Abstract
The cells of the immune system are highly dynamic, constantly sensing and adapting to changes in their surroundings. Complex metabolic pathways govern leukocytes’ ability to fine-tune their responses to external threats. Mammalian target of rapamycin complex 1 and hypoxia inducible factor are important hubs of these pathways and play a critical role coordinating cell activation and proliferation and cytokine production. For this reason, these molecules are attractive therapeutic targets in inflammatory disease. Insight into perturbations in immune cell metabolic pathways and their impact on inflammatory bowel disease (IBD) progression are starting to emerge. However, it remains to be determined whether the aberrations in immune metabolism that occur in gut resident immune cells contribute to disease pathogenesis or are reflected in the peripheral blood of patients with IBD. In this review, we explore what is known about the metabolic profile of T cells, monocytes, macrophages, dendritic cells, and natural killer cells in IBD and discuss the potential of manipulating immune cell metabolism as a novel approach to treating IBD.
Keywords: immunometabolism, mTORC1, HIF, glycolysis, oxidative phosphorylation
INTRODUCTION
Inflammatory bowel disease (IBD) is a complex, immune-mediated inflammatory disease characterized by epithelial barrier dysfunction, dysbiosis, and dysregulation of the mucosal immune response.1 It comprises 2 disorders: Crohn’s disease (CD), in which inflammation can affect any area of the gastrointestinal tract, and ulcerative colitis (UC), which is limited to the colon.1 There is no cure for IBD, and treatment consists of medical and surgical interventions to reduce the inflammatory burden and to induce and maintain disease remission. Anti-inflammatory medications (corticosteroids, aminosalicylates), immunosuppressants (azathioprine, mercaptopurine, methrotrexate) and biologic drugs (anti-tumor necrosis factor [TNF] drugs, other anti-interleukin drugs) are the mainstay of treatment. However, a significant proportion of patients do not respond to these medications or others in the expanding repertoire of novel targeted biologic therapies. In addition, patients often experience adverse drug effects or secondary loss of response, meaning that a significant proportion of patients ultimately require surgery. As we approach the era of personalized medicine, considering how to integrate our knowledge of dysregulated immune metabolism with disease pathogenesis in IBD may provide a unique opportunity to tailor treatment based on an individual patient’s metabolic profile.1
Cellular metabolism comprises numerous biochemical reactions to process microenvironmental molecules, thus allowing energy creation and the generation of secondary products required for cell regulation.2 Numerous factors impact on immune cell metabolism, including mitochondria plasticity; expression of metabolic enzymes; rate of glycolysis; and levels of glucose, glutamine, and other metabolites in the environment.3 The emerging research field, termed immunometabolism, investigates the link between these factors and a variety of important functions of the immune system such as cell activation and migration, cytokine production, and cellular plasticity.2
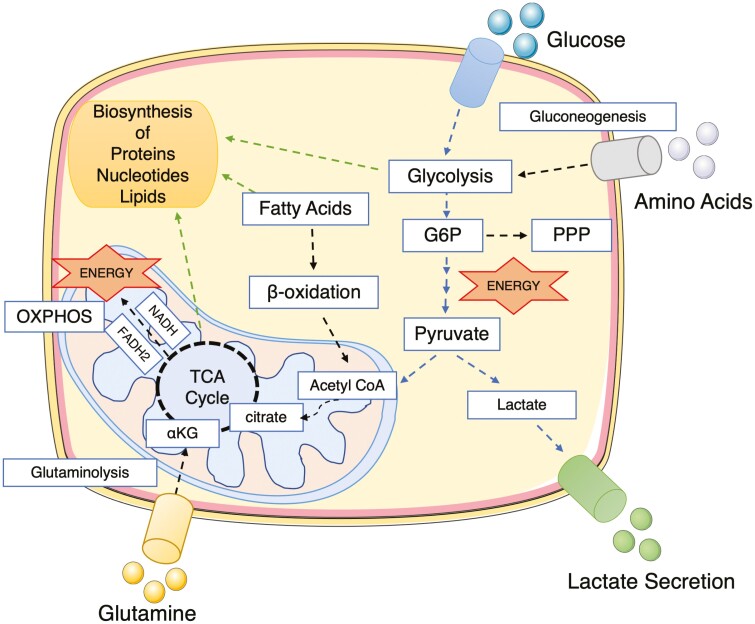
Immune responses are characterized by robust cellular proliferation with specific changes in cellular metabolism to provide energy in the form of adenosine triphosphate (ATP) plus the necessary biosynthetic materials for optimal cell function. Glucose is the most efficient fuel source for cell energy production. It is broken down into pyruvate by a metabolic process called glycolysis and is further reduced to lactate in the cytosol or is oxidized as acetyl coenzyme A in the mitochondria, entering the tricarboxylic acid (TCA) cycle.4 The cofactors nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide generated by the TCA cycle are used to drive oxidative phosphorylation (OXPHOS), producing large quantities of ATP.2 Amino acids and fatty acids can also fuel OXPHOS. Amino acids can be a direct substrate in the TCA cycle or can be further metabolized to support nucleotide and fatty acids synthesis.2, 4 The pentose phosphate pathway (PPP) is a secondary metabolic pathway that functions in parallel with glycolysis. The PPP is important for the generation of nucleotides, amino acids, and cofactors for metabolic reactions and the regulation of the cellular redox-state (Fig. 1).2, 4
FIGURE 1.
Overview of the main players in immunometabolism. Glucose is transported into the cell and metabolized by glycolysis into pyruvate. The PPP branches from G6P and is important for NADPH generation (necessary for fatty acid synthesis and glutathione-mediated scavenging of reactive oxygen species) and synthesis of ribonucleotides. Pyruvate is converted to acetyl-CoA in the mitochondria or to lactate in the cytosol and is secreted from the cell. Acetyl-CoA feeds into TCA in a reaction with oxaloacetate to citrate. The TCA cycle results in the production of reducing equivalents (NADH, FADH2), which transfer electrons into the electron transport chain, generating a proton gradient across the inner mitochondrial membrane. This proton gradient drives the ATP synthase complex to generate ATP. This metabolic process of energy production is known as OXPHOS. Fatty acids can also fuel the TCA cycle when converted into acetyl-CoA though β-oxidation. Amino acids can participate in different metabolic pathways; for instance, glutamine can be converted into α-ketoglutarate though glutaminolysis and enter as a component of the TCA cycle. Amino acids such as alanine can also feed glycolysis. Intermediates of metabolic pathways can generate biosynthetic precursors for the biosynthesis of proteins, nucleotides, and lipids, which are important for cell growth and function. For instance, acetyl-CoA provides building blocks for the synthesis of fatty acids, amino acids, and cholesterol. Acetyl-CoA indicates acetyl-coenzyme A; αKG, α-ketoglutarate; FADH2, flavin adenine dinucleotide; G6P, glucose-6-phosphate; NADH, nicotinamide adenine dinucleotide plus hydrogen; NADPH, nicotinamide adenine dinucleotide phosphate.
Notably, enzymes that regulate cell metabolism are important for cytokine production. For instance, hexokinase, the first enzyme in the glycolysis cascade, regulates interleukin (IL)-1β production.5 Serum and fecal samples from patients with IBD have shown high levels of pyruvate kinase M2 (PKM2), which has been proposed as a potential serum biomarker in IBD.6,7 Studies have shown that PKM2 catalyzes the last step within glycolysis and promotes signal transducer and activator of transcription 3 phosphorylation, inducing IL-1β and IL-6 secretion by macrophages.8 The glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) also regulates the expression of inflammatory cytokines. When it is not participating in glycolysis, GAPDH binds TNF-α mRNA in monocytes, repressing TNF-α translation and protein synthesis. Therefore, when cells are highly glycolytic, TNF-α mRNA is free from GAPDH and translation is allowed.8 Finding new ways to block glycolysis and the glycolytic enzymes that promote the production of proinflammatory cytokines may prove clinically beneficial in inflammatory diseases.9
It is becoming clear that the metabolic status of immune cells can influence their phenotype, polarization to a pro- or anti-inflammatory state, and the resulting efficacy of the immune response.4 Effector lymphocytes increase their glycolytic machinery to proliferate, whereas naïve and memory cells mostly rely on OXPHOS and fatty acid oxidation for cell maintenance.4 This shift to glycolysis is a key metabolic change in activated immune cells, and the manipulation of metabolic signaling has been proposed as a way to control immunity. Furthermore, recent findings show that intermediates of the TCA cycle can impact immune cell phenotype and function.10, 11 These discoveries and how they relate to inflammatory processes and treatment options in IBD are discussed throughout this review.
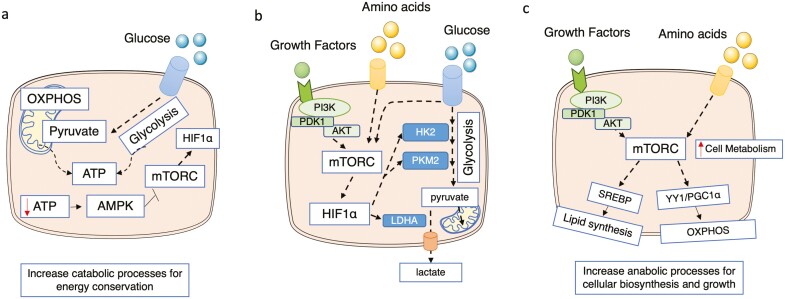
The protein kinase mammalian target of rapamycin complex 1 (mTORC1), adenosine monophosphate-activated protein kinase (AMPK), and the transcription factor hypoxia-inducible factor 1α (HIF-1α) are known as the master regulators of cell metabolism.4 Generally speaking, increased activation of mTORC1 and HIF-1α is associated with proinflammatory responses and increased glycolysis, and AMPK activation usually corresponds to memory or an anti-inflammatory cell phenotype and OXPHOS.12-15 Low concentrations of ATP activate AMPK, which increases the catabolic processes enabling energy conservation and blocks the pathways that utilize ATP, eg, mTORC1. The activation of mTORC1 increases HIF-1α expression, which directly impacts glycolytic enzymes, eg, hexokinase 2, PKM2, and lactate dehydrogenase A. Furthermore, mTORC1 increases other anabolic processes required for cellular biosynthesis and growth, including lipid synthesis through regulation of the sterol regulatory element-binding protein (SREBP) transcription factor and OXPHOS through the transcription factor yin-yang 1 and the peroxisome-proliferator-activated receptor coactivator 1α axis in the mitochondria (Fig. 2).
FIGURE 2.
The interplay between the AMPK, mTORC1, and HIF-1α signaling pathways. (A) During ATP depletion, AMPK is activated, increasing catabolic processes, which generate ATP, and reducing anabolic processes, which utilize ATP, to restore energy homeostasis. The AMPK activation blocks the activity of mTORC1, a metabolic regulator that promotes cellular anabolism. As HIF-1α levels are increased by mTORC1 in many cells, the activation of AMPK will also repress HIF-1α signaling. (B) Activation of mTORC1 through the signaling pathways PI3K/AKT/mTORC1 and nutrient availability promotes glycolysis through the expression of HIF-1α dependent glycolytic genes. mTORC1 activates HIF-1α signaling, which impacts glycolysis. For instance, HIF-1α upregulates HK2, PKM2, and glucose transporters such as SLC2A1 (Glut1). LDHA, an enzyme responsible for the conversion of pyruvate into lactate, is also regulated by HIF-1α. (C) mTORC1 senses changes in growth factors and amino acids. Once activated, mTORC1 promotes an increase of cell metabolism by regulating glycolysis (see B), OXPHOS through an increase of the YY1/PGC1α axis, and lipid metabolism through the regulation of SREBP transcription factors. AKT indicates also known as protein kinase B; HK2: hexokinase 2; LDHA: lactate dehydrogenase A; SLC2A1, solute carrier family 2 member 1 (also known as Glut1); PGC1α: peroxisome-proliferator-activated receptor coactivator 1α; PI3K, phosphoinositide 3-kinase; YY1: transcription factor yin-yang 1.
Interestingly, many of these metabolic regulators are related to IBD pathogenesis. For instance, the HIF transcription factors (HIF-1α and HIF-2α) are sensors of environmental oxygen levels and are important regulators of intestinal homeostasis.16 It has been posited that an overall reduction in HIF-1α but increased HIF-2α expression in the mucosa of patients with IBD supports tissue damage.16 Mammalian target of rapamycin (mTOR) is a protein kinase present in all eukaryotes that senses environmental changes (eg, nutrient availability, immunoregulatory factors, cytokines) regulating cell fate and metabolism.17 It can be subdivided into 2 complexes: mTORC1 and mTORC2, differing in signaling activation and physiological results. Whereas mTORC2 is less well studied than mTORC1, recent research has shown its importance in cell regulation.18 The mTORC1 protein is highly activated in the gut mucosa of patients with IBD, and the inhibition of mTORC1 signaling in animal models of colitis has beneficial effects.18-21 The mechanism of action of one of the most commonly used anti-inflammatory drugs to treat UC, mesalamine (a 5-aminosalicylate), is through inhibition of the mTORC1 signaling pathway.19 Moreover, the mTORC1 inhibitors tacrolimus, everolimus, and sirolimus (rapamycin) have been tested alone or in combination with other drugs in different clinical trials for the treatment of IBD, showing promising results (eg, clinical trials NCT02675153, NCT01418131, and EudraCT 2017-003131-11). Finally, AMPK is a master regulator of energy cell homeostasis, metabolism, and epigenetic modifications. It plays an important role in maintaining gut barrier function and suppresses inflammation in multiple cell types. Therefore, targeting AMPK signaling in patients with IBD may improve the management of the disease.22, 23Table 1 summarizes the drugs used to treat IBD that target the mTORC1 or AMPK signaling pathways.
TABLE 1.
Table Summarizing Known Drugs That Target Metabolism to Treat IBD
| Drug | Impact Upon Metabolic Signaling | Consequence for Metabolic Pathways |
|---|---|---|
| Immunosuppressants (eg, methotrexate) | Activates AMPK, blocks mTORC1 | Increases OXPHOS |
| Corticosteroids | Blocks mTORC1 | Inhibits glycolysis |
| Aminosalicylates | Blocks mTORC1 | Inhibits glycolysis |
| Tacrolimus, everolimus, sirolimus | Blocks mTORC1 | Inhibits glycolysis |
Research into other inflammatory diseases such as rheumatoid arthritis (RA) has led to huge advances in the understanding of the contribution of immune cells’ metabolic profile to disease pathogenesis. In RA, T cells have suppressed mitochondrial function and increased glycolysis, HIF-1α expression, and mTORC1 activity.24 Leakage of mitochondrial DNA from damaged mitochondria promotes activation of the inflammasome, perpetuating inflammation.24 The macrophages and dendritic cells of patients with RA also express elevated levels of proteins that shift cell metabolism to a proinflammatory state, eg, HIF-1α, mTORC1, and the glycolytic enzyme PKM2. Changes in the nutrient availability in the microenvironment of the synovium, eg, the accumulation of lactate and succinate, and changes in amino acid availability contribute to sustaining inflammation.25 The mechanism of action of methotrexate, a drug used to dampen the inflammatory response in both RA and IBD, is through AMPK activation, which promotes the downregulation of the glycolytic pathway, thus suppressing T cell activation and cytokine production.26 The direct inhibition of glycolysis in immune cells using 2-deoxy-glucose has also shown positive outcomes in regulating inflammation in RA disease models.26, 27
Other inflammatory diseases such as psoriasis, systemic lupus erythematous, and obesity are also treated with drugs targeting cell metabolism, eg, metformin, dimethyl fumarate, and rapamycin.26 Moreover, immune cell metabolism has been targeted in the fight against cancer.28 Cancer cells are known to consume the available nutrients in the microenvironment and to release immunosuppressive cytokines, consequently impeding immune cell metabolism and function, limiting cancer cell elimination.28 One of the approaches for cancer treatment is to genetically improve patient cell metabolism through the augmentation of metabolic pathways that support T-cell effector functions. Adoptive transfers of T cells expressing chimeric antigen receptors with improved metabolism have shown to boost therapeutic efficacy against tumors.28, 29 We can speculate that the metabolic manipulation of anti-inflammatory cells, eg, T regulatory cells (Treg), can be beneficial to improve stability and anti-inflammatory effects for application in cell therapy in IBD and other inflammatory diseases.30
Cholesterol and fatty acids can modulate immune cells’ inflammatory responses, which directly impact inflammation. Studies have shown that IBD is strongly associated with defects in lipid metabolism often accompanied by microbial dysbiosis and corresponding alterations in metabolic byproducts.31, 32 In immune cells, lipogenesis is regulated at the transcriptional level by the SREBPs and enzymes for fatty acid biosynthesis such as fatty acid synthase. Disrupting lipid synthesis or SREBPs inhibits the development of effector T-cell subsets and the activation of CD8+ cytotoxic T lymphocytes and natural killer (NK) cells. Immune cell lipid metabolism in patients with IBD is not well understood; however, the serum of patients with IBD has a significantly altered profile of circulating lipid metabolites compared with that of healthy control patients. Therefore, lipid metabolites may have potential as biomarkers for monitoring disease progression.32
Microbial metabolites (short-chain fatty acids [SCFAs]) and some dietary fatty acids have therapeutic potential in the control of inflammation, making them the subject of intense research.2 Diets high in omega-3 fatty acids and/or oleic acid may support the resolution of inflammation.33 Available data on how microbial metabolites influence immune cell metabolism are limited, but it is clear that SCFAs have a significant effect, suppressing proinflammatory cytokine production and boosting anti-inflammatory responses.34 The SCFAs can favor macrophage differentiation toward an anti-inflammatory phenotype though shifting cell reliance on glycolysis to OXPHOS.35, 36 Moreover, butyrate enhances the antimicrobial function of macrophages via the inhibition of mTORC1 that upregulates glycolysis in these cells.37,38
Discovering how changes in microbiota and the resulting metabolic byproducts impact inflammation could inform dietary or microbiome-based interventions that would keep patients in remission for longer. Recently, Effenberger et al39 showed that the microbiota of patients in remission synthesized more butyrate than that of patients with inadequate response to treatment, indicating that quantification of SCFA production by the microbiota of patients with IBD may be used as a predictive parameter for therapeutic efficacy. Therefore, investigating the underlying mechanisms of how these metabolites regulate immune responses and metabolism is relevant to discovering crucial immune cell pathways dysregulated in disease and to tailor treatments to maximize their efficacy in patients.40
METABOLIC ADAPTATION OF IMMUNE CELLS
Lymphocytes are highly dynamic cells that respond rapidly to microbial antigens and inflammatory signals.41 Upon activation, lymphocytes increase their expression of glucose and amino acid transporters and switch their metabolism to aerobic glycolysis, a process known as the Warburg effect.42 During aerobic glycolysis, ATP is generated in addition to large quantities of intermediates necessary for the synthesis of nucleotides, amino acids, and lipids, which are required for cell growth, proliferation, and function.42 The production of reactive oxygen species, the generation of NAD phosphate/NAD plus hydrogen, and an increase in calcium uptake occur during OXPHOS and are also essential for the effector functions of activated lymphocytes.42 Lymphocyte subsets have distinct metabolic profiles to meet the demands of different effector functions. For instance, CD4+ T helper (Th) cells, such as Th1, Th2, Th17, or CD8+ cytotoxic T lymphocytes are more glycolytic than regulatory and naïve T cells. Subpopulations of T cells also have different levels of mTOR activity.43
The metabolism of myeloid cells also differs depending on their environment and stimuli, which impacts their function. The stimulation of Toll-like receptor 4 with lipopolysaccharide (LPS) induces a quick switch of monocytes and macrophages from OXPHOS to glycolysis; however, the stimulation of other Toll-like receptors, eg, Toll-like receptor 2, by Pam3CysSK4 increases OXPHOS and mitochondrial activity.44 In vitro, macrophages are classified into M1 (proinflammatory) and M2 (anti-inflammatory) macrophages, and each subset has a distinct metabolic profile. Whereas M1 macrophage polarization relies on anaerobic glycolysis, M2 macrophage polarization depends on fatty acid metabolism and OXPHOS.45 Metabolism also plays a significant role in reprogramming dendritic cells’ (DCs) state of maturation. Immunogenic DCs have increased glycolysis and PPP pathway flux, and tolerogenic DCs have increased OXPHOS. Interestingly, the differentiation of human DCs from monocytes by culturing in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 is tightly linked to mTORC1 regulation, increased OXPHOS, and fatty acid metabolism.46
Circulating human NK cells can be divided into 2 distinct subpopulations based on CD56 expression on their surface, being referred to as CD56bright (cytokine producers) and CD56dim cells (cytotoxic cells).47, 48 These NK cell subpopulations also differ in their metabolism and reliance on mTORC1. Upon cytokine activation, CD56bright cells are more metabolically active than CD56dim calls, which correlates with the necessity of CD56bright cells to produce large quantities of cytokines.47 All these metabolic differences are relevant for specific cell function, and any impact on metabolic pathways can change cell fate.18
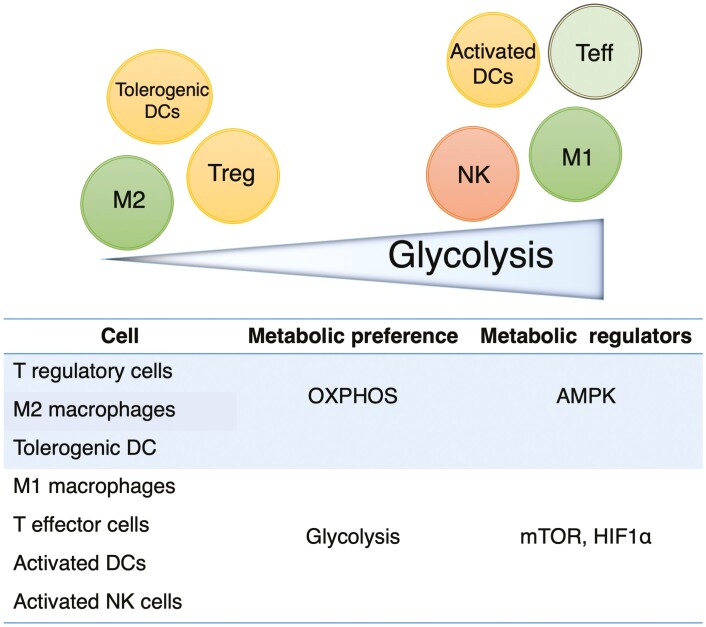
A summary of the metabolic preferences of the different immune cells and the associated signaling molecules that promote metabolism are shown in Fig. 3. In the following sections, we summarize some data on how metabolism has been or can be targeted in different immune cell types to regulate inflammation during IBD.
FIGURE 3.
Metabolic preference of different cell subsets. T regulatory cells, M2 macrophages, and tolerogenic DCs have reduced glycolytic metabolism, relying mainly on oxidative phosphorylation for their survival and to rapidly respond to stimulus. AMPK is one of the main signaling molecules regulating their metabolism. Activated, proinflammatory cells like M1 macrophages, T effector cells, activated DCs, and activated NK cells have increased glucose uptake and high rates of glycolysis, allowing rapid growth and optimal effector functions. mTOR complexes and HIF-1α play a critical role in maintaining this metabolic phenotype. Teff indicates T effector cells (Th1, Th2, Th17, CD8+T cells).
IMMUNE METABOLISM IN IBD
T Cells
There has been tremendous progress in the characterization of the metabolic pathways that control T-cell function in health and disease.49 The T cells play a fundamental role in the pathogenesis of IBD. An imbalance between the pro- and anti-inflammatory T cells may initiate and promote the inflammatory process in patients with IBD.50 Patients with active IBD have increased infiltration of proinflammatory T cells into inflamed gut tissue, whereas the activity of anti-inflammatory Treg cells is impaired.50 The initial stimulus to cytokine production and the promotion of proinflammatory T cell accumulation in the gut mucosa is unknown; however, targeting T-cell metabolism can be a beneficial strategy to modulate cell differentiation, motility, and survival irrespective of antigen specificity.49
Studies have shown that mTORC1 signaling regulates glycolysis and thus multiple facets of T-cell biology including differentiation, costimulatory receptor expression (eg, CD28), and migration.17 Blocking mTORC1 activity with rapamycin has been shown to have protective effects in the IL-10 knockout colitis disease model.51 Rapamycin treatment has limited the number of infiltrating CD4+ T cells in the colonic lamina propria and reduced interferon (IFN)-γ production, promoting disease amelioration.51 Targeted drugs specifically interacting with the mTOR complex have been developed. For example, AZD8055 inhibits both mTORC1 and mTORC2 and has been shown to be more effective than rapamycin in blocking mTOR activity.52 Hu et al52 showed that AZD8055 promotes Treg cell differentiation while suppressing Th1 and Th17 responses and proinflammatory cytokine production, limiting colitis in an animal model.
Patients with IBD have reduced levels of Treg cells in the circulation, so boosting Treg numbers, suppressive capacity, and ability to home in on the gut represents a promising strategy for the regulation of gut inflammation.53 Gut mucosa is hypoxic, and the local expression of HIFs regulate genes involved in the cellular adaptation to hypoxia.54 Research has shown that HIF-1α is an important metabolic switch controlling Treg cell differentiation. In addition, Treg cells require HIF-1α stabilization for the relocation of pyruvate to the mitochondria to fuel OXPHOS.55, 56 Furthermore, HIF-1α stability is required for Treg-cell effector function, which restrains gut inflammation.57, 58 Oxygen-sensing prolyl hydroxylases (PHDs) regulate the stability of HIF-1α. With normal levels of oxygen, PHDs hydroxylate HIF-1α, resulting in proteasomal degradation of the transcription factor subunit. The reduction of oxygen availability limits PHD activity, allowing HIF-1α accumulation. Targeting PHDs to promote HIF-1α stabilization is a promising target to promote Treg cell differentiation and improve cells’ anti-inflammatory profile to treat intestinal inflammation.54, 57, 58
The hexosamine pathway, a branch of glycolysis that generates substrates for protein glycosylation, is important in the regulation of immune cell function. Mucosal T cells isolated from patients with UC have defective glycosylation, which correlates to excessive gut inflammation and disease activity.59 Dias et al59 showed that mucosal T cells isolated from patients with UC with active disease supplemented in vitro with β1,6-N-acetylglucosamine, a monosaccharide that feeds the hexosamine pathway, were sufficient to limit the production of TNF-α, IFN-γ, and IL-17; limit the T-cell receptor signaling pathway; and increase T-cell apoptosis. These data suggest that supplementation with β1,6-N-acetylglucosamine could be evaluated as an adjuvant therapy in the treatment of patients with active UC by boosting mucosal T-cell metabolism.59
The aryl hydrocarbon receptor (AhR) regulates metabolic enzymes in immune cells. Activation of the AhR signaling pathway induces FOXP3 expression and downregulates the proinflammatory activity of Th17 cells.60 Mesalamine, a commonly used anti-inflammatory drug used to treat IBD, promotes Treg cell differentiation and accumulation in the colon of mice though activation of the AhR.61 Lv et al62 showed that another AhR agonist, norisoboldine, a natural compound isolated from the root of Lindera aggregata, can reduce glycolysis and glucose uptake, thus enhancing Treg cell differentiation and alleviating colitis in mice. Further details on how the activation of AhR limits cell metabolism may provide novel strategies to treat inflammation in IBD.60
Certain microbial metabolites induce Treg cell differentiation, enhancing both FOXP3 and IL-10 expression and limiting IL-17 secretion by Th17 cells in the gut.31, 34 Chang et al63 recently reported that ascorbate may play a key role with patients with CD with decreased ascorbate metabolism, which may contribute to limited mucosal healing. Ascorbate has been shown to dampen CD4+ T-cell function by limiting glycolysis.63 Full understanding of the mechanisms controlling the interplay between microbial metabolites and T cells in the gut may facilitate the development of novel anti-inflammatory drugs.63
Monocytes and Macrophages
Monocytes and macrophages are key cells of the innate immune response and play a vital role in IBD pathogenesis. Peripheral blood monocytes can be divided into 3 subcategories based on the expression of CD14 and CD16 on the cell surface: classical (CD14++CD16+), nonclassical (CD14dimCD16+), and intermediate (CD14+CD16+) monocytes.64 These 3 monocyte populations have distinct effector functions accompanied by significant differences in their metabolic profile.65 Transcriptional studies performed by Schmidl et al66 showed that whereas classical monocytes express genes involved in anaerobic energy production and carbohydrate metabolism, nonclassical and intermediate monocytes express genes related to OXPHOS and mitochondria function. This metabolic contrast between monocyte subsets is linked to their phenotype and function. Classical monocytes are known to have a superior phagocytosis potential, which requires energy, and intermediate and nonclassical monocytes are known for their cytokine production and patrolling function, where long-term survival is necessary.
Patients with IBD have altered distribution of the different subsets of monocytes and macrophages in their blood and colon.67-70 Patients with CD have increased numbers of circulating intermediate monocytes, and higher concentrations of CD14+HLADRint monocytes, M1 macrophages, and activated DCs are found in the inflamed colon. This unbalanced distribution of monocyte populations during active disease may be related to the available metabolites and cytokines in the colonic microenvironment that induce a proinflammatory metabolic state, perpetuating the inflammation. Further investigation is required to understand how to normalize monocyte subset distribution and control their function to help dampen inflammatory responses.
Tissue macrophages are derived from either embryonic stem cells or recruited blood monocytes.64 Different subpopulations of macrophages can be found in the intestine, each bearing a specific role in tissue homeostasis, and little is known of their metabolic preferences.64, 71 Targeting therapies to monocyte/macrophage metabolism should consider the different subpopulations and their specific function in the gut or peripheral blood to avoid adverse clinical outcomes.71 Large quantities of monocytes and M1 macrophages are found in the inflamed gut tissue of patients with IBD, promoting and perpetuating the local inflammatory response.67, 72 Manipulating the metabolism of proinflammatory macrophages to differentiate into an anti-inflammatory subtype may be beneficial for patients with IBD. Research has shown that HIF-1α and mTOR regulate glycolysis and macrophage function.45 The inhibition of HIF-1α or mTOR in macrophages leads to disease amelioration in models of colitis.73,74 Ip et al75 showed that IL-10 suppresses macrophage activation in colitis by blocking mTOR activity and suppressing glycolysis. Furthermore, IL-10 enhances the mitochondria function of macrophages because it promotes OXPHOS but favors the autophagy of cells with dysfunctional mitochondria.75 Unfortunately, clinical trials supplementing IL-10 in IBD have shown discrepant results, indicating that patients respond differently to IL-10. Improvements to cytokine delivery and the development of better combination therapies and potentially metabolic profiling will be required to allow a meaningful clinical impact.76
Itaconate and α-ketoglutarate are metabolites originating from the TCA cycle. These metabolites are known for their anti-inflammatory effects on macrophages by inhibiting the secretion of proinflammatory cytokines like IL-6 and IL-1β and increasing IL-10 production in response to LPS.45 More research is needed to better understand the role of these metabolites as immune effector molecules, but they represent a novel therapeutic opportunity to control monocyte/macrophage function in inflammatory disease.10, 11, 77
Monocyte activation and migration to the site of inflammation are intrinsically linked to glycolysis. Lee et al38 showed that inhibiting glycolysis with 2-deoxy-D-glucose impaired the adherence of LPS-stimulated monocytes to wells that were precoated with fibrinogen. Gerner et al78 showed that monocyte infiltration can also be limited by the administration of an inhibitor of nicotinamide phosphoribosyl transferase, which reduces NAD availability and thus directly inhibits many bioenergetic processes. Circulating and tissue monocytes from patients with IBD secrete large quantities of proinflammatory cytokines.64, 79 The inhibition of NAD in monocytes impairs IL-6 and CD86 expression and promotes IL-10 secretion,78 identifying this as an important pathway to investigate further in IBD.
The NLR family pyrin domain containing 3 (NLRP3) is a component of the inflammasome and is highly expressed by macrophages. Cytokines, pathogen-associated molecular patterns, or damage-associated molecular patterns activate the NLRP3 inflammasome and support the production of the proinflammatory cytokine IL-1β.80 Recently, scientists found a connection between the activation of the NLRP3 inflammasome and glycolysis. The inhibition of hexokinase or fructose-2,6-biphosphatase 3, an enzyme that catalyzes a rate-limiting step in glycolysis, is sufficient to limit IL-1β secretion by macrophages, indicating a promising strategy to limit cytokine secretion.5, 8
Tofacitinib is a Janus kinase inhibitor that was recently approved for the treatment of patients with UC. Cordes et al81 showed that tofacitinib manipulates the monocyte phenotype to a more anti-inflammatory subtype. Given that LPS activation of monocytes triggers the Janus kinase/signal transducers and activators of transcription (JAK/STAT pathway), which in turn activates mTOR signaling, upregulating glycolysis and supporting cytokine secretion, it is not difficult to hypothesize that tofacitinib may have a significant impact on monocyte metabolism.82
The advances that have been made in understanding the metabolic regulation of monocytes/macrophages in recent years will no doubt support the development of new therapeutic strategies to treat IBD by the means of manipulating cell fate through metabolic control.
DCs
The DCs are at the interface of innate and adaptive immunity and as such are central mediators of microbial homeostasis because they mediate the detection and subsequent elimination of intestinal pathogens.64, 83, 84 In healthy individuals, intestinal DCs have a regulatory phenotype, but in patients with IBD, DCs are dysregulated and secrete large quantities of proinflammatory cytokines upon LPS stimulation and have a higher expression of receptors implicated in T-cell activation.85 Moreover, increased quantities of DCs are found in the inflamed gut mucosa of patients with IBD, and depleting DCs is sufficient to control colitis in a murine model of disease.67, 86 The DCs can be divided into numerous subpopulations with different progenitors. Each DC subtype has different metabolic requirements reflecting their function.46 The DCs resemble macrophages in their metabolic profile, with mTORC1, HIF-1α, and mitochondrial fitness playing a significant role in cell differentiation and polarization.46 Resting DCs rely on OXPHOS and AMPK signaling to maintain their quiescent metabolic state. After activation, DCs switch to glycolysis, which facilitates the generation of biosynthetic precursors for cell growth and function.46
Notably, DC metabolism is intrinsically linked to their ability to control T-cell polarization. Therefore, targeting DC metabolism is an attractive approach to modulate immunity in patients with IBD.84 However, the metabolic profile of DCs in IBD is not clear. Based on available data, we can speculate that DCs localized in the gut of patients with IBD have metabolic profiles resembling those of activated DCs, with high glycolysis and an increased capacity to activate effector CD4+ and CD8+ T cells.84
It has been shown that glycolysis and the suppression of AMPK signaling increases IL-12 production by DCs, thereby promoting Th1 polarization.84 Studies have suggested that Th2 priming depends on the inhibition of mTOR signaling and lipid metabolism in DCs, but further studies are required.84 In addition, mTOR inhibition in DCs can inhibit antigen uptake, reducing their ability to prime antigen-specific T-cell responses.87 This effect could be linked to the importance of mTOR in the control of cell metabolism and mitochondrial function in DCs, which is necessary for proper antigen processing and presentation.88 However, mTOR activity is important for some DC subsets to express an immunoregulatory phenotype with increased secretion of IL-10.89 Investigation of the different profiles of DCs in patients with CD vs UC would be of value to evaluate the potential of targeting differential DC metabolism in personalized IBD treatments.
NK Cells
The NK cells are innate lymphocytes best known for their role in antitumor and antiviral immunity.90 However, NK cells also have a vital immune regulatory function and may play a significant role in IBD pathogenesis by the recruiting of immune cells to the area of inflammation.91 Usually, the elimination of NK cells reduces chronic inflammation.90 For instance, patients with CD treated using mercaptopurine have reduced numbers of NK cells in both circulation and in the gut mucosa. However, patients treated with mercaptopurine have an increased risk of lymphoma.92 This increased risk of adverse off-target effects could be associated with reduced NK cell numbers observed in the circulation of these patients.93 Therefore, short-term reductions in NK cells may have beneficial clinical effects, but chronic elimination could be detrimental.
The NK cell effector functions, including optimal cytokine production, require energy and are dependent on mTORC1 signaling.47 Further understanding of specific metabolic pathways that regulate cytokine secretion by NK cells involved in IBD pathogenesis is desired but not easily achieved because there is a huge amount of information not yet understood about NK cell metabolic regulation.91, 94 The IFN-γ production by NK cells requires upregulation of glycolysis, OXPHOS, and mTORC1 signaling.47,94 The role of metabolism in the production of other proinflammatory cytokines by NK cells is not clear; however, preliminary data in our laboratory has shown that blocking mTORC1 with rapamycin significantly blocked NK cell TNF-α production (data not published). Further investigation of mTORC1 activity and cytokine production by NK cells and innate lymphoid cells isolated from inflamed gut mucosa would be helpful to understand other consequences of increased mTORC1 activity in IBD.91 Differences between tissue resident and circulating NK cells have been discussed elsewhere,95 and considering these differences will be important for the effective translation of any NK cell–based therapy to the clinic.
Intestinal epithelial cells are constantly releasing immune-suppressive cytokines including transforming growth factor (TGF)-β to dampen the proinflammatory effect of immune cells located in the gut mucosa.96 The dysregulation of TGF-β signaling is associated with the development of colitis in murine models of disease. In humans, although TGF-β can be found in high concentrations in the gut mucosa, its activity is strongly impaired mainly because of the high expression of SMAD7, an inhibitor of TGF-β signaling.96 Studies have shown that TGF-β significantly impairs peripheral-blood NK cell effector functions and metabolism though the canonical TGF-β signaling pathway.97 Interestingly, chronic exposure of NK cells to TGF-β inhibits NK cell metabolism and mTORC1 function.97,98 Mongersen is a SMAD7 antisense oligonucleotide showing promising results in clinical trials with patients with CD.96 A study found that Mongersen promoted mucosal healing and led to patient remission; however, drug safety, efficacy, and specific impacts on NK cells are yet to be fully determined.96
FINAL REMARKS
Inflammation and metabolism are inextricably linked. Targeting immune cell metabolism may yield effective strategies to control inflammation. Patients with IBD have high numbers of activated immune cells producing proinflammatory cytokines, in both the circulation and inflamed mucosa. In turn, high levels of cytokines in the tissue microenvironment promote an altered metabolic configuration in resident cells, consequently diverting a healthy functioning cell to a disease promoter, thus perpetuating chronic inflammation.
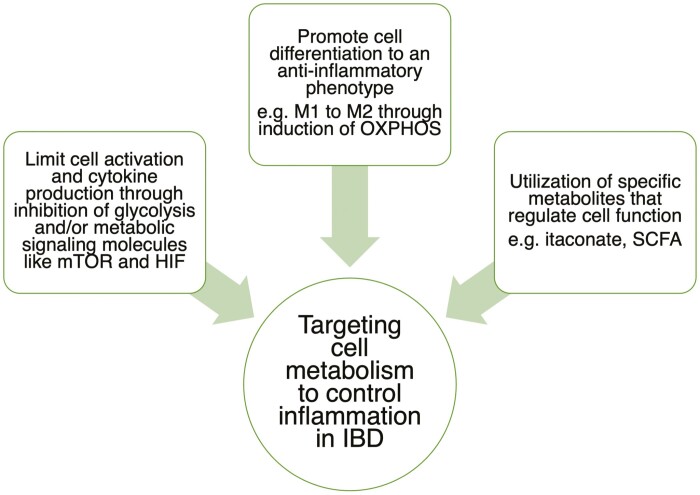
Continued investigation and further understanding of the metabolic signature of immune cells in IBD will enable the optimization of treatments. Clinical and demographic parameters such as patient symptoms, medication, disease location/severity, and genetic predisposition may have a strong impact on the metabolic profile of immune cells isolated from the peripheral blood and tissue of patients with IBD. This information should provide insights into which metabolic pathway could be manipulated. Many current IBD therapies lack specificity and are associated with significant off-target effects, highlighting the importance of exploring novel approaches such as metabolic signaling pathways to improve care for these patients (Fig. 4). Understanding the underlying metabolic mechanisms regulating cell motility and cytokine secretion in health and disease will contribute hugely to the development of novel drugs to regulate the inflammatory response.
FIGURE 4.
Summary of how metabolism can be targeted to control inflammation in patients with IBD.
Supported by: This work was supported by the AbbVie Newman Fellowship in Inflammatory Bowel Disease 2019.
ACKNOWLEDGMENTS
We thank Dr. David Finlay for figure revision. Images from the Servier Medical ART database were used when making the figures.
REFERENCES
- 1. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 2. O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munford H, Dimeloe S. Intrinsic and extrinsic determinants of T cell metabolism in health and disease. Front Mol Biosci. 2019;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finucane OM, Sugrue J, Rubio-Araiz A, et al. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci Rep. 2019;9:4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almousa AA, Morris M, Fowler S, et al. Elevation of serum pyruvate kinase M2 (PKM2) in IBD and its relationship to IBD indices. Clin Biochem. 2018;53:19–24. [DOI] [PubMed] [Google Scholar]
- 7. Jeffery J, Lewis SJ, Ayling RM. Fecal dimeric M2-pyruvate kinase (tumor M2-PK) in the differential diagnosis of functional and organic bowel disorders. Inflamm Bowel Dis. 2009;15:1630–1634. [DOI] [PubMed] [Google Scholar]
- 8. Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 9. Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr Top Med Chem. 2018;18:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zasłona Z, O’Neill LAJ. Cytokine-like roles for metabolites in immunity. Mol Cell. 2020;78:814–823. [DOI] [PubMed] [Google Scholar]
- 12. Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. 2015;27:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gnanaprakasam JNR, Sherman JW, Wang R. MYC and HIF in shaping immune response and immune metabolism. Cytokine Growth Factor Rev. 2017;35:63–70. [DOI] [PubMed] [Google Scholar]
- 14. Biddlestone J, Bandarra D, Rocha S. The role of hypoxia in inflammatory disease (review). Int J Mol Med. 2015;35:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kedia-Mehta N, Finlay DK. Competition for nutrients and its role in controlling immune responses. Nat Commun. 2019;10:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah YM. The role of hypoxia in intestinal inflammation. Mol Cell Pediatr. 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salmond RJ. mTOR regulation of glycolytic metabolism in T cells. Front Cell Dev Biol. 2018;6:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khare V, Dammann K, Asboth M, et al. Overexpression of PAK1 promotes cell survival in inflammatory bowel diseases and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou M, Xu W, Wang J, et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. Ebiomedicine. 2018;35:345–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin X, Sun Q, Zhou L, et al. Colonic epithelial mTORC1 promotes ulcerative colitis through COX-2-mediated Th17 responses. Mucosal Immunol. 2018;11:1663–1673. [DOI] [PubMed] [Google Scholar]
- 22. Sun X, Yang Q, Rogers CJ, et al. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahara M, Takaki A, Hiraoka S, et al. Berberine improved experimental chronic colitis by regulating interferon-γ- and IL-17A-producing lamina propria CD4+ T cells through AMPK activation. Sci Rep. 2019;9:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weyand CM, Wu B, Goronzy JJ. The metabolic signature of T cells in rheumatoid arthritis. Curr Opin Rheumatol. 2020;32:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fearon U, Hanlon MM, Wade SM, et al. Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis. Clin Exp Immunol. 2019;197:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pålsson-McDermott EM, O’Neill LAJ. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30:300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abboud G, Choi SC, Kanda N, et al. Inhibition of glycolysis reduces disease severity in an autoimmune model of rheumatoid arthritis. Front Immunol. 2018;9:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu X, Gnanaprakasam JNR, Sherman J, et al. A metabolism toolbox for CAR T therapy. Front Oncol. 2019;9:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clough JN, Omer OS, Tasker S, et al. Regulatory T-cell therapy in Crohn’s disease: challenges and advances. Gut. 2020;69:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bilotta AJ, Cong Y. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: implication in precision medicine. Precis Clin Med. 2019;2:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scoville EA, Allaman MM, Brown CT, et al. Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics. 2018;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shores DR, Binion DG, Freeman BA, et al. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2192–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ji J, Shu D, Zheng M, et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep. 2016;6:24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Z, Tang H, Chen P, et al. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther. 2019;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulthess J, Pandey S, Capitani M, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–445.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee MKS, Al-Sharea A, Shihata WA, et al. Glycolysis is required for LPS-induced activation and adhesion of human CD14+CD16- monocytes. Front Immunol. 2019;10:2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Effenberger M, Reider S, Waschina S, et al. Microbial butyrate synthesis indicates therapeutic efficacy of azathioprine in IBD patients. J Crohns Colitis. 2021;15:88–98. [DOI] [PubMed] [Google Scholar]
- 40. Postler TS, Ghosh S. Understanding the Holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26:110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buck MD, Sowell RT, Kaech SM, et al. Metabolic instruction of immunity. Cell. 2017;169:570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearce EL, Poffenberger MC, Chang CH, et al. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lachmandas E, Boutens L, Ratter JM, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol. 2016;2:16246. [DOI] [PubMed] [Google Scholar]
- 45. Viola A, Munari F, Sánchez-Rodríguez R, et al. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wculek SK, Khouili SC, Priego E, et al. Metabolic control of dendritic cell functions: digesting information. Front Immunol. 2019;10:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keating SE, Zaiatz-Bittencourt V, Loftus RM, et al. Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J Immunol. 2016;196:2552–2560. [DOI] [PubMed] [Google Scholar]
- 48. Poli A, Michel T, Patil N, et al. Revisiting the functional impact of NK cells. Trends Immunol. 2018;39:460–472. [DOI] [PubMed] [Google Scholar]
- 49. MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smids C, Horjus Talabur Horje CS, Drylewicz J, et al. Intestinal T cell profiling in inflammatory bowel disease: linking T cell subsets to disease activity and disease course. J Crohns Colitis. 2018;12:465–475. [DOI] [PubMed] [Google Scholar]
- 51. Matsuda C, Ito T, Song J, et al. Therapeutic effect of a new immunosuppressive agent, everolimus, on interleukin-10 gene-deficient mice with colitis. Clin Exp Immunol. 2007;148:348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu S, Cheng M, Fan R, et al. Beneficial effects of dual TORC1/2 inhibition on chronic experimental colitis. Int Immunopharmacol. 2019;70:88–100. [DOI] [PubMed] [Google Scholar]
- 53. Himmel ME, Yao Y, Orban PC, et al. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miska J, Lee-Chang C, Rashidi A, et al. HIF-1α is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of tregs in glioblastoma. Cell Rep. 2019;27:226–237.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Higashiyama M, Hokari R, Hozumi H, et al. HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J Leukoc Biol. 2012;91:901–909. [DOI] [PubMed] [Google Scholar]
- 59. Dias AM, Correia A, Pereira MS, et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc Natl Acad Sci U S A. 2018;115:E4651–E4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu C, Xie Q, Zhao B. The role of AhR in autoimmune regulation and its potential as a therapeutic target against CD4 T cell mediated inflammatory disorder. Int J Mol Sci. 2014;15:10116–10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oh-Oka K, Kojima Y, Uchida K, et al. Induction of colonic regulatory T cells by mesalamine by activating the aryl hydrocarbon receptor. Cell Mol Gastroenterol Hepatol. 2017;4:135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lv Q, Wang K, Qiao S, et al. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018;9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chang YL, Rossetti M, Vlamakis H, et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019;12:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caër C, Wick MJ. Human intestinal mononuclear phagocytes in health and inflammatory bowel disease. Front Immunol. 2020;11:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kapellos TS, Bonaguro L, Gemünd I, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmidl C, Renner K, Peter K, et al. ; FANTOM consortium . Transcription and enhancer profiling in human monocyte subsets. Blood. 2014;123:e90–e99. [DOI] [PubMed] [Google Scholar]
- 67. Liu H, Dasgupta S, Fu Y, et al. Subsets of mononuclear phagocytes are enriched in the inflamed colons of patients with IBD. BMC Immunol. 2019;20:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jones GR, Bain CC, Fenton TM, et al. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol. 2018;9:2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ortega Moreno L, Fernandez-Tome S, Chaparro M, et al. Profiling of human circulating dendritic cells and monocyte subsets discriminates between type and mucosal status in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2021;27:268–274. [DOI] [PubMed] [Google Scholar]
- 70. Slevin SM, Dennedy MC, Connaughton EP, et al. Infliximab selectively modulates the circulating blood monocyte repertoire in Crohn’s disease. Inflamm Bowel Dis. 2016;22:2863–2878. [DOI] [PubMed] [Google Scholar]
- 71. Na YR, Stakenborg M, Seok SH, et al. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531–543. [DOI] [PubMed] [Google Scholar]
- 72. Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bäcker V, Cheung FY, Siveke JT, et al. Knockdown of myeloid cell hypoxia-inducible factor-1α ameliorates the acute pathology in DSS-induced colitis. PLoS One. 2017;12:e0190074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lyons J, Ghazi PC, Starchenko A, et al. The colonic epithelium plays an active role in promoting colitis by shaping the tissue cytokine profile. PLoS Biol. 2018;16:e2002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ip WKE, Hoshi N, Shouval DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol. 2013;19:3931–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. 2018;9:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gerner RR, Klepsch V, Macheiner S, et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut. 2018;67:1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schwarzmaier D, Foell D, Weinhage T, et al. Peripheral monocyte functions and activation in patients with quiescent Crohn’s disease. PLoS One. 2013;8:e62761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mao L, Kitani A, Strober W, Fuss IJ. The role of NLRP3 and IL-1β in the pathogenesis of inflammatory bowel disease. Front Immunol. 2018;9:2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cordes F, Lenker E, Spille LJ, et al. Tofacitinib reprograms human monocytes of IBD patients and healthy controls toward a more regulatory phenotype. Inflamm Bowel Dis. 2020;26:391–406. [DOI] [PubMed] [Google Scholar]
- 82. Langston PK, Shibata M, Horng T. Metabolism supports macrophage activation. Front Immunol. 2017;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stagg AJ. Intestinal dendritic cells in health and gut inflammation. Front Immunol. 2018;9:2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Patente TA, Pelgrom LR, Everts B. Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization. Curr Opin Immunol. 2019;58:16–23. [DOI] [PubMed] [Google Scholar]
- 85. Baumgart DC, Thomas S, Przesdzing I, et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin Exp Immunol. 2009;157:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abe K, Nguyen KP, Fine SD, et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci U S A. 2007;104:17022–17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stallone G, Infante B, Di Lorenzo A, et al. mTOR inhibitors effects on regulatory T cells and on dendritic cells. J Transl Med. 2016;14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bonifaz L, Cervantes-Silva M, Ontiveros-Dotor E, et al. A role for mitochondria in antigen processing and presentation. Immunology. 2014;144:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ohtani M, Hoshii T, Fujii H, et al. Cutting edge: mTORC1 in intestinal CD11c+ CD11b+ dendritic cells regulates intestinal homeostasis by promoting IL-10 production. J Immunol. 2012;188:4736–4740. [DOI] [PubMed] [Google Scholar]
- 90. Schuster IS, Coudert JD, Andoniou CE, et al. “Natural Regulators”: NK cells as modulators of T cell immunity. Front Immunol. 2016;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Poggi A, Benelli R, Venè R, et al. Human gut-associated natural killer cells in health and disease. Front Immunol. 2019;10:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–218. [DOI] [PubMed] [Google Scholar]
- 94. O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19:282–290. [DOI] [PubMed] [Google Scholar]
- 95. Sojka DK, Plougastel-Douglas B, Yang L, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sedda S, Marafini I, Dinallo V, et al. The TGF-β/Smad system in IBD pathogenesis. Inflamm Bowel Dis. 2015;21:2921–2925. [DOI] [PubMed] [Google Scholar]
- 97. Zaiatz-Bittencourt V, Finlay DK, Gardiner CM. Canonical TGF-β signaling pathway represses human NK cell metabolism. J Immunol. 2018;200:3934–3941. [DOI] [PubMed] [Google Scholar]
- 98. Slattery K, Gardiner CM. NK cell metabolism and TGFβ—implications for immunotherapy. Front Immunol. 2019;10:2915. [DOI] [PMC free article] [PubMed] [Google Scholar]