ABSTRACT
Extremely drug-resistant (XDR) Acinetobacter baumannii causes challenging nosocomial infections. We report the case of a patient with XDR A. baumannii pneumonia and septic shock successfully treated with cefiderocol and a novel antibiotic obtained via expanded access protocol. With focused research and drug development efforts, the poor outcomes associated with these infections may be mitigated.
KEYWORDS: Acinetobacter baumanii, Gram-negative resistance, novel antibiotics, nosocomial infections, durlobactam, Gram-negative bacteria, antibiotic resistance, antimicrobial agents, beta-lactamases, beta-lactams, sulbactam-durlobactam
INTRODUCTION
Extremely drug-resistant (XDR) Acinetobacter baumannii is a dreaded entity which may cause nosocomial bacteremia and ventilator-associated pneumonia (VAP). These pathogens escape effective antimicrobial therapy through numerous intrinsic and acquired resistance mechanisms. The incidence of infections caused by XDR A. baumannii is increasing worldwide through adaptive selection of resistant isolates and horizontal transmission of resistance mechanisms and is facilitated by inappropriate and excessive use of broad-spectrum antimicrobials. Importantly, there are limited evidence-based therapeutic options for patients with XDR A. baumannii, leading to significant morbidity and mortality globally. Furthermore, clinicians are hindered by a present lack of national and international clinical practice guidelines for the management of XDR A. baumannii infections.
We present a case of a patient with severe COVID-19 who developed septic shock due to VAP caused by XDR A. baumannii. Given the failure of multiple antimicrobial regimens, a paucity of effective options, and impending mortality, salvage treatment with cefiderocol plus sulbactam-durlobactam was initiated through an expanded access protocol. The patient achieved subsequent clinical cure with this novel combination, the first published report to illustrate treatment with sulbactam-durlobactam.
CASE PRESENTATION
A 55-year-old female was transferred to our institution with hypoxic respiratory failure due to COVID-19 infection. Prior to hospitalization, the patient had well-controlled metabolic syndrome, including diabetes mellitus treated with metformin (A1c, 6.4%), hypertension treated with amlodipine and lisinopril, and obesity successfully treated with gastric bypass. At the time of transfer on hospital day (HD) 1, the patient had been intubated for 1 week at an outside hospital for acute respiratory distress syndrome (ARDS) and right-sided pneumothorax. For COVID-19, she had received remdesivir, dexamethasone, convalescent plasma, and prone positioning but continued to have high ventilator requirements and fraction of inspired O2 (FiO2) of 75% and positive-end expiratory pressure (PEEP) of 10 cm H2O; she was not on antibiotics. Initial evaluation otherwise revealed a critically ill patient, sedated, intubated, and mechanically ventilated, with urinary and intravascular catheters in place, as well as a right-sided thoracostomy tube. She also required vasopressor support with norepinephrine. Admission microbiologic evaluation showed normal respiratory flora growing in her sputum and negative blood and urine cultures. Legionella and pneumococcal urinary antigens were negative. Inflammatory biomarkers and acute-phase reactants, including C-reactive protein, d-dimer, ferritin, and lactate dehydrogenase, were elevated as expected for severe COVID-19.
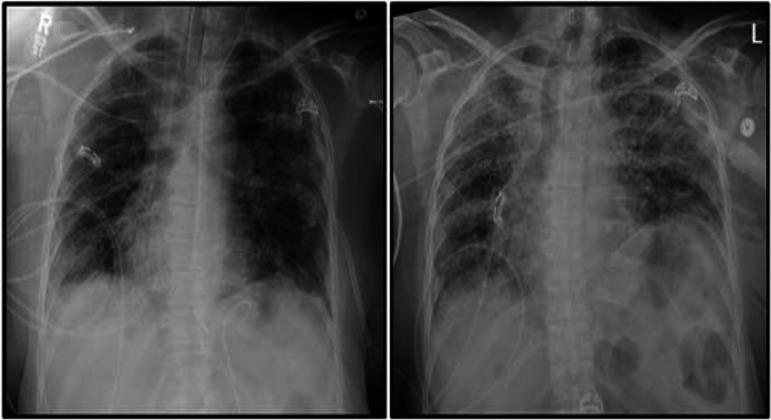
Clinically, she slowly improved over 2 weeks, culminating in extubation by HD 13. However, within 24 h, the patient clinically worsened, with refractory hypoxia requiring reintubation and hypotension necessitating vasopressor support. She had developed new, copious tracheal secretions, and repeat radiographs revealed the development of extensive bilateral interstitial opacities (Fig. 1). Accordingly, empirical antimicrobial therapy for VAP was initiated with vancomycin and meropenem. Sputum cultures, which had initially harbored only respiratory flora, now grew Acinetobacter baumannii and Pseudomonas aeruginosa. Whereas the P. aeruginosa was pan-susceptible, the A. baumannii isolate was revealed to be pan-resistant by Vitek 2 (bioMérieux SA, Marcy-l'Étoile, France). Repeat blood cultures remained negative. The patient suffered cardiac arrest on HD 15, requiring advanced cardiac life support. This was further complicated by extension of right-sided pneumothorax and development of pneumomediastinum.
FIG 1.
Chest radiographs on hospital days 2 (left) and 13 (right) showing development of multifocal ventilator-associated pneumonia during hospitalization.
CHALLENGE QUESTION
Which of the following novel antibiotics maintains activity against carbapenem-resistant Acinetobacter spp.?
A. Ceftazidime-avibactam
B. Meropenem-vaborbactam
C. Ceftolozane-tazobactam
D. Cefiderocol
TREATMENT AND OUTCOME
Three days after reintubation and beginning meropenem and vancomycin—and upon receipt of the pan-resistant A. baumannii susceptibility profile—salvage combination therapy was initiated as follows: meropenem, 2 g intravenously (i.v.) every 8 h (q8h) given as extended infusion over 3 h; high-dose ampicillin-sulbactam, 3 g i.v. q4h; and polymyxin B, 1,000,000 units i.v. q12h. However, despite this maximal regimen and aggressive pulmonary hygiene measures, there was little improvement in her septic shock or respiratory failure over the next 6 days. Her course was characterized by ongoing fevers, vasopressor requirement, near-maximal respiratory parameters, and worsening respiratory secretions, which became tenacious and tinged with blood. Despite the addition of i.v. eravacycline, 1 mg/kg q12h on HD 21, our patient made no significant improvement over the following 48 h.
At this time, given her ongoing decline and anticipated mortality, an emergency investigational new drug (EIND) application was submitted to the Food and Drug Administration (FDA) for sulbactam-durlobactam (SUL-DUR). This drug is currently in a phase 3 clinical trial that examines its efficacy and safety in patients with serious infections caused by A. baumannii-calcoaceticus complex used in combination with imipenem-cilastatin (registered at ClinicalTrials.gov as NCT03894046 (https://clinicaltrials.gov/ct2/show/NCT03894046). This β-lactam and β-lactamase inhibitor combination has demonstrated in vitro and in vivo activity against XDR A. baumannii isolates resistant to sulbactam, carbapenems, colistin, and other antibiotics (1, 2). While appropriate consents, EIND application, and expanded use protocols were being processed, on HD 23, treatment was transitioned to monotherapy with cefiderocol (CFD), 2 g i.v. q8h, which is a siderophore cephalosporin also with reported activity against XDR Acinetobacter spp. The decision for combination therapy was rooted in maximizing the probability of having at least one active agent—pending specialized, confirmatory susceptibility testing and given the nature of this ongoing severely—challenging clinical case. With EIND approval, i.v. SUL-DUR was promptly initiated (1g/1g q6h infused over 3 h) on HD 24 less than 48 h later, less than 24 h after starting cefiderocol. There was no significant change in the patient’s clinical status during the short lag period between cefiderocol and SUL-DUR initiation.
Our patient's clinical status steadily improved on treatment with cefiderocol and SUL-DUR: respiratory secretions decreased in both volume and consistency, fevers resolved, and vasopressors weaned off—all within 72 h of adding SUL-DUR. One week into treatment with CFD and SUL-DUR, further antimicrobial susceptibility data became available, with a CFD MIC of 0.5 mg/liter and SUL-DUR MIC of 4 per broth microdilution. Over the 14-day course of therapy, the patient's respiratory status became unlabored, ventilatory support decreased to minimal, and the patient underwent tracheostomy prior to discharge to a long-term acute care facility for physical therapy and reconditioning on HD 38. There were no apparent adverse effects to either SUL-DUR or CFD. She subsequently arrived home several weeks later. Five months after discharge, the patient continues to do well, walking 12,000 steps daily, and has resumed her prehospitalization routine.
Acinetobacter baumannii, part of the Acinetobacter calcoaceticus-baumannii complex, may be a cause of nosocomial infection, commonly occurring in patients with preexisting comorbidities (3). Too often, A. baumannii is a problematic pathogen due to its multitude of resistance mechanisms, intrinsic and acquired (3). Such features include the capacity for enzymatic modifications of antibiotics, efflux pumps, target site mutations, and changes in permeability (3). The most notable resistance mechanism associated with carbapenem-resistant isolates involves enzymatic hydrolysis of β-lactam antibiotics by β-lactamases, specifically class D carbapenemases of the OXA family (3). Several OXA enzymes have been identified in A. baumannii. OXA-51 is considered a weak carbapenemase, while OXA-23, OXA-40, OXA-58, OXA-143, and OXA-253 are considered more difficult to treat (3). A relative newcomer antibiotic combination, ceftazidime-avibactam, restores activity against class A, C, and D β-lactamases, rendering it useful against many other Gram-negative pathogens; however, its activity among OXA enzymes is limited to OXA-48. It thus tends to be an inadequate option when treating patients with carbapenem-resistant A. baumannii (CRAB) infections. Similarly, other extended-spectrum antimicrobials such as ceftolozane-tazobactam, imipenem-cilastatin, and meropenem-vaborbactam, restore activity against only class A and C β-lactamases. According to the Center for Diseases Control (CDC) Antibiotic Resistance Threats Report, CRAB was escalated to an “urgent” threat from the previously designated “serious” threat in 2013 (4). This change was partly due to the increasing ease of resistance transmissibility within A. baumannii and corresponding paucity of effective antimicrobials (4).
The latest Infectious Diseases Society of America (IDSA) guidelines for the management of hospital-acquired and ventilator-associated pneumonia (HAP and VAP, respectively) suggest treatment of susceptible isolates of A. baumannii with either a carbapenem or ampicillin-sulbactam (5). For more resistant isolates, the use of i.v. polymyxins is recommended if such susceptibility is preserved (5). However, there is a gap in the current guidelines for the treatment of A. baumannii resistant to both carbapenems and polymyxins. This deficiency in our pharmacologic repertoire has led to intense interest in the use of other broad-spectrum and emergency-use antimicrobials for the treatment of these infections.
As evidenced by our clinical case, one drug of interest is cefiderocol. A novel siderophore cephalosporin, cefiderocol is one of the only antimicrobials with activity against CRAB infections. Cefiderocol restores activity against all four classes of β-lactamases. Known for its unique mechanism of action, cefiderocol complexes with ferric iron to form an iron complex. It is then able to utilize a Gram-negative organism’s iron uptake mechanism and porin channels to enter the periplasmic space, where it exhibits bactericidal inhibition of cell wall synthesis through binding of penicillin-binding protein 3 (PBP3). Cefiderocol also demonstrates in vitro activity against several members of the family Enterobacterales (formerly Enterobacteriaceae), resistant strains of Pseudomonas aeruginosa, and A. baumannii isolates harboring notable class D β-lactamases, including OXA-23, OXA-24, OXA-40, OXA-51, and OXA-58 (6). An accumulating body of evidence indicates a role for cefiderocol in the treatment of highly resistant Gram-negative bacteria, including those caused by carbapenem-resistant A. baumannii. Three fairly recent reports—including two prospective, randomized, controlled trials (CREDIBLE-CR and APEKS-NP)—suggest that cefiderocol may be a viable option specifically for invasive infections caused by such pathogens, particularly when other antimicrobials may be unavailable due to resistance (7–9). The drug may not be a panacea, however, considering the increased all-cause mortality observed in at least one study, including those infections caused by A. baumannii (7).
A promising investigational treatment option for CRAB infection is sulbactam-durlobactam (also known as ETX2514SUL). Sulbactam is a cell wall synthesis inhibitor, potently inhibiting essential penicillin-binding proteins in Acinetobacter spp. and thus leading to cell death. The degradation of sulbactam by a variety of β-lactamases present in contemporary Acinetobacter spp. clinical isolates, however, limits its efficacy (10–12). Durlobactam is a non-β-lactam β-lactamase inhibitor with an extended spectrum of activity compared to other β-lactamase inhibitors currently on the market. Most notably, durlobactam is a potent inhibitor of class D carbapenemases of the OXA family, which are prevalent in A. baumannii, including our aforementioned clinical isolate. In both in vitro studies and in vivo studies of animal models of infection, durlobactam effectively protects sulbactam from hydrolysis by β-lactamases, restoring activity against XDR A. baumannii (1). Sulbactam-durlobactam is currently being investigated exclusively in combination with imipenem-cilastatin in the ATTACK trial. A multinational, pathogen-targeted, randomized, active-controlled, comparator-controlled phase 3 study, ATTACK is evaluating the efficacy and safety of an SUL-DUR-based regimen in patients for the treatment of bloodstream infections (BSI) or hospital-associated or ventilator-associated bacterial pneumonia (HABP and VABP, respectively) due to A. baumannii-calcoaceticus complex (registered at ClinicalTrials.gov under the identifier NCT03894046) (https://clinicaltrials.gov/ct2/show/NCT03894046).
Whole-genome sequencing analysis of the A. baumannii isolate from this patient revealed that the isolate belongs to sequence type 2 (ST2) (Institute Pasteur scheme), which has been associated with global spread of carbapenem resistance (13). In addition to the presence of macrolide [Mph(E)], aminoglycoside [ArmA, AadA1, APH(6)-Id, and APH(3′)], tetracycline (TetB), chloramphenicol (CatB), and sulfonamide (Sul1) resistance determinants, this isolate was found to encode genes for ADC-30, OXA-66, and OXA-72 β-lactamases, which correlate with the carbapenem resistance observed for this isolate. No specific mutation was identified to explain the observed colistin resistance. In vitro susceptibility to SUL-DUR was also determined. The MIC for sulbactam alone was 32 mg/liter, while the SUL-DUR MIC was 4 mg/liter. The proposed breakpoint for SUL-DUR susceptibility is ≤4 mg/liter, suggesting that SUL-DUR would be efficacious in the treatment of this clinical isolate (2). Due to the concomitant dosing of cefiderocol, it is impossible to know which agent was the primary driver of microbial eradication; however, it is possible that durlobactam, in addition to providing protection to sulbactam, was also able to enhance the activity of cefiderocol. However, there is no current proposed synergistic mechanism to explain this phenomenon beyond the known β-lactamase activity of durlobactam.
Given the ever-growing threat of antimicrobial resistance, we believe that the novel combination of sulbactam-durlobactam holds immense promise as an optimized treatment for XDR Acinetobacter baumannii infections. We look forward to additional forthcoming clinical data that will better characterize this agent’s potential place in modern antimicrobial therapy.
Data availability.
This whole-genome shotgun project has been deposited at DDBJ/ENA/GenBank under accession number JAHXCA000000000. The version described in this paper is version JAHXCA010000000.
ACKNOWLEDGMENTS
We extend a sincere thank you to Entasis Therapeutics for supplying sulbactam-durlobactam through their expanded access program and to Samir Moussa of Entasis Therapeutics, who performed sulbactam-durlobactam susceptibility testing and whole-genome sequencing of this A. baumannii clinical isolate. A final thanks to David Altarac, Chief Medical Officer of Entasis Therapeutics, for without his support and guidance, this successful patient outcome nor manuscript likely would have been impossible.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. Expert clinicians then provide a commentary on the case.
REFERENCES
- 1.Durand-Réville TF, Guler S, Comita-Prevoir J, Chen B, Bifulco N, Huynh H, Lahiri S, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Velez-Vega C, Olivier NB, McLaughlin R, Gao N, Thresher J, Palmer T, Andrews B, Giacobbe RA, Newman JV, Ehmann DE, de Jonge B, O'Donnell J, Mueller JP, Tommasi RA, Miller AA. 2017. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2:17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 2.McLeod SM, Moussa SH, Hackel MA, Miller AA. 2020. In vitro activity of sulbactam-durlobactam against Acinetobacter baumannii-calcoaceticus complex isolates collected globally in 2016 and 2017. Antimicrob Agents Chemother 64:e02534-19. doi: 10.1128/AAC.02534-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez MS, Bonomo RA, Tolmasky ME. 2020. Carbapenemases: transforming Acinetobacter baumanii into a yet more dangerous menace. Biomolecules 10:720. doi: 10.3390/biom10050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 5.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Jr., Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shionogi & Co., Ltd. 2019. Cefiderocol package insert. Shionogi & Co., Ltd, Florham Park, NJ. [Google Scholar]
- 7.Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, Portsmouth S, Torre-Cisneros J, Toyoizumi K, Wunderink RG, Nagata TD. 2021. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 8.Falcone M, Tiseo G, Nicastro M, Leonildi A, Vecchione A, Casella C, Forfori F, Malacarne P, Guarracino F, Barnini S, Menichetti F. 2021. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant Gram-negative infections in intensive care unit patients. Clin Infect Dis 72:2021–2024. doi: 10.1093/cid/ciaa1410. [DOI] [PubMed] [Google Scholar]
- 9.Wunderink RG, Matsunaga Y, Ariyasu M, Clevenbergh P, Echols R, Kaye KS, Kollef M, Menon A, Pogue JM, Shorr AF, Timsit J-F, Zeitlinger M, Nagata TD. 2021. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 21:213–225. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 10.Durand-Réville TF, Guler S, Comita-Prevoir J, Chen B, Bifulco N, Huynh H, Lahiri S, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Velez-Vega C, Olivier NB, McLaughlin R, Gao N, Thresher J, Palmer T, Andrews B, Giacobbe RA, Newman JV, Ehmann DE, de Jonge B, O'Donnell J, Mueller JP, Tommasi RA, Miller AA. 2017. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2:17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 11.Kuo S-C, Lee Y-T, Yang Lauderdale T-L, Huang W-C, Chuang M-F, Chen C-P, Su S-C, Lee K-R, Chen T-L. 2015. Contribution of Acinetobacter-derived cephalosporinase-30 to sulbactam resistance in Acinetobacter baumannii. Front Microbiol 6:231. doi: 10.3389/fmicb.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance—treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamidian M, Nigro SJ. 2019. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom 5:e000306. doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This whole-genome shotgun project has been deposited at DDBJ/ENA/GenBank under accession number JAHXCA000000000. The version described in this paper is version JAHXCA010000000.