lines) in individual ferrets were assayed using nasal washes collected at the indicated time points after donor inoculation or contact exposure. (B) The AUCs for virus shedding from ferrets are presented as individual animal shedding curve values, which were calculated in GraphPad Prism 8.0. P values were calculated using two-way ANOVA with Šidák’s multiple-comparison post hoc test; the associated bars are color coded to indicate the groups being compared. **, P < 0.01.
Infectious virus was recovered from nasal washes from BXA-treated donors at 2 to 6 dpi, with the titers being comparable to those in washes from the vehicle-treated donor group (Fig. 7). Transmission was detected in all DC and AC ferrets, although virus shedding was delayed in one of three DC ferrets (which became virus positive only on 9 dpc) and in two of three AC animals (which became virus positive starting at 11 and 13 dpc, respectively). The accumulated virus loads in the BXA-treated donors and the corresponding contact animals did not differ significantly except in one DC ferret that had a lower AUC for virus titers (Fig. 7B). However, the virus shedding in the URTs of these contact ferrets was less than that in the corresponding contacts in the vehicle-treated group. Therefore, as with BR/08, BXA treatment could not inhibit virus replication in the URTs of ferrets inoculated with PH/13 virus in competitive-mixture experiments. However, in contrast to the results with the BR/08 competitive mixture, BXA treatment did not block dissemination of PH/13 to naive AC ferrets (see Fig. S4B).
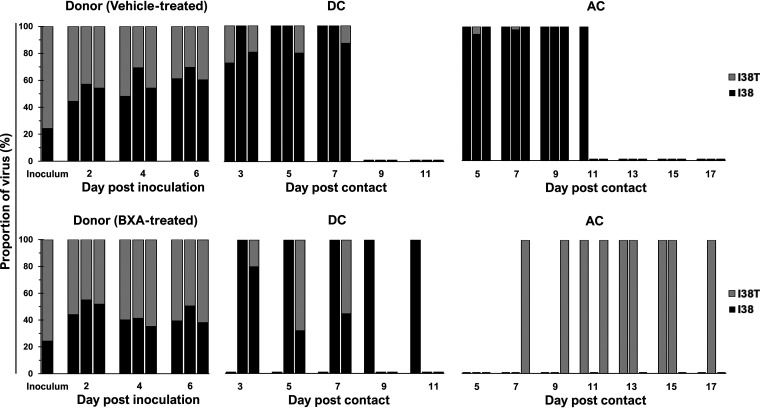
Dynamics of viral subpopulations in ferrets inoculated with the BXA-Sen and BXA-Res PH/13 virus-mixture.
The PH/13 virus mixture inoculum also contained smaller proportions of PH/13-I38 relative to PH/13-I38T (24% versus 75%) (Fig. 8; see also Fig. S5). Mixed-virus subpopulation in proportions (52% versus 48%) almost equivalent to that expected was established in untreated donors at 2 dpi. Although the average proportion of PH/13-I38 gradually increased toward the end of virus shedding, approximately 36% of shed virus still harbored the I38T genotype (see Fig. S5). Mixed-virus subpopulations were detected at initial shedding in two of three DC ferrets and in one of three AC animals in various proportions dominated by PH/13-I38 (Fig. 8). Eventually, PH/13-I38 outcompeted PH/13-I38T on the final day of shedding except in one DC ferret, demonstrating the growth fitness advantage of the BXA-Sen virus over the BXA-Res variant.
FIG 8.
Proportions of BXA-Sen and BXA-Res virus subpopulations in nasal washes collected from individual ferrets inoculated with a PH/13 competitive-mixture. All virus-positive ferret nasal washes were processed for amplification of the full-length PA gene by RT-PCR. The proportions of the respective PA I38 and I38T subpopulations were determined by using the Illumina sequencing platform to call variants in the nucleotides encoding the amino acid at position 38 of the PA protein. High-quality reads were mapped to the consensus PA sequence of the PH/13 virus. The data shown are the virus proportions (expressed as percentages of the total reads) in individual ferrets.
In BXA-treated donors, the target mixed-virus proportion (50% versus 50%) was established at 2 dpi (Fig. 8; see also Fig. S5). By 6 dpi, 57% of the mixed-virus subpopulation contained I38T, indicating that the BXA-Res variant moderately outcompeted the BXA-Sen virus by the end of infection under BXA treatment (Fig. 8; see also Fig. S5). In the contacts, PH/13-I38 was shed throughout infection in two DC ferrets, whereas the remaining animal contained a mixed-virus subpopulation slightly dominated by PH/13-I38T on the last day of shedding (with a ratio of 45% versus 55%). In contrast, all AC ferrets exclusively shed the BXA-Res virus throughout infection (Fig. 8). Therefore, the moderate replicative advantage of PH/13-I38T in BXA-treated donor ferrets may have favored transmission of the BXA-Res variant to AC but not DC ferrets.
DISCUSSION
Baloxavir represents a novel strategy for managing influenza and adds significantly to our arsenal of available antivirals. However, the emergence of variants with reduced baloxavir susceptibility might undermine its clinical efficacy. Evaluating the likelihood of emergence and spread of such variants is necessary to mitigate the associated risks to public health. Compared to IAVs, IBVs have been under-represented in many preclinical studies and detected at lower proportions in clinical studies (14, 23, 36–39). Therefore, robust evidence of the clinical impact of baloxavir on IBV infections is unavailable. Here, we have addressed the clinical relevance of the PA I38T substitution in IBVs in the context of baloxavir treatment by applying two approaches in a ferret model, assessing the outcomes with single-virus and virus mixture inoculations.
A randomized clinical phase 2 study reported dose-dependent increases in BXA plasma concentrations among Japanese patients at 24 h after a single oral dose of 10, 20, or 40 mg of baloxavir (23). However, our PK profiling in ferrets indicates that a single BXA 8-mg/kg dose did not induce BXA plasma concentrations higher than those obtained in 4 mg/kg (30). This suggests that a dosing regimen of BXA higher than 8 mg/kg will be needed if elevated plasma concentrations in ferrets are desired. We also noted that concentration levels in the present study tend to decrease faster within 48 h of administration. Potential cause for this variation in PK profile includes animal host variability (e.g., heterogeneity of outbred ferrets, sex, and age of the ferrets used) and differences in experimental setup. Accordingly, clinical trials also showed that the PK profile of the standard single oral dose of baloxavir in humans differs between Asian and non-Asian patients (32). Despite these, our dosing regimen still maintained plasma concentrations above the target therapeutic level for 120 h prompting us to assess whether such level would be effective against IBV infection as it is against IAV infection (30).
With single-virus inoculations, the morbidity and disease manifestations in ferrets inoculated with the BXA-Sen and BXA-Res IBVs were comparable. Therefore, the antiviral efficacy was assessed by measuring the reduction in virus shedding in the URT and the ability of BXA treatment to limit transmission via the DC and AC routes. In the present study, subcutaneous administration of BXA reduced the virus titers in the URTs of ferrets inoculated with BXA-Sen virus at 24 h after treatment initiation, which corresponds to the peak plasma concentration of BXA. With this route of BXA delivery in ferrets, the PK profile closely resembles that of orally administered baloxavir prodrug in humans compared to that of oral dosing in ferrets, which leads to rapid clearance (29, 30). Although overall virus titers were generally lower, BXA did not shorten the duration of BR/08-I38 shedding and, consequently, failed to block virus transmission to contacts. Conversely, a single dose of BXA at 4 mg/kg efficiently inhibited shedding of the A(H1N1)pdm09 virus in donor ferrets, which limited onward transmission (30). Note, however, that the virus inoculum doses used in those studies were lower and that contacts experienced shorter exposures, with DC ferrets still contracting the virus. We did not observe apparent differences in the number of DC and AC ferrets that became infected upon exposure to vehicle- or BXA-treated donors. Such results were not surprising considering that the exposure between the donors and contact ferrets was almost immediate after treatment, especially for DC animals. In A(H1N1)pdm09-infected ferrets, the infection frequency of DC animals was also inconsistent when they were exposed to donors immediately after BXA treatment (32). However, it was posited that the rapid reduction of A(H1N1)pdm09 virus transmission by the BXA treatment remained significant compared to oseltamivir or placebo regardless of the timing of cohousing. It will be interesting to see whether delaying or limiting the exposure period of contact ferrets to donors would also improve the outcome of IBV transmission under BXA treatment. The decrease in virus titers in our BXA-treated donors was less evident than with the A(H1N1)pdm09 virus, recapitulating in vitro data showing that IBVs are less susceptible to BXA (20, 21). In clinical trials, reduced virus shedding after BXA treatment is less pronounced with IBV infections compared to IAV infections (25), suggesting further optimization of the BXA regimen for IBV may be required to maximize clinical benefit.
Some drug-resistant influenza viruses have decreased transmissibility and, therefore, reduced fitness compared to the corresponding wild-type viruses (40). However, consistent with our previous findings, the PA I38T substitution did not compromise virus transmissibility in ferrets, although it did attenuate virus replication (20). BXA did not inhibit shedding of the BR/08-I38T virus in single-virus inoculations, affirming the BXA-Res phenotype. Together, these results indicate that IBVs that acquire resistance to BXA as a consequence of the I38T substitution remain transmissible between ferrets in the presence or absence of BXA treatment. I38T-bearing IAVs have been documented in untreated patients in day care and in household settings (41–43). Similarly, I38T IAV variants could sustain a chain of three sequential generations of DC transmission in the ferret animal model (35). Sustained interhost transmission of drug-resistant viruses in the absence of selection pressure can lead to widespread circulation, as has happened with emergent IAVs that are resistant to ion-channel inhibitors, thereby rendering those drugs ineffective for influenza treatment (40).
The frequencies of PA I38X variants after baloxavir treatment are high in seasonal IAV-infected populations, particularly in pediatric patients (16, 26). In contrast, the detection rate of I38-substituted IBVs has been low to date. We found that treatment-emergent IBVs carrying the I38T substitution may arise late in infection in response to BXA selection pressure. However, their emergence was not associated with infectious virus rebound or extended duration of shedding. The transient detection of these variants suggests that their appearance is unsustainable. Any low-frequency resistance mutation that is initiated by infection with a small subpopulation of virions could be lost because of certain population bottlenecks as viruses transmit (44). Therefore, the incidence of BXA resistance associated with the I38T substitution in IBVs is likely to remain at or near the de novo rate. Results may also partially explain why IBVs harboring PA markers for reduced baloxavir susceptibility are not frequently detected in drug-treated patients.
Compared to single-virus inoculations, competitive-mixture experiments facilitated a better head-to-head fitness comparison between the BXA-Sen and BXA-Res viruses. In this model, one strain could outgrow the other over time because it replicates faster before immune clearance (34). In addition, assessments of competitive fitness under drug pressure may depict the virus-virus interactions within patients harboring virus mixtures while undergoing baloxavir treatment. The replicative fitness advantage of the BR/08-I38 virus over the BR/08-I38T virus is apparently limited, since BXA-Res subpopulations remained present up to the last day of virus shedding in donors and contacts. This limited within-host replicative advantage of BXA-Sen virus over its BXA-Res counterpart was also noted in an A(H3N2) virus (45). Although BXA-Res viruses in the mixture might evolve from the BXA-Sen virus population (and vice versa), the results of single-virus inoculations suggest that reversion to I38 or acquisition of the I38T substitution is inefficient. With BXA treatment, the BXA-Res subpopulation gradually increased over time, but the selection pressure was still insufficient for that subpopulation to outgrow the BXA-Sen virus subpopulation. Instead, BXA treatment halted the dissemination of both the BXA-Sen and BXA-Res viruses to AC ferrets in the competitive-mixture setting. The precise mechanism by which BR/08 viruses and, particularly, the BXA-Res variant were inhibited requires further investigation. It is plausible that this result was due to the combined effects of virus-virus interaction within the ferret host, BXA treatment (AC transmission was not interrupted in vehicle treatment) and the relatively low replicative ability of the BR/08 virus background.
The impact of the I38T substitution on fitness can vary depending on the virus background (35). In contrast to the results with BR/08 viruses, neither DC transmission nor AC transmission were inhibited by BXA treatment when the mixed-virus inoculum had the Yamagata-lineage PH/13 virus background. PH/13 generally replicates to higher titers compared to BR/08 in vitro and in mice (20, 46). Similarly, the PH/13 virus mixture yielded higher titers in the URTs of ferrets than did the BR/08 virus mixture. Therefore, we hypothesize that the inherently superior replicative capacity of PH/13 was a determining factor in the sustained aerosol transmission in the competitive-mixture model under BXA treatment. The differential impact of key NAI resistance-associated NA substitutions on virus fitness and drug susceptibilities was also noted when identical markers were introduced into these IBVs representing the two distinct lineages (46). We proposed that the overall genetic composition of the viruses and the contributions of other gene segments may variably affect the impact of drug resistance-conferring substitutions on viral fitness (33). Since replicative competence might be an integral feature of the PH/13 virus, this must be considered when assessing the outcomes of IBV transmission experiments. Furthermore, the uniform dissemination of the BXA-Res PH/13 virus to all AC animals implies that the I38T variant may arise and be selected in drug-treated patients if it constitutes >60% of the mixed-virus subpopulation. Although competitive-mixture experiments provide a better insight into the relative fitness of two different strains compared to the more traditional method of inoculating ferrets with pure populations of sensitive or resistant viruses (34), these experiments also highlight the complexity of examining virus fitness (33). Given that only limited numbers of such competitive-mixture animal studies under antiviral treatment are available (47, 48), experimental designs that will accurately assess the impact of drug-resistant substitutions on virus fitness to closely predict their potential to spread widely throughout the community are needed.
Overall, we have demonstrated that a single-dose BXA treatment in ferrets has the potential to reduce IBV replication and impede forward transmission via the aerosol route. However, such restrictions were not as robust compared to results obtained against IAV infection in this animal model (32). These results, to some extent, correlate with the lesser activity of BXA against IBVs than against IAVs in vitro (20–22). Therefore, future studies should aim to assess and optimize current BXA treatment strategy in managing IBV infections for improved antiviral efficacy and maximum clinical benefit. We further conclude that IBVs harboring the PA I38T substitution are moderately less fit than their wild-type virus counterparts and that they might not readily emerge among treated individuals. Accordingly, the clinical and epidemiologic concerns regarding these variants are currently limited. However, risks to public health remain because the variant viruses retain their transmissibility if they do disseminate within a household or in other close-contact settings. Therefore, the appropriate use of baloxavir, particularly in managing IBV infections, and continued close monitoring for the emergence of variants with reduced drug susceptibility are critically important.
MATERIALS AND METHODS
Ethics statement.
Protocols and procedures were approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee (IACUC) in accordance with the Animal Welfare Act and the National Research Council’s Guide for the Care and Use of Laboratory Animals. All animal experiments were conducted at St. Jude Children’s Research Hospital in Memphis, TN, under applicable laws and guidelines after approval from the IACUC.
Cells, antiviral compound, and viruses.
Madin-Darby canine kidney (MDCK) and human embryonic kidney cells (ATCC, Manassas, VA) were maintained in fetal bovine serum-supplemented Eagle minimal essential medium (MEM; Life Technologies, Grand Island, NY) and Opti-MEM (Gibco-Thermo Scientific, Carlsbad, CA), respectively. BXA (MedChem Express, Monmouth, NJ) suspension was freshly prepared and directly suspended in 0.5% (wt/vol) methylcellulose (Fujifilm, Osaka, Japan).
Recombinant IBVs (BR/08-I38, BR/08-I38T, PH/13-I38, and PH/13-I38T) were generated and rescued as described previously (20). Sequence analysis of plasmid constructs and rg-WT viruses revealed that they reflect viral sequences obtained from the Global Initiative on Sharing All Influenza Data (accession numbers: BR/08 = EPI556423 to EPI556425, EPI163725, EPI309756 to EPI309757, EPI173276, and EPI217339; PH/13 = EPI529338 to EPI529445). Viruses were propagated in MDCK cells at 33°C for 72 h in serum-free MEM containing 1.5 μg/ml of TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-treated trypsin (Worthington Biochem, Lakewood, NJ). Full-length viral genome sequences for all virus stocks were amplified and analyzed by Sanger sequencing. PA genes were further subjected to NGS analysis to confirm the genotype at PA residue 38.
Ferret inoculation, antiviral treatment, and transmission experiments.
Young male ferrets (4 to 5 months of age, weighing 750 to 1,000 g) were obtained from Triple F Farms (Sayre, PA) and verified as being seronegative for currently circulating human IAVs and IBVs. Donor ferrets (n = 3/group) were housed individually in a multilevel cage system. Under isoflurane anesthesia, donors were inoculated intranasally (0.250 ml/nostril) with 105 TCID50 of infectious virus in sterile 1× phosphate-buffered saline (PBS). The inoculum consisted of either a single virus or equal proportions (1:1) of BXA-Sen (I38) and BXA-Res (I38T) viruses based on their infectivity (5.0 × 104 TCID50/virus). At 1 dpi, donors were treated with a single dose of BXA (8 mg/kg in 0.5% methylcellulose) administered subcutaneously at four sites (0.5 ml/site) on the dorsal side of the ferret under isoflurane sedation (29). Control virus-infected, vehicle-treated ferrets received 0.5% methylcellulose. Upon recovery from sedation, a naive DC ferret was exposed to each donor, and they were cohoused together on the same day. To examine transmission via the airborne route, an AC ferret was additionally placed in a cage adjacent to that containing the donor-DC pair, separated by a double set of steel-bar dividers (7.6 cm apart). This arrangement prevented physical contact but allowed the free flow of respiratory droplets (19). The DC and AC ferrets remained exposed to the donors throughout the experiment. All animals were observed daily to determine changes in their general health condition (including any signs of lethargy and respiratory events such as sneezing, nasal discharge, or congestion), weight, and temperature (measured with subcutaneous implantable temperature transponders [Bio Medic Data Systems, Inc., Seaford, DE]) relative to baseline levels.
Nasal wash samples were collected at 2 dpi and every second day thereafter under ketamine anesthesia (25 mg/kg) administered intramuscularly. Sneezing was induced by intranasal instillation of 1 ml of sterile PBS. The infectious titers (TCID50/ml) of viruses in nasal washes and stock viruses were determined in MDCK cells (49). At 21 dpi, sera were collected, and seroconversion to a homologous challenge virus was determined by a hemagglutination inhibition assay with 0.5% turkey red blood cells. For each animal in each experimental group, the AUC values were calculated from time points that were positive for virus detection in nasal washes between 2 and 6 dpi for donor animals, 3 and 11 dpc for DC ferrets, and 3 and 17 dpc for AC ferrets. Differences in the AUCs for virus shedding were calculated and compared statistically to the comparator groups by two-way analysis of variance (ANOVA), followed by Šidák’s multiple-comparison post hoc test (using GraphPad Prism 8.0).
Deep-sequencing analysis.
Viral RNA was extracted from virus stocks and from ferret nasal washes with a QIAamp RNeasy kit (Qiagen, Valencia, CA) and used as a template for amplifying the full PA gene by single-step RT-PCR for NGS (50). Libraries of purified amplicons were prepared using a Nextera XT DNA Library Preparation kit (Illumina, San Diego, CA). The libraries were then run on the MiSeq platform, using a 2 × 250 kit for a total of approximately 500 cycles. Sequences were analyzed using CLC Genomics Workbench (CLCbio v12), and variant analysis was conducted using the quality-based basic variant detection algorithm. The variant significance threshold was set to 5% variant frequency if the sequence coverage was more than 1,000 reads at the position where the variant was found and to 10% if the coverage was between 100 and 1,000 reads.
BXA pharmacokinetic analysis in ferrets.
Young male ferrets (n = 4) received a single injection of BXA at four locations on the dorsal side (8 mg/kg in 2 ml of suspension, given at 0.5 ml/injection site/animal). Whole blood (∼0.2 ml) was collected via the jugular vein at 1, 4, 8, 24, 48, 72, 96, and 120 h after dosing in microcentrifuge tubes containing EDTA dipotassium salt dihydrate (K2EDTA-2H2O), and plasma was obtained by centrifugation at 7,000 rpm for 2 min. The plasma concentration of BXA was determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS), using an LC-20ADXR system (Shimadzu Corporation, Kyoto, Japan) and a QTRAP 5500 system (Sciex, Framingham, MA). Plasma samples were prepared by protein precipitation, and chromatographic separation was performed on a Kinetex EVO C18 column (2.6-μm particle size, 50 mm × 2.1 mm internal diameter) (Phenomenex, Torrance, CA) at room temperature. Precursor/product transitions (m/z) of 484/247 and 468/339 were monitored for BXA and an internal standard, L405930, respectively. The method qualification and bioanalytical runs all passed acceptance criteria for non-GLP assay performance. A linear model (1/X2 weighting) fitted the BXA calibrators across the 1 to 100 ng/ml range, with a correlation coefficient (R) of ≥0.9951. Sample dilution integrity was confirmed. The intrarun precision and accuracy for BXA were ≤8.42% CV and 100 to 107%, respectively.
ACKNOWLEDGMENTS
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contracts HHSN272201400006C and 75N93021C00016, and by ALSAC.
We thank Keith A. Laycock for excellent editing of the manuscript, Shivantika Bisen and Melissa Penaflor for technical assistance, and the staff of the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children’s Research Hospital for help with the next-generation sequencing.
We have no personal or financial affiliation with a commercial entity that might pose a conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson M-A, Bresee JS, Azziz-Baumgartner E, Cheng P-Y, Dawood F, Foppa I, Olsen S, Haber M, Jeffers C, MacIntyre CR, Newall AT, Wood JG, Kundi M, Popow-Kraupp T, Ahmed M, Rahman M, Marinho F, Sotomayor Proschle CV, Vergara Mallegas N, Luzhao F, Sa L, Barbosa-Ramírez J, Sanchez DM, Gomez LA, Vargas XB, Acosta Herrera A, Llanés MJ, The Global Seasonal Influenza-Associated Mortality Collaborator Network , et al. 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391:1285–1300., 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2018. Influenza (seasonal). World Health Organization, Geneva, Switzerland. http://www.who.in//ews-roo/act-sheet/etai/nfluenza-(seasonal). [Google Scholar]
- 3.Koutsakos M, Nguyen THO, Barclay WS, Kedzierska K. 2016. Knowns and unknowns of influenza B viruses. Future Microbiol 11:119–135. 10.2217/fmb.15.120. [DOI] [PubMed] [Google Scholar]
- 4.van de Sandt CE, Bodewes R, Rimmelzwaan GF, de Vries RD. 2015. Influenza B viruses: not to be discounted. Future Microbiol 10:1447–1465. 10.2217/fmb.15.65. [DOI] [PubMed] [Google Scholar]
- 5.Burnham AJ, Baranovich T, Govorkova EA. 2013. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral Res 100:520–534. 10.1016/j.antiviral.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, Deleon-Carnes M, Emery SL, Drew CP, Shieh WJ, Uyeki TM, Zaki SR. 2012. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis 205:895–905. 10.1093/infdis/jir861. [DOI] [PubMed] [Google Scholar]
- 7.Gubareva L, Mohan T. 2020. Antivirals targeting the neuraminidase. Cold Spring Harb Perspect Med 2020:a038455. 10.1101/cshperspect.a038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hirotsu N, Kashiwagi S. 2006. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003-2004 and 2004-2005 influenza seasons. Clin Infect Dis 43:439–444. 10.1086/505868. [DOI] [PubMed] [Google Scholar]
- 9.Lee N, Hui DS, Zuo Z, Ngai KLK, Lui GCY, Wo SK, Tam WWS, Chan MCW, Wong BCK, Wong RYK, Choi KW, Sin WWY, Lee ELY, Tomlinson B, Hayden FG, Chan PKS. 2013. A prospective intervention study on higher-dose oseltamivir treatment in adults hospitalized with influenza A and B infections. Clin Infect Dis 57:1511–1519. 10.1093/cid/cit597. [DOI] [PubMed] [Google Scholar]
- 10.Sugaya N, Mitamura K, Yamazaki M, Tamura D, Ichikawa M, Kimura K, Kawakami C, Kiso M, Ito M, Hatakeyama S, Kawaoka Y. 2007. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 44:197–202. 10.1086/509925. [DOI] [PubMed] [Google Scholar]
- 11.Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, St George K, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM, for the Oseltamivir-Resistance Working Group . 2009. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301:1034–1041. 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 12.Hurt AC, Ernest J, Deng YM, Iannello P, Besselaar TG, Birch C, Buchy P, Chittaganpitch M, Chiu SC, Dwyer D, Guigon A, Harrower B, Kei IP, Kok T, Lin C, McPhie K, Mohd A, Olveda R, Panayotou T, Rawlinson W, Scott L, Smith D, D’Souza H, Komadina N, Shaw R, Kelso A, Barr IG. 2009. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia, and South Africa. Antiviral Res 83:90–93. 10.1016/j.antiviral.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, Schweige B, Opp M, Paget J, van-de-Kassteele J, Hay A, Zambon M, on behalf of the European Influenza Surveillance Scheme . 2009. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg Infect Dis 15:552–560. 10.3201/eid1504.181280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A, for the Baloxavir Marboxil Investigators Group . 2018. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 379:913–923. 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 15.Noshi T, Kitano M, Taniguchi K, Yamamoto A, Omoto S, Baba K, Hashimoto T, Ishida K, Kushima Y, Hattori K, Kawai M, Yoshida R, Kobayashi M, Yoshinaga T, Sato A, Okamatsu M, Sakoda Y, Kida H, Shishido T, Naito A. 2018. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res 160:109–117. 10.1016/j.antiviral.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Ison MG, Portsmouth S, Yoshida Y, Shishido T, Mitchener M, Tsuchiya K, Uehara T, Hayden FG. 2020. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 20:1204–1214. 10.1016/S1473-3099(20)30004-9. [DOI] [PubMed] [Google Scholar]
- 17.Ikematsu H, Hayden FG, Kawaguchi K, Kinoshita M, de Jong MD, Lee N, Takashima S, Noshi T, Tsuchiya K, Uehara T. 2020. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med 383:309–320. 10.1056/NEJMoa1915341. [DOI] [PubMed] [Google Scholar]
- 18.Aschenbrenner DS. 2021. Xofluza Now Indicated to Prevent Influenza. Am J Nurs 121:26–27. 10.1097/01.NAJ.0000734116.32090.78. [DOI] [PubMed] [Google Scholar]
- 19.Genentech. 2021. Xofluza prescribing information. Genentech, South San Francisco, CA. https://www.gene.com/download/pdf/xofluza_prescribing.pdf. [Google Scholar]
- 20.Jones JC, Pascua PNQ, Fabrizio TP, Marathe BM, Seiler P, Barman S, Webby RJ, Webster RG, Govorkova EA. 2020. Influenza A and B viruses with reduced baloxavir susceptibility display attenuated in vitro fitness but retain ferret transmissibility. Proc Natl Acad Sci USA 117:8593–8601. 10.1073/pnas.1916825117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koszalka P, Tilmanis D, Roe M, Vijaykrishna D, Hurt AC. 2019. Baloxavir marboxil susceptibility of influenza viruses from the Asia-Pacific, 2012–2018. Antiviral Res 164:91–96. 10.1016/j.antiviral.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Takashita E, Morita H, Ogawa R, Nakamura K, Fujisaki S, Shirakura M, Kuwahara T, Kishida N, Watanabe S, Odagiri T. 2018. Susceptibility of influenza viruses to the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Front Microbiol 9:3026. 10.3389/fmicb.2018.03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe A, Ishida T, Hirotsu N, Kawaguchi K, Ishibashi T, Shishido T, Sato C, Portsmouth S, Tsuchiya K, Uehara T. 2019. Baloxavir marboxil in Japanese patients with seasonal influenza: dose response and virus type/subtype outcomes from a randomized phase 2 study. Antiviral Res 163:75–81. 10.1016/j.antiviral.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Omoto S, Speranzini V, Hashimoto T, Noshi T, Yamaguchi H, Kawai M, Kawaguchi K, Uehara T, Shishido T, Naito A, Cusack S. 2018. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep 8:9633. 10.1038/s41598-018-27890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ince WL, Smith FB, O’Rear JJ, Thomson MJ. 2020. Treatment-emergent influenza virus polymerase acidic substitutions independent of those at I38 associated with reduced baloxavir susceptibility and virus rebound in trials of baloxavir marboxil. J Infect Dis 222:957–961. 10.1093/infdis/jiaa164. [DOI] [PubMed] [Google Scholar]
- 26.Uehara T, Hayden FG, Kawaguchi K, Omoto S, Hurt AC, De Jong MD, Hirotsu N, Sugaya N, Lee N, Baba K, Shishido T, Tsuchiya K, Portsmouth S, Kida H. 2020. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 221:346–355. 10.1093/infdis/jiz244. [DOI] [PubMed] [Google Scholar]
- 27.Gubareva LV, Mishin VP, Patel MC, Chesnokov A, Nguyen HT, De La Cruz J, Spencer S, Campbell AP, Sinner M, Reid H, Garten R, Katz JM, Fry AM, Barnes J, Wentworth DE. 2019. Assessing baloxavir susceptibility of influenza viruses circulating in the United States during the 2016/17 and 2017/18 seasons. Euro Surveill 24:1800666. 10.2807/1560-7917.ES.2019.24.3.1800666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takashita E, Daniels RS, Fujisaki S, Gregory V, Gubareva LV, Huang W, Hurt AC, Lackenby A, Nguyen HT, Pereyaslov D, Roe M, Samaan M, Subbarao K, Tse H, Wang D, Yen HL, Zhang W, Meijer A. 2020. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2017–2018. Antiviral Res 175:104718. 10.1016/j.antiviral.2020.104718. [DOI] [PubMed] [Google Scholar]
- 29.Kitano M, Matsuzaki T, Oka R, Baba K, Noda T, Yoshida Y, Sato K, Kiyota K, Mizutare T, Yoshida R, Sato A, Kamimori H, Shishido T, Naito A. 2020. The antiviral effects of baloxavir marboxil against influenza A virus infection in ferrets. Influenza Other Respir Viruses 14:710–719. 10.1111/irv.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LYY, Zhou J, Frise R, Goldhill DH, Koszalka P, Mifsud EJ, Baba K, Noda T, Ando Y, Sato K, Yuki AI, Shishido T, Uehara T, Wildum S, Zwanziger E, Collinson N, Kuhlbusch K, Clinch B, Hurt AC, Barclay WS. 2020. Baloxavir treatment of ferrets infected with influenza A(H1N1)pdm09 virus reduces onward transmission. PLoS Pathog 16:e1008395. 10.1371/journal.ppat.1008395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh DY, Hurt AC. 2016. Using the ferret as an animal model for investigating influenza antiviral effectiveness. Front Microbiol 7:80. 10.3389/fmicb.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshimichi H, Ishibashi T, Kawaguchi N, Sato C, Kawasaki A, Wajima T. 2018. Safety, tolerability, and pharmacokinetics of the novel anti-influenza agent baloxavir marboxil in healthy adults: phase I study findings. Clin Drug Invest 38:1189–1196. 10.1007/s40261-018-0710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govorkova EA, Ilyushina NA, Marathe BM, McClaren JL, Webster RG. 2010. Competitive fitness of oseltamivir-sensitive and -resistant highly pathogenic H5N1 influenza viruses in a ferret model. J Virol 84:8042–8050. 10.1128/JVI.00689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurt AC, Nor’e SS, McCaw JM, Fryer HR, Mosse J, McLean AR, Barr IG. 2010. Assessing the viral fitness of oseltamivir-resistant influenza viruses in ferrets, using a competitive-mixtures model. J Virol 84:9427–9438. 10.1128/JVI.00373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee LY, Zhou J, Koszalka P, Frise R, Farrukee R, Baba K, Miah S, Shishido T, Galiano M, Hashimoto T, Omoto S, Uehara T, Mifsud EJ, Collinson N, Kuhlbusch K, Clinch B, Wildum S, Barclay WS, Hurt AC. 2021. Evaluating the fitness of PA/I38T-substituted influenza A viruses with reduced baloxavir susceptibility in a competitive mixtures ferret model. PLoS Pathog 17:e1009527. 10.1371/journal.ppat.1009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirotsu N, Sakaguchi H, Sato C, Ishibashi T, Baba K, Omoto S, Shishido T, Tsuchiya K, Hayden FG, Uehara T, Watanabe A. 2020. Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis 71:971–981. 10.1093/cid/ciz908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komeda T, Takazono T, Hosogaya N, Ogura E, Fujiwara M, Miyauchi H, Ajisawa Y, Iwata S, Watanabe H, Honda K, Kitanishi Y, Hara K, Mukae H. 2021. Comparison of household transmission of influenza virus from index patients treated with baloxavir marboxil or neuraminidase inhibitors: a health insurance claims database study. Clin Infect Dis 72:e859–e867. 10.1093/cid/ciaa1622. [DOI] [PubMed] [Google Scholar]
- 38.Komeda T, Takazono T, Hosogaya N, Miyazaki T, Ogura E, Iwata S, Miyauchi H, Honda K, Fujiwara M, Ajisawa Y, Watanabe H, Kitanishi Y, Hara K, Mukae H. 2020. Comparison of hospitalization incidence in influenza outpatients treated with baloxavir marboxil or neuraminidase inhibitors: a health insurance claims database study. Clin Infect Dis 2020:ciaa1870. 10.1093/cid/ciaa1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portsmouth S, Hayden FG, Kawaguchi K, Ishibashi T, Kinoshita M, Shishido T, Tsuchiya K, Uehara T. 2021. Baloxavir treatment in adolescents with acute influenza: subgroup analysis from the CAPSTONE-1 Trial. J Pediatric Infect Dis Soc 10:477–484. 10.1093/jpids/piaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes EC, Hurt AC, Dobbie Z, Clinch B, Oxford JS, Piedra PA. 2021. Understanding the impact of resistance to influenza antivirals. Clin Microbiol Rev 34:e00224-20. 10.1128/CMR.00224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takashita E, Kawakami C, Ogawa R, Morita H, Fujisaki S, Shirakura M, Miura H, Nakamura K, Kishida N, Kuwahara T, Ota A, Togashi H, Saito A, Mitamura K, Abe T, Ichikawa M, Yamazaki M, Watanabe S, Odagiri T. 2019. Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalized child without prior baloxavir treatment, Japan, January 2019. Euro Surveill 24:1900170. 10.2807/1560-7917.ES.2019.24.12.1900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashita E, Ichikawa M, Morita H, Ogawa R, Fujisaki S, Shirakura M, Miura H, Nakamura K, Kishida N, Kuwahara T, Sugawara H, Sato A, Akimoto M, Mitamura K, Abe T, Yamazaki M, Watanabe S, Hasegawa H, Odagiri T. 2019. Human-to-human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir, Japan, February 2019. Emerg Infect Dis 25:2108–2111. 10.3201/eid2511.190757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai M, Yamashita M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Kiso M, Murakami J, Yasuhara A, Takada K, Ito M, Nakajima N, Takahashi K, Lopes TJS, Dutta J, Khan Z, Kriti D, van Bakel H, Tokita A, Hagiwara H, Izumida N, Kuroki H, Nishino T, Wada N, Koga M, Adachi E, Jubishi D, Hasegawa H, Kawaoka Y. 2020. Influenza A variants with reduced susceptibility to baloxavir isolated from Japanese patients are fit and transmit through respiratory droplets. Nat Microbiol 5:27–33. 10.1038/s41564-019-0609-0. [DOI] [PubMed] [Google Scholar]
- 44.McCrone JT, Lauring AS. 2018. Genetic bottlenecks in intraspecies virus transmission. Curr Opin Virol 28:20–25. 10.1016/j.coviro.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chesnokov A, Patel MC, Mishin VP, De La Cruz JA, Lollis L, Nguyen HT, Dugan V, Wentworth DE, Gubareva LV. 2020. Replicative fitness of seasonal influenza A viruses with decreased susceptibility to baloxavir. J Infect Dis 221:367–371. 10.1093/infdis/jiz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pascua PNQ, Marathe BM, Bisen S, Webby RJ, Govorkova EA. 2020. Influenza B viruses from distinct genetic lineages are variably impaired by neuraminidase inhibitor resistance-associated substitutions. Antiviral Res 173:104669. 10.1016/j.antiviral.2019.104669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis 178:1257–1262. 10.1086/314440. [DOI] [PubMed] [Google Scholar]
- 48.Mishin VP, Hayden FG, Gubareva LV. 2005. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob Agents Chemother 49:4515–4520. 10.1128/AAC.49.11.4515-4520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed L, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 50.Zhou B, Lin X, Wang W, Halpin RA, Bera J, Stockwell TB, Barr IG, Wentworth DE. 2014. Universal influenza B virus genomic amplification facilitates sequencing, diagnostics, and reverse genetics. J Clin Microbiol 52:1330–1337. 10.1128/JCM.03265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures and tables. Download AAC.01137-21-s0001.pdf, PDF file, 2.0 MB (2MB, pdf)