ABSTRACT
The Burkholderia cepacia complex (Bcc) and Burkholderia gladioli are opportunistic pathogens that most commonly infect persons with cystic fibrosis or compromised immune systems. Members of the Burkholderia genus are intrinsically multidrug resistant (MDR), possessing both a PenA carbapenemase and an AmpC β-lactamase, rendering treatment of infections due to these species problematic. Here, we tested the β-lactam–β-lactamase inhibitor combination imipenem-relebactam against a panel of MDR Bcc and B. gladioli strains. The addition of relebactam to imipenem dramatically lowered the MICs for Bcc and B. gladioli: only 16% of isolates tested susceptible to imipenem, while 71.3% were susceptible to the imipenem-relebactam combination. While ceftazidime-avibactam remained the most potent combination drug against this panel of Bcc and B. gladioli strains, imipenem-relebactam was active against 71.4% of the ceftazidime-avibactam-resistant isolates. Relebactam demonstrated potent inactivation of Burkholderia multivorans PenA1, with an apparent Ki (Ki app) value of 3.2 μM. Timed mass spectrometry revealed that PenA1 formed a very stable adduct with relebactam, without any detectable desulfation for as long as 24 h. Based on our results, imipenem-relebactam may represent an alternative salvage therapy for Bcc and B. gladioli infections, especially in cases where the isolates are resistant to ceftazidime-avibactam.
KEYWORDS: β-lactamases, Burkholderia, β-lactam, PenA, carbapenemase, β-lactamase inhibitor, imipenem, relebactam, antibiotic resistance, avibactam, ceftazidime
INTRODUCTION
Burkholderia spp. are Gram-negative bacteria that can infect humans, animals, and/or plants (1, 2). Major human pathogens include species within the Burkholderia cepacia complex (Bcc) and Burkholderia gladioli, which can cause chronic infections in persons with cystic fibrosis (CF), chronic granulomatous disease, or immunodeficiency. The Bcc includes >20 closely related species that are opportunistic and often of nosocomial origin (2). Infections caused by Bcc and B. gladioli are difficult to treat due to inherent antimicrobial drug resistance (e.g., polymyxin resistance). β-Lactam antibiotics, such as meropenem and ceftazidime, are often used to treat infections caused by Bcc and B. gladioli.
β-Lactams are the largest, most prescribed class of antibiotics and inhibit the transpeptidase domains of penicillin-binding proteins (PBPs), leading to altered cell wall metabolism and eventual bacterial cell death. The most common β-lactam resistance mechanism in Gram-negative bacteria is the production of β-lactamases, which hydrolyze the amide bonds of β-lactams, thus inactivating them and preventing them from inhibiting their PBP targets. β-Lactamases are structurally grouped into four classes: A, B, C, and D. Class A, C, and D enzymes possess a serine nucleophile, and class B β-lactamases require Zn2+ for catalysis. Within these classes, each β-lactamase possesses a unique spectrum of activity toward penicillins, cephalosporins, monobactams, and carbapenems (3). The conventional approach to circumventing β-lactamases is the addition of a β-lactamase inhibitor to a β-lactam; thus, the β-lactamase is inhibited, and the β-lactam is able to inactivate the PBP target (4, 5). Much as with β-lactams, the inhibition profiles of β-lactamase inhibitors differ across β-lactamase classes, and in some cases, β-lactamase inhibitors are substrates for β-lactamases (e.g., KPC-2 versus clavulanic acid) (3, 6).
Bcc and B. gladioli express two chromosomally encoded inducible β-lactamases: a class A and a class C β-lactamase (7–10). PenR, a LysR-type transcriptional regulator (LTTR), regulates the expression of these β-lactamase genes (bla) in response to β-lactam exposure (7, 10, 11). PenA1, the class A β-lactamase of Burkholderia multivorans ATCC 17616, possesses a very broad hydrolytic spectrum (e.g., ampicillin, ceftazidime, aztreonam, imipenem, clavulanic acid, sulbactam) (12). The class C β-lactamase of B. multivorans ATCC 17616, AmpC1, contributes only minimally to β-lactam resistance due to its poor hydrolytic activity (10). The PenA-like and AmpC-like β-lactamases of Bcc and B. gladioli have different β-lactam hydrolysis spectra, likely due to heterogeneity in their primary amino acid sequences (7, 10, 12, 13).
Clavulanic acid, sulbactam, and tazobactam were the first β-lactamase inhibitors approved for clinical use in the 1980s to 1990s and were partnered with various β-lactam antibiotics (e.g., amoxicillin, piperacillin) (5). B. multivorans PenA1 is able to hydrolyze clavulanic acid, sulbactam, and tazobactam, and cells producing blapenA1 test nonsusceptible to these historic β-lactam–β-lactamase inhibitor combinations (12). In the past decade, a resurgence has occurred in the β-lactamase inhibitor field with the advent of diazabicyclooctanes (DBOs) and boronates (4, 14). Ceftazidime-avibactam was the first contemporary β-lactam–β-lactamase inhibitor combination to be approved in the United States by the Food and Drug Administration (FDA), in 2015. Avibactam is a novel bridged DBO β-lactamase inhibitor with a non-β-lactam scaffold that possesses inhibitory activity toward class A, class C, and some class D β-lactamases (15). Avibactam has been shown to inactivate PenA1 (16), restore susceptibility to ceftazidime when tested in vitro in combination against multidrug-resistant (MDR) Bcc and B. gladioli, and successfully treat patients (16–25). However, several studies using the ceftazidime breakpoints established by the Clinical and Laboratory Standards Institute (CLSI) have revealed that some clinical isolates of Bcc and B. gladioli (10 to 46% of Bcc and 76% of B. gladioli isolates) are nonsusceptible to ceftazidime-avibactam (16, 19, 24, 26–30). Thus, combating Bcc and B. gladioli with alternative regimens remains a critical undertaking.
In 2019, the FDA approved another β-lactam–β-lactamase combination, imipenem/cilastatin-relebactam. Imipenem is a carbapenem β-lactam partner, as opposed to ceftazidime, which is the cephalosporin partner of avibactam. The cilastatin inhibits human renal dehydropeptidase, which would otherwise degrade imipenem. Relebactam possesses the same core DBO scaffold as avibactam with a piperidine side chain and demonstrates inhibitory activity toward class A and class C β-lactamases (31–33). Imipenem/cilastatin-relebactam is indicated for adult use in treating complicated intra-abdominal and urinary tract infections, including pyelonephritis, as well as hospital-acquired and ventilator-acquired bacterial pneumonia (34). Imipenem/cilastatin-relebactam is designated for the treatment of a variety of susceptible Gram-negative pathogens (e.g., Enterobacterales and Pseudomonas aeruginosa) (34). In this study, the in vitro antimicrobial activity of imipenem-relebactam was compared to those of imipenem, ceftazidime, and ceftazidime-avibactam against a challenge panel of Bcc and B. gladioli strains. Moreover, the inhibitory power of relebactam against PenA1 and AmpC1 was evaluated.
RESULTS
Imipenem-relebactam is more potent than ceftazidime alone and is effective against ceftazidime-avibactam-resistant strains.
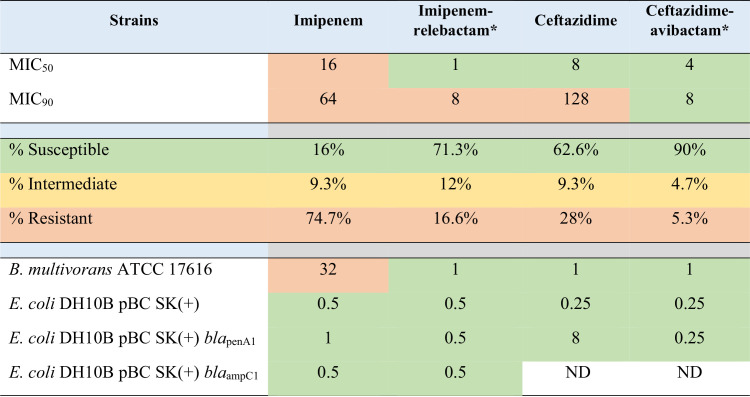
Clinical isolates of Bcc (i.e., Burkholderia ambifaria, B. arboris, B. cenocepacia, B. cepacia, B. contaminans, B. diffusa, B. dolosa, B. multivorans, B. pseudomultivorans, B. pyrrocinia, B. seminalis, B. stabilis, B. ubonensis, and B. vietnamiensis) and B. gladioli were subjected to antimicrobial susceptibility testing using agar dilution methodology against imipenem and imipenem-relebactam. B. multivorans ATCC 17616 was tested on every MIC panel to control for variability between experiments. A total of 15 independent experiments were conducted to test 150 strains (Table 1 and Fig. 1; see also Table S1 in the supplemental material).
TABLE 1.
In vitro activities of imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam against150 Bcc and B. gladioli strains and controlsa
Imipenem breakpoints (susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; resistant, ≥8 μg/ml) for P. aeruginosa were used to assign phenotypes for imipenem and the combination with relebactam. Ceftazidime breakpoints (susceptible, ≤8 μg/ml; intermediate, 16 μg/ml; resistant, ≥32 μg/ml) for Bcc were used to assign phenotypes for ceftazidime and the combination with avibactam. Categories are color coded as follows: green, susceptible; yellow, intermediate; orange, resistant. ND, not determined.
Relebactam and avibactam were tested at a fixed concentration of 4 μg/ml.
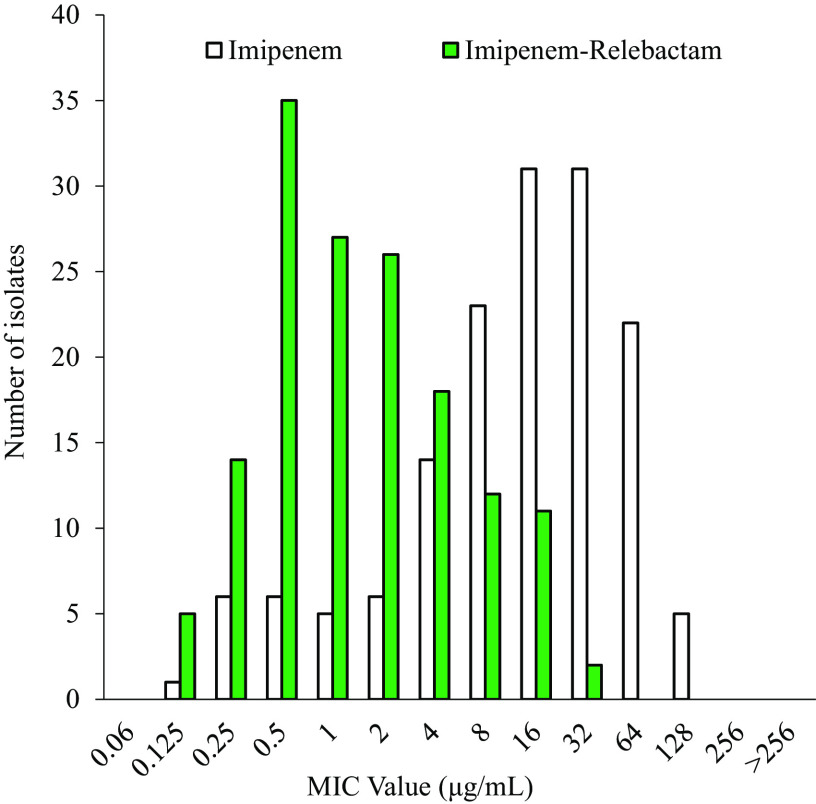
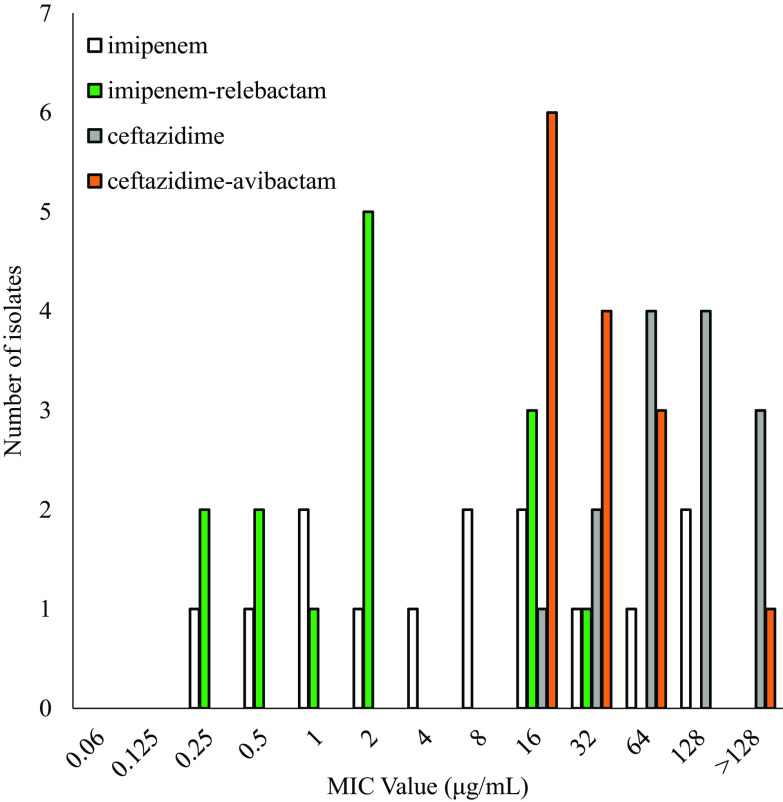
FIG 1.
In vitro activity of imipenem alone (white bars) compared to that of imipenem combined with relebactam (green bars).
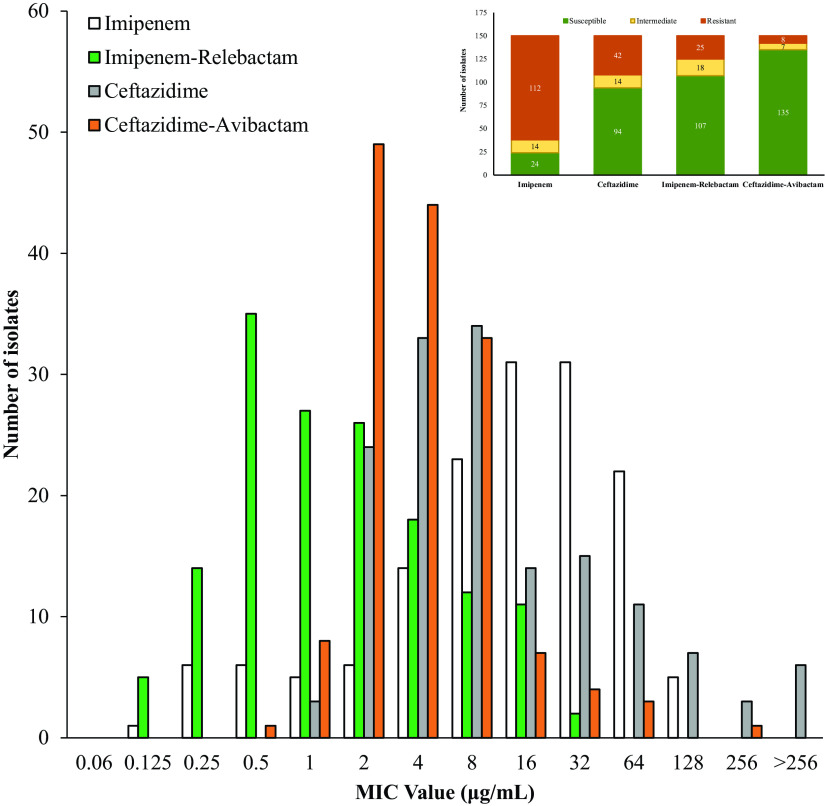
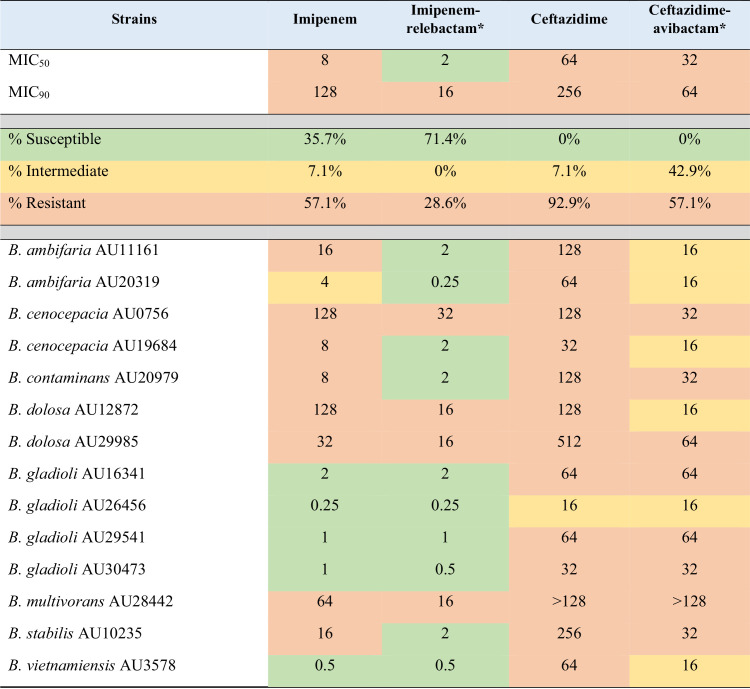
The addition of relebactam to imipenem dramatically lowered the MICs for Bcc and B. gladioli: only 16% of isolates tested susceptible to imipenem, while 71.3% were susceptible to the imipenem-relebactam combination (Table 1 and Fig. 1). Moreover, imipenem-relebactam lowered MICs greatly from those of ceftazidime, a first-line treatment agent for Burkholderia sp. infections, since only 62.6% of isolates were susceptible to ceftazidime (Table 1). Adding avibactam to ceftazidime increased susceptibility to 90% (Table 1). Thus, ceftazidime-avibactam was comparatively more potent against this panel of isolates (Fig. 2). However, imipenem-relebactam was effective against 71.4% of 14 ceftazidime-avibactam-resistant Bcc and B. gladioli clinical isolates (Table 2 and Fig. 3). Only 4 of the 14 isolates (B. cenocepacia AU0756, B. dolosa AU12872, B. dolosa AU29985, and B. multivorans AU28442) were resistant to all of the agents tested (Table 2 and Fig. 3).
FIG 2.
In vitro activity comparison for imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam. (Inset) Numbers of isolates resistant (orange), intermediate (yellow), or susceptible (green) to imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam.
TABLE 2.
In vitro activities against Burkholderia sp. isolates with ceftazidime and ceftazidime-avibactam MICs of >8 μg/mla
Imipenem breakpoints (susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; resistant, ≥8 μg/ml) for P. aeruginosa were used to assign phenotypes for imipenem and the combination with relebactam. Ceftazidime breakpoints (susceptible, ≤8 μg/ml; intermediate, 16 μg/ml; resistant, ≥32 μg/ml) for B. cepacia were used to assign phenotypes for ceftazidime and the combination with avibactam. Categories are color coded as follows: green, susceptible; yellow, intermediate; orange, resistant.
Relebactam and avibactam were tested at a fixed concentration of 4 μg/ml. The ceftazidime and ceftazidime-avibactam data presented here were obtained previously as part of another study (16).
FIG 3.
Comparison of in vitro activities of imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam against 14 strains with ceftazidime and ceftazidime-avibactam MICs of >8 μg/ml.
To determine if susceptibility patterns are influenced by species, a comparison was conducted. B. gladioli and B. vietnamiensis isolates were more susceptible to imipenem, at 100% and 70%, respectively, than the other Burkholderia species that were tested. The addition of relebactam increased susceptibility to 100% for the B. vietnamiensis strains (Table S2). However, the sample size of this subanalysis is small, so generalizations on potency versus species cannot be made at this time.
Relebactam inactivates the PenA1 carbapenemase, while imipenem inhibits AmpC1.
Relebactam demonstrated potent inactivation of B. multivorans PenA1, with an apparent Ki (Ki app) value of 3.2 μM, which is comparable to that of avibactam (Ki app = 0.5 μM) (Table 3) (16). The acylation rate (k2/K) was nearly 300-fold lower for relebactam than for avibactam, while the koff rate for relebactam was 7-fold higher than that for avibactam. The off-rate is evident even when one measures the on-rate as the final steady-state velocities for the progress curves with relebactam sloping upward instead of plateauing (data not shown).
TABLE 3.
Steady-state inhibitor kinetic values against B. multivorans PenA1a
| Parameter (unit) | Value for: |
|
|---|---|---|
| Relebactam | Avibactamb | |
| K i app | 3.2 ± 0.5 μM | 500 ± 50 nM |
| k2/K (M−1 s−1) | (7.2 ± 2.0) × 103 | (2 ± 1) × 106 |
| koff (s−1) | (1.4 ± 0.5) × 10−2 | (2 ± 1) × 10−3 |
| t1/2 (min) | 0.83 | 5.8 |
| tn (15 min)c | 30 | 1 |
Individual data points were collected in triplicate, while all experiments were completed at a minimum in duplicate.
Data from reference 16.
tn (15 min), turnover number at 15 min.
Relebactam was a poor inhibitor of B. multivorans AmpC1, with a Ki app value of >800 μM; similarly, avibactam does not inhibit AmpC1, with a Ki app value of >600 μM (10). Conversely, imipenem demonstrated measurable inhibition of AmpC1, with a Ki app value of 13 ± 1 μM.
Relebactam forms a stable adduct with PenA1 and AmpC1.
To discern any changes to relebactam during its reaction with PenA1 or AmpC1, timed mass spectrometry of PenA1 or AmpC1 incubated with relebactam was conducted. The molecular masses of PenA1 and AmpC1 alone were 29,412 ± 5 Da and 39,750 ± 5 Da, respectively (Fig. 4). By examining the reaction course of PenA1 and relebactam at a 1:1 ratio at 1 min, 15 min, 60 min, and 24 h, a single major peak of 29,761 ± 5 Da was observed, corresponding to the addition of 349 Da, essentially identical to relebactam’s molecular mass of 348.38 Da (Fig. 4). Thus, PenA1 formed a very stable adduct with relebactam, without any detectable desulfation under the conditions tested (Fig. 4). Despite the high Ki app values for AmpC1 and relebactam, by increasing the amount of relebactam relative to that of AmpC1 (1:100), an AmpC1–relebactam complex was obtained as early as 15 min and remained stable at 24 h (Fig. 4).
FIG 4.
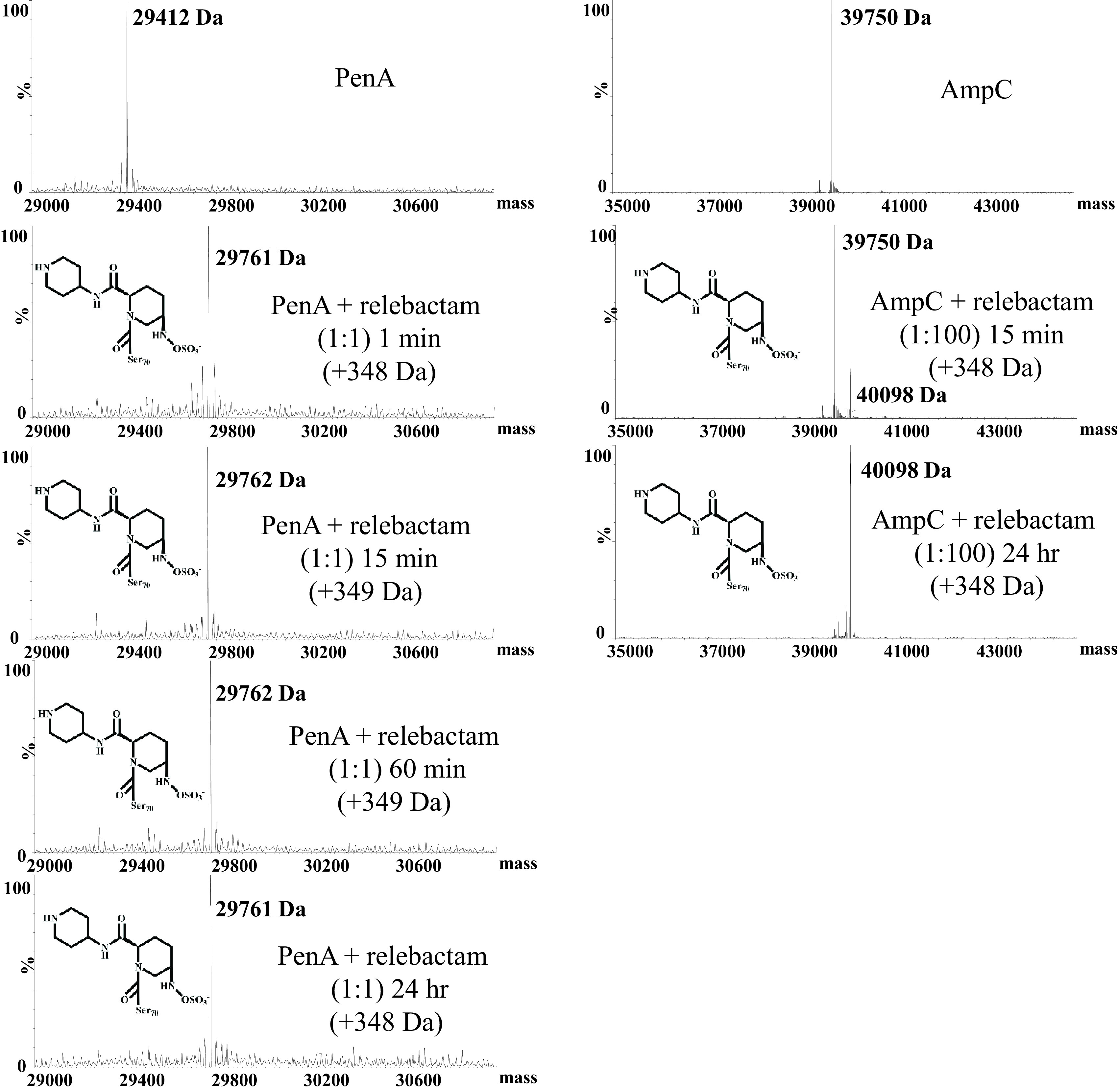
Timed mass spectrometry of PenA1 and AmpC1 with relebactam.
Imipenem-relebactam maintains induction of blapenA1, while relebactam alone does not influence expression.
Imipenem is a known inducer of blapenA1. In order to determine the effects of imipenem-relebactam and relebactam on the expression of blapenA1 and blaampC1, B. multivorans ATCC 17616 cells were grown and exposed to sub-MICs of the test agents for 1 h. The cells were lysed and subjected to immunoblotting with an anti-PenA1 peptide antibody and an anti-AmpC1 antibody in order to detect the PenA1 and AmpC1 β-lactamases, respectively. An anti-RecA antibody was used as a loading control. Induction of blapenA1 was observed upon exposure to imipenem or imipenem-relebactam, but not upon exposure to relebactam alone (Fig. 5). Induction of blaampC1 was not detected under any of the conditions (Fig. 5).
FIG 5.
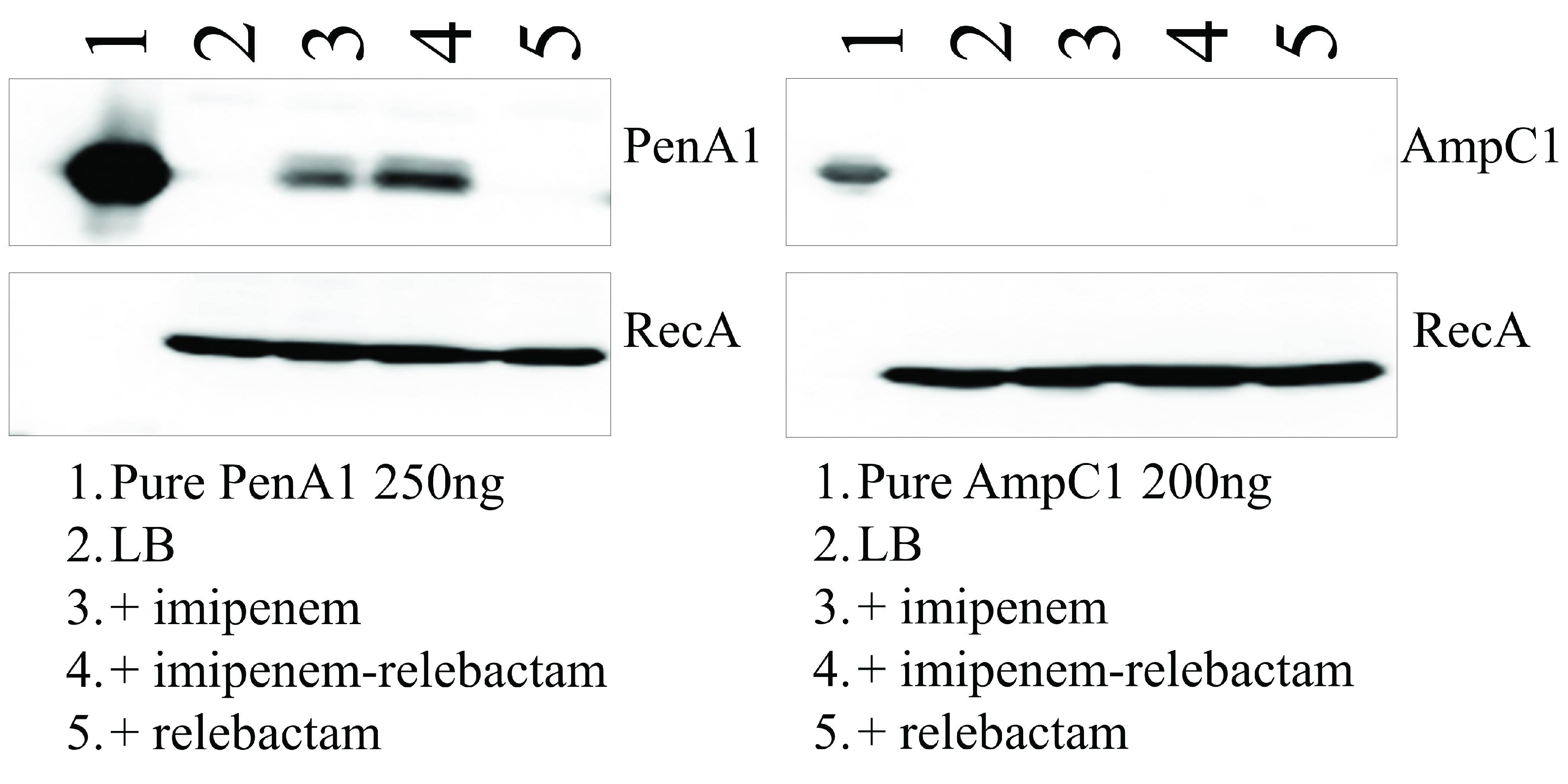
Immunoblotting for PenA1 and AmpC1 following induction by imipenem (1 μg/ml), imipenem-relebactam (1 μg/ml-4 μg/ml), or relebactam (4 μg/ml) using B. multivorans ATCC 17616. RecA was used as a control.
DISCUSSION
By use of agar dilution susceptibility testing, the activity of imipenem-relebactam was evaluated against a challenge panel of Bcc and B. gladioli strains. The addition of relebactam lowered imipenem MICs overall, with only 16.6% of isolates remaining resistant. Imipenem-relebactam was superior to the first-line agent ceftazidime, which is commonly used to treat Burkholderia sp. infections. Ceftazidime-avibactam outperformed imipenem-relebactam overall. However, against ceftazidime-avibactam-resistant isolates, imipenem-relebactam demonstrated potent activity, with 71.4% testing susceptible. Unlike relebactam alone, imipenem and imipenem-relebactam are able to induce the expression of blapenA1. Furthermore, the kinetics with PenA1 and relebactam revealed that despite inhibition of the enzyme, the on-rate was lower and the off-rate was higher, which, along with the induction of blapenA1, may explain why imipenem-relebactam is not as potent against Bcc as ceftazidime-avibactam. Moreover, despite the lack of inhibitory activity against AmpC1, once relebactam formed a complex with AmpC1, the complex was stable for as long as 24 h. In summary, imipenem-relebactam represents a potential alternative therapy for infections caused by Bcc and B. gladioli, especially in cases where the isolates are resistant to ceftazidime-avibactam.
MATERIALS AND METHODS
Antibiotics and reagents.
Imipenem and relebactam were provided by Merck. Nitrocefin was purchased from Thermo Scientific Oxoid. The data reported for ceftazidime and avibactam were from a previous study (16).
Bacterial strains.
A total of 150 MDR clinical strains, including 140 Bcc (14 different species) and 10 B. gladioli strains, were obtained from the Burkholderia cepacia Research Laboratory and Repository (University of Michigan) (7, 16, 35). These 150 isolates were recovered from respiratory specimens from 150 individuals with CF receiving care in 68 cities in 36 states in the United States. Each Bcc isolate was identified to the species level by using species-specific PCR, recA restriction fragment length polymorphism (RFLP), and/or DNA sequencing of the recA gene (36, 37). If the species could not be determined, the isolate is listed as Bcc Indeterminate.
In vitro susceptibility test methods.
MICs for the bacterial isolates were determined by the cation-adjusted Mueller-Hinton (MH) agar dilution method. The MIC measurements were performed by using a Steers Replicator that delivered 10 μl at 104 CFU of overnight culture grown in MH broth at 37°C and diluting in fresh MH broth. Relebactam was tested at 4 μg/ml in combination with increasing concentrations of imipenem. MIC results were interpreted using CLSI breakpoints, where available (30).
Enzyme expression, purification, and steady-state kinetics.
E. coli DE3 Origami 2 cells carrying pGEX-6p2 blapenA1 and pGEX-6p2-blaampC1 were used for protein expression and purification, as described previously (10, 12). Briefly, cells were grown in Super Optimal Broth, and then isopropyl-β-d-1-thiogalactopyranoside was added to induce expression. Cells were pelleted and were frozen at −20°C. Subsequently, the cells were lysed, and the β-lactamase was purified and verified by electrospray ionization mass spectrometry, as described previously (10, 12). Kinetic parameters were obtained as described below; individual data points were obtained in triplicate, while each experiment was conducted at least in duplicate.
Kinetic parameters for PenA1 and AmpC1 were obtained using an Agilent 8453 Diode Array spectrophotometer in 10 mM phosphate-buffered saline, pH 7.4 (PBS), at room temperature using previously described methods (15, 38).
The apparent Ki (Ki app) value was determined for PenA1 and AmpC1 using a direct competition assay under steady-state conditions. PenA1 and AmpC1 were both maintained at 10 nM, while the relebactam concentration was varied. Nitrocefin was used as the reporter substrate at a fixed concentration of 100 μM. The β-lactamase, relebactam, and nitrocefin were mixed manually, and the initial reaction velocity was monitored. Data were linearized by plotting the inverse initial reaction velocities (1/v0) versus inhibitor concentration (I). Ki app was determined by dividing the value for the y-intercept by the slope of the line and accounting for the use of nitrocefin as a reporter.
To obtain the acylation rate (k2/K), progress curves were obtained by mixing PenA1 at 5 nM with increasing concentrations of relebactam and using nitrocefin at 100 μM as a reporter substrate. Progress curves were fit to the following equation to obtain observed rate constant (kobs) values:
Here, vf is final velocity, v0 is initial velocity, and A0 is initial absorbance at a λ of 482 nm. The data were plotted as kobs versus [relebactam]. The k2/K value was obtained by correcting the value obtained for the slope of the line for the use of nitrocefin as an indicator substrate.
The off-rate (koff) of relebactam for PenA1 was determined by incubating 5 μM PenA1 with 25 μM relebactam for 5 min at room temperature, diluting the mixture to 1:10,000, and adding 100 μM nitrocefin. Progress curves measuring nitrocefin hydrolysis were collected for 1 h, and the data were fit to the equation given above to obtain koff. PenA1 alone and nitrocefin alone were used as controls.
Timed mass spectrometry.
The masses of intact PenA1 and AmpC1 with and without relebactam were measured by mass spectrometry on a Waters Synapt G2-Si quadrupole time-of-flight mass spectrometer. The Synapt G2-Si mass spectrometer was calibrated with a sodium iodide solution using a 50-to-2,000 m/z mass range. PenA1 at 10 μM was incubated with 10 μM relebactam at a 1:1 ratio for 15 min, 1 h, and 24 h in PBS. PenA1 and AmpC1 were also incubated alone as controls. AmpC1 at 10 μM was incubated with 1,000 μM relebactam at a 1:100 ratio for 15 min and 24 h in PBS. At the desired time points, the reactions were terminated by the addition of a final concentration of 0.1% formic acid and 1% acetonitrile. The samples were run using a Waters Acquity H class ultraperformance liquid chromatograph (UPLC) and an Acquity UPLC BEH C18 column (particle size, 1.7 μm; inside diameter, 2.1 mm; length, 100 mm). The aqueous phase consisted of 0.1% (vol/vol) formic acid in water (A), and the organic phase was made up of acetonitrile with 0.1% formic acid (B); the flow rate was maintained at 0.5 ml/min. Initial parameters consisted of 90% A and 10% B. At 1 min, a gradient of 19% A and 81% B was started, and it lasted until min 4. At 4 min, the gradient was adjusted to 15% A and 85% B and was maintained for 30 s. Thereafter, the gradient was adjusted to 10% A and 90% B for 30 s, and by min 5, the initial conditions were reinitiated. The tune settings were as follows: capillary voltage at 3.5 kV, sampling cone at 35, source offset at 35, source temperature at 100°C, desolvation temperature at 500°C, cone gas at 100 liters/h, desolvation gas at 800 liters/h, and nebulizer bar at 6.0. The spectra were analyzed using MassLynx, v4.1, and were deconvoluted using the MaxEnt1 program.
Immunoblotting.
B. multivorans ATCC 17616 was grown in lysogeny broth to log phase at an optical density at 600 nm (OD600) between 0.6 and 0.7. The cells were treated with sub-MICs of imipenem (1 μg/ml) or imipenem-relebactam (0.5 μg/ml to 4 μg/ml) for 1 h. Subsequently, the cells were pelleted and lysed to prepare crude extracts, as described previously (39). These crude extracts, as well as purified full-length PenA protein, were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% (wt/vol) nonfat dry milk in 20 mM Tris-Cl with 150 mM NaCl (pH 7.4) (TBS) for 1 h and were probed in 5% nonfat dry milk in TBS with 1 μg/ml of a polyclonal anti-PenA peptide antibody or anti-AmpC antibody and 1 μg/ml of a polyclonal anti-RecA antibody. All antibodies were raised in rabbits by New England Peptide using a selected PenA 18-amino-acid peptide, the AmpC protein, and the RecA protein as the antigens, respectively (7, 10). The membranes were washed five times for 10 min with TBS with 0.05% Tween 20 (TBST). For protein detection, blots were incubated for 1 h with a 1:5,000 dilution of a horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody in 5% nonfat dry milk in TBS. The blots were washed five times for 10 min with TBST and were developed using the ECL-Plus developing kit (GE Healthcare Life Sciences) according to the manufacturer’s instructions. A Fotodyne Luminary/FX system was used to capture images.
ACKNOWLEDGMENTS
The research reported in this publication was supported by the Merck Investigator Studies Program (award 58726). Moreover, research was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs and the Veterans Affairs Merit Review Program (BX002872; to K.M.P.-W.) from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government. These studies were also partly supported by grants from the Cystic Fibrosis Foundation to J.J.L. and K.M.P.-W.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Vial L, Chapalain A, Groleau MC, Deziel E. 2011. The various lifestyles of the Burkholderia cepacia complex species: a tribute to adaptation. Environ Microbiol 13:1–12. 10.1111/j.1462-2920.2010.02343.x. [DOI] [PubMed] [Google Scholar]
- 2.Gautam V, Singhal L, Ray P. 2011. Burkholderia cepacia complex: beyond Pseudomonas and Acinetobacter. Indian J Med Microbiol 29:4–12. 10.4103/0255-0857.76516. [DOI] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 54:969–976. 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp-Wallace KM. 2019. The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections. Expert Opin Pharmacother 20:2169–2184. 10.1080/14656566.2019.1660772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drawz SM, Bonomo RA. 2010. Three decades of β-lactamase inhibitors. Clin Microbiol Rev 23:160–201. 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp-Wallace KM, Bethel CR, Distler AM, Kasuboski C, Taracila M, Bonomo RA. 2010. Inhibitor resistance in the KPC-2 β-lactamase, a preeminent property of this class A β-lactamase. Antimicrob Agents Chemother 54:890–897. 10.1128/AAC.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becka SA, Zeiser ET, Marshall SH, Gatta JA, Nguyen K, Singh I, Greco C, Sutton GG, Fouts DE, LiPuma JJ, Papp-Wallace KM. 2018. Sequence heterogeneity of the PenA carbapenemase in clinical isolates of Burkholderia multivorans. Diagn Microbiol Infect Dis 92:253–258. 10.1016/j.diagmicrobio.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joris B, Galleni M, Frere JM, Labia R. 1994. Analysis of the penA gene of Pseudomonas cepacia 249. Antimicrob Agents Chemother 38:407–408. 10.1128/AAC.38.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prince A, Wood MS, Cacalano GS, Chin NX. 1988. Isolation and characterization of a penicillinase from Pseudomonas cepacia 249. Antimicrob Agents Chemother 32:838–843. 10.1128/AAC.32.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becka SA, Zeiser ET, Barnes MD, Taracila MA, Nguyen K, Singh I, Sutton GG, LiPuma JJ, Fouts DE, Papp-Wallace KM. 2018. Characterization of the AmpC β-lactamase from Burkholderia multivorans. Antimicrob Agents Chemother 62:e01140-18. 10.1128/AAC.01140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trepanier S, Prince A, Huletsky A. 1997. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob Agents Chemother 41:2399–2405. 10.1128/AAC.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papp-Wallace KM, Taracila MA, Gatta JA, Ohuchi N, Bonomo RA, Nukaga M. 2013. Insights into β-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem 288:19090–19102. 10.1074/jbc.M113.458315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirel L, Rodriguez-Martinez JM, Plesiat P, Nordmann P. 2009. Naturally occurring class A β-lactamases from the Burkholderia cepacia complex. Antimicrob Agents Chemother 53:876–882. 10.1128/AAC.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp-Wallace KM, Bonomo RA. 2016. New β-lactamase inhibitors in the clinic. Infect Dis Clin North Am 30:441–464. 10.1016/j.idc.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem 288:27960–27971. 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papp-Wallace KM, Becka SA, Zeiser ET, Ohuchi N, Mojica MF, Gatta JA, Falleni M, Tosi D, Borghi E, Winkler ML, Wilson BM, LiPuma JJ, Nukaga M, Bonomo RA. 2017. Overcoming an extremely drug resistant (XDR) pathogen: avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from cystic fibrosis patients. ACS Infect Dis 3:502–511. 10.1021/acsinfecdis.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlowsky JA, Hackel MA, Bouchillon SK, Sahm DF. 2020. In vitro activity of WCK 5222 (cefepime-zidebactam) against worldwide collected Gram-negative bacilli not susceptible to carbapenems. Antimicrob Agents Chemother 64:e01432-20. 10.1128/AAC.01432-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TT, Condren M, Walter J. 2020. Ceftazidime-avibactam for the treatment of multidrug resistant Burkholderia cepacia complex in a pediatric cystic fibrosis patient. Pediatr Pulmonol 55:283–284. 10.1002/ppul.24557. [DOI] [PubMed] [Google Scholar]
- 19.Caverly LJ, Spilker T, Kalikin LM, Stillwell T, Young C, Huang DB, LiPuma JJ. 2019. In vitro activities of β-lactam–β-lactamase inhibitor antimicrobial agents against cystic fibrosis respiratory pathogens. Antimicrob Agents Chemother 64:e01595-19. 10.1128/AAC.01595-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dacco V, Claut L, Piconi S, Castellazzi L, Garbarino F, Teri A, Colombo C. 2019. Successful ceftazidime-avibactam treatment of post-surgery Burkholderia multivorans genomovar II bacteremia and brain abscesses in a young lung transplanted woman with cystic fibrosis. Transpl Infect Dis 21:e13082. 10.1111/tid.13082. [DOI] [PubMed] [Google Scholar]
- 21.Spoletini G, Etherington C, Shaw N, Clifton IJ, Denton M, Whitaker P, Peckham DG. 2019. Use of ceftazidime/avibactam for the treatment of MDR Pseudomonas aeruginosa and Burkholderia cepacia complex infections in cystic fibrosis: a case series. J Antimicrob Chemother 74:1425–1429. 10.1093/jac/dky558. [DOI] [PubMed] [Google Scholar]
- 22.Los-Arcos I, Len O, Martin-Gomez MT, Gonzalez-Lopez JJ, Saez-Gimenez B, Deu M, Nuvials X, Ferrer R, Roman A, Gavalda J. 2019. Lung transplantation in two cystic fibrosis patients infected with previously pandrug-resistant Burkholderia cepacia complex treated with ceftazidime-avibactam. Infection 47:289–292. 10.1007/s15010-018-1261-y. [DOI] [PubMed] [Google Scholar]
- 23.Canton-Bulnes ML, Hurtado Martinez A, Lopez-Cerero L, Arenzana Seisdedos A, Merino-Bohorquez V, Garnacho-Montero J. 2019. A case of pan-resistant Burkholderia cepacia complex bacteremic pneumonia, after lung transplantation treated with a targeted combination therapy. Transpl Infect Dis 21:e13034. 10.1111/tid.13034. [DOI] [PubMed] [Google Scholar]
- 24.Massip C, Mathieu C, Gaudru C, Miaut V, Floch P, Oswald E, Segonds C, Guet-Revillet H. 2019. In vitro activity of seven β-lactams including ceftolozane/tazobactam and ceftazidime/avibactam against Burkholderia cepacia complex, Burkholderia gladioli and other non-fermentative Gram-negative bacilli isolated from cystic fibrosis patients. J Antimicrob Chemother 74:525–528. 10.1093/jac/dky423. [DOI] [PubMed] [Google Scholar]
- 25.Tamma PD, Fan Y, Bergman Y, Sick-Samuels AC, Hsu AJ, Timp W, Simner PJ, Prokesch BC, Greenberg DE. 2018. Successful treatment of persistent Burkholderia cepacia complex bacteremia with ceftazidime-avibactam. Antimicrob Agents Chemother 62:e00213-17. 10.1128/AAC.02213-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dalem A, Herpol M, Echahidi F, Peeters C, Wybo I, De Wachter E, Vandamme P, Pierard D. 2018. In vitro susceptibility of Burkholderia cepacia complex isolated from cystic fibrosis patients to ceftazidime-avibactam and ceftolozane-tazobactam. Antimicrob Agents Chemother 62:e00590-18. 10.1128/AAC.00590-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farfour E, Trochu E, Devin C, Cardot Martin E, Limousin L, Roux A, Picard C, Jolly E, Vasse M, Lesprit P. 2018. Trends in ceftazidime-avibactam activity against multidrug-resistant organisms recovered from respiratory samples of cystic fibrosis patients. Transpl Infect Dis 20:e12955. 10.1111/tid.12955. [DOI] [PubMed] [Google Scholar]
- 28.Lagace-Wiens P, Walkty A, Karlowsky JA. 2014. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13–25. 10.2147/CE.S40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mushtaq S, Warner M, Livermore DM. 2010. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 65:2376–2381. 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI supplement 6. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 31.Blizzard TA, Chen H, Kim S, Wu J, Bodner R, Gude C, Imbriglio J, Young K, Park YW, Ogawa A, Raghoobar S, Hairston N, Painter RE, Wisniewski D, Scapin G, Fitzgerald P, Sharma N, Lu J, Ha S, Hermes J, Hammond ML. 2014. Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin. Bioorg Med Chem Lett 24:780–785. 10.1016/j.bmcl.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 32.Barnes MD, Bethel CR, Alsop J, Becka SA, Rutter JD, Papp-Wallace KM, Bonomo RA. 2018. Inactivation of the Pseudomonas-derived cephalosporinase-3 (PDC-3) by relebactam. Antimicrob Agents Chemother 62:e02406-17. 10.1128/AAC.02406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papp-Wallace KM, Barnes MD, Alsop J, Taracila MA, Bethel CR, Becka SA, van Duin D, Kreiswirth BN, Kaye KS, Bonomo RA. 2018. Relebactam is a potent inhibitor of the KPC-2 β-lactamase and restores imipenem susceptibility in KPC-producing Enterobacteriaceae. Antimicrob Agents Chemother 62:e00174-18. 10.1128/AAC.00174-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merck Sharp & Dohme. 2019. Prescribing information: RECARBRIO (imipenem c, and relebactam) for injection, for intravenous use. Merck Sharp & Dohme Corp., Whitehouse Station, NJ.
- 35.Zeiser ET, Becka SA, Wilson BM, Barnes MD, LiPuma JJ, Papp-Wallace KM. 2019. “Switching partners”: piperacillin-avibactam is a highly potent combination against multidrug-resistant Burkholderia cepacia complex and Burkholderia gladioli cystic fibrosis isolates. J Clin Microbiol 57:e00181-19. 10.1128/JCM.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahenthiralingam E, Bischof J, Byrne SK, Radomski C, Davies JE, Av-Gay Y, Vandamme P. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J Clin Microbiol 38:3165–3173. 10.1128/JCM.38.9.3165-3173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne GW, Vandamme P, Morgan SH, LiPuma JJ, Coenye T, Weightman AJ, Jones TH, Mahenthiralingam E. 2005. Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl Environ Microbiol 71:3917–3927. 10.1128/AEM.71.7.3917-3927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papp-Wallace KM, Winkler ML, Gatta JA, Taracila MA, Chilakala S, Xu Y, Johnson JK, Bonomo RA. 2014. Reclaiming the efficacy of β-lactam–β-lactamase inhibitor combinations: avibactam restores the susceptibility of CMY-2-producing Escherichia coli to ceftazidime. Antimicrob Agents Chemother 58:4290–4297. 10.1128/AAC.02625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papp-Wallace KM, Taracila MA, Smith KM, Xu Y, Bonomo RA. 2012. Understanding the molecular determinants of substrate and inhibitor specificities in the carbapenemase KPC-2: exploring the roles of Arg220 and Glu276. Antimicrob Agents Chemother 56:4428–4438. 10.1128/AAC.05769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2. Download AAC.01332-21-s0001.pdf, PDF file, 0.3 MB (308.6KB, pdf)