Introduction
Over the last decade, therapies for moderate-to-severe ulcerative colitis (UC) have expanded to target not only tumor necrosis factor-alpha (TNF-α; infliximab, adalimumab, golimumab) agents but also α 4β 7 integrin (vedolizumab), interleukins 12 and 23 (ustekinumab), and Janus kinase inhibitors (tofacitinib). However, head-to-head clinical trials comparing these agents are limited,1 and optimal positioning of biologics is an area of ongoing investigation.
A recent meta-analysis identified infliximab as the preferred first-line therapy for induction of clinical remission in patients who are biologic-naïve and either tofacitinib or ustekinumab as preferred agents after anti-TNF failure.2 However, many patients considering tofacitinib and ustekinumab, which were approved for UC after vedolizumab, have had anti-integrin failure as well. The OCTAVE trials of tofacitinib induction and maintenance therapy did not report the number of patients previously exposed to both anti-TNFs and vedolizumab.3 In the UNIFI trials of ustekinumab induction and maintenance therapy, only 17% of patients had prior exposure to both drug classes, among whom approximately 10% achieved remission at week 8.4 Nevertheless, the efficacies of tofacitinib and ustekinumab in this refractory population within real-world settings are poorly understood. We therefore sought to compare tofacitinib vs ustekinumab among patients with UC with prior anti-TNF and anti-integrin failure.
METHODS
Study Design and Patient Enrollment
This retrospective cohort study included adults with prior anti-TNF and anti-integrin failure initiating tofacitinib or ustekinumab for UC (ICD-10 code K51x) between January 1, 2015 and July 1, 2020 in the outpatient setting at the Brigham and Women’s Hospital or Massachusetts General Hospital (both in Boston, MA). Patients initiating treatment for non-UC indications and patients with prior colectomy were excluded. Electronic health records were reviewed for clinical data. The Simple Clinical Colitis Activity Index (SCCAI), which is documented in clinic notes in our health system, was used to track disease activity. This study was approved by the institutional review board of the Brigham and Women’s Hospital.
Independent Variables
Independent variables at the time of treatment initiation included age; sex; race; UC duration; extraintestinal manifestations; substance use (cigarette smoking, cannabis, and opioids); UC extent (Montreal classification); last Mayo endoscopic subscore; number of prior biologic exposures; prior or current azathioprine, 6-mercaptopurine, or methotrexate use; and current corticosteroid use (prednisone, methylprednisolone, or oral budesonide) and the most recent values of the following variables within 12 weeks before drug initiation: body mass index (BMI), serum albumin, C-reactive protein, fecal calprotectin, SCCAI score, and daily bowel frequency.
Outcomes
The primary outcome was steroid-free clinical remission at 12 to 16 weeks after drug initiation defined by an SCCAI score ≤2 and no use of prednisone, methylprednisolone, or oral budesonide. Secondary outcomes included steroid-free clinical response at 12 to 16 weeks (reduction in baseline SCCAI score by ≥2 points), colectomy-free drug survival (time to treatment discontinuation or colectomy because of treatment inefficacy), and adverse events. Patients with treatment discontinuation because of inefficacy before 12 to 16 weeks were considered nonresponders. Additional descriptive outcomes are listed in the Supplementary Methods.
Statistical Analysis
Continuous data were compared using the Student t test or Wilcoxon rank sum test based on normality. Categorical data were compared using the Fisher exact test. Logistic regression was used to calculate unadjusted odds ratios (ORs) for the association between tofacitinib vs ustekinumab with steroid-free remission and response. Propensity scores (PSs) were estimated using a multivariable logistic regression model in which tofacitinib vs ustekinumab was regressed on independent variables. Kernel weighting was implemented using the psmatch2 and pstest commands in Stata (Stata/IC 15.1, StataCorp, College Station, TX). Covariate balance was assessed using Rubin’s B, Rubin’s R, and absolute standardized bias for each covariate (Supplementary Methods).5 The weighted sample was then used in logistic regression models to calculate ORs for the association between treatment and steroid-free remission and response.
Kaplan-Meier curves stratified by treatment were constructed for colectomy-free drug survival. Patients were censored at loss of follow-up or treatment discontinuation for reasons other than inefficacy (eg, nonadherence). The log-rank test was used to compare survival distributions between tofacitinib and ustekinumab and time to adverse event. All statistical analyses were performed using Stata/IC 15.1 (StataCorp, College Station, TX).
RESULTS
We identified 81 patients with UC who initiated tofacitinib (n = 45) 10 mg twice daily or ustekinumab (n = 36) 90 mg every 8 weeks (after weight-based induction) after anti-TNF and anti-integrin treatment failure. Median follow-up was 675 days (interquartile range, 537-1106 days) for tofacitinib and 407 days (interquartile range, 207-1191 days) for ustekinumab. Baseline characteristics by treatment are presented in Table 1. Among patients with available SCCAI data after treatment initiation (n = 76), there were similar rates of steroid-free remission (43.9% tofacitinib vs 40.0% ustekinumab; P = 0.82) and steroid-free response (46.3% tofacitinib vs 48.6% ustekinumab; P = 1.00) by treatment at 12 to 16 weeks. Additional descriptive outcomes are presented in Table 2. Univariable logistic regression showed no significant associations between tofacitinib vs ustekinumab and steroid-free remission (OR, 1.17; 95% confidence interval [CI], 0.47-2.93) or steroid-free response (OR, 0.91; 95% CI, 0.37-2.26).
TABLE 1.
Baseline Characteristics: Tofacitinib vs Ustekinumab
| Characteristics* | Tofacitinib (n = 45) | Ustekinumab (n = 36) | P † |
|---|---|---|---|
| Female, fraction (%) | 25/45 (55.6) | 19/36 (52.8) | 0.83 |
| Age, y, mean (SD) | 44.2 (14.9) | 41.7 (11.5) | 0.42 |
| UC duration, y, mean (SD) | 10.6 (5.9) | 13.6 (8.6) | 0.06 |
| BMI, mean (SD) | 25.7 (5.8) | 25.6 (5.5) | 0.92 |
| Race, fraction (%) | 0.48 | ||
| White | 41/45 (91.1) | 33/36 (91.7) | |
| Black | 2/45 (4.4) | 0/36 (0.0) | |
| Asian | 2/46 (4.4) | 3/36 (8.3) | |
| Disease extent, fraction (%) | 0.91 | ||
| Proctitis | 1/45 (2.2) | 1/36 (2.8) | |
| Left-sided | 18/45 (40.0) | 13/36 (36.1) | |
| Pancolitis | 26/45 (57.8) | 22/36 (61.1) | |
| Last Mayo endoscopic subscore, fraction (%) | 0.42 | ||
| Normal or mild (≤1) | 7/45 (15.6) | 11/36 (30.6) | |
| Moderate (2) | 24/45 (53.3) | 16/36 (44.4) | |
| Severe (3) | 14/45 (31.1) | 9/36 (26.0) | |
| Extraintestinal manifestation, fraction (%) | 15/45 (33.3) | 16/36 (44.4) | 0.36 |
| >2 prior biologics, fraction (%) | 29/45 (64.4) | 17/36 (47.2) | 0.18 |
| >3 prior biologics, fraction (%) | 8/45 (17.8) | 6/36 (16.7) | 1.00 |
| Prior immunomodulator,‡ fraction (%) | 37/45 (82.2) | 28/36 (77.8) | 0.78 |
| Current immunomodulator, fraction (%) | 1/45 (2.2) | 8/36 (22.2) | <0.01 |
| Current corticosteroids,§ fraction (%) | 25/45 (55.6) | 23/36 (63.9) | 0.50 |
| Current smoking, fraction (%) | 2/45 (4.4) | 0/36 (0.0) | 0.50 |
| Current cannabis, fraction (%) | 4/45 (8.9) | 5/36 (13.9) | 0.50 |
| Current opioids, fraction (%) | 3/45 (6.7) | 3/36 (8.3) | 1.00 |
| SCCAI, mean (SD) | 6.1 (5.2) | 5.3 (4.3) | 0.25 |
| Laboratory values | |||
| Albumin, mean (SD), g/dL | 4.0 (0.4) | 4.1 (0.4) | 0.18 |
| C-reactive protein, mean (SD), mg/L, n = 45, 35 | 16.1 (26.6) | 12.4 (25.3) | 0.54 |
| Fecal calprotectin, median (IQR), µg/g, n = 13, 13 | 435 (320-940.3) | 604 (214-1394.4) | 0.86 |
Denominators for outcomes vary because of unavailable data.
*Baseline characteristics represent the most recent clinical data available within 3 months before ustekinumab initiation.
† Calculated using Student t test, Wilcoxon rank sum test, or Fisher exact test.
‡Includes azathioprine, 6-mercaptopurine, and methotrexate.
§Includes prednisone, methylprednisolone, and oral budesonide preparations.
BMI indicates body mass index; IQR, interquartile range.
TABLE 2.
Clinical Outcomes: Tofacitinib vs Ustekinumab
| Outcomes | Tofacitinib | Ustekinumab | P * |
|---|---|---|---|
| Steroid-free clinical remission 12-16 wks, fraction (%) | 18/41 (43.9) | 14/35 (40.0) | 0.82 |
| Steroid-free clinical response 12-16 wks, fraction (%) | 19/41 (46.3) | 17/35 (48.6) | 1.00 |
| Colectomy-free drug survival, fraction (%)† | 22/45 (48.9) | 23/36 (63.9) | 0.75 |
| Adverse drug reaction or infection, fraction (%)† | 5/45 (11.1) | 2/36 (5.6) | 0.57 |
| Steroid-free clinical remission 52 wks, fraction (%) | 15/25 (60.0) | 6/11 (54.6) | — |
| Endoscopic remission, fraction (%)† | 7/25 (28.0) | 3/18 (16.7) | — |
| Dose de-escalation, fraction (%)‡ | 17/45 (37.8) | — | — |
| Dose escalation, fraction (%)‡ | — | 12/36 (33.3) | — |
*Calculated using Fisher exact test or log-rank test. The last 4 outcomes are intended to be descriptive, so P values are not calculated.
† These outcomes are assessed over all available follow-up of 675 days (IQR, 537-1106 days) for tofacitinib and 407 days (IQR, 207-1191 days) for ustekinumab.
‡These outcomes were assessed at any point over all available follow-up. In the tofacitinib group, 17 patients underwent dose de-escalation to a total daily dose of 10 mg (n = 15) or 15 mg (n = 2). In the ustekinumab group, 12 patients underwent dose escalation to every 4 weeks (n = 6) or every 6 weeks (n = 6).
IQR indicates interquartile range.
The PS was estimated using a logistic regression of tofacitinib vs ustekinumab on all independent variables. A high standard of matching was confirmed with a Rubin’s B of 18.9%, a Rubin’s R of 1.64, and <10% absolute standardized bias across all covariates (Supplementary Fig. 1).5, 6 The PS incorporated the following baseline covariates: age category (18-25, 26-59, or ≥60 years), UC duration category (≤10 or >10 years), female sex, black race, body mass index category (<25.0, 25.0-29.9, ≥30.0), extraintestinal manifestations, current smoking, current cannabis use, current opioid use, current steroid use, SCCAI score, >3 prior biologic exposures, pancolitis, last Mayo endoscopic subscore >1, last serum albumin category (≤4 or ≥4 g/dL, dichotomized by median value), and last C-reactive protein category (≤ 5.3 or ≥5.3 mg/L, dichotomized by median value). Fecal calprotectin was excluded because of missing data in >50% of the cohort. The final kernel-weighted cohort included 72 patients (ie, 9/81 were excluded: 5 with incomplete baseline covariates, 4 outside the range of common support). There was no significant difference between tofacitinib vs ustekinumab for steroid-free remission (OR, 1.65; 95% CI, 0.60-4.54) or steroid-free response (OR, 0.79; 95% CI, 0.28-2.24) at 12 to 16 weeks after kernel weighting.
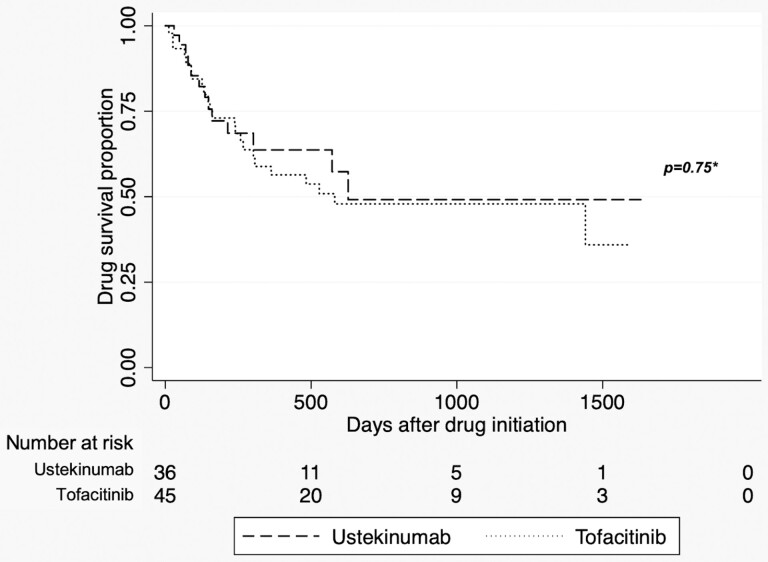
Drug discontinuation or total colectomy because of treatment failure occurred in 23/45 (51.1%) patients in the tofacitinib group and 13/36 (36.1%) patients in the ustekinumab group over available follow-up. Kaplan-Meier analysis stratified by treatment indicated no difference in colectomy-free drug survival (P = 0.75, log-rank test; Fig. 1). Adverse events were similar between groups (11.1% tofacitinib vs 5.6% ustekinumab, P = 0.57 by log-rank test), which included norovirus infection, deep vein thrombosis, liver injury, refractory nausea/vomiting, and shingles for tofacitinib and rash and urinary tract infection for ustekinumab.
FIGURE 1.
Kaplan-Meier analysis: colectomy-free drug survival stratified by treatment. Numbers on curves represent censoring at loss of follow-up. *Log-rank test.
Discussion
There remains little guidance for clinicians regarding the positioning of biologics for UC, an area of increasing complexity with the expansion of therapeutic options. In our treatment-refractory population, both tofacitinib and ustekinumab were effective at inducing steroid-free remission in approximately 40% of patients at 12 to 16 weeks. When comparing treatments, we observed no difference in the unadjusted odds of achieving remission and no difference in the drug survival curves on Kaplan-Meier analysis. After PS weighting to reduce treatment selection bias, the lack of associations between treatment and outcomes persisted.
This is the first study to describe and compare outcomes for patients initiating tofacitinib or ustekinumab after failure of 2 biologic classes. However, our results suggest that both agents may be reasonable considerations in this population. Although this study emphasizes short-term outcomes, the few patients with 52 weeks of follow-up (n = 25 for tofacitinib, n = 11 for ustekinumab) experienced higher rates of clinical remission than at 12 to 16 weeks. Evaluation of posttreatment endoscopic data over a median of 675 days of follow-up for ustekinumab and 407 days for tofacitinib suggested relatively low rates of endoscopic remission. However, the decision to undergo endoscopy was at the discretion of providers, who may be more likely to pursue this evaluation in patients with clinically active disease. A prospective study design would be needed to accurately assess endoscopic remission.
Despite our relatively small cohort, we observed expected adverse events occur in the tofacitinib group (eg, shingles, deep vein thrombosis, liver injury) that could be viewed as being of concern to patients and providers. In contrast, events in the ustekinumab group (urinary tract infection and rash) were mild and relatively nonspecific, consistent with prior data showing the long-term safety of this therapy.7 We speculate that larger, prospective comparisons of these drugs may identify greater safety with ustekinumab.
This retrospective study has several limitations. Our study was not powered to detect small differences in outcomes; the unweighted sample had an 80% power to detect a 30% difference in outcomes (Supplementary Methods). Therefore, we cannot conclude that tofacitinib and ustekinumab are equally efficacious. The strengths of the study include the use of multiple statistical methods to compare treatment efficacy, including logistic regression, Kaplan-Meier analysis, and PS weighting. The PS weighting successfully balanced baseline covariates to mitigate confounding by indication, which could not be addressed using traditional multivariable models that would suffer from overfitting. However, we acknowledge that unmeasured confounding may still exist.
Conclusions
Both tofacitinib and ustekinumab seem to be effective third-line classes of therapy in patients with refractory UC. However, the comparative effectiveness and safety of these agents and biomarkers that may help select individuals for each therapy remain unknown. Prospective studies and head-to-head clinical trials of tofacitinib vs ustekinumab are needed to address these gaps in knowledge.
Supplementary Material
Acknowledgments
We thank Harvard Catalyst for providing statistical support.
Author contributions: RSD: study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, drafting of manuscript; JM and HG: acquisition of data; JRA: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision.
Supported by: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007533 to RSD).
Conflicts of interest: JRA serves as a consultant for Takeda, Janssen, Pfizer, Pandion, Servatus, Finch Therapeutics, Iterative Scopes, and Artugen and has grant support from Merck. RD, JM, and HG have no financial or personal conflicts of interest to disclose.
References
- 1. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. ; VARSITY Study Group . Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381:1215–1226. [DOI] [PubMed] [Google Scholar]
- 2. Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2179–2191.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 4. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 5. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Services Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 6. Morgan CJ. Reducing bias using propensity score matching. J Nucl Cardiol. 2018;25:404–406. [DOI] [PubMed] [Google Scholar]
- 7. Panaccione R, Danese S, Sandborn WJ, et al. Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy. Aliment Pharmacol Ther. 2020;52:1658–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.