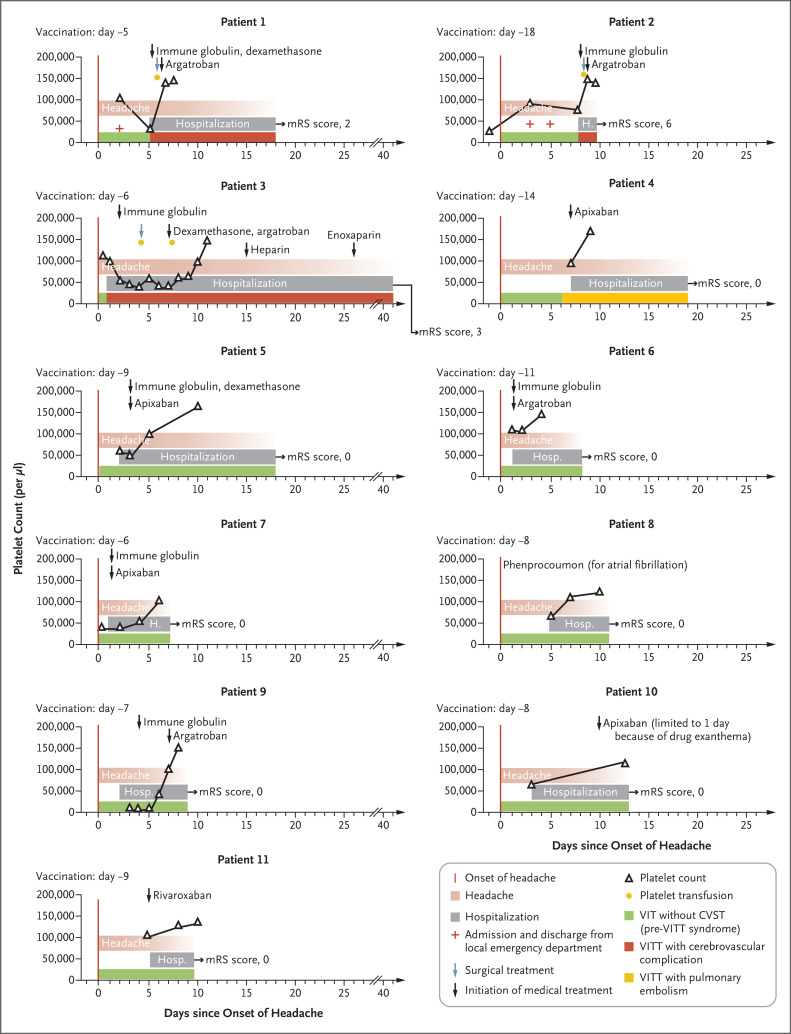
To the Editor: Vaccine-induced immune thrombotic thrombocytopenia (VITT), a serious adverse event after vaccination with ChAdOx1 nCoV-19 (AstraZeneca) or Ad26.COV2.S (Johnson & Johnson–Janssen), is caused by platelet factor 4 (PF4)–dependent, platelet-activating antibodies.1-3 High-dose immune globulins and anticoagulation are the main treatments.4,5 In this report, we present evidence that vaccine-induced thrombocytopenia (VIT) without associated cerebral venous sinus thrombosis (CVST) or other thromboses and with severe headache as the heraldic symptom may precede VITT (“pre-VITT syndrome”).
Eleven patients presented with severe headache in the absence of CVST 5 to 18 days after ChAdOx1 nCoV-19 vaccination. All the patients had thrombocytopenia, high d-dimer levels, and high levels of anti–PF4–heparin IgG antibodies. During follow-up, intracranial hemorrhage occurred in three patients (Patients 1, 2, and 3), with radiologic evidence of new CVST in Patients 2 and 3 (Figure 1, and Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Only two patients (Patients 2 and 4) were initially admitted with conditions that met the criteria for VITT; both patients had pulmonary embolism, and additional splanchnic vein thrombosis was present in Patient 2. In Patient 2, anticoagulation treatment had been initiated several days earlier for pulmonary embolism (without diagnosis of VITT) but was stopped after the onset of headache, shortly before CVST developed. In two patients (Patients 1 and 3), peripheral thromboses were eventually identified during follow-up. Thrombotic complications did not develop in seven of the patients (Patients 5 through 11); all but one of these patients received high-dose immune globulin, glucocorticoids, or therapeutic-dose anticoagulation within 5 days after headache onset. In contrast, in all four patients with subsequent thrombosis (Patients 1 through 4), therapeutic-dose anticoagulation either was not started until 6 to 9 days after headache onset or was stopped prematurely before the development of CVST.
Figure 1. Clinical and Laboratory Data for Patients with VIT and Severe Headache (Pre-VITT Syndrome).
Shown are the time courses of the manifestation of pre-VITT syndrome (defined by headache onset), hospital admission (including emergency department admission and discharge in Patients 1 and 2), platelet counts, and cerebrovascular complications (in Patients 1, 2, and 3), as well as medical and neurosurgical treatment (decompressive craniectomy). In each graph, the number of days since the onset of headache is shown on the x axis and platelet counts on the y axis. Outcomes were assessed with the modified Rankin scale (mRS); scores on the scale range from 0 to 6, with higher scores indicating greater disability (0 indicates no symptoms, and 6 indicates death). CVST denotes cerebral venous sinus thrombosis, VIT vaccine-induced thrombocytopenia, and VITT vaccine-induced immune thrombotic thrombocytopenia.
Although the combination of thrombocytopenia and severe headache due to CVST has been recognized as the typical presentation of VITT,1,2 the experience with these 11 patients suggests that VIT with severe headache, elevated d-dimer levels, and strongly positive results on anti–PF4–heparin IgG enzyme-linked immunosorbent assay may precede VITT.
Our findings have immediate implications for clinical practice: in this pre-VITT syndrome, severe headache may not develop as a symptom secondary to CVST but instead may precede CVST by several days, potentially in association with microthrombosis in smaller cortical veins. Consequently, patients who present with severe headache 5 to 20 days after adenovirus vector vaccination against coronavirus disease 2019 should undergo immediate testing for thrombocytopenia and d-dimer levels and, if available, testing for anti–PF4–heparin IgG antibodies. When these antibodies are present at high titers, patients are at imminent risk for CVST, and it is likely that this condition can be prevented with immediate treatment, such as with intravenous immune globulin. The decision to initiate therapeutic-dose anticoagulation is a difficult one; the risk of emerging thrombosis, including CVST, has to be balanced against the risk of intracranial hemorrhage on an individual basis (e.g., with consideration of platelet count and fibrinogen levels).
Supplementary Appendix
Disclosure Forms
This letter was published on September 15, 2021, at NEJM.org.
Footnotes
Supported by Deutsche Forschungsgemeinschaft project number 374031971–TRR 240 (to Prof. Greinacher) and EXC-2049–390688087 NeuroCure under the German Excellence Strategy (to Prof. Endres) and by the Domagk-Programm of the Universitätsmedizin Greifswald (to Dr. Schönborn).
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Long B, Bridwell R, Gottlieb M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am J Emerg Med 2021;49:58-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussel JB, Connors JM, Cines DB, et al. Thrombosis with thrombocytopenia syndrome (also termed vaccine-induced thrombotic thrombocytopenia), version 1.6. American Society of Hematology, August 12, 2021. (https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia).
- 5.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced thrombotic thrombocytopenia. N Engl J Med 2021;385:720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.