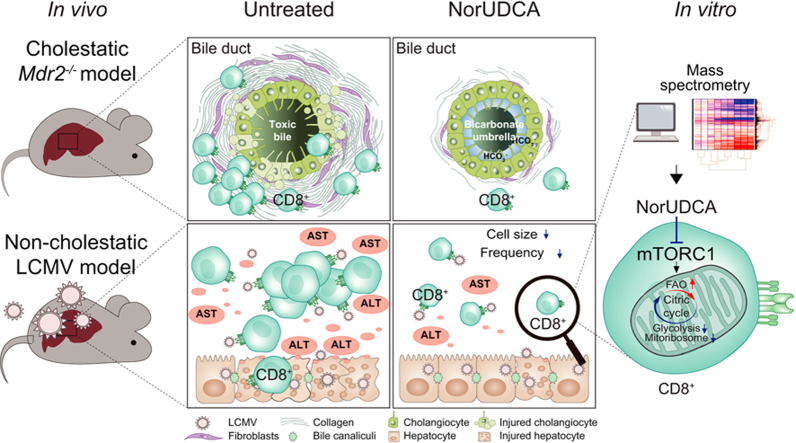
Abstract
Background & Aims
24-Norursodeoxycholic acid (NorUDCA) is a novel therapeutic bile acid used to treat immune-mediated cholestatic liver diseases, such as primary sclerosing cholangitis (PSC), where dysregulated T cells including CD8+ T cells contribute to hepatobiliary immunopathology. We hypothesized that NorUDCA may directly modulate CD8+ T cell function thus contributing to its therapeutic efficacy.
Methods
NorUDCA’s immunomodulatory effects were first studied in Mdr2-/- mice, as a cholestatic model of PSC. To differentiate NorUDCA’s immunomodulatory effects on CD8+ T cell function from its anticholestatic actions, we also used a non-cholestatic model of hepatic injury induced by an excessive CD8+ T cell immune response upon acute non-cytolytic lymphocytic choriomeningitis virus (LCMV) infection. Studies included molecular and biochemical approaches, flow cytometry and metabolic assays in murine CD8+ T cells in vitro. Mass spectrometry was used to identify potential CD8+ T cell targets modulated by NorUDCA. The signaling effects of NorUDCA observed in murine cells were validated in circulating T cells from patients with PSC.
Results
NorUDCA demonstrated immunomodulatory effects by reducing hepatic innate and adaptive immune cells, including CD8+ T cells in the Mdr2-/- model. In the non-cholestatic model of CD8+ T cell-driven immunopathology induced by acute LCMV infection, NorUDCA ameliorated hepatic injury and systemic inflammation. Mechanistically, NorUDCA demonstrated strong immunomodulatory efficacy in CD8+ T cells affecting lymphoblastogenesis, expansion, glycolysis and mTORC1 signaling. Mass spectrometry identified that NorUDCA regulates CD8+ T cells by targeting mTORC1. NorUDCA’s impact on mTORC1 signaling was further confirmed in circulating PSC CD8+ T cells.
Conclusions
NorUDCA has a direct modulatory impact on CD8+ T cells and attenuates excessive CD8+ T cell-driven hepatic immunopathology. These findings are relevant for treatment of immune-mediated liver diseases such as PSC.
Lay summary
Elucidating the mechanisms by which 24-norursodeoxycholic acid (NorUDCA) works for the treatment of immune-mediated liver diseases, such as primary sclerosing cholangitis, is of considerable clinical interest. Herein, we uncovered an unrecognized property of NorUDCA in the immunometabolic regulation of CD8+ T cells, which has therapeutic relevance for immune-mediated liver diseases, including PSC.
Keywords: Primary Sclerosing Cholangitis, Immune-mediated liver disease, bile acid, mass spectrometry, CD8+ T cells, metabolism, (phospho-) proteome
Graphical abstract
Highlights
-
•
NorUDCA reduces the number of hepatic innate and adaptive immune cells, including CD8+ T cells, in the Mdr2-/- model of sclerosing cholangitis.
-
•
Independent of its anticholestatic effects, NorUDCA exhibits direct immunomodulatory properties in CD8+ T cells.
-
•
NorUDCA targets mTORC1 to modulate the (phospho-) proteomic and metabolic landscape of CD8+ T cells.
-
•
Circulating CD8+ T cells from PSC patients show enhanced clonal expansion and mTORC1 activity which are restricted by NorUDCA.
Introduction
CD8+ T cells are critical players in cell-mediated adaptive immunity, protecting against microbial infections and cancer.1 However, when CD8+ T cell responses and tissue infiltration become dysregulated and excessive, CD8+ T cells can turn into immunopathological factors driving hepatic inflammation and damage.2 As such, CD8+ T cell-mediated cytotoxicity and tissue damage play an important role in viral, autoimmune or immune-mediated liver diseases.3,4 Therefore, identifying novel therapies to control detrimental CD8+ T cell inflammatory reactions is pivotal to counteract the increasing burden of autoimmune liver diseases and the corresponding need for liver transplantation, reflecting the limited effectiveness of current therapeutic options.5
24-Norursodeoxycholic acid (NorUDCA) is a novel therapeutic bile acid (BA) whose pharmacological mechanisms profoundly differ from its biochemical parent compound ursodeoxycholic acid (UDCA) which has been used with variable success in a wide range of cholestatic and metabolic liver diseases.6 In a recent phase II study, NorUDCA has demonstrated promising results in treating primary sclerosing cholangitis (PSC),7 an immune-mediated cholestatic liver disease for which effective medical therapy is lacking.8 Dysregulated T cells including CD8+ T cells are thought to contribute to the immunopathogenesis of PSC.4 Therefore, we hypothesized that NorUDCA’s therapeutic mechanism of action may be attributed to potential immunomodulatory potency on CD8+ T cells besides its well-established anticholestatic efficacy.9
We first explored whether NorUDCA possesses immunomodulatory efficacy on CD8+ T cells in vivo in the cholestatic model of Mdr2-/- mice mimicking PSC. Second, to differentiate from its anticholestatic effects, NorUDCA’s direct impact on CD8+ T cell effector function was also investigated in a non-cholestatic model based on acute non-cytolytic lymphocytic choriomeningitis virus (LCMV) infection, in which hepatic injury and systemic inflammation are predominantly mediated by effector CD8+ T cells.[10], [11], [12] Further mechanistic studies were performed using molecular and biochemical approaches, flow cytometry and metabolic assays in primary murine CD8+ T cells in vitro. Mass spectrometry (MS) was used to identify potential targets modulated by NorUDCA in CD8+ T cells. Human peripheral T cells from healthy volunteers and patients with PSC were used to validate the signaling effects of NorUDCA observed in murine systems.
Materials and methods
Mdr2(Abcb4)-/- mouse model
Multidrug resistance gene 2 Mdr2(Abcb4)-/- (FVB/N) male mice were fed with NorUDCA- or UDCA (0.5%, w/w)-containing diets for 12 days starting at the age of 8 weeks after birth, a time point when sclerosing cholangitis is already fully established.13 For comparison, wild-type (WT) FVB/N and Mdr2(Abcb4)-/- (FVB/N) male mice received standard chow diet. Experiments were approved by the Federal Ministry for Science and Research at Medical University of Vienna (MUV) (BMWFW-66.009/0008-WF/V/3b/2015).
LCMV clone13 mouse model
Eight-week-old C57BL/6J male mice (Janvier labs, France) were pre-fed with standard chow or a 0.5% (w/w) NorUDCA-/UDCA-supplemented diet for 10 days or treated with rapamycin (600 μg/kg) i.p. 1 day before being infected14,15 with 2x106 focus-forming units of LCMV strain clone 13 i.v.16 Mice were continued on a NorUDCA-/UDCA-containing diet or standard chow or treated with rapamycin i.p. daily14,15 and sacrificed at day 10 following infection, a time point when CD8+ T cell inflammation response reaches a peak in vivo.11 Uninfected naïve mice on standard chow diet were studied as controls. Viral titers were determined by focus-forming assay as previously described.16 Experiments were approved by the Federal Ministry for Science and Research at Medical University of Vienna (BMWFW-66.009/0361-WF/V/3b/2017).
CD8+ T cell isolation, activation and proliferation assay
CD8+ T cells from peripheral lymph nodes and spleens of C57BL/6J male mice were purified by negative depletion (purity >90%) and submitted to activation and proliferation assays as described in the supplementary material.
Flow cytometric analysis
Extracellular and intracellular staining of T cells from in vivo and in vitro models for flow cytometry were performed as described in the supplementary material.
Calcium influx
Calcium influx assay was performed as described in the supplementary material.
Histopathology and multicolor immunofluorescence staining
Histopathology and multicolor immunofluorescence staining was performed as described previously.13
Serum analysis
Serum biochemistry was performed as described previously.9
Compound treatments
Concentrations of NorUDCA (500 μM) and UDCA (50 μM) for in vitro experiments were selected to mimic the in vivo serum BA level of mice on a 0.5% (w/w) NorUDCA- or UDCA-supplemented diet9 (Fig. 2B). Concentrations of NorUDCA and UDCA selected for in vitro experiments were additionally assessed by viability assays of CD8+ T cells (Fig. S4B). Other compounds were used at the indicated concentrations: Rapamycin (mTORC1 inhibitor; 100 nM), Ly294002 (PI3K inhibitor; 10 μM).
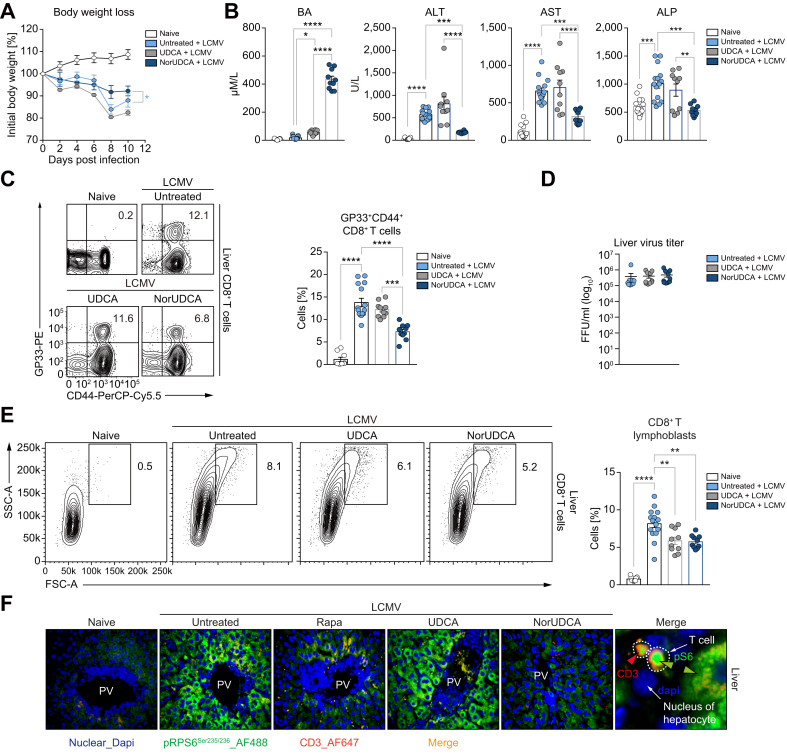
Fig. 2.
NorUDCA suppresses cell size and frequency of intrahepatic effector CD8+ T cells in LCMV clone 13 model.
(A) Changes in body weight over time. (B) Serum BA, ALT, AST, ALP. (C) Representative plots and quantitative analysis of intrahepatic virus-specific CD8+ T cells. (D) Liver LCMV virus titer. (E) Representative plots and quantitative analysis of intrahepatic CD8+ T cells. Cells recognizing the LCMV-GP33 peptide in the context of MHC class I presentation are labeled as GP33+. (F) Representative high-power-field multicolor immunofluorescence staining of CD3 and pRPS6Ser235/236 of liver slides of indicated groups. Data are summary of 3 independent experiments. At least 3 biologically independent animals were used per group during experiments. Quantitative data are presented as mean±SE. p values were calculated by one-way ANOVA corrected with Tukey post-hoc test and in A were calculated by two-way ANOVA. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transaminase; BA, bile acid; LCMV, lymphocytic choriomeningitis virus; NorUDCA, 24-norursodeoxycholic acid; PV, portal vein; Rapa, rapamycin.
Gene expression analysis
RNA isolation from primary murine CD8+ T cell culture, cDNA synthesis and real-time PCR were performed as described previously.9 Gene expression was calculated relative to Hprt. Primer sequences are provided in Table S2.
Immunoblot analysis
Immunoblot analysis was performed using standard protocols as previously described17 using antibodies shown in Table S1.
Seahorse metabolic assay
Oxygen consumption rates and extracellular acidification rates of activated CD8+ T cells ± NorUDCA were measured as previously described.18
Proteomics sample preparation and phosphopeptide enrichment
Primary murine CD8+ T cells activated with anti-CD3 and anti-CD28 for 24 h ± NorUDCA or rapamycin were harvested in 3 biological replicates for each condition and prepared as described in the supplementary materials.
Proteomics and phosphoproteomics data analysis
Proteomics and phosphoproteomics data analysis were performed as described in the supplementary materials.
Human T cell experiments
Three independent experiments with peripheral T cells obtained from patients with PSC (also suffering from associated inflammatory bowel disease) and age- and sex-matched healthy volunteers were performed in parallel following the Declaration of Helsinki and approved by the Ethics Committee of the MUV (747/2011 and 2001/2018). Experimental details were described in the supplementary materials.
Quantification and statistical analysis
All values are expressed as mean ± SEM and were statistically analyzed as detailed in the figure legends using GraphPad Prism v.7.0 (La Jolla, CA, USA). A p value of less than 0.05 was considered statistically significant and indicated as follows: ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001.
For further experimental details please visit the supplementary materials.
Results
NorUDCA exhibits immunomodulatory effects on CD8+ T cells in the cholestatic Mdr2-/- mouse model of sclerosing cholangitis
Previously we reported that 4-week NorUDCA feeding reversed sclerosing cholangiopathy in the cholestatic Mdr2-/- mice by inducing bicarbonate-rich hypercholeresis which counteracts BA toxicity and reinforces the biliary “bicarbonate umbrella”.9,19 However, the potential impact of NorUDCA on hepatic immune cell composition in Mdr2-/- mice has not yet been explored. Therefore, we performed short-term (12 days) NorUDCA feeding on 8-week-old Mdr2-/- mice with fully established biliary injury and fibrosis, to investigate if NorUDCA possesses immunomodulatory effects, in a setting where the disease phenotype in Mdr2-/- mice has not yet been completely reversed by NorUDCA to allow sufficient hepatic immune cell numbers for analysis. Its parent compound UDCA was used for comparison.
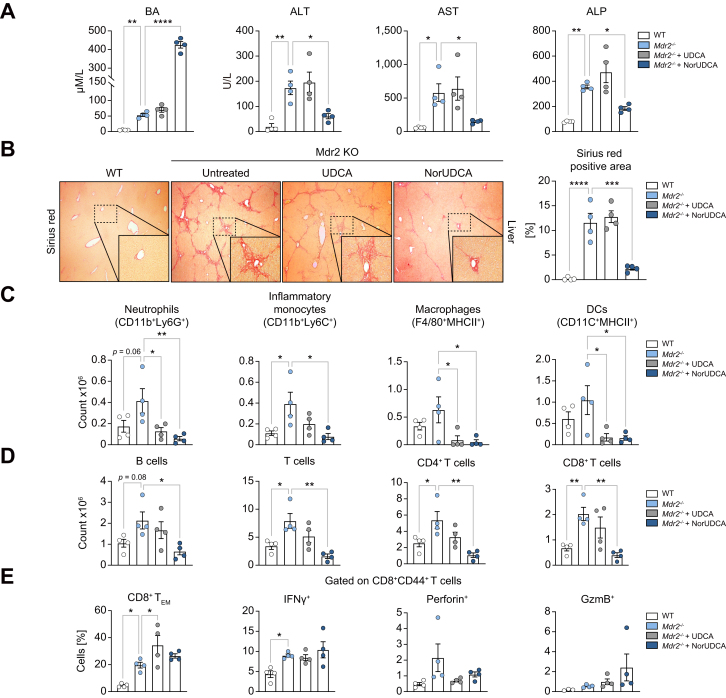
Increased plasma BA levels indicated NorUDCA and UDCA absorbance in Mdr2-/- mice, notably NorUDCA induced around 7-fold higher concentrations than UDCA (Fig. 1A). Mdr2-/- mice showed elevated hepatobiliary injury and fibrosis which were attenuated by NorUDCA in contrast to UDCA (Fig. 1A,B). Besides an increased number of hepatic innate immune cells, we also observed increased numbers of hepatic adaptive immune cells, including B and T cells (both CD4+ and CD8+ T cells) in Mdr2-/- mice compared to WT mice in line with published reports20 (Fig. 1C,1D, see Fig. S1A for gating strategy). NorUDCA demonstrated general anti-inflammatory effects by reducing the number of hepatic innate and adaptive immune cells (Fig. 1C, D). Since T-lymphocytes, especially effector CD8+ T cells critically contribute to the pathogenesis of the Mdr2-/- model,20 we particularly analyzed the impact of NorUDCA on effector CD8+ T cells. NorUDCA decreased the number of hepatic CD8+ T cell populations as well as interferon-γ-producing and perforin-producing CD8+ T cells in Mdr2-/- mice (Fig. 1D, Fig. S1B). We next investigated whether NorUDCA affects effector differentiation of hepatic CD8+ T cells by analyzing naïve/effector composition and effector functions based on relative frequencies. While the absolute CD8+ T cell numbers are decreased, NorUDCA-treated Mdr2-/- mice showed normal differentiation of hepatic effector CD8+ T cells, as reflected by similar frequencies of effector memory cells expressing key cytokines and cytolytic molecules (Fig. 1E). Collectively, NorUDCA shows potent immunomodulatory efficacy in Mdr2-/- mice, especially on CD8+ T cells.
Fig. 1.
Short-term NorUDCA treatment demonstrates immunomodulatory efficacy on CD8+ T cells in cholestatic Mdr2-/- model of spontaneous sclerosing cholangiopathy.
(A) Serum BA, ALT, AST, ALP. (B) Representative Sirius red staining of liver sections and corresponding quantitative analysis are shown. (C,D) Quantitative analysis of hepatic innate and adaptive immune cell distribution of indicated groups. (E) Impact of BA treatment on hepatic effector differentiation of CD8+ T cells in Mdr2-/- mice. Data are representative of 2 independent experiments. 4 biologically independent animals were used per group during experiments. Quantitative data are presented as mean±SE. p values were calculated by one-way ANOVA corrected for multiple comparisons with Dunnett test using Mdr2-/- mice as reference. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗p <0.001, ∗∗∗∗p <0.0001. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BA, bile acid; DC, dendritic cells; GzmB, GranzymeB; NorUDCA, 24-norursodeoxycholic acid; TEM, TEffector Memory.
NorUDCA reduces frequency and cell size of intrahepatic effector CD8+ T cells with alleviated hepatic injury in LCMV clone 13 model
The immunomodulatory efficacy of NorUDCA on CD8+ T cells in Mdr2-/- mice incited us to further explore NorUDCA’s direct impact on CD8+ T cell biology in a mouse model of CD8+ T cell-driven liver injury lacking overt cholestasis (to counteract the potentially confounding indirect anticholestatic effects of NorUDCA). Therefore, we studied the direct effect of NorUDCA on effector CD8+ T cell immune responses in a murine model of LCMV clone 13 infection, specifically focusing on the acute phase of the infection on day 10 when escalation of hepatic injury had been widely reported.[10], [11], [12]
In line with previous reports,10,12,16,18 we observed a significant loss of body weight and elevated level of hepatic injury parameters in mice following LCMV infection, which were attenuated by NorUDCA, while UDCA showed only minimal effects (Fig. 2A, 2B). Increased plasma BA levels indicated NorUDCA and UDCA absorption in vivo; of note, NorUDCA induced around 10-fold higher BA concentrations than UDCA in naïve mice (Fig. 2B).
We observed that the frequency of intrahepatic CD8+ T cells positive for the major LCMV epitope glycoprotein-33 (GP33) in NorUDCA-treated infected mice was lower than untreated or UDCA-treated infected mice (Fig. 2C, gating strategy see Fig. S2A). Despite the change of frequency of intrahepatic virus-specific CD8+ T cells in NorUDCA-treated infected mice, the effector function of virus-specific CD8+ T cells remained unchanged, as evidenced by the comparable expression of cytolytic molecules like granzyme B and perforin in intrahepatic virus-specific effector CD8+ T cells of infected mice with and without NorUDCA treatment (Fig. S2B). Despite the lower number of intrahepatic virus-specific CD8+ T cells, the hepatic viral loads were not affected by NorUDCA treatment in infected mice (Fig. 2D).
Of note, we observed that the cell size of intrahepatic CD8+ T cells isolated from NorUDCA-treated infected mice was smaller than that of untreated-infected mice (Fig. 2E). Cell size is controlled by the mammalian target of rapamycin (mTOR), a conserved serine/threonine kinase that integrates environmental and physiological signals.21 The mTOR kinase associates with various regulatory proteins and forms 2 distinct complexes, mTORC1 and mTORC2, which are modulated by several upstream signaling pathways.21 mTORC1 and mTORC2 have distinct functions in regulating downstream cellular processes, specifically cell growth, expansion, ribosome biogenesis and glycolysis for mTORC1 and cell survival for mTORC2.22 mTORC1 promotes initiation and elongation of translation via ribosomal protein S6 kinase 1 (S6K1) which phosphorylates ribosomal protein S6 (RPS6). Phosphorylation of RPS6 at Ser235/236 (pRPS6Ser235/236) is widely used as readout for mTORC1 activity. We included rapamycin as an mTORC1 inhibitor control (at a concentration used in a previous LCMV infection study15). We detected an increase of hepatic infiltrating pRPS6Ser235/236-positive CD3+ T cells in mice upon LCMV infection, which were decreased when treated with rapamycin or NorUDCA (Fig. 2F). This suggests that mTORC1 is repressed by rapamycin and NorUDCA. Additionally, we also detected a downregulation of pRPS6Ser235/236 in hepatocytes from rapamycin- or NorUDCA-treated infected mice (Fig. 2F), suggesting that the inhibitory efficacy of rapamycin and NorUDCA is not limited to T cells but also expands to other cell types. In contrast, pRPS6Ser235/236 levels were not altered in UDCA-treated infected mice, indicating that UDCA does not impact mTORC1 signaling in infected mice (Fig. 2F). In summary, our results indicate that NorUDCA treatment during the acute phase of LCMV infection led to a reduction in effector CD8+ T cell-driven inflammation and injury.
NorUDCA modulates effector CD8+ T cells systemically in the LCMV clone 13 model
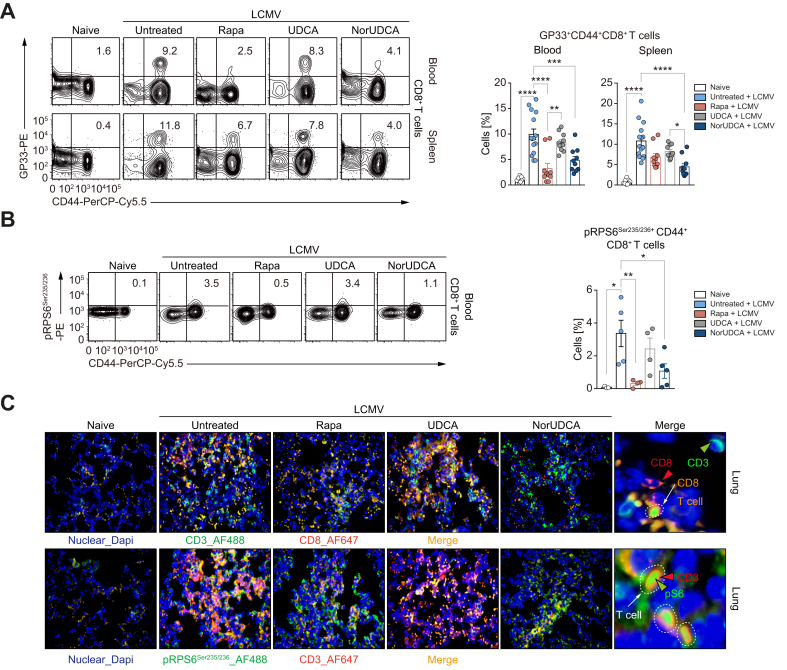
To determine whether the observed immunomodulatory activity of NorUDCA observed in the LCMV model is restricted to the liver or can be extended to extrahepatic tissues, we analyzed NorUDCA’s impact on CD8+ T cells from the blood and spleen. Similar to our observations in the liver, NorUDCA reduced the frequency of virus-specific effector CD8+ T cells in both the blood and spleens of infected mice, while UDCA had no impact (Fig. 3A). Further we found that, like rapamycin, NorUDCA decreased the phosphorylation (pSer235/236) of mTORC1’s downstream target RPS6 in circulating effector CD8+ T cells of infected mice (Fig. 3B).
Fig. 3.
NorUDCA exerts strong systemic immunometabolic modulatory properties in LCMV clone 13 model.
(A) Representative plots of virus-specific CD8+ T cells from peripheral blood and spleen. Cells recognizing the LCMV-GP33 peptide in the context of MHC class I presentation are labeled as GP33+. Quantitative analysis is shown alongside. (B) pRPS6Ser235/236 expression on blood CD8+ T cells. Quantitative analysis is shown alongside. (C) Representative high-power field multicolor immunofluorescence staining of CD3/CD8 and CD3/pRPS6Ser235/236 (lower) of lung slides of indicated groups. Data are pooled from 3 independent experiments. At least 3 biologically independent animals were used per group during experiments. Quantitative data are presented as mean±SE. p values were calculated by one-way ANOVA corrected with Tukey post-hoc test. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗p <0.001, ∗∗∗∗p <0.0001. LCMV, lymphocytic choriomeningitis virus; NorUDCA, 24-norursodeoxycholic acid; Rapa, rapamycin.
Moreover, we also investigated whether NorUDCA impacts on mTORC1 signaling in effector CD8+ T cells ex vivo. To mimic the in vivo activation, we isolated splenocytes from infected mice treated with or without NorUDCA, UDCA or rapamycin and re-stimulated the cells with LCMV-GP33 peptides. pRPS6Ser235/236 was assessed in effector CD8+ T cells (Fig. S3A shows the experimental scheme). pRPS6Ser235/236 levels were lower in splenic effector CD107a/b+CD8+ T cells (CD107a/b is a degranulation marker indicative of effector CD8+ T cell function) from NorUDCA-treated infected mice upon re-stimulation, reflecting reduced mTORC1 activity (Fig. S3B).
Of note, upon ex vivo re-stimulation, splenic effector CD8+ T cells from NorUDCA-treated infected mice expressed reduced levels of tumor necrosis factor-α. However, interferon-γ expression, which is essential for maintaining efficient protective immunity against LCMV,23 was comparable (Fig. S3C), indicating intact antiviral immunity. This was further supported by the unchanged splenic virus titer in NorUDCA-treated infected mice (Fig. S3D).
Additionally, we analyzed CD8+ T cell infiltration in the lung as another extrahepatic organ. Multicolor immunofluorescence microscopy revealed that NorUDCA not only reduced infiltrating CD8+ T cells but also T cells displaying pRPS6Ser235/236 in the lung parenchyma of infected mice, further suggesting a systemic modulatory effect of NorUDCA on mTORC1 during CD8+ T cell differentiation in vivo (Fig. 3C).
NorUDCA reduces CD8+ T cell blasting and expansion associated with attenuated mTORC1 signaling in vitro
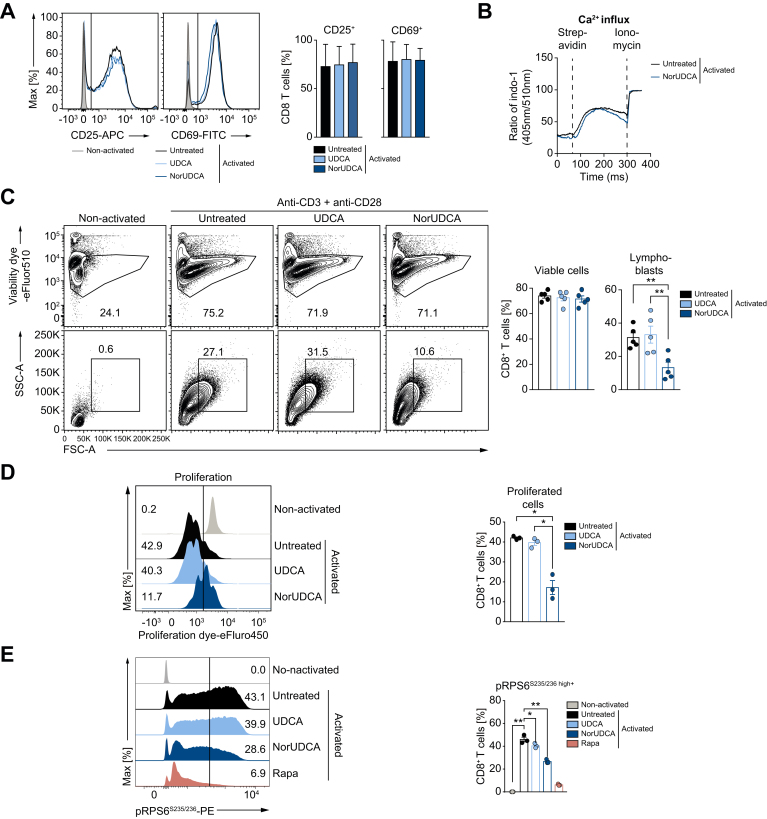
To gain further mechanistic insights about NorUDCA’s direct impact on CD8+ T cell signaling, function and metabolism (see Fig. S4A for gating strategy) we activated primary murine CD8+ T cells from spleen and lymph nodes in vitro in the presence of NorUDCA or UDCA with the concentrations matching the in vivo concentrations in our LCMV model system without affecting cell viabilities (Fig. 2B, Fig. S4B).
Upon antigen recognition, naïve CD8+ T cells undergo a complex activation, blasting (increasing cell size), proliferation and differentiation process leading to establishment of a large pool of various effector CD8+ T cells required to mount effective immunity.1 Both NorUDCA and UDCA showed no effects on T cell receptor-proximal CD8+ T cell activation as reflected by unchanged upregulation of T cell activation markers CD25 and CD69 and normal Ca2+ influx (Fig. 4A, 4B). However, CD8+ T cell blasting (Fig. 4C) and clonal expansion (Fig. 4D) were reduced by NorUDCA without affecting cell viability (Fig. 4C) compared to cells without treatment or treated with UDCA. In accordance with our in vivo mTORC1 signaling finding, NorUDCA decreased the frequency of CD8+ T cells displaying high level of pRPS6S235/236 (Fig. 4E), indicating that NorUDCA impairs mTORC1 activity by acting directly on CD8+ T cells during their differentiation to effector cells.
Fig. 4.
NorUDCA attenuates murine CD8+ T cells blasting and expansion with blunted mTORC1 signaling.
(A) Primary murine CD8+ T cells were activated for 24 h using anti-CD3 and anti-CD28 mAbs ± compound treatment as indicated. Histograms show CD25 and CD69 expression. Quantification is shown alongside. (B) Graph represents calcium influx in activated CD8+ T cells ± NorUDCA. (C) Primary murine CD8+ T cells were activated for 36 h under indicated condition. Numbers indicate frequencies of viable cells or lymphoblasts. Quantitative analysis is shown alongside. (D) Proliferation of primary murine CD8+ T cells treated as in (C) by dilution of intracellular proliferation dye. Numbers show frequency of proliferated cells. Quantitative analysis is shown alongside. (E) Histograms depict pRPS6S235/236 in CD8+ T cells treated as in (C). Numbers in the histograms indicate frequencies of cells with high pRPS6S235/236 expression. Quantitative analysis is shown alongside. Data are representative of 3 independent experiments. Quantitative data are presented as mean ± SE. p values were calculated by one-way ANOVA corrected with Tukey post-hoc test. ∗p <0.05, ∗∗p <0.01. mAbs, monoclonal antibodies; NorUDCA, 24-norursodeoxycholic acid; Rapa, rapamycin.
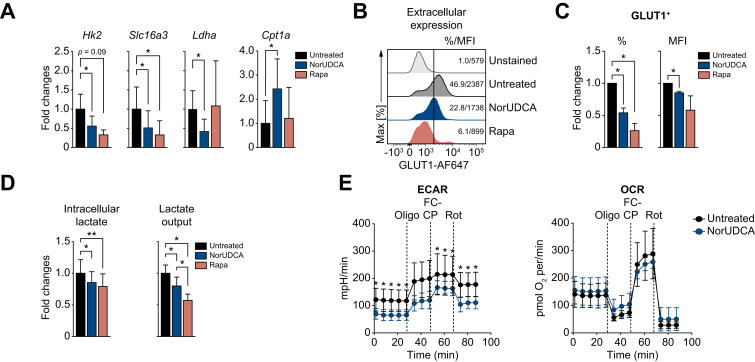
NorUDCA redirects activation-induced metabolic reprograming in CD8+ T cells
Since mTORC1 is required for the expression of glucose transporters and enzymes that control glycolysis in CD8+ T cells,17 we explored whether NorUDCA alters the metabolic profile of CD8+ T cells. In vitro we found that NorUDCA repressed the expression of genes promoting glycolysis in activated CD8+ T cells (Fig. 5A). Surface glucose transporter 1 (GLUT1 or SLC2A1) expression on CD8+ T cells was also reduced by NorUDCA (Fig. 5B, 5C). Furthermore, NorUDCA reduced intracellular synthesis and output of lactate in CD8+ T cells, an end product of the glycolytic pathway (Fig. 5D). To explore whether inhibition of glycolysis enforces fatty acid β-oxidation (FAO) in CD8+ T cells,24 we tested the expression of genes regulating FAO in CD8+ T cells. NorUDCA upregulated carnitine palmitoyltransferase 1a (Cpt1a) expression (Fig. 5A), a rate-limiting enzyme in FAO.24
Fig. 5.
NorUDCA demonstrates immunometabolic actions in modulating murine CD8+ T cell in vitro.
(A) Quantitative RT-qPCR analysis of expression of indicated genes (normalized to Hprt and untreated groups) in primary murine CD8+ T cells activated for 24 h ± compound treatments as indicated. (B) Representative histograms depicting GLUT1 extracellular expression of cells cultured as in (A) and treated as indicated. (C) Quantitative analysis is shown alongside. (D) Quantification of intra- and extracellular output of lactate from cells cultured as in (A). (E) Real-time changes in the ECAR (left) and OCR (right) of activated CD8+ T cells after treatment with Oligo, FCCP, and Rot ± NorUDCA. Data are either representative or show the summary of 3 independent experiments. Summary data are presented as mean ± SE. p values were calculated by one-way ANOVA corrected with Tukey post-hoc test. ∗p <0.05, ∗∗p <0.01. ECAR, extracellular acidification rate; FCCP, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone; NorUDCA, 24-norursodeoxycholic acid; OCR, oxygen consumption rate; Oligo, oligomycin; Rapa, rapamycin; Rot, rotenone; RT-qPCR, reverse transcription quantitative PCR.
To further assess the functional impact of NorUDCA on glycolysis and mitochondrial respiratory activity, we performed extracellular metabolic flux measurements to determine real-time changes in extracellular acidification rate (ECAR) and oxygen consumption rate (OCR). NorUDCA decreased the glycolytic activity in CD8+ T cells, indicated by a lower level of ECAR, while OCR (indicator for mitochondria oxidative phosphorylation) was not affected in this setting (Fig. 5E)).
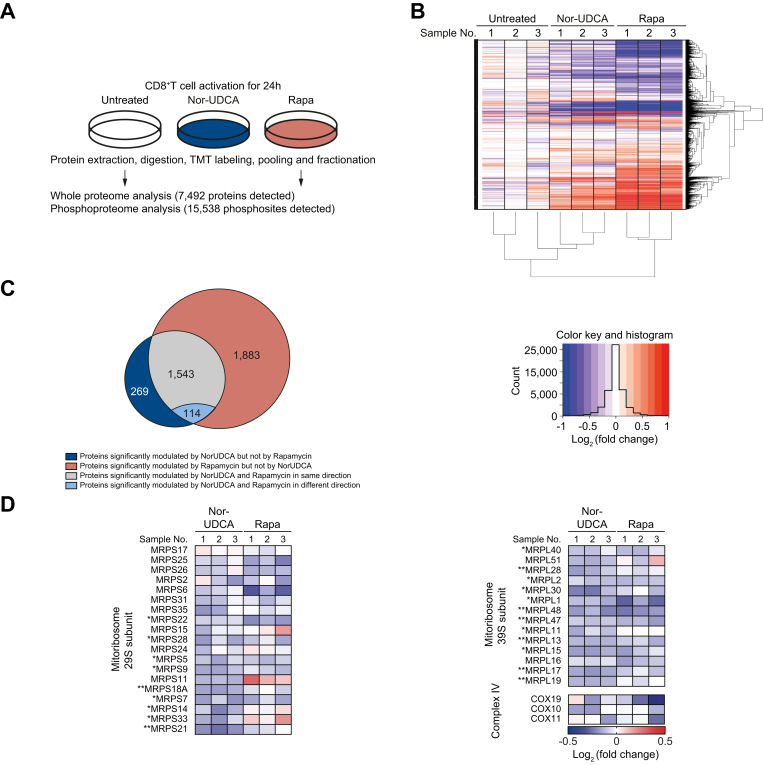
NorUDCA reshapes mTORC1 associated (phospho-)proteome and metabolic landscape in CD8+ T cells upon activation
To mechanistically understand how NorUDCA impacts on mTORC1, we performed quantitative high-resolution MS using murine CD8+ T cells treated with NorUDCA or rapamycin. We first focused on comparing NorUDCA with rapamycin regarding their modulation of the mTORC1-associated (phospho-) proteome in CD8+ T cells. In total 7,492 proteins and 15,538 phosphorylated sites per biological replicate were identified (Fig. 6A). Cluster analysis of differentially expressed proteins demonstrated that NorUDCA had similarities with rapamycin in reshaping the overall proteome of CD8+ T cells (Fig. 6B). Analysis revealed that 80.1% (1,543 of 1,926 proteins) of the proteins significantly modulated by NorUDCA were also modulated by rapamycin in the same direction, further revealing mTORC1 as a main pathway targeted by NorUDCA. Interestingly, 19.1% (383 of 1,926 proteins) of proteins were uniquely modulated by NorUDCA (Fig. 6C). Further functional annotations by Hallmark, GO (gene ontology) and KEGG databases identified metabolic pathways shared by NorUDCA and rapamycin, as well as pathways uniquely affected by NorUDCA (Fig. S5, Table S3).
Fig. 6.
NorUDCA shows similar impacts on CD8+ T cell proteomic profile and reduces mitoribosome protein abundance in CD8+ T cells compared to rapamycin.
(A) Scheme depicting the experimental approach. (B) Hierarchical cluster analysis of differentially regulated proteins under indicated conditions are shown in the heat map represented color-coded expression levels following Log2 value of ratio between protein content of NorUDCA- or rapamycin-treated CD8+ T cells and that of untreated-cells (n = 3). (C) Venn diagrams display the overlap of identified proteins between NorUDCA- and rapamycin-treated groups. (D) Heat map of mitoribosome protein abundance changes upon NorUDCA or rapamycin treatment (both normalized to untreated group) of CD8+ T cells cultured as in (A). Data were obtained from proteomics analysis using biological replicates from 3 independent experiments. Asterisks next to protein labeling represent significance of comparison between NorUDCA and untreated groups. Data show the summary of 3 independent experiments. p values in (D) were calculated among the comparison between untreated- and NorUDCA-treated groups by two-way ANOVA. ∗p <0.05, ∗∗p <0.01. NorUDCA, 24-norursodeoxycholic acid; Rapa, rapamycin.
We next studied mTORC1-associated metabolic proteomes modulated by NorUDCA in CD8+ T cells. In agreement with the findings described above, we detected a reduction of hexokinase 2 (Fig. S6) and additional proteins crucial for glycolysis in MS data set of NorUDCA-treated CD8+ T cells (Fig. S6). Moreover, NorUDCA enhanced protein abundance of FA transporters and enzymes, such as long-chain FA transport protein 4 (SLC27A4), fatty-acyl-CoA synthase, CPT1α and CPT2 (Fig. S6). In contrast, FA synthesis was reduced as revealed by the decreased abundance of FA synthase and increased phosphorylation of acetyl-CoA carboxylase at the S117 residue, which inhibits enzyme activity (Fig. S6). Taken together, these data indicate that NorUDCA redirects the CD8+ T cell nutrient sensing program from glycolysis toward fatty acid utilization, as a consequence of mTORC1 suppression.
Interestingly, MS analysis revealed that the abundance of several mTORC1-dependent mitoribosomes was reduced in NorUDCA-treated CD8+ T cells (Fig. 6D). Mitoribosomes play a critical role in translation of mitochondria-encoded electron transport components to facilitate oxidative phosphorylation during T cell activation.25 Our MS data showed that mitoribosome biogenesis was disrupted by NorUDCA, which might counteract the upregulated FAO machinery, and might explain why OCR remained unchanged by NorUDCA. Additionally, mTORC1-regulated COX10,25 which is important for expression and assembly of electron transport components complex IV, also showed a tendency for reduction by NorUDCA (Fig. 6D). Overall, our MS analysis revealed a modulation of mTORC1-associated metabolism in CD8+ T cells by NorUDCA.
The activity of mTORC1 is regulated by a complex regulatory network.21 When we tested potential candidate upstream signaling molecules regulating mTORC1, we found that Ras-Erk-P90 RSK signaling was suppressed by NorUDCA, while classic mTORC1 upstream axes involving phosphatidylinositol-3 kinase and pAKTT308 were not reduced (Fig. S7A-C). (Phospho-) proteome analysis further confirmed the inhibition of mTORC1 by NorUDCA In murine-activated CD8+ T cells (Fig. S8).
Additionally, intracellular phosphatidic acid that can induce direct mTORC1 activation by competing with FK506-binding protein 38 for binding with FK506 protein, a well-known endogenous inhibitor of mTORC1,26 was reduced by NorUDCA in activated CD8+ T cells (Fig. S7D). Collectively, our studies revealed that NorUDCA directly affects both mTORC1 and upstream pathways controlling mTORC1 activation; therefore, our data indicate mTORC1 as a potential central hub for NorUDCA’s signaling action.
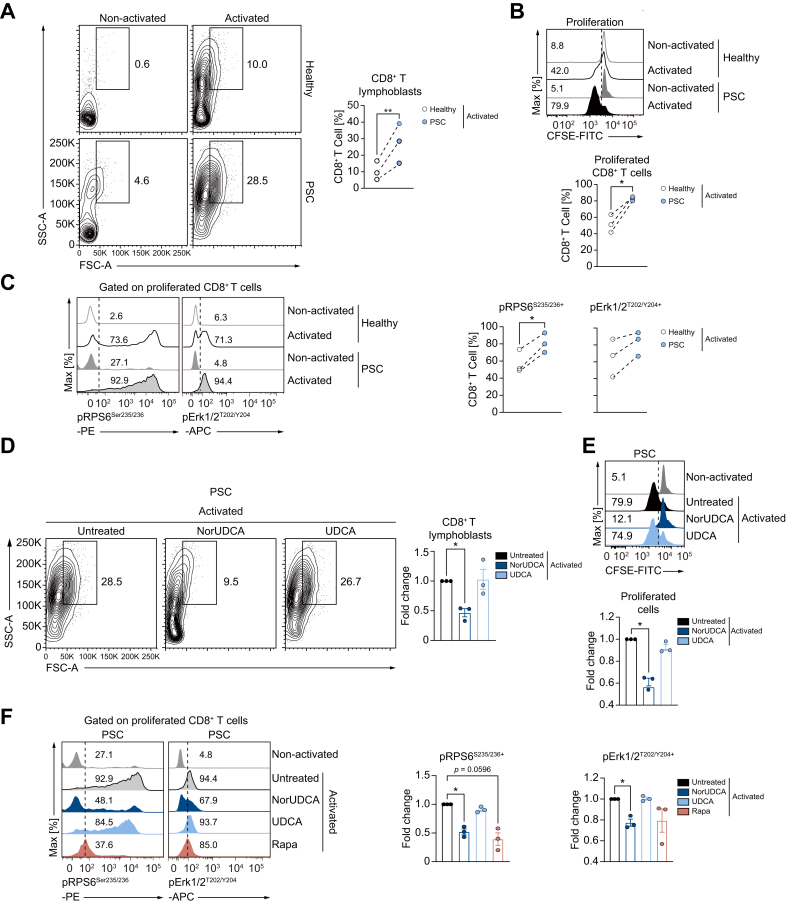
NorUDCA suppresses blasting and expansion with blunted mTORC1 kinase activity in PSC CD8+ T cells
Finally, we investigated whether some of our key findings obtained with murine cells apply to human peripheral CD8+ T cells, especially peripheral CD8+ T cells from patients with PSC, where dysregulated T cells are associated with disease progression.4 We examined the impact of NorUDCA on CD8+ T cell activation, blasting, clonal expansion and mTORC1 in vitro by ex vivo activation of isolated peripheral T cells from healthy volunteers and patients in the absence or presence of NorUDCA (Fig. 7, gating strategy see Fig. S9A). UDCA was used for comparison. Rapamycin was used as control for mTORC1 inhibition.
Fig. 7.
NorUDCA reduces lymphoblastogenesis and expansion with suppressed mTORC1 kinase activities in PSC CD8+ T cells.
(A-C) Peripheral human T cells from patients with PSC and age- and sex-matched healthy volunteers were activated in parallel under indicated conditions. Representative plots showing lymphoblastogenesis and expansion of gated CD8+ T cells under indicated conditions. pErk1/2T202/Y204 and pRPS6Ser235/236 on proliferated PSC CD8+ T cells as indicated. Numbers indicate frequencies. Quantitative analysis is shown alongside. (D-F) Effects of compounds on lymphoblastogenesis and expansion of CD8+ T cells from patients with PSC are shown. Treatment effects on pErk1/2T202/Y204 and pRPS6Ser235/236 on proliferated PSC CD8+ T cells as indicated. Numbers show frequency of positive cells. Quantitative analysis is shown alongside. Data represent 3 independent experiments from patients with PSC and healthy volunteers run in parallel (connected by broken line in Fig. 7A-C). Quantitative data are presented as mean±SE. p values were calculated by paired t test or one-way ANOVA corrected with Tukey post-hoc test when more than 2 groups were included. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001. NorUDCA, 24-norursodeoxycholic acid; PSC, primary sclerosing cholangitis, Rapa, rapamycin.
In contrast to CD8+ T cells from healthy volunteers, CD8+ T cells from patients with PSC exhibited enhanced lymphoblastogenesis, proliferation and mTORC1 signaling (Fig. 7A-C). In line with our murine observations, NorUDCA strongly decreased cell size and clonal expansion of CD8+ T cells in patients with PSC, while not affecting cell viability and activation (Fig. 7D, 7E, Fig. S9B, 9C). We next explored whether NorUDCA affects Erk-mTORC1 signaling in PSC CD8+ T cells by assessing expression of pErk1/2T202/Y204 and pRPS6Ser235/236. After NorUDCA treatment, we detected blunted Erk-mTORC1 signaling in proliferating CD8+ T cells together with reduced lymphoblastogenesis and proliferation in patients with PSC, while UDCA showed no effect (Fig. 7D-F).
Discussion
Elucidating the therapeutic mechanisms of NorUDCA (recently renamed as norucholic acid27) – a novel therapeutic approach to immune-mediated liver diseases such as PSC7 – is of considerable clinical interest. Previously, we have reported that NorUDCA strongly reversed biliary fibrosis and injury in the Mdr2-/- model of sclerosing cholangitis.9 Our study reveals that NorUDCA, in addition to its strong anticholestatic efficacy, decreases the number of hepatic innate and adaptive immune cells including CD8+ T cells in Mdr2-/- mice, indicating a pronounced immunomodulatory effect. Since Mdr2-/- mice suffer from defective bile formation13 and NorUDCA has substantial effects on bile composition by inducing cholehepatic shunting with bicarbonate-rich choleresis,19,28,29 it remains unclear whether NorUDCA acts directly on immune cells, or indirectly by improving the toxic bile microenvironment.
Our data in a non-cholestatic, immune (CD8+ T cell)-mediated model of hepatic and systemic immunopathology induced by acute infection of LCMV clone 13,[10], [11], [12] now clearly demonstrate that NorUDCA directly modulates CD8+ T cells and attenuates excessive CD8+ T cell-linked hepatic immunopathology. One key mechanism we uncovered is that NorUDCA can directly impact on blasting, proliferation and metabolism of CD8+ T cells. Given the complexity of immune regulation, we certainly cannot rule out additional beneficial mechanisms induced by NorUDCA, such as modulation of innate and humoral immunity which definitely requires further independent studies. However, focusing on CD8+ T cells, we further identified mTORC1 as a major target of NorUDCA in CD8+ T cells. With comprehensive MS analysis we uncovered that the majority of the proteins and functionally annotated pathways modulated by NorUDCA were changed by the classic mTORC1 inhibitor rapamycin in the same direction, thus substantiating our finding that mTORC1 is a major target of NorUDCA in CD8+ T cells.
mTORC1 inhibition provides a critical immunometabolic link between the control of CD8+ T cell immune responses,17,30 and the regulation of glycolysis and FAO.22 Following mTORC1 inhibition, NorUDCA reshapes metabolic reprogramming in CD8+ T cells, reducing glycolysis and enhancing the FAO machinery, which ultimately leads to reduced cell size and expansion of CD8+ T cells. Comparing MS data of NorUDCA- and rapamycin-treated CD8+ T cells, we also found an array of metabolic pathways uniquely altered by NorUDCA, such as metabolism of organic acids, organonitrogen compounds and cellular catabolic processes. This implies that NorUDCA might also possess pleiotropic immunometabolic or signaling properties not exclusively resulting from direct mTORC1 inhibition. Our data thus indicate exciting entry points for future full-scale metabolic studies on NorUDCA which will further advance our mechanistic understanding and extend clinical applications of NorUDCA.
mTORC1 hyperactivation is implicated in the pathogenesis of several autoimmune diseases.31 One original discovery derived from our current study is that similar to the feature of many autoimmune states, we observed that circulating CD8+ T cells from patients with PSC exhibit a hyperproliferative phenotype with enhanced mTORC1 activity, in contrast to CD8+ T cells from healthy volunteers. Importantly, in vitro NorUDCA not only suppressed mTORC1 in CD8+ T cells from patients with PSC as efficiently as rapamycin, but also decreased Erk activity in CD8+ T cells from patients with PSC while rapamycin showed no impact. NorUDCA’s property of modulating several signaling nodes in the mTORC1 transduction network might indicate better pharmacological efficacy for treating PSC, although rapamycin or other mTORC1 inhibitors have not yet been investigated in PSC.8 Further studies are required to demonstrate that mTORC1 hyperactivation accounts for the pathological role of CD8+ T cells in driving PSC and to confirm whether targeting mTORC1 in CD8+ T cells has therapeutic potential.
Finally, our study might also provide a hint towards a better understanding of the different therapeutic activities of UDCA and NorUDCA in patients with PSC. Our side-by-side comparison revealed that UDCA entirely lacked the signaling effects and immunometabolic modulatory functions observed for NorUDCA. In line with previous studies,9,19 UDCA reached about 10-fold lower concentrations than NorUDCA in WT mice, since UDCA is unable to undergo cholehepatic shunting.28,29 When testing whether our findings could be translated into circulating CD8+ T cells of patients with PSC, we found that NorUDCA, but not UDCA, suppressed mTORC1 signaling at concentrations reached in vivo in murine model systems. Since NorUDCA, due to cholehepatic shunting, reaches substantially higher concentrations than UDCA in vivo, it should be considered that the observed mechanistic differences in vitro could either result from the modified BA structure or the different concentrations used or a combination of both. We also acknowledge that the observed differences in concentrations of UDCA and NorUDCA in murine in vivo models might not fully reflect the different therapeutic mechanisms of UDCA and NorUDCA in patients.6 However, our findings offer novel mechanistic insights and indicate new pathways by which NorUDCA regulates hepatic inflammation. Additionally, it raises exciting possibilities that targeted chemical modifications of BA structures (e.g. removal of a single methyl-group in NorUDCA19) may add novel signaling and immunomodulatory activities to BAs and create additional therapeutic avenues.
Taken together, we demonstrate the pronounced immunomodulatory efficacy of NorUDCA on CD8+ T cells resulting in alleviated hepatic inflammation. Our findings may have imminent therapeutic implications for treatment of PSC, where NorUDCA has shown promising results7 while UDCA has no established efficacy.6 Further studies may be warranted to explore the array of potential therapeutic applications of NorUDCA in immune-mediated liver diseases.
Abbreviations
BA, bile acid; Cpt1a/2, carnitine palmitoyltransferase 1a/2; ECAR, extracellular acidification rate; FAO, fatty acid β-oxidation; GP33, LCMV epitope glycoprotein-33; LCMV, lymphocytic choriomeningitis virus; Mdr2(Abcb4)-/-, multidrug resistance gene 2; MS, mass spectrometry; mTOR, mammalian target of rapamycin; NorUDCA, 24-norursodeoxycholic acid; OCR, oxygen consumption rate; PSC, primary sclerosing cholangitis; RPS6, ribosomal protein S6; TEM, effector memory T cell; UDCA, ursodeoxycholic acid.
Financial support
This study has been funded by Austrian Science Foundation (FWF) through projects F3517, F7310, I2755 and the Doctoral Program “Inflammation and Immunity” (DK-IAI W1212) (to M.T.). The work in the laboratory W.E. was supported by the FWF projects P26193, P29790, F7005 and DK-IAI W1212. N.B. and L.S. were funded by the Austrian Science Fund (FWF): P24265, P30885 and F7004. S.S. and A.F.G. were funded by Austrian Science Fund (FWF): P27747. A.B. received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. 677006, "CMIL"). A.L. and P.H. were supported by a DOC fellowship of the Austrian Academy of Sciences. H.S. and A.O.-R. have received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No 683356-FOLSMART.
Authors’ contributions
C.Z., N.B., W.E. and M.T. designed the research; C.Z. and N.B. performed most of the experiments and analyzed the data; C.Z., N.B., A.C.M. and P.M. performed proteomic and (phospho-) proteomic experiments and analyzed the data; C.Z., N.B., H.B., C.V., T.C., C.D.F., A.B., D.H., T.P., L.S., M.A., A.F.G., M.K., P.H., J.R. and S.S. performed in vivo experiments; C.Z., N.B., A. O-R., P.S., C.D., E.H., T.W. and H.Stockinger. performed in vitro human T cell experiments and analyzed the data. C.Z., H.B., H.S. and T.S. measured serum liver biochemistry. C.Z., N.B., A.L., A.G., S.S., L.S., P.H. and T.C. performed metabolic assays. C.Z., N.B., W.E. and M.T. wrote the manuscript. All authors read and corrected the manuscript.
Data availability statement
All data are available upon reasonable request.
Conflict of interest
M. Trauner has served as speaker for Falk Foundation, Gilead, Intercept and MSD; he has advised for Albireo, BiomX, Boehringer Ingelheim, Falk Pharma GmbH, Genfit, Gilead, Intercept, Jannsen, MSD, Novartis, Phenex, Regulus and Shire. He further received travel grants from Abbvie, Falk, Gilead and Intercept and research grants from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD Takeda and UltraGenyx. He is also co-inventor of patents on the medical use of NorUDCA filed by the Medical Universities of Graz and Vienna. All the other authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.06.036.
Contributor Information
Nicole Boucheron, Email: Nicole.boucheron@meduniwien.ac.at.
Michael Trauner, Email: michael.trauner@meduniwien.ac.at.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Pearce E.L., Poffenberger M.C., Chang C.H., Jones R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards D.M., Kyewski B., Feuerer M. Re-examining the nature and function of self-reactive T cells. Trends Immunol. 2016;37:114–125. doi: 10.1016/j.it.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maini M.K., Boni C., Lee C.K., Larrubia J.R., Reignat S., Ogg G.S. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liaskou E., Jeffery L.E., Trivedi P.J., Reynolds G.M., Suresh S., Bruns T. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014;147 doi: 10.1053/j.gastro.2014.04.003. 221-232 e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb G.J., Rana A., Hodson J., Akhtar M.Z., Ferguson J.W., Neuberger J.M. Twenty-year comparative analysis of patients with autoimmune liver diseases on transplant waitlists. Clin Gastroenterol Hepatol. 2018;16 doi: 10.1016/j.cgh.2017.09.062. 278-287 e277. [DOI] [PubMed] [Google Scholar]
- 6.Beuers U., Trauner M., Jansen P., Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Fickert P., Hirschfield G.M., Denk G., Marschall H.U., Altorjay I., Farkkila M. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Fickert P., Wagner M., Marschall H.U., Fuchsbichler A., Zollner G., Tsybrovskyy O. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Straub T., Pircher H. Enhancing immunity prevents virus-induced T-cell-mediated immunopathology in B cell-deficient mice. Eur J Immunol. 2019;49:782–789. doi: 10.1002/eji.201847962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinkernagel R.M., Haenseler E., Leist T., Cerny A., Hengartner H., Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornberg M., Kenney L.L., Chen A.T., Waggoner S.N., Kim S.K., Dienes H.P. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front Immunol. 2013;4:475. doi: 10.3389/fimmu.2013.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fickert P., Fuchsbichler A., Wagner M., Zollner G., Kaser A., Tilg H. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Araki K., Turner A.P., Shaffer V.O., Gangappa S., Keller S.A., Bachmann M.F. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengsch B., Johnson A.L., Kurachi M., Odorizzi P.M., Pauken K.E., Attanasio J. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baazim H., Schweiger M., Moschinger M., Xu H., Scherer T., Popa A. CD8(+) T cells induce cachexia during chronic viral infection. Nat Immunol. 2019;20:701–710. doi: 10.1038/s41590-019-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay D.K., Rosenzweig E., Sinclair L.V., Feijoo-Carnero C., Hukelmann J.L., Rolf J. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lercher A., Bhattacharya A., Popa A.M., Caldera M., Schlapansky M.F., Baazim H. Type I interferon signaling disrupts the hepatic urea cycle and alters systemic metabolism to suppress T cell function. Immunity. 2019;51 doi: 10.1016/j.immuni.2019.10.014. 1074-1087 e1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halilbasic E., Fiorotto R., Fickert P., Marschall H.U., Moustafa T., Spirli C. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2-/- mice. Hepatology. 2009;49:1972–1981. doi: 10.1002/hep.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravichandran G., Neumann K., Berkhout L.K., Weidemann S., Langeneckert A.E., Schwinge D. Interferon-gamma-dependent immune responses contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol. 2019;71:773–782. doi: 10.1016/j.jhep.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollizzi K.N., Patel C.H., Sun I.H., Oh M.H., Waickman A.T., Wen J. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest. 2015;125:2090–2108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartholdy C., Christensen J.P., Wodarz D., Thomsen A.R. Persistent virus infection despite chronic cytotoxic T-lymphocyte activation in gamma interferon-deficient mice infected with lymphocytic choriomeningitis virus. J Virol. 2000;74:10304–10311. doi: 10.1128/jvi.74.22.10304-10311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Windt G.J., Pearce E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan H., Yang K., Li Y., Shaw T.I., Wang Y., Blanco D.B. Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity. 2017;46:488–503. doi: 10.1016/j.immuni.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon M.S., Sun Y., Arauz E., Jiang Y., Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J Biol Chem. 2011;286:29568–29574. doi: 10.1074/jbc.M111.262816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norucholic acid. WHO drug information. 2020. p. 34. [Google Scholar]
- 28.Yoon Y.B., Hagey L.R., Hofmann A.F., Gurantz D., Michelotti E.L., Steinbach J.H. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann A.F., Zakko S.F., Lira M., Clerici C., Hagey L.R., Lambert K.K. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology. 2005;42:1391–1398. doi: 10.1002/hep.20943. [DOI] [PubMed] [Google Scholar]
- 30.Thomson A.W., Turnquist H.R., Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suto T., Karonitsch T. The immunobiology of mTOR in autoimmunity. J Autoimmun. 2020;110:102373. doi: 10.1016/j.jaut.2019.102373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request.