Abstract
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, immune-mediated diseases of the gastrointestinal (GI) tract. Their etiology is complex and involves immune (eg, cytokines) and nonimmune (eg, environment) mediated contributions, causing inflammatory damage to the GI tract. Though cytokines contribute a major role in the inflammatory process of both CD and UC, there are some key differences in which cytokines are involved in the pathobiology of CD and UC. Over the past several years, new biologic-directed therapies have focused on controlling specific aspects of inflammation associated with both conditions. Although these treatments have benefited patients overall, approximately 30% of patients still do not respond to induction (initial) therapy, and up to 50% of patients lose response to treatment over a year. Many of these therapies are administered parenterally and have been associated with adverse events such as serious infections or malignancy. Therefore, there is a significant unmet medical need for these patients to minimize symptoms and promote GI healing. There are several therapeutic agents in the pipeline, including oral, small molecules, which hold much promise. One group of small molecules known as Janus kinase (JAK) inhibitors offers an additional option for treatment of chronic inflammatory conditions, based on currently available data. The article will focus on the potential benefits of JAK inhibitors as oral, small molecules, such as the potential role of selectivity, and potential risks.
Keywords: biologic drugs, inflammatory bowel disease treatment, JAK, Crohn’s disease, ulcerative colitis
INTRODUCTION
The inflammatory bowel diseases (IBDs) of Crohn’s disease (CD) and ulcerative colitis (UC) are multifactorial, chronic, relapsing, immune-mediated conditions of the gastrointestinal (GI) tract.1, 2 Although the clinical classification of these conditions is limited to these 2 primary forms, IBD is complex in etiology, clinical presentation, and therapeutic responsiveness. Complexity of this disease is due to the pathologic manifestation of a diverse series of triggers including genetic predisposition, environmental exposures, and the gut microbiota.2 Specific triggers of CD and UC involve a combination of immune- and nonimmune-mediated factors including chemokines, cytokines, and inflammasomes playing a role in the development of these conditions.1 It is not fully understood which of these factors are the initiators of inflammation and which are compounders; however, as a disorder of immune-dysregulation, there is an accepted recognition of the role of the innate immune system to initiate inflammation, followed by the adaptive immune system to maintain this inflammation, resulting in disease progression.1 The first line of defense against pathogenic infection as a potential etiology and many other aspects of the innate and adaptive immune responses are unbalanced3 and lead to a chronic inflammatory state, resulting in the damage observed in both CD and UC. This article is a review of JAK pathway pathophysiology and how the newly available agents in this drug class offer a new target for the treatment of IBD.
METHODS
Literature search strategy included reviewing all original research articles published in the last 10 years generated from an automated, internal (Gilead) OVID literature search for Janus kinase (JAKs) inhibitors and α4β7 integrins, in addition to a PubMed search for articles relating to inflammatory bowel disease and/or abstracts from IBD-related conferences. Search terms included but were not limited to inflammatory bowel disease, tofacitinib, upadacitinib, filgotinib, ozanimod, TD-1473, ustekinumab, vedolizumab, immunogenicity, adherence, compliance, selectivity, adverse events/effects, serious adverse events/effects malignancy, nonmelanoma skin cancer, herpes zoster, serious infection, cytokines, small oral molecules, thromboembolism, deep vein thrombosis, and pulmonary embolism.
Molecular Basis of Action
Cytokines play an important role in maintaining homeostasis of the GI tract. Though many of the components of innate and adaptive immune system produce cytokines that are responsible for the initial inflammatory process, which is designed to be protective in nature, they also play an important role to help “turn off” any protective inflammatory response once the insult has been resolved.1 Crohn’s disease and UC are distinct pathologies with different clinical and pathological features, so it makes sense that different cytokines are produced within the inflamed gut of these patients.4 Because the cytokines released in IBD are implicated in the development of IBD and its complications,4 blocking these pro-inflammatory cytokines has become a focus for the treatment of IBD.4, 5 The discovery of these cytokines as key drivers of this immune-mediated disease has led to the discovery of many drug therapies targeting these pathways.6 Medical treatment of IBD focuses on controlling active and chronic GI inflammation, prevention of disease progression, and induction of clinical, biochemical, endoscopic and histological remission.1 To date, the major focus of therapeutic development is targeting various aspects of the immune system based on the identification of specific immune-mediated inflammatory targets identified in IBD.1 Therefore, both cytokine production and intracellular signaling are important targets for the treatment of IBD.5
The Status of Therapeutic Options in IBD
Current treatment of IBD is based largely upon a trial of treatment approach. Biologics, mainly tumor necrosis factor (TNF) alpha inhibitors, have become the mainstay of treatment as first-line for moderate to severe UC and CD because these agents target the cytokines involved in IBD and thus have revolutionized the treatment of these conditions7; however, they do not induce complete remission in all patients, reflecting the complexity of inflammatory diseases.7 Lack of response to therapy can be as high as 50%.8 The cumulative relapse rates vary between 67% and 83% after 10 years.8 Even for the more recently introduced biological therapies, a significant number of patients have an inadequate response due to primary nonresponse (around 30%) or loss of response over the time after initial remission9 (secondary nonresponders, around 50% of responders) or develop an adverse event that leads to drug discontinuation.8 Biologic limitations include route of administration (intravenous or subcutaneous) and the development of antidrug antibodies (ADAs).10–14
Immunogenicity (detection of ADA) develops in 10%–20% of patients receiving anti-TNF therapy, and the development of these antibodies can lead to loss of response.10 Immunogenicity associated with vedolizumab and ustekinumab ranges from 2% to 5%.11–13 The variability and/or loss of clinical response to a range of therapeutic options has partially driven the need for new drug classes and mechanisms of action. This in addition to a lack of personalized medicine options for patients has made predicting clinical response to any specific therapeutic regimen a challenge for many patients. This is where novel, small molecules such as Janus kinase inhibitors may play a role in the treatment of IBD.
Potential Targets of JAK Inhibitors
Janus kinases are a family of 4 intracellular tyrosine kinases known as JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), and they interact with a family of 7 signal transducers and activators of transcription (STATs),15 which include STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6.16 JAK1, JAK2 and JAK3 are ubiquitously expressed, whereas JAK3 is mainly localized in hematopoietic cells.17
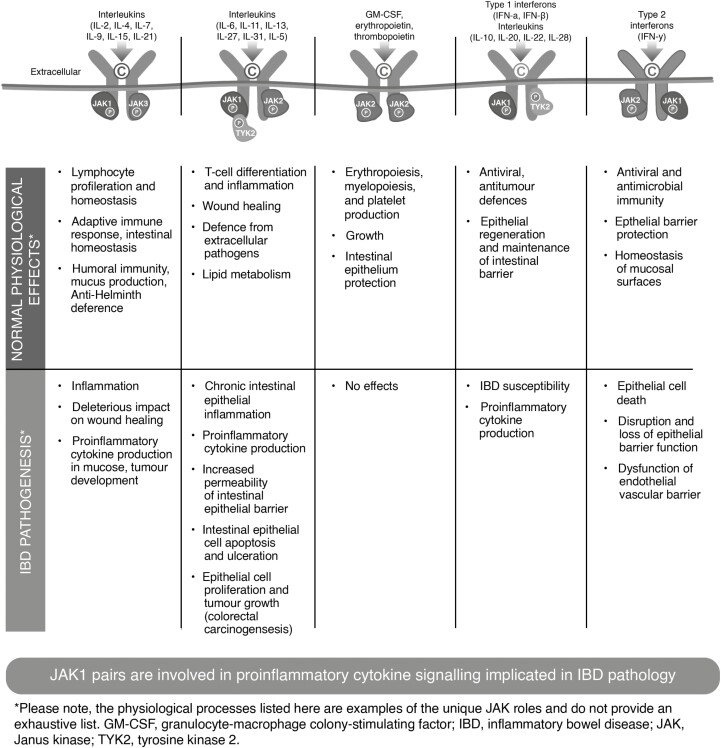
The JAK-STAT pathway has many important functions including playing an active role in innate immunity, adaptive immunity, and hematopoiesis and participating in cellular processes like cell growth, survival, differentiation, and migration of various immune cells.15 Due to this multifactorial role in human physiology, the JAK-STAT signaling pathway plays a key role in several inflammatory diseases17 such as IBD. The JAK-STAT pathway operates downstream of more than 50 to 60 cytokines, growth factors, and hormones and is regarded as a central communication node for the immune system.18, 19 An important element of JAK function is that they pair with each other.20 Because JAKs are activated in pairs, the different combinations of JAKs are associated with different cytokine receptors; thus the inhibition of each type of JAK leads to inhibition of signaling of a specific subset of cytokines and different effects (Fig. 1).8, 19–36Table 1 displays the cytokines and their respective JAK pairing.15, 19, 37
FIGURE 1.
Figure shows the interaction between different cytokines and JAK pairings and resultant physiologic effects in both healthy and disease states. Normal cytokine signaling through JAK pairs is essential for bodily functions such as providing immunity against pathogens and hematopoiesis. In inflammatory bowel disease, an exaggerated immune response leads to excessive signaling through the JAK-STAT pathway, resulting in overexpression of proinflammatory cytokines and a cycle of chronic, relapsing gastrointestinal inflammation and potential damage.
TABLE 1.
Cyokines and Their Associated JAKs
| Cytokine Receptors | Associated JAKs |
|---|---|
| IL-2 | JAK-1, JAK-3 |
| IL-4 | JAK-1, JAK-3 |
| IL-7 | JAK-1, JAK-3 |
| IL-9 | JAK-1, JAK-3 |
| IL-13 | JAK-1, JAK-3, TYK-2 |
| IL-15 | JAK-1, JAK-3 |
| IL-21 | JAK-1, JAK-3 |
| GM-CSF | JAK 2 |
| IL-3 | JAK 2 |
| IL-5 | JAK 2 |
| IL-6 | JAK-1, JAK-2, TYK-2 |
| IL-11 | JAK-1, JAK-2, TYK-2 |
| IL-27 | JAK-1, JAK-2, TYK-2 |
| IL-12 | JAK-2, TYK-2 |
| IL-23 | JAK-2, TYK-2 |
| IL-35 | JAK-1, JAK-2 |
| EPO | JAK-2 |
| TPO | JAK-2 |
| G-CSF | JAK-2 |
| Growth Hormone | JAK-2 |
| Leptin | JAK-2 |
| IFN α/β | JAK-1, TYK-2 |
| IFN-γ | JAK-1, JAK-2 |
| IL-28 | JAK-1, TYK-2 |
| IL-29 | JAK-1, TYK-2 |
| IL-10 | JAK-1, JAK-2, TYK-2 |
| IL-19 | JAK-1, JAK-2, TYK-2 |
| IL-20 | JAK-1, JAK-2, TYK-2 |
| IL-22 | JAK-1, JAK-2, TYK-2 |
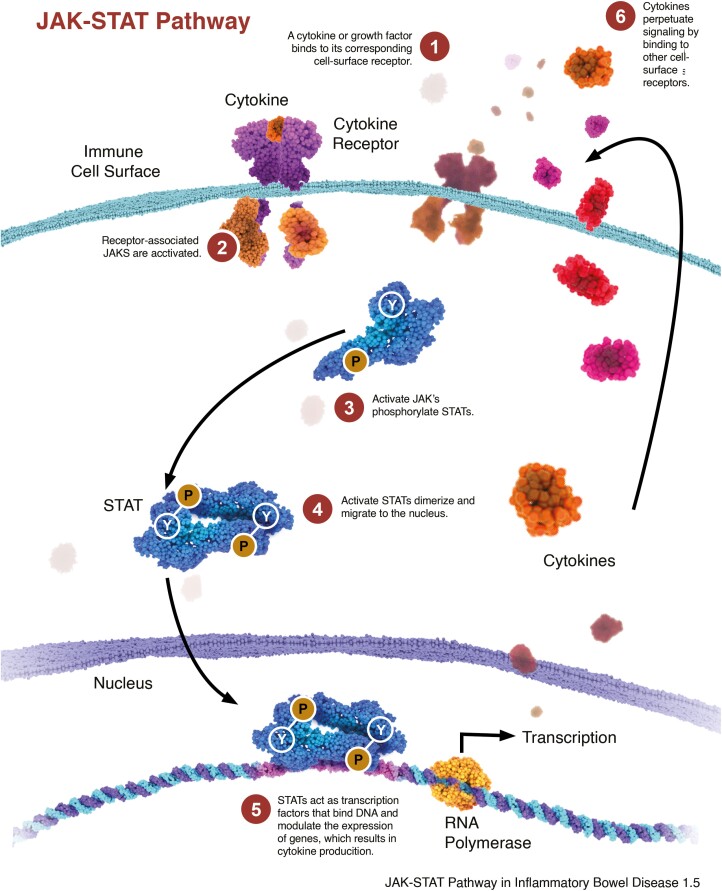
The steps involved in the JAK-STAT pathway10 are as follows (Fig. 2): First, Cytokine binds to its specific cell-surface receptor, causing receptor dimerization and activation of the associated JAKs. Next, activated JAKs are phosphorylated, which then act as docking site for STATS. Once docked, STATs are also phosphorylated by the activated receptor-associated JAKs. Then phosphorylated STATs dissociate from the receptor chains, dimerize with each other, and translocate to the cell nucleus where gene transcription occurs. This activated transcription/translation produces proteins (eg, cytokines) that mediate immune responses and inflammation, completing the inflammation feedback loop.
FIGURE 2.
Figure illustrates the steps involved in the JAK-STAT pathway.
Genetic variants in or expression of certain JAK-STAT cytokines have been associated with CD and/or UC.2, 16, 38, 39 The JAK-STAT cytokines primarily affiliated with CD are interleukin (IL)-12 and interferon (IFN),-γ 39 and those primarily affiliated with UC are IL-5, IL-9, IL-13, and IL-33.19, 39 The JAK-STAT cytokines implicated in both CD and UC are IL-6, IL-21, and IL-23.19, 39 Because there are some differences in the cytokines involved in each condition, there may be a difference in therapeutic response to agents that block different cytokines.20 Several other cytokines, such as TNF and IL-17A/F, are also involved in the pathobiology of IBD but are not mediated through the JAK-STAT pathway.19, 39
Interferon-γ signals via JAK1/JAK2; and IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, and IL-21 signal through JAK1/JAK3.4, 19, 37 Interferon-α, IFNβ, and the cytokines IL-10, IL-19, IL-20, and IL-22 signal through JAK1/TYK2.4, 19, 36 There is potential that a dual JAK1/TYK2 inhibitor may enhance efficacy by inhibition of TYK2-mediated IL-23 signaling.40 The cytokines that signal through JAK1/JAK2/TYK2 include IL-6, IL-11, IL-27,37 IL-12, and IL-23.8
JAK1 has been suggested to dominate in IL-2 induced JAK1/JAK3 and IL-6 induced JAK1/JAK2/TYK2 signaling pathways; therefore, selective JAK1 inhibitors could represent a specific target, allowing higher doses and potentially achieving efficacy of inflammatory conditions19 while avoiding dose-limiting pharmacodynamics (eg, adverse effects) observed with less specific JAK inhibitors.8 JAK3 will always pair with JAK1 and is only associated with cytokines IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 (γ-common chain).20
JAK2 is unique when compared with the other JAKs because it can pair with itself, unlike the other kinases.15 JAK2 controls the signaling of cytokines IL-3 and IL-5 and the growth factors of granulocyte-macrophage stimulating colony factor (GMSCF), erythropoietin (EPO), and thrombopoietin.8, 20 JAK2 also regulates IL-12 and IL-23.4 Moreover, activation of JAK2 contributes to IL-23 but not to IL-12 signaling, suggesting that IL-12 might be the most appropriate stimulus for evaluating TYK2 coding variation.36 Because JAK2-dependent EPO signaling results in decreases in hemoglobin levels,20 this side effect seems to be the main reason for not exploiting the pharmacodynamic potential of pan-JAK inhibitors with systemic activity.8
Because JAK2 activity is essential for hematopoiesis,20 inhibition will likely result in low blood cell counts.30 JAK2 inhibition increases platelet counts, and rare cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported.36
Tyrosine kinase 2 mediates a small number of cytokine pathways20 and is associated with IFN-γ, IL-12, and IL-23 receptors in conjunction with JAK2.4 Therefore, the potential selective inhibition of TYK2 mainly regulating IL-12, IL-23, and IFN signaling, with limited effect on transducing signaling for other cytokines, could lead to less systemic side effects.36 Deficiencies in TYK2 would impact IFN-γ, IFN-α/β IL-12, and IL-23 signaling20; however, TYK2-dependent signaling is not yet fully delineated.20
Therapeutic Targets of JAK in IBD
Because JAK enzymes interact with many of the cytokines that are implicated in IBD, they are an attractive therapeutic option and key drug target20 and may offer an advantage over biologics such as TNFα inhibitors, anti-α4β7 integrin inhibitors, or those that block only IL-12/23.
This central role in downstream signaling for cytokines makes JAKs an attractive target for inflammatory diseases such as IBD.8 The JAK inhibitors can block cytokines released by both the innate and adaptive immune responses,8, 41 which are activated in CD and UC; therefore, targeting this pathway seems an appropriate treatment strategy. A principle of JAK inhibition that differs from cytokines inhibition using biologics is that the objective is not to specifically block the JAK pathway completely but to reversibly reduce the activity of one or more JAK isoforms, thus having the advantage of being able to rapidly reverse the effects.42 Janus kinases also vary with respect to their degree of selectivity, and it is hypothesized that JAK1 selective agents may result in fewer adverse effects.36 However, selectivity can be associated with lower efficacy compared with nonselective inhibitors.8, 19 As more clinical data become available, clinicians will be able to determine the impact of selectivity of JAK inhibitors in IBD or other inflammatory diseases, with respect to efficacy and safety.36
Small molecules, such as JAK inhibitors, can rapidly enter the systemic circulation,8 which may translate into a fast onset of action. This effect was demonstrated by tofacitinib in a post hoc analysis of OCTAVE-1 induction and OCTAVE-2 induction, both randomized, double-blind, placebo-controlled trials, where mean analysis changes were significantly greater in patients with UC who received tofacitinib vs placebo, with reductions from baseline stool frequency, total number of bowel movements, and rectal bleeding score by day 3.43
The potential of small molecules such as JAKs to rapidly induce symptomatic relief could theoretically result in less use of corticosteroids. Although corticosteroids have become important to manage patient symptoms initially,43 chronic use is not without consequence due to several known adverse events associated with corticosteroids and loss of response.43 Having a therapeutic option that can minimize or avoid the use of steroids can only prove beneficial to patients with IBD43 (excluding cases of severe acute UC).44
Because small molecules can be administered orally, they have the potential to improve adherence.
Once-daily dosing was associated with significantly better adherence rates than twice-daily dosing for chronic diseases.45 In 3 studies that evaluated different dosing schedules of treatment for UC with adherence and persistence to different formulations of mesalamine, once-daily dosing was associated with better adherence compared with more than once-daily dosing.46–48 In one of these studies, the authors attributed reduced relapse rates to improved adherence.47 However, more data are needed to evaluate the effects of newer therapies on adherence and persistence and whether it will result in improved outcomes. Effectiveness and tolerability to treatment are also important factors in patient compliance to treatment.45
Additional benefits of oral molecules such as JAKs include no immunogenicity (no ADA), no need for therapeutic drug monitoring, and no infusion site reactions (infliximab was reported up to 2.8%, vedolizumab <5%, and ustekinumab 2.3%–6.9%).49 Lastly, there is the potential that these agents may be effective in patients who have extraintestinal manifestations (EIMs) associated with IBD.
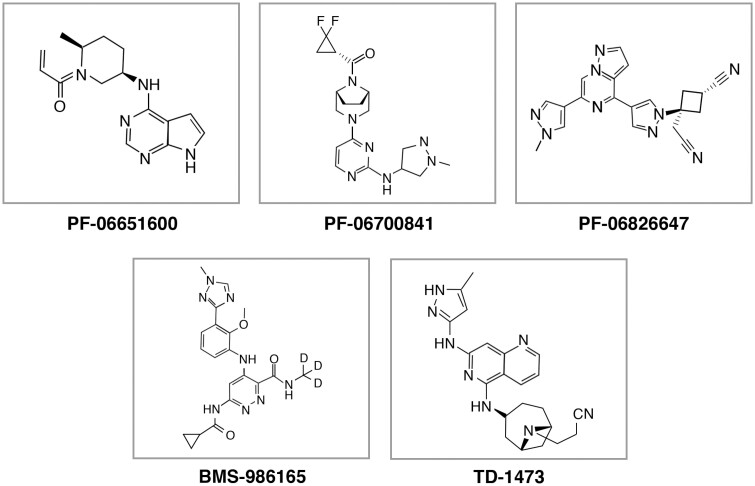
The only small molecule that is currently approved for the treatment of UC is the JAK1/JAK3 inhibitor, tofacitinib. Additional JAK inhibitors being investigated for UC and/or CD include filgotinib and upadacitinib, both JAK-1 selective inhibitors8; PF-06651600, a JAK-3 selective inhibitor40; PF-06700841, a TYK2/JAK1 inhibitor40;PF-06826647, a TYK2 inhibitor50; BMS-986165, a potent binder of TYK240; and the gut-selective pan-JAK inhibitor, TD-14738, 40 (see Fig. 3).51–58 Results from studies evaluating these agents show promise for the treatment of UC and/or CD.
FIGURE 3.
Figure provides the chemical structures of JAK inhibitors currently under investigation for Crohn’s disease and/or ulcerative colitis.
There are some potential disadvantages to oral therapies. Janus kinases affect multiple cytokines, and nonselective agents may exert a broad range of systemic effects and “off-target” toxicity.18 Because small molecules are administered orally, there is the potential for drug-drug interactions, and several of these agents undergo hepatic metabolism by the cytochrome P450 system.59, 60 In addition, improved adherence of oral therapies to parenterally administered drugs have not been demonstrated in the context of IBD. Some patients may benefit from increased supervision coupled with increased interactions with health care providers at infusion centers; this may help promote adherence and/or compliance.45
Other potential concerns for JAK inhibitors include risk of increased infection and risk of malignancy due to their multiple effect on the immune system.18 Though the safety profile of JAK inhibitors is considered acceptable, there may be potential differences with respect to selectivity, and these adverse effects may also be dose-dependent.36 In addition, some JAKs may claim selectivity, but as their dose increases, this selectivity may be lost.36 Tofacitinib primarily inhibits JAK-1 and JAK-3, but at higher doses, inhibition of JAK-2 has been observed20, 36; TYK2 is also inhibited to a lesser extent in biochemical assays.40
Perhaps the most prominent infection noted in patients who received JAK inhibitors is herpes zoster (HZ). An observational analysis using US health plan data (abatacept, rituximab, TNF blockers and tocilizumab) reported an approximate doubling in the rate of HZ with tofacitinib compared with biologics in rheumatoid arthritis (RA) patients.61 In the OCTAVE Induction 1 and 2 trials, HZ infection occurred in 3 patients (0.6%) and 2 patients (0.5%), respectively, in the 10-mg tofacitinib groups and in 1 (0.8%) patient and no patients in the placebo groups. In the OCTAVE Induction 1 trial, HZ infection occurred in 3 patients (0.6%) in the 10-mg group and in 1 patient (0.8%) in the placebo group. In OCTAVE Induction 2, HZ infection occurred in 2 patients (0.5%) in the 10-mg group and no patients in the placebo group.62 No cases of HZ infection were serious adverse events or resulted in discontinuation; most affected one dermatome or 2 adjacent dermatomes.62 In the phase 2/3 UC clinical trials program for tofacitinib, overall 65 (5.6%) patients developed HZ.63 Eleven patients had multidermatomal involvement, and 1 developed encephalitis (resolved upon standard treatment). Five (7.7%) events led to treatment discontinuation. Incidence rates were highest in patients age 65 years and older, Asian patients, patients with prior TNF failure, and patients using 10 mg of tofacitinib twice daily.63
In the OCTAVE Induction 1 and 2 trials, serious infections occurred in 6 patients (1.3%) and 1 patient (0.2%), respectively, in the 10-mg tofacitinib groups and in no patients in the placebo groups.62 In the OCTAVE Sustain trial, serious infections occurred in 2 patients (1.0%) in the 5-mg tofacitinib group, 1 patient (0.5%) in the 10-mg tofacitinib group, and 2 patients (1.0%) in the placebo group. The rate of serious infections was higher with tofacitinib in the induction trials but similar across treatment groups in the maintenance trial.63
In CELEST, a randomized, double-blind, placebo-controlled phase 2 CD study of upadacitinib, during the induction period, 1 patient receiving 24 mg of upadacitinib twice daily (N = 36) had a nonserious HZ event, and 2 patients experienced HZ events during the maintenance period; 1 patient received 12 mg twice daily (n = 59), and another patient received 24 mg twice daily.63 Each event resolved with antiviral treatment. In CELEST, the incidence of serious infections was reported in 9 patients during induction and in 6 patients during maintenance, with 3 mg of upadacitinib twice daily being the most often cited dose.64
Among patients with CD who received filgotinib in a randomized, double-blind, placebo-controlled phase 2 study (FITZROY), 1 patient (N = 30) who initially received 200 mg of filgotinib as induction therapy and then had the dose reduced to100 mg during the maintenance therapy reported HZ.65 There were no HZ cases in the induction phase of the study where all patients received 200 mg of filgotinib once daily (N = 130) or placebo (N = 44). Serious treatment-emergent adverse effects were reported in 9% (14 of 152) of patients treated with filgotinib and 4% (3 of 67) patients treated with placebo. Although the exact mechanism by which HZ reactivation occurs in the context of JAK inhibition is unclear, the downregulation of innate antiviral signaling through type 1 and 2 interferons are likely to be involved.66
Early research showed tofacitinib affected the baseline levels of low-density lipoprotein (LDL) and total cholesterol.67 Moreover, normalization of HDL cholesterol was found in patients given tofacitinib. Although dose-dependent increases in cholesterol have been reported by several investigators, the negative clinical impact of this change has not been observed. Sands et al68 reported on data collected from 1157 patients randomized to receive tofacitinib as part of the UC clinical program (one phase 2 and two phase 3 [OCTAVE 1 and 2] induction studies, OCTAVE Sustain, and an ongoing long-term extension study); they reported greater increases from baseline in total cholesterol, high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol in patients given tofacitinib compared with placebo at week 8.68 Lipid concentrations were increased in patients given tofacitinib vs patients given placebo through week 61. Overall, ratios of LDL cholesterol to HDL-c and total cholesterol to HDL-c did not change significantly over the 61-week period. No clinically meaningful changes in lipid ratios or in the composite cardiac risk Reynolds ratio (RRS) were observed after administration of tofacitinib.68
In CELEST, significant elevations in total, low-density, and high-density cholesterol and decreases in triglyceride levels were observed in the upadacitinib 24-mg twice-daily arm compared with the placebo group at week 16; total and low-density cholesterol levels were also significantly elevated in the 12-mg twice-daily group vs placebo.64 Nonsignificant differences in laboratory values were observed between dose groups at week 52. In FITZROY, exposure to 200 mg of filgotinib once a day resulted in an 11% increase in mean HDL and a 12% increase in mean LDL at week 20. In contrast, a 4% increase in mean HDL from baseline was seen in those with equivalent placebo exposure, along with a 13% increase in mean LDL. These changes correspond to a 3% increase in the LDL to HDL ratio in patients treated with filgotinib at week 20 vs a 10% increase in the placebo group.65 Although recommendations for lipid and cholesterol monitoring have not been established, an assessment during treatment would seem reasonable.
There is also a theoretical concern regarding risk of malignancy including nonmelanoma skin cancer (NMSC)18 due to the long-term blocking of the JAK-STAT pathway. In the induction trials for OCTAVE, NMSC occurred in 2 patients (N = 938) who both received 10 mg of tofacitinib twice daily69; no patients reported malignancy during the induction phase. In OCTAVE Sustain, NMSC occurred in 3 patients (N = 196) on 10 mg of tofacitinib twice daily and 1 patient in the placebo group (N = 198).70 Malignancy was reported in in 1 patient on placebo in the maintenance phase.69, 70 In OCTAVE Sustain (N = 1157), overall 16 patients reported NMSC, and 13 patients reported malignancy.70 An earlier report noted that 10 of 11 patients with NMSC had previous exposure to thiopurines, and 6 of 11 had prior NMSC.69 During induction in CELEST, there was 1 case of NMSC in the 24-mg upadacitinib twice daily group (N = 36)64 in a patient with prior exposure to azathioprine. During the maintenance phase, 2 malignancies were reported: 1 patient receiving 6 mg of upadacitinib twice daily (N = 37) with prior exposure to 6-mercaptopurine and a family history of malignancy and another patient receiving 24 mg of upadacitinib once daily (N = 35) with no exposure to thiopurines or a family history of cancer.
Malignancies, including NMSC, were not reported in the FITZROY publication.65 Although reassuring, long-term data with these agents are limited in comparison with biologics such as TNF inhibitors, and postmarketing surveillance is essential in evaluating the risk of malignancies with JAKs.18
An Italian group has published 1 report of a normal pregnancy outcome while taking baricitinib for rheumatoid arthritis, although it was unintentionally given during the pregnancy.71 Similarly, Mahadavan et al reported in this journal the outcomes of tofacitinib exposure in pregnancy to be similar to reference populations in nonexposed patients.72 A PubMed search regarding the use of upadacitinib and pregnancy outcomes did not yield any results. The label for upadacitinib states that the limited human data on use of upadacitinib in pregnant women are not sufficient to evaluate a drug-associated risk for major birth defects or miscarriage. Based on animal studies, upadacitinib has the potential to adversely affect a developing fetus.60 Likewise, no reports of pregnancy outcomes and filgotininb exposure were identified in a PubMed search or in FITZROY.65, 73, 74
Tofacitinib recently underwent a label change regarding risks for thrombotic events when higher doses (10 mg twice daily) are administered.59 Rheumatoid arthritis patients 50 years of age and older with at least 1 cardiovascular (CV) risk factor treated with 10 mg of tofacitinib twice a day had a higher rate of all-cause mortality, including sudden CV death, compared with those treated with 5 mg of tofacitinib given twice daily or TNF blockers in a large, ongoing, postmarketing safety study. Patients with rheumatologic diseases already have an increased risk of thrombotic events,19 and CV disease is the leading cause of death in patients with RA, accounting for almost 31% mortality.75
In the tofacitinib UC clinical development program, 1 patient had a DVT and 4 patients had a PE while taking 10 mg of tofacitinib twice daily.76 All patients had venous thromboembolism risk factors alongside UC. These data were generated from phase 2 and 3 induction or maintenance or open-label extension studies. Among the 1157 patients who received tofacitinib as part of the UC clinical program, 4 patients reported an adjudicated major adverse cardiac event (MACE); 3 of the 4 patients had 4 or more cardiovascular risk factors.68 The labeling for tofacitinib recommends avoiding use of tofacitinib in patients that may be at increased risk of thrombosis; when treating patients with UC, the label recommends using the lowest effective dose of tofacitinib for the shortest duration needed to achieve/maintain therapeutic response.
In CELEST, 2 patients with risk factors for CV events had myocardial infarction (MI) events that were adjudicated. One event occurred during induction in a patient receiving 12 mg of upadacitinib twice daily (N = 36) and the other during maintenance in a patient receiving 3 mg of upadacitinib twice daily (N = 60).64 One patient receiving 3 mg twice daily developed a mesenteric vein thrombophlebitis during the induction period. No events of DVT or PE were observed. The label for upadacitinib notes that thrombosis, including DVT, PE, and arterial thrombosis, occurred in patients treated with JAK inhibitors used to treat inflammatory conditions.60 In the FITZROY publication, there were no reports of DVT, venous thromboembolism, pulmonary embolism, or MACE.65 However, 1 patient enrolled in the study did experience nonserious treatment emergent adverse effects (TEAEs) of severe pain in extremity and severe embolism (pulmonary).73 Both were considered possibly related to filgotinib and led to temporary discontinuation of treatment. Both TEAEs resolved during the study.73 Patients with IBD are also at risk for CV events (primarily myocardial infarction and stroke) compared with the normal population.77 Olivera et al recently published a meta-analysis of venous thrombosis events of 17 studies including 7 tofacitinib, 3 upadacitinib, 3 filgotinib, and 4 baricitinib trials.78 Overall, 24,128 patients were included, and the 10 controlled trials revealed an incidence rate of these thrombotic events of 0.31 per 100 patient years.78 In addition, as this population ages, the impact of JAK inhibitors on these patients remains unknown. However, the FDA label for each drug should be reviewed and considered for each patient treated, as with all medications.
SUMMARY
The pathobiology of IBD is complex and involves an imbalance of inflammatory cytokines, interplay between cytokines, gut microbiota, environment, and genetics that has an impact on the overall disease. A significant unmet need in IBD therapy is the identification and targeted treatment of primarily and secondarily nonresponsive IBD. Selecting the most appropriate therapy for patients will become more difficult as more therapies become available. Although currently there is only 1 “novel” oral therapy impacting inflammatory cytokines available for the treatment of UC (and none in CD), in the next few years, there will be a variety of small oral molecules from which to choose, ranging from JAK inhibitors, with a range of selectivity, to agents that block leukocyte trafficking or sphingosine-1-phosphate receptors for treating both UC and CD.
Disease severity (symptoms, endoscopy findings, and assessing the risk for complications) is also an important consideration when determining initial therapy for either CD or UC. Patient comorbidities such as risk for CV disease or presence of EIMs should also be taken into consideration when deciding on a treatment option, either to avoid potential adverse events or to optimize therapy. These patients may also be at risk for potential drug-drug interactions, as many of these investigational agents are metabolized through the cytochrome P450 system and may require dosage adjustment. Of those that are not impacted by drug-drug interactions, there may be a potential for combination therapy; however, the safety of these combinations remains unknown.
Strategies such as treat-to-target, which focuses on achieving both clinical remission and mucosal healing,77, 79 are summarized elsewhere80, 81 and will impact patient management decisions. Managing primary, secondary, and tertiary complications of IBD is also important and is summarized by Weaver et al. Recently, a tool was developed to help calculate the risk of colectomy in patients with UC.82 Earlier therapies for CD or UC focused on Crohn’s Disease Activity Index or the Mayo Clinic Score, respectively. Symptom relief was the primary focus, and mucosal healing was secondary. However, there is a shift in this philosophy as more recent studies focus on a dual primary end point of clinical remission and endoscopic (mucosal) healing.79, 80
There are several potential benefits for small molecules, including oral administration, rapid onset of action, potential for increased adherence, lack of immunogenicity, treatment of EIMs, and the potential for long-lasting therapeutic benefit. Small molecules, as a class, have benefited from our improved understanding of inflammatory mechanisms in a variety of target tissues. As for the antibody-based biologics, patients obtain the ultimate benefit of these targeted therapies.
Janus kinase inhibitors are encouraging treatment strategies because they impact multiple cytokines which are implicated in both UC and Crohn’s disease and can have an advantage to other agents that include no ADA development and oral administration. Thus far, data demonstrate effectiveness and safety of JAK inhibitors and other oral agents among patients with moderate to severely active disease. However, these agents are still relatively new, and there are limited long-term data with respect to biologics in IBD. Disadvantages such as the potential for risk of serious infections or “off-target effects” with long-term use remain significantly unknown. Lastly, the evolving field of OMICS, (including proteomics and genomics), and “precision medicine” will no doubt play a significant role in the future of selecting the most optimal therapy for patients, not just in IBD but in a range of other inflammatory diseases.
ACKNOWLEDGMENTS
The authors would like to thank Ethan Grant (Gilead Sciences), Cara Marsh (Gilead Sciences), Slavka Baronikova (Galapagos), John Gonzalez (Galapagos), and Hannah Mace (Aspire Scientific) for their review of the manuscript.
Conflicts of Interest: EP is employed by Gilead Sciences as a Principal Medical Scientist.
BY has received grant support from Procter and Gamble.
REFERENCES
- 1. Holleran G, Lopetuso L, Petito V, et al. The innate and adaptive immune system 2as targets for biologic therapies in inflammatory bowel disease. Int J Mol Sci. 2017;18:1–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goethel A, Croitoru K, Philpott DJ. The interplay between microbes and the immune response in inflammatory bowel disease. J Physiol. 2018;596:3869–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Retnakumar SV, Muller S. Pharmacological autophagy regulators as therapeutic agents for inflammatory bowel diseases. Trends Mol Med. 2019;25:516–537. [DOI] [PubMed] [Google Scholar]
- 4. Marafini I, Sedda S, Dinallo V, et al. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther. 2019;19:1207–1217. [DOI] [PubMed] [Google Scholar]
- 5. Hvas CL, Bendix M, Dige A, et al. Current, experimental, and future treatments in inflammatory bowel disease: a clinical review. Immunopharmacol Immunotoxicol. 2018;40:446–460. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16:843–862. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz DM, Bonelli M, Gadina M, et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández-Clotet A, Castro-Poceiro J, Panés J. JAK inhibition: the most promising agents in the IBD pipeline? Curr Pharm Des. 2019;25:32–40. [DOI] [PubMed] [Google Scholar]
- 9. Ma C, Battat R, Dulai PS, et al. Innovations in oral therapies for inflammatory bowel disease. Drugs. 2019;79:1321–1335. [DOI] [PubMed] [Google Scholar]
- 10. Moss AC, Brinks V, Carpenter JF. Review article: immunogenicity of anti-TNF biologics in IBD - the role of patient, product and prescriber factors. Aliment Pharmacol Ther. 2013;38:1188–1197. [DOI] [PubMed] [Google Scholar]
- 11. Battat R, Ma C, Jairath V, et al. Benefit-risk assessment of vedolizumab in the treatment of Crohn’s disease and ulcerative colitis. Drug Saf. 2019;42:617–632. [DOI] [PubMed] [Google Scholar]
- 12. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 13. Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis. 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
- 14. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olivera P, Danese S, Peyrin-Biroulet L. JAK inhibition in inflammatory bowel disease. Expert Rev Clin Immunol. 2017;13:693–703. [DOI] [PubMed] [Google Scholar]
- 16. D’Amico F, Fiorino G, Furfaro F, et al. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs. 2018;27:595–599. [DOI] [PubMed] [Google Scholar]
- 17. Musumeci F, Greco C, Giacchello I, et al. An update on JAK inhibitors. Curr Med Chem. 2019;26:1806–1832. [DOI] [PubMed] [Google Scholar]
- 18. Bechman K, Yates M, Galloway JB. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol Res. 2019;147:104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Virtanen AT, Hairkarainen T, Raivola J, et al. Selective JAKinibs: Prospects in inflammatory and autoimmune diseases. Bio Drugs. 2019;33:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57:5023–5038. [DOI] [PubMed] [Google Scholar]
- 21. Coskun M, Salem M, Pedersen J, et al. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8. [DOI] [PubMed] [Google Scholar]
- 22. O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heneghan AF, Pierre JF, Kudsk KA. JAK-STAT and intestinal mucosal immunology. Jakstat. 2013;2:e25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soendergaard C, Bergenheim FH, Bjerrum JT, et al. Targeting JAK-STAT signal transduction in IBD. Pharmacol Ther. 2018;192:100–111. [DOI] [PubMed] [Google Scholar]
- 25. Flamant M, Rigaill J, Paul S, et al. Advances in the development of Janus Kinase inhibitors in inflammatory bowel disease: future prospects. Drugs. 2017;77:1057–1068. [DOI] [PubMed] [Google Scholar]
- 26. Banerjee S, Biehl A, Gadina M, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abraham C, Dulai PS, Vermeire S, et al. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:374–388.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neurath MF. IL-23 in inflammatory bowel disease and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8. [DOI] [PubMed] [Google Scholar]
- 30. De Vries LCS, Wildenberg ME, De Jonge WJ, et al. The future of Janus Kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2017;11:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bevivino G, Monteleone G. Advances in understanding the role of cytokines in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2018;12:907–915. [DOI] [PubMed] [Google Scholar]
- 32. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizoguchi A, Yano A, Himuro H, et al. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langer V, Vivi E, Regensburger D, et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J Clin Invest. 2019;129:4691–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haep L, Britzen-Laurent N, Weber TC, et al. Interferon gamma counteracts the angiogenic switch and induced vascular permeability in dextran sulfate sodium colitis in mice. J Crohns Colitis. 2015;21:2360–2371. [DOI] [PubMed] [Google Scholar]
- 36. Danese S, Argollo M, Le Berre C, et al. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68:1893–1899. [DOI] [PubMed] [Google Scholar]
- 37. Boland BS, Vermeire S. Janus Kinase antagonists and other novel small molecules for the treatment of Crohn’s disease. Gastroenterol Clin North Am. 2017;46:627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 39. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–278. [DOI] [PubMed] [Google Scholar]
- 40. Currie KS, Patel L, Sedillo KF. Small-molecule agents for the treatment of inflammatory bowel disease. Bioorg Med Chem Lett. 2019;29:2034–2041. [DOI] [PubMed] [Google Scholar]
- 41. Nielsen OH, Seidelin JB, Ainsworth M, et al. Will novel oral formulations change the management of inflammatory bowel disease? Expert Opin Investig Drugs. 2016;25:709–718. [DOI] [PubMed] [Google Scholar]
- 42. Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanauer SB, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduced symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17:139–147. [DOI] [PubMed] [Google Scholar]
- 44. Feuerstein JD, Isaacs KL, Schneider Y, et al. ; AGA Institute Clinical Guidelines Committee . AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moran K, Null K, Huang Z, et al. Retrospective claims analysis indirectly comparing medication adherence and persistence between intravenous biologics and oral small-molecule therapies in inflammatory bowel diseases. Adv Ther. 2019;36:2260–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kane S, Huo D, Magnanti K. A pilot feasibility study of once daily versus conventional dosing mesalamine for maintenance of ulcerative colitis. Clin Gastroenterol Hepatol. 2003;1:170–173. [DOI] [PubMed] [Google Scholar]
- 47. Hawthorne AB, Stenson R, Gillespie D, et al. One-year investigator blind randomized multicenter trial comparing Asacol 2.4 g once daily with 800 mg three times daily for maintenance of remission in ulcerative colitis. Inflamm Bowel Dis. 2012;18:1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lachaine J, Yen L, Beauchemin C, et al. Medication adherence and persistence in the treatment of Canadian ulcerative colitis patients: analyses with the RAMQ database. BMC Gastroenterol. 2013;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pfizer. Product Pipeline. https://www.pfizer.com/science/drug-product-pipeline. Accessed July 15, 2020.
- 51. Image for PF-06651600 . https://www.medchemexpress.com/PF-06651600.html. Accessed November 27, 2020.
- 52. Chemical name for PF-06651600 . https://pubchem.ncbi.nlm.nih.gov/compound/pf-06651600. Accessed July 10, 2020.
- 53. Image for PF-06700841 . https://www.medchemexpress.com/PF-06700841_P-Tosylate.html. Accessed November 27, 2020.
- 54. Chemical name for PF-06700841 . https://pubchem.ncbi.nlm.nih.gov/compound/PF-06700841-_P-Tosylate#section=Names-and-Identifiers. Accessed July 10, 2020.
- 55. Image for PF-06826647 . https://www.medchemexpress.com/tyk2-in-8.html. Accessed November 27, 2020.
- 56. Chemical name for PF-06826647 . https://www.medkoo.com/products/34149. Accessed July 10, 2020.
- 57. Image for BMS-986165 . https://www.medchemexpress.com/BMS-986165.html. Accessed November 27, 2020.
- 58. Chemical name for BMS-986165 . https://www.medkoo.com/products/30386. Accessed July 10, 2020.
- 59. FDA. Highlights of Prescribing Information: Xeljanz (December 2019). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203214s025lbl.pdf. Accessed July 15, 2020.
- 60. FDA. Highlights of Prescribing Information: Rinvoq (July 2020). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211675s001lbl.pdf. Accessed July 15, 2020.
- 61. Curtis JR, Xie F, Yun H, et al. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for UC. New Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 63. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes Zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sandborn WJ, Feagan BG, Loftus EV Jr, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology. 2020;158:2123–2138.e8. [DOI] [PubMed] [Google Scholar]
- 65. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. [DOI] [PubMed] [Google Scholar]
- 66. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234–243. [DOI] [PubMed] [Google Scholar]
- 67. Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis vs healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:123–132.e3. [DOI] [PubMed] [Google Scholar]
- 69. Lichtenstein GR, Ciorba MA, Rogler G, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of malignancy rates from the OCTAVE clinical program. Gastroenterology Suppl. 2018;154:s385–s386. Abstract Sa1763. [Google Scholar]
- 70. Sandborn WJ, Panes J, Panaccione R, et al. Tofacitinib for the treatment of ulcerative colitis: up to 5.4 years of safety from the global clinical trials. Gastroenterology Suppl. 2019;156:s1097. Abstract Tu1717. [Google Scholar]
- 71. Costanzo G, Firinu D, Losa F, et al. Baricitinib exposure during pregnancy in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2020;12:1759720X19899296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mahadevan U, Dubinsky MC, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Galapagos. Data on File. GLPG0634-CL-211 Clinical Study Report v1.0. Double-blind, randomized, placebo-controlled, multi-centre study to investigate the efficacy and safety of GLPG0634 in subjects with active Crohn’s disease with evidence of mucosal ulceration. October 28, 2016.
- 74. Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Charles-Schoeman C, DeMasi R, Valdez H, et al. Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019;50:1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Koutroumpakis E, Ramos-Rivers C, Regueiro M, et al. Association between long-term lipid profiles and disease severity in a large cohort of patients with inflammatory bowel disease. Dig Dis Sci. 2016;61:865–871. [DOI] [PubMed] [Google Scholar]
- 78. Olivera PA, Lasa JS, Bonovas S, et al. Safety of Janus Kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158:1554–1573.e12. [DOI] [PubMed] [Google Scholar]
- 79. Weaver KN, Long MD. Preventive medicine in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2019;17:824–828. [DOI] [PubMed] [Google Scholar]
- 80. Agrawal M, Colombel JF. Treat-to-target in inflammatory bowel diseases, what is the target and how do we treat? Gastrointest Endosc Clin N Am. 2019;29:421–436. [DOI] [PubMed] [Google Scholar]
- 81. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 82. Dalal RS, Osterman MR, Bucher AM, et al. A user-friendly tool to identify colectomy risk in patients with ulcerative colitis. Inflam Bowel Dis. 201925:1550–1558. [DOI] [PubMed] [Google Scholar]