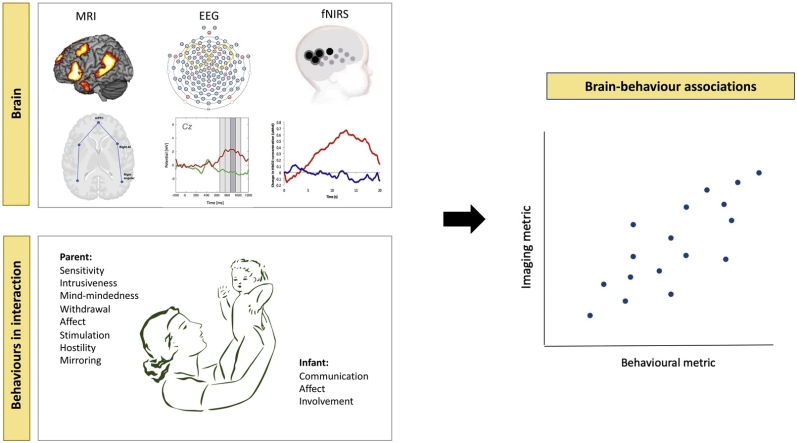
Graphical abstract
Keywords: Social interactions, Infancy, Brain development, Brain-Behaviour associations, Caregiver-infant interactions, Quality of interaction, Electroencephalography (EEG), Functional magnetic resonance imaging (fMRI), Brain network connectivity, Frontal EEG asymmetry, Event-related potential (ERP)
Highlights
-
•
Associations between caregiver-infant behaviours during social interactions and brain development outcomes were investigated.
-
•
Caregivers' and infants' behaviours in interactions related to children’s structural, functional and connectivity measures.
-
•
Concurrent associations between behavioural and brain measures were apparent as early as three months postnatally.
-
•
Long-term associations between behaviours in early interactions and brain development outcomes were observed decades later.
-
•
Individual differences in early interactions and associated brain development is an important avenue for further research.
Abstract
From birth, interactions with others are an integral part of a person’s daily life. In infancy, social exchanges are thought to be critical for optimal brain development. This systematic review explores this association by drawing together infant studies that relate adult-infant behaviours – coded from their social interactions - to children’s brain measures collected during a neuroimaging session in infancy, childhood, adolescence or adulthood. In total, we identified 55 studies that explored associations between infants’ social interactions and neural measures. These studies show that several aspects of caregiver-infant behaviours are associated with, or predict, a variety of neural responses in infants, children and adolescents. The presence of both concurrent and long-term associations - some of which are first observed just a few months postnatally and extend into adulthood - open an important research avenue and motivate further longitudinal studies.
1. Introduction
Interactions with others are an integral part of a person’s daily life from the moment of birth. In recent years much research has been dedicated to furthering our understanding of how such interactions are underpinned by, and influence, specialised brain functions. With recent advances in neuroimaging methods for the study of infants, a new avenue for exploration has opened. This systematic review aims to investigate links between infant’s social experiences and early brain development, by identifying which behaviours in social interaction are most associated with specific changes in brain structure and functions.
Humans are inherently a social species. In addition to being born with a set of predispositions to navigate the social world (Johnson et al., 1991; DeCasper and Fifer, 1980; Vouloumanos et al., 2010; Simion et al., 2008), the vast repertoire of infant’s social and communicative skills develops with experience within the context of interaction with other humans, in particular, with their caregivers (Legerstee, 2009).
Our early years of life are seen as a “window of opportunity” (and a “point of vulnerability”) for several reasons (Andersen, 2003). Most of the basic architecture supporting structural and functional organisation of the brain is present by the second birthday, followed by slow-paced “fine-tuning” and reorganisations of the major circuits and networks (review Gilmore et al., 2018). Specifically, the basic “wiring” of the brain in the form of major white matter tracts and white-matter structural networks is already established by the time of birth. So too are the highly correlated variations in grey-matter volumes observed across primary sensorimotor regions (Gilmore et al., 2018; Geng et al., 2017; Cao et al., 2017). Similarly, the developmental patterns of functional connectivity reveal somewhat mature network configurations of auditory, visual and sensorimotor functional networks before birth (Fransson et al., 2007; Lin et al., 2008; Gao et al., 2015). Early experiences and gene expression postnatally can further refine these primary circuits to be more efficient, leading towards a gradual improvement of other functional networks supporting various cognitive and perceptual functions (i.e. social processing) (Alcauter et al., 2014). Rooted in these primary sensory networks, the protracted experience-dependent development of higher-order parietal, frontal, and temporal brain areas as well as the maturation of white matter tracts (myelination) takes place postnatally (Gilmore et al., 2018; Geng et al., 2017; Emerson et al., 2016; Gao et al., 2015; Zielinski et al., 2010; Gogtay et al., 2004).
The early experiences that a child receives - both prenatally and postnatally (Miguel et al., 2019) - are embedded within the caregiving environment and may contribute to neural differentiation necessary for further neural reorganisations (Champagne and Curley, 2005). For example, neural attunement to specific types of stimuli that are repeatedly presented in the environment is heavily influenced by caregivers (see Maurer and Werker, 2013 for review). If a mother speaks in more than one language to her child, that child’s brain will specialise differently as compared to infants exposed to only one language (Costa and Sebastián-Gallés, 2014). Furthermore, in dyads where caregivers are deaf or blind and parent-child interactive experiences differ relative to the normative experiences within their own population, subsequent alterations in infants’ functional brain development are observed (e.g. Vernetti et al., 2018; Mercure et al., 2020).
Adverse patterns of social experiences – in extreme cases institutionalization, neglect and abuse – are associated with structural and functional brain atypicalities (reviews McLaughlin et al., 2019; Belsky and De Haan, 2011; Teicher et al., 2003), for example, reduced cerebellar, grey and white matter volumes are already evident by early childhood (e.g. Sheridan et al., 2012; Eluvathingal et al., 2006; Bauer et al., 2009). However, even variations in normative caregiving environment can affect brain development in children who have not been exposed to extreme adversity. For example, a study with adults demonstrated that supportive parenting during early adolescence buffered against the adversities of poverty as reflected in the adult’s resting-state functional connectivity in both central executive and emotion-regulation networks (Brody et al., 2019). In contrast, negative or aggressive parenting was linked to alterations in the development of the prefrontal cortex in adolescents (Whittle et al., 2016).
The investigation of associations between early social-environmental risk factors and later brain structure and function in infancy has only been achievable in recent decades through advances in infant-friendly neuroimaging techniques. Novel analytical and technological advances - i.e. wearable/portable neuroimaging devices, large scale data collection, and computer-automated behavioural coding – have widened the scope of this research towards studies that use multiple age points (i.e. longitudinal framework) and more varied and ecologically valid contexts (i.e. home testing). The objective of this paper is to provide a systematic overview of infant studies that utilized neuroimaging and behavioural observation techniques to study the impact of social interactions on the brain.
2. Methods
This systematic review was conducted across research articles that investigated both measures of infant social interactions and sequential measures of the same participants’ brain activity or structure.
In this review, we chose to use behavioural observations of infants’ social interactions as the most objective sampling of the early social environment. Rather than distal predictors i.e. maltreatment or neglect, behaviours during interaction serve as an approximation of how a child and/or a parent behave naturally in everyday life and these concrete observable actions, or a constellation of behaviours, are investigated in relation to neural measures.
Studies described in this review included cross-cultural studies and exploratory studies, and due to the high exclusion rate of infant participants in neuroimaging studies reported throughout the literature (Stets et al., 2012; Lloyd-Fox et al., 2010), no limits on sample size were set. In detail, research articles were included if: (1) they measured at least one episode of real-time social interaction of an infant/toddler with an adult – either caregiver or experimenter - between the age of 0–24 months; (2) behaviours of either infant, caregiver or both in interaction were either videoed to be then coded by researchers, or coded by experimenters in-vivo as they occurred; (3) they measured at least one aspect of brain structure or function of the participant, either at the same timepoint as the interaction episode or as a follow-up later in childhood; (4) they measured healthy populations or a clinical population (with or without a control group); and (5) they were published in the English language.
Moreover, in this review, we included research papers that performed more structured interactions. For example, a popular Strange Situation Procedure (Ainsworth et al., 1978) involves a very concrete scenario for a parent to follow and allows the researcher to classify an infant into an attachment category based on their naturalistic reaction to separation and reunion with the caregivers.
Research articles were excluded if: (1) the measures of social interactions were based solely on self- or diagnostic reports; (2) brain measure was collected during the interaction, incl. hyperscanning studies; (3) the design was an intervention study; (4) the article was a review paper, a protocol or a book chapter; (5) the paper did not have a full journal article (e.g. abstract only); (6) the article was not peer-reviewed; (7) the article was a non-human study, a case study, a questionnaire study or a case-control study.
Four electronic databases were searched for full research articles: Scopus, PsychInfo, PubMed and Web of Science. These were searched systematically across the period of January - March 2020, and then repeated in full in May-June 2021. In both cases, key search terms were utilised to encompass the following domains: (1) neuroimaging techniques and brain - keyword examples include “electroencephalography”, “brain volume”, “neural processing” or “resting state”; and (2) social interaction - keyword examples include “face-to-face”, “mother-infant communication” or “interactive experience”. The second search was much more comprehensive in that it did not limit the papers to particular years, included more exhaustive keywords for each of the searched domains and excluded the domain of keywords that specified papers published with the infant population and, instead, expanded the participant age ranges where possible.
Given that keywords relating to “social interaction” can particularly be wide-ranging, a more extensive double-search strategy deemed necessary. For a more detailed strategy for each of the searches for each database, see Supplementary Material.
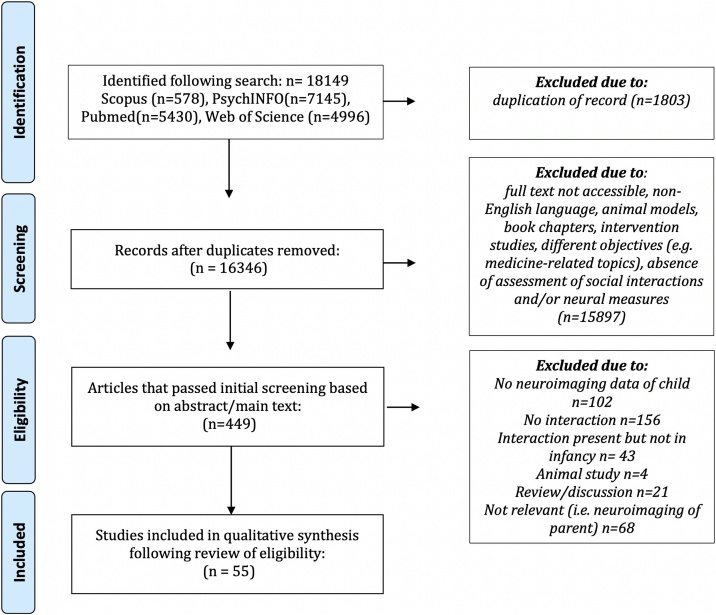
Once articles were identified and all duplicates across the databases removed, all articles were reviewed based on the title, then, abstract and, if doubts remained, the full-text was accessed to determine eligibility further. A diagram summarizing the literature search process is provided in Fig. 1 following a standard reporting style (Moher et al., 2009).
Fig. 1.
An overview of the steps undertaken during the literature search for the systematic review.
3. Results
3.1. Method search
In total, n = 16,346 original records were identified across all four databases. Of these articles, n = 449 were read for eligibility and n = 55 articles were identified that explored the associations between a child’s brain measures and variables derived directly from the recording of adult-infant social interactions. The literature search has been run twice, and, while all articles that were discovered in the initial search came out in the second search, an additional n = 28 articles were added. Out of these research articles, n = 15 research articles were the result of research criteria expansion to include articles published before 2000 and new articles published in 2020–2021 (n = 7 articles) and articles that used the Strange Situation Procedure as the sole measure of social interaction (n = 8 articles).
3.2. Study characteristics
General information on the identified 55 studies can be found in Table 1. Participants were predominantly White (n = 34) and educated (n = 33). Only five studies included lower income families (Hanford et al., 2018; Lyons-Ruth et al., 2016; Jones et al., 2009; Diego et al., 2006; Field et al., 2003). In addition to studies of the typical population, this review included two studies that compared infants with, and without, an increased familial likelihood of developing autism spectrum disorders (ASD) (Eggebrecht et al., 2017; Elsabbagh et al., 2015), and eleven studies investigated depressed mothers, out of which one study (Diego et al., 2002) did not have a control group.
Table 1.
Characteristics of 55 empirical studies.
| Characteristics | n= /55 | % |
|---|---|---|
| Race | ||
| White | 34 | 62% |
| African-American/Spanish | 6 | 11% |
| Asian | 5 | 9% |
| Not mentioned | 11 | 20% |
| Socio-economic Status/ Education | ||
| Mid-to-high SES/educated | 33 | 60% |
| Low SES | 5 | 9% |
| Not mentioned | 17 | 31% |
| Population | ||
| Typical population | 41 | 75% |
| Depressed mothers | 11 | 20% |
| Elevated risk for developing ASD | 2 | 4% |
| Preterm babies | 1 | 2% |
| Sample size | ||
| <50 | 24 | 44% |
| 51−100 | 16 | 29% |
| 101−200 | 9 | 16% |
| >201 | 6 | 11% |
| Study design | ||
| Longitudinal | 13 | 24 % |
| Cross-sectional | 23 | 42% |
| Follow-up | 19 | 35 % |
Importantly, at least 30 of the research articles were identified as outputs of larger longitudinal projects, and seven such projects/studies – (1) Wang et al. (2019); Lee et al. (2019); Rifkin-Graboi et al. (2019; 2015); Wen et al. (2017); (2) Bernier et al. (2016); Swingler et al. (2017); (3) Tharner et al., 2011; Cortes Hidalgo et al., 2019; (4) Sethna et al. (2017; 2019); (5) Swingler et al. (2007; 2010); (6) Moutsiana et al. (2014; 2015) and (7) Hane et al. (2006; 2010) – had their samples taken from the same pool of participants.
With regards to the study design, n = 23 studies had brain and behavioural observation data collected at the same age point, often during one session. In a further n = 19 studies, brain or behavioural data was collected once during a follow-up - months, years or decades later. Thirteen studies followed the longitudinal design and repeated the same measures of either brain (Lee et al., 2019; Wang et al., 2019; Swingler et al., 2017; Bernier et al., 2016) or behaviour (Broomell et al., 2019; Diaz et al., 2019; Pratt et al., 2019; Licata et al., 2015; Beckwith and Parmelee, 1986) or both (Hardin et al., 2021; Eggebrecht et al., 2017; Jones et al., 1997, 2004). Seven of these studies included a time point during which brain and behaviour data were available concurrently (Hardin et al., 2021; Broomell et al., 2019; Diaz et al., 2019; Eggebrecht et al., 2017; Swingler et al., 2017; Bernier et al., 2016; Jones et al., 1997, 2004). More information on children’s brain imaging and adult-child interaction sessions can be found in Table 2.
Table 2.
A summary of the characteristics of the neuroimaging and behavioural sessions included in this review.
| Characteristics | N | % | Characteristics | N | % | |
|---|---|---|---|---|---|---|
| Child’s age during the brain session | Child’s age during the interaction | |||||
| 0−5 mths | 18 | 33% | 0−5 mths | 16 | 29% | |
| 6−12mths | 24 | 40% | 6−12 mths | 28 | 51% | |
| 13−24mths | 5 | 8% | 13−24 mths | 16 | 29% | |
| >2yrs | 13 | 22% | ||||
| Neuroimaging technique | Interaction setting | |||||
| fMRI | 20 | 36% | Home - free interaction | 5 | 9% | |
| fNIRS | 1 | 2% | Lab - free interaction | 35 | 64% | |
| EEG | 32 | 58 % | Lab - structured interaction | 18 | 33% | |
| MEG | 1 | 2% | ||||
| Ultrasound imaging | 1 | 2% | Interaction partner | |||
| Brain measure | mother | 42 | 76 % | |||
| Structural studies | 13 | 24 % | father | 1 | 2% | |
| Resting-state | 25 | experimenter | 2 | 4% | ||
| EEG asymmetry | 13 | 24 % | caregiver and experimenter | 11 | 20% | |
| EEG frontal power | 5 | 9% | ||||
| Functional network | 8 | 15% | Coded behaviours of: | |||
| Other | 2 | 4% | mother | 38 | 69 % | |
| Task-related activations | 15 | father | 1 | 2% | ||
| faces | 3 | 5% | infant | 30 | 55 % | |
| facial expressions | 4 | 7% | dyad | 6 | 11% | |
| eye-gaze | 1 | 2% | ||||
| prosody | 2 | 4% | ||||
| emotion processing | 5 | 9% | ||||
In short, the age of infants during the interaction episodes ranged from 2 to 24 months with a few studies including multiple social interaction episodes at later ages (e.g. Diaz et al., 2019; Licata et al., 2015). Most adult-infant interactions were taking place in the laboratory settings, with only five studies setting being participants’ homes (Bernier et al., 2019; Leblanc et al., 2017; Hane et al., 2006; 2010; Beckwith and Parmelee, 1986).
While most of the interactions were unstructured, in which infants and adults were interacting as they normally would in naturalistic settings, in n = 18 studies an experimental situation, often in addition to the unstructured interaction, was introduced. Examples of experimental situations include the “Strange Situation Procedure” (Biro et al., 2021; Peltola et al., 2020; Cortes Hidalgo et al., 2019; Quevedo et al., 2017; Lyons-Ruth et al., 2016; Moutsiana et al., 2014, 2015; Tharner et al., 2011), “Still-face procedure” (Catalina Camacho et al., 2019), separation paradigm (Swingler et al., 2007, 2010; Gunnar and Nelson, 1994) and divided attention task (Mize and Jones, 2012; Dawson et al., 1999). While all these procedures allow infants’ behaviours to be investigated as they are happening, these prescriptive situations of interaction are unlikely to happen in everyday life. Table 3 provides an overview of the methods of investigations employed across studies.
Table 3.
Caregiver-infant interaction domains, methods of investigation to study infant, caregiver and dyadic behaviours and publications that have employed these methods to study interactions.
| Domain | Defined | Method of Investigation | Relevant publication(s) |
|---|---|---|---|
| INFANT | |||
| Affect | Positive or negative affect, based on body movements, vocalizations and facial expressions | Global Rating Scale (GRS; Murray et al., 1996) | Sethna et al., 2017 |
| Bosquet Enlow et al., 2011 | Catalina Camacho et al., 2019 | ||
| Gunnar et al., 1992 | Gunnar and Nelson, 1994 | ||
| Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) | Elsabbagh et al., 2015 | ||
| Kochanska, 1997, 1998 | Hane and Fox, 2006 | ||
| Custom | Dawson et al., 1999 | ||
| Hanford et al., 2018; | |||
| Affect recovery | Affect following a mildly stressful situation | Still-Face Paradigm; Bosquet Enlow et al., 2011 | Catalina Camacho et al., 2019 |
| Affectionate touch | Combination of deep touch (stroking, rubbing, caressing, and wiping), light touch (running the tip of the fingers on the caregiver), and touch (resting hand on caregiver) | Adapted version of Moszkowski and Stack, 2007 | Hardin et al., 2021 |
| Attachment | Specific, preferential, and enduring emotional tie between an infant and a caregiver, reflected in infants' behaviours such as separation distress, greeting at re-union and tendency to turn to caregiver when distressed. | Strange Situation Procedure (SSP; 1978; Main and Solomon, 1990) | Biro et al., 2021 |
| Cortes Hidalgo et al., 2019 | |||
| Lyons-Ruth et al., 2016 | |||
| Moutsiana et al., 2014, 2015 | |||
| Quevedo et al., 2017 | |||
| Peltola et al., 2020 | |||
| Tharner et al., 2011; | |||
| Rifkin-Graboi et al., 2019 | |||
| Attachment Behavior Q-Sort (AQS; Waters and Deane, 1985) | Leblanc et al., 2017 | ||
| Bernier et al., 2019 | |||
| Attention | The amount and quality of visual contact with an interest in the caregiver directly or through mutual focus | Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) | Elsabbagh et al., 2015 |
|
Bids for attention |
Behaviours used by baby to attract attention in the context of caregivers’ inattention: touch, vocalizations, offering toys, attention, grabbing, negative affect |
Divided attention task; Custom |
Dawson et al., 1999 |
| Mize and Jones, 2012 | |||
| Communication | Infant’s level of engagement and communication (e.g., vocal and non-vocal behaviour directed towards the partner) | Global Rating Scale (GRS; Murray et al., 1996) | Sethna et al., 2017, 2019 |
| Inhibition | Level of restraint of exploration: proximity to caregiver during play and when stranger approaches; latency to approach a stranger and a novel toy. | Custom | Jones et al., 2009 |
| “Interactive behaviour” | Measured using positive facial affect, directed attention, and positive vocalizations. | Custom | Jones et al., 2004 |
| Initiating joint attention | An act used to direct another’s attention to an object, event, or topic of a communicative act | Communication and Symbolic Behavioral Scales-Developmental Profile (CSBS-DP; Wetherby et al., 2002) | Eggebrecht et al., 2017 |
| Involvement | Child’s attempts to engage the mother in play in a non-urgent and relaxed way | Emotional Availability Scales (EAS; Biringen, 2008) | Licata et al., 2015 |
| Liveliness | Level of voluntary physical activity, particularly that initiated by the infant | Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) | Elsabbagh et al., 2015 |
| Reactivity, regulation | Baby’s strategies to regulate during a distressing situation. Orienting: attempting to engage with the partner. Distraction: attending to or attempting to manipulate an object. |
Custom | Swingler et al., 2007, 2014 |
| Responding to joint attention | Ability to coordinate the focus of attention by responding to e.g., gaze shift, head turn, and pointing gesture | Custom | Elison et al., 2012 |
| Responsive-ness | Degree to which the child reacts in a positive, non-urgent way to the mother, focusing on emotional rather than behavioural responsiveness as well as genuine, positive affect displayed by the child. | Adapted version of Jörg et al., 1994 | Holz et al., 2018 |
| Emotional Availability Scales (EAS; Biringen, 2008) | Licata et al., 2015 | ||
| Withdrawal | Falling asleep or being disengaged in interaction | Gunnar et al., 1992 | Gunnar and Nelson, 1994 |
|
CAREGIVER | |||
| Affect | Affective state characterized by level of caregivers’ enjoyment, effort and vitality, degree of self-consciousness, and the extent of anxiety in the interaction. | Global Rating Scale (GRS; Murray et al., 1996); | Sethna et al., 2017 |
| Extent to which the caregiver expressed positive emotions during the task through her tone of voice and facial expressions. | Calkins et al., 2004 | Bernier et al., 2016 | |
| Kraybill and Bell, 2013 | |||
| Adapted version of Gartstein et al., 2008, 2018 | Gartstein et al., 2020 | ||
| Perone and Gartstein, 2019 | |||
| Positive and negative affect of the caregiver | Feldman et al., 2011 | St. John et al., 2017 | |
| Affectionate touch | Combination of deep affectionate touch (firm patting, stroking, massaging with the whole hand) and light affectionate touch (affectionate kissing, caressing, or stroking, grazing, hugging) | Adapted version of Touch Scoring Instrument (Polan and Ward, 1994) | Hardin et al., 2021 |
| Communication | Mind-Mindedness – comments about infant internal states or intentionality | Meins et al., 2001 | Dégeilh et al., 2018 |
| Custom | Hanford et al., 2018 | ||
| Infant-directed speech | Feldman et al., 2011 | St. John et al., 2017 | |
| Disruptive communication | Atypical Maternal Behavior Instrument for Assessment and Classification (Lyons-Ruth et al., 1999) | Lyons-Ruth et al., 2016 | |
| Directiveness or support for autonomy | The degree to which infants’ behaviours are his/her focus rather than caregivers’ agenda | Whipple et al., 2010 | Dégeilh et al., 2018 |
| Emotional Availability Scales (EAS, Biringen, 2008) | Licata et al., 2015 | ||
| Intensity | Overall loudness and complexity of interaction | Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) | Elsabbagh et al., 2015 |
| Zhao et al., 2019 | |||
| Interaction style – depressed mothers | (i) intrusive: rough physical contact (i.e. poking), staccato actions and vocalizations; tense or fake facial expressions; (ii) withdrawn: flat affect, rare touching and vocalizing; disengaged behavior, looking away from the infant; | Adapted version of Field et al., 1990 | Diego et al., 2002 |
| Diego et al., 2006 | |||
| Field et al., 2003 | |||
| Jones et al., 1997 | |||
| Intrusiveness | Display of over-controlling behaviour or focus on her own agenda, ignoring the infant's behaviour or cues | Calkins et al., 2004 | Bernier et al., 2016 |
| Broomell et al., 2019 | |||
| Diaz et al., 2019 | |||
| Ainsworth et al., 1974 | Huffmeijer et al., 2020 | ||
| Indexed as caregiver taking toy from infant | Feldman et al., 2011 | St. John et al., 2017 | |
| Mirroring | Imitation of infants’ facial movements | Murray et al., 2016 | Rayson et al., 2017 |
| Parenting | Positive parenting: warmth, positivity, and sensitivity in interaction Negative parenting: hostility and intrusiveness; |
Adapted version of Early Parenting Coding System (Shaw et al., 2006; Hipwell et al., 2015) | Hanford et al., 2018 |
| General quality of parenting based on talking, looking at infant & social play | Beckwith and Cohen, 1984 | Beckwith and Parmelee, 1986 | |
| Constructed based on (i) Acceptance-Rejection, (ii) Sensitivity-Insensitivity, (iii) Degree of Availability, and (iv) Appropriateness of Pace in Feeding & (v) non-intrusive. | Adapted version of Ainsworth, 1976; Park et al., 1997 | Hane and Fox, 2006, 2010 | |
| Quality of parenting based on positive facial affect, directed attention and positive vocalization | Custom | Jones et al., 2004 | |
| Responsiveness | All behaviours executed in response to the infant behaviours (vocal, facial or motor) | Adapted version of Jörg et al., 1994 | Holz et al., 2018 |
| Sensitivity | Caregivers’ response to the infant’s communication cues; the extent to which it is contingent and appropriate to the infant’s needs and experiences; | Global Rating Scale (GRS; Murray et al., 1996) | Sethna et al., 2017, 2019 |
| Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) | Elsabbagh et al., 2015 | ||
| Zhao et al., 2019 | |||
| Coding Interactive Behavior (CIB; Feldman, 2012) | Pratt et al., 2019 | ||
| Mini Maternal Behavior Q-Sort (MBQS) e.g. Moran et al (2009a, 2009b), Tarabulsy et al (2009). | Lee et al., 2019 | ||
| Rifkin-Graboi et al., 2015, 2019 | |||
| Wang et al., 2019 | |||
| Wen et al., 2017 | |||
| Ainsworth et al., 1974 | Huffmeijer et al., 2020 | ||
| Calkins et al., 2004 | Bernier et al., 2016 | ||
| Diaz et al., 2019 | |||
| Adapted version of Calkins et al. (2004) | Swingler et al., 2014, 2017 | ||
| Emotional Availability Scales (EA; Biringen, 2008) | Licata et al., 2015 | ||
| Adapted version of Gartstein et al., 2008, 2018 | Perone and Gartstein, 2019 | ||
| Custom | Gartstein et al., 2020 | ||
| Combination of the measures of (i) Cooperation/ Attunement (ability to accurately interpret infants’ cues and to adjust the interaction correspondingly); (ii) Positivity (positive affect and attitude, no overt signs of feeling overwhelmed or criticism towards infant); (iii) Accessibility/. Availability (consistent attentiveness toward the infant, even when engaged in other tasks). | Adapted version of Maternal Behavior Q-Sort (MBQS); e.g. Moran et al (2009a, 2009b), Tarabulsy et al (2009). | Bernier et al., 2019 | |
| Composed of: (i) sensitivity to non-distress, (ii) positive regard and (iii) intrusiveness (reversed) | NICHD Early Child Care Research Network, 1999 | Nolvi et al., 2020 | |
| Composed of: (i) sensitivity; (ii) structuring (iii) non-intrusiveness, (iv) nonhostility. | Adapted version of Emotional Availability Scales (EA; Biringen, 2008) | Taylor-Colls and Pasco Fearon, 2015 | |
| Stimulation | Extent to which the caregiver directly stimulated infant’s body for the purpose of heightening the infant’s level of arousal | Adapted version of Jörg et al., 1994 | Holz et al., 2018 |
| Attempts to attract the infant’s attention or to establish contact with him/her. | Custom | Gartstein et al., 2020 | |
|
DYADIC | |||
| Affect synchrony | Number of caregiver-child positive affect episodes within approach-proximal position. | Adapted version of Feldman and Eidelman, 2004 | Pratt et al., 2019 |
| Directedness | Dyadic activity or assessment of who directs most of the activity – parent or child | Custom | Gartstein et al., 2020 |
| Dyadic intensity | Intensity, not quantity, of mutual engagement at its most optimal | Custom | Gartstein et al., 2020 |
| Mutuality | Degree of dyadic sharedness and reciprocity of experience | Field et al., 1990 | Jones et al., 2004 |
| Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) | Elsabbagh et al., 2015 | ||
| Reciprocity | Attunement and turn-taking; characterised by high coordination and synchrony |
Beckwith and Cohen, 1984 |
Beckwith and Parmelee, 1986 |
| Manchester Assessment of Caregiver-Infant Interaction (MACI; Wan et al., 2012, 2013) |
Elsabbagh et al., 2015 |
||
| Perone and Gartstein, 2019 | |||
In this review, caregivers were the primary interactive partners with only two studies (4% of studies) investigating infants’ joint attention behaviours when interacting with the experimenters (Eggebrecht et al., 2017; Elison et al., 2012). Therefore, for simplicity, going forward, all subsequent “adult-infant interaction” episodes will be referred to as “caregiver-infant interactions”. Since none of the studies in this review relied on parental or individuals’ self-reports, caregiver-infant interactions were assessed by researchers. In all but three studies (Bernier et al., 2019; Leblanc et al., 2017; Beckwith and Parmelee, 1986), caregiver-infant interactions were first videoed and then coded offline to capture behaviours of interest. Moreover, following a standard procedure for coding videos, a portion of videos was second-coded by a second researcher to ensure reliability. The three studies that did not follow this procedure were coded by researchers online as caregiver-infant interaction was taking place but were nevertheless checked for reliability.
As rich as infants’ daily social experiences are, limitless options are available when it comes to coding behaviours in caregiver-infant interactions. In this review, different terms such as e.g. “maternal sensitivity during the free play”, “infants’ interactive experiences” or “infants’ behaviours in interaction” were used to refer to different aspects of caregiver-infant behaviours that were studied. Taken together, behaviours in caregiver-infant interactions were focused on either various behaviours of infants or caregivers or both (combined dyadically). A more detailed overview of behaviours that were coded in the context of studies presented in this review can be seen in Table 3. To note, some behaviours have more than one definition across studies.
Sometimes researchers coded very specific behaviours (e.g. number of infants’ smiles) that occurred within a specific timeframe (e.g. Dégeilh et al., 2018; Swingler et al., 2007). Sometimes the temporal order and/or duration were considered and discrete micro-level codes capturing behaviours of interest (e.g. gaze) were recorded (e.g. Pratt et al., 2019). However, in most of the studies, behaviours were rated using global rating scales that incorporated context to the observed behaviour and the overall impression was scored (e.g. maternal intrusiveness). In short, the most popular behaviour that was captured in adult-infant interaction was maternal sensitivity (n = 21). However, a few of the other behaviours such as “positive parenting” and “responsiveness” touched upon very similar concepts.
With regard to the brain imaging sessions, participants’ ages ranged from neonatal up to 29 years of age (Lyons-Ruth et al., 2016) depending on the study design (i.e. cross-sectional, longitudinal, follow-up). The most widely used neuroimaging technique was encephalography (EEG) (n = 32) followed by (functional) magnetic resonance imaging (fMRI, MRI, DTI) (n = 20), while functional near-infrared spectroscopy (fNIRS), magnetic encephalography (MEG) and ultrasound brain imaging were used in only one study each.
Several sub-themes have emerged dependent on which brain measures were used: (i) structural brain studies (n = 13); (ii) resting-state/functional connectivity studies (n = 25), which can be further separated into frontal EEG asymmetry (n = 13), network-connectivity (n = 8), frontal power (n = 5) and other (n = 2); and (iii) task-evoked functional activation studies of infant’s processing of various social stimuli (n = 15), which can be further separated into faces (n = 3), facial expressions (n = 4), emotional tone (n = 2), eye-gaze (n = 1) and emotion processing and regulation (n = 5). A brief description of each study - its goals, measures and results - can be found in Table 4. For clarity, Table 4 is divided into these three sub-themes. Importantly, only relevant measures and time points – those consistent with the aims of this review in capturing adult-infant interactions in the first two years of life and brain data collection - are shown in Table 4. For information on the full list of measures, visits and results see each paper individually.
Table 4.
Studies investigating the associations between adult-infant social interactions and brain development outcomes.
| Citation |
N incl. |
Domain |
Interaction |
Brain |
Relevant findings |
||
|---|---|---|---|---|---|---|---|
| Age | Behaviours coded | Age | Technique | ||||
|
Structural brain studies | |||||||
| Tharner et al. (2011) | 629 | Attachment & subcortical structure volume | 14 mths | SSP: infant attachment | 1 mth | Ultra-sound | ↑Ganglio-thalamic ovoid predicted ↓risk of disorganised attachment. No link to volume of lateral ventricles. |
| Elison et al. (2012) | 12 | Neural priors of joint attention | 9-10 mths | Infant: responding to joint attention | 6 mths | DTI | Fractional anisotropy in the R uncinate fasciculus (connects the amygdala to the vmPFC & anterior temporal pole) predicted individual differences in responding to joint attention |
| Moutsiana et al. (2015) | 75 | Attachment & hippocampal & amygdala volumes | 18 mths | SSP: infant attachment | 22 yrs | MRI | Insecurely attached infant have ↑ amygdala volumes compared to securely attached infants; no link to hippocampal volumes. |
| Rifkin-Graboi et al. (2015) Study 1 | 20 | Maternal sensitivity on hippocampal & amygdala volumes | 6 mths | Mother: sensitivity | 6 mths | MRI | Maternal sensitivity predicted hippocampal volumes bilaterally |
| Lyons-Ruth et al. (2016) | 18 | Attachment & amygdala volume | 19 mths | SSP: infant attachment; Mother: disrupted communication | 29 yrs | MRI | ↑ L amygdala volume linked to maternal communication (esp. withdrawal & contradictory comments) & infant attachment |
| Sethna et al. (2017) | 39 | Maternal/ infant behaviours & regional brain volumes | 3–6 mths | Mother: affect, sensitivity; Infant: communication, fretfulness |
3–6 mths | MRI | ↑ maternal affect linked to ↑ grey & white matter volumes. ↑maternal sensitivity linked to↑ subcortical grey matter volume;↑infant communication linked to ↓ cerebellar volume (with ↑cerebellum in older male infants); infant fretfulness: no associations |
| Leblanc et al. (2017) | 33 | Attachment & grey matter volume/thick-ness | 15 mths | Infant: Attachment (AQS) | 10-11 yrs | MRI | Securely attached infants have ↑grey matter volumes in the R STS/STG, MTG, TPJ; no relationship with cortical thickness. |
| Sethna et al. (2019) | 28 | Paternal/ infant behaviour & regional brain volumes | 3–6 mths | Father:, sensitivity; Infant: communication | 3–6 mths | MRI | ↑ paternal sensitivity associated with ↓ cerebellar volumes in infants who have high communication |
| Rifkin-Graboi et al. (2019) | 83 | Neurophenotype and link between maternal & infant behaviours | 6, 18 mths | Mother: sensitivity; Infant: attachment | ∼1 mth | MRI | No brain region volume predicted attachment; in neonates who have ↑ L HPC, ↓ maternal sensitivity linked to↑ attachment disorganisation |
| Bernier et al. (2019) | 33 | Maternal sensitivity & hippocampal/amygdala volumes | 13, 15 mths | Mother: sensitivity (cooperation, positivity, accessibility); Infant: attachment |
10-11 yrs | MRI | Overall sensitivity score was not predictive of any brain volume but sub-components↑ maternal accessibility linked to ↓ R amygdala volume; ↑ maternal positivity linked to↓ bilateral hippocampal volume; No relationship with attachment. |
| Cortes Hidalgo et al. (2019) | 588 | Attachment & brain morphology | 14 mths | SSP: infant attachment | 9–11 yrs | MRI | Disorganised attachment linked to ↑ hippocampal volume; No associations with amygdala or other global measures; No associations with secure attachment. |
| Lee et al. (2019) | 65–115 | Maternal sensitivity & hippocampal/ amygdala volumes/micrstructure | 6 mths | Mother: sensitivity | 4.5, 6 yrs | MRI | ↑ maternal sensitivity linked to ↓ amygdala volumes in 6 y.o. boys not girls, associated with amygdala & hippocampal microstructure at 4.5 & 6 y.o, no links with hippocampal volume. |
| Nolvi et al. (2020) | 53, 36 | Neonatal brain volume, caregiving quality & executive skills | 6 mths | Mother: sensitivity (non-distress, positive regard & intrusiveness) | ∼1 mth | MRI | Positive relationship between maternal sensitivity & child cognition observed only in neonates with large or average (not small) total brain volume. Same pattern observed for hippocampal & ACC volumes. |
|
Resting-state/functional connectivity studies | |||||||
| Beckwith and Parmelee (1986) | 53 | Maternal care as protective factor in preterm babies | 1,8, 24 mths | Mother: attention, contingency; talking; Dyad: reciprocity, mutual regard |
∼1 mth | EEG | ↓ EEG sleep patterns linked to↓ developmental scores during 1st year of life independent of care, but by age of 5 & 8 years only in ↓ responsive families, were these patterns linked to↓ developmental scores. |
| Jones et al. (1997) | 34, 24 | Maternal depression & infant frontal EEG asymmetry | 3, 6 mths | Mother: intrusiveness, withdrawal | 3,6 mths | EEG | Frontal EEG asymmetry changed for infants of intrusive mothers relative to withdrawn mothers to become more left. |
| Dawson et al. (1999) | 117 | Infant EEG patterns and maternal depression on behaviour | 13–15 mths | Infant: affect, hostility, touches, gaze, vocalizations | 13–15 mths | EEG | In infants of depressed mothers:↓ L frontal activity linked to↓ affect to mother, ↑ negative affect, ↑ comfort seeking,↓ vocalizing. In infants of non-depressed mothers: ↓ L frontal activity linked to ↑ touching |
| Diego et al. (2002) Study 1 | 27 | Infant frontal EEG asymmetry & interactive style | 3 mths | Mother: intrusiveness, withdrawal | 3 mths | EEG | Infants of depressed withdrawn mothers had right frontal EEG asymmetry (right α power < left α power) than infants of depressed intrusive mothers |
| Field et al. (2003) | 140 | Maternal interactive style & infant EEG asymmetry | 3 mths | Mother: intrusiveness, withdrawal, “good interaction” | Birth | EEG | Infants of depressed mothers who showed “good interaction” had relative right frontal EEG activation (right α power < left α power) to infants of non-depressed mothers but less right than in infants of withdrawn & intrusive mothers; |
| Jones et al. (2004) | 70, 53 | Factors linked to breastfeeding | 1, 3 mths | Infant/mother: affect, gaze, vocalization; Dyad: mutuality | 1,3 mths | EEG | Infants of depressed mothers who had been breastfed until their 3rd month of life were less likely to show the right frontal EEG asymmetry patterns |
| Diego et al. (2006) | 66 | Infant frontal EEG asymmetry & maternal interactive styles | 3–6 mths | Mother: intrusiveness, withdrawal | 3–6 mths | EEG | Infants of depressed withdrawn mothers had right frontal EEG asymmetry (right α power < left α power) than infants of depressed intrusive mothers at 3-6 months but not as neonates |
| Hane et al. (2006) | 144–185 | Maternal/ infant behaviours & infant stress reactivity | 9 mths | In-home visit; Infant: affect. Mother: sensitivity, intrusiveness | 9 mths | EEG | ↓ maternal sensitivity (1 SD below normal) linked to right frontal EEG asymmetry (right α power < left α power),↑ fearfulness & ↓ sociality; infant temperament had no effect. |
| Jones et al. (2009) | 40 | EEG frontal activity & infant inhibition | 12 mths | Infant: inhibition | 12 mths | EEG | Infants of depressed mothers showed ↑relative right frontal EEG asymmetry (right α power < left α power) & were less inhibited |
| Hane et al. (2010) | 185 | Maternal behaviours & infant development | 9 mths | Mother: pace, acceptance, sensitivity, availability, intrusiveness, cooperation | 3 yrs | EEG | Relative to those who had high quality of caregiving, low caregiving quality was linked to right frontal EEG asymmetry, ↑inhibition, ↑ aggression,↑ behavioural problems |
| Mize and Jones (2012) | 30 | Infant’s emotional response & brain activity in response to maternal inattention | 13 mths | Infant: gaze, proximity to mother, touch, arousal, affect | 13 mths | EEG | Approach behaviours in response to maternal inattention were linked to ↑ left frontal EEG activity (left α power < right α power) |
| Kraybill and Bell (2013) | 56 | Maternal behaviour, & infant executive functions | 10 mths | Mother: affect | 10 mths | EEG | No relationship between maternal behaviour & infant frontal EEG activity; both independently predicted child’s executive functions. |
| Swingler et al. (2014) | 223 | Infant regulatory behaviours, EEG asymmetry & maternal sensitivity | 5 mths | Infant: affect, orientation to mother & distraction; Mother: sensitivity. | 5 mths | EEG | ↑ maternal sensitivity linked to↑ infant’s orientation to mother regardless of the EEG asymmetry & ↑ infant negative reactions but only if infant demonstrated↑ right frontal asymmetry (right α power < left α power). Infants with left frontal EEG asymmetry (left α power < right α power) & highly sensitive mothers used more distraction more often to regulate |
| Licata et al. (2015) | 28 | Child's frontal EEG asymmetry & emotional availability | 7 mths4 yrs | Mother: sensitivity, structuring; Child: responsiveness involvement | 14 mths | EEG | In 14-month-olds ↑ EEG right frontal asymmetry (right α power < left α power) related to ↓ child's involvement in interactions at 4 years but at 7 months. |
| Rifkin-Graboi et al. (2015) Study 2 | 17 | Maternal sensitivity & HPC/ amygdala functional connectivity | 6 mths | Mother: sensitivity | 6 mths | fMRI | ↑ maternal sensitivity linked to↑ functional connectivity in(a) R HPC network & vmPFC, dlPFC, L fusiform, R middle temporal cortex; (b) L HPC connectivity & L fusiform, L STS, L lateral occipital cortex.↓ maternal sensitivity linked to ↑connectivity with (a) R HPC network & R lingual gyrus, R PCC; (b) L HPC connectivity & L entorhinal cortex; (c) amygdala & inferior and middle temporal, entorhinal cortices. |
| Bernier et al. (2016) | 197 | Maternal behaviours & infant frontal resting power | 5 mths | Mother: sensitivity, intrusiveness, affect, physical stimulation | 5, 10 24 mths | EEG | ↑ maternal positive affect & ↓ physical stimulation linked to infants’ ↑resting α & Θ frontal EEG power that increased between 5 & 24 mths. |
| Swingler et al. (2017) | 331–370 | Impact of maternal behaviours on infant's attention | 5 mths | Mother: positive intrusiveness, affect | 5, 10 mths | EEG | ↑ maternal intrusiveness linked to ↑ baseline neural activation left medial frontal site from 5 to 10 months. No associations with maternal positive affect & frontal EEG changes were found. |
| Eggebrecht et al. (2017) | 116, 98 | Joint attention & neural measures | 12, 24 mths | Infant: initiation of joint attention | 12, 24 mths | fMRI | Number of joint attention initiations related to connections between visual & dorsal attention network as well as DMN |
| St. John et al. (2017) | 65 | Caregiving, maternal & infant cortisol levels & EEG asymmetry | 6 mths | Mother: affect, motherese, intrusiveness | 12 mths | EEG | ↑ maternal salivary cortisol level at 6 mon predicted ↓ 12 mon olds α (6-9Hz) EEG power independently from maternal caregiving. |
| Wen et al. (2017) | 111 | Effect of maternal depression/sensitivity on infant frontal EEG asymmetry | 6 mths | Mother: sensitivity | 6 mths | EEG | Significant effects of postnatal maternal depression & maternal sensitivity predicted greater right EEG frontal asymmetry (right α power < left α power) in infants who spent at least 50% of their waking hours with mothers. |
| Dégeilh et al. (2018) | 28 | Maternal behaviours & resting functional connectivity of brain networks | 13, 15 mths | Mother: MM (13 mon); support for autonomy (15 mon) | 10 yrs | fMRI | ↑ MM predicted ↑ negative connectivity between DMN (vmPFC, right angular gyrus) and SN (AI);↑Autonomy support predicted ↑ negative connectivity between DMN (AI) and SN (dACC, bilateral AI). |
| Hanford et al. (2018) | 46 | Effect of infant affect & maternal behaviours on neural networks | 3 mths | Infant: affect Mother: warmth, hostility, intrusiveness, involvement, sensitivity, MM | 3 mths | fMRI | ↑ maternal MM linked to ↑positive associations between infant positive emotionality & nodal metrics within prefrontal & occipital cortices, bilateral insula; and ↑ negative associations between infant positive emotionality & nodal metrics within R supramarginal gyrus and L STS. ↑ positive caregiving linked to ↓ negative relationship between infant positive emotionality & L OFC;↓ negative caregiving linked to↑ relationship between infant negative emotionality & R inferior parietal lobule; |
| Perone and Gartstein (2019) | 51 | Infant frontal-posterior functional connectivity & caregiver-infant interaction | 6–11 mths | Mother: responsiveness, emotional tone, reciprocity. | 6–11 mths | EEG | ↓ frontal-posterior Θ connectivity linked to↑ levels of responsiveness, ↑ reciprocity, ↑positive affect;↑ levels of frontal-posterior α connectivity linked to ↑ expression of positive emotional tone; ↑ frontal-posterior γ connectivity linked to ↑ responsive interactions. |
| Catalina Camacho et al. (2019) | 38 | Neural regions that support affective behaviours | 6 mths | Still-Face Paradigm: affect, emotion recovery | 6 mth | MRI | Negative affect: no relationship; ↑positive affect recovery ↑rCBF in limbic regions (IFG, right medial OFC). |
| Diaz et al. (2019) | 410 | Effect of extrinsic & intrinsic factors on children’s negative affect | 5,24 mths | Mother: sensitivity, intrusiveness; Infant: affect | 5 mths | EEG | ↑ Maternal sensitivity at 5 and 24 mon linked to ↓ infant’s negative affect at 24 months but only if they demonstrate left frontal EEG asymmetry at 5 mths;↑ maternal intrusiveness at 5- and 24-mths linked to ↑infant’s negative affect, but only for infants with right frontal EEG asymmetry (right α power < left α power) at 5 mths. |
| Wang et al. (2019) | 61, 76 | Impact of maternal sensitivity on child's HPC connectivity | 6 mths | Mother: sensitivity | 4, 6 yrs | fMRI | ↑maternal sensitivity at 4 y.o linked to↑ functional connectivity between R aHPC & R precentral, bilateral postcentral gyri, & ↓ functional connectivity between aHPC and L DLPFC. ↑maternal sensitivity at 6 y.o. linked to ↑connectivity between R aHPC functional connectivity and bilateral calcarine, R lingual and L cuneus cortex. |
| Broomell et al. (2019) | 401 | Maternal intrusiveness & neural power on executive function | 5, 10 mths | Mother: intrusiveness | 5 mths | EEG | Association between infant frontal EEG alpha power & 4-year executive functions was significant only for infants who had mothers low in intrusiveness during free play and not significant for those who had mothers high in intrusiveness during free play |
| Hardin et al. (2021) | 91, 76 | Effect of maternal depression & breastfeeding on touch patterns & cortical maturation | 1, 3 mths | Infant & mother: different types of touch | 1, 3 mths | EEG | Frontal EEG asymmetry, breastfeeding & positive affect in infants predicted infant affectionate touch patterns during interaction. |
|
Task-evoked functional activation studies | |||||||
| Gunnar and Nelson (1994) | 23 | familiar/novel faces & infant emotions | 12 myths | Separation – infant: affect, comforting, sleep/ withdrawal | 12 mths | EEG | Infants with ↑ late positive waveforms to the infrequent familiar face were ↑distressed during separation |
| Diego et al. (2002) Study 2 | 18,19 | Maternal behaviours & infant processing of facial expressions. | 3 mths | Mother: physical, affective, vocal behaviours to determine intrusive or withdrawal style. | 3 mths | EEG | Infants of intrusive depressed mothers showed right frontal asymmetry (right α power < left α power) when viewing stranger's surprised relative to happy expressions. Infants of withdrawn mothers failed to show changes in frontal EEG asymmetry to any facial expressions. |
| Swingler et al. (2007) | 30 | Infant behaviours & infant's processing of faces | 6 mths | Infant: affect, distress, looking for mother, reaching & proximity seeking with mother | 6 mths | EEG | ↑ proximity seeking linked to ↑ Nc component when viewing strangers relative to mother's face |
| Swingler et al. (2010) | 30 | Effect of infant separation behaviours on face processing | 6 mths | Infant: distress, looking for mother | 6 mths | EEG | ↑ infant distress linked to↑ P400 & Nc responses to the mother’s face; ↑ infant's visual search for mother linked to ↑ P400 & Nc latencies to the stranger’s face. |
| Moutsiana et al. (2014) | 54 | Attachment security & neural systems underlying emotion regulation | 18 mths | SSP: infant attachment | 22 yrs | fMRI | Insecurely vs. securely attached infants showed ↑ activation in PFC & ↓ co-activation of nucleus accumbens with PFC. |
| Taylor-Colls and Pasco Fearon (2015) | 40 | Impact of caregivers’ behaviours on infants’ processing of facial expressions | 7 mths | Mother: sensitivity, structuring, non-intrusiveness, non-hostility | 7 mths | EEG | ↑ maternal sensitivity (summed score) linked to ↑ Nc amplitude for happy faces relative to neutral faces while no association was present for fearful faces. |
| Elsabbagh et al. (2015) | 84 | Interactive measures & eye-gaze processing in infants with high- & low- (h/l) familial likelihood of developing ASD | 7 mths | Mother: sensitivity & non-directiveness; Infant: attentiveness, positive affect and liveliness. Dyad: mutuality, engagement | 7 mths | EEG | hASD infants (with no diagnosis of ASD) who displayed more positive affect and mutuality exhibit higher P400 latency difference to gaze stimuli; association not found in lASD.↑ maternal sensitivity in low-risk group, but not high-risk group, was associated with ↑ P100 latency differentiation of gaze to self vs away. |
| Rayson et al. (2017) | 19 | Maternal mirroring & infants’ matching of facial expressions | 2 mths | Mother: mirroring | 9 mths | EEG | ↑maternal mirroring of a particular facial expression at two months predicted ↑ infant mu ERD during observation of the same expression later at nine months. |
| Quevedo et al. (2017) | 171 | Attachment & reward processing | 18 mths | SPP: infant attachment | 20 yrs | fMRI | Insecurely attached infants showed ↑ activity in reward- and emotion- related structures such as basal ganglia, amygdala, as well as emotion regulation structures in response to positive & negative outcomes |
| Holz et al. (2018) | 172 | Maternal care & reward processing | 3 mths | Mother: stimulation, responsiveness; Infant: responsiveness | 25 yrs | fMRI | ↑ maternal stimulation was associated with ↑ activity in the caudate head in individuals with familial risk, reverse in no-risk participants. No effect of infant & maternal responsiveness. |
| Zhao et al. (2019) | 29 | Maternal behaviours & infant responses to prosody | 6 mths | Mother: sensitivity, non-directiveness | 6 mths | fNIRS | ↑ activation to angry vs. neutral prosody over temporal areas was correlated with ↑ maternal directiveness; no associations with maternal sensitivity were present. |
| Pratt et al. (2019) | 57 | Maternal sensitivity & neural basis of attachment | 9 mths6 yrs | Mother: affect, proximity, sensitivity; Child: gaze, affect, proximity; Dyad: affect synchrony. |
11 yrs | MEG | ↑ maternal sensitivity across childhood (combined at 9 mths & 6 yrs) & affect synchrony at 6yrs correlated with ↑ β activations over temporal cortex (STS/STG, MTG, ITG, insula) to observing own interaction in preadolescence. |
| Huffmeijer et al. (2020) | 22 | Maternal behaviours & infant processing of prosody & frontal asymmetry | 6 mths | Mother: sensitivity & intrusiveness | 7 mths | EEG | maternal sensitivity & intrusiveness were not related to infant frontal EEG asymmetry; ↑ intrusive mothers linked to ↑ ERP amplitudes to angry vs happy utterances |
| Gartstein et al. (2020) | 50 | Infant EEG asymmetry during emotion-eliciting tasks & interactive factors | 9 mths | Mother: sensitivity/ responsiveness, reciprocity, synchrony, intensity, directedness | 9 mths | EEG | Infants who shifted to stronger frontal activation of the left hemisphere had ↑ reciprocity when interacting with mothers. Those infants who shifted towards the right hemisphere had mothers who were directive & intense. |
| Peltola et al. (2020) | 61 | Attachment patterns & responses to fearful/ non-fearful faces | 7, 14 mths | SSP: infant attachment; Mother: sensitivity | 7 mths | EEG | Maternal sensitivity at 7mth was not related to face processing. In insecurely attached infants, no ERP differences to fearful vs non-fearful faces. Securely attached infants showed robust N290 ERP response of fearful vs. non-fearful faces. |
| Biro et al. (2021) | 70 | Attachment & neural response to prosociality | 12 mths | SSP: infant attachment | 10 mths | EEG | Infants with disorganised attachment showed a lack of right-sided frontal asymmetry compared to secure & insecure infants. |
Note. aHPC = anterior hippocampus; AI = anterior insula; AQS = Attachment Behaviour Q-Sort; ASD = Autism Spectrum Disorder; dACC = dorsal anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; DMN = default mode network; DTI = diffuse sensor imaging; EEG = electroencephalography; ERD = event-related desynchronization; ERP = event-related potential; fMRI = functional magnetic resonance imaging; GM = grey matter; HPC = hippocampus; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; L = left; MEG = magnetic encephalography; MM = mind-mindedness; MTG = medial temporal gyrus; Nc = negative component; OFC = orbito-frontal cortex; PCC = posterior cingulate cortex; R = right; SN = salience network; SSP = strange situation procedure; STG = superior temporal gyrus; STS = superior temporal sulcus; TPJ = temporo parietal gyrus; vmPFC = ventro-medial prefrontal cortex.
4. Summary of findings
4.1. Structural brain studies
Structural brain studies suggest that individual differences in infant attachment patterns and maternal sensitivity were linked to the amygdala (Lyons-Ruth et al., 2016; Moutsiana et al., 2015) and hippocampal volumes (Cortes Hidalgo et al., 2019; Rifkin-Graboi et al., 2015) concurrently in the first six months of life and in the longer-term. Evidence was however not replicable (Bernier et al., 2019; Lee et al., 2019) for both amygdala (Cortes Hidalgo et al., 2019) and hippocampal volumes (Moutsiana et al., 2015; Lyons-Ruth et al., 2016). Instead, the measures of amygdala and hippocampal microstructure at 4.5 and 6 years of age, rather than the overall volume, were linked to the measures of maternal sensitivity collected in infancy (Lee et al., 2019). Alternatively, these inconsistencies might be driven by the pre-existing individual differences in the volume of limbic system structures present at birth (Nolvi et al., 2020; Tharner et al., 2011; Rifkin-Graboi et al., 2019). Specifically, high thalamus and basal ganglia volumes measured at birth were linked to reduced risk of insecure attachment 14 months later (Tharner et al., 2011). Moreover, maternal sensitivity coded when infants were 6 months old predicted executive functions in children 2 and 5 years of age but only in infants who had large/average hippocampal volume at birth (Nolvi et al., 2020). Finally, in neonates who have large left hippocampus, low maternal sensitivity was linked to attachment disorganisation at 18 months (Rifkin-Graboi et al., 2019).
While limbic structures are commonly linked to emotion-related processes and, thus, are often investigated in the context of caregiving, Leblanc et al. (2017), whose measure of attachment was continuous rather than categorical, discovered that securely attached 15-month-old infants had increased grey matter volume in the “social brain network” (right STS/STG, MTG and TPJ) when they were 10−11 years of age. Interestingly, Sethna and colleagues (2017; 2019) discovered that better infant communication coded at 3- to 6-months of age was concurrently linked to smaller cerebellar volumes, especially in older males. Moreover, smaller cerebellar volumes were also observed in infants of highly sensitive fathers (Sethna et al., 2019) but only if infants were highly communicative, potentially reflecting the role of the cerebellum in effective communication during face-to-face interactions. Building on the topic of communication, in Elison et al. (2012), a large portion of the observed infants’ joint attention skills was explained by the microstructural organisation of white matter connecting the amygdala, fronto-insular and ventro-medial prefrontal cortex and temporal poles.
To sum up, individual differences in attachment and maternal sensitivity were linked, albeit inconsistently, to volumes of limbic structures (Lyons-Ruth et al., 2016; Moutsiana et al., 2015; Cortes Hidalgo et al., 2019; Rifkin-Graboi et al., 2015; Bernier et al., 2019; Lee et al., 2019), social brain structures (Leblanc et al., 2017) and cerebellum (Sethna et al., 2017). However, some studies also showed that individual differences in the volumes of limbic system structures at birth (Nolvi et al., 2020; Rifkin-Graboi et al., 2019; Tharner et al., 2011) contributed to subsequent behavioural associations.
4.2. Resting-state/functional connectivity studies
Studies within this sub-theme covered such research topics as frontal EEG asymmetry (Hardin et al., 2021; Diaz et al., 2019; Wen et al., 2017; Licata et al., 2015; Swingler et al., 2014; Mize and Jones, 2012; Hane et al., 2010; Jones et al., 2009; Diego et al., 2006; Hane and Fox, 2006; Jones et al., 2004; Field et al., 2003; Jones et al., 1997), frontal EEG power (Broomell et al., 2019; Swingler et al., 2017; Bernier et al., 2016; Kraybill and Bell, 2013; Dawson et al., 1999;), functional network connectivity (Perone and Gartstein, 2019; Wang et al., 2019; Catalina Camacho et al., 2019; Hanford et al., 2018; Dégeilh et al., 2018; Eggebrecht et al., 2017; Rifkin-Graboi et al., 2015), and functional network connectivity measured during social interaction (St. John et al., 2017) and sleep (Beckwith and Parmelee, 1986).
4.2.1. Frontal EEG asymmetry
EEG asymmetry refers to a difference in the amount of activity in one hemisphere relative to another, typically calculated over frontal brain areas in the alpha (6−9 Hz in infants) power band. Since greater alpha power is related to the deactivation of underlying cortical areas, greater alpha power over the left frontal area relative to the right is assumed to reflect greater activity in the right frontal area and vice versa. Left frontal asymmetry (left α power < right α power), is associated with positive emotions and approach behaviours, whereas right frontal asymmetry (right α power < left α power) is typically associated with more negative emotions and withdrawal behaviours (Coan and Allen, 2003).
Frontal EEG asymmetry in infants has a long history of being investigated in the context of maternal depression. Diego et al. (2006) and Field et al. (2003) showed that neonates of depressed mothers showed right frontal EEG asymmetry (right α power < left α power) compared to neonates of non-depressed mothers. However, while the presence of depression in mothers seemed to be linked to the emergence of frontal EEG asymmetry in their infants, maternal interactive style postnatally played a crucial role in its trajectory of development. Between birth and 3−6 months of age, infants of depressed mothers who had an intrusive style of interaction showed a shift toward greater left frontal EEG asymmetry (right α power > left α power) while infants whose mother were withdrawn in interaction showed even stronger increases in right frontal EEG asymmetry (right α power < left α power) (Diego et al., 2006, 2002; Jones et al., 1997). Further, those infants whose mothers have “good” interaction style (neither intrusive nor withdrawn) were positioned intermediately between non-depressed and depressed mothers with intrusive/ withdrawn interaction styles (Field et al., 2003). In addition to interactive styles, Wen et al. (2017) demonstrated associations between right frontal EEG asymmetry with postnatal depression and low maternal sensitivity only in those infants who spent at least 50 % of their waking hours with mothers.
While the understanding of risk factors and postnatal influences on frontal EEG asymmetry is undoubtedly of great importance, knowing how individual differences in frontal EEG asymmetry and maternal care translate into infants’ behaviour is central to understanding the impact on subsequent learning and functioning. In non-clinical populations, only those interactions evidencing extremely low caregiving scores (i.e. low sensitivity, high intrusiveness) at 9 months of age were related to children’s increased right frontal asymmetry (right α power < left α power) at 9 months (Hane and Fox, 2006) and 3 years of age (Hane et al., 2010). Behaviourally, at both time points those children were more hesitant to approach novel people and toys (high inhibition), were less sociable and more aggressive when interacting with their caregivers. Interestingly, this pattern was reversed for infants of depressed caregivers, so that increased relative right frontal EEG asymmetry (right α power < left α power) at 12 months was concurrently associated with decreased inhibition (Jones et al., 2009).
Breastfeeding – as a special interaction between infants and mothers – was also linked to frontal EEG asymmetry. Namely, breastfeeding was associated with decreased right frontal EEG asymmetry in infants of depressed mothers (Jones et al., 2004), and together with infants’ use of positive affect in interaction predicted the use of more affectionate touching during play (Hardin et al., 2021).
Finally, supporting the hypothesis that frontal EEG asymmetry is linked to approach-avoidance tendencies, those 13-month-old infants who showed more approach-motivated behaviours when mother was not attending to them had increased left frontal EEG asymmetry (right α power > left α power) (Mize and Jones, 2012). Similarly, Licata et al. (2015) found that decreased frontal α power in the left relative to right hemisphere measured at 14 months of age was related to increased child’s involvement in interactions with their mothers. However, this association was observed when involvement was measured in 4-year-olds but not when infants were 7 months of age. Finally, both Diaz et al. (2019) and Swingler et al. (2014) reported that high maternal sensitivity was linked to lower negative affect in infants, but only in those infants who showed left frontal EEG asymmetry (left α power < right α power).
To sum up, the emergence of frontal EEG asymmetry in infants is linked to prenatal risk-factors such as maternal depression (Diego et al., 2006; Field et al., 2003). However, the developmental course of frontal EEG asymmetry is associated with environmental factors such as time spent with mothers (Wen et al., 2017), sensitivity (Hane and Fox, 2006; 2010) and interactive style (Diego et al., 2006, 2002; Jones et al., 1997). Behaviourally, frontal EEG asymmetry is manifested in infants’ approach-avoidance tendencies (Mize and Jones, 2012; Hane et al., 2006; 2010) and moderates the relationship between infants’ and caregivers’ behaviours (Diaz et al., 2019; Swingler et al., 2014;), implying the existence of infants’ differential susceptibility to maternal behaviours depending on their neurophenotype.
4.2.2. Functional networks
In recent years, researchers have begun to investigate the relationship between maternal (Perone and Gartstein, 2019; Wang et al., 2019; Dégeilh et al., 2018; Rifkin-Graboi et al., 2015) and infant (Catalina Camacho et al., 2019; Hanford et al., 2018; Eggebrecht et al., 2017) behaviours during social interactions and functional network connectivity in infants.
One research group (Wang et al., 2019; Rifkin-Graboi et al., 2015) investigated the relationship between maternal sensitivity and functional connectivity of hippocampus and amygdala. Measured concurrently at 6 months of age (Rifkin-Graboi et al., 2015), their findings indicated that high maternal sensitivity was associated with increased functional connectivity between hippocampus and regions involved in emotion regulation (vmPFC), cognition (dLPFC) and communication (middle temporal cortex, fusiform and STS). Later, these measures of maternal sensitivity predicted decreased functional connectivity between the right anterior hippocampal network (aHPC) and the cognitive control network (dlPFC) as well as increased functional connectivity to the somatosensory cortex (Wang et al., 2019) in 4.5-year-old children, and increased functional connectivity to the visual processing network in 6-year-old children. Caregiver-infant interactive behaviours such as responsiveness, emotional tone and reciprocity were investigated in the context of neural rhythms that are involved in attention and regulatory processes (Perone and Gartstein, 2019). Results showed that all aforementioned behaviours collected during caregiver-infant interactions were linked to lower frontal-posterior theta (3−6 Hz) range connectivity in 6- to 11-month-olds; positive emotional tone to higher fronto-posterior alpha (6−9 Hz) connectivity and more responsive interactions to higher gamma (30−50 Hz).
Dégeilh et al. (2018) and Hanford et al. (2018) sought to characterize the relationships between caregiving and functional integration of multiple large-scale networks in infants. Hanford et al. (2018) used network-based approaches to quantify connectivity in terms of nodal efficiency (or how well a single node communicates with other nodes) and nodal redundancy (or how resilient the connection is to insults to any individual node). While no maternal or infant behaviours – measured concurrently with the neural data at 3 months - influenced these nodal matrices independently, results suggested that maternal behaviours influenced the relationships between infants’ behaviours and network measures. Specifically, higher mental state talk associated with stronger positive associations between infant positive affect and nodal matrices in areas of the bilateral insula, prefrontal and occipital cortex that are involved in emotion regulation, interoception and visual processing. In this study, caregivers’ behaviours (a combined measure of hostility and intrusiveness), led to the association between infant negative affect and the right inferior parietal lobule, which has often been implicated in increased vigilance in infancy. Similarly, Dégeilh et al. (2018) discovered that high maternal mind-mindedness and support for their infant’s autonomy, measured when infants were 13 and 15 months of age, predicted stronger negative connectivity between large-scale networks including the default mode network (DMN) and salience network (SN) when they were followed up at 10 years of age. The authors suggest that this negative relationship in connectivity – with one network being active while the other is inactive at any given time - reflects more mature brain development.
Taken together, it seems that caregivers’ behaviours measured during caregiver-infant interaction play a role in the development of several functional connectivity networks, most notably those involved in emotion regulation and cognition. It is particularly interesting to report that individual differences in infants’ ability to self-regulate during mildly stressful interactions were positively associated with cerebral blood flow in prefrontal regions (the inferior frontal gyri and oribitofrontal cortex) associated with automatic regulation, indicating more mature functioning of those structures (Catalina Camacho et al., 2019). Similarly, infants’ individual differences in their initiations of joint attention were linked to increased functional connections between visual and dorsal attention network, and between visual and default mode networks at 12 months of age (Eggebrecht et al., 2017) indicative of more mature neurodevelopment.
4.2.3. Frontal EEG power
As in the MRI study by Catalina Camacho et al. (2019), who showed how activations in the prefrontal regions are linked to more mature brain regions involved in emotion regulation, Dawson et al. (1999) used EEG to investigate frontal brain activity in 14- to 15-month-old infants during emotion regulation. Specifically, those infants who showed increased generalised frontal brain activation showed higher levels of negative affect, tantrums and aggression, potentially reflecting the difficulty in regulating intense emotions.
In addition to emotion regulation, the prefrontal cortex has been inextricably linked to executive functions (e.g. Cuevas and Bell, 2014). In the context of studies presented in this review, two maternal behaviours stood out and were associated with infant frontal EEG power. Specifically, Bernier et al. (2016) discovered that high maternal positive affect and low physical stimulation during play with their infants at 5 months (that combined as one factor during factor analysis) predicted higher frontal alpha (6−9 Hz) and theta (4−6 Hz) EEG resting-state power across several frontal regions, including frontal pole, medial and lateral frontal cortex. This association was present when infants were 10- and 24- months, but not concurrently when infants were 5 months old (similarly from the same longitudinal dataset Swingler et al. (2017) reported no concurrent associations between maternal positive affect and medial frontal EEG power in 5-month-olds). This finding in 10-month-olds however was not supported by Kraybill et al. (2013) who found no associations between maternal positive affect measures at 10 months and infants’ frontal EEG power. Despite the inconsistency of findings in relation to the neural measures, maternal positive affect did seem to relate to the behavioural measures of attention and executive functions in infants before the first year of life (Swingler et al., 2017; Kraybill and Bell, 2013).
Interestingly, associations between frontal EEG power and maternal intrusiveness followed quite the opposite pattern. Behaviourally, no direct associations between maternal intrusiveness and executive functions were discovered for 10-month-olds and 4-year-olds (Swingler et al., 2017; Broomell et al., 2019). However, measured at 5 months of age high intrusiveness was associated with changes in EEG power at the left medial frontal area at 10 months of age (Swingler et al., 2017). It also moderated the relationship between infants’ frontal EEG power and executive functions, so that the relationship existed only for those infants whose mothers were low on intrusiveness and did not exist for those who were high on intrusiveness during the free play (Broomell et al., 2019). Interestingly, when measured during a structured peek-a-boo task, maternal intrusiveness measured at 5 and 10 months of age predicted better executive skills in pre-schoolers, while carrying no associations with frontal EEG power (Broomell et al., 2019), indicating the central role of context when evaluating caregiver behaviours in relation to infants’ neural measures.
To conclude, overall, studies supported the role of frontal regions in emotion recognition and cognition. Two maternal behaviours derived from caregiver-infant interactions were linked to infants’ frontal EEG activity. Maternal positive affect was more consistently linked to infants’ behavioural performance, while somewhat inconsistent associations with frontal EEG power have been reported (Swingler et al., 2017; Kraybill and Bell, 2013 but Bernier et al., 2016 reported associations for older infants). For maternal intrusiveness, associations with frontal EEG power were evidenced while no direct associations with infants’ behaviours were noted (Swingler et al., 2017; Broomell et al., 2019).
4.2.4. Other studies
St. John et al. (2017) measured the effects of maternal salivary cortisol levels and caregiving behaviours (i.e. affect, motherese and intrusiveness) on infant salivary cortisol levels at 6 months of age and relative associations with neural development at 12 months of age. The measurement of both caregiving behaviours and maternal/infant cortisol levels allowed them to investigate the role of caregiving as a mechanism underlying associations between physiological stress and infants’ neural and physiological measures. Resting-state EEG connectivity data was collected while infants were engaged in a contingent social interaction with an experimenter. Results showed that higher maternal cortisol levels measured at the age of 6 months were associated with increased infant cortisol levels at 6 months and decreased 6−9 Hz (alpha) power in all regions of the brain at 12 months of age independently from maternal caregiving behaviours.
A longitudinal EEG study by Beckwith and Parmelee (1986) measured continuity in the EEG signal during sleep in preterm babies in their first days of life and the relation to caregiving. In healthy neonates at term (37 weeks gestation), background EEG rhythm becomes continuous and displays similar patterns of organisation across awake and active sleep with discontinuous organisation, known as trace alternant, displayed during quiet sleep. This study found that preterm infants with lower levels of trace alternant patterns had low developmental scores during the first year of life independent of caregiving. Furthermore, when followed into mid-childhood (5 and 8 years), only those children whose mothers were low on responsiveness during their first two years of life, had low developmental outcomes.
4.3. Task-evoked functional experiments
In this literature review, we identified fifteen research papers in which the relationship between behaviours of mothers and infants - derived directly from caregiver-child interactions - and infant’s cortical processing of different social stimuli were explored.
4.3.1. Face processing
To summarise, the separation paradigm was used in these studies to investigate how infants’ distress during separation from a caregiver was related to face processing. Two studies used the negative central (Nc) event related potential – an EEG marker linked to novel/salient stimuli (De Haan et al., 2004)– discovering that 6-month-olds who showed many proximity-seeking behaviours to mothers reacted to a stranger’s face (relative to their mothers’ face) with a larger Nc amplitude over frontal lateral areas (Swingler et al., 2007). Additionally, infants who were more distressed when separated from their mothers showed higher Nc and face-sensitive P400 components when looking at her face compared to the stranger’s face (Swingler et al., 2010).
4.3.2. Facial expressions
Four studies investigated infants’ processing of facial expression and associations with interactive measures. Rayson et al. (2017) investigated event-related desynchronization in EEG mu frequency (central site) when 9-month-old infants observed happy and sad emotions as well as non-emotional mouth openings. Stronger mu desynchronization was elicited by the same expression later in infancy when high maternal mirroring of infant’s facial expressions was observed by mothers when their infants were 2 months of age. Specifically, infants whose mothers mirrored mouth openings or smiles more often showed greater right hemisphere mu desynchronization when observing happy faces and mouth opening stimuli at 9 months of age, indicating that maternal mirroring facilitates the development of an action-perception mechanism for faces. Diego et al. (2002) focused on the interactive style (i.e. withdrawn or intrusive) of clinically depressed mothers to investigate how their 3-month- old infants process happy, surprised and sad facial expressions in images of strangers’- and own- mothers’ faces. Infants of intrusive depressed mothers displayed a shift from left to right frontal EEG asymmetry when presented with strangers’ surprised and sad facial expressions, and showed a significant increase in cortisol salivary levels. Such a physiological profile indicates that infants of intrusive mothers were more stressed by the interaction. No such effects were observed for infants of depressed withdrawn mothers who showed higher baseline levels of right frontal EEG asymmetry and tended to look away from strangers’ faces.
In an EEG study, Taylor-Colls and Fearon (2015) discovered that 7-month-old infants exhibited larger Nc amplitudes to fearful compared to happy and neutral faces. Moreover, infants whose mothers scored highly on the sensitivity scale – a composite of sensitivity, structuring, non-intrusiveness and non-hostility – during interactions showed increased Nc amplitude to happy relative to neutral faces. Since the Nc component is associated with increased attentiveness, often linked to the processing of salient, albeit negative, information (De Haan et al., 2004), these findings might indicate that the experience of sensitive caregivers’ responses led infants to encode positive facial expressions as more rewarding. When it comes to the findings by Peltola et al. (2020), no associations between maternal behaviours and 7-month-olds’ processing of fearful and non-fearful faces were discovered. Instead, infants who went on to develop secure attachment at 14 months of age showed robust N290 ERP response to fearful vs non-fearful faces at 7 months of age.
4.3.3. Eye-gaze processing
Elsabbagh et al. (2015) focused on 7-month-olds at elevated risk for autism spectrum disorders (ASD) and showed that those infants – both with and without increased familial likelihood of developing ASD - who expressed more positive affect in interactions with mothers tended to show higher face-sensitive P400 responses to gaze stimuli directed at them relative to those directed away. When considering maternal behaviours during the interactions, an association between behaviour and infant brain responses was only found in infants with a low familial likelihood of developing ASD. High maternal sensitivity was associated with higher P100 differentiation of gaze directed towards, versus away, from self. Note that while dyadic mutuality between infant and mother was also found to be associated with the ERP results it was omitted in the final model in favour of infant positive affect to account for correlation (collinearity) issues.
4.3.4. Prosody
Two studies considered the relationship between infants processing of prosody and maternal sensitivity (Zhao et al., 2019; Huffmeijer et al., 2020), directiveness (Zhao et al., 2019) and intrusiveness (Huffmeijer et al., 2020) as measured during caregivers’ interactions with their 6-month-old infants. In an fNIRS study, Zhao et al. (2019) found that angry vocalisations evoked stronger haemodynamic responses (increases in oxy-haemoglobin concentration) relative to neutral vocalisations in the left anterior superior temporal cortex in infants whose mothers were more directive, thus, more intrusive, demanding, controlling and critical. In an EEG study, Huffmeijer et al. (2020) revealed that infants of intrusive mothers showed a lower positive P2 amplitude over the fronto-central site in response to angry relative to happy utterances. Furthermore, neither maternal sensitivity nor infant frontal EEG asymmetry was associated with infant’s responses to vocal emotional stimuli.
4.3.5. Emotion processing and regulation
Behaviours observed in caregiver-infant interactions (Gartstein et al., 2020; Holz et al., 2018) and infants’ attachment patterns (Quevedo et al., 2017; Moutsiana et al., 2014; Biro et al., 2021) were related to the processing of emotional information - using pictures (Moutsiana et al., 2014), monetary rewards (Quevedo et al., 2017; Holz et al., 2018) and social situations (Pratt et al., 2019; Gartstein et al., 2020; Biro et al., 2021) as stimuli. Overall, insecure attachment and low maternal stimulation in interactions measured in infancy were related to the inefficiency in regulating affect associated with rewards in adults between 20–25 years of age (Moutsiana et al., 2014; Quevedo et al., 2017; Holz et al., 2018). This inefficiency was reflected in greater activations in cortical midline structures (including the prefrontal cortex and anterior cingulate cortex) and basal ganglia (including caudate head and nucleus accumbens).
When processing social stimuli, individual differences in maternal sensitivity measured at 9 months and 6 years of age (summed score) correlated with beta (14−25 Hz) activations in temporal and insular areas in 11-year-olds to own- versus other- mother-child interaction videos (Pratt et al., 2019). Associations in infancy have also been shown by Biro et al. (2021) and Gartstein et al. (2020). Specifically, 9-month-old infants who reacted less negatively to a mildly distressful condition such as the still-face paradigm had more synchronous and reciprocal interactions with caregivers. Those infants who shifted towards more withdrawal (increased right frontal activity) had mothers who were more directed and intense (Gartstein et al., 2020). More disorganised infants (in terms of their attachment pattern) – relative to secure or insecure infants - showed a reduction or absence of right-sided frontal asymmetry when watching mildly distressing social encounters (Biro et al., 2021).
To summarise, infants’ pattern of interactions with mothers was associated with infants’ processing of faces (Swingler et al., 2007, 2010; Gunnar and Nelson, 1994), emotional expressions (Peltola et al., 2020), rewards (Moutsiana et al., 2014; Quevedo et al., 2017; Holz et al., 2018) and social scenes (Biro et al., 2021). Moreover, maternal use of mirroring in interaction and high sensitivity were linked to the processing of facial expressions (Rayson et al., 2017; Taylor-Colls and Pasco Fearon, 2015), directed gaze (Elsabbagh et al., 2015) and emotional cues (Pratt et al., 2019), while intrusiveness was associated with the processing of prosody (Huffmeijer et al., 2020; Zhao et al., 2019) and regulation of emotions (Gartstein et al., 2020).
5. Discussion
The main goal of this paper was to review research studies that investigated the relationship between adult-infant behaviours - collected and quantified during their videoed social interactions - and measures of the child’s brain structure and function, collected at any point at earlier, concurrent or later visits. Overall, 55 research papers were identified as investigating associations between infant brain measures and data derived from caregiver-infant interactions.
The articles included in this review were unconstrained to allow the inclusion of any form of neural or social interaction measure and with any research population. Hence, it was possible to see that various measures of social interactions were associated with a diverse array of neural measures in children, ranging from regional brain volumes (MRI volumes) - to differential processing of socio-emotional stimuli (EEG/ERP studies) - to connectivity measures of specific as well as large scale functional networks. Taken together, these findings confirm that experience-dependent wiring of a child’s developing brain is at least to some extent linked to behaviours observed in caregiver-infant interactions.
Interestingly, the neural data collected in the aforementioned studies are heterogenous partly due to the different tools and paradigms that were utilised, while caregiver/infant behaviours that were derived from the interactions are more consistent across studies. Following animal models (e.g. Curley and Champagne, 2016) and numerous behavioural and physiological evidence in humans (e.g. Luby et al., 2013), the quality of caregiving was the primary focus of papers included in this review. With fathers being interactive partners in only one of the studies found (Sethna et al., 2019), most studies investigated interactions with mothers. As such, maternal behaviours were coded from caregiver-infant interactions in 38/55 (69 %) of the studies, with maternal sensitivity being the most investigated behaviour (in 21 studies).
Defined as prompt and appropriate comments and/or actions towards infants’ cues, maternal sensitivity was either scored on one scale or derived through several other sub-scales i.e. warmth, non-intrusiveness and responsiveness. Studies showed that maternal sensitivity – or its derivatives – are linked to both structural and connectivity measures of the limbic system collected concurrently (Rifkin-Graboi et al., 2015) and during a follow-up (Lee et al., 2019; Wang et al., 2019). Moreover, high maternal sensitivity was linked to individual differences in infants’ processing of happy vs neutral facial expressions (Taylor-Colls and Pasco Fearon, 2015), self- vs other-directed gaze (Elsabbagh et al., 2015), and activity within the temporal cortex (STS/STG, MTG, ITG, insula) when observing emotional stimuli (Pratt et al., 2019).
In addition to maternal sensitivity, maternal positive affect during interaction independently predicted connectivity within frontal and limbic areas (Perone and Gartstein, 2019; Bernier et al., 2016, 2019). Further, maternal use of mental-state talk was a significant predictor of individual differences observed in the maturation of large-scale functional connectivity networks (Dégeilh et al., 2018; Hanford et al., 2018). In contrast, maternal intrusiveness - seen as detrimental for infants’ attention (Swingler et al., 2017) – was particularly linked to differential processing of angry prosody (Zhao et al., 2019; Huffmeijer et al., 2020). In infants of depressed mothers, maternal intrusiveness was associated with decreases in right frontal EEG asymmetry in infants of depressed mothers when compared to infants of depressed mothers who were withdrawn (Diego et al., 2002; 2006; Field et al., 2003; Jones et al., 1997).
A smaller but substantial number of studies (30/55 studies; 55%) coded infants’ behaviours during interactions. Most studies (11 studies) investigated attachment patterns and found links to multiple neural measures such as structural volume changes within the limbic system (Moutsiana et al., 2015; Lyons-Ruth et al., 2016; Cortes Hidalgo et al., 2019; Quevedo et al., 2017), grey matter volume within the social brain network (Leblanc et al., 2017) and individual differences in activity within emotion and reward systems, including prefrontal cortex and amygdala, (Moutsiana et al., 2014; Quevedo et al., 2017) in adulthood. Direct associations between the children’s neural measures and infants’ behaviours in interaction were primarily demonstrated in the context of frontal EEG asymmetry and corresponding approach-avoidance tendencies in children (Hane and Fox, 2006; 2010; Jones et al., 2009; Mize and Jones, 2012; Licata et al., 2015; Dawson et al., 1999). Three more studies discussed the individual differences in the neural basis of joint attention (Eggebrecht et al., 2017; Elison et al., 2012) and emotion regulation (Catalina Camacho et al., 2019).
Several papers addressed the moderating role of maternal or infant behaviours on children’s brain-behaviour associations, covering several neural measures such as structural volumes of the cerebellum (Sethna et al., 2019), frontal EEG asymmetry (Diaz et al., 2019; Swingler et al., 2014), functional network connectivity (Hanford et al., 2018) and frontal EEG activity (Broomell et al., 2019).
While infant and caregiver behaviours have been investigated in isolation, an important area of research identified within this review is that of dyadic behaviours and interactions. The “transactional model” of development proposes the ongoing interactional process in which a child can influence the environmental stimuli and responses (Sameroff, 1993). Within such a model, infants’ own behaviours and social capacities can influence the flow of interaction with the caregiver and, thus, behaviours of caregiver and infant ought to be investigated in relation to each other, and in relation to the neural measures. Dyadic behaviours cannot be reduced to one individual as the timing and pace of both infant/child and caregiver are crucial for this form of interaction. One such example, the amount of turn-taking that occurs between partners, has been found to associate with increased left inferior frontal activation (Romeo et al., 2018), which is a brain region that plays a key role in language processing. In this review, behaviours that simultaneously covered two people i.e. mutuality, reciprocity or dyadic intensity were investigated in six studies (Perone and Gartstein, 2019; Elsabbagh et al., 2015; Pratt et al., 2019; Gartstein et al., 2020; Beckwith and Parmelee, 1986; Jones et al., 2004). Significant associations were reported in three of these studies (Perone and Gartstein, 2019; Gartstein et al., 2020; also Pratt et al., 2019 but note in this study significant associations were found only when assessed later in childhood between 6 and 11 years, and not with infant measures of dyadic interaction). The remaining three studies either omitted dyadic variables in the final model or did not report any associations – neither positive nor negative (Elsabbagh et al., 2015; Beckwith and Parmelee, 1986; Jones et al., 2004). Nevertheless, dyadic measurements are a promising avenue for future research.
Interestingly, associations between neural patterns of structure and function and observed behaviours during caregiver-infant interactions were found strikingly early (Sethna et al., 2017, 2019; Hanford et al., 2018), and, furthermore, even the earliest behaviours carried long-term associations, months (Diego et al., 2006; Rayson et al., 2017), and sometimes decades later (Holz et al., 2018). For example, maternal stimulation during interactions with her infant at 3 months of age was related to functional neural activation within the reward processing network observed 25 years later (Holz et al., 2018).
The role of caregivers, particularly in the first half a year of life, appeared to be particularly invaluable given that caregivers’ behaviours moderated the relationship between infants’ own behavioural and neural measures (e.g. Hanford et al., 2018; Broomell et al., 2019). Furthermore, studies presented in this review suggest that infants exhibit differential susceptibility early in life, so that some infants benefit from certain experiences (including caregiving experiences) more than other infants (e.g. Sethna et al., 2019; Diaz et al., 2019; Swingler et al., 2014; Nolvi et al., 2020). For example, only those infants who were highly communicative at a young age benefited from paternal sensitivity when it comes to associations with cerebellar volumes (Sethna et al., 2019), though note that only one study exists with regard to the investigation of paternal behaviours, so this area warrants further research. In a further study, maternal sensitivity was found to be associated with affect in infants, but only for those who displayed left frontal EEG asymmetry (left α power < right α power) (Diaz et al., 2019).
Building on this, a broader discussion with regards to brain development can be introduced. Until recently, researchers aimed to capture the general principles of brain development by relying on group-level analyses. This approach of studying variability across specific periods of development is crucial but is complementary to the investigation of individual variability across children of the same age. In particular, with the emergence of projects targeting global contexts, interest in understanding the role of individual environment and individual brain architecture, and how that translates into individual differences observed in behaviour and cognitive abilities has arisen.
In this review, we can clearly see that individual differences in social experiences were associated with individual differences observed at the neural level. The presence of associations – concurrent and long-term ones - indicates that postnatally the brain accommodates and reacts to environmental stimuli. With respect to specific brain regions, studies have shown that primary networks such as motor and visual-related networks established at birth typically yield more consistent functional architecture and have low variability among individuals (Gao et al., 2014). As a backbone on which other skills build upon, these primary sensorimotor networks possess a high degree of commonality between individuals, and one might expect that the social environment would have little influence on their development. Yet, during the first few postnatal months these networks also show re-organisation and refinement to process information more effectively (Alcauter et al., 2014), meaning that postnatal experiences might have an influence. In this review, two studies supported this hypothesis (Wang et al., 2019; Hanford et al., 2018). For example, in infants whose mothers use more mental state talk during interactions at 3 months of age, positive affect is concurrently linked to increased connectivity to the occipital cortex (Hanford et al., 2018). Notably, higher-order cognitive networks and association areas with their slow-paced protracted development demonstrate higher inter-subject variability and are more indicative of individuals’ experiences in social interactions (e.g. Mueller et al., 2013; Gao et al., 2014). In this review we showed that positive caregiving was predictive of more mature network connectivity between the executive control and default mode networks at 10 years of age (Dégeilh et al., 2018). Although not being the measures of connectivity per se, a large proportion of studies presented in this review connected individual differences in behaviours observed during the interaction to infants’ frontal EEG activity (e.g. Jones et al., 2009; Bernier et al., 2016; Broomell et al., 2019).
With protracted development of higher-order areas providing opportunities for environmental influences, the foundation for these trajectories could be established as early as during the perinatal period. Namely, high variability in higher order areas (i.e. cingulate gyrus, prefrontal and parietal lobes) has been observed already at birth (Gao et al., 2014; Stoecklein et al., 2020; Wang et al., 2021; Xu et al., 2019) suggesting that higher order areas capture individuals’ uniqueness throughout the lifespan. These individual differences in neonatal neurophenotypes may underlie differential susceptibility to both adverse and positive aspects of the early environment resulting in subsequent variability observed on the neural and behavioural levels. For example, individual variability in hippocampus volumes or the volumes of anterior cingulate cortex at birth (Nolvi et al., 2020; Rifkin-Graboi et al., 2019) moderated the subsequent associations between maternal and infant outcomes. These differences could be a result of intrauterine and/or genetic influences, and future studies should try to research how prenatal factors and individual variability in brain development explains individual differences in behaviour independent from postnatal factors.
At present, it is difficult to infer causality between brain measures and caregiver/infant behaviours because to characterise these individual trajectories, longitudinal study designs are central. To note, thirteen studies presented in this review collected the same brain and/or behavioural data at more than one age point. The majority of the studies (76%) measured brain-behavioural relationships only once either concurrently or during follow-ups. Studies with one testing time point are limited in their claims about causality and provide no information on the developmental changes that so rapidly occur in young brains. For example, in the study by Rifkin-Graboi et al. (2015) maternal sensitivity was associated with bilateral hippocampal volumes measured concurrently. In this case, the relationship between these variables tells us nothing about the directionality, or potential causal factors. Potentially maternal sensitivity led to increases in bilateral hippocampal volumes or it could be the case that infants with increased hippocampal volumes behave in a certain way allowing their cues to be more recognizable and easier for mothers to respond to. Even those studies that introduced temporal precedence in trying to address the cause-and-effect question face challenges (Shadish et al., 2002). For example, Moutsiana et al. (2015) showed that insecurely attached infants have larger amygdala volumes at the age of 22 years. However, it could be the case that had they also measured amygdala volumes in infancy, they would have found them to already be higher within those infants, and the volumes in fact remained relatively consistent throughout development, and, thus, resulted in the association that is observed in the study. Concurrent measures, if available, could inform about the stability of behaviour or neural data over time, as well as provide information about any cross-lagged relationships. For example, by measuring brain and behaviour concurrently over time, Jones et al. (1997) showed that at the age of three months infants of depressed mothers who have interactive and intrusive style show relative right frontal EEG asymmetry indicative of withdrawal behaviours compared to infants of non-depressed mothers. However, by the age of 6 months, infants of depressed mothers who were intrusive showed a relative shift towards left frontal EEG asymmetry. With repeated behavioural and neural measures over two time points, one can more confidently say that at 3 months of age the impact of maternal behaviours was not yet evident but that by the age of 6 months the environmental influence of mother’s intrusive behaviours on infants’ brain activity (frontal EEG asymmetry) was more prominent. Collecting brain-behaviour data concurrently has also been invaluable in interpreting some of the individual differences observed in infants’ functional activations. For example, the neural and observational data collected by Swingler et al. (2007; 2010) suggests that infants who have an established secure pattern of interaction with mothers (and show higher Nc amplitude to strangers’ faces), and, thus, are not particularly influenced by separation, allocate more attention to exploring the unfamiliar external environment. In contrast, those who are distal from mothers and/or are more heavily influenced by separation, continue to hold sustained attention on mothers’ faces (higher Nc amplitude), as if, reflecting their insecurity.
5.1. Limitations and recommendations for future studies
Although this review has highlighted several interesting behaviour-brain correlates of social processing, future studies should attempt to conduct longitudinal investigations to elucidate the associations between neural development and measures of social interactions over time.
Infancy is a period of rapid change and researchers should move from group-level analysis, and use individual differences as variables of interest and not as statistical noise or errors. Individual differences should be collected and analysed using methods for longitudinal data analysis that would allow modelling of individual slopes and latent changes, and include more than two time points to uncover any non-linear effects that caregiver-infant behaviours may have on the developing brain.
On a related note, reliable modelling and analysis of brain-behaviour association data require large sample sizes. The median sample size in the research papers presented in this review is ∼ 60 participants, ranging from 12 (Elison et al., 2012) to 629 (Tharner et al., 2011) participants. Although this median sample size is larger than typically reported in neuroimaging-only studies (Szucs and Ioannidis, 2020), it may still be underpowered to detect small-to-medium within-subject effects. This especially holds if individual differences in brain and behaviour are investigated, given that correlation stability is achieved with increases in sample sizes (Schönbrodt and Perugini, 2013; Klapwijk et al., 2021). One way to deal with this problem would be to calculate sample sizes justified by expected effect sizes but no such calculations were reported in articles presented in this review. Another way to manage the requirement for large sample sizes is to utilise publicly available datasets. One example of such dataset is the UNC/UNM Baby Connectome Project (https://babyconnectomeproject.org) (Howell et al., 2019) led by the University of North Carolina and University of Minnesota that collects the 0- to 5- year- olds’ data. In addition to the brain data, this project (Howell et al., 2019) offers a wide range of behavioural and neuropsychological assessment data including Mullen Scales of Early Learning (MSEL; Mullen, 1995) and Infant Behavior Questionnaire-Revised (IBQ-R) (Gartstein and Rothbart, 2003). Another example is the Developing Human Connectome Project (dHCP; http://www.developingconnectome.org) led by King’s College London, Imperial College London and Oxford University that collects the fMRI data across the perinatal period. Finally, the Pediatric Imaging Neurocognition and Genetics study (PING; http://pingstudy.ucsd.edu/) collects the neural and genetic data of individuals between 3 and 21 years of age. While these advances towards open science are promising in further advancement of the field, there are still some limitations when it comes to studying infants’ social environment including caregiver-infant interactions. For example, within Europe, video materials constitute personal data and are protected to the highest degree following General Data Protection Regulation (GDPR), preventing their release in open-source databases. To address this, as video data cannot be shared in this way, sharing agreements between Institutions are encouraged, thus enhancing collaborative efforts and multidisciplinary approaches to data analysis.
Given large sample size and adequate modelling techniques, the questions of causality between brain and behaviours may be addressed. Another direction to achieve this is to use an intervention approach such as that used in the study by Bick et al. (2019) who delivered a training program to caregivers at risk for adversity. Following a randomized clinical trial method, training was undertaken with children from 1- to 29- months of age, and involved vivo and video-based feedback on the parents’ positive behaviour in interactions. An 8-year follow-up showed that children assigned to training showed greater higher frequency power in the beta range (12−20 Hz), often linked to conscious thought and logical thinking in adults. Considering that this review describes behaviours that occur naturally in caregiver-infant interactions, parent-centred interventions are a promising avenue for future investigation.
Given that most of the studies did not have pre-registered analyses, it is possible that some of the effects reported resulted from post-hoc exploratory analyses. Pre-registration of future studies is recommended, especially given the variety of different interaction and neural measures that are sometimes recorded.
Building on this, we would like to highlight the complexity of coding of adult-infant interactions and the need for full descriptions to allow replication in future studies. Firstly, definitions and the level of detail offered in articles regarding the coding schemes differ significantly between publications, resulting in ambiguities. For example, three studies (Bernier et al., 2016; Broomell et al., 2019; Diaz et al., 2019) coded “maternal intrusiveness” following the coding scheme that defined it as “a display of over-controlling behaviours by mothers or focus on her own agenda, ignoring the infant’s behaviour or cues”. In contrast, while the behaviours measured formed a similar construct, in Dégeilh et al. (2018) they coded for “maternal directiveness”, defined as “the degree to which infants’ behaviours are his/her focus rather than caregivers’ agenda”. Furthermore, while Holz et al. (2018) clearly differentiated “responsiveness” as “any behaviour done by mother in response to her infants’ behaviour”, Perone and Gartstein (2019) combined it with the “sensitivity” construct and rather than include “any” maternal behaviour in response to infants chose to focus on a very specific quality. Secondly, many studies changed or adapted coding manuals to their studies and, while these adaptations are often necessary, it might be useful to provide a more detailed guide on what exactly has been changed in relation to other coding manuals and how these changes might impact the interpretation of findings. Thirdly, few studies composed and/or de-composed their constructs (Bernier et al., 2019; Taylor-Colls and Pasco Fearon, 2015; Hane et al., 2006; 2010; Hanford et al., 2018). While such practices might help to reduce the number of behaviours that are studied (and aid in multiple comparison problem), they do need to have solid statistical grounds.
Despite the extensive screening process that was undertaken in this review, it is possible that we missed some relevant articles due to the breadth of terms associated with social interactions. Our review also potentially suffers from publication bias, with an unknown number of studies with null effects failing to be published.
Several studies in this review were identified as “preliminary” (e.g. Sethna et al., 2017, 2019; Dégeilh et al., 2018; Bernier et al., 2019; Rifkin-Graboi et al., 2015; Zhao et al., 2019) necessitating further replications of the findings. A small number of research papers presented in this review analysed the same dataset from multiple angles (e.g. Sethna et al., 2017, 2019; Swingler et al., 2007, 2010), thereby, providing a richer data source and allowing for a better understanding of associations between brain and behaviour.
Articles presented in this review also highlighted that greater focus on the study of neonates and infants below the age of 3 months is required to disentangle the effects of biological predispositions and rapidly emerging environmental associations. As such, more studies on prenatal factors and individual differences in brain development are required to explain individual differences in behaviour independent from, and in relation to, postnatal factors. More diverse samples are required to collect a wider range of behaviours in varying contexts. Instead of investigating distal predictors of brain development (e.g. Jednoróg et al., 2012; Hanson et al., 2013), we recommend a further focus directed towards caregiver-infant interactions as a mechanism underlying these associations. As evident in the studies on caregiver-infant interactions, the effects of maternal clinical diagnoses (e.g. depression or schizophrenia), poverty and stress on a child are at least partially manifested through parental changes in caregiving. For example, families from low socio-economic settings use fewer words, less complex sentences and less diverse language when communicating with their infants (Hart and Risley, 1995; Huttenlocher et al., 2010), while depressed mothers are less sensitive and attuned to their infants in interactions (Murray et al., 1996). Current research has predominantly focused on studies of healthy, rich and educated families (i.e. WEIRD sampling; Henrich et al., 2010) and therefore investigation of these brain-behavioural correlates across more diverse settings and populations is recommended. With some studies leaving their demographics unmentioned, only n = 11 studies presented in this review studied non-White population and n = 5 studies included low-SES families (Table 1),
On this note, this review demonstrated that the vast majority of interactive measures focus on maternal caregiving and infant behaviours. While being undoubtedly crucial, future studies should devote more attention to other partners including the father, non-biological and biological caregivers, as well as upon dyadic, rather than individual, behaviours to investigate their roles in driving brain development.
It is important to acknowledge that most of measures of caregiver-infant interactions identified in this review were based on 5-to-20-minute recordings, the majority of which were conducted within a research setting. This is by no means representative of infants’ daily experiences with their caregivers. While even these short periods of time are enough to elucidate the relationships presented in this review, recent advances in wearable technologies, as well as automatic programs to code interactions, may enable researchers to record more ecologically valid interactions in home and community settings across longer time windows and multiple situations.
Alongside advances in wearable monitoring devices, recent neuroimaging advances have allowed for pioneering investigations of simultaneous concurrent brain activity in both infant and caregiver. Therefore, a new avenue has opened for investigation of longitudinal associations between partners’ behaviours and brain responses during naturalistic ecologically valid caregiver-infant interactions, and future research should focus on harnessing the combined potential of these approaches.
Finally, given such a large choice of methods, populations, individual differences between different ages and within the same age and heterogeneity of findings, it is crucial to start bringing all the findings together by drawing out separate neural systems most affected by caregiver-infant behaviours.
6. Conclusion
This review reveals the existence of an important, yet understudied, research field within developmental psychology, namely, that coded indices of caregiver-infant interactions are associated with a wide range of child’s neural measures collected concurrently and at later time points, spanning into adolescence. Behavioural interaction indices were found to be associated with a range of structural, connectivity and functional brain correlates of social processing across the first years of life. Early emergence of these brain-behaviour associations in infancy and their strong predictive value for later development and outcomes makes this an important direction for future research.
Funding sources
This work was supported by: a European Union’s Horizon 2020 research and innovation programme under the SAPIENS Marie Skłodowska-Curie Actions (No 814302) to DI, MJ and SLF; a Medical Research Council Programme Grant (MR/T003057/1) to MJ: and a UKRI Future Leaders Fellowship (grant MR/S018425/1) to SLF. The funding bodies were not involved in the preparation of the manuscript.
Declaration of Competing Interest
The authors declare no competing interests.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2021.09.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ainsworth M.D.S. Princeton, NJ: Educational Testing Service; 1976. Technical manual for the Systems for Coding Infant Attachment and Reciprocal Maternal Behaviors. [Google Scholar]
- Ainsworth M.D.S., Bell S.M., Stayton D.J. In: The Integration of a Child into the Social World. Richards M.P.M., editor. Cambridge University Press; Cambridge, UK: 1974. Infant mother attachment and social development: socialization as a product of reciprocal responsiveness to signals; pp. 99–135. [Google Scholar]
- Ainsworth M.S., Blehar M.C., Waters E., Wall S. Lawrence Erlbaum Associates Ltd; Hove, UK: 1978. Patterns of Attachment: a Psychological Study of the Strange Situation. [Google Scholar]
- Alcauter S., Lin W., Smith J.K., Short S.J., Goldman B.D., Reznick J.S., Gilmore J.H., Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J. Neurosci. 2014;34(27):9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1-2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Bauer P.M., Hanson J.L., Pierson R.K., Davidson R.J., Pollak S.D. Cerebellar Vol. and cognitive functioning in children who experienced early deprivation. Biol. Psychiatry. 2009;66(12):1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith L., Cohen S.E. In: Ed.), Home Environment and Early Cognitive Development. Gottfried A., editor. Academic Press; New York: 1984. Home environment and cognitive competence in preterm children during the first 5 years; pp. 235–271. [Google Scholar]
- Beckwith L., Parmelee A.H., Jr EEG patterns of preterm infants, home environment, and later IQ. Child Dev. 1986;57(3):777–789. [PubMed] [Google Scholar]
- Belsky J., De Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J. Child Psychol. Psychiatry. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Bernier A., Calkins S.D., Bell M.A. Longitudinal associations between the quality of mother-infant interactions and brain development across infancy. Child Dev. 2016;87(4):1159–1174. doi: 10.1111/cdev.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A., Dégeilh F., Leblanc É., Daneault V., Bailey H.N., Beauchamp M.H. Mother-infant interaction and child brain morphology: a multidimensional approach to maternal sensitivity. Infancy. 2019;24(2):120–138. doi: 10.1111/infa.12270. [DOI] [PubMed] [Google Scholar]
- Bick J., Palmwood E.N., Zajac L., Simons R., Dozier M. Early parenting intervention and adverse family environments affect neural function in middle childhood. Biol. Psychiatry. 2019;85(4):326–335. doi: 10.1016/j.biopsych.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringen Z. The Emotional Availability (EA) Scales. 4th edition. Colorado State University; Boulder, Colorado: 2008. Unpublished manual. [Google Scholar]
- Biro S., Peltola M.J., Huffmeijer R., Alink L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Frontal EEG asymmetry in infants observing separation and comforting events: the role of infants’ attachment relationship. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M., Kitts R.L., Blood E., Bizarro A., Hofmeister M., Wright R.J. Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation. Infant Behav. Dev. 2011;34(4):487–503. doi: 10.1016/j.infbeh.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Nusslock R., Barton A.W., Miller G.E., Chen E., Holmes C., McCormick M., Sweet L.H. The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychol. Sci. 2019;30(7):1040–1049. doi: 10.1177/0956797619847989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomell A., Smith C.L., Calkins S.D., Bell M.A. Context of maternal intrusiveness during infancy and associations with preschool executive function. Infant Child Dev. 2019;29(1):e2162. doi: 10.1002/icd.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S.D., Hungerford A., Dedmon S.E. Mothers’ interactions with temperamentally frustrated infants. Infant Ment. Health J. 2004;25:219–239. doi: 10.1002/imhj.20002. [DOI] [Google Scholar]
- Cao M., Huang H., He Y. Developmental connectomics from infancy through early childhood. Trends Neurosci. 2017;40(8):494–506. doi: 10.1016/j.tins.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina Camacho M., King L.S., Ojha A., Garcia C.M., Sisk L.M., Cichocki A.C., Humphreys K.L., Gotlib I.H. Cerebral blood flow in 5‐ to 8‐month‐olds: regional tissue maturity is associated with infant affect. Dev. Sci. 2019;23(5) doi: 10.1111/desc.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Curley J.P. How social experiences influence the brain. Curr. Opin. Neurobiol. 2005;15(6):704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B. In: The Asymmetrical Brain. Hugdahl K., Davidson R.J., editors. MIT Press; 2003. The state and trait nature of frontal EEG asymmetry in emotion; pp. 565–615. [Google Scholar]
- Cortes Hidalgo A.P., Muetzel R., Luijk M.P.C.M., Bakermans-Kranenburg M.J., El Marroun H., Vernooij M.W., van IJzendoorn M.H., White T., Tiemeier H. Observed infant-parent attachment and brain morphology in middle childhood– A population-based study. Dev. Cogn. Neurosci. 2019;40 doi: 10.1016/j.dcn.2019.100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., Sebastián-Gallés N. How does the bilingual experience sculpt the brain?. Nature reviews. Neuroscience. 2014;15(5):336–345. doi: 10.1038/nrn3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K., Bell M.A. Infant attention and early childhood executive function. Child Dev. 2014;85(2):397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley J.P., Champagne F.A. Influence of maternal care on the developing brain: mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016;40:52–66. doi: 10.1016/j.yfrne.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Frey K., Self J., Panagiotides H., Hessl D., Yamada E., Rinaldi J. Frontal brain electrical activity in infants of depressed and nondepressed mothers: relation to variations in infant behavior. Dev. Psychopathol. 1999;11(3):589–605. doi: 10.1017/S0954579499002229. [DOI] [PubMed] [Google Scholar]
- de Haan M., Belsky J., Reid V., Volein A., Johnson M.H. Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. J. Child Psychol. Psychiatry. 2004;45(7):1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- DeCasper A.J., Fifer W.P. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Dégeilh F., Bernier A., Leblanc É., Daneault V., Beauchamp M.H. Quality of maternal behaviour during infancy predicts functional connectivity between default mode network and salience network 9 years later. Dev. Cogn. Neurosci. 2018;34:53–62. doi: 10.1016/j.dcn.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A., Swingler M.M., Tan L., Smith C.L., Calkins S.D., Bell M.A. Infant frontal EEG asymmetry moderates the association between maternal behaviour and toddler negative affectivity. Infant Behav. Dev. 2019;55:88–99. doi: 10.1016/j.infbeh.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego M.A., Field T., Hart S., Hernandez-Reif M., Jones N., Cullen C., Schanberg S., Kuhn C. Facial expressions and EEG in infants of intrusive and withdrawn mothers with depressive symptoms. Depress. Anxiety. 2002;15(1):10–17. doi: 10.1002/da.1079. [DOI] [PubMed] [Google Scholar]
- Diego M.A., Field T., Jones N.A., Hernandez-Reif M. Withdrawn and intrusive maternal interaction style and infant frontal EEG asymmetry shifts in infants of depressed and non-depressed mothers. Infant Behav. Dev. 2006;29(2):220–229. doi: 10.1016/j.infbeh.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht A.T., Elison J.T., Feczko E., Todorov A., Wolff J.J., Kandala S., Adams C.M., Snyder A.Z., Lewis J.D., Estes A.M., Zwaigenbaum L., Botteron K.N., McKinstry R.C., Constantino J.N., Evans A., Hazlett H.C., Dager S., Paterson S.J., Schultz R.T., Styner M.A., Gerig G., Das S., Kostopoulos P., Schlaggar B.L., Petersen S.E., Piven J., Pruett J.R. Joint attention and brain functional connectivity in infants and toddlers. Cereb. Cortex. 2017;27(3):1709–1720. doi: 10.1093/cercor/bhw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison J.T., Wolff J.J., Heimer D.C., Paterson S.J., Gu H., Hazlett H.C., Styner M., Gerig G., Piven J. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev. Sci. 2012;16(2):186–197. doi: 10.1111/desc.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Bruno R., Wan M.W., Charman T., Johnson M.H., Green J., Team B.A.S.I.S. Infant neural sensitivity to dynamic eye gaze relates to quality of parent-infant interaction at 7-months in infants at risk for autism. J. Autism Dev. Disord. 2015;45(2):283–291. doi: 10.1007/s10803-014-2192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal T.J., Chugani H.T., Behen M.E., Juhász C., Muzik O., Maqbool M., Chugani D.C., Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Emerson R.W., Gao W., Lin W. Longitudinal study of the emerging functional connectivity asymmetry of primary language regions during infancy. J. Neurosci. 2016;36(42):10883–10892. doi: 10.1523/jneurosci.3980-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Bio-behavioral synchrony: a model for integrating biological and microsocial behavioral processes in the study of parenting. Parent. Sci. Pract. 2012;12(2-3):154–164. doi: 10.1080/15295192.2012.683342. [DOI] [Google Scholar]
- Feldman R., Eidelman A.I. Parent-infant synchrony and the social-emotional development of triplets. Dev. Psychol. 2004;40(6):1133–1147. doi: 10.1037/0012-1649.40.6.1133. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Dev. Sci. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Field T., Healy B.T., Goldstein S., Guthertz M. Behavior-state matching and synchrony in mother-infant interactions of nondepressed versus depressed dyads. Dev. Psychol. 1990;26(1):7–14. doi: 10.1037/0012-1649.26.1.7. [DOI] [Google Scholar]
- Field T., Diego M., Hernandez-Reif M., Schanberg S., Kuhn C. Depressed mothers who are “good interaction” partners versus those who are withdrawn or intrusive. Infant Behav. Dev. 2003;26(2):238–252. doi: 10.1016/s0163-6383(03)00020-1. [DOI] [Google Scholar]
- Fransson P., Skiöld B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Aden U. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. U.S.A. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Elton A., Zhu H., Alcauter S., Smith J.K., Gilmore J.H., Lin W. Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J. Neurosci. 2014;34(34):11288–11296. doi: 10.1523/jneurosci.5072-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Smith J.K., Gilmore J.H., Lin W. Development of human brain cortical network architecture during infancy. Brain Struct. Funct. 2015;220(2):1173–1186. doi: 10.1007/s00429-014-0710-3. https://doi-org.ezp.lib.cam.ac.uk/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein M.A., Rothbart M.K. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav. Dev. 2003;26(1):64–86. doi: 10.1016/S0163-6383(02)00169-8. [DOI] [Google Scholar]
- Gartstein M.A., Crawford J., Robertson C.D. Early markers of language and attention: mutual contributions and the impact of parent-infant interactions. Child Psychiatry Hum. Dev. 2008;39(1):9–26. doi: 10.1007/s10578-007-0067-4. [DOI] [PubMed] [Google Scholar]
- Gartstein M.A., Hancock G.R., Iverson S.L. Positive affectivity and fear trajectories in infancy: contributions of mother-child interaction factors. Child Dev. 2018;89(5):1519–1534. doi: 10.1111/cdev.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein M.A., Warwick H., Campagna A.X. Electroencephalogram frontal asymmetry changes during emotion‐eliciting tasks and parent–child interaction dynamics. Soc. Dev. 2020;30(2):496–514. doi: 10.1111/sode.12484. [DOI] [Google Scholar]
- Geng X., Li G., Lu Z., Gao W., Wang L., Shen D., Zhu H., Gilmore J.H. Structural and maturational covariance in early childhood brain development. Cereb. Cortex. 2017;27(3):1795–1807. doi: 10.1093/cercor/bhw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J., Knickmeyer R., Gao W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. https://doi-org.ezp.lib.cam.ac.uk/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Nelson C.A. Event-related potentials in year-old infants: relations with emotionality and cortisol. Child Dev. 1994;65(1):80–94. [PubMed] [Google Scholar]
- Gunnar M.R., Larson M.C., Hertsgaard L., Harris M.L., Brodersen L. The stressfulness of separation among nine-month-old infants: effects of social context variables and infant temperament. Child Dev. 1992;63(2):290–303. [PubMed] [Google Scholar]
- Hane A.A., Fox N.A. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychol. Sci. 2006;17(6):550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hane A.A., Henderson H.A., Reeb-Sutherland B.C., Fox N.A. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: a follow-up study. Dev. Psychobiol. 2010;52(6):558–567. doi: 10.1002/dev.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford L.C., Schmithorst V.J., Panigrahy A., Lee V., Ridley J., Bonar L., Versace A., Hipwell A.E., Phillips M.L. The Impact of Caregiving on the Association Between Infant Emotional Behavior and Resting State Neural Network Functional Topology. Front. Psychol. 2018;9:1968. doi: 10.3389/fpsyg.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0080954. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J.S., Jones N.A., Mize K.D., Platt M. Affectionate touch in the context of breastfeeding and maternal depression influences infant neurodevelopmental and temperamental substrates. Neuropsychobiology. 2021;80(2):158–175. doi: 10.1159/000511604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B., Risley T.R. Paul H Brookes Publishing; 1995. Meaningful Differences in the Everyday Experience of Young American Children. [Google Scholar]
- Henrich J., Heine S., Norenzayan A. The weirdest people in the world? Behav. Brain Sci. 2010;33(2-3):61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- Hipwell A.E., Guo C., Phillips M.L., Swain J.E., Moses-Kolko E.L. Right Frontoinsular cortex and Subcortical activity to INFANT cry is associated with Maternal mental State Talk. J. Neurosci. 2015;35(37):12725–12732. doi: 10.1523/jneurosci.1286-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz N.E., Boecker-Schlier R., Jennen-Steinmetz C., Hohm E., Buchmann A.F., Blomeyer D., Baumeister S., Plichta M.M., Esser G., Schmidt M., Meyer-Lindenberg A., Banaschewski T., Brandeis D., Laucht M. Early maternal care may counteract familial liability for psychopathology in the reward circuitry. Soc. Cogn. Affect. Neurosci. 2018;13(11):1191–1201. doi: 10.1093/scan/nsy087. https://doi-org.ezp.lib.cam.ac.uk/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Styner M.A., Gao W., Yap P.-T., Wang L., Baluyot K., Yacoub E., Chen G., Potts T., Salzwedel A., Li G., Gilmore J.H., Piven J., Smith J.K., Shen D., Ugurbil K., Zhu H., Lin W., Elison J.T. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. NeuroImage. 2019;185:891–905. doi: 10.1016/j.neuroimage.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffmeijer R., Bakermans-Kranenburg M.J., Gervain J. Maternal intrusiveness predicts infants’ event-related potential responses to angry and happy prosody independent of infant frontal asymmetry. Infancy. 2020;25(3):246–263. doi: 10.1111/infa.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher J., Waterfall H., Vasilyeva M., Vevea J., Hedges L.V. Sources of variability in children’s language growth. Cogn. Psychol. 2010;61(4):343–365. doi: 10.1016/j.cogpsych.2010.08.002. https://doi-org.ezp.lib.cam.ac.uk/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C., Dehaene-Lambertz G., Ramus F. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Dziurawiec S., Ellis H., Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Jones N.A., Field T., Fox N.A., Davalos M., Malphurs J., Carraway K., Schanberg S., Kuhn C. Infants of intrusive and withdrawn mothers. Infant Behav. Dev. 1997;20(2):175–186. doi: 10.1016/s0163-6383(97)90020-5. [DOI] [Google Scholar]
- Jones N.A., McFall B.A., Diego M.A. Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: the mediating role of infant temperament. Biol. Psychol. 2004;67(1-2):103–124. doi: 10.1016/j.biopsycho.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Jones N.A., Field T., Almeida A. Right frontal EEG asymmetry and behavioral inhibition in infants of depressed mothers. Infant Behav. Dev. 2009;32(3):298–304. doi: 10.1016/j.infbeh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Jörg M., Dinter R., Rose F. Category system for microanalysis of early mother–child interaction. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie. 1994;22:97–106. [PubMed] [Google Scholar]
- Klapwijk E., van den Bos W., Tamnes C., Raschle N., Mills K. Opportunities for increased reproducibility and replicability of developmental neuroimaging. Dev. Cogn. Neurosci. 2021;47 doi: 10.1016/j.dcn.2020.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G. Multiple pathways to conscience for children with different temperaments: from toddlerhood to age 5. Dev. Psychol. 1997;33(2):228–240. doi: 10.1037//0012-1649.33.2.228. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Mother–child relationship, child fearfulness, and emerging attachment: a short-term longitudinal study. Dev. Psychol. 1998;34(3):480–490. doi: 10.1037/0012-1649.34.3.480. [DOI] [PubMed] [Google Scholar]
- Kraybill J.H., Bell M.A. Infancy predictors of preschool and post‐kindergarten executive function. Dev. Psychobiol. 2013;55(5):530–538. doi: 10.1002/dev.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc É., Dégeilh F., Daneault V., Beauchamp M.H., Bernier A. Attachment security in infancy: a preliminary study of prospective links to brain morphometry in late childhood. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Poh J.S., Wen D.J., Tan H.M., Chong Y.S., Tan K.H., Gluckman P.D., Fortier M.V., Rifkin-Graboi A., Qiu A. Maternal care in infancy and the course of limbic development. Dev. Cogn. Neurosci. 2019;40 doi: 10.1016/j.dcn.2019.100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerstee M. The role of dyadic communication in social cognitive development. Adv. Child Dev. Behav. 2009;37:1–53. doi: 10.1016/s0065-2407(09)03701-x. [DOI] [PubMed] [Google Scholar]
- Licata M., Paulus M., Kühn-Popp N., Meinhardt J., Sodian B. Infant frontal asymmetry predicts child emotional availability. Int. J. Behav. Dev. 2015;39(6):492–496. doi: 10.1177/0165025415576816. [DOI] [Google Scholar]
- Lin W., Zhu Q., Gao W., Chen Y., Toh C.H., Styner M., Gerig G., Smith J.K., Biswal B., Gilmore J.H. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am. J. Neuroradiol. 2008;29(10):1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Nishino T., Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K., Bronfman E., Parsons E. Atypical attachment in infancy and early childhood among children at developmental risk. IV. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monogr. Soc. Res. Child Dev. 1999;64(3):67–220. doi: 10.1111/1540-5834.00034. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K., Pechtel P., Yoon S.A., Anderson C.M., Teicher M.H. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav. Brain Res. 2016;308:83–93. doi: 10.1016/j.bbr.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main M., Solomon J. In: Attachment in the Preschool Years: Theory, Research and Intervention. Greenberg D.C.M.T., Cicchetti D., Cummings E.M., editors. University of Chicago Press; Chicago, IL: 1990. Procedures for identifying infants as disorganized-disoriented during the ainsworth strange situation; pp. 121–160. [Google Scholar]
- Maurer D., Werker J.F. Perceptual narrowing during infancy: a comparison of language and faces. Dev. Psychobiol. 2013;56(2):154–178. doi: 10.1002/dev.21177. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annual Review of Developmental Psychology. 2019;1(1):277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins E., Fernyhough C., Fradley E., Tuckey M. Rethinking maternal sensitivity: mothers’ comments on infants’ mental processes predict security of attachment at 12 months. J. Child Psychol. Psychiatry. 2001;42(5):637–648. doi: 10.1111/1469-7610.00759. [DOI] [PubMed] [Google Scholar]
- Mercure E., Evans S., Pirazzoli L., Goldberg L., Bowden-Howl H., Coulson-Thaker K., Beedie I., Lloyd-Fox S., Johnson M.H., MacSweeney M. Language experience impacts brain activation for spoken and signed language in infancy: insights from unimodal and bimodal bilinguals. Neurobiol. Language (Cambridge, Mass.) 2020;1(1):9–32. doi: 10.1162/nol_a_00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel P.M., Pereira L.O., Silveira P.P., Meaney M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019;61(10):1127–1133. doi: 10.1111/dmcn.14182. [DOI] [PubMed] [Google Scholar]
- Mize K.D., Jones N.A. Infant physiological and behavioral responses to loss of maternal attention to a social-rival. Int. J. Psychophysiol. 2012;83(1):16–23. doi: 10.1016/j.ijpsycho.2011.09.018. https://doi.org/10. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran G., Pederson D.R., Bento S. The Selected Works of Greg Moran. 2009. Maternal behavior Q-sort (MBQS) – overview, available materials and support.http://works.bepress.com/gregmoran/48 Retrieved from. [Google Scholar]
- Moran G. The Selected Works of Greg Moran. 2009. mini-MBQS-v revised mini-MBQS 25 item for video coding.http://works.bepress.com/gregmoran/ Retrieved from. [Google Scholar]
- Moszkowski R.J., Stack D.M. Infant touching behaviour during mother–infant face-to-face interactions. Infant Child Dev. 2007;16(3):307–319. doi: 10.1002/icd.510. [DOI] [Google Scholar]
- Moutsiana C., Fearon P., Murray L., Cooper P., Goodyer I., Johnstone T., Halligan S. Making an effort to feel positive: insecure attachment in infancy predicts the neural underpinnings of emotion regulation in adulthood. J. Child Psychol. Psychiatry. 2014;55(9):999–1008. doi: 10.1111/jcpp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsiana C., Johnstone T., Murray L., Fearon P., Cooper P.J., Pliatsikas C., Goodyer I., Halligan S.L. Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. J. Child Psychol. Psychiatry. 2015;56(5):540–548. doi: 10.1111/jcpp.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Wang D., Fox M.D., Yeo B.T., Sepulcre J., Sabuncu M.R., Shafee R., Lu J., Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77(3):586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. American Guidance Service; Circle Pines, NM: 1995. Mullen Scales of Early Learning. [Google Scholar]
- Murray L., Fiori-Cowley A., Hooper R., Cooper P. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512–2526. doi: 10.2307/1131637. [DOI] [PubMed] [Google Scholar]
- Murray L., De Pascalis L., Bozicevic L., Hawkins L., Sclafani V., Ferrari P.F. The functional architecture of mother-infant communication, and the development of infant social expressiveness in the first two months. Sci. Rep. 2016;6:39019. doi: 10.1038/srep39019. https://doi-org.ezp.lib.cam.ac.uk/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network Child care and mother–child interaction in the first three years of life. Dev. Psychol. 1999;35(6):1399–1413. doi: 10.1037/0012-1649.35.6.1399. [DOI] [PubMed] [Google Scholar]
- Nolvi S., Rasmussen J.M., Graham A.M., Gilmore J.H., Styner M., Fair D.A., Entringer S., Wadhwa P.D., Buss C. Neonatal brain volume as a marker of differential susceptibility to parenting quality and its association with neurodevelopment across early childhood. Dev. Cogn. Neurosci. 2020;45 doi: 10.1016/j.dcn.2020.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Belsky J., Putnam S., Crnic K. Infant emotionality, parenting, and 3-year inhibition: exploring stability and lawful discontinuity in a male sample. Dev. Psychol. 1997;33(2):218–227. doi: 10.1037//0012-1649.33.2.218. [DOI] [PubMed] [Google Scholar]
- Peltola M.J., van IJzendoorn M.H., Yrttiaho S. Attachment security and cortical responses to fearful faces in infants. Attach. Hum. Dev. 2020;22(2):174–188. doi: 10.1080/14616734.2018.1530684. [DOI] [PubMed] [Google Scholar]
- Perone S., Gartstein M.A. Relations between dynamics of parent-infant interactions and baseline EEG functional connectivity. Infant Behav. Dev. 2019;57 doi: 10.1016/j.infbeh.2019.101344. [DOI] [PubMed] [Google Scholar]
- Polan H.J., Ward M.J. Role of the mother’s touch in failure to thrive: a preliminary investigation. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33(8):1098–1105. doi: 10.1097/00004583-199410000-00005. [DOI] [PubMed] [Google Scholar]
- Pratt M., Zeev-Wolf M., Goldstein A., Feldman R. Exposure to early and persistent maternal depression impairs the neural basis of attachment in preadolescence. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;93:21–30. doi: 10.1016/j.pnpbp.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Quevedo K., Waters T.E.A., Scott H., Roisman G.I., Shaw D.S., Forbes E.E. Brain activity and infant attachment history in young men during loss and reward processing. Dev. Psychopathol. 2017;29(2):465–476. doi: 10.1017/S0954579417000116. [DOI] [PubMed] [Google Scholar]
- Rayson H., Bonaiuto J.J., Ferrari P.F., Murray L. Early maternal mirroring predicts infant motor system activation during facial expression observation. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-12097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L.W., Sanmugam S., Broekman B.F.P., Chen H. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5(10) doi: 10.1038/tp.2015.133. e668–e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Tan H.M., Shaun G.K., Sim L.W., Sanmugam S., Chong Y.S., Tan K.H., Shek L., Gluckman P.D., Chen H., Fortier M., Meaney M., Qiu A. An initial investigation of neonatal neuroanatomy, caregiving, and levels of disorganized behavior. Proc. Natl. Acad. Sci. 2019;116(34):16787–16792. doi: 10.1073/pnas.1900362116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.R., Leonard J.A., Robinson S.T., West M.R., Mackey A.P., Rowe M.L., Gabrieli J.D.E. Beyond the 30-Million-Word gap: children’s conversational exposure is associated with language-related brain function. Psychol. Sci. 2018;29(5):700–710. doi: 10.1177/0956797617742725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff A.J. In: Handbook of Infant Mental Health. Zeanah C.H., editor. Guilford; New York: 1993. Models of development and developmental risk; pp. 3–13. [Google Scholar]
- Schönbrodt F., Perugini M. At what sample size do correlations stabilize? J. Res. Pers. 2013;47(5):609–612. doi: 10.1016/j.jrp.2013.05.009. [DOI] [Google Scholar]
- Sethna V., Pote I., Wang S., Gudbrandsen M., Blasi A., McCusker C., Daly E., Perry E., Adams K., Kuklisova-Murgasova M., Busuulwa P., Lloyd-Fox S., Murray L., Johnson M.H., Williams S., Murphy D., Craig M.C., McAlonan G.M. Mother-infant interactions and regional brain volumes in infancy: an MRI study. Brain Struct. Funct. 2017;222(5):2379–2388. doi: 10.1007/s00429-016-1347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna V., Siew J., Pote I., Wang S., Gudbrandsen M., Lee C., Perry E., Adams K.P.H., Watson C., Kangas J., Stoencheva V., Daly E., Kuklisova-Murgasova M., Williams S.C.R., Craig M.C., Murphy D.G.M., McAlonan G.M. Father-infant interactions and infant regional brain volumes: a cross-sectional MRI study. Dev. Cogn. Neurosci. 2019;40 doi: 10.1016/j.dcn.2019.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish W.R., Cook T.D., Campbell D.T. Mifflin and Company; Houghton: 2002. Experimental and Quasi-experimental Designs for Generalized Causal Inference. [Google Scholar]
- Shaw D.S., Schonberg M., Sherrill J., Huffman D., Lukon J., Obrosky D., Kovacs M. Responsivity to offspring’s expression of emotion among childhood-onset depressed mothers. J. Clin. Child Adolesc. Psychol. 2006;35(4):490–503. doi: 10.1207/s15374424jccp3504_1. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., Fox N.A., Zeanah C.H., McLaughlin K.A., Nelson C.A. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl. Acad. Sci. 2012;109(32):12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion F., Regolin L., Bulf H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. U.S.A. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John A.M., Kao K., Liederman J., Grieve P.G., Tarullo A.R. Maternal cortisol slope at 6 months predicts infant cortisol slope and EEG power at 12 months. Dev. Psychobiol. 2017;59(6):787–801. doi: 10.1002/dev.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stets M., Stahl D., Reid V.M. A meta-analysis investigating factors underlying attrition rates in infant ERP studies. Dev. Neuropsychol. 2012;37(3):226–252. doi: 10.1080/87565641.2012.654867. [DOI] [PubMed] [Google Scholar]
- Stoecklein S., Hilgendorff A., Li M., Förster K., Flemmer A.W., Galiè F., Wunderlich S., Wang D., Stein S., Ehrhardt H., Dietrich O., Zou Q., Zhou S., Ertl-Wagner B., Liu H. Variable functional connectivity architecture of the preterm human brain: impact of developmental cortical expansion and maturation. Proc. Natl. Acad. Sci. U.S.A. 2020;117(2):1201–1206. doi: 10.1073/pnas.1907892117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler M.M., Sweet M.A., Carver L.J. Relations between mother-child interactions and the neural correlates of face processing in 6-Month-Olds. Infancy. 2007;11(1):63–86. doi: 10.1207/s15327078in1101_3. [DOI] [Google Scholar]
- Swingler M.M., Sweet M.A., Carver L.J. Brain–behavior correlations: Relationships between mother–stranger face processing and infants’ behavioral responses to a separation from mother. Dev. Psychol. 2010;46(3):669–680. doi: 10.1037/a0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler M.M., Perry N.B., Calkins S.D., Bell M.A. Maternal sensitivity and infant response to frustration: the moderating role of EEG asymmetry. Infant Behav. Dev. 2014;37(4):523–535. doi: 10.1016/j.infbeh.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler M.M., Perry N.B., Calkins S.D., Bell M.A. Maternal behavior predicts infant neurophysiological and behavioral attention processes in the first year. Dev. Psychol. 2017;53(1):13–27. doi: 10.1037/dev0000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D., Ioannidis J. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage. 2020 doi: 10.1016/j.neuroimage.2020.117164. [DOI] [PubMed] [Google Scholar]
- Tarabulsy G.M., Provost M.A., Bordeleau S., Trudel-Fitzgerald C., Moran G., Pederson D.R., Trabelsi M., Lemelin J.P., Pierce T. Validation of a short version of the maternal behavior Q-set applied to a brief video record of mother-infant interaction. Infant Behav. Dev. 2009;32(1):132–136. doi: 10.1016/j.infbeh.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Taylor-Colls S., Pasco Fearon R.M. The effects of parental behavior on infants’ neural processing of emotion expressions. Child Dev. 2015;86(3):877–888. doi: 10.1111/cdev.12348. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Polcari A., Anderson C.M., Navalta C.P., Kim D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27(1-2):33–44. doi: 10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Tharner A., Herba C.M., Luijk M.P., van Ijzendoorn M.H., Bakermans-Kranenburg M.J., Govaert P.P., Roza S.J., Jaddoe V.W., Hofman A., Verhulst F.C., Tiemeier H. Subcortical structures and the neurobiology of infant attachment disorganization: a longitudinal ultrasound imaging study. Soc. Neurosci. 2011;6(4):336–347. doi: 10.1080/17470919.2010.538219. [DOI] [PubMed] [Google Scholar]
- Vernetti A., Ganea N., Tucker L., Charman T., Johnson M.H., Senju A. Infant neural sensitivity to eye gaze depends on early experience of gaze communication. Dev. Cogn. Neurosci. 2018;34:1–6. doi: 10.1016/j.dcn.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouloumanos A., Hauser M.D., Werker J.F., Martin A. The tuning of human neonates’ preference for speech. Child Dev. 2010;81:517–527. doi: 10.1111/j.1467-8624.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- Wan M.W., Green J., Elsabbagh M., Johnson M., Charman T., Plummer F., BASIS Team Parent-infant interaction in infant siblings at risk of autism. Res. Dev. Disabil. 2012;33(3):924–932. doi: 10.1016/j.ridd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Wan M.W., Green J., Elsabbagh M., Johnson M., Charman T., Plummer F., Team B.A.S.I.S. Quality of interaction between at-risk infants and caregiver at 12-15 months is associated with 3-year autism outcome. J. Child Psychol. Psychiatry. 2013;54(7):763–771. doi: 10.1111/jcpp.12032. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang H., Wee C.Y., Lee A., Poh J.S., Chong Y.S., Tan K.H., Gluckman P.D., Yap F., Fortier M.V., Rifkin-Graboi A., Qiu A. Maternal sensitivity predicts anterior hippocampal functional networks in early childhood. Brain Struct. Funct. 2019;224(5):1885–1895. doi: 10.1007/s00429-019-01882-0. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xu Y., Zhao T., Xu Z., He Y., Liao X. Individual uniqueness in the neonatal functional connectome. Cereb. Cortex. 2021;31(8):3701–3712. doi: 10.1093/cercor/bhab041. [DOI] [PubMed] [Google Scholar]
- Waters E., Deane K. In: Monographs of the Society for Research in Child Development, 50, nos. 1-2. Bretherton I., Waters E., editors. 1985. Defining and assessing individual differences in attachment relationships: Q-methodology and the organization of behavior in infancy and early childhood; pp. 41–65. [Google Scholar]
- Wen D.J., Soe N.N., Sim L.W., Sanmugam S., Kwek K., Chong Y.S., Gluckman P.D., Meaney M.J., Rifkin-Graboi A., Qiu A. Infant frontal EEG asymmetry in relation with postnatal maternal depression and parenting behavior. Transl. Psychiatry. 2017;7(3) doi: 10.1038/tp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby A.M., Allen L., Cleary J., Kublin K., Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. J. Speech Lang. Hear. Res. 2002;45(6):1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Whipple N., Bernier A., Mageau G.A. Broadening the study of INFANT security OF attachment: maternal Autonomy-support in the context of Infant Exploration. Soc. Dev. 2010;20(1):17–32. doi: 10.1111/j.1467-9507.2010.00574.x. [DOI] [Google Scholar]
- Whittle S., Vijayakumar N., Dennison M., Schwartz O., Simmons J.G., Sheeber L. Observed measures of negative parenting predict brain development during adolescence. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Cao M., Liao X., Xia M., Wang X., Jeon T., Ouyang M., Chalak L., Rollins N., Huang H., He Y. Development and emergence of individual variability in the functional connectivity architecture of the preterm human brain. Cerebral cortex (New York, N.Y.: 1991) 2019;29(10):4208–4222. doi: 10.1093/cercor/bhy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Chronaki G., Schiessl I., Wan M.W., Abel K.M. Is infant neural sensitivity to vocal emotion associated with mother-infant relational experience? PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212205. https:// doi.org/10.1371/journal.pone.0212205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski B.A., Gennatas E.D., Zhou J., Seeley W.W. Network-level structural covariance in the developing brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107(42):18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.