Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, clinical practice variation, coronavirus disease 2019, mechanical ventilation
OBJECTIVES:
As coronavirus disease 2019 is a novel disease, treatment strategies continue to be debated. This provides the intensive care community with a unique opportunity as the population of coronavirus disease 2019 patients requiring invasive mechanical ventilation is relatively homogeneous compared with other ICU populations. We hypothesize that the novelty of coronavirus disease 2019 and the uncertainty over its similarity with noncoronavirus disease 2019 acute respiratory distress syndrome resulted in substantial practice variation between hospitals during the first and second waves of coronavirus disease 2019 patients.
DESIGN:
Multicenter retrospective cohort study.
SETTING:
Twenty-five hospitals in the Netherlands from February 2020 to July 2020, and 14 hospitals from August 2020 to December 2020.
PATIENTS:
One thousand two hundred ninety-four critically ill intubated adult ICU patients with coronavirus disease 2019 were selected from the Dutch Data Warehouse. Patients intubated for less than 24 hours, transferred patients, and patients still admitted at the time of data extraction were excluded.
MEASUREMENTS AND MAIN RESULTS:
We aimed to estimate between-ICU practice variation in selected ventilation parameters (positive end-expiratory pressure, Fio2, set respiratory rate, tidal volume, minute volume, and percentage of time spent in a prone position) on days 1, 2, 3, and 7 of intubation, adjusted for patient characteristics as well as severity of illness based on Pao2/Fio2 ratio, pH, ventilatory ratio, and dynamic respiratory system compliance during controlled ventilation. Using multilevel linear mixed-effects modeling, we found significant (p ≤ 0.001) variation between ICUs in all ventilation parameters on days 1, 2, 3, and 7 of intubation for both waves.
CONCLUSIONS:
This is the first study to clearly demonstrate significant practice variation between ICUs related to mechanical ventilation parameters that are under direct control by intensivists. Their effect on clinical outcomes for both coronavirus disease 2019 and other critically ill mechanically ventilated patients could have widespread implications for the practice of intensive care medicine and should be investigated further by causal inference models and clinical trials.
As coronavirus disease 2019 (COVID-19) is a novel disease, treatment strategies continue to be debated (1, 2). Although this may be somehow unsettling, it also provides the intensive care community with a unique opportunity.
The population of COVID-19 patients requiring invasive mechanical ventilation is relatively homogeneous compared with other ICU populations, such as those with unselected acute respiratory distress syndrome (ARDS). We hypothesized that the novelty of COVID-19 and the uncertainty over its similarity with non-COVID ARDS (3) resulted in substantial practice variation between hospitals allowing for an analysis of differences in mechanical ventilation strategies between ICUs. We also hypothesized that this practice variation was larger in the first wave of critically ill COVID patients than later in the pandemic (the second wave). Persisting practice variation might identify potentially modifiable factors to improve treatment of COVID-19 patients and help define the research agenda for improving the practice of mechanical ventilation in general.
Forty-seven ICUs initiated the Dutch Data Warehouse (DDW), a large-scale ICU data sharing collaboration (4). The DDW now contains over 400 million data points on more than 2,000 critically ill COVID-19 patients in 25 hospitals in the Netherlands and allows for detailed analyses on routinely collected clinical data.
The objective of this study was to use the high-resolution longitudinal data from the DDW to quantify the magnitude of variation in ventilation practices between ICUs and show variation in ventilation practice in the first and second waves of COVID-19 patients as well as differences between the two waves.
MATERIALS AND METHODS
The Medical Ethics Review Committee at Amsterdam University Medical Centers, location VU University Medical Center waived the need for patient informed consent and approved of an opt-out procedure for the collection of COVID-19 patient data during the COVID-19 crisis under approval number 2020.156. This report adheres to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines (5).
To explore potential practice variation, we selected all COVID-19–positive intubated ICU patients admitted during the first and second COVID-19 waves but excluded patients still admitted during data extraction, patients intubated for less than 24 hours, and patients transferred between hospitals, in order to isolate the influence of individual hospitals and exclude COVID-19 patients intubated for non-ARDS reasons. (Supplementary Fig. 1, http://links.lww.com/CCX/A815) The cutoff dates for the two waves were determined by visual inspection (Supplementary Fig. 2, http://links.lww.com/CCX/A815), where the end of wave 1 was set based on a plateau in monthly patient counts. The first wave was, therefore, defined as from February 2020 to July 2020 and the second wave as from August 2020 to December 2020. The first and second waves were split and analyzed separately. Additionally, as this variance may change over time, a separate analysis was performed on a subset of hospitals that delivered data in both waves.
Using multilevel linear mixed-effects modeling, we calculated the between-ICU variability for parameters related to mechanical ventilation that are directly influenced by intensivists: positive end-expiratory pressure (PEEP), Fio2, tidal volume, set respiratory rate, minute volume, and the percentage of time per day in prone position. These variables were sampled per hour, forward-filled up to 8 hours, and averaged as 24-hour means on days 1, 2, 3, 7, and 14 of intubation, starting from the moment of intubation.
The primary outcome was the intraclass correlation coefficient (ICC), representing the proportion of variance attributable to systematic differences between ICUs after adjusting for covariates that may influence ventilator settings directly or indirectly. The ICC was calculated as the variance of the ICU random effect divided by the total (ICU + residual) variance. For better clinical interpretation, practice variation was also expressed on the scales of the respective variables (e.g., cm H2O for PEEP) as the 95% prediction interval of the ICU random effect.
To adjust for case-mix differences as possible drivers of between-ICU practice variation, the following covariates were included: age, gender, body mass index, comorbidities at time of ICU admission as static covariates, as well as Pao2/Fio2 ratio, pH, ventilatory ratio, and dynamic respiratory system compliance during controlled ventilation at the respective time-points (see Supplementary Table 1, http://links.lww.com/CCX/A815). The academic status of the hospital was included as a static covariate for an exploratory analysis in the Supplementary Material (http://links.lww.com/CCX/A815). Comorbidities were grouped based on similarity or used separately. The dynamic respiratory system compliance of the first 3 hours of each day was used to best reflect the status of the lung before treatment changes over the next 24 hours.
Missing covariates were imputed through multiple imputation by chained equations using predictive mean matching resulting in five imputed datasets for each outcome. A prediction matrix for covariates was created by selecting relevant predictors through stepwise selection by Akaike information criterion based on a generalized linear model for binary variables or linear models for continuous variables. Missing observations of dependent (ventilation) variables were not imputed.
A multilevel model was used to account for sampling populations of both patients and hospitals. Linear mixed-effects models were fitted with hospitals as the second level, and linear models were fitted without hospitals as a restricted model. The average log-likelihood ratio across the imputed datasets was used for calculating the D2 statistic and p value to indicate the influence of grouping per hospital.
Coefficients were averaged and pooled over the repeated analyses. Parameters and standard errors were aggregated across the imputed datasets, and the resulting variance was used to calculate the ICC. After adjusting for covariates, the ICC indicates the proportion of variance explained by the grouping structure and thus attributable to the hospitals. Software packages and libraries used are listed in Supplementary Table 2 (http://links.lww.com/CCX/A815).
RESULTS
Data were available for 1,294 patients. A total of 807 patients were admitted to 25 participating ICUs between February and July 2020, with a median number of patients per ICU of 29 (interquartile range [IQR], 25–38) (Supplementary Fig. 3, http://links.lww.com/CCX/A815). In the second wave, 487 patients were admitted to 14 ICUs between August and December 2020, with a median number of patients per ICU of 36.5 (IQR, 25.25–44.75) (Supplementary Fig. 4, http://links.lww.com/CCX/A815). Potential ad hoc ICUs within hospitals were regarded as one single unit per hospital.
The baseline patient demographics, comorbidities, and severity of illness indicators were comparable over both the duration of the admissions and across the two waves. ICU mortality was 30.5% in the first wave and 30.2% in the second wave. (Supplementary Tables 3–5, http://links.lww.com/CCX/A815) The proportion of patients originating from academic hospitals was increased for the second wave for which we performed an exploratory analysis (Supplementary Figs. 5 and 6, http://links.lww.com/CCX/A815.) Data availability for covariates and outcome parameters are reported in the Online Supplement and Supplementary Tables 6–8, (http://links.lww.com/CCX/A815).
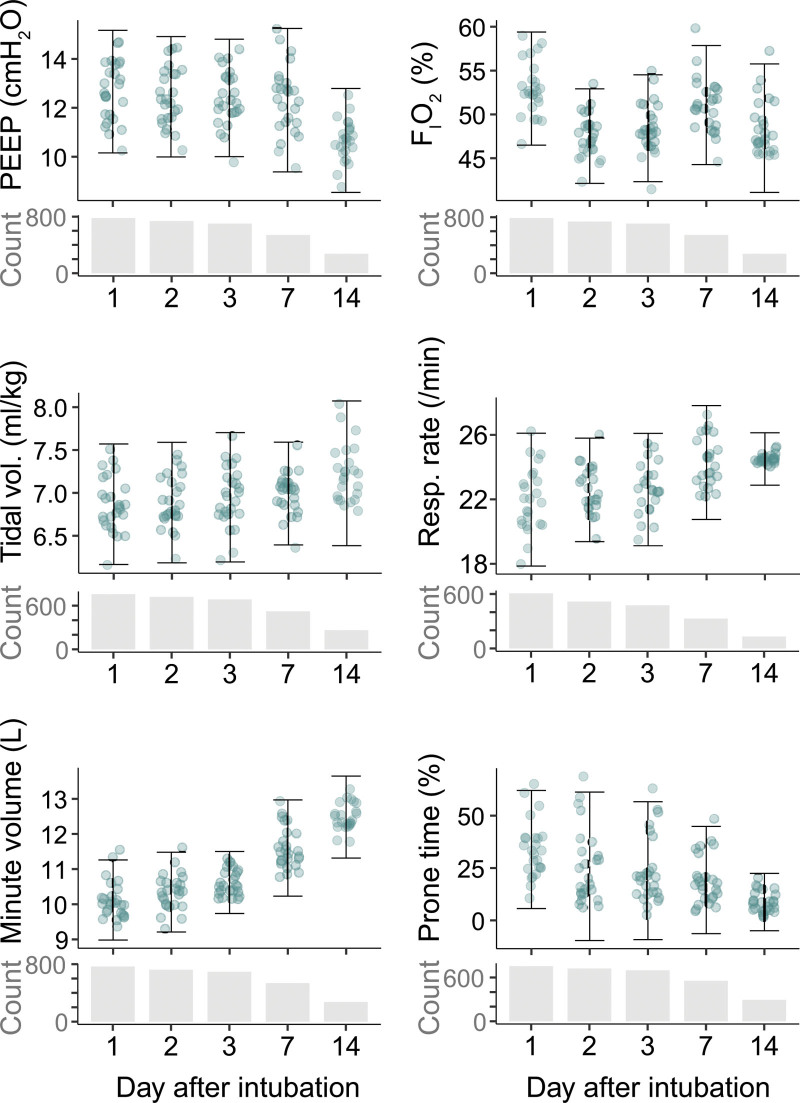
The adjusted between-ICU variance in ventilation variables during wave 1 is shown in Figure 1. After correcting for patient factors, significant (p ≤ 0.001) practice variation was found in all ventilation variables on days 1, 2, 3, and 7 of intubation. Hospital unconditional means after adjusting for covariates are shown in Figure 2 and illustrate the spread of average ventilator treatment strategies at their respective scales. Average values per parameter per analysis day are reported in Supplementary Table 9 (http://links.lww.com/CCX/A815). A detailed description of the mixed-effects model including variance, ICC, patient counts, and statistical significance is available in Supplementary Table 10 (http://links.lww.com/CCX/A815).
Figure 1.
Between-ICU practice variation in ventilator settings during wave 1. Heatmap of practice variation expressed as the intraclass correlation coefficient (ICC). The ICC is the fraction of residual between-patient variability (after adjustment for covariates) that is attributable to between-ICU differences. For example, more than 35% of between-patient variability in set respiratory rate on day 1 is attributable to systematic differences between hospitals. IBW = ideal body weight, PEEP = positive end-expiratory pressure.
Figure 2.
Practice variation expressed on the scales of the ventilator settings. Individual data points are the hospital unconditional means after adjusting for covariates. The interval bounds are model-based estimates of the 2.5 and 97.5 percentiles of the random effects, which represent the between-ICU variability in mean ventilator setting after adjusting for covariates and discounting random variance. The number of included patients is shown for each variable for each time point. PEEP = positive end-expiratory pressure.
The heat map of the comparison of the first wave versus the second wave is shown in Figure 3. For this comparison, only hospitals delivering data in both waves were included. Practice variation during wave 2 remained comparable with wave 1 for the same selection of hospitals except for the variance in PEEP and time spent in prone position, which was decreased in the second wave.
Figure 3.
Between-ICU practice variation in ventilator settings for hospitals in both waves. Heatmap comparing practice variation expressed as the intraclass correlation coefficient (ICC) for hospitals having delivered data in both wave 1 and wave 2. IBW = ideal body weight, PEEP = positive end-expiratory pressure.
DISCUSSION
In this study of mechanically ventilated COVID-19 patients admitted to 25 different Dutch ICUs, we found substantial variation in ventilation practices after adjusting for patient baseline and respiratory system characteristics. This practice variation differs between ventilator settings and evolves over admission time. The variation was comparable for the same selection of hospitals across the first two waves of COVID patients, except for PEEP and time spent in prone position, indicating that hospitals selectively changed to a more uniform approach for their respective ventilation strategies during 2020. Statistically significant practice variation remained for all parameters except tidal volume, minute volume, and time spent in prone position at the seventh day of intubation.
The data points in Figure 2 are unconditional means and, thus, denote ventilation settings in a patient that is average with respect to all adjustment variables. For such “average” patients, the mean set respiratory rate on day 1 varied between 18/min in one ICU to 26/min in another ICU—a relative difference of 44%. The relative difference between the average applied PEEP in different ICUs was 45% on day 1 and even more on day 7. There was extreme variation in the average percentage of time per patient-day in a prone position, which ranged from an average of nearly 0% in some ICUs to more than 50% in others.
Practice variation may be due to suboptimal adherence to best practice standards or, importantly, due to fundamental uncertainty surrounding the optimal ventilation choices. In the latter case, the observed practice variation constitutes equipoise (6): patients are treated differently depending on where they are admitted, with possibly important consequences for outcomes. This should provide a major impetus for further research into the respective domains of uncertainty. The results from this study can be used to identify such domains.
Practice variation has recently been described in non-COVID ARDS, showing variance in tidal volume and ventilation pressures (7). We have now shown practice variation is still clearly demonstrable in a relatively homogenous underlying disease mechanism that shows evolvement over the duration of the pandemic.
Furthermore, the studied hospitals show an average tidal volume per kg–predicted body weight between 6.5 and 7.5 mL/kg, whereas the international Large observational study to UNderstand the Global impact of Severe Acute respiratory Failure study showed actual clinical practice to use an average tidal volume of 7.5 mL/kg in ARDS patients (8). Deviation from a strict tidal volume of 6 mL/kg could potentially be made to prioritize optimization of other clinical aspects although we included the common influencing factors for these decisions.
This study comes with limitations. First, the estimated practice variation may still be influenced by unmeasured confounders, although we believe the most important determinants of ventilation choices are included in the model. Second, routinely collected data were used necessitating imputation strategies and resulted in the omission of analysis day 14 when comparing wave 1 with wave 2. Third, data to analyze variance between different ICUs in the same hospital or between individual intensivists were lacking. Finally, due to highly variable reporting of the inspiratory/expiratory ratio and detailed ventilator modes, these parameters could not be interpreted and were, therefore, excluded from analysis.
CONCLUSIONS
This is the first study to clearly demonstrate significant practice variation between ICUs related to mechanical ventilation parameters that are under direct control by intensivists. The magnitude of practice variation decreased between the first and second COVID-19 waves for some ventilation parameters, indicating that ventilation practices within hospitals do change within a year in light of new information. However, significant variation still remains. The effect of practice variation on clinical outcomes for both COVID-19 and other critically ill mechanically ventilated patients could have widespread implications for the practice of intensive care medicine and should be investigated further by causal inference models and clinical trials.
ACKNOWLEDGMENTS
The Dutch ICU Data Sharing Against COVID-19 Collaborators:
From collaborating hospitals having shared data:
Remko van den Akker, Intensive Care, Adrz, Goes, The Netherlands, r.vandenakker@adrz.nl
Tom A. Rijpstra, MD, Department of Intensive Care, Amphia Ziekenhuis, Breda, The Netherlands, trijpstra@amphia.nl
M. C. Reuland, MD, Department of Intensive Care Medicine, Amsterdam UMC, Universiteit van Amsterdam, Amsterdam, The Netherlands, m.c.reuland@amsterdamumc.nl
Klaas Sierk Arnold, MD, Anesthesiology, Antonius Ziekenhuis Sneek, Sneek, The Netherlands, k.arnold@antonius-sneek.nl
Arend Jan Meinders, MD, Department of Internal Medicine and Intensive Care, St Antonius Hospital, Nieuwegein, The Netherlands, a.meinders@antoniusziekenhuis.nl
Nicolas Schroten, MD, Intensive Care, Albert Schweitzerziekenhuis, Dordrecht, The Netherlands, nicolasschroten@gmail.com
Laura van Manen, MD, Department of Intensive Care, BovenIJ Ziekenhuis, Amsterdam, The Netherlands, l.vanmanen@bovenij.nl
Leon Montenij, MD, PhD, Department of Anesthesiology, Pain Management and Intensive Care, Catharina Ziekenhuis Eindhoven, Eindhoven, The Netherlands, leon.montenij@catharinaziekenhuis.nl
Julia Koeter, MD, Intensive Care, Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands, j.koeter@cwz.nl
J. W. Fijen, MD, PhD, Department of Intensive Care, Diakonessenhuis Hospital, Utrecht, The Netherlands, jwfijen@diakhuis.nl
Jasper van Bommel, MD, PhD, Department of Intensive Care, Erasmus Medical Center, Rotterdam, The Netherlands, j.vanbommel@erasmusmc.nl
Roy van den Berg, Department of Intensive Care, ETZ Tilburg, Tilburg, The Netherlands, r.vandenberg@etz.nl
Martha de Bruin, MD, Department of Intensive Care, Franciscus Gasthuis & Vlietland, Rotterdam, The Netherlands, m.debruin4@franciscus.nl
Roger van Rietschote, Business Intelligence, Haaglanden MC, Den Haag, The Netherlands, roger.van.rietschote@haaglandenmc.nl
Ellen van Geest, Department of ICMT, Haga Ziekenhuis, Den Haag, The Netherlands, e.vangeest@hagaziekenhuis.nl
Koen S. Simons, MD, PhD, Department of Intensive Care, Jeroen Bosch Ziekenhuis, Den Bosch, The Netherlands, k.simons@jbz.nl
Anisa Hana, MD, PhD, Intensive Care, Laurentius Ziekenhuis, Roermond, The Netherlands, anisa.hana@lzr.nl
Joost Labout, MD, PhD, ICU, Maasstad Ziekenhuis Rotterdam, The Netherlands, laboutj@maasstadziekenhuis.nl
Michael Kuiper, Intensive Care, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands, m.kuiper@mcl.nl
Albertus Beishuizen, MD, PhD, Department of Intensive Care, Medisch Spectrum Twente, Enschede, The Netherlands, b.beishuizen@mst.nl
Bart van de Gaauw, MD, PhD, Martiniziekenhuis, Groningen, The Netherlands, b.vandergaauw@mzh.nl
Roos Renckens, MD, PhD, Department of Internal Medicine, Northwest Clinics, Alkmaar, The Netherlands, r.renckens@nwz.nl
B. van den Bogaard, MD, PhD, ICU, OLVG, Amsterdam, The Netherlands, b.vandenbogaard@olvg.nl
Peter Pickkers, Department of Intensive Care Medicine, Radboud University Medical Centre, Nijmegen, The Netherlands, peter.pickkers@radboudumc.nl
Pim van der Heiden, MD, PhD, Intensive Care, Reinier de Graaf Gasthuis, Delft, The Netherlands, pvdheiden@hotmail.com
Dennis Geutjes, Department of Information Technology, Slingeland Ziekenhuis, Doetinchem, The Netherlands, d.geutjes@slingeland.nl
Claudia (C. W.) van Gemeren, MD, Intensive Care, Spaarne Gasthuis, Haarlem en Hoofddorp, The Netherlands, c.van.gemeren@spaarnegasthuis.nl
Emma Rademaker, MD, MSc, Department of Intensive Care, UMC Utrecht, Utrecht, The Netherlands, e.rademaker-2@umcutrecht.nl
Frits H. M. van Osch, PhD, Department of Clinical Epidemiology, VieCuri Medisch Centrum, Venlo, The Netherlands, fvosch@viecuri.nl
Johan Lutisan, MD, ICU, WZA, Assen, The Netherlands, johan.lutisan@wza.nl
Jacomar J. M. van Koesveld, MD, ICU, IJsselland Ziekenhuis, Capelle aan den IJssel, The Netherlands, jvkoesveld@ysl.nl
Bart P. Grady, MD, PhD, Department of Intensive Care, Ziekenhuisgroep Twente, Almelo, The Netherlands, b.grady@zgt.nl
Martijn de Kruif, MD, PhD, Department of Pulmonology, Zuyderland MC, Heerlen, The Netherlands, m.dekruif@zuyderland.nl
From the Laboratory for Critical Care Computational Intelligence:
Martin E. Haan, MD, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, m.e.haan@amsterdamumc.nl
Luca Roggeveen, MD, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, l.roggeveen@amsterdamumc.nl
Dagmar M. Ouweneel, PhD, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, d.m.ouweneel@amsterdamumc.nl
Ronald Driessen, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, r.driessen@amsterdamumc.nl
Jan Peppink, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, jan.peppink@amsterdamumc.nl
G. J. Zijlstra, MD, PhD, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, g.j.zijlstra@amsterdamumc.nl
A. J. van Tienhoven, MD, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, a.vantienhoven@amsterdamumc.nl
Evelien van der Heiden, MD, Department of Intensive Care Medicine, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, e.vanderheiden@amsterdamumc.nl
Jan Jaap Spijkstra, MD, PhD, Department of Intensive Care Medicine, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, jj.spijkstra@amsterdamumc.nl
Hans van der Spoel, MD, Department of Intensive Care Medicine, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, ji.vanderspoel@amsterdamumc.nl
Angelique de Man, MD, PhD, Department of Intensive Care Medicine, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, ame.deman@amsterdamumc.nl
Heder J. de Vries, MD, Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, h.vries@amsterdamumc.nl
Fuda van Diggelen, MSc, Quantitative Data Analytics Group, Department of Computer Sciences, Faculty of Science, VU University, Amsterdam, The Netherlands, fuda.van.diggelen@vu.nl
Ali el Hassouni, PhD, Quantitative Data Analytics Group, Department of Computer Sciences, Faculty of Science, VU University, Amsterdam, The Netherlands, a.el.hassouni@vu.nl
David Romero Guzman, PhD, Quantitative Data Analytics Group, Department of Computer Sciences, Faculty of Science, VU University, Amsterdam, The Netherlands, d.w.romeroguzman@vu.nl
Sandjai Bhulai, PhD, Analytics and Optimization Group, Department of Mathematics, Faculty of Science, Vrije Universiteit, Amsterdam, The Netherlands,
s.bhulai@vu.nl
From Pacmed:
Sebastiaan J. J. Vonk, MSc, Pacmed, Amsterdam, The Netherlands, bas.vonk@pacmed.nl
Mattia Fornasa, PhD, Pacmed, Amsterdam, The Netherlands, mattia.fornasa@pacmed.nl
Tomas Machado, Pacmed, Amsterdam, The Netherlands, tomas.machado@pacmed.nl
Adam Izdebski, Pacmed, Amsterdam, The Netherlands, adam.izdebski@pacmed.nl
Taco Houwert, MSc, Pacmed, Amsterdam, The Netherlands, taco.houwert@pacmed.nl
Hidde Hovenkamp, MSc, Pacmed, Amsterdam, The Netherlands, hidde@pacmed.nl
Roberto Noorduijn Londono, MSc, Pacmed, Amsterdam, The Netherlands, roberto.noorduijn@pacmed.nl
Davide Quintarelli, MSc, Pacmed, Amsterdam, The Netherlands, davide.quintarelli@pacmed.nl
Martijn G. Scholtemeijer, MD, Pacmed, Amsterdam, The Netherlands, martijn.scholtemeijer@pacmed.nl
Aletta A. de Beer, MSc, Pacmed, Amsterdam, The Netherlands, aletta.debeer@pacmed.nl
Giovanni Cinà, PhD, Pacmed, Amsterdam, The Netherlands, giovanni.cina@pacmed.nl
Willem E. Herter, BSc, Pacmed, Amsterdam, The Netherlands, willem@pacmed.nl
Michael de Neree tot Babberich, Pacmed, Amsterdam, The Netherlands, michael.deneree@pacmed.nl
Olivier Thijssens, MSc, Pacmed, Amsterdam, The Netherlands, olivier.thijssens@pacmed.nl
Lot Wagemakers , Pacmed, Amsterdam, The Netherlands, lot.wagemakers@pacmed.nl
Hilde G. A. van der Pol, Pacmed, Amsterdam, The Netherlands, hilde.vanderpol@pacmed.nl
Tom Hendriks, Pacmed, Amsterdam, The Netherlands, tom.hendriks@pacmed.nl
Julie Berend, Pacmed, Amsterdam, The Netherlands, julieberend1@gmail.com
Virginia Ceni Silva, Pacmed, Amsterdam, The Netherlands, vivicenisilva@gmail.com
Robert F. J. Kullberg, MD, Pacmed, Amsterdam, The Netherlands, bobkullberg@gmail.com
From RCCnet:
Leo Heunks, MD, PhD, Department of Intensive Care Medicine, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands, l.heunks@amsterdamumc.nl
Nicole Juffermans, MD, PhD, ICU, OLVG, Amsterdam, The Netherlands, n.p.juffermans@amsterdamumc.nl
Arjen J. C. Slooter, MD, PhD, Department of Intensive Care Medicine, UMC Utrecht, Utrecht University, Utrecht, The Netherlands, a.slooter-3@umcutrecht.nl
From other collaborating partners:
Martijn Beudel, MD, PhD, Department of Neurology, Amsterdam UMC, Universiteit van Amsterdam, Amsterdam, The Netherlands, m.beudel@amsterdamumc.nl
Nicolet F. de Keizer, PhD, Department of Clinical Informatics, Amsterdam UMC, Amsterdam, The Netherlands, n.f.keizer@amsterdamumc.nl
1 Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
2 Department of Clinical Epidemiology, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
3 Pacmed, Amsterdam, The Netherlands.
4 Department of Intensive Care, Admiraal De Ruyter Ziekenhuis, Goes, The Netherlands.
5 Department of Intensive Care, Amphia Ziekenhuis, Breda, The Netherlands.
6 Department of Intensive Care Medicine, Laboratory for Critical Care Computational Intelligence, Amsterdam Medical Data Science, Amsterdam UMC, Amsterdam, The Netherlands.
7 Department of Anesthesiology, Antonius Ziekenhuis Sneek, Sneek, The Netherlands.
8 Department of Anesthesiology and Intensive Care, St. Antonius Hospital, Nieuwegein, The Netherlands.
9 Department of Intensive Care, Albert Schweitzerziekenhuis, Dordrecht, The Netherlands.
10 Department of Intensive Care, Bovenij Ziekenhuis, Amsterdam, The Netherlands.
11 Department of Intensive Care, Catharina Ziekenhuis Eindhoven, Eindhoven, The Netherlands.
12 Department of Intensive Care, Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands.
13 Department of Intensive Care, Diakonessenhuis Hospital, Utrecht, The Netherlands.
14 Department of Intensive Care, Erasmus Medical Center, Rotterdam, The Netherlands.
15 Department of Intensive Care, ETZ Tilburg, Tilburg, The Netherlands.
16 Department of Intensive Care, Franciscus Gasthuis & Vlietland, Rotterdam, The Netherlands.
17 Department of Intensive Care, Haaglanden Medisch Centrum, Den Haag, The Netherlands.
18 Department of Intensive Care, HagaZiekenhuis, Den Haag, The Netherlands.
19 Department of Intensive Care, Ikazia Ziekenhuis Rotterdam, Rotterdam, The Netherlands.
20 Department of Intensive Care, Jeroen Bosch Ziekenhuis, Den Bosch, The Netherlands.
21 Department of Intensive Care, Laurentius Ziekenhuis, Roermond, The Netherlands.
22 Department of Intensive Care, Maasstad Ziekenhuis Rotterdam, Rotterdam, The Netherlands.
23 Department of Intensive Care, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands.
24 Department of Intensive Care, Medisch Spectrum Twente, Enschede, The Netherlands.
25 Department of Intensive Care, SEH, BWC, Martiniziekenhuis, Groningen, The Netherlands.
26 Department of Intensive Care Medicine, Northwest Clinics, Alkmaar, The Netherlands.
27 Department of Intensive Care, OLVG, Amsterdam, The Netherlands.
28 Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, The Netherlands.
29 Department of Intensive Care, Reinier de Graaf Gasthuis, Delft, The Netherlands.
30 Department of Anesthesia and Intensive Care, Slingeland Ziekenhuis, Doetinchem, The Netherlands.
31 Department of Intensive Care, Spaarne Gasthuis, Haarlem en Hoofddorp, The Netherlands.
32 Department of Intensive Care, UMC Utrecht, Utrecht, The Netherlands.
33 Department of Intensive Care, VieCuri Medisch Centrum, Venlo, The Netherlands.
34 Department of Intensive Care, WZA, Assen, The Netherlands.
35 Department of Intensive Care, IJsselland Ziekenhuis, Capelle aan den IJssel, The Netherlands.
36 Department of Intensive Care, Ziekenhuisgroep Twente, Almelo, The Netherlands.
37 Department of Intensive Care, Ziekenhuis Gelderse Vallei, Ede, The Netherlands.
38 Department of Intensive Care, Zuyderland MC, Heerlen, The Netherlands.
39 Department of Intensive Care Medicine, Het Van Weel-Bethesda Ziekenhuis, Dirksland, The Netherlands.
40 Department of Intensive Care, Bravis Ziekenhuis, Bergen op Zoom en Roosendaal, The Netherlands.
41 Department of Intensive Care, Flevoziekenhuis, Almere, The Netherlands.
42 Department of Intensive Care, LUMC, Leiden, The Netherlands.
43 Department of Intensive Care, MUMC+, University Maastricht, Maastricht, The Netherlands.
44 Department of Intensive Care, Streekziekenhuis Koningin Beatrix, Winterswijk, The Netherlands.
45 Department of Intensive Care Medicine, Hospital St Jansdal, Harderwijk, The Netherlands.
46 Department of Intensive Care, Tergooi Hospital, Hilversum, The Netherlands.
47 Department of Intensive Care Medicine, afdeling Intensive Care, ziekenhuis Tjongerschans, Heerenveen, The Netherlands.
48 Department of Intensive Care, Treant Zorggroep, Emmen, The Netherlands.
49 Department of Computer Science, Quantitative Data Analytics Group, Department of Computer Science, Faculty of Science, VU University, Amsterdam, The Netherlands.
Supplementary Material
Footnotes
The Dutch ICU Data Sharing Against COVID-19 Collaborators are listed in the Acknowledgments.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the Netherlands Organization for Health Research and Development under project number 10430012010003.
The authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Remko van den Akker, Tom A. Rijpstra, M. C. Reuland, Klaas Sierk Arnold, Arend Jan Meinders, Nicolas Schroten, Laura van Manen, Leon Montenij, Julia Koeter, J. W. Fijen, Jasper van Bommel, Roy van den Berg, Martha de Bruin, Roger van Rietschote, Ellen van Geest, Koen S. Simons, Anisa Hana, Joost Labout, Michael Kuiper, Albertus Beishuizen, Bart van de Gaauw, Roos Renckens, B. van den Bogaard, Peter Pickkers, Pim van der Heiden, Dennis Geutjes, Claudia (C. W.) van Gemeren, Emma Rademaker, Frits H. M. van Osch, Johan Lutisan, Jacomar J. M. van Koesveld, Bart P. Grady, Martijn de Kruif, Martin E. Haan, Luca Roggeveen, Dagmar M. Ouweneel, Ronald Driessen, Jan Peppink, G. J. Zijlstra, A. J. van Tienhoven, Evelien van der Heiden, Jan Jaap Spijkstra, Hans van der Spoel, Angelique de Man, Heder J. de Vries, Fuda van Diggelen, Ali el Hassouni, David Romero Guzman, Sandjai Bhulai, Sebastiaan J. J. Vonk, Mattia Fornasa, Tomas Machado, Adam Izdebski, Taco Houwert, Hidde Hovenkamp, Roberto Noorduijn Londono, Davide Quintarelli, Martijn G. Scholtemeijer, Aletta A. de Beer, Giovanni Cinà, Willem E. Herter, Michael de Neree tot Babberich, Olivier Thijssens, Lot Wagemakers, Hilde G. A. van der Pol, Tom Hendriks, Julie Berend, Virginia Ceni Silva, Robert F. J. Kullberg, Leo Heunks, Nicole Juffermans, Arjen J. C. Slooter, Martijn Beudel, and Nicolet F. de Keizer
REFERENCES
- 1.Menk M, Estenssoro E, Sahetya SK, et al. Current and evolving standards of care for patients with ARDS. Intensive Care Med. 2020; 46:2157–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: First update. Crit Care Med. 2021; 49:e219–e234 [DOI] [PubMed] [Google Scholar]
- 3.Tobin MJ. Pondering the atypicality of ARDS in COVID-19 is a distraction for the bedside doctor. Intensive Care Med. 2021; 47:361–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleuren LM, de Bruin DP, Tonutti M, et al. ; Dutch ICU Data Sharing Collaborators. Large-scale ICU data sharing for global collaboration: The first 1633 critically ill COVID-19 patients in the Dutch Data Warehouse. Intensive Care Med. 2021; 47:478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 6.London AJ. Self-defeating codes of medical ethics and how to fix them: Failures in COVID-19 response and beyond. Am J Bioeth. 2021; 21:4–13 [DOI] [PubMed] [Google Scholar]
- 7.Qadir N, Bartz RR, Cooter ML, et al. Variation in early management practices in moderate-to-severe ARDS in the United States: The severe ARDS - generating evidence study. Chest. 2021; 160:1304–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.