Abstract
Background
Despite high vaccine coverage and effectiveness, the incidence of symptomatic infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been increasing in Israel. Whether the increasing incidence of infection is due to waning immunity after the receipt of two doses of the BNT162b2 vaccine is unclear.
Methods
We conducted a 6-month longitudinal prospective study involving vaccinated health care workers who were tested monthly for the presence of anti-spike IgG and neutralizing antibodies. Linear mixed models were used to assess the dynamics of antibody levels and to determine predictors of antibody levels at 6 months.
Results
The study included 4868 participants, with 3808 being included in the linear mixed-model analyses. The level of IgG antibodies decreased at a consistent rate, whereas the neutralizing antibody level decreased rapidly for the first 3 months with a relatively slow decrease thereafter. Although IgG antibody levels were highly correlated with neutralizing antibody titers (Spearman’s rank correlation between 0.68 and 0.75), the regression relationship between the IgG and neutralizing antibody levels depended on the time since receipt of the second vaccine dose. Six months after receipt of the second dose, neutralizing antibody titers were substantially lower among men than among women (ratio of means, 0.64; 95% confidence interval [CI], 0.55 to 0.75), lower among persons 65 years of age or older than among those 18 to less than 45 years of age (ratio of means, 0.58; 95% CI, 0.48 to 0.70), and lower among participants with immunosuppression than among those without immunosuppression (ratio of means, 0.30; 95% CI, 0.20 to 0.46).
Conclusions
Six months after receipt of the second dose of the BNT162b2 vaccine, humoral response was substantially decreased, especially among men, among persons 65 years of age or older, and among persons with immunosuppression.
As the rollout of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1,2 is expanding worldwide, data on the durability of protection are limited. A randomized, controlled trial and real-world studies have shown vaccine efficacy of 94 to 95% with the BNT162b2 vaccine (Pfizer–BioNTech) and vaccine effectiveness in preventing symptomatic coronavirus disease 2019 (Covid-19) 7 days or more after receipt of the second dose of vaccine.1,3-5 Real-world effectiveness and immunogenicity data describing the antibody kinetics over time after vaccination are beginning to appear, but a complete picture of the duration of immunity is not yet available. We recently reported that breakthrough infection in BNT162b2-vaccinated persons was correlated with neutralizing antibody titers.6 However, a threshold titer that can predict breakthrough infection has not been defined.
The BNT162b2 vaccine elicits high IgG and neutralizing antibody responses 7 to 14 days after receipt of the second dose. Lower antibody levels have been shown to develop in older persons, men, and persons with an immunosuppressed condition, which suggests that antibody titers in these populations may decrease earlier than in other populations.7,8 A decrease in anti-spike (S) antibody levels by a factor of two was observed from the peak (at 21 to 40 days) to 84 days after receipt of the second dose of the BNT162b2 vaccine among 197 vaccinated persons.9 Here, we report the results of a large-scale, real-world, longitudinal study involving health care workers that was conducted to assess the kinetics of immune response among persons with different demographic characteristics and coexisting conditions throughout the 6-month period after receipt of the second dose of the BNT162b2 vaccine.
Methods
Study Design and Population
We conducted this prospective longitudinal cohort study involving health care workers at Sheba Medical Center, a large tertiary medical center in Israel that includes 1600 beds and 14,739 health care workers, including employees, students, volunteers, and retired personnel. The distribution of the health care workers at Sheba Medical Center is as follows: 18% are physicians, 27% are nurses or nurse aids, 21% are paramedical personnel, and 34% are administrative or logistic employees. The study protocol was approved by the institutional review board at Sheba Medical Center.
All the participants were health care workers who had been invited to participate in the study and provide peripheral-blood samples for serologic assays before receipt of the first vaccine dose and then monthly (every 28 days, within a window of ±14 days) for 6 months after receipt of the second vaccine dose. Written informed consent was obtained from all study participants.
Eligibility criteria included an age of 18 years or older, no SARS-CoV-2 infection before receipt of the first vaccine dose (determined on the basis of either a negative anti–SARS-CoV-2 IgG test or the absence of a positive polymerase-chain-reaction [PCR] assay result for SARS-CoV-2, with no history of suspected clinical SARS-CoV-2 infection), and at least one serologic assay result after receipt of the second dose of vaccine. Data on PCR testing are provided in Supplementary Methods Section S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. The end of the study for any participant was defined as 175 days after receipt of the second vaccine dose, a positive SARS-CoV-2 PCR or antinucleocapsid (anti-N) antibody result, or loss to follow-up.
All the health care workers at Sheba Medical Center were required to report a daily health status on arrival at the hospital. If any Covid-19–associated symptom or exposure to a SARS-CoV-2–infected person at work, at home, or in the community was reported, a PCR test for SARS-CoV-2 was required.6,10 In addition, monthly serologic follow-up was conducted during the study period. Participants with a substantial increase in IgG antibody levels or neutralizing antibody titers (≥4 times) between consecutive tests were tested for anti-N antibody to rule out a Covid-19 breakthrough infection and, if positive, were withdrawn from the study.
Participants were notified of their personal test results. Participants whose IgG and neutralizing antibody titers decreased to below the test cutoff level tended not to return for follow-up visits. These and other missing outcomes were accommodated through the linear mixed model that was used in the analysis (described below).
Study Design of Serologic Assays
Antibodies were tested during the baseline period (defined as days 4 through 17 after receipt of the second vaccine dose) and every 4 weeks thereafter: during days 18 through 42 (period 1), days 43 through 70 (period 2), days 71 through 98 (period 3), days 99 through 126 (period 4), days 127 through 154 (period 5), and days 155 through 175 (period 6). Because we could not perform neutralizing antibody assays in all study participants, we selected a subgroup that included higher proportions of persons with risk factors of interest, such as an age of 65 years or older and coexisting conditions. Criteria for the selection of participants for the neutralizing antibody subgroup are listed in Supplementary Methods Section S2. The peak period was defined as the interval of time with the highest titers after receipt of the second dose.
A correlation between neutralizing antibody titers and infectivity was recently reported6 and suggested that although the specific threshold titer that can predict breakthrough infection is still undefined, neutralizing antibodies may be used as a correlate of protection. We therefore assessed the probability of having a titer below the cutoff for diagnostic positivity on the neutralizing antibody test (i.e., 16), as well as four titrations above it: 32, 64, 128, and 256. We assessed titers of IgG and neutralizing antibody at two primary time points: the peak period (as defined above) and the end of study (at 175 days).
Data on age and sex were available for all study participants. A computer-based questionnaire about demographic characteristics and coexisting conditions was sent electronically to all study participants. The questionnaire and definitions of the study variables are provided in Tables S2 and S3. Participants who did not respond to the questionnaire were not included in the mixed-model analysis.
Serologic Assays
Samples from vaccinated participants were tested for antibodies against SARS-CoV-2 receptor-binding domain with the Access SARS-CoV-2 IgG assay (Beckman Coulter).11,12 Anti-N antibodies were tested with the Platelia SARS-CoV-2 Total Ab Assay (Bio-Rad) according to manufacturer instructions. The SARS-CoV-2 pseudovirus neutralization assay was performed as described previously7 with the use of a green fluorescent protein reporter–based pseudotyped virus with a vesicular stomatitis virus backbone coated with SARS-CoV-2 S protein. The lower level of diagnostic detection for IgG is 0.62, and the lower level that is considered neutralizing is 16. Additional information about antibody testing is provided in Supplementary Methods Section S3.
Statistical Analysis
We used linear mixed models to examine the IgG and neutralizing antibody kinetics over the 6-month period after receipt of the second vaccine dose and to associate these changes with the demographic characteristics and coexisting conditions of the participants. The dependent variable was either the IgG or neutralizing antibody level, which was log-transformed. Fixed-effect covariates included sex, age group (18 to 44 years, 45 to 64 years, or ≥65 years), and age-by-sex interaction. Time was modeled as a constant up to 30 days after receipt of the second dose and as a linear trend thereafter. For neutralizing antibody levels, the slope of the linear trend was allowed to change at day 70 after receipt of the second dose. Interactions of the initial time slope (from day 30 onward) with age group and sex were also included. In addition to this basic model, body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) and coexisting conditions were added so that we could examine their relationships to antibody kinetics, and interactions of time with each of these covariates were retained in the model only if they were significant at the 5% level after Bonferroni adjustment for multiple comparisons. Individual participant level and time trend were included as random effects. Missing data regarding IgG or neutralizing antibody levels were accommodated within these models under the “missing at random” assumption. Participants with missing values for the covariates were excluded from the analysis. Additional details regarding the mixed-models analysis are provided in Supplementary Methods Sections S4 and S5.
The estimated effects of covariates are presented as ratios of means with 95% confidence intervals on the original scale of the IgG and neutralizing antibodies. A two-sided P value of less than 0.05 was considered to indicate statistical significance. On the basis of each fitted model, the estimated probability of having a titer below the specified different neutralizing antibody titers at 6 months after receipt of the second dose (with the 95% confidence interval) was calculated for different participant profiles by means of computer simulation.
Scatter plots of log-transformed IgG and neutralizing antibody levels and the distributions of log-transformed IgG and neutralizing antibody levels according to time since the receipt of the second dose were created with the use of GraphPad Prism software, version 5.0 (GraphPad Software). Correlations between IgG and neutralizing antibody levels for each period were assessed by Spearman’s rank correlation. Statistical analysis was performed with the use of SAS software, version 9.4 (SAS Institute), and the linear mixed-models analyses were performed with the use of R software, version 3.6.2 (R Foundation for Statistical Computing).
Results
Study Population and Serologic Assays
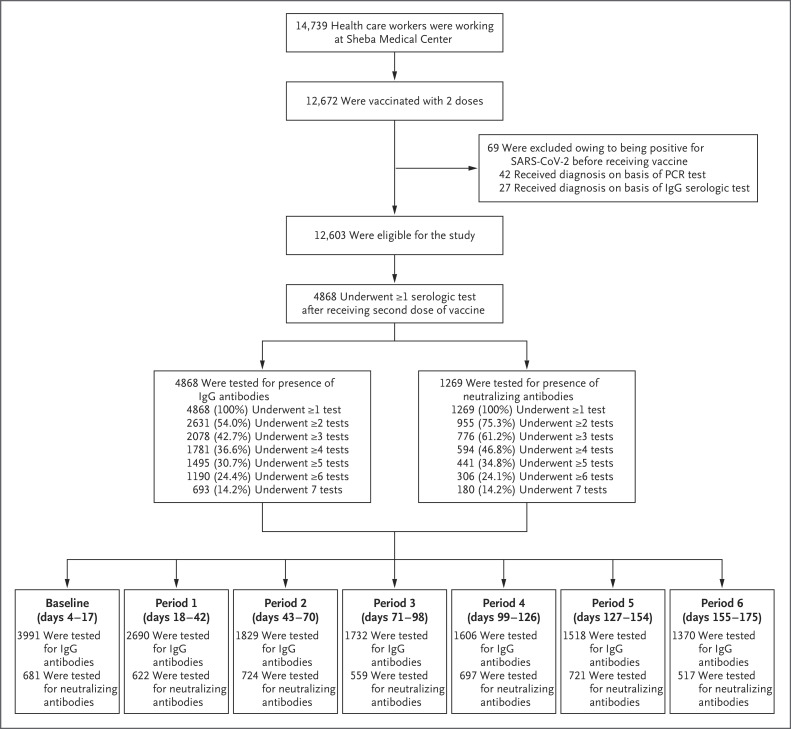
The study was conducted from December 19, 2020, to July 9, 2021. Of the 12,603 vaccinated health care workers who were eligible for the study, 4868 were recruited for study participation (Figure 1). During the study period, 20 participants had a breakthrough SARS-CoV-2 infection (defined as a positive PCR result for SARS-CoV-2), and 5 had a positive anti-N result. A total of 14,736 IgG assays and 4521 neutralizing antibody assays were performed. The numbers of persons with repeated IgG tests and neutralizing antibody assays are shown in Figure 1. IgG levels were evaluated at least once for all study participants during the 6 months of follow-up and at least twice for 2631 participants (54.0%). The neutralizing antibody subgroup included 1269 participants (26.1%) who underwent at least one neutralizing antibody test; 955 of these participants (75.3%) were tested at least twice. Data on age and sex were available for all study participants. Overall, 3808 participants (78.2%) responded to the computer-based questionnaire and were included in the mixed-model analysis.
Figure 1. Recruitment of Participants, Testing, and Follow-up.
This study involved a prospective cohort of health care workers who had received the BNT162b2 vaccine and underwent at least one serologic assay after receipt of the second dose of vaccine. During the study period (December 19, 2020, to July 9, 2021), participants were followed monthly for 6 months after receipt of the second dose. PCR denotes polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
The demographic characteristics and data on coexisting conditions in the study participants are provided in Table S1, in both the overall population and the neutralizing antibody subgroup. The mean (±SD) age of the participants was 46.9±13.7 years in the overall population and 52.7±14.2 years in the neutralizing antibody subgroup. The distributions of the demographic characteristics and coexisting conditions among the participants according to study period and IgG and neutralizing antibody assays are provided in Tables S4 and S5.
SARS-CoV-2 Antibody Kinetics after Receipt of Second Vaccine Dose
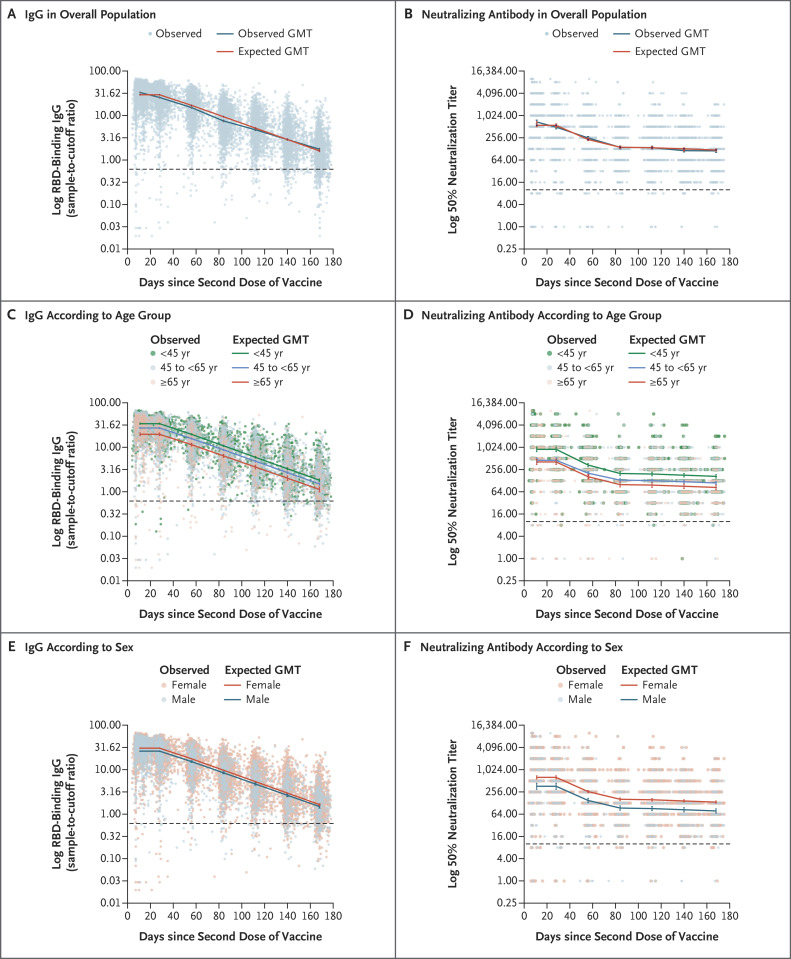
Antibody response and kinetics were assessed for 6 months after receipt of the second vaccine dose (Figure 2A and 2B and S1 and Table S6). The highest titers after the receipt of the second vaccine dose (peak) were observed during days 4 through 30, so this was defined as the peak period. The expected geometric mean titer (GMT) for IgG for the peak period, expressed as a sample-to-cutoff ratio, was 29.3 (95% confidence interval [CI], 28.7 to 29.8). A substantial reduction in the IgG level each month, which culminated in a decrease by a factor of 18.3 after 6 months, was observed. Neutralizing antibody titers also decreased significantly, with a decrease by a factor of 3.9 from the peak to the end of study period 2, but the decrease from the start of period 3 onward was much slower, with an overall decrease by a factor of 1.2 during periods 3 through 6. The GMT of neutralizing antibody, expressed as a 50% neutralization titer, was 557.1 (95% CI, 510.8 to 607.7) in the peak period and decreased to 119.4 (95% CI, 112.0 to 127.3) in period 6.
Figure 2. Distribution of Antibodies 6 Months after Receipt of Second Dose of the BNT162b2 Vaccine.
Panels A and B show the geometric mean titers (GMTs) of IgG and neutralizing antibody, respectively, in the entire study population, and Panels C through F show GMTs according to age group and sex. Antibodies were tested monthly throughout seven periods after receipt of the second dose of vaccine. Dots represent individual observed serum samples. The dashed line in each panel indicates the cutoff for diagnostic positivity. 𝙸 bars indicate 95% confidence intervals. RBD denotes receptor-binding domain.
Differential Decay According to Age and Sex
IgG and neutralizing antibody kinetics showed differences in immunogenicity according to age group and sex (Figure 2C through 2F). The rate of IgG decay in all subgroups defined according to age and sex was constant throughout the 6-month period, whereas neutralization was substantially reduced up to period 3, followed by a slower decrease thereafter. Participants 65 years of age or older had lower IgG and neutralizing antibody levels than persons 18 to less than 45 years of age during the peak period and also had a greater decrease, up to approximately 3 months (end of period 2), in the neutralizing antibody titer (Figure 2C and 2D, and see Supplementary Results Sections S1 and S2).
Predictors of Peak and End-of-Study Antibody Titers
In the peak and end-of-study periods, significantly lower IgG titers were associated with older age, male sex, the presence of two or more coexisting conditions (i.e., hypertension, diabetes, dyslipidemia, or heart, lung, kidney, or liver disease), the presence of autoimmune disease, and the presence of immunosuppression. Significantly lower neutralizing antibody titers were associated with older age, male sex, and the presence of immunosuppression in both periods, and significantly higher neutralizing antibody titers were associated with a BMI of 30 or higher (obesity) as compared with a BMI of less than 30 in both study periods. Our results show that although the IgG and neutralizing antibody titers were significantly lower in participants with two or more specific coexisting conditions than in those with no specific coexisting condition during the peak period, no significant differences in neutralizing antibody titers were observed at the end of study. In addition, participants with autoimmune disease had a significantly lower IgG titer but not neutralizing antibody titer during both the peak and end-of-study periods than did those without autoimmune disease. An age-by-sex interaction was found; the difference by which the titers in men 45 years of age or older were lower than the titers in men younger than 45 years of age was larger than the difference between the corresponding female groups.
At the end of study, the mixed-model analysis showed decreases in IgG and neutralizing antibody concentrations of 38% and 42%, respectively, among persons 65 years of age or older as compared with participants 18 to less than 45 years of age and of 37% and 46%, respectively, among men 65 years of age or older as compared with women in the same age group (Table 1). Participants with immunosuppression had decreases in the IgG and neutralizing antibody concentrations of 65% and 70%, respectively, as compared with participants without immunosuppression. Obese participants (those with a BMI of ≥30) had a 31% increase in neutralizing antibody concentrations as compared with nonobese participants (Table 1).
Table 1. Mixed-Model Analysis of Variables Associated with IgG and Neutralizing Antibody Titers after Receipt of the Second Vaccine Dose.*.
| Variable | Peak Titer | End-of-Study Titer | ||
|---|---|---|---|---|
| IgG (N = 3808) |
Neutralizing Antibody (N = 1149) |
IgG (N = 3808) |
Neutralizing Antibody (N = 1149) |
|
| ratio of mean titer (95% CI) | ||||
| Age group† | ||||
| <45 yr | Reference | Reference | Reference | Reference |
| 45 to <65 yr | 0.80 (0.77–0.84) | 0.52 (0.43–0.64) | 0.81 (0.78–0.85) | 0.66 (0.57–0.76) |
| ≥65 yr | 0.61 (0.56–0.66) | 0.59 (0.47–0.75) | 0.62 (0.57–0.68) | 0.58 (0.48–0.70) |
| Sex† | ||||
| Female | Reference | Reference | Reference | Reference |
| Male | 0.98 (0.98–0.98) | 0.64 (0.52–0.80) | 0.99 (0.97–1.00) | 0.64 (0.55–0.75) |
| Coexisting condition | ||||
| Body-mass index ≥30 | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 1.05 (0.99–1.11) | 1.31 (1.14–1.51) | 0.99 (0.93–1.05) | 1.31 (1.14–1.51) |
| No. of specific coexisting conditions‡ | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.02 (0.96–1.08) | 0.88 (0.70–1.11) | 1.02 (0.96–1.08) | 0.96 (0.84–1.17) |
| ≥2 | 0.82 (0.75–0.89) | 0.59 (0.44–0.79) | 0.82 (0.75–0.89) | 0.88 (0.71–1.09) |
| Autoimmune disease§ | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.88 (0.81–0.95) | 1.15 (0.94–1.39) | 0.88 (0.81–0.95) | 1.15 (0.94–1.39) |
| Immunosuppression§ | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.35 (0.29–0.42) | 0.30 (0.20–0.46) | 0.35 (0.29–0.42) | 0.30 (0.20–0.46) |
| Interactions between age and sex | ||||
| Age <45 yr | ||||
| Female sex | Reference | Reference | Reference | Reference |
| Male sex | 0.89 (0.84–0.95) | 0.53 (0.36–0.79) | 0.96 (0.89–1.03) | 0.81 (0.60–1.08) |
| Age 45 to <65 yr | ||||
| Female sex | Reference | Reference | Reference | Reference |
| Male sex | 0.88 (0.82–0.94) | 0.79 (0.54–1.15) | 0.87 (0.81–0.94) | 0.62 (0.50–0.77) |
| Age ≥65 yr | ||||
| Female sex | Reference | Reference | Reference | Reference |
| Male sex | 0.70 (0.61–0.80) | 0.64 (0.45–0.89) | 0.63 (0.54–0.73) | 0.54 (0.41–0.73) |
The peak period was defined as days 4 through 30 after receipt of the second dose of vaccine, and the end of study as day 175 after receipt of the second dose.
Shown is the marginal effect from the mixed model without the age-by-sex interaction.
Specific coexisting conditions included hypertension, diabetes, dyslipidemia, heart disease, lung disease, kidney disease, and liver disease.
Any participant with an autoimmune disease who also received an immunosuppressive drug was also considered to have immunosuppression.
For IgG levels, the correlation between individual participants’ peak levels and their slopes of the decrease was positive but weak (0.17; 95% CI, 0.11 to 0.24); the rates of decay were not strongly related to initial levels. However, for neutralizing antibody, the correlation was strongly negative (−0.63; 95% CI, −0.70 to −0.55). After adjustment for other factors, participants with a higher initial level tended to have a decrease that was faster up to approximately 70 days after receipt of the second dose. Beyond that time, rates of decay were modest and did not vary much among participants.
We used the mixed model to predict the probability in different subgroups of reaching a neutralizing antibody titer lower than the test cutoff for diagnostic positivity (i.e., <16) by 6 months after receipt of the second dose. We also used the model to predict the probability of a decrease to below different neutralizing antibody titers (<32, <64, <128, or <256) (Table 2). Among healthy women and men in the three age groups (18 to <45 years, 45 to <65 years, and ≥65 years of age), the probability of having a neutralizing antibody titer of less than 256 at 175 days after receipt of the second dose were as follows: 0.68, 0.79, and 0.81, respectively, among women and 0.75, 0.89, and 0.92, respectively, among men. The probability of having a neutralizing antibody titer of less than 16 in these three age groups (18 to <45 years, 45 to <65 years, and ≥65 years of age) were as follows: 0.02, 0.05, and 0.06, respectively, among women and 0.04, 0.11, and 0.15, respectively, among men. Overall (regardless of sex and age group), obese participants were at lower risk for having lower neutralizing antibody titers than nonobese participants. Participants with immunosuppression were more likely than healthy participants to have a below-average neutralizing antibody titer (Table 2).
Table 2. Probability of Having a Titer below Different Neutralizing Antibody Titers at 175 Days after Receipt of the Second Vaccine Dose, According to Sex and Age.
| Sex and Titer | Probability among Healthy Persons (95% CI)* |
Probability among Persons with BMI ≥30 (95% CI) |
Probability among Persons with Immunosuppression (95% CI)† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 18 to <45 Yr | 45 to <65 Yr | ≥65 Yr | 18 to <45 Yr | 45 to <65 Yr | ≥65 Yr | 18 to <45 Yr | 45 to <65 Yr | ≥65 Yr | |
| percent | |||||||||
| Female sex | |||||||||
| <16 | 0.02 (0.02–0.04) | 0.05 (0.04–0.07) | 0.06 (0.04–0.09) | 0.01 (0.01–0.02) | 0.03 (0.02–0.04) | 0.04 (0.02–0.06) | 0.18 (0.10–0.29) | 0.28 (0.17–0.41) | 0.30 (0.18–0.45) |
| <32 | 0.09 (0.07–0.11) | 0.15 (0.12–0.18) | 0.17 (0.13–0.22) | 0.06 (0.04–0.08) | 0.10 (0.08–0.13) | 0.12 (0.08–0.17) | 0.38 (0.25–0.52) | 0.51 (0.36–0.65) | 0.53 (0.38–0.68) |
| <64 | 0.23 (0.19–0.27) | 0.34 (0.29–0.39) | 0.36 (0.30–0.44) | 0.16 (0.12–0.21) | 0.26 (0.21–0.31) | 0.28 (0.21–0.36) | 0.62 (0.47–0.75) | 0.73 (0.60–0.84) | 0.75 (0.62–0.86) |
| <128 | 0.45 (0.40–0.50) | 0.58 (0.53–0.62) | 0.60 (0.53–0.68) | 0.36 (0.30–0.42) | 0.48 (0.42–0.54) | 0.51 (0.42–0.60) | 0.82 (0.70–0.90) | 0.89 (0.80–0.95) | 0.90 (0.82–0.96) |
| <256 | 0.68 (0.64–0.73) | 0.79 (0.75–0.83) | 0.81 (0.75–0.86) | 0.60 (0.53–0.66) | 0.72 (0.66–0.76) | 0.74 (0.66–0.80) | 0.93 (0.87–0.97) | 0.97 (0.93–0.99) | 0.97 (0.93–0.99) |
| Male sex | |||||||||
| <16 | 0.04 (0.02–0.06) | 0.11 (0.08–0.16) | 0.15 (0.10–0.21) | 0.02 (0.01–0.04) | 0.08 (0.05–0.11) | 0.10 (0.06–0.16) | 0.24 (0.12–0.38) | 0.44 (0.28–0.60) | 0.50 (0.34–0.67) |
| <32 | 0.12 (0.08–0.18) | 0.28 (0.21–0.34) | 0.34 (0.25–0.42) | 0.08 (0.05–0.13) | 0.20 (0.15–0.26) | 0.25 (0.18–0.34) | 0.45 (0.29–0.62) | 0.67 (0.52–0.81) | 0.73 (0.58–0.85) |
| <64 | 0.29 (0.21–0.38) | 0.50 (0.43–0.58) | 0.57 (0.48–0.66) | 0.22 (0.14–0.30) | 0.41 (0.32–0.49) | 0.48 (0.38–0.58) | 0.68 (0.53–0.82) | 0.85 (0.74–0.93) | 0.88 (0.79–0.95) |
| <128 | 0.52 (0.42–0.62) | 0.73 (0.67–0.79) | 0.78 (0.71–0.85) | 0.43 (0.33–0.53) | 0.65 (0.57–0.72) | 0.71 (0.62–0.79) | 0.86 (0.75–0.94) | 0.95 (0.90–0.98) | 0.96 (0.92–0.99) |
| <256 | 0.75 (0.66–0.82) | 0.89 (0.85–0.92) | 0.92 (0.88–0.95) | 0.66 (0.57–0.76) | 0.84 (0.78–0.89) | 0.88 (0.82–0.92) | 0.95 (0.90–0.98) | 0.99 (0.97–0.997) | 0.99 (0.98–0.998) |
Healthy persons were defined as participants without hypertension, diabetes, dyslipidemia, heart disease, lung disease, liver disease, immunosuppression, or autoimmune disease and with a BMI of less than 30.
Immunosuppression included organ transplantation, biologic therapy, chemotherapy, glucocorticoids, splenectomy, and human immunodeficiency virus infection.
Correlation between IgG and Neutralizing Antibody Levels
We assessed the correlation between IgG and neutralizing antibody levels. Although a strong correlation between IgG and neutralizing antibody titers was maintained throughout the 6 months after receipt of the second dose of vaccine (Spearman’s rank correlation between 0.68 and 0.75) (Fig. S2), the regression relationship between the IgG and neutralizing antibody levels depended on the time since the second dose of vaccine, a finding that was probably due to the different kinetics between IgG and neutralizing antibody levels (Figure 2).
Discussion
In this prospective longitudinal study, we found a significant waning of humoral responses within 6 months after receipt of the second dose of BNT162b2 vaccine in a large cohort of 4868 participants. We observed a continuous decrease in anti-S IgG titers at a relative stable rate within 6 months. The decrease in neutralizing antibody titers was brisk initially, in the period of up to 70 to 80 days, but slowed thereafter. Antibody titers were associated with age, sex, and coexisting conditions. Particularly vulnerable populations with lower neutralizing titers were older men and participants with immunosuppression.
Published work about many vaccines, such as those against measles, mumps, and rubella, has shown a small decrease each year of 5 to 10% in the neutralizing antibody levels.13,14 We found that a significant and rapid decrease in humoral response to the BNT162b2 vaccine was observed within months after vaccination.
Neutralizing antibodies have been shown to correlate with protection.6,15 Yet, neutralizing assays are complex and time-consuming. Thus, the correlation between anti-S IgG and neutralizing antibody levels reported here are useful. Although we found a consistently strong correlation, the regression relationship between IgG and neutralizing antibody was dependent on time. Thus, relating IgG levels to neutralizing ability depends on time since the second dose.
Using a mixed model, we analyzed the association of age, sex, and coexisting conditions with immunogenicity, both at the peak and at 6 months after receipt of the second dose. We found that antibody levels in both periods were higher in women than in men and decreased with age, as has been previously shown for the first month after receipt of the second dose.7,16 Similar to the findings in other reports,17-19 a significantly lower antibody response was found consistently through the observation period among participants with immunosuppression, who had neutralizing antibody titers that were lower by a factor of 5 than those among participants without immunosuppression.
Obese persons (BMI, ≥30) had a significantly higher neutralizing antibody titer during long-term follow-up than nonobese participants. Obesity is associated with severe Covid-19,20 and disease severity is associated with a higher Covid-19 humoral immune response. A recent study showed that SARS-CoV-2 neutralizing antibodies are positively associated with BMI.21 Yet, it is still unclear whether vaccinated obese persons are at higher or lower risk for breakthrough infection and whether the relatively high humoral response to the vaccine is protective.
Several studies on the durability of humoral response in persons who have recovered from SARS-CoV-2 infection showed that both IgG and neutralizing antibody levels decrease only modestly at 8 to 10 months after the infection.22,23 This striking difference in antibody kinetics between convalescent persons and vaccinated persons may be the reason for the substantially lower incidence of breakthrough infection among previously infected persons than among vaccinated persons.24,25 Overall, the accumulating evidence from our study and others22-25 shows that long-term humoral response and vaccine effectiveness in previously infected persons were superior to that in recipients of two doses of vaccine.
Our study was conducted in a cohort of health care workers, who were mostly healthy persons and therefore may not represent the general population. To overcome this limitation, although IgG tests were performed in the entire study population, neutralizing antibody tests were performed in a subgroup that included higher proportions of older persons or of persons with coexisting conditions in order to better represent the general population.
Our data provide important insights into the longitudinal dynamics of the immune response to BNT162b2 vaccination. As this pandemic continues to evolve, the importance of determining immune correlates of protection after vaccination becomes clearer. Strategies to prolong host immunity need to be evaluated in order to protect the population against SARS-CoV-2 and its variants.
Acknowledgments
We thank Dr. Gert Zimmer, of the Institute of Virology and Immunology, Mittelhäusern, Switzerland, for providing the green fluorescent protein reporter–based pseudotyped virus with a vesicular stomatitis virus backbone coated with severe acute respiratory syndrome coronavirus 2 spike protein; and Ms. Yael Becker-Ilany and Ms. Hanaa Jaber for technical assistance.
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on October 6, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis 2021. May 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. DOI: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med 2021;9:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terpos E, Trougakos IP, Apostolakou F, et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol 2021;96(7):E257-E259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021;398:385-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regev-Yochay G, Amit S, Bergwerk M, et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg Health Eur 2021;7:100150-100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oved K, Olmer L, Shemer-Avni Y, et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine 2020;29:100651-100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indenbaum V, Koren R, Katz-Likvornik S, et al. Testing IgG antibodies against the RBD of SARS-CoV-2 is sufficient and necessary for COVID-19 diagnosis. PLoS One 2020;15(11):e0241164-e0241164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis 2008;197:950-956. [DOI] [PubMed] [Google Scholar]
- 14.Seagle EE, Bednarczyk RA, Hill T, et al. Measles, mumps, and rubella antibody patterns of persistence and rate of decline following the second dose of the MMR vaccine. Vaccine 2018;36:818-826. [DOI] [PubMed] [Google Scholar]
- 15.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205-1211. [DOI] [PubMed] [Google Scholar]
- 16.Bates TA, Leier HC, Lyski ZL, et al. Age-dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA 2021. July 21 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med 2021;174:1336-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021. July 8 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Lu Y, Huang Y-M, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism 2020;113:154378-154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soffer S, Glicksberg BS, Zimlichman E, et al. The association between obesity and peak antibody titer response in COVID-19 infection. Obesity (Silver Spring) 2021;29:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371(6529):eabf4063-eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanshylla K, Di Cristanziano V, Kleipass F, et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe 2021;29(6):917-929.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson M, Stec M, Rewane A, Landay A, Cloherty G, Moy J. SARS-CoV-2 antibody responses in infection-naïve or previously infected individuals after 1 and 2 doses of the BNT162b2 vaccine. JAMA Netw Open 2021;4(8):e2119741-e2119741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.