ABSTRACT
Hospitalized patients with SARS-CoV-2 infection (COVID-19) often receive antibiotics for suspected bacterial coinfection. We estimated the incidence of bacterial coinfection and secondary infection in COVID-19 using clinical diagnoses to determine how frequently antibiotics are administered when bacterial infection is absent. We performed a retrospective cohort study of inpatients with COVID-19 present on admission to hospitals in the Premier Healthcare Database between April and June 2020. Bacterial infections were defined using ICD-10-CM diagnosis codes and associated “present on admission” coding. Coinfections were defined by bacterial infection present on admission, while secondary infections were defined by bacterial infection that developed after admission. Coinfection and secondary infection were not mutually exclusive. A total of 18.5% of 64,961 COVID-19 patients (n = 12,040) presented with bacterial infection at admission, 3.8% (n = 2,506) developed secondary infection after admission, and 0.9% (n = 574) had both; 76.3% (n = 49,551) received an antibiotic while hospitalized, including 71% of patients who had no diagnosis of bacterial infection. Secondary bacterial infection occurred in 5.7% of patients receiving steroids in the first 2 days of hospitalization, 9.9% receiving tocilizumab in the first 2 days of hospitalization, and 10.3% of patients receiving both. After adjusting for patient and hospital characteristics, bacterial coinfection (adjusted relative risk [aRR], 1.15; 95% confidence interval [CI], 1.11 to 1.20) and secondary infection (aRR 1.93; 95% CI, 1.82 to 2.04) were both independently associated with increased mortality. Although 1 in 5 inpatients with COVID-19 presents with bacterial infection, secondary infections in the hospital are uncommon. Most inpatients with COVID-19 receive antibiotic therapy, including 71% of those not diagnosed with bacterial infection.
KEYWORDS: COVID-19, antibiotics, bacterial coinfection, secondary infection
INTRODUCTION
Hospitalized patients with SARS-CoV-2 infection (COVID-19) are often suspected of having cooccurring bacterial infection. Thus, 57 to 72% of patients admitted with COVID-19 receive antibiotics (1–4). However, microbiologically confirmed bacterial coinfection only occurs in 1% to 8% of patients presenting with COVID-19 (2–6). Secondary bacterial infection develops after hospital admission in an additional 3 to 14% (1, 5). When present, bacterial coinfection or secondary infection significantly increases morbidity and mortality from COVID-19 (6–8).
Existing studies define bacterial coinfections and secondary infections based on positive microbiologic test results, which likely underestimate the true incidence of bacterial infections (9). Sputum culture is an insensitive diagnostic test that relies on the patient’s ability to produce a quality specimen (9,10). Blood cultures are infrequently positive in the setting of bacterial pneumonia, and their yield is reduced when collected after the initiation of antibiotics (11). For these reasons and others, guidelines do not recommend routine collection of either sputum or blood cultures among patients with community-acquired pneumonia, including guidelines specifically for the management of COVID-19 pneumonia (12).
The purposes of this study were (i) to estimate the incidence of bacterial coinfection and secondary infection in the setting of COVID-19 based on clinical diagnoses rather than microbiologic testing and (ii) to examine patterns of antibiotic use based on whether bacterial infection was diagnosed at the time of treatment. Previous studies describing the incidence of bacterial infection in COVID-19 based on microbiologic testing primarily took place during the first wave of the pandemic. We focused on data from this period to allow comparison between our results and what has been previously reported.
RESULTS
Among 64,961 patients with COVID-19 present on admission (POA) at 605 hospitals contributing to Premier, 21.7% (n = 14,163) received a diagnosis consistent with bacterial infection. A proportion of 18.5% of patients (n = 12,040) were admitted with bacterial coinfection at admission, 3.9% (n = 2,506) developed secondary bacterial infection after admission, and 0.9% (n = 574) were diagnosed with both. The proportions of patients with bacterial coinfection and secondary infection in demographic and clinical subgroups are shown in Table 1. Risk of bacterial coinfection or secondary infection and patient-level characteristics are listed in Table 2.
TABLE 1.
Characteristics of inpatients with COVID-19 present on admission, by presence or absence of bacterial infectiona
| Characteristic | No. (%) with bacterial infection at admission |
No. (%) with bacterial infection after admission |
||
|---|---|---|---|---|
| Absent (n = 52,921) | Present (n = 12,040) | Absent (n = 62,455) | Present (n = 2,506) | |
| Age | ||||
| 18–30 yr old | 2,830 (5.4) | 380 (3.2)* | 3,154 (5.1) | 56 (2.2)* |
| 31–40 yr old | 4,243 (8.0) | 558 (4.6)* | 4,686 (7.5) | 115 (4.6)* |
| 41–50 yr old | 6,196 (11.7) | 958 (8.0)* | 6,915 (11.1) | 239 (9.5)* |
| 51–60 yr old | 9,807 (18.5) | 1,627 (13.5)* | 10,921 (17.5) | 513 (20.5)* |
| 61–70 yr old | 11,406 (21.6) | 2,533 (21.0)* | 13,220 (21.2) | 719 (28.7)* |
| >70 yr old | 18,439 (34.8) | 5,984 (49.7)* | 23,559 (37.7) | 864 (34.5)* |
| Gender | ||||
| Male | 28,708 (54.3) | 5,662 (47.0)* | 32,930 (52.7) | 1,440 (57.5)* |
| Female | 24,130 (45.6) | 6,364 (52.9)* | 29,428 (47.1) | 1,066 (42.5)* |
| Race | ||||
| Black | 12,146 (23.0) | 2,725 (22.6)* | 14,311 (22.9) | 560 (22.4)** |
| White | 22,334 (42.2) | 6,032 (50.1)* | 27,306 (43.7) | 1,060 (42.3)** |
| Other | 13,687 (25.9) | 2,575 (21.4)* | 15,631 (25.0) | 631 (25.2)** |
| Unknown | 4,754 (9.0) | 708 (5.9)* | 5,207 (8.3) | 255 (10.2)** |
| Hispanic ethnicity | 11,074 (20.9) | 1,808 (15.0)* | 12,375 (19.8) | 507 (20.2) |
| Elixhauser comorbidity index score | ||||
| 0–2 | 22,341 (42.2) | 2,981 (24.8)* | 24,658 (39.5) | 664 (26.5)* |
| 3–4 | 17,945 (33.9) | 4,201 (34.9)* | 21,267 (34.1) | 879 (35.1)* |
| 5–6 | 9,301 (17.6) | 3,262 (27.1)* | 11,953 (19.1) | 610 (24.3)* |
| >6 | 3,334 (6.3) | 1,596 (13.3)* | 4,577 (7.3) | 353 (14.1)* |
| Source of admission | ||||
| Home | 41,991 (79.4) | 8,608 (71.5)* | 48,726 (78.0) | 1,873 (74.7)* |
| Long-term care | 2,466 (4.6) | 1,242 (10.3)* | 3,589 (5.8) | 99 (4.0)* |
| Hospital transfer | 3,527 (6.7) | 1,032 (8.6)* | 4,239 (6.8) | 320 (12.8)* |
| Teaching hospital | 34,229 (64.5) | 7,658 (63.6)** | 40,033 (64.1) | 1,854 (74.0)* |
| Urban hospital | 49,206 (93.0) | 11,082 (92.0) | 57,975 (92.8) | 2,313 (92.3)* |
| Hospital bed size | ||||
| 0–299 beds | 16,136 (30.5) | 3,669 (30.5)* | 19,241 (30.8) | 564 (22.5)* |
| 300–499 beds | 15,788 (29.8) | 3,989 (33.1)* | 19,060 (30.5) | 717 (28.6)* |
| 500+ beds | 20,997 (39.7) | 4,382 (17.3)* | 24,154 (38.7) | 1,225 (48.9)* |
| Hospital region | ||||
| Midwest | 7,946 (15.0) | 2,179 (18.1)* | 9,731 (15.6) | 394 (15.7)** |
| Northeast | 28,456 (53.8) | 5,858 (48.7)* | 32,925 (52.7) | 1,379 (55.0)** |
| South | 13,845 (26.2) | 3,350 (27.8)* | 16,598 (26.6) | 597 (23.8)** |
| West | 2,674 (5.1) | 653 (5.4)* | 3,191 (5.1) | 136 (5.4)** |
Values are reported as the frequency, n, followed by the column percentage in parentheses. For characteristics with multiple levels, such as age, Elixhauser comorbidity index score, or hospital bed size, an overall chi-square test was performed across all levels rather than a separate comparison at each level. In this study, bacterial infection at admission was used as a proxy for bacterial coinfection. Bacterial infection after admission was used as a proxy for secondary infection. *, significantly different at the level of P < 0.001 compared to patients without bacterial infection; **, significantly different at the level of P < 0.05 compared to patients without bacterial infection.
TABLE 2.
Absolute risk of bacterial infection at or after admission, by patient characteristicsa
| Characteristic | Bacterial infection at admission | Bacterial infection after admission |
|---|---|---|
| Age | ||
| 18–30 yr old | 14.1 (12.6, 15.6) | 2.1 (1.5, 2.8) |
| 31–40 yr old | 13.5 (12.4, 14.6) | 2.5 (2.0, 3.1) |
| 41–50 yr old | 15.1 (14.0, 16.2) | 3.2 (2.7, 3.7) |
| 51–60 yr old | 15.2 (14.5, 15.9) | 4.2 (3.8, 4.6) |
| 61–70 yr old | 18.0 (17.3, 18.6) | 4.7 (4.3, 5.2) |
| >70 yr old | 21.8 (21.1, 22.4) | 3.7 (3.4, 4.0) |
| Gender | ||
| Male | 16.6 (16.2, 17.0) | 4.1 (3.8, 4.4) |
| Female | 20.4 (19.9, 21.0) | 3.5 (3.2, 3.8) |
| Race | ||
| Black | 17.2 (16.6, 17.9) | 3.5 (3.1, 3.8) |
| White | 19.5 (18.9, 20.2) | 3.7 (3.4, 4.0) |
| Other | 16.9 (16.1, 17.8) | 3.9 (3.5, 4.3) |
| Unknown | 16.7 (15.5, 17.9) | 4.3 (3.6, 5.0) |
| Hispanic ethnicity | 16.6 (15.8, 17.4) | 4.2 (3.7, 4.6) |
| Elixhauser index | ||
| 0–2 | 16.0 (15.0, 17.1) | 4.4 (3.6, 5.3) |
| 3–4 | 19.1 (18.5, 19.7) | 4.1 (3.7, 4.6) |
| 5–6 | 20.0 (19.0, 20.9) | 3.4 (3.1, 3.8) |
| >6 | 18.7 (17.0, 20.3) | 3.1 (2.5, 3.8) |
| Admission source | ||
| Home | 18.1 (17.6, 18.7) | 3.9 (3.6, 4.2) |
| Long-term care | 21.3 (20.2, 22.5) | 6.1 (5.4, 6.8) |
| Hospital transfer | 25.3 (23.9, 26.7) | 2.9 (2.3, 3.5) |
| Immunosuppression by hospital day 2 | ||
| Corticosteroids | 5.2 (4.7, 5.8) | |
| Tocilizumab | 7.8 (6.2, 9.3) | |
| Both | 12.2 (9.6, 14.7) | |
| Neither | 3.3 (3.1, 3.6) |
Cell values represent estimated marginal risk in percentage points predicted by a multivariable log-binomial regression model, assuming the distribution of other covariates was equal to their distribution in the overall sample. Accompanying 95% confidence intervals in parentheses were bootstrapped with 100 repetitions. Multivariable log-binomial regression was adjusted for age, gender, source of admission, race/ethnicity, hospital characteristics, and Elixhauser comorbidities. In this study, bacterial infection at admission was used as a proxy for bacterial coinfection. Bacterial infection after admission was used as a proxy for secondary infection.
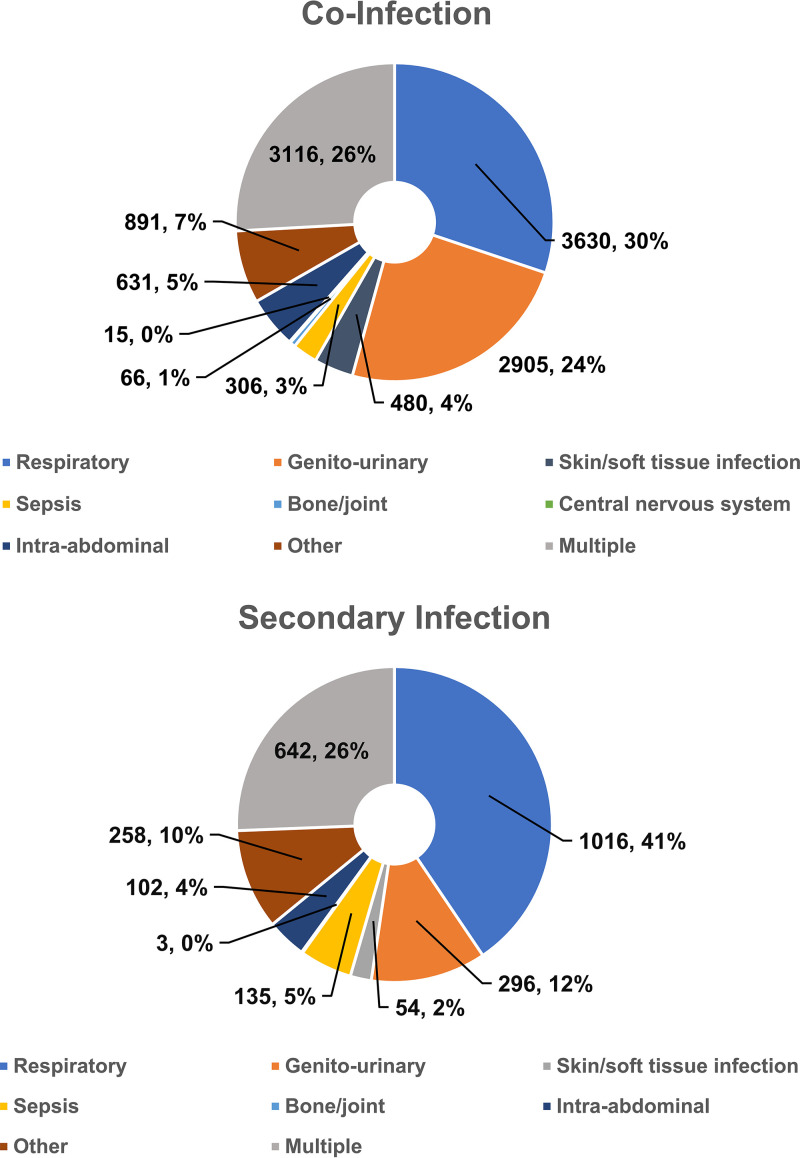
The most common subcategories of bacterial coinfection at admission were genitourinary (8.5% of the total sample, n = 5,548), respiratory (6.5%, n = 4,234), other (5.5%, n = 3,598; examples include “other bacterial infections of unspecified site,” “abscess of mediastinum”), and bacterial sepsis (1.5%, n = 976) (Fig. 1). The most common subcategories of bacterial secondary infection after admission were respiratory (2.1% of the overall sample, n = 1,372), other (1.3%, n = 827), genitourinary (1.0%, n = 660), and bacterial sepsis (0.5%, n = 315). The most common specific diagnoses associated with bacterial coinfection and secondary infection are listed in Table 3. Risk factors for bacterial coinfection and secondary infection are described in the supplemental material.
FIG 1.
Subcategories of bacterial infection in the setting of COVID-19 present on admission. These figures are intended to represent the source of infection. The category for “multiple” infectious sources includes patients who had infections in >1 nonsepsis categories. A patient with pneumonia and a urinary tract infection would be counted as having multiple infections only. Sepsis was only included as a primary category for patients without another source of infection. A patient with pneumonia and sepsis would be counted as having a respiratory infection only.
TABLE 3.
Most common diagnoses consistent with bacterial infection among patients presenting with COVID-19
| ICD 10 code | Description | Frequencya [no. (%)] |
|---|---|---|
| Bacterial coinfections present on admission | ||
| N39.0 | Urinary tract infection, site not specified | 4,679 (7.2) |
| J15.9 | Unspecified bacterial pneumonia | 2,756 (4.2) |
| B96.20 | Unspecified Escherichia coli as the cause of diseases classified elsewhere | 1,354 (2.1) |
| N30.00 | Acute cystitis without hematuria | 451 (0.7) |
| J15.6 | Pneumonia due to other Gram-negative bacteria | 432 (0.7) |
| B96.1 | Klebsiella pneumoniae as the cause of diseases classified elsewhere | 428 (0.7) |
| R78.81 | Bacteremia | 354 (0.5) |
| B96.89 | Other bacterial agents as the cause of diseases classified elsewhere | 352 (0.5) |
| B95.2 | Enterococcus as the cause of diseases classified elsewhere | 342 (0.5) |
| B96.4 | Proteus (mirabilis) (morganii) causing diseases classified elsewhere | 310 (0.5) |
| Bacterial secondary infection not present on admission | ||
| N39.0 | Urinary tract infection, site not specified | 590 (0.9) |
| J95.851 | Ventilator associated pneumonia | 452 (0.7) |
| J15.9 | Unspecified bacterial pneumonia | 354 (0.5) |
| J15.212 | Pneumonia due to methicillin-resistant Staphylococcus aureus | 160 0.2) |
| B96.20 | Unspecified Escherichia coli as the cause of diseases classified elsewhere | 156 0.2) |
| B95.2 | Enterococcus as the cause of diseases classified elsewhere | 140 (0.2) |
| J15.211 | Pneumonia due to methicillin-susceptible Staphylococcus aureus | 140 (0.2) |
| J15.6 | Pneumonia due to other Gram-negative bacteria | 133 (0.2) |
| J15.1 | Pneumonia due to Pseudomonas | 125 (0.2) |
| R78.81 | Bacteremia | 125 (0.2) |
Reported proportions represent the number of inpatients with a given diagnosis out of the total sample of inpatients with COVID-19 present on admission. For perspective, the total number of patients in our sample with bacterial coinfection was 12,040; 38.9% of inpatients presenting with bacterial coinfection received a diagnosis of N39.0 for urinary tract infection, site not specified. A total of 2,506 patients in our sample developed bacterial secondary infection; 23.5% of inpatients who developed bacterial secondary infection were diagnosed with N39.0 for urinary tract infection, site not specified.
A proportion of 22.0% of patients with COVID-19 POA (n = 14,303) died in-hospital or were discharged to hospice. Unadjusted mortality was 19.2% among patients without bacterial infection (n = 9,755 out of 50,903), 30.6% among patients with bacterial coinfection (n = 3,687 out of 12,040), and 44.3% among patients with bacterial secondary infection (n = 1,109 out of 2,506). After adjusting for baseline patient- and hospital-level characteristics, bacterial coinfection (adjusted relative risk [aRR], 1.15; 95% confidence interval [CI], 1.11 to 1.20) and secondary infection (aRR, 1.93; 95% CI, 1.82 to 2.04) were both independently associated with increased adjusted mortality risk.
Antibiotic use among patients with and without bacterial infections.
A proportion of 76.3% of patients with COVID-19 POA (n = 49,551) received at least one antibiotic during hospitalization. A proportion of 33.1% (n = 21,475) received an antibiotic with activity against Pseudomonas aeruginosa, and 32.7% (n = 21,228) received an antibiotic with activity against methicillin-resistant Staphylococcus aureus (MRSA). The most common antibiotics were ceftriaxone (48.5% of the total sample, n = 31,510), azithromycin (46.0%, n = 29,875), and vancomycin (22.9%, n = 14,861). Patients who received an antibiotic were treated for a median of 7 days of therapy (DOT; interquartile range [IQR], 3 to 12). Antipseudomonal agents and anti-MRSA agents were administered for a median of 4 DOT (IQR, 2 to 8) and 3 DOT (IQR, 1 to 6), respectively.
A total of 36,049 patients received an antibiotic without a diagnosis of bacterial infection (70.8% of those without a diagnosis of bacterial infection), including 21,491 who had neither bacterial infection nor a nonspecific pneumonic or septic syndrome (62.9% of patients in this category). Among these patients, the median DOT was 5 (IQR, 2 to 8). A proportion of 16.7% of patients without bacterial infection, sepsis, or pneumonia (n = 5,715) received treatment with an antipseudomonal agent (median, 2 DOT; IQR, 1 to 5), and 18.4% (n = 6,292) received an anti-MRSA agent (median, 2 DOT; IQR, 1 to 5).
Bacterial secondary infections after immunosuppression.
A proportion of 33.2% of patients with COVID-19 POA (n = 21,570) received an oral or intravenous steroid, including 19.6% (n = 12,709) who received early steroids (i.e., in first 2 calendar days of hospitalization). Among the subset who received early steroids, 5.7% (n = 723) developed bacterial secondary infection. After adjusting for patient and hospital characteristics, early steroids were associated with increased risk of bacterial secondary infection (aRR, 1.65; 95% CI, 1.48 to 1.84; see the supplemental material).
A proportion of 6.7% of patients with COVID-19 POA (n = 4,364) received tocilizumab, including 2.2% (n = 1,445) who received early tocilizumab. Among the subset who received early tocilizumab, 9.9% (n = 143) developed bacterial secondary infection. After adjusting for patient and hospital characteristics, early tocilizumab was associated with increased risk of bacterial secondary infection (aRR, 2.66; 95% CI, 2.14 to 3.33).
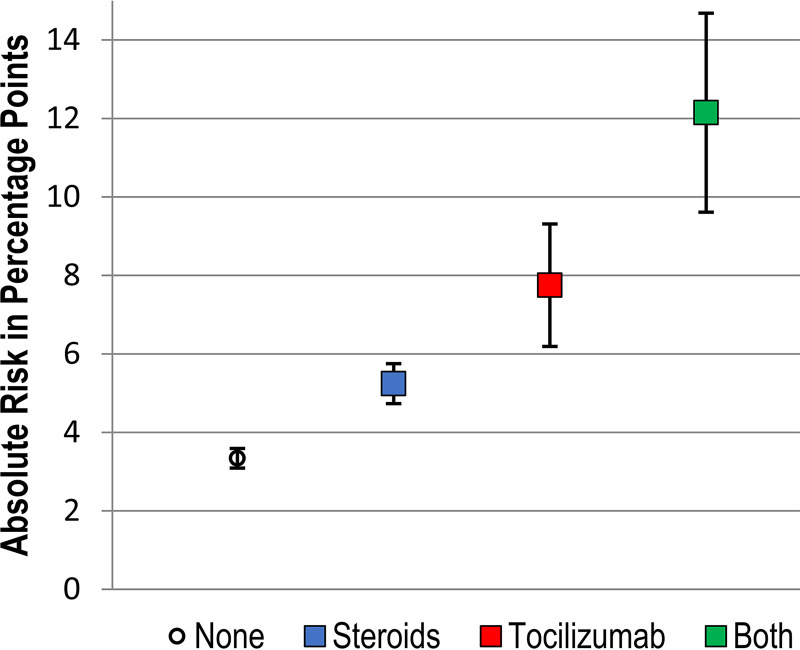
A proportion of 4.9% of patients in the total sample (n = 3,203) received both steroids and tocilizumab, including 1.2% (n = 773) who received both by hospital day 2. Among the subset who received both classes of immunosuppression early in hospitalization, 10.4% (n = 80) developed secondary bacterial infections. When considering both immunosuppressive therapies, early steroids (aRR, 1.60; 95% CI, 1.42 to 1.81) and early tocilizumab (aRR, 2.74; 95% CI, 2.11 to 3.57) were each independently associated with increased risk of bacterial secondary infection, but no interaction effects were detected. After risk adjustment for covariates (and assuming that other covariates are otherwise equal between groups), absolute risk of secondary infection was estimated to be 3.3% (95% CI, 3.1 to 3.6%) among patients not receiving early immunosuppression, 5.2% (95% CI, 4.7 to 5.8%) among patients receiving early steroids, 7.8% (95% CI, 6.2 to 9.3%) among patients receiving early tocilizumab, and 12.2% (95% CI, 9.6 to 14.7%) among patients receiving both agents in the first 2 days of hospitalization (see the supplemental material for full models and Fig. 2 for graphical display of absolute risk).
FIG 2.
Absolute risk of bacterial secondary infection based on exposure to immunosuppression in the first two days of hospitalization. Percentages represent estimated marginal risk of bacterial infection after admission predicted by a multivariable log-binomial regression model, assuming the distribution of other covariates was equal to their distribution in the overall sample; 95% confidence intervals were bootstrapped with 500 repetitions. Multivariable log-binomial regression was adjusted for age, gender, source of admission, race/ethnicity, hospital characteristics, and Elixhauser comorbidities.
DISCUSSION
In this retrospective cohort study, 18.5% of patients who were hospitalized with COVID-19 POA during the first wave of the COVID-19 pandemic were diagnosed with concurrent bacterial coinfection, and 3.8% were diagnosed with bacterial secondary infection after admission. Antibiotic use was widespread, even in the absence of bacterial infection, pneumonia, or sepsis. This study reflects one of the largest to date to examine bacterial infections and antibiotic use among patients with COVID-19, and the first large study to examine clinical diagnoses of bacterial infection rather than microbiologically confirmed cases.
We observed a higher rate of bacterial coinfections at admission than has been previously reported during the same time period (1–6). This discrepancy between our study and the existing literature is likely explained by our use of diagnosis codes to identify bacterial infections rather than results from microbiologic testing. Studies that define bacterial infections based upon microbiologic test results likely underestimate the true incidence, because cultures are not obtained in every case and may be falsely negative in the setting of antibiotic use or improper specimen collection. In contrast, though subjective clinician diagnoses have been associated with both overdiagnosis and underdiagnosis (13, 14), discharge diagnoses are relatively accurate. Although an admitting diagnosis of bacterial infection may be incorrect in 27 to 43% of cases (13–15), discharge diagnoses are associated with a positive predictive value for bacterial infection of ≥80% (16, 17). Nonetheless, we suspect that our findings may overestimate the incidence of bacterial infection, given potential financial incentives for hospitals to code severe bacterial illness as POA. The true incidence of bacterial coinfection among COVID-19 inpatients is likely between the rate reported in our study and what has been reported based on microbiologic testing.
As has been widely observed (1–4), we found that antibiotics were commonly prescribed in the context of viral infection due to SARS-CoV-2 during the first wave of the COVID-19 pandemic. Our study builds on the existing literature, however, because of our expansive definition for bacterial infection. After excluding any patient with a possible bacterial infection, including those with undefined sepsis syndromes or unspecified pneumonia, antibiotics were still administered in 3 out of 5 cases. Use of broad-spectrum agents with activity against Pseudomonas or MRSA far exceeded the expected prevalence of pneumonia due to these organisms (18). Our study was not designed to evaluate the appropriateness of antibiotic therapy, but we suspect our findings reflect antibiotic overuse driven by fear and uncertainty when the optimal clinical management of COVID-19 was still largely unknown.
Corticosteroids are the cornerstone of evidence-based treatment for hospitalized patients with severe or critical COVID-19 (19). Although the evidence to support use of tocilizumab is evolving, there is likely a role for tocilizumab in the management of hospitalized patients with severe, progressive COVID-19 (20). In our cohort, administration of these agents early in hospitalization either alone or in combination was associated with increased likelihood of bacterial secondary infection. When both agents were used in the first 2 days of hospitalization, the absolute risk of secondary bacterial infection was 12.1%. Although our analysis cannot establish causal relationships and is likely confounded by unobservable factors related to severity of illness, these findings should nonetheless serve to caution providers. Bacterial secondary infections are not uncommon among hospitalized patients with COVID-19 receiving immunosuppression, and careful attention is needed to ensure they are recognized early and managed appropriately.
For this study, we developed a comprehensive list of diagnoses comprising bacterial infections for which patients might be prescribed antibiotics. Although code sets provided by AHRQ in the CCSR were used as the basis of our list, many diagnoses included in relevant CCSR categories were nonspecific and needed to be excluded. Further research is needed to develop and validate standardized code sets to identify bacterial infections from administrative data.
Limitations.
The main limitation of this study is that all patients diagnosed with bacterial infection may not actually have bacterial infection, and we were unable to perform chart review to confirm bacterial infection based on clinical criteria. Diagnosis codes reflect clinicians’ suspicion for bacterial infection and, thus, likely overestimate the incidence of urinary infections, which are commonly misdiagnosed in the setting of asymptomatic bacteriuria (21). Use of microbiologic data to estimate the incidence of urinary tract infections would be subject to this same bias. Overall, we suspect that diagnosis codes represent a useful approximation when averaged across centers. The next limitation is that classification of bacterial coinfection or secondary infection relied on present on admission coding, which is commonly used in association with health care performance metrics but nonetheless may be influenced by anticipated reimbursement (22, 23). POA coding is accurate in about 70% of cases of community-acquired pneumonia, although differences in accuracy have been reported based on the hospital and diagnosis (24, 25). In cases where POA coding was inaccurate, coinfections may have been misclassified as secondary infections or vice versa. Another limitation is that we were unable to account for medications that patients might have received outside of the hospital encounter, including immunosuppression or antibiotics prescribed prior to admission and antibiotics continued after discharge. Thus, counts of inpatient antibiotic DOT likely underestimate total antibiotic exposures. Additionally, we did not include fungal infections in our analysis and therefore are unable to estimate the incidence of invasive fungal disease among patients receiving immunosuppression. Finally, inclusion in our sample depended on being discharged within the study period. Although the median duration of hospitalization for COVID-19 at that time was about 9 days (26), our study may not have captured severely ill patients who were admitted toward the end of June 2020 or remain hospitalized for a prolonged period. However, estimates of mortality based on Premier data collected during this period are consistent with other published studies and do not demonstrate evidence of bias (27).
Conclusions.
Antibiotic treatment was likely overused among patients hospitalized with COVID-19 during the first wave of the pandemic. A proportion of 76% of inpatients with COVID-19 during this period were prescribed antibiotics, despite only 22% being diagnosed with bacterial infection.
MATERIALS AND METHODS
Data source.
We conducted a retrospective observational cohort study of patients who were discharged from hospitals contributing to the Premier Healthcare Database. The data were extracted on 20 July 2020. Contributing hospitals cover highly geographically diverse areas across the United States and capture approximately one of every four U.S. hospital discharges. Premier internally validates all data (28). In addition to research performed by traditional academic institutions, the Premier Healthcare Database has been used by the National Institutes of Health and Centers for Disease Control and Prevention (CDC) to conduct studies related to the clinical epidemiology of COVID-19 (29–31). This study did not include personally identifiable information and was exempt from institutional review board review.
Study sample and COVID-19 case definition.
All adult inpatients with COVID-19 present on admission (POA) discharged in April to June 2020 at contributing hospitals were included. COVID-19 POA was defined by the presence of an ICD-10-CM diagnosis code of U07.1, designated POA. The U07.1 code was introduced in April 2020 and represents either a positive test for SARS-CoV-2 or a clinician’s statement that a patient has COVID-19 (32). Compared to laboratory data, U07.1 has been validated as highly accurate for identifying hospital admissions related to COVID-19 (33). For patients who arrived by acute care transfer from another hospital, POA refers to a diagnosis that was present at time of admission to the accepting hospital.
Definitions of bacterial coinfection and secondary bacterial infection.
Bacterial infections were identified using sets of ICD-10-CM diagnosis codes adapted from relevant categories in the AHRQ Healthcare Utilization Project’s Clinical Classification Software Refined (CCSR; see the supplemental material) (34). Final sets of diagnosis codes were reviewed independently by two infectious disease physicians (J. Baghdadi and K. C. Cofey). A third infectious disease physician was available to adjudicate disagreements (A. D. Harris). Diagnosis codes were included if they indicated (i) a specific bacterial pathogen, (ii) bacterial infection generally, or (iii) an infection commonly presumed to be bacterial in origin (e.g., osteomyelitis). Diagnosis codes for chronic infections, mycobacterial infections, and fungal infections were excluded. Several diagnosis codes for acute infection were also excluded on the basis that they may be used in cases of either bacterial or viral illness, such as “other specific sepsis.”
Bacterial infections were classified as coinfection or secondary infection relative to the current admission based on whether bacterial diagnoses were marked POA by managing providers. All patients in the study sample had COVID-19 POA. Coinfections were identified by a diagnosis of bacterial infection designated POA, meaning present at the same time as presentation with COVID-19 infection. Secondary infections were identified by bacterial infection that developed during hospitalization but after admission, meaning not present at the time of presentation with COVID-19. Thus, patients who presented to one hospital without bacterial coinfection developed secondary infection and then were transferred to a second hospital would appear from the perspective of the second hospital to have bacterial coinfection. Patients with POA and non-POA bacterial diagnoses were included as having both bacterial coinfection and secondary infection.
Patient and hospital variables.
Hospital-level covariates included teaching status, urban versus rural location, and geographic region. To represent the burden of COVID-19 on the admitting hospital, a variable was constructed to capture the percentage of monthly admissions related to COVID-19. To represent intensive care utilization, a variable was constructed to represent the proportion of admitted patients during a given month requiring mechanical ventilation. Patient-level covariates included demographics, source of admission, and POA comorbidities. POA comorbidities were identified by mapping encounter-level diagnosis codes to the Elixhauser comorbidity index (35).
Medication use data.
Daily inpatient medication data were extracted from charges for the hospital encounter. Antibiotic use was quantified by days of therapy (DOT). If a patient received two different antibiotics on a single hospital day, 2 DOT were attributed. Specific antibiotic categories of interest included agents with activity against Pseudomonas aeruginosa and agents with activity against methicillin-resistant Staphylococcus aureus (MRSA) (36). Corticosteroids or tocilizumab were defined as “early,” meaning likely administered before the development of hospital-acquired infections, if they were administered by hospital day 2. This cutoff was selected to ensure that early immunosuppression clearly preceded hospital-onset infections, which are typically defined as occurring after hospital day three or four (37). Outpatient or discharge medications were not available.
Outcomes.
The primary outcome was a composite of in-hospital death, death in hospice, or discharge to hospice. Secondary outcomes included development of bacterial coinfection and bacterial secondary infection.
Statistical methods.
Multivariable mixed-effects log-binomial regression models were fit using methodology proposed by Zou et al. to estimate the adjusted relative risk (aRR) (38). Absolute risk of bacterial secondary infection was estimated based on marginal risk predicted by multivariable log-binomial regression modeling, assuming the distribution of other covariates was equal to their distribution in the overall sample. CIs at 95% for absolute risk estimates were bootstrapped with 100 repetitions. Except for use of corticosteroids or tocilizumab in the first 48 h of admission, covariates in multivariable models were limited to characteristics that were present or known at time of admission to the hospital. Stata/IC version 14.1 was used for all analyses.
ACKNOWLEDGMENTS
J.D.B. received support from the University of Maryland Baltimore Institute for Clinical & Translational Research/Clinical and Translational Science Award (grant numbers 1KL2TR003099-03 and 1UL1TR003098-03).
We have no conflicts to disclose.
J.D.B., K.E.G., and A.D.H. received funding from Merck for a separate research project related to antibiotic use.
Footnotes
Supplemental material is available online only.
Contributor Information
Jonathan D. Baghdadi, Email: jbaghdadi@som.umaryland.edu.
Anthony D. Harris, Email: aharris@som.umaryland.edu.
REFERENCES
- 1.Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, Soucy J-PR, Daneman N. 2020. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 26:1622–1629. 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, Satta G, Cooke G, Holmes A. 2020. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 71:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karami Z, Knoop BT, Dofferhoff ASM, Blaauw MJT, Janssen NA, van Apeldoorn M, Kerckhoffs APM, van de Maat JS, Hoogerwerf JJ, Ten Oever J. 2021. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dis (Lond) 53:102–110. 10.1080/23744235.2020.1839672. [DOI] [PubMed] [Google Scholar]
- 4.Vaughn VM, et al. 2020. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis 72:e533–e541. 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. 2020. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 26:1395–1399. 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Fernandez-Pittol M, Pitart C, Inciarte A, Bodro M, Morata L, Ambrosioni J, Grafia I, Meira F, Macaya I, Cardozo C, Casals C, Tellez A, Castro P, Marco F, García F, Mensa J, Martínez JA, Soriano A, COVID-19 Researchers Group. 2021. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 27:83–88. 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaillancourt M, Jorth P. 2020. The unrecognized threat of secondary bacterial infections with COVID-19. mBio 11:20. 10.1128/mBio.01806-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy TF. 2008. Placebo-controlled trials of treatments for community-acquired pneumonia: review of the literature and discussion of feasibility and potential value. Clin Infect Dis 47(Suppl 3):S145–S149. 10.1086/591396. [DOI] [PubMed] [Google Scholar]
- 10.García-Vázquez E, Marcos MA, Mensa J, de Roux A, Puig J, Font C, Francisco G, Torres A. 2004. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med 164:1807–1811. 10.1001/archinte.164.16.1807. [DOI] [PubMed] [Google Scholar]
- 11.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, CDC EPIC Study Team. 2015. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427. 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metlay JP, Waterer GW. 2020. Treatment of community-acquired pneumonia during the coronavirus disease 2019 (COVID-19) pandemic. Ann Intern Med 173:304–305. 10.7326/M20-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra A, Nicks B, Maniago E, Nouh A, Limkakeng A. 2010. A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med 28:862–865. 10.1016/j.ajem.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Caterino JM, Leininger R, Kline DM, Southerland LT, Khaliqdina S, Baugh CW, Pallin DJ, Stevenson KB. 2017. Accuracy of current diagnostic criteria for acute bacterial infection in older adults in the emergency department. J Am Geriatr Soc 65:1802–1809. 10.1111/jgs.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon LB, Waxman MJ, Ragsdale L, Mermel LA. 2013. Overtreatment of presumed urinary tract infection in older women presenting to the emergency department. J Am Geriatr Soc 61:788–792. 10.1111/jgs.12203. [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. 2007. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol 60:397–409. 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Patkar NM, Curtis JR, Teng GG, Allison JJ, Saag M, Martin C, Saag KG. 2009. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol 62:321–327. 10.1016/j.jclinepi.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metersky ML, Frei CR, and, Mortensen EM. 2016. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology 21:157–163. 10.1111/resp.12651. [DOI] [PubMed] [Google Scholar]
- 19.Wootton D. 2021. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, Savic S, Youngstein T, Del Sorbo L, Cubillo Gracian A, De La Zerda DJ, Ustianowski A, Bao M, Dimonaco S, Graham E, Matharu B, Spotswood H, Tsai L, Malhotra A. 2021. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 384:1503–1516. 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridkin S, Baggs J, Fagan R, Magill S, Pollack LA, Malpiedi P, Slayton R, Khader K, Rubin MA, Jones M, Samore MH, Dumyati G, Dodds-Ashley E, Meek J, Yousey-Hindes K, Jernigan J, Shehab N, Herrera R, McDonald CL, Schneider A, Srinivasan A, Centers for Disease Control and Prevention (CDC). 2014. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 22.Gohil SK, Cao C, Phelan M, Tjoa T, Rhee C, Platt R, Huang SS. 2016. Impact of policies on the rise in sepsis incidence, 2000–2010. Clin Infect Dis 62:695–703. 10.1093/cid/civ1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Needleman J, Buerhaus PI, Vanderboom C, Harris M. 2013. Using present-on-admission coding to improve exclusion rules for quality metrics: the case of failure-to-rescue. Med Care 51:722–730. 10.1097/MLR.0b013e31829808de. [DOI] [PubMed] [Google Scholar]
- 24.Goldman LE, Chu PW, Osmond D, Bindman A. 2011. The accuracy of present-on-admission reporting in administrative data. Health Serv Res 46:1946–1962. 10.1111/j.1475-6773.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houchens RL, Elixhauser A, Romano PS. 2008. How often are potential patient safety events present on admission? Jt Comm J Qual Patient Saf 34:154–163. 10.1016/S1553-7250(08)34018-5. [DOI] [PubMed] [Google Scholar]
- 26.Cates J, Lucero-Obusan C, Dahl RM, Schirmer P, Garg S, Oda G, Hall AJ, Langley G, Havers FP, Holodniy M, Cardemil CV. 2020. Risk for in-hospital complications associated with COVID-19 and influenza—Veterans Health Administration, United States, October 1, 2018–May 31, 2020. MMWR Morb Mortal Wkly Rep 69:1528–1534. 10.15585/mmwr.mm6942e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman KE, et al. 2020. Impact of sex and metabolic comorbidities on COVID-19 mortality risk across age groups: 66,646 inpatients across 613 US hospitals. Clin Infect Dis 10.1093/cid/ciaa1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Premier Healthcare Sciences. 2020. Premier Healthcare Database white paper: data that informs and performs. Premier, Inc, Washington, DC. [Google Scholar]
- 29.Lavery AM, Preston LE, Ko JY, Chevinsky JR, DeSisto CL, Pennington AF, Kompaniyets L, Datta SD, Click ES, Golden T, Goodman AB, Mac Kenzie WR, Boehmer TK, Gundlapalli AV. 2020. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission-United States, March-August 2020. MMWR Morb Mortal Wkly Rep 69:1695–1699. 10.15585/mmwr.mm6945e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennington AF, Kompaniyets L, Summers AD, Danielson ML, Goodman AB, Chevinsky JR, Preston LE, Schieber LZ, Namulanda G, Courtney J, Strosnider HM, Boehmer TK, Mac Kenzie WR, Baggs J, Gundlapalli AV. 2021. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19—United States, March–September 2020. Open Forum Infect Dis 8:ofaa638. 10.1093/ofid/ofaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadri SS, Demirkale CY, Sun J, Busch LM, Strich JR, Rosenthal N, Warner S. 2021. Real-world inpatient use of medications repurposed for coronavirus disease 2019 in United States hospitals, March–May 2020. Open Forum Infect Dis 8:ofaa616. 10.1093/ofid/ofaa616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. 2020. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf. Accessed 28 January 2021.
- 33.Kadri SS, Gundrum J, Warner S, Cao Z, Babiker A, Klompas M, Rosenthal N. 2020. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. JAMA 324:2553–2554. 10.1001/jama.2020.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.HCUP. 2020. Clinical classifications software refined (CCSR) for ICD-10-CM diagnoses. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccsrefined.jsp.
- 35.Elixhauser A, Steiner C, Harris DR, Coffey RM. 1998. Comorbidity measures for use with administrative data. Med Care 36:8–27. 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Goodman KE, Cosgrove SE, Pineles L, Magder LS, Anderson DJ, Dodds Ashley E, Polk RE, Quan H, Trick WE, Woeltje KF, Leekha S, Harris AD. 2021. Significant regional differences in antibiotic use across 576 US hospitals and 11,701,326 adult admissions. Clin Infect Dis 73:213–217. 10.1093/cid/ciaa570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock C, Thom KA, Harris AD, Li S, Morgan D, Milstone AM, Caffo B, Joshi M, Leekha S. 2016. A multicenter longitudinal study of hospital-onset bacteremia: time for a new quality outcome measure? Infect Control Hosp Epidemiol 37:143–148. 10.1017/ice.2015.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou G. 2004. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702–706. 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S7. Download AAC.01341-21-s0001.pdf, PDF file, 0.6 MB (614.6KB, pdf)