ABSTRACT
Despite decades of research, tuberculosis remains a leading cause of death from a single infectious agent. Spectinamides are a promising novel class of antituberculosis agents, and the lead spectinamide 1810 has demonstrated excellent efficacy, safety, and drug-like properties in numerous in vitro and in vivo assessments in mouse models of tuberculosis. In the current dose ranging and dose fractionation study, we used 29 different combinations of dose level and dosing frequency to characterize the exposure-response relationship for spectinamide 1810 in a mouse model of Mycobacterium tuberculosis infection and in healthy animals. The obtained data on 1810 plasma concentrations and counts of CFU in lungs were analyzed using a population pharmacokinetic/pharmacodynamic (PK/PD) approach as well as classical anti-infective PK/PD indices. The analysis results indicate that there was no difference in the PK of 1810 in infected compared to healthy, uninfected animals. The PK/PD index analysis showed that bacterial killing of 1810 in mice was best predicted by the ratio of maximum free drug concentration to MIC (fCmax/MIC) and the ratio of the area under the free concentration-time curve to the MIC (fAUC/MIC) rather than the cumulative percentage of time that the free drug concentration is above the MIC (f%TMIC). A novel PK/PD model with consideration of postantibiotic effect could adequately describe the exposure-response relationship for 1810 and supports the notion that the in vitro observed postantibiotic effect of this spectinamide also translates to the in vivo situation in mice. The obtained results and pharmacometric model for the exposure-response relationship of 1810 provide a rational basis for dose selection in future efficacy studies of this compound against M. tuberculosis.
KEYWORDS: Mycobacterium tuberculosis, exposure-response, mathematical modeling, pharmacodynamics, pharmacokinetics
TEXT
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis (Mtb) that kills around 1.5 million people annually, more than any other infectious disease (1). Treatment of TB remains a major impediment due to its associated complexities such as prolonged duration, severe side effects, and emergence of multidrug resistant (MDR) and extensively drug-resistant (XDR) strains (2). Spectinamides are novel semisynthetic analogues of spectinomycin with potent anti-TB activity. In multiple murine models, spectinamides have proven efficacious and well tolerated (3). In in vitro experiments, spectinamides were also found to be active against MDR and XDR Mtb (4). Recently, spectinamide 1810 has been nominated as a preclinical candidate owing to its promising pharmacological and safety profile (5, 6).
Pharmacokinetic (PK)/pharmacodynamic (PD) modeling and simulation tools have been widely used to guide the selection of efficacious dosing regimens in the development of antibacterial agents (7, 8), including antituberculosis agents (9–14). At the preclinical stage, typical animal studies are conducted in mouse models of infection administered with a range of dose levels and dosing frequencies, and then efficacy is measured in terms of reduction in the number of CFU relative to baseline. The number of CFU is a quantitative measure of bacterial burden of multiplying bacterial states in the infected tissues. While widely used as a biomarker to assess antimycobacterial activity of drug compounds, its inability to quantify nonmultiplying bacterial states is a limitation that needs to be considered in its interpretation (9). While integrating the exposure of antibacterial agents with its putative effect in target tissues, it is a prerequisite to characterize the natural growth kinetics of bacteria in the target tissues. The model selection and its complexity are largely driven by bacterial physiology as well as the availability of sufficient in vitro and in vivo data to support estimating the differential growth kinetics of various subpopulations.
Numerous publications in the field of anti-bacterial pharmacotherapy are focused on the investigation of PK/PD indices as major drivers for antibacterial efficacy (15, 16). Typically, a dose fractionation study is conducted in vivo by administration of smaller fractions of doses with varying dosing frequency and multiple total daily dose levels. Based on these studies, the exposure-response relationship for the PK/PD indices, maximum drug concentration divided by MIC (Cmax/MIC), area under the concentration time curve divided by MIC (AUC/MIC), and the cumulative percentage of time when drug concentration remains above MIC (%TMIC) are determined. The relationship between PK/PD indices and the response as a reduction in bacterial burden reveals the main exposure drivers for antibacterial activity and thus guides dosage regimen design. Rifamycins, for example, show Cmax/MIC- and AUC/MIC-driven killing of Mtb, whereas pretomanid shows %TMIC-driven killing of Mtb (17, 18).
Postantibiotic effect (PAE) is a residual antibacterial activity which persists beyond the removal of antibacterial agent from the system. The plausible mechanisms leading to PAE are delayed recovery after reversible, nonlethal damage to cell structures, prolonged persistence of the drug at a binding site or within the subcellular target space, and the need for the bacteria to synthesize new enzymes or other essential molecules in a time-consuming process before initiation of regrowth (19–21). A variety of antimycobacterial agents, including isoniazid, rifampin, linezolid, moxifloxacin, and pyrazinamide, have been reported to exhibit PAE (22–24). Spectinamides have also been shown to exhibit substantial PAE (up to 137 h) in in vitro experiments, with 20 h for spectinamide 1810 against Mycobacterium bovis at 10× MIC (6). The majority of the reports have investigated PAE as an in vitro phenomenon, but postulated PAE mechanisms suggest that PAE can also be expected in in vivo settings (25–27). To the best of our knowledge, modeling and simulation tools have not yet been explored for the estimation of PAE in in vivo experiments.
In this study, we characterized the exposure-response relationship of the lead spectinamide 1810 in its efficacy against Mtb. Here, we used dose ranging and dose fractionation studies to characterize the dose-concentration-effect relationship of spectinamide 1810 in murine models of Mtb infection, established PK/PD indices to identify the main pharmacokinetic drivers for antimycobacterial activity of spectinamide 1810, and applied PK/PD modeling to quantify and characterize the in vivo PAE of this compound.
RESULTS
The dose fractionation and dose ranging studies were designed to characterize the exposure-response relationship of spectinamide 1810 in Mtb-infected BALB/c mice by performing PK and efficacy assessments in the groups of animals. The study was successfully completed in 147 of 150 infected animals from the treatment groups, and 146 provided evaluable samples for log CFU measurement and PK analysis. In addition, 84 mice provided plasma concentrations for PK analysis in healthy animals.
Pharmacokinetic analysis.
Based on the observed biphasic disposition of spectinamide 1810 after subcutaneous (s.c.) administration, a two-compartment model with first-order absorption was selected to describe the pharmacokinetics of spectinamide 1810. The model was parameterized in terms of a first-order absorption rate constant (K), and clearance (CL/F), central volume of distribution (Vc/F), intercompartmental clearance (Q/F), and peripheral volume of distribution (Vp/F), corrected for bioavailability (F). Estimation of between-animal variability was limited to clearance. The analysis was performed for healthy and infected animals separately as well as jointly. The corresponding parameter estimates along with their precision and between-animal variability for healthy and infected animals are provided in Table 1. The analyses as well as a concurrently performed covariate modeling approach for disease status with and without the presence of Bayesian priors derived from healthy animals and comparison of post hoc estimates for healthy and infected animals indicated that there was no relevant difference in PK parameters for spectinamide 1810 between healthy and Mtb-infected BALB/c mice. The basic goodness-of-fit plots for the final model are provided in Fig. S1 in the supplemental material. The visual predictive check plots using final model were created for peak concentrations (0.25 h) and trough concentrations (8 h) in infected animals and are shown in Fig. 1 and Fig. S2, respectively. In addition, the visual predictive check for healthy animals is depicted in Fig. S3.
TABLE 1.
Parameter estimates of final population PK modela
| Parameters | Typical estimate (% RSE) |
Random variabilityb (% RSE) [% shrinkage] |
||
|---|---|---|---|---|
| Healthy | Infected | Healthy | Infected | |
| Ka (h−1) | 14.0 (12) | 15.4 (8) | ||
| Vc/F (liters/kg) | 0.205 (5) | 0.217 (3) | ||
| Vp/F (liters/kg) | 0.145 (16) | 0.160 (12) | ||
| CL/F (liters/h/kg) | 0.701 (2) | 0.697 (2) | 14.6 (30) [17] | 13.2 (24) [59] |
| Q/F (liters/h/kg) | 0.0105 (16) | 0.0089 (6) | ||
| RUV | 12.3 (35) [44] | 60.0 (8) [4] | ||
Abbreviations: Ka, absorption rate constant; Vc/F, volume of central compartment corrected for bioavailability; Vp/F, volume of peripheral compartment corrected for bioavailability; CL/F, clearance corrected for bioavailability; Q/F, intercompartmental clearance corrected for bioavailability; RSE, relative standard error.
Expressed as percent coefficient of variation of between-animal variability or residual unexplained variability (RUV).
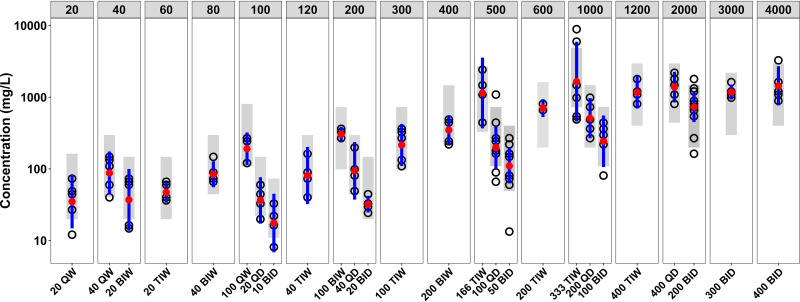
FIG 1.
Spectinamide 1810 peak plasma concentrations in infected mice for the different dosing regimens with same weekly dose (top line). Doses are in milligrams per kilogram. Open circles represent observed peak plasma concentrations, and blue lines represent 95% confidence intervals around the observed values. The gray shaded area is the 95% confidence interval for population PK model-based simulated values.
Spectinamide 1810 underwent rapid absorption from the subcutaneous administration site with a short absorption half-life of approximately 3 min. Its overall volume of distribution, around 0.35 liter/kg, indicates that the distribution space was mainly limited to the extracellular fluid volume, which is expected based on its overall high hydrophilicity (clog P, −3.03). Plasma concentrations declined with an alpha half-life of 0.20 h at therapeutic concentrations and a terminal half-life of 9.7 h at concentrations 1 to 2 orders of magnitude below therapeutically relevant concentrations. Similar to other cationic amphiphilic drugs, the prolonged terminal elimination half-life of spectinamide 1810 at very low concentrations is likely related to a high binding affinity for cellular phospholipids and the slow release from these binding sites (28).
Pharmacodynamic analysis.
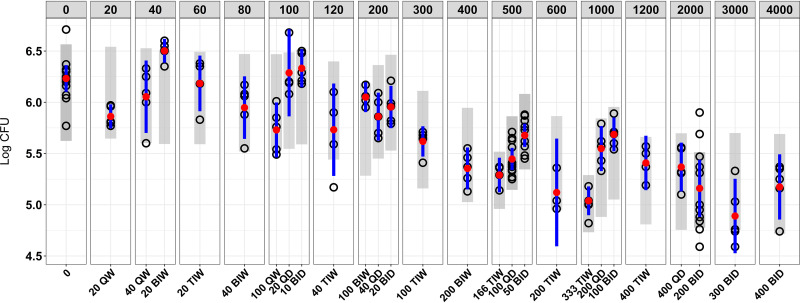
After 4 weeks of therapy of mice chronically infected with Mtb, spectinamide 1810 exhibited a dose-dependent decrease in the bacterial counts. The plot of weekly dose (Fig. 2) with various combinations of dose and dosing frequency versus log CFU indicates a dose-dependent increase in reduction of bacterial counts with higher weekly doses but also suggests that higher doses with lower dosing frequency resulted in better efficacy. For example, a weekly dose of 500 mg/kg when given as 166 mg/kg three times a week (TIW) resulted in better efficacy than 100 mg/kg daily (QD), which in turn was better than 50 mg/kg twice a day (BID). Similar trends with some exceptions were also observed with dose fractionation of other weekly doses, where intermittent dosing provided improved efficacy relative to more frequent dosing.
FIG 2.
Bacterial burden (log CFU) in the lungs of Mtb-infected mice for the different dosing regimens with same weekly dose (top line). Doses are provided in milligrams per kilogram. Open circles represent observed log CFU values, red circles are mean values of the observed CFU data, and blue lines represent 95% confidence intervals around the observed values. The gray shaded area is the 95% confidence interval for PK/PD model-based simulated values.
Identification of the PK drivers for efficacy.
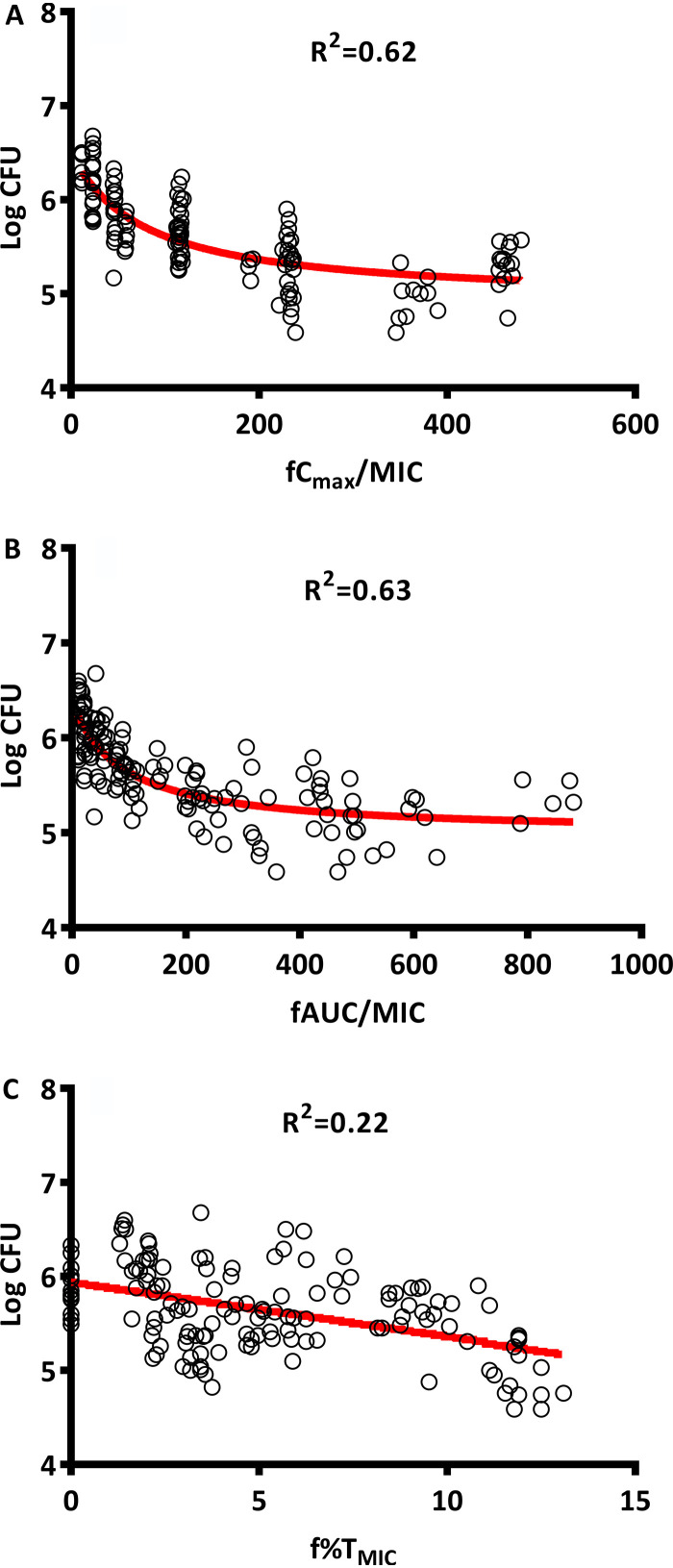
To further address the question of whether increased dose fractionation is advantageous for spectinamide 1810 therapy, the relationship of bacterial killing relative to PK/PD indices of frequently used antibacterial therapy was investigated. Figure 3 demonstrates the relationship between PK/PD indices and reduction in log CFU for spectinamide 1810 based on the results of the dose fractionation study. AUC/MIC for free, unbound drug (fAUC/MIC) and fCmax/MIC showed a strong correlation with log CFU reduction (R2 = 0.63 and 0.62, respectively), whereas f%TMIC exhibited only a weak relationship (R2 = 0.22). This indicates that the antibacterial effect of spectinamide 1810 against mycobacteria was largely driven by exposure and peak concentrations, rather than exceeding a minimum drug level.
FIG 3.
Relationship between PK/PD indices and log CFU. (A) Log CFU versus fCmax/MIC; (B) log CFU versus fAUC/MIC; (C) log CFU versus f%TMIC.
PK/PD modeling with PAE estimation.
The developed one-population bacterial growth model adequately described the natural growth kinetics of Mtb in infected mice. The in vivo net growth rate constant (Kgs) for Mtb was estimated to be 0.0357 h−1 (Table 2), and the corresponding mean generation time (doubling time) was calculated as 28.0 h, which is within the range reported in the literature (13 to 80 h) (29–31).
TABLE 2.
Parameter estimates describing the in vivo natural growth kinetics of Mtb in BALB/c micea
| Parameter | Typical estimate (% RSE) [% shrinkage] | Half-life (days) |
|---|---|---|
| Kgs (h−1) | 0.0357 (4) | 0.809 |
| Log Nmax | 6.11 (1) | |
| Log CFUinitialb | 1.77 (3) | |
| RUV | 0.437 (11) [4] |
Abbreviations: Kgs, first-order growth rate constant; log Nmax, decadic logarithm of the maximum number of bacteria; log CFUinitial, inoculum size on day 1; RUV, standard deviation of residual unexplained variability in log CFU; RSE, relative standard error.
Initial inoculum in experiments for natural growth curve assessment. In studies 1 and 2, the values were 2.28 and 2.07, respectively.
For quantitatively characterizing spectinamide 1810’s antibacterial activity, the model-predicted PAE concentration based on the central compartment concentrations was linked to drug effect as a kill rate of the bacteria via a sigmoidal maximum-effect (Emax)-type model.
Initially, the PK/PD analysis was conducted only on the data from study 1, as both studies showed a substantial, unintended difference in bacterial load at the initiation of treatment between studies 1 and 2 (7.08 versus 5.37 log CFU). Different PAE models were explored. Estimation of either a combination of extension of the peak plasma concentration over time and a subsequent slow first-order elimination or a slow first-order elimination from the effect compartment resulted in comparable fits with similar OFV. However, both approaches provided a better fit (ΔOFV, ∼24) compared to using an extension of the peak plasma concentration alone. Based on the principle of parsimony, we decided to select the model with a decline in PAE effect determined by a first-order elimination rate constant (KPAE) alone for the further data analysis. The data from study 2 were subsequently included in the analysis. In order to account for study-specific differences, study was tested as a covariate either on the fixed-effect parameters EC50 (concentration at half-maximal kill rate) and Kkillmax (maximum kill rate induced by spectinamide 1810) or on random-effect parameters. Study specific effects on fixed effect parameters were determined using the relationship
| (1) |
where P represents the typical value of the parameter in the population, STUDY is an indicator variable equal to 0 for study 1 and 1 for study 2, Θ1 is the typical value of P for study 1, and Θ2 is the multiplicative factor describing the increase or decrease in P in study 2. The incorporation of study-specific differences on Kkillmax and estimating different random error distributions for each study provided the best fit and maximum drop in objective function compared to analyzing the data without accounting for study differences. The resulting parameter point estimates as well as bootstrap-derived medians and confidence intervals are presented in Table 3, and the corresponding basic goodness-of-fit plots for the model without PAE and the final model with PAE are shown in Fig. S4 and S5 in the supplemental material, respectively. A visual predictive check showing the congruence between the actual log CFU data and the PK/PD model-predicted simulations for the different dose levels and dosing regimens is integrated in Fig. 2.
TABLE 3.
Parameter estimates with bootstrap result for final PK/PD modela
| Parameter | Typical estimate (% RSE) | Half-life (days) | Bootstrap-derived median (90% CI) |
|---|---|---|---|
| Kkillmax (h−1) | 0.0374 (5.3) | 0.772 | 0.0370 (0.0350–0.0410) |
| EC50 (μg/ml) | 79.6 (60.2) | 79.6 (42.4–141.3) | |
| γ | 1.58 (23.1) | 1.64 (1.06–2.42) | |
| KPAE (h−1) | 0.0142 (74.6) | 2.03 | 0.0147 (0.0048–0.0254) |
| Study-specific coefficient for Kkillmaxb | 1.15 (4.0) | 1.15 (1.08–1.23) | |
| RUV1 | 0.284 (12.1) | 0.279 (25.0–30.7) | |
| RUV2 | 0.173 (27.2) | 16.3 (12.4–20.8) |
Abbreviations: Kkillmax, maximum kill rate induced by spectinamide 1810; EC50, concentration of spectinamide 1810 at half of Kkillmax; γ, Hill coefficient; KPAE, first-order rate constant for dissipation of postantibiotic effect; RUV1 and RUV2, standard deviations of residual unexplained variability in log CFU for study 1 and study 2, respectively.
Fold difference in Kkillmax in study 2 relative to study 1.
DISCUSSION
Characterization of the exposure-response relationship is a crucial step in characterizing the pharmacologic profile of novel antibiotic agents. In this study with PK and PD assessments in the same animals, we integrated the exposure and anti-infective response data of an extensive dose fractionation study for the lead spectinamide compound 1810 to derive a model-based PK/PD assessment of its response in a mouse model of Mtb infection. In this context, we successfully implemented a novel, empirical modeling framework that supported PAE as part of the antibacterial activity of spectinamide 1810. Additionally, we also used a PK/PD index-based approach to guide the dose selection process, which indicated that spectinamide 1810 exhibits concentration- and exposure-dependent killing in vivo. The findings from both analysis approaches showed a superior efficacy of higher doses given less frequently over that of more frequent smaller doses while maintaining a constant weekly dose.
The collection of series of blood samples for PK assessments from infected animals is challenging and usually prohibitive due to potential effects on in vivo efficacy. In the current analysis, we therefore combined a sparse sampling design in infected animals with a population PK analysis approach that used concentration data from densely sampled healthy animals to stabilize the pharmacokinetic analysis for infected animals. While this is an approach that has so far only rarely been used in preclinical development, it is frequently applied in clinical drug development for populations where only limited sampling is available, for example, to bridge PK data from adult to pediatric populations (32, 33). We used the same methodology to bridge PK studies with rich sampling in healthy animals to those with sparse sampling in infected animals. The approach steered the modeling process to derive reliable parameters for healthy as well as infected animals. Spectinamide 1810 is mainly eliminated by the renal route in unchanged form, similar to other spectinamides (4), and to our knowledge there is no published indication that kidney function is affected by Mtb infection in mice. This supports our finding of no difference in the PK behavior of spectinamide 1810 in healthy and infected animals.
The evaluation of PK/PD indices indicates that bacterial killing in mice was best predicted by fCmax/MIC and fAUC/MIC rather than f%TMIC. This observation is in line with our previous in vitro observations for spectinamide 1599, a precursor molecule of spectinamide 1810 (34). Similar patterns were also observed for existing first line anti-TB agents. Monotherapy studies conducted on preclinical models showed that microbial killing by isoniazid, rifampin, pyrazinamide, and ethambutol was linked with either fCmax/MIC, fAUC/MIC, or both (18, 35–40). In contrast, for pretomanid, f%TMIC correlated best with bactericidal activity in dose fractionation studies performed in mice (17).
Several modeling approaches have been reported to describe the natural growth of Mtb in vivo. These include simpler models in which bacterial growth is pragmatically assumed to occur in a homogenous bacterial population and is limited by a maximum bacterial number with capacity-limited or logistic growth functions (7, 34). More complex models with two subpopulations have also been explored under the assumption that a growing population under unfavorable conditions gets converted into a dormant, resting, or slowly growing population (41, 42). A multistate model for TB consisting of fast-growing, slowly growing, and nonreplicating bacterial states was applied for pharmacometric analysis of rifampin (10). Most of these models were used to characterize the PK/PD relationship for in vitro time-kill experiments or in a few cases for in vivo studies partially supported by parameters obtained from in vitro experiments. To allow characterization of the complex dynamics of these models, time series of multiple bacterial counts are needed that provide a time course of CFU count development over a prolonged period. In the present study, however, all bacterial count samples from different dose groups were collected at the same time after termination of therapy. Therefore, our data did not contain sufficient information to support these more complex modeling approaches, and a simpler one-population model was used. This model was able to adequately describe the growth kinetics of Mtb and drug effect of spectinamide 1810 in our analysis.
One of the related limitations of our analysis and the resulting one population model, however, is the lack of information on whether spectinamide 1810 potentially exhibits biphasic killing behavior with different activity against persister populations of mycobacteria, as has been reported for isoniazid (43). As such, model validity may be limited beyond the studied dose levels, dosing regimens, or treatment duration. The wide range of dosing regimens investigated in this study, however, provides a solid basis for the pharmacologic characterization of spectinamide 1810.
Many antibacterial agents are known to exhibit a PAE. To the best of our knowledge, PAE has so far not been implemented in PK/PD modeling strategies. One of the potential mechanisms considered for PAE is a longer persistence of drug in subcellular fractions of the affected bacteria relative to the surrounding tissue environment or host plasma. Based on that notion, we designed a PK/PD model with a hypothetical PAE compartment for antibacterial activity. Inclusion of the PAE compartment significantly improved the model fit of the data (Fig. S4 and S5) and rationalized the efficacy patterns observed in the different dose groups of the dose fractionation studies.
The model-derived half-life for the decline of the in vivo PAE, 48.8 h, is substantially longer than the previously reported in vitro PAE of 20 h. Multiple factors could contribute to this discrepancy, including strain differences used for the assessment, drug distribution processes in vivo, multiple bacterial subpopulations with different metabolic states encountered in vivo, and potential effects of the host immune system.
While investigating potential study-specific differences in our two substudies, we found that study 2 had a 15% higher Kkillmax and that it resulted in slightly better efficacy at comparable doses. Differences in bacterial load at the initiation of treatment between studies 1 and 2 (7.08 versus 5.37 log CFU) could have been the potential reason for achieving different kill rates; however, future studies to understand the effect of bacterial load on efficacy are warranted for spectinamide 1810. A study designed to evaluate the influence of route of administration and inoculum size on the efficacy of first-line anti-TB agents demonstrated superior therapeutic outcomes measured in terms of reduction of bacterial load in lungs and extrapulmonary sites in intratracheally infected mice with comparatively smaller inocula than intravenously infected mice (44).
Being a lead candidate, it is expected that in the near future, the lead spectinamide 1810 will undergo further screening for efficacy in other preclinical models. The comprehensive analysis and characterization of the exposure-response relationship of spectinamide 1810 in a murine TB model presented here provide the basis for such studies by establishing a rationale framework for in vivo antimycobacterial activity based on integrated pharmacokinetic/pharmacodynamic knowledge.
MATERIALS AND METHODS
Chemicals and reagents.
Spectinamide 1810 [2-(5-hydroxypyridin-2-yl)-N-(3′-dihydro-3′-deoxy-3′(R)-amino spectinomycin] acetamide and internal standard [3′-dihydro-3′-deoxy-3′(R)-isopropylacetylamino spectinomycin] were synthesized as previously described (6). Acetonitrile, methanol, high-performance liquid chromatography (HPLC)-grade water, formic acid, and nonafluoropentanoic acid were purchased from Fisher Scientific (Pittsburgh, PA).
Animal experimentation.
Single- and multiple-dose studies on the pharmacokinetics of spectinamide 1810 in healthy mice after subcutaneous (s.c.) administration were performed at the University of Tennessee Health Science Center following approval by the local institutional animal care and use committee.
Female BALB/c mice, 8 weeks old and weighing 18 to 22 g, were purchased from Charles River Laboratories (Wilmington, MA) and acclimatized for a 72-h period prior to use. The rationale for using female mice was based on the facts that the murine efficacy model in this gender is an established and validated model for TB drug efficacy studies and that this allows cross comparison to previous studies with the spectinamide drug class and other anti-TB agents. Healthy, noninfected animals (total, 84) were dosed with daily doses of 50 or 200 mg/kg either as single doses or multiple doses for five consecutive days. Groups of three mice were sacrificed at a predefined time point after dosing, and blood samples were obtained by cardiac puncture under isoflurane anesthesia followed by euthanasia. For single-dose studies, samples were collected at 0.083, 0.25, 0.5, 1, 3, 8, and 24 h, and for multiple-dose studies, they were collected predose and at 0.083, 0.25, 1, 3, and 8 h after administration of the last dose. Plasma was separated immediately from blood by centrifugation (∼3,750 × g for 10 min at 4°C) and stored at −70°C until liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
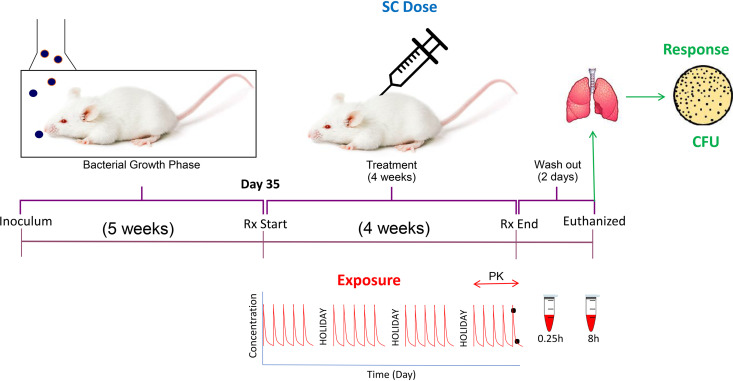
Two dose ranging and dose fractionation studies with simultaneous PK and efficacy assessments in the same animals were conducted in a dedicated biosafety level 3 (BSL-3) facility at Colorado State University according to the guidelines and with approval of the Colorado State University Institutional Animal Care and Use Committee. Figure 4 depicts a schematic representation of the overall study design. Female BALB/c mice (total 196) purchased from Charles River Laboratories (Wilmington, MA) were infected with Mtb (ATCC 35801/TMC 107/Erdman; considered sensitive to spectinamide 1810; MIC, 1.6 mg/liter) with a low-dose aerosol infection as previously described, using an inoculum of 2.0 × 106 CFU/ml in a GlasCol chamber to achieve deposition of ∼100 CFU in the lungs per mouse (45). At day 34 postinfection, s.c. administration with spectinamide 1810 was initiated for groups of five mice in study 1 and six mice in study 2 with dosing regimens as described in Tables 4 and 5 and continued for 4 weeks with drug holidays on weekends (i.e., 5 days of consecutive dosing and 2 days of no treatment per week). For practicability, the assessments were performed in two separate studies. In total, 29 dose groups ranging from total daily doses of 20 mg/kg to 800 mg/kg and total weekly doses from 20 mg/kg to 4,000 mg/kg were administered. Dosing frequencies ranged from once per week (QW) over twice (BIW) and three times (TIW) per week to daily (QD) and twice daily (BID).
FIG 4.
Schematic representation of dose fractionation and dose ranging study of spectinamide 1810 conducted in Mtb-infected female BALB/c mice.
TABLE 4.
Dosing regimens evaluated in the dose fractionation study (in terms of total weekly dose)a
| Dosing frequency | Regimen used to achieve a total weekly dose (mg/kg) of: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | 120 | 200 | 300 | 400 | 500 | 600 | 1,000 | 1,200 | 2,000 | 3,000 | 4,000 | |
| Study 1 | ||||||||||||||||
| 5× per wk (M–F) twice | 10 BID | 20 BID | 50 BID | 100 BID | 200 BID | 300 BID | 400 BID | |||||||||
| 5× per wk (M–F) | 20 QD | 40 QD | 100 QD | 200 QD | 400 QD | |||||||||||
| 3× per wk (M, W, F) | 20 TIW | 40 TIW | 100 TIW | 200 TIW | 400 TIW | |||||||||||
| 2× per wk (M, R) | 20 BIW | 40 BIW | 100 BIW | 200 BIW | ||||||||||||
| 1× per wk (M) | 20 QW | 40 QW | 100 QW | |||||||||||||
| Study 2 | ||||||||||||||||
| 5× per wk (M–F) twice | 50 BID | 200 BID | ||||||||||||||
| 5× per wk (M–F) | 100 QD | |||||||||||||||
| 3× per wk (M, W, F) | 166 TIW | 333 TIW | ||||||||||||||
Individual dose per kilogram of body weight administered per dosing occasion and dosing frequency. For comparison with regard to dose fractionation, equal weekly doses are given. Abbreviations: M, Monday; T, Tuesday; W, Wednesday; R, Thursday; F, Friday; BID, twice daily; QD, once daily; TIW, three times weekly; BIW, twice weekly; QW, once weekly.
TABLE 5.
Dosing regimens evaluated in the dose fractionation study (in terms of total daily dose)a
| Dosing frequency | Regimen used to achieve a daily dose (mg/kg) of: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 100 | 166 | 200 | 333 | 400 | 600 | 800 | |
| Study 1 | |||||||||
| 5× per wk (M–F) twice | 10 BID | 20 BID | 50 BID | 100 BID | 200 BID | 300 BID | 400 BID | ||
| 5× per wk (M–F) | 20 QD | 40 QD | 100 QD | 200 QD | 400 QD | ||||
| 3× per wk (M, W, F) | 20 TIW | 40 TIW | 100 TIW | 200 TIW | 400 TIW | ||||
| 2× per wk (M, R) | 20 BIW | 40 BIW | 100 BIW | 200 BIW | |||||
| 1× per wk (M) | 20 QW | 40 QW | 100 QW | ||||||
| Study 2 | |||||||||
| 5× per wk (M–F) twice | 50 BID | 200 BID | |||||||
| 5× per wk (M–F) | 100 QD | ||||||||
| 3× per wk (M, W, F) | 166 TIW | 333 TIW | |||||||
Abbreviations: M, Monday; T, Tuesday; W, Wednesday; R, Thursday; F, Friday; BID, twice daily; QD, once daily; TIW, three times weekly; BIW, twice weekly; QW, once weekly.
Spectinamide 1810 was formulated in Plasma-Lyte (Baxter, Deerfield, IL) and water with different ratios for each group in order to maintain ideal osmotolerance close to physiological values, and 0.2 ml of the formulation was administered subcutaneously using a 29-gauge insulin syringe. Two blood samples per mouse were collected by submandibular bleed in Microtainer plasma separator tubes containing lithium-heparin (BD, Franklin Lakes, NJ), one at 0.25 h and another at 8 h postadministration during the last week of dosing. The selection of sampling times was based on a mixture of practicability (the same sampling time points for all dosing regimens), feasibility (limitation of undue stress on the study animals that were also used for the efficacy assessment), and previous experience by our group with limited sampling strategies and optimal designs (32). Plasma was separated immediately by centrifugation (∼3,750 × g for 10 min at 4°C) and stored at −70°C until LC-MS/MS analysis. After 4 weeks of treatment followed by a 2-day washout period, lungs were harvested, homogenized for dilution, and plated on Middlebrook 7H11 agar plates supplemented with oleic acid-albumin-dextrose-catalase (OADC). Plates were incubated at 37°C, and CFU were enumerated after at least 21 days of incubation. In each study, to enumerate the bacterial uptake from the low-dose aerosol infection, 6 mice were sacrificed on day 1 postinfection. On day 34 postinfection, 5 mice were sacrificed to determine bacterial load at the start of therapy. On day 62 postinfection, 5 untreated BALB/c mice and 7 BALB/c mice dosed with vehicle were sacrificed to determine bacterial load in the untreated group and placebo group, respectively. This information was used for describing the natural bacterial growth in the host. The viable CFU counts were logarithmically converted prior to further data analysis. No observation was below the limit of counting.
Quantification of spectinamide 1810 concentrations in plasma.
To allow quantification of spectinamide 1810 in the plasma specimens obtained from infected animals outside the BSL-3 environment, samples were sterilized by exposure to gamma irradiation (approximately 1 megarad) according to a validated protocol. To account for potential partial degradation of the analyte by irradiation, quality control samples with known concentrations of analyte (high, medium, and low) were evenly placed among test samples during sterilization and used to correct any loss of analyte in the plasma specimens collected from the infected animals. The approximate level of degradation was consistent within one batch of irradiated samples and was on average a reduction in concentration by 31.8%.
Plasma samples from both healthy and infected animals were processed by protein precipitation. To 25-μl aliquots of specimens, 100 μl methanol containing 10 ng/ml internal standard [3′-dihydro-3′-deoxy-3′(R)-isopropylacetylamino spectinomycin] was added. This was followed by vortexing for 1 min and centrifugation at 10,000 × g for 10 min at 4°C. The supernatant was separated, and 5 μl of supernatant was analyzed using high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Chromatographic separations were carried out using a Shimadzu Nexera XR (LC-20ADXR) liquid chromatograph (Shimadzu Corporation, Kyoto, Japan) consisting of two pumps, an online degasser, system controller, and an autosampler, using a Waters Symmetry 3.5-μm C8, 50- by 2.1-mm column (Waters, Milford, MA). The mobile phase was solvent A (water with 1.6% nonafluoropentanoic acid and 0.7% formic acid) and solvent B (90% acetonitrile with 0.8% nonafluoropentanoic acid and 0.35% formic acid) in a gradient mode as follows: 0 to 0.5 min, 20% B; 0.5 to 1.6 min, 20 to 90% B; 1.6 to 2 min, 90% B; 2 to 2.5 min, 90 to 20% B; and 2.5 to 3 min, 20% B at a flow rate of 0.5 ml/min. Analytes were detected with a Sciex 5500 triple-quadruple mass spectrometer (Applied Biosystems, Foster City, CA) with electrospray ionization in multiple-reaction-monitoring mode using the compound-specific transitions of m/z 469.2/207.1 for spectinamide 1810 and m/z 418.3/207.1 for internal standard.
A calibration curve was generated from 11 calibration standards by plotting peak area ratio (analyte peak area/internal standard [IS] peak area) against the concentration of an analyte using weighted least-square linear regression analyses with a 1/x weighting factor. The calibration curve ranged from 1.95 to 2,000 ng/ml and was validated for accuracy and precision with spiked samples of mouse plasma (quality control). The peak area ratios of analyte to internal standard were linear over the concentration range tested, with correlation coefficients of >0.996. Accuracy (deviation of the analyzed quality control samples from nominal values) ranged between 87.1 and 102.9% over the entire range of the calibration curve, and precision (coefficient of variation of repeated measurements of the quality control samples) ranged between 1.4 and 4.4%.
Population pharmacokinetic analysis.
The PK assessments available in this analysis included data sets from healthy, uninfected animals with numerous sampling time points after dosing as well as data obtained from infected animals with limited sampling. The healthy animals provided one PK sample per animal by destructive sampling, while the infected animals provided two PK samples per animal by submandibular sampling. Each infected animal also provided one PD sample (CFU count) by destructive sampling. To facilitate a reliable characterization of spectinamide 1810 in all studied conditions and dosing scenarios, we employed a population PK analysis approach using nonlinear mixed-effects modeling and integrated Bayesian priors to stabilize the analysis despite the data set with limited samples per animal. Bayesian approaches were successfully implemented in previous studies for the estimation of PK parameters from limited data by incorporating prior information obtained from more informative studies (46, 47).
In a first step, a population PK model was developed to describe the pharmacokinetics of spectinamide 1810 from a total of 84 observations from 84 healthy animals as assessed in the single- and multiple-dose PK studies. The obtained parameter estimates along with their distributions served as Bayesian priors in the subsequent analysis of the 1810 pharmacokinetics in infected animals. In the second step, 282 concentration-time data from 146 infected animals were modeled using the Bayesian priors via the PRIOR NWPRI subroutine in the NONMEM software package and the healthy-animal data to improve robustness and stability of the parameter estimates as previously described (see the supplemental material for more details) (48, 49). The concentration data were natural log transformed and modeled using a log-transform-both-sides (LTBS) approach (50). The evaluation of the structural PK model was undertaken based on graphical exploratory data analysis, basic goodness-of-fit plots, and previous PK knowledge of compounds from the spectinamide series (4, 51).
For all models evaluated in this analysis, standard population modeling methodology was used (52). Between-animal variability was modeled as a log-normal distribution, and residual unexplained variability (RUV) was characterized as an additive error, which relates to a proportional error when an LTBS approach is used.
Identification of exposure drivers for efficacy.
Based on the individual PK parameters obtained by post hoc estimation from the population PK model and the known low plasma protein binding of spectinamide 1810 in mice (31%) (6), the PK/PD indices fCmax/MIC, fAUC/MIC, and f%TMIC for unbound concentrations were calculated at steady state, using the previously determined MIC of 1.6 mg/liter for spectinamide 1810 (6). Cmax was the model-predicted maximum concentration achieved after administration of the last dose; AUC and time for which drug concentration was above MIC were calculated for the time period equal to the longest dosing interval, i.e., 1 week (168 h). For calculating Cmax and AUC, individual PK parameters were obtained for each animal by post hoc estimation from the final population PK model, the steady-state concentration-time profile of spectinamide 1810 was simulated (step size, 0.01 h) for each animal using the respective individual PK parameters and dosing regimen, and Cmax and AUC were derived by noncompartmental analysis methodology. Log CFU versus PK/PD indices were plotted, and corresponding coefficients of determination were calculated.
PK/PD model-based exposure response analysis. (i) Bacterial growth kinetic model.
Since only one bacterial load measurement could be collected per animal and all the samples were collected at the same time after 4 weeks of treatment, there was a lack of time course data for bacterial killing. Therefore, a simple one population bacterial growth kinetic model as expressed in equation 2 was used to describe natural growth kinetics of Mtb in chronically infected mice. A logistic growth function was applied to capture the net growth kinetics between bacterial growth and host mediated killing (53–55).
| (2) |
Here, Kgs represents the net rate between growth rate and host-related kill rate of bacteria. N is the number of bacteria at any given time, which eventually approaches a maximum (Nmax). The parameters describing natural growth kinetics of Mtb were estimated based on the time course of log CFU obtained from current and previous studies performed at Colorado State University using the same mouse and bacterial strains.
(ii) PK/PD modeling with PAE estimation.
Once the natural bacterial growth model had been established, the next step was to integrate the drug effect into it. Based on the individual post hoc estimates from the population PK analysis, a sequential PK/PD analysis was performed by linking exposure to efficacy. Different modeling approaches were explored. These included direct as well indirect link approaches using sigmoidal Emax functions that link drug concentrations to bacterial kill rates (7, 56).
In vitro experiments have previously shown that many spectinamides exhibit a long PAE ranging between 19 and 137 h (4, 6). For spectinamide 1810, preliminary results suggest a PAE of 20 h (6). Based on that knowledge, we explored the potential of an in vivo PAE by integrating and testing different PAE components in the bacterial killing component of the PK/PD model. This was modeled by creating a hypothetical effect compartment concentration that was identical to the central compartment in the model until the peak concentration in each dosing interval but then either persisted at peak concentration for a defined time period, declined after the peak but with a slower decline than in plasma, or a combination thereof. The major difference from a classic effect compartment approach (57) lies in the lack of delay in concentration and effect compartment concentration prior to peak concentrations. This hypothesized dynamic behavior was inspired by one of the theories that PAE is driven by prolonged persistence of a drug at a binding site or within the subcellular target space in the bacteria (21, 58).
The CFU data were log transformed by taking the decadic logarithm to CFU. Similar to the PK analysis, an LTBS approach was also used for the PK/PD model, and RUV was characterized using an additive error. As a single sample was available per animal, it was not feasible to separately estimate between-animal variability; hence, only the RUV characterized as additive error was estimated in the PD part of the model. Study-specific fixed-effect parameters and random effects like RUV were estimated to assess the study specific differences. Basic goodness-of-fit plots, objective function value (OFV), and the Akaike information criterion (AIC) were considered while comparing structural models. The AIC was used to compensate for better fit due to model complexity using the following equation (52):
| (3) |
where NPR is the total number of parameters estimated in each model, including all fixed- and random-effects parameters, and OFV is the objective function value of the model fit.
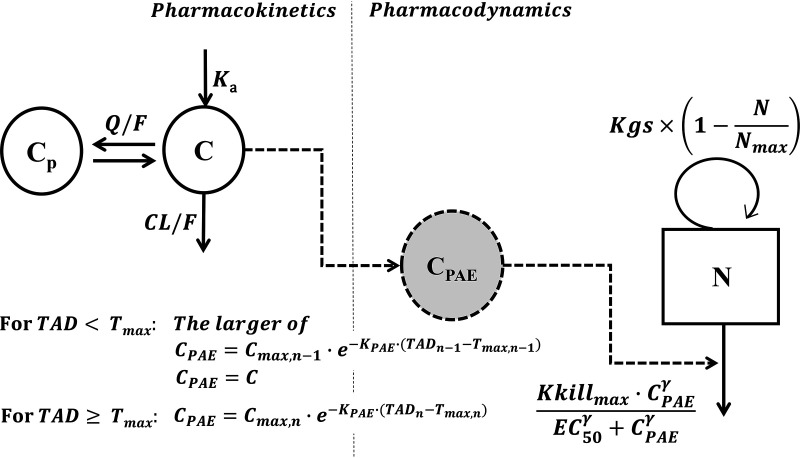
The final model selected that most adequately described the exposure-response relationship for spectinamide 1810 was an integrated PK/PD model with PAE effect, where the killing effect of spectinamide 1810 is linked to plasma concentrations via a PAE model component in which drug decline after peak plasma concentrations is delayed following a first-order process characterized by a rate constant (KPAE), as shown in Fig. 5. The parameters related to the bacterial natural growth model were fixed during the PK/PD analysis to the previously established values, and only the drug effect parameters were estimated. With the addition of drug effect to the natural growth model, equation 2 was then modified to equation 4 (53–55):
| (4) |
where CPAE represents the concentration in the hypothetical PAE compartment at time t, Kkillmax is the maximum kill rate induced by spectinamide 1810, EC50 is the concentration at half-maximal kill rate, and γ is the Hill coefficient.
FIG 5.
PK/PD model to establish the relationship between exposure and response. Abbreviations: Ka, absorption rate constant; Vc/F, volume of central compartment corrected for bioavailability; Vp/F, volume of peripheral compartment corrected for bioavailability; CL/F, clearance corrected for bioavailability; Q/F, intercompartmental clearance corrected for bioavailability. C, CP, and CPAE represent spectinamide 1810 concentration in central, peripheral, and hypothetical PAE compartments, respectively; TAD, time after dose; Tmax, time corresponding to maximum plasma concentration (Cmax) of spectinamide 1810; Kkillmax, maximum kill rate induced by spectinamide 1810; EC50, concentration of spectinamide 1810 at half of Kkillmax; γ, Hill function; KPAE, first-order rate constant for dissipation of post antibiotic effect; Kgs, first-order net growth rate constant; N, number of bacteria at any given time which eventually approaches a maximum (Nmax); n, nth dosing interval; n − 1, dosing interval preceding the nth dosing interval.
In the initial stages of model development, models were evaluated based on OFV and basic goodness-of-fit plots, such as observed versus predicted values, and distribution of weighted and conditional weighted residuals across time and predicted values. In the later stages, simulation-based methods, including visual predictive check and bootstrap analysis, were used for model qualification. In the visual predictive check, the final model was used to simulate 1,000 replicates of the original data, and the median and the 5th and 95th percentiles of the simulated concentrations and CFU values were compared with the observed data. Likewise, a nonparametric bootstrap analysis was executed with 1,000 replicates to assess the robustness of the final parameter estimates.
Modeling and simulations were performed using the software packages NONMEM (v.7.4; Icon Development Solutions, Ellicott City, MD) with the First Order Conditional Estimation algorithm with η-ε interaction, PsN (v.4.9.0) (59), and Pirana (v.2.9.8; Certara, Princeton, NJ). Plots were generated using GraphPad Prism (v.7; GraphPad Software, San Diego, CA) or the ggplot2 package in R (v.3.5.1; R Foundation for Statistical Computing, Vienna, Austria). AUC was calculated using the metrumrg package in R (Metrum Research Group, Tariffville, CT).
ACKNOWLEDGMENTS
This research was supported by the National Institute of Allergy and Infectious Diseases and the Office of the Director of the National Institutes of Health (grants R01AI090810, R01AI120670, and S10OD016226), and ALSAC, St. Jude Children's Research Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Furin J, Cox H, Pai M. 2019. Tuberculosis. Lancet 393:1642–1656. 10.1016/S0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
- 2.Rabahi MF, Silva Junior J, Ferreira ACG, Tannus-Silva DGS, Conde MB. 2017. Tuberculosis treatment. J Bras Pneumol 43:472–486. 10.1590/S1806-37562016000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson GT, Scherman MS, Bruhn DF, Liu J, Hastings C, McNeil MR, Butler MM, Bowlin TL, Lee RB, Lee RE, Lenaerts AJ. 2017. Spectinamides are effective partner agents for the treatment of tuberculosis in multiple mouse infection models. J Antimicrob Chemother 72:770–777. 10.1093/jac/dkw467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman MS, Vaddady PK, Zheng Z, Qi J, Akbergenov R, Das S, Madhura DB, Rathi C, Trivedi A, Villellas C, Lee RB, Rakesh Waidyarachchi SL, Sun D, McNeil MR, Ainsa JA, Boshoff HI, Gonzalez-Juarrero M, Meibohm B, Bottger EC, Lenaerts AJ. 2014. Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med 20:152–158. 10.1038/nm.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budha NR, Lee RE, Meibohm B. 2008. Biopharmaceutics, pharmacokinetics and pharmacodynamics of antituberculosis drugs. Curr Med Chem 15:809–825. 10.2174/092986708783955509. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Bruhn DF, Lee RB, Zheng Z, Janusic T, Scherbakov D, Scherman MS, Boshoff HI, Das S, Rakesh Waidyarachchi SL, Brewer TA, Gracia B, Yang L, Bollinger J, Robertson GT, Meibohm B, Lenaerts AJ, Ainsa J, Bottger EC, Lee RE. 2017. Structure-activity relationships of spectinamide antituberculosis agents: a dissection of ribosomal inhibition and native efflux avoidance contributions. ACS Infect Dis 3:72–88. 10.1021/acsinfecdis.6b00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathi C, Lee RE, Meibohm B. 2016. Translational PK/PD of anti-infective therapeutics. Drug Discov Today Technol 21-22:41–49. 10.1016/j.ddtec.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 63:e02307-18. 10.1128/AAC.02307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clewe O, Aulin L, Hu Y, Coates AR, Simonsson US. 2016. A multistate tuberculosis pharmacometric model: a framework for studying anti-tubercular drug effects in vitro. J Antimicrob Chemother 71:964–974. 10.1093/jac/dkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Ortega F, Rullas J, Alameda L, Angulo-Barturen I, Ferrer S, Simonsson US. 2017. The multistate tuberculosis pharmacometric model: a semi-mechanistic pharmacokinetic-pharmacodynamic model for studying drug effects in an acute tuberculosis mouse model. J Pharmacokinet Pharmacodyn 44:133–141. 10.1007/s10928-017-9508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande D, Pasipanodya JG, Srivastava S, Bendet P, Koeuth T, Bhavnani SM, Ambrose PG, Smythe W, McIlleron H, Thwaites G, Gumusboga M, Van Deun A, Gumbo T. 2018. Gatifloxacin pharmacokinetics/pharmacodynamics-based optimal dosing for pulmonary and meningeal multidrug-resistant tuberculosis. Clin Infect Dis 67:S274–S283. 10.1093/cid/ciy618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrichs MT, Drusano GL, Brown DL, Maynard MS, Sy SKB, Rand KH, Peloquin CA, Louie A, Derendorf H. 2019. Dose optimization of moxifloxacin and linezolid against tuberculosis using mathematical modeling and simulation. Int J Antimicrob Agents 53:275–283. 10.1016/j.ijantimicag.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Strydom N, Tyagi S, Soni H, Tasneen R, Nuermberger EL, Savic RM. 2020. Mechanistic modeling of Mycobacterium tuberculosis infection in murine models for drug and vaccine efficacy studies. Antimicrob Agents Chemother 64:e01727-19. 10.1128/AAC.01727-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drusano GL, Duncanson B, Scanga CA, Kim S, Schmidt S, Neely MN, Yamada WM, Vicchiarelli M, Peloquin CA, Louie A. 2020. Developing new drugs for Mycobacterium tuberculosis therapy: what information do we get from preclinical animal models? Antimicrob Agents Chemother 64:e01376-20. 10.1128/AAC.01376-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaddady PK, Lee RE, Meibohm B. 2010. In vitro pharmacokinetic/pharmacodynamic models in anti-infective drug development: focus on TB. Future Med Chem 2:1355–1369. 10.4155/fmc.10.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen EI, Cars O, Friberg LE. 2011. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother 55:4619–4630. 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad Z, Peloquin CA, Singh RP, Derendorf H, Tyagi S, Ginsberg A, Grosset JH, Nuermberger EL. 2011. PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob Agents Chemother 55:239–245. 10.1128/AAC.00849-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peloquin CA, Velasquez GE, Lecca L, Calderon RI, Coit J, Milstein M, Osso E, Jimenez J, Tintaya K, Sanchez Garavito E, Vargas Vasquez D, Mitnick CD, Davies G. 2017. Pharmacokinetic evidence from the HIRIF trial to support increased doses of rifampin for tuberculosis. Antimicrob Agents Chemother 61:e00038-17. 10.1128/AAC.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacKenzie FM, Gould IM. 1993. The post-antibiotic effect. J Antimicrob Chemother 32:519–537. 10.1093/jac/32.4.519. [DOI] [PubMed] [Google Scholar]
- 20.Li RC, Lee SW, Kong CH. 1997. Correlation between bactericidal activity and postantibiotic effect for five antibiotics with different mechanisms of action. J Antimicrob Chemother 40:39–45. 10.1093/jac/40.1.39. [DOI] [PubMed] [Google Scholar]
- 21.Spivey JM. 1992. The postantibiotic effect. Clin Pharm 11:865–875. [PubMed] [Google Scholar]
- 22.Chan CY, Au-Yeang C, Yew WW, Hui M, Cheng AF. 2001. Postantibiotic effects of antituberculosis agents alone and in combination. Antimicrob Agents Chemother 45:3631–3634. 10.1128/AAC.45.12.3631-3634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan CY, Au-Yeang C, Yew WW, Leung CC, Cheng AF. 2004. In vitro postantibiotic effects of rifapentine, isoniazid, and moxifloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother 48:340–343. 10.1128/AAC.48.1.340-343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui M, Au-Yeang C, Wong KT, Chan CY, Yew WW, Leung CC. 2008. Post-antibiotic effects of linezolid and other agents against Mycobacterium tuberculosis. Int J Antimicrob Agents 31:395–396. 10.1016/j.ijantimicag.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhanel GG, Craig WA. 1994. Pharmacokinetic contributions to postantibiotic effects. Focus on aminoglycosides. Clin Pharmacokinet 27:377–392. 10.2165/00003088-199427050-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zhanel GG, Hoban DJ, Harding GK. 1991. The postantibiotic effect: a review of in vitro and in vivo data. DICP 25:153–163. 10.1177/106002809102500210. [DOI] [PubMed] [Google Scholar]
- 27.Davoodi S, Daryaee F, Chang A, Walker SG, Tonge PJ. 2020. Correlating drug-target residence time and post-antibiotic effect: insight into target vulnerability. ACS Infect Dis 6:629–636. 10.1021/acsinfecdis.9b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhura DB, Lee R, Meibohm B. 2013. Pharmacokinetic profile of spectinomycin in rats. Pharmazie 68:675–676. [PMC free article] [PubMed] [Google Scholar]
- 29.Budha NR, Lee RB, Hurdle JG, Lee RE, Meibohm B. 2009. A simple in vitro PK/PD model system to determine time-kill curves of drugs against mycobacteria. Tuberculosis (Edinb) 89:378–385. 10.1016/j.tube.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul S, Laochumroonvorapong P, Kaplan G. 1996. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J Infect Dis 174:105–112. 10.1093/infdis/174.1.105. [DOI] [PubMed] [Google Scholar]
- 31.Chanwong S, Maneekarn N, Makonkawkeyoon L, Makonkawkeyoon S. 2007. Intracellular growth and drug susceptibility of Mycobacterium tuberculosis in macrophages. Tuberculosis (Edinb) 87:130–133. 10.1016/j.tube.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Meibohm B, Laer S, Panetta JC, Barrett JS. 2005. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J 7:E475–E487. 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laer S, Elshoff JP, Meibohm B, Weil J, Mir TS, Zhang W, Hulpke-Wette M. 2005. Development of a safe and effective pediatric dosing regimen for sotalol based on population pharmacokinetics and pharmacodynamics in children with supraventricular tachycardia. J Am Coll Cardiol 46:1322–1330. 10.1016/j.jacc.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 34.Vaddady PK, Trivedi A, Rathi C, Madhura DB, Liu J, Lee RE, Meibohm B. 2019. Dynamic time-kill curve characterization of spectinamide antibiotics 1445 and 1599 for the treatment of tuberculosis. Eur J Pharm Sci 127:233–239. 10.1016/j.ejps.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons MA, Lenaerts AJ. 2015. Computational pharmacokinetics/pharmacodynamics of rifampin in a mouse tuberculosis infection model. J Pharmacokinet Pharmacodyn 42:375–389. 10.1007/s10928-015-9419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. 2015. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 211(Suppl 3):S96–S106. 10.1093/infdis/jiu610. [DOI] [PubMed] [Google Scholar]
- 37.Jayaram R, Shandil RK, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Kantharaj E, Balasubramanian V. 2004. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 48:2951–2957. 10.1128/AAC.48.8.2951-2957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 47:2118–2124. 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad Z, Fraig MM, Bisson GP, Nuermberger EL, Grosset JH, Karakousis PC. 2011. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob Agents Chemother 55:1527–1532. 10.1128/AAC.01524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickinson JM, Ellard GA, Mitchison DA. 1968. Suitability of isoniazid and ethambutol for intermittent administration in the treatment of tuberculosis. Tubercle 49:351–366. 10.1016/s0041-3879(68)80016-9. [DOI] [PubMed] [Google Scholar]
- 41.Davies GR, Brindle R, Khoo SH, Aarons LJ. 2006. Use of nonlinear mixed-effects analysis for improved precision of early pharmacodynamic measures in tuberculosis treatment. Antimicrob Agents Chemother 50:3154–3156. 10.1128/AAC.00774-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillespie SH, Gosling RD, Charalambous BM. 2002. A reiterative method for calculating the early bactericidal activity of antituberculosis drugs. Am J Respir Crit Care Med 166:31–35. 10.1164/rccm.2112077. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis 200:1136–1143. 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 44.de Steenwinkel JE, ten Kate MT, de Knegt GJ, Verbrugh HA, van Belkum A, Hernandez-Pando R, Bakker-Woudenberg IA. 2011. Course of murine tuberculosis and response to first-line therapy depends on route of infection and inoculum size. Int J Tuber Lung Dis 15:1478–1484. 10.5588/ijtld.11.0012. [DOI] [PubMed] [Google Scholar]
- 45.Driver ER, Ryan GJ, Hoff DR, Irwin SM, Basaraba RJ, Kramnik I, Lenaerts AJ. 2012. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:3181–3195. 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemoto A, Masaaki M, Yamaoka K. 2017. A Bayesian approach for population pharmacokinetic modeling of alcohol in Japanese individuals. Curr Ther Res Clin Exp 84:42–49. 10.1016/j.curtheres.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dansirikul C, Morris RG, Tett SE, Duffull SB. 2006. A Bayesian approach for population pharmacokinetic modelling of sirolimus. Br J Clin Pharmacol 62:420–434. 10.1111/j.1365-2125.2005.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gisleskog PO, Karlsson MO, Beal SL. 2002. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn 29:473–505. 10.1023/a:1022972420004. [DOI] [PubMed] [Google Scholar]
- 49.Chan Kwong AHP, Calvier EAM, Fabre D, Gattacceca F, Khier S. 2020. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: overview and guidance with a focus on the NONMEM PRIOR subroutine. J Pharmacokinet Pharmacodyn 47:431–446. 10.1007/s10928-020-09695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLeay SC, Vis P, van Heeswijk RP, Green B. 2014. Population pharmacokinetics of bedaquiline (TMC207), a novel antituberculosis drug. Antimicrob Agents Chemother 58:5315–5324. 10.1128/AAC.01418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rathi C, Lukka PB, Wagh S, Lee RE, Lenaerts AJ, Braunstein M, Hickey A, Gonzalez-Juarrero M, Meibohm B. 2019. Comparative pharmacokinetics of spectinamide 1599 after subcutaneous and intrapulmonary aerosol administration in mice. Tuberculosis (Edinb) 114:119–122. 10.1016/j.tube.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mould DR, Upton RN. 2013. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2:e38. 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalla Costa T, Nolting A, Rand K, Derendorf H. 1997. Pharmacokinetic-pharmacodynamic modelling of the in vitro antiinfective effect of piperacillin-tazobactam combinations. Int J Clin Pharmacol Ther 35:426–433. [PubMed] [Google Scholar]
- 54.Yano Y, Oguma T, Nagata H, Sasaki S. 1998. Application of logistic growth model to pharmacodynamic analysis of in vitro bactericidal kinetics. J Pharm Sci 87:1177–1183. 10.1021/js9801337. [DOI] [PubMed] [Google Scholar]
- 55.Jumbe N, Louie A, Leary R, Liu W, Deziel MR, Tam VH, Bachhawat R, Freeman C, Kahn JB, Bush K, Dudley MN, Miller MH, Drusano GL. 2003. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J Clin Invest 112:275–285. 10.1172/JCI16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meibohm B, Derendorf H. 1997. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther 35:401–413. [PubMed] [Google Scholar]
- 57.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. 1979. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther 25:358–371. 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 58.Vogelman BS, Craig WA. 1985. Postantibiotic effects. J Antimicrob Chemother 15(Suppl A):37–46. 10.1093/jac/15.suppl_a.37. [DOI] [PubMed] [Google Scholar]
- 59.Lindbom L, Pihlgren P, Jonsson EN, Jonsson N. 2005. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.01744-20-s0001.pdf, PDF file, 0.7 MB (674.1KB, pdf)