Abstract
Background
Waning of vaccine protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or coronavirus disease 2019 (Covid-19) is a concern. The persistence of BNT162b2 (Pfizer–BioNTech) vaccine effectiveness against infection and disease in Qatar, where the B.1.351 (or beta) and B.1.617.2 (or delta) variants have dominated incidence and polymerase-chain-reaction testing is done on a mass scale, is unclear.
Methods
We used a matched test-negative, case–control study design to estimate vaccine effectiveness against any SARS-CoV-2 infection and against any severe, critical, or fatal case of Covid-19, from January 1 to September 5, 2021.
Results
Estimated BNT162b2 effectiveness against any SARS-CoV-2 infection was negligible in the first 2 weeks after the first dose. It increased to 36.8% (95% confidence interval [CI], 33.2 to 40.2) in the third week after the first dose and reached its peak at 77.5% (95% CI, 76.4 to 78.6) in the first month after the second dose. Effectiveness declined gradually thereafter, with the decline accelerating after the fourth month to reach approximately 20% in months 5 through 7 after the second dose. Effectiveness against symptomatic infection was higher than effectiveness against asymptomatic infection but waned similarly. Variant-specific effectiveness waned in the same pattern. Effectiveness against any severe, critical, or fatal case of Covid-19 increased rapidly to 66.1% (95% CI, 56.8 to 73.5) by the third week after the first dose and reached 96% or higher in the first 2 months after the second dose; effectiveness persisted at approximately this level for 6 months.
Conclusions
BNT162b2-induced protection against SARS-CoV-2 infection appeared to wane rapidly following its peak after the second dose, but protection against hospitalization and death persisted at a robust level for 6 months after the second dose. (Funded by Weill Cornell Medicine–Qatar and others.)
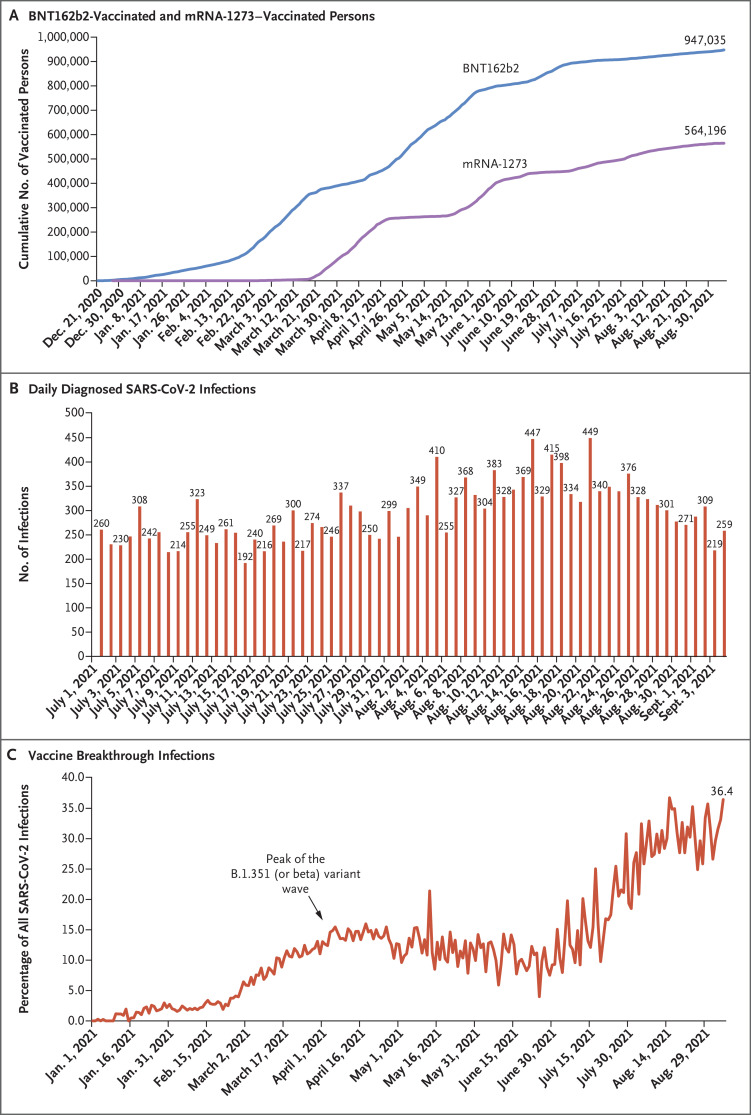
Qatar launched a mass coronavirus disease 2019 (Covid-19) immunization campaign on December 21, 2020, first using the BNT162b21 (Pfizer–BioNTech) messenger RNA (mRNA) vaccine2 and 3 months later adding the mRNA-12733 (Moderna) vaccine.4 Immunization with both vaccines followed the Food and Drug Administration–approved protocol,1,3 and vaccine coverage increased steadily from December 2020 until the time of this writing (Figure 1A). Vaccine rollout proceeded in phases in which vaccination was prioritized first to frontline health care workers, persons with severe or multiple chronic conditions, and persons 70 years of age or older. Vaccination was then gradually extended by one age group at a time and to select professional groups (e.g., teachers), with age being the principal criterion for vaccine eligibility throughout the rollout (Fig. S1A in the Supplementary Appendix, available with the full text of this article at NEJM.org). As of September 7, 2021, it was estimated that at least 90% of persons 12 years of age or older had received at least one vaccine dose and that at least 80% had received both doses.5 This appeared to be the highest mRNA vaccine coverage worldwide.6
Figure 1. Time Trend of Vaccinated Persons, SARS-CoV-2 Infections, and Vaccine Breakthrough Infections in Qatar.
SARS-CoV-2 denotes severe acute respiratory syndrome coronavirus 2.
As vaccination was scaled up, the country had two back-to-back waves of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from January through June 2021, which were dominated by the B.1.1.7 (or alpha)7 and B.1.351 (or beta)7 variants.2,4,8-10 Appreciable community transmission of the B.1.617.2 (or delta)7 variant was first detected toward the end of March 2021; by the summer of 2021, B.1.617.2 had become the dominant variant.8-10 Despite the high vaccine coverage, the incidence of SARS-CoV-2 infection increased slowly from July to August 2021, before starting to decline at the end of August (Figure 1B). In this study, we assessed the real-world effectiveness of the BNT162b2 vaccine against SARS-CoV-2 infection and Covid-19–related hospitalization and death after receipt of the first and second doses.
Methods
Study Population, Data Sources, and Study Design
This study was conducted in the resident population of Qatar. Covid-19 laboratory testing, vaccination, clinical infection data, and related demographic details were extracted from the integrated, nationwide, digital health information platform that hosts the national, federated SARS-CoV-2 databases. These databases are complete, with no missing information for polymerase-chain-reaction (PCR) testing, Covid-19 vaccinations, Covid-19–related hospitalizations, or basic demographic details since the start of the epidemic.
Vaccine effectiveness against SARS-CoV-2 infection was estimated with the use of the test-negative, case–control study design, a standard design for assessing vaccine effectiveness against influenza11,12 and SARS-CoV-2.2,4,11-16 Key to this design is its control of bias arising from misclassification of infection and differences in health care–seeking behavior between vaccinated and unvaccinated persons.11,12 Case participants (PCR-positive persons) and controls (PCR-negative persons) were matched one to one according to sex, 10-year age group, nationality, reason for SARS-CoV-2 PCR testing, and calendar week of PCR test. Matching of case participants and controls was performed to control for known differences in the risk of exposure to SARS-CoV-2 infection in Qatar.17-20
Effectiveness was estimated against documented infection (defined as a PCR-positive swab, regardless of the reason for PCR testing or the presence of symptoms), as well as against any severe,21 critical,21 or fatal22 case of Covid-19. Classification of Covid-19 case severity (acute-care hospitalizations),21 criticality (intensive care unit hospitalizations),21 and fatality22 was conducted in accordance with World Health Organization guidelines, and assessments were made by trained medical personnel with the use of individual chart reviews. (Details are provided in Sections 1 and 2 in the Supplementary Appendix.)
The study was approved by the institutional review boards at Hamad Medical Corporation and Weill Cornell Medicine–Qatar, with waiver of informed consent. Reporting of the study followed Strengthening the Reporting of Observational Studies in Epidemiology guidelines (Table S1). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the article.
Laboratory Methods and Classification of Infections
Details regarding laboratory methods for reverse-transcriptase–quantitative PCR (RT-qPCR) testing are provided in Section 3 in the Supplementary Appendix. Methods for classification of infections according to variant type with the use of RT-qPCR variant screening23 of random positive clinical samples8,10 are provided in Section 4 in the Supplementary Appendix.
Statistical Analysis
Sociodemographic characteristics of study samples were described with the use of frequency distributions and measures of central tendency. The odds ratio, comparing odds of vaccination among case participants and controls, and its associated 95% confidence interval were derived with the use of conditional logistic regression that accounted for the matching in the study design. This matching and analysis approach aims to minimize the potential bias that may result from variation in epidemic phase,11,24 gradual rollout of vaccination during the study,11,24 or other confounders.17-20,25,26 Confidence intervals were not adjusted for multiplicity. Interactions were not investigated. Vaccine effectiveness at different time points and the associated 95% confidence interval were then calculated by applying the following equation11,12: vaccine effectiveness=1−odds ratio of vaccination among case participants as compared with controls. Details regarding the statistical analysis are provided in Section 1 in the Supplementary Appendix.
A sensitivity analysis was conducted by adjusting the conditional logistic regression for previous infection and health care worker status, because health care workers were prioritized for vaccination and may have had a higher risk of infection exposure. Several additional sensitivity analyses were conducted by modifying the study inclusion and exclusion criteria to investigate whether the effectiveness estimates could have been biased. Effectiveness was also estimated with the use of a multivariable logistic-regression analysis of associations with a PCR-positive test — that is, by applying a different method from that of the main analysis of matched test-negative, case–control study design. Descriptions of these sensitivity analyses are provided in Section 5 in the Supplementary Appendix. Statistical analyses were conducted with the use of STATA/SE software, version 17.0.27
Results
Study Population
Between December 21, 2020, and September 5, 2021, a total of 947,035 persons received at least one dose of BNT162b2, and 907,763 completed the two-dose regimen (Figure 1A). The median date of the first dose was April 21, 2021, and the median date of the second dose was May 10, 2021. The median time between the first and second doses was 21 days (interquartile range, 21 to 22 days), and 97.4% of persons received their second dose within 30 days after the first dose. During this period, 564,196 persons received at least one dose of mRNA-1273, and 494,859 completed the two-dose regimen (Figure 1A).
Figure S2 shows the population-selection process used to investigate BNT162b2 effectiveness against SARS-CoV-2 infection. The distribution of included PCR-positive case participants according to calendar month is shown in Figure S1B. Demographic characteristics and reasons for PCR testing of samples used to estimate vaccine effectiveness are presented in Table 1 and Tables S2 and S3. The median age of the study participants was 31 years, approximately 69% were male, and participants came from diverse national origins. Study samples were representative of the distinct demographic characteristics of the population of Qatar.17,28
Table 1. Demographic Characteristics of the Participants and Reasons for PCR Testing among Samples Used to Estimate BNT162b2 Vaccine Effectiveness.*.
| Characteristics | 0–13 Days after First Dose | ≥14 Days after First Dose and No Second Dose | First Month after Second Dose | |||
|---|---|---|---|---|---|---|
| Case Participants: PCR-Positive (N=115,326) |
Controls: PCR-Negative (N=115,326) |
Case Participants: PCR-Positive (N=113,830) |
Controls: PCR-Negative (N=113,830) |
Case Participants: PCR-Positive (N=115,913) |
Controls: PCR-Negative (N=115,913) |
|
| Median age (IQR) — yr | 31 (21–39) | 31 (21–39) | 31 (21–39) | 31 (21–39) | 31 (21–39) | 31 (22–39) |
| Age group — no. (%) | ||||||
| <20 yr | 26,915 (23.3) | 26,915 (23.3) | 26,813 (23.6) | 26,813 (23.6) | 26,822 (23.1) | 26,822 (23.1) |
| 20–29 yr | 24,879 (21.6) | 24,879 (21.6) | 24,567 (21.6) | 24,567 (21.6) | 24,770 (21.4) | 24,770 (21.4) |
| 30–39 yr | 36,749 (31.9) | 36,749 (31.9) | 36,150 (31.8) | 36,150 (31.8) | 36,896 (31.8) | 36,896 (31.8) |
| 40–49 yr | 19,154 (16.6) | 19,154 (16.6) | 18,820 (16.5) | 18,820 (16.5) | 19,382 (16.7) | 19,382 (16.7) |
| 50–59 yr | 6,083 (5.3) | 6,083 (5.3) | 5,959 (5.2) | 5,959 (5.2) | 6,350 (5.5) | 6,350 (5.5) |
| 60–69 yr | 1,229 (1.1) | 1,229 (1.1) | 1,195 (1.0) | 1,195 (1.0) | 1,326 (1.1) | 1,326 (1.1) |
| ≥70 yr | 317 (0.3) | 317 (0.3) | 326 (0.3) | 326 (0.3) | 367 (0.3) | 367 (0.3) |
| Sex | ||||||
| Male | 79,255 (68.7) | 79,255 (68.7) | 78,148 (68.7) | 78,148 (68.7) | 79,750 (68.8) | 79,750 (68.8) |
| Female | 36,071 (31.3) | 36,071 (31.3) | 35,682 (31.3) | 35,682 (31.3) | 36,163 (31.2) | 36,163 (31.2) |
| Nationality† | ||||||
| Bangladeshi | 8,323 (7.2) | 8,323 (7.2) | 8,229 (7.2) | 8,229 (7.2) | 8,326 (7.2) | 8,326 (7.2) |
| Egyptian | 6,734 (5.8) | 6,734 (5.8) | 6,629 (5.8) | 6,629 (5.8) | 6,859 (5.9) | 6,859 (5.9) |
| Filipino | 10,713 (9.3) | 10,713 (9.3) | 10,535 (9.3) | 10,535 (9.3) | 10,674 (9.2) | 10,674 (9.2) |
| Indian | 29,914 (25.9) | 29,914 (25.9) | 29,536 (25.9) | 29,536 (25.9) | 30,325 (26.2) | 30,325 (26.2) |
| Nepalese | 10,505 (9.1) | 10,505 (9.1) | 10,367 (9.1) | 10,367 (9.1) | 10,496 (9.1) | 10,496 (9.1) |
| Pakistani | 5,793 (5.0) | 5,793 (5.0) | 5,735 (5.0) | 5,735 (5.0) | 5,837 (5.0) | 5,837 (5.0) |
| Qatari | 17,375 (15.1) | 17,375 (15.1) | 17,087 (15.0) | 17,087 (15.0) | 17,147 (14.8) | 17,147 (14.8) |
| Sri Lankan | 3,756 (3.3) | 3,756 (3.3) | 3,732 (3.3) | 3,732 (3.3) | 3,784 (3.3) | 3,784 (3.3) |
| Sudanese | 3,249 (2.8) | 3,249 (2.8) | 3,242 (2.8) | 3,242 (2.8) | 3,285 (2.8) | 3,285 (2.8) |
| Other nationalities‡ | 18,964 (16.4) | 18,964 (16.4) | 18,738 (16.5) | 18,738 (16.5) | 19,180 (16.5) | 19,180 (16.5) |
| Reason for PCR testing | ||||||
| Clinical suspicion | 40,768 (35.4) | 40,768 (35.4) | 40,261 (35.4) | 40,261 (35.4) | 41,138 (35.5) | 41,138 (35.5) |
| Contact tracing | 18,772 (16.3) | 18,772 (16.3) | 18,481 (16.2) | 18,481 (16.2) | 18,666 (16.1) | 18,666 (16.1) |
| Routine health care testing | 14,479 (12.6) | 14,479 (12.6) | 14,351 (12.6) | 14,351 (12.6) | 14,536 (12.5) | 14,536 (12.5) |
| Survey | 27,972 (24.3) | 27,972 (24.3) | 27,484 (24.1) | 27,484 (24.1) | 27,883 (24.1) | 27,883 (24.1) |
| Individual request | 12,914 (11.2) | 12,914 (11.2) | 12,838 (11.3) | 12,838 (11.3) | 13,233 (11.4) | 13,233 (11.4) |
| Other | 421 (0.4) | 421 (0.4) | 415 (0.4) | 415 (0.4) | 457 (0.4) | 457 (0.4) |
Data for other time-since-vaccination analyses are shown in Tables S2 and S3 in the Supplementary Appendix. Case participants and controls were matched one to one according to sex, 10-year age group, nationality, reason for polymerase-chain-reaction (PCR) testing, and calendar week of PCR test. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
Nationalities were chosen to represent the most populous groups in Qatar.
The category of other nationalities includes 108 other nationalities in Qatar for 0 to 13 days after the first dose, 106 other nationalities for 14 or more days after the first dose and no second dose, and 108 other nationalities for the first month after the second dose.
Only approximately 35% of case participants received a diagnosis of SARS-CoV-2 infection on the basis of symptoms (Table 1). The remaining case participants received a diagnosis on the basis of PCR testing for other reasons, including contact tracing, surveys or random testing campaigns, individual requests, and routine health care testing.
Vaccine Breakthrough Infections
As of the end of the study (September 5, 2021), a total of 8203 SARS-CoV-2 BNT162b2 breakthrough infections had been recorded among participants who received one dose of this vaccine, and 10,543 such infections had been recorded among participants who received two doses. The percentage of all daily diagnosed SARS-CoV-2 infections that were vaccine (BNT162b2 or mRNA-1273) breakthrough infections increased gradually over time and reached 36.4% on September 5, 2021 (Figure 1C). Most vaccine breakthrough infections (77.2%) were recorded for the BNT162b2 vaccine.
As of August 30, 2021, a total of 377 and 106 severe Covid-19 cases (acute-care hospitalizations21) had been recorded among participants who had received either one or two doses of BNT162b2, respectively. Similarly, 32 and 10 critical Covid-19 cases (ICU hospitalizations21) and 34 and 15 fatal Covid-19 cases (Covid-19–related deaths22) had also been recorded, respectively. Details are provided in Section 2 in the Supplementary Appendix.
Vaccine Effectiveness against Any SARS-CoV-2 Infection
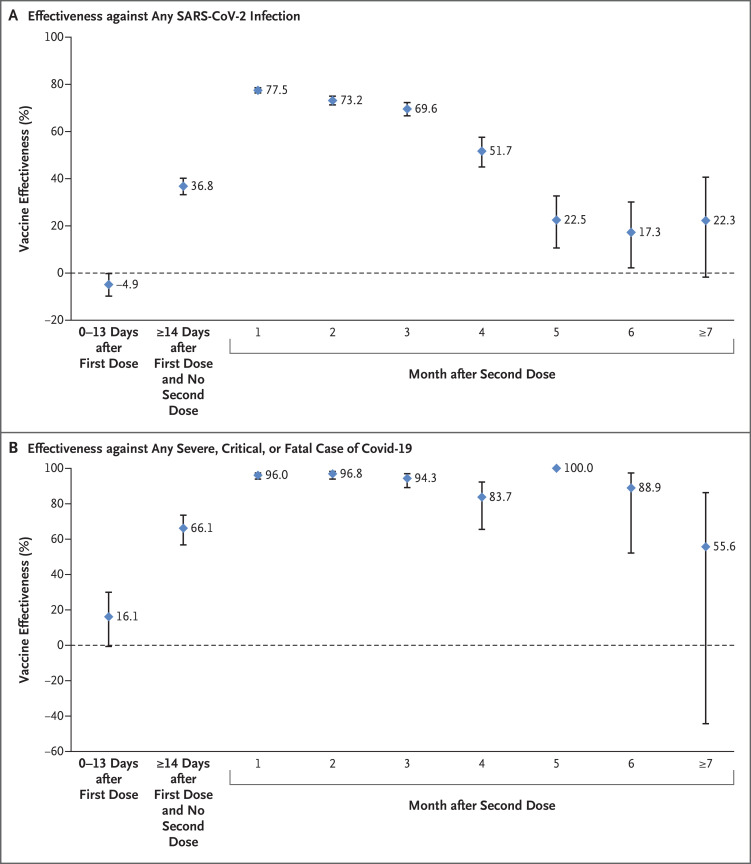
Estimated BNT162b2 effectiveness against any SARS-CoV-2 infection was negligible for the first 2 weeks after the first dose, increased to 36.8% (95% confidence interval [CI], 33.2 to 40.2) in the third week after the first dose, and reached its peak at 77.5% (95% CI, 76.4 to 78.6) in the first month after the second dose (Table 2 and Figure 2A). However, effectiveness declined gradually, starting from the first month after the second dose. The decline accelerated after the fourth month, and effectiveness reached a low level of approximately 20% in months 5 through 7 after the second dose. A sensitivity analysis that adjusted for previous infection and health care worker status confirmed the main analysis results (Table 3).
Table 2. Effectiveness of the BNT162b2 Vaccine against Any SARS-CoV-2 Infection and against Any Severe, Critical, or Fatal Case of Covid-19.*.
| Substudies† | Effectiveness against Infection | Effectiveness against Hospitalization and Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Participants: PCR-Positive‡ |
Controls: PCR-Negative‡ |
Effectiveness % (95% CI)§ | Case Participants: Severe, Critical, or Fatal Disease‡¶ |
Controls: PCR-Negative‡ |
Effectiveness % (95% CI)§ | |||||
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | |||
| 0–13 Days after first dose | 4228 | 111,098 | 4053 | 111,273 | −4.9 (−9.8 to −0.2) |
245 | 4001 | 286 | 3960 | 16.1 (−0.6 to 30.0) |
| ≥14 Days after first dose and no second dose | 2358 | 111,472 | 3567 | 110,263 | 36.8 (33.2 to 40.2) |
102 | 4032 | 272 | 3862 | 66.1 (56.8 to 73.5) |
| Month after second dose | ||||||||||
| 1 | 2915 | 112,998 | 9986 | 105,927 | 77.5 (76.4 to 78.6) |
32 | 4082 | 585 | 3529 | 96.0 (93.9 to 97.4) |
| 2 | 1450 | 111,874 | 4304 | 109,020 | 73.2 (71.3 to 75.0) |
23 | 4062 | 323 | 3762 | 96.8 (93.9 to 98.3) |
| 3 | 800 | 111,388 | 2128 | 110,060 | 69.6 (66.7 to 72.3) |
17 | 4026 | 181 | 3862 | 94.3 (89.1 to 97.0) |
| 4 | 492 | 111,070 | 856 | 110,706 | 51.7 (45.0 to 57.6) |
10 | 3996 | 51 | 3955 | 83.7 (65.5 to 92.3) |
| 5 | 548 | 110,991 | 646 | 110,893 | 22.5 (10.6 to 32.7) |
0 | 3990 | 33 | 3957 | 100.0‖ |
| 6 | 460 | 111,007 | 512 | 110,955 | 17.3 (2.2 to 30.1) |
8 | 3988 | 24 | 3972 | 88.9 (52.1 to 97.4) |
| ≥7 | 135 | 110,942 | 162 | 110,915 | 22.3 (−1.7 to 40.7) |
6 | 3983 | 11 | 3978 | 55.6 (−44.3 to 86.3) |
Covid-19 denotes coronavirus disease 2019, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
In each analysis for a specific time-since-vaccination stratum, we included only participants vaccinated in this specific time-since-vaccination stratum and those unvaccinated. Only matched pairs of PCR-positive and PCR-negative persons, in which both members of the pair were either unvaccinated or fell within each time-since-vaccination stratum, were included in the corresponding estimate of vaccine effectiveness. Thus, the number of case participants (and controls) varied across time-since-vaccination analyses.
Case participants and controls were matched one to one according to sex, 10-year age group, nationality, reason for PCR testing, and calendar week of PCR test.
Vaccine effectiveness was estimated with the use of the test-negative, case–control study design.11,12
Severity,21 criticality,21 and fatality22 were defined according to World Health Organization guidelines.
The confidence interval could not be estimated because there were zero events among vaccinated participants.
Figure 2. Effectiveness of the BNT162b2 Vaccine.
Data are presented as effectiveness point estimates, with 𝙸 bars indicating the corresponding 95% confidence intervals. Covid-19 denotes coronavirus disease 2019.
Table 3. Sensitivity Analysis.*.
| Substudies† | Effectiveness against Infection | Effectiveness against Hospitalization and Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Participants: PCR-Positive‡ |
Controls: PCR-Negative‡ |
Effectiveness % (95% CI)§ | Case Participants: Severe, Critical, or Fatal Disease‡¶ |
Controls: PCR-Negative‡ |
Effectiveness % (95% CI)§ | |||||
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | |||
| 0–13 Days after first dose | 4228 | 111,098 | 4053 | 111,273 | −11.9 (−17.3 to −6.7) |
245 | 4001 | 286 | 3960 | 7.5 (−11.9 to 23.6) |
| ≥14 Days after first dose and no second dose | 2358 | 111,472 | 3567 | 110,263 | 32.2 (28.2 to 35.9) |
102 | 4032 | 272 | 3862 | 65.0 (55.0 to 72.8) |
| Month after second dose | ||||||||||
| 1 | 2915 | 112,998 | 9986 | 105,927 | 75.8 (74.6 to 77.0) |
32 | 4082 | 585 | 3529 | 95.9 (93.6 to 97.3) |
| 2 | 1450 | 111,874 | 4304 | 109,020 | 69.7 (67.4 to 71.8) |
23 | 4062 | 323 | 3762 | 96.3 (92.9 to 98.0) |
| 3 | 800 | 111,388 | 2128 | 110,060 | 63.7 (59.9 to 67.1) |
17 | 4026 | 181 | 3862 | 93.4 (87.5 to 96.5) |
| 4 | 492 | 111,070 | 856 | 110,706 | 39.1 (29.9 to 47.0) |
10 | 3996 | 51 | 3955 | 80.8 (56.9 to 91.4) |
| 5 | 548 | 110,991 | 646 | 110,893 | 11.4 (−3.5 to 24.1) |
0 | 3990 | 33 | 3957 | 100.0‖ |
| 6 | 460 | 111,007 | 512 | 110,955 | 9.2 (−9.1 to 24.5) |
8 | 3988 | 24 | 3972 | 81.8 (18.5 to 95.9) |
| ≥7 | 135 | 110,942 | 162 | 110,915 | −4.4 (−41.3 to 22.9) |
6 | 3983 | 11 | 3978 | 44.1 (−86.5 to 83.3) |
Shown is the effectiveness of the BNT162b2 vaccine against any SARS-CoV-2 infection and against any severe, critical, or fatal case of COVID-19 after adjustment for previous infection and health care worker status.
In each analysis for a specific time-since-vaccination stratum, we included only participants vaccinated in this specific time-since-vaccination stratum and those unvaccinated. Only matched pairs of PCR-positive and PCR-negative persons, in which both members of the pair were either unvaccinated or fell within each time-since-vaccination stratum, were included in the corresponding estimate of vaccine effectiveness. Thus, the number of case participants (and controls) varied across time-since-vaccination analyses.
Case participants and controls were matched one to one according to sex, 10-year age group, nationality, reason for PCR testing, and calendar week of PCR test.
Vaccine effectiveness was estimated with the use of the test-negative, case–control study design.11,12
Severity,21 criticality,21 and fatality22 were defined according to World Health Organization guidelines.
The confidence interval could not be estimated because there were zero events among vaccinated participants.
Vaccine Effectiveness against Any SARS-CoV-2 Infection According to Age and Variant
BNT162b2 effectiveness was assessed according to age (<60 years and ≥60 years) to investigate whether declining effectiveness over time could have been confounded by age. Results for both age groups were largely similar in scale (although effectiveness was slightly lower for those ≥60 years of age, with some differences in the rate at which protection was acquired) and in the pattern of declining effectiveness (Table S4), and results were similar to those for all participants in all age groups (Table 2).
The above measures largely reflect the effectiveness of BNT162b2 against B.1.351,2,29 which was by far the dominant variant during most of these time periods.2,4,8-10 However, with the steady increase in the incidence of B.1.617.2 during the summer of 20218-10,30 and the steady decrease in the incidence of B.1.351 during this time,8-10 effectiveness measures 5 or more months after the second dose increasingly reflected effectiveness against B.1.617.2.
BNT162b2 effectiveness was assessed against B.1.1.7, B.1.351, and B.1.617.2 infections separately to investigate whether declining effectiveness could have been confounded by exposure to different variants over time. Estimated effectiveness against infection with each variant (Table S5) showed a pattern similar to that seen against any SARS-CoV-2 infection (Table 2).
Vaccine Effectiveness against Symptomatic and Asymptomatic Infections
Estimated BNT162b2 effectiveness against symptomatic infection and asymptomatic infection showed the same pattern of increasing effectiveness after the first dose, with peak effectiveness in the first month after the second dose and a gradual decline in effectiveness in the following months that accelerated after the fourth month for symptomatic infection and after the third month for asymptomatic infection (Table 4). However, effectiveness against symptomatic infection was consistently higher than that against asymptomatic infection. The peak effectiveness against symptomatic infection was 81.5% (95% CI, 79.9 to 83.0), whereas that against asymptomatic infection was 73.1% (95% CI, 70.3 to 75.5).
Table 4. Effectiveness of the BNT162b2 Vaccine against Symptomatic SARS-CoV-2 Infection and Asymptomatic SARS-CoV-2 Infection.
| Substudies* | Effectiveness against Symptomatic Infection† | Effectiveness against Asymptomatic Infection‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Participants: PCR-Positive§ |
Controls: PCR-Negative§ |
Effectiveness % (95% CI)¶ | Case Participants: PCR-Positive§ |
Controls: PCR-Negative§ |
Effectiveness % (95% CI)¶ | |||||
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | |||
| 0–13 Days after first dose | 1954 | 38,814 | 1864 | 38,904 | −5.5 (−12.9 to 1.4) |
984 | 26,988 | 962 | 27,010 | −2.6 (−12.8 to 6.7) |
| ≥14 Days after first dose and no second dose | 1074 | 39,187 | 1922 | 38,339 | 47.9 (43.6 to 51.9) |
516 | 26,968 | 645 | 26,839 | 22.2 (12.1 to 31.2) |
| Month after second dose | ||||||||||
| 1 | 867 | 40,271 | 3756 | 37,382 | 81.5 (79.9 to 83.0) |
781 | 27,102 | 2202 | 25,681 | 73.1 (70.3 to 75.5) |
| 2 | 650 | 39,402 | 1948 | 38,104 | 72.5 (69.6 to 75.1) |
381 | 27,038 | 899 | 26,520 | 66.9 (61.9 to 71.3) |
| 3 | 372 | 39,030 | 1040 | 38,362 | 70.6 (66.4 to 74.3) |
239 | 26,982 | 402 | 26,819 | 47.7 (37.3 to 56.3) |
| 4 | 258 | 38,829 | 486 | 38,601 | 57.0 (48.6 to 64.0) |
120 | 26,956 | 132 | 26,944 | 11.5 (−17.1 to 33.2) |
| 5 | 316 | 38,776 | 344 | 38,748 | 12.0 (−6.1 to 27.1) |
99 | 26,956 | 117 | 26,938 | 24.3 (−7.1 to 46.5) |
| 6 | 230 | 38,800 | 251 | 38,779 | 12.8 (−9.1 to 30.3) |
125 | 26,958 | 124 | 26,959 | −2.1 (−52.0 to 31.4) |
| ≥7 | 75 | 38,761 | 97 | 38,739 | 27.8 (−1.4 to 48.7) |
29 | 26,948 | 25 | 26,952 | −33.3 (−181.8 to 36.9) |
In each analysis for a specific time-since-vaccination stratum, we included only participants vaccinated in this specific time-since-vaccination stratum and those unvaccinated. Only matched pairs of PCR-positive and PCR-negative persons, in which both members of the pair were either unvaccinated or fell within each time-since-vaccination stratum, have been included in the corresponding estimate of vaccine effectiveness. Thus, the number of case participants (and controls) varied across time-since-vaccination analyses.
A symptomatic infection was defined as a PCR-positive test conducted because of clinical suspicion due to the presence of symptoms compatible with a respiratory tract infection.
An asymptomatic infection was defined as a PCR-positive test conducted with no reported presence of symptoms compatible with a respiratory tract infection — that is, the PCR testing was done as part of a survey or a random testing campaign.
Case participants and controls were matched one to one according to sex, 10-year age group, nationality, reason for PCR testing, and calendar week of PCR test.
Vaccine Effectiveness against Covid-19–Related Hospitalization and Death
Estimated BNT162b2 effectiveness against any severe, critical, or fatal disease due to any SARS-CoV-2 infection was negligible for the first 2 weeks after the first dose. It increased rapidly to 66.1% (95% CI, 56.8 to 73.5) in the third week after the first dose and reached 96% or higher in the first 2 months after the second dose (Table 2 and Figure 2B). Unlike effectiveness against infection, effectiveness against hospitalization and death did not decline over time, except possibly in the seventh month after the second dose when there was a hint of a decline, but the case numbers were small. The sensitivity analysis that adjusted for previous infection and health care worker status confirmed the main analysis results (Table 3). Effectiveness according to age group (Table S4) showed similar results.
Effectiveness was also estimated against severe disease, critical disease, and fatal disease individually (Table S6), as opposed to against a composite outcome of any severe, critical, or fatal disease (Table 2). Estimated effectiveness against each of these individual disease outcomes was similar to that against the composite disease outcome, with no evident decline in effectiveness for 6 months after the second dose.
Additional Analyses
Additional sensitivity analyses were conducted to investigate the effects of potential bias in these real-world effectiveness estimates (Section 5 in the Supplementary Appendix). All analyses generated consistent results, with the same pattern of declining effectiveness in the months after the second dose (Tables S7 through S10) as was observed in the main analysis (Table 2).
For additional validation of study findings, effectiveness was estimated with the use of a different method from that of the matched test-negative, case–control study design. Estimates were derived with the use of multivariable logistic-regression analysis of associations with a PCR-positive test during the study (Table S11). This analysis also generated results similar to those of the main analysis (Table 2).
Discussion
BNT162b2-induced protection against infection builds rapidly after the first dose, peaks in the first month after the second dose, and then gradually wanes in subsequent months. The waning appears to accelerate after the fourth month, to reach a low level of approximately 20% in subsequent months. Although the protection against asymptomatic infection diminished more quickly than that against symptomatic infection, as would be expected in a vaccine that prevents symptoms given infection,31,32 no evidence was found for an appreciable waning of protection against hospitalization and death, which remained robust — generally at 90% or higher — for 6 months after the second dose. Implications of these findings on infection transmission remain to be clarified, but vaccine breakthrough infections were found recently, in this same population, to be less infectious than primary infections in unvaccinated persons.33
Because the immunization campaign prioritized vaccination of persons with severe or multiple chronic conditions and prioritized vaccination according to age group, this pattern of waning of protection could theoretically be confounded by effects of age and coexisting conditions. However, this possibility was not supported by our results, because a similar pattern of waning of protection was observed for all ages. Old age may (partially) serve as a proxy for coexisting conditions, and the number of persons with severe or multiple chronic conditions is small among the young, working-age population of Qatar.17,28 The national list of vaccine prioritization included only 19,800 persons of all age groups with serious coexisting conditions to be prioritized in the first phase of vaccine rollout.
Infection incidence was driven by different variants over time; thus, it is possible that waning of protection could be confounded by exposure to different variants at different time points. However, this seems unlikely. By far the dominant variant during the study was B.1.351,2,4,8-10 and a similar pattern of waning of protection was observed for B.1.1.7, B.1.351, and B.1.617.2.
Vaccinated persons presumably have a higher rate of social contact than unvaccinated persons and may also have lower adherence to safety measures.34-36 This behavior could reduce real-world effectiveness of the vaccine as compared with its biologic effectiveness, possibly explaining the waning of protection. Public health restrictions have been easing gradually in Qatar but differently for vaccinated and unvaccinated persons. Many social, work, and travel activities now require evidence of vaccination (a “health pass”) that is administered through a mandatory mobile app (the Ehteraz app). Risk compensation may be even higher with increasing time since receipt of the second dose — that is, there could be a progressive normalization of behavior.35-37 However, risk compensation is perhaps more likely to affect the overall level of estimated effectiveness than the observed rapid waning of protection over time, unless such risk compensation increases rapidly with time after the second dose.
PCR testing in Qatar is done on a mass scale, with approximately 5% of the population being tested every week.5 Approximately 75% of those who receive a diagnosis of SARS-CoV-2 infection at present do so not because of the appearance of symptoms but because of routine testing. It is possible that many asymptomatic infections were diagnosed among vaccinated participants that otherwise would have been missed. The higher ascertainment of infection may have lowered the effectiveness estimates. This idea is supported by the observed lower effectiveness against asymptomatic infection.
Emerging evidence supports the findings of this study. An increasing number of studies suggest substantial waning of BNT162b2 effectiveness.38-42 The findings are also supported by recent reports from Israel and the United States that indicate declining BNT162b2 effectiveness against infection with elapsed time and according to calendar month.42-46 Our findings, along with the greater immunogenicity of a schedule with a longer dose interval,47 may also explain the observed low effectiveness against B.1.617.2 in countries where the second dose was implemented 3 weeks after the first dose, such as in Israel,43 Qatar,30 and the United States,46 where B.1.617.2 has been dominant at a time when a nonnegligible proportion of the population had their second dose in January or February of 2021. However, higher effectiveness against B.1.617.2 has been observed in countries where a delayed interval schedule has been implemented, such as in Canada15 and the United Kingdom,13,14 where B.1.617.2 became dominant at a time when a negligible proportion of the population had their second dose in January or February of 2021.
This study has limitations. Individual-level data on coexisting conditions were not available; therefore, they could not be explicitly factored into our analysis. However, adjusting for age may have served, in part, as a proxy. With the young population of Qatar,17,28 only a small proportion of the study population may have had serious coexisting conditions. Only 9% of the population are 50 years of age or older,17,28 and 60% are young, expatriate craft and manual workers involved in mega-development projects.18,19,48 Our findings may not be generalizable to other countries where elderly persons constitute a sizable proportion of the total population.
Effectiveness was assessed with the use of an observational, test-negative, case–control study design,11,12 rather than a randomized, clinical trial design, in which cohorts of vaccinated and unvaccinated persons were followed. We were unable to use a cohort study design owing to depletion of the unvaccinated cohorts by the high vaccine coverage. However, the cohort study design that was applied earlier to the same population of Qatar yielded findings similar to those reported for the test-negative, case–control design,2,4 which supports the validity of this standard approach in assessing vaccine effectiveness for respiratory tract infections.2,4,11-15 The results of this study are also consistent with our previous estimates of vaccine effectiveness immediately after the first and second doses.2,29 We note that the earlier estimates involved (mostly) symptomatic infections with low PCR cycle threshold values, whereas the present study estimates involve (mostly) asymptomatic infections of both high and low PCR cycle threshold values.
Nonetheless, one cannot rule out the possibility that in real-world data, bias could arise in unexpected ways or from unknown sources, such as subtle differences in test-seeking behavior or changes in the pattern of testing with the introduction of other testing approaches, such as rapid antigen testing. For example, inclusion of PCR testing before travel or at port of entry was found to introduce a negative bias — that is, lowering the effectiveness estimates (Table S10) — perhaps because of different test-seeking behaviors of those vaccinated as compared with those unvaccinated, as a consequence of the travel privileges granted only to vaccinated persons.49
Vaccine effectiveness for participants at 0 to 13 days after the first dose was just below zero, possibly suggesting a negative bias. However, this has also been observed elsewhere for both Covid-19 vaccines50-52 and other vaccines.53 This effect may reflect differences in social behavior at or after vaccination or an immunologic effect.53
Notwithstanding these limitations, consistent findings of this study were reached that indicated a large effect size for the waning of vaccine protection over time, regardless of the reason for PCR testing and whether there were symptoms. Moreover, with the mass scale of PCR testing in Qatar,5 the likelihood of bias is perhaps minimized. Indeed, the different sensitivity and additional analyses that were conducted to investigate effects of potential bias, such as by modifying the inclusion and exclusion criteria, all yielded findings that indicated a rapid waning of vaccine protection.
In this study, we found that BNT162b2-induced protection against infection peaked in the first month after the second dose and then gradually waned month by month, before reaching low levels 5 to 7 months after the second dose. Meanwhile, BNT162b2-induced protection against hospitalization and death persisted with hardly any waning for 6 months after the second dose. These findings suggest that a large proportion of the vaccinated population could lose its protection against infection in the coming months, perhaps increasing the potential for new epidemic waves.
Acknowledgments
We thank the staffs of Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine–Qatar for the diligent efforts and contributions that made this study possible.
Supplementary Appendix
Disclosure Forms
This article was published on October 6, 2021, at NEJM.org.
Footnotes
Supported by the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine–Qatar ; the Ministry of Public Health; and Hamad Medical Corporation. The Qatar Genome Program provided the reagents for the viral genome sequencing.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021;385:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021;27:1614-1621. [DOI] [PubMed] [Google Scholar]
- 5.Qatar Ministry of Public Health. National Covid-19 vaccination program data, 2021. (https://covid19.moph.gov.qa/EN/Pages/Vaccination-Program-Data.aspx).
- 6.Coronavirus (COVID-19) vaccinations. Our World in Data, 2021. (https://ourworldindata.org/covid-vaccinations).
- 7.World Health Organization. Tracking SARS-CoV-2 variants. 2021. (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/).
- 8.Qatar viral genome sequencing data: data on randomly collected samples. GISAID, 2021. (https://www.gisaid.org/phylodynamics/global/nextstrain/).
- 9.Benslimane FM, Al Khatib HA, Al-Jamal O, et al. One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. May 20, 2021. (https://www.medrxiv.org/content/10.1101/2021.05.19.21257433v2). preprint. [DOI] [PMC free article] [PubMed]
- 10.Hasan MR, Kalikiri MKR, Mirza F, et al. Real-time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int J Infect Dis 2021. September 12 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 11.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31:2165-2168. [DOI] [PubMed] [Google Scholar]
- 12.Verani JR, Baqui AH, Broome CV, et al. Case-control vaccine effectiveness studies: preparation, design, and enrollment of cases and controls. Vaccine 2017;35:3295-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021;397:2461-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasreen S, He S, Chung H, et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. July 3, 2021. (https://www.medrxiv.org/content/10.1101/2021.06.28.21259420v1). preprint.
- 16.Dean NE, Hogan JW, Schnitzer ME. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. DOI: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep 2021;11:6233-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Thani MH, Farag E, Bertollini R, et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis 2021;8(8):ofab221-ofab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeremijenko A, Chemaitelly H, Ayoub HH, et al. Herd immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities, Qatar. Emerg Infect Dis 2021;27:1343-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyle PV, Chemaitelly H, Ben Hadj Kacem MA, et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. iScience 2021;24:102646-102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 clinical management: living guidance. Geneva: World Health Organization, January 25, 2021. (https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1). [Google Scholar]
- 22.International guidelines for certification and classification (coding) of COVID-19 as cause of death. Geneva: World Health Organization, April 20, 2020. (https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1). [Google Scholar]
- 23.Vogels C, Fauver J, Grubaugh N. Multiplexed RT-qPCR to screen for SARS-COV-2 B.1.1.7, B.1.351, and P.1 variants of concern V.3. February 9, 2021. (https://www.protocols.io/view/multiplexed-rt-qpcr-to-screen-for-sars-cov-2-b-1-1-br9vm966).
- 24.Jacoby P, Kelly H. Is it necessary to adjust for calendar time in a test negative design? Responding to: Jackson ML, Nelson JC. The test negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31(April (17)):2165-8. Vaccine 2014;32:2942-2942. [DOI] [PubMed] [Google Scholar]
- 25.Pearce N. Analysis of matched case-control studies. BMJ 2016;352:i969-i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 27.Stata statistical software: release 17. College Station, TX: StataCorp. 2021. (https://www.stata.com/). [Google Scholar]
- 28.Planning and Statistics Authority. Qatar monthly statistics. State of Qatar (https://www.psa.gov.qa/en/pages/default.aspx).
- 29.Abu-Raddad LJ, Chemaitelly H, Yassine HM, et al. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J Travel Med 2021. May 28 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. August 11, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.11.21261885v1). preprint. [DOI] [PubMed]
- 31.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2021;21(2):e26-e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams LR, Ferguson NM, Donnelly CA, Grassly NC. Measuring vaccine efficacy against infection and disease in clinical trials: sources and magnitude of bias in COVID-19 vaccine efficacy estimates. July 31, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.30.21260912v1). preprint. [DOI] [PMC free article] [PubMed]
- 33.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of vaccination and of prior infection on infectiousness of vaccine breakthrough infections and reinfections. July 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.28.21261086v1). preprint.
- 34.Makhoul M, Ayoub HH, Chemaitelly H, et al. Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. Vaccines (Basel) 2020;8:668-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usherwood T, LaJoie Z, Srivastava V. A model and predictions for COVID-19 considering population behavior and vaccination. Sci Rep 2021;11:12051-12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson O, Campos-Mercade P, Meier AN, Wengström E. Anticipation of COVID-19 vaccines reduces social distancing. January 15, 2021. (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3765329). preprint. [DOI] [PMC free article] [PubMed]
- 37.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? BMJ 2006;332:605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. August 5, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.03.21261496v1). preprint. [DOI] [PMC free article] [PubMed]
- 39.Thomas SJ, Moreira ED, Kitchin N, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. July 28, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1). preprint. [DOI] [PMC free article] [PubMed]
- 40.Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2 breakthrough infections to time-from-vaccine, preliminary study. July 31, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.29.21261317v1). preprint. [DOI] [PMC free article] [PubMed]
- 41.Pouwels KB, Pritchard E, Matthews PC, et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. August 24, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.18.21262237v1). preprint. [DOI] [PMC free article] [PubMed]
- 42.Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med. DOI: 10.1056/NEJMc2112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Israeli Ministry of Health. COVID-19 vaccine effectiveness against the Delta variant: Israel’s Ministry of Health report. 2021. (https://www.gov.il/BlobFolder/reports/vaccine-efficacy-safety-follow-up-committee/he/files_publications_corona_two-dose-vaccination-data.pdf).
- 44.Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status — New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. August 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v3). preprint.
- 47.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021;398:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Planning and Statistics Authority. Labor force sample survey. State of Qatar, 2017. (https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Social/LaborForce/2017/statistical_analysis_labor_force_2017_En.pdf).
- 49.Bertollini R, Chemaitelly H, Yassine HM, Al-Thani MH, Al-Khal A, Abu-Raddad LJ. Associations of vaccination and of prior infection with positive PCR test results for SARS-CoV-2 in airline passengers arriving in Qatar. JAMA 2021;326:185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hitchings MDT, Ranzani OT, Scaramuzzini Torres MS, et al. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P.1 variant transmission in Brazil: a test-negative case-control study. April 7, 2021. (https://www.medrxiv.org/content/10.1101/2021.04.07.21255081v1). preprint. [DOI] [PMC free article] [PubMed]
- 51.Hunter PR, Brainard J. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose: a reanalysis of a study of ‘real-world’ vaccination outcomes from Israel. February 3, 2021. (https://www.medrxiv.org/content/10.1101/2021.02.01.21250957v1). preprint.
- 52.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021;397:1725-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daum RS, Siber GR, Ballanco GA, Sood SK. Serum anticapsular antibody response in the first week after immunization of adults and infants with the Haemophilus influenzae type b-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Infect Dis 1991;164:1154-1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.