Abstract
Stroke remains the leading cause of long-term disability in the US. Although therapy can achieve limited improvement of paretic arm use and performance, weakness and abnormal muscle synergies—which cause unintentional elbow, wrist, and finger flexion during shoulder abduction—contribute significantly to limb disuse and compound rehabilitation efforts. Emerging wearable exoskeleton technology could provide powered abduction support for the paretic arm, but requires a clinically feasible, robust control scheme capable of differentiating multiple shoulder degrees-of-freedom. This study examines whether pattern recognition of sensor data can accurately identify user intent for 9 combinations of 1- and 2- degree-of-freedom shoulder tasks. Participants with stroke (n=12) used their paretic and non-paretic arms, and healthy controls (n=12) used their dominant arm to complete tasks on a lab-based robot involving combinations of abduction, adduction, and internal and external rotation of the shoulder. We examined the effect of arm (paretic, non-paretic), load level (25% vs 50% maximal voluntary torque), and dataset (electromyography, load cell, or combined) on classifier performance. Results suggest that paretic arm, lower load levels, and using load cell or EMG data alone reduced classifier accuracy. However, this method still shows promise. Further work will examine classifier–user interaction during active control of a robotic device and optimization/minimization of sensors.
Keywords: Linear discriminant analysis, Pattern recognition, Stroke, Robotic therapy
I. INTRODUCTION
Stroke remains the leading cause of serious long-term disability in the US [1]. An estimated 7 million individuals in the United State, and 82.9 million individuals worldwide, have experienced a stroke [1] and approximately 30–66% live with permanent upper extremity impairment [2, 3]. In addition to limb weakness (paresis), involuntary co-activation of upper limb muscles after a stroke impedes coordinated use of the paretic arm [4]. This motor discoordination—sometimes described as primitive, automatic, reflexive, obligatory, stereotypical, or whole-limb movement patterns, and referred to here as abnormal flexion or extension synergies—significantly increases difficulty in accomplishing activities of daily living (ADLs) [5]. Abnormal synergies may stem from a neurophysiological reliance on more diffuse corticobulbar-spinal pathways to compensate for damage to, or loss of corticospinal projections due to stroke [6].
Flexion synergy in the upper extremity is observed when lifting the arm against gravity (shoulder abduction), which causes unintentional co-activation of elbow, wrist, and finger flexors, limiting functional use of the affected limb [7]. The complete flexion pattern includes scapular retraction and elevation, shoulder abduction, and external rotation, elbow flexion, forearm supination, and flexion of wrist and digits [8]. However, with support or reduction of proximal shoulder effort, flexion synergy intensity decreases [7], with a subsequent increase in reach distance [5, 9].
Conversely, upper-extremity extension synergy occurs when adducting the humerus against resistance, causing involuntary elbow extension. The full extension synergy includes protraction of the scapula, shoulder adduction and internal rotation, elbow extension, and forearm pronation [8]. The effects of extension synergy on wrist and finger activity are not as predictable, but most commonly still result in flexion of both the wrist and fingers. However, extension synergy is typically not as important or detrimental as flexion synergy, as there is rarely a need to adduct the humerus against resistance.
Both flexion and extension synergies present in proportion to effort. For flexion synergy, small yet significant improvements in unsupported reach distance have been obtained using a training paradigm that takes advantage of this relationship [10, 11]. By progressively increasing the abduction load as reaching goals are met, individuals with stroke are able to reach further without external physical support. However, the maximal potential benefits of this rehabilitation strategy are unknown, possibly due to unknown effects of dosage and limitations on participation/intervention time.
A wearable device that reduces shoulder effort based on user intent and need may provide a novel solution to address activity limitations caused by weakness and abnormal synergies, allowing the user to better engage with their environment, and facilitating other therapeutic interventions to restore elbow, wrist, and hand function. Such devices have been proposed [12–14], and some have recently become commercially available for healthy individuals [15, 16], but appropriate control strategies for individuals with stroke with abnormal synergies are lacking.
Machine learning techniques such as support vector machines, neural networks, and linear discriminant analysis (LDA) have been used with sensor data—e.g., force, acceleration, muscle activity (i.e., electromyography, EMG—to predict user intent [17].In individuals with amputation, EMG pattern recognition has been used to determine user intent to control powered upper [18, 19] and lower [20–22] limb prostheses. LDA-based classifiers have enabled control of multiple degree-of-freedom (DOF) myoelectric prosthese; are generally considered to be accurate, robust, and computationally efficient [23–25]; and have been cleared for commercial sale for individuals with amputation [26, 27].
Successful application of wearable robotic technology in individuals with spinal cord injury [28] or with amputation [23] has paved the way for use in other populations, including individuals with stroke. However, accurately and reliably controlling a wearable robotic device will be a challenging problem in individuals with stroke due to pathological muscle activation.
Incorporating user intent information into the control strategy overcomes the disadvantages of passive or pre-planned movement patterns (i.e., “slacking” and restricted freedom of movement, respectively) by requiring user effort and active participation [29]. Pattern recognition of EMG during shoulder movement was explored as a control system for persons with amputation, and classification error rates below 10% were achieved in a healthy control population [30, 31]. However, for individuals with stroke, classification of user intent for simply opening and closing the paretic hand was negatively affected by lifting at the shoulder at only 25% of maximum effort [32, 33]. EMG from the paretic forearm predicts intended movement with a broad range of outcomes including high [34], mixed [35], and low [36] error rates. EMG has also been used to predict goal-directed reaching with sufficient accuracy in a healthy control population but insufficient accuracy for individuals with stroke[37]. Our previous work [38] demonstrated low classification error (<10%) for 8 isometric shoulder and elbow tasks in a majority of participants with stroke. However, some participants had high error rates, possibly because in our experimental design only a single-DOF was being tested or controlled at any one time. It is possible that participants were consciously or unconsciously completing different tasks using a similar multi-DOF strategy to maximize strength readings. For example, they could have simultaneously maximized external rotation and abduction effort and used that strategy for both external rotation and abduction testing, making the torque and muscle activation patterns similar and difficult to distinguish.
Our present work aims to understand how abnormal synergies due to stroke affect the ability of an LDA-based classifier to discriminate between different 1- and 2-DOF shoulder tasks. We extended our preliminary analysis [39] to evaluate performance of classifiers using data from non-paretic arms of individuals with stroke as well as age- and gender-matched healthy controls. Additionally, we evaluated the effect of lifting-load(25% or 50% maximal voluntary torque) and dataset type (EMG, load cell, or a combined data set) on classification error rate.
We hypothesized that, due to muscle co-activation patterns caused by abnormal synergy after a stroke, classification accuracy of control participants would be higher than that for the paretic arm of the participants with stroke. Classifier performance on the non-paretic (or less-affected [40]) arms was hypothesized to be higher than paretic but lower than control classifier performance. We also hypothesized that classification error for the paretic arm would be higher at higher levels of proximal shoulder effort due to increased synergy presentation. Finally, we hypothesized that the accuracy of classifiers using EMG would be lower than those using raw load cell data, and that classifiers using a single data set would be lower than those using a combined data set.
II. METHODS
A. Participants
All participants provided informed consent to participate in the protocol, which was approved by the Northwestern University Institutional Review Board (IRB #: STU00205835). We recruited 12 age- and gender- matched control participants and 14 participants with chronic (> 1 year) hemiparetic stroke with moderate to severe motor impairments, as determined by a score on the upper-extremity portion of the Fugl-Meyer assessment (FMA-UE) of > 10 and < 45. These individuals exhibit flexion synergy at levels of effort less than limb weight (approximately 50% shoulder abduction strength) and could benefit from using a powered assistive device for the shoulder. Two participants with stroke were excluded from the study: one was unable to accomplish the dual-task protocol and the other had no external rotation strength. Consequently, 12 participants with stroke (50% female, who were, on average, 60.8±10.3 years old, with an FMA-UE score of 26.9 ± 8.4, and 16.8 ± 8.3 years post-stroke, along with 12 control participants (50% female, mean age 59.1 years old ± 9.9) completed the study. For participants with stroke, both arms were tested (non-paretic then paretic); for control participants, only the dominant arm was tested.
B. Setup and instrumentation
To measure maximal isometric strength, participants were seated in a rigid chair (Biodex, Shirley, NY; Model 830–110). Torso movement was minimized by securing them with a lap belt and two chest straps and placing their feet on a foot rest. A fiberglass cast was applied to their anatomically neutral forearm, wrist, and hand to rigidly and securely attach their arm to a 6-DOF load cell (JR3 Inc., Woodland, CA, USA; Model 45E15A). This custom setup was adjusted to place the participant’s arm in 90° of abduction, 45° of horizontal adduction, and 90° of elbow flexion. A licensed physical therapist applied bipolar EMG electrodes (Delsys, Cambridge, MA, USA; 16 channel Bagnoli), with 1cm interelectrode spacing, over 11 muscles—deltoid (anterior, intermediate, and posterior), upper-trapezius, supraspinatus, infraspinatus, latissimus dorsi, teres complex, pectoralis major sternal fibers, biceps brachii, and triceps lateral head)—located using guidelines set forth in Anatomical Guide for the Electromyographer [41] using palpation and anatomical landmarks. A ground electrode was placed over the acromion. Maximal torque measurements (Section II.C) were collected in this setup to minimize wear on the ACT3D robot (Section II.D)
C. Isometric maximal voluntary torque measurements
Maximal isometric voluntary torques were determined for six movements: shoulder abduction, adduction, external and internal rotation, and elbow flexion and extension and used to inform the second part of the experiment. Verbal encouragement was provided to ensure maximal effort. Forces, moments, and EMG were collected at 1 kHz.
D. Dual task setup on ACT3D
Participants were then moved to a customized ACT3D haptic master robot [42, 43] with a 6-DOF load cell (JR3 Inc., Woodland, CA, USA; Model 51E20A) attached under the end effector. The load cell allows precise control of abduction and adduction (vertical) loading while simultaneously measuring forces and moments in three orthogonal directions. Participants were secured in the rigid chair as described in Section II.B and connected to the ACT3D with a custom setup that centered the medial epicondyle (center of rotation of elbow) over the load cell. The custom device clamped the medial and lateral epicondyles between foam pads, and secured the cast to the device at the forearm (Fig. 1A, top). The arm position was similar to that described for isometric torque measurements. Movement in the transverse plane was constrained and a “ceiling” and “floor” were created to prevent elbow movement beyond 5 cm above and below 90° of abduction, respectively. This enabled movement in the vertical direction equating to approximately ± 10° of abduction/adduction while minimizing change of alignment of the medial epicondyle over the load cell (0.4 cm).
Fig. 1.
(A) Top: depiction of setup with participant connected to ACT3D robot. Arrows indicate that the robot can move and control the load applied in the vertical direction. This participant provided written agreement to the use of this image. Middle: real-time feedback of internal/external rotation isometric torque, Bottom: data acquisition and conditioning details. (B) Top: Raw force and moment data received from load cell mounted under participant’s elbow. Middle: Raw EMG for infraspinatus of a control participant during a 25% adduction, external rotation trial. Bottom: Steps involved in classification of raw data.
E. Dual task protocol
Participants were required to abduct (lift, +) and adduct (depress, −) at 0, ± 25, and ± 50% of their maximum joint torque determined by isometric testing. For 0%, the entire limb weight was supported by the ACT3D, which is equivalent to no effort. Each trial lasted 10 seconds. For the first 5 seconds the participant was asked to move and maintain their arm between the ceiling and floor, with proprioceptive and visual feedback from their limb. At 5 seconds the participant was provided a verbal and visual cue via a graphical user interface (Fig. 1A middle) to begin either maximal isometric external or internal rotation while continuing to keep their arm off the horizontal surfaces. The order of testing conditions was randomized. A minimum of 3 and a maximum of 10 trials of each condition were completed with the goal of having 3 trials with isometric humeral rotation maximums within 10% of each other and at least 3 seconds of active rotation. This resulted in data for 3 trials of 10 conditions (0, ± 25, ± 50% abduction/adduction for both external and internal rotation).
F. Data Processing
The Delsys EMG collection system bandpass filtered signals between 20–450 Hz, and force and moment data were transformed to joint torques. External and internal rotation was calculated for each trial. For the subsequent analysis, we used the three trials from each condition in which the greatest humeral rotational torques were achieved and the arm was maintained off of the horizontal surfaces during the periods of interest (1.5s-4.5s and 6.5–9.5s). Representative joint torques and normalized EMG data for each group are presented in Fig. 2. The purpose of two time segments was to extract pure abduction-, adduction-, or no- loading time (occurs during first 5 seconds) as opposed to dual-task time (occurs during last 5 seconds). Different load-levels (0, 25, and 50% of abduction and adduction) were used in attempt to elicit different degrees of the abnormal synergy so we could run an analysis of the effect of lifting effort on classification accuracy.
Fig. 2.
Visual comparison of representative trials of participants from each arm type for 25% adduction (first 5 seconds) followed by external rotation (last 5 seconds). Top row is calculated shoulder joint torques (blue = adduction(−)/abduction(+), red = internal rotation(−)/external rotation(+), yellow = horizontal adduction (−)/abduction(+). Bottom 3 rows are normalized root mean squared EMG over 200 ms windows for the posterior deltoid, infraspinatus, and pectoralis major muscles.
G. Classification
Two sets of LDA-based classifiers were created from each dataset type: EMG data, raw load cell data, and a combined dataset (EMG and raw load cell data, appended post feature extraction). One set of classifiers was created using the 0% and ±25% lifting condition data and the second set of classifiers was created using the 0% and ±50% lifting condition data. The data from the 0% lift condition was used as a baseline in both sets of classifiers to create no movement, external rotation only, and internal rotation only classes.
Data collected between 1.5s to 4.5s and between 6.5 and 9.5s in each trial were extracted and labeled according to the task being accomplished during each time period. Data were windowed into 200ms windows using 20ms steps (180ms overlap). This allowed the load cell data recorded at 50 Hz and the EMG data recorded at 1000 Hz to be combined after windowing without down-sampling. Four time-domain features (mean absolute value, zero-crossing, slope sign change, and waveform length) [44] were extracted from EMG data and mean absolute values were extracted for load cell data. These features have proven accurate and robust in other myoelectric control applications [45].
Classifiers were trained and tested using a trial-wise leave-one-out cross validation, i.e., two of the three trials were used to create a classifier and tested against the third. Each combination was tested and classification accuracies for each dataset were averaged within and across participants for comparison.
H. Statistics
Two linear mixed-effects models (equation 1) were used to test the effect of the fixed factors: arm type (non-paretic vs paretic and non-paretic vs control), load level (25%, 50%), and dataset type (EMG, load cell, combined) on classification error rate. Two models were used since only the dominant arm of the control group was tested making the design unbalanced. One benefit of using a linear model is that it allows an analysis of the effect of each factor (magnitude and direction) as opposed to a pure comparison of group means. A mixed-effect model was chosen to allow individuals to be treated as random effects so each could have their own response. The model also included arm * load and arm* dataset interaction terms to detect any interaction between these terms. This would be expected if, as we hypothesized, load level or dataset behaved differently for each arm-group; specifically we expected that load level and paretic arm would have an interaction with heavier lift, yielding greater error rates due to increased presentation of abnormal synergy. These models and post-hoc statistical analysis were completed using MiniTab ®Statistical Software (Minitab, Inc., State College, PA, USA). Main effects (arm, load, dataset) are implicit in this model.
| (1) |
III. RESULTS
A. Linear mixed-effects model (LMEM)
A summary of results from the two models is presented in Tables I (paretic vs non-paretic) and II (control vs non-paretic). No interaction terms were significant for either model. Significant differences exist between non-paretic and paretic arms (p = <0.00001) and lift at 25% vs 50% (p=0.00083) with estimated pattern recognition classification error differences of −8.6% and 3.63%, respectively. Thus classification error for paretic arms increased by 8.6% compared to non-paretic arms and lifting at 50% maximum joint torque improved classification by 3.63% over lifting at 25%. The model also predicted the model coefficient for the combined dataset classifier as better than the load cell data classifier by 4.09%, and the load cell classifier was better than the EMG classifier by 3.14%. Similar results were found in the comparison between the control group and the non-paretic arm group (Table II). The classifiers built using the control arm, 50% load level, and the combined dataset performed better than all other classifiers.
TABLE I.
NON-PARETIC VS PARETIC LMEM RESULTS
| NP vs P | F | p-value | Compare | Δ coef |
|---|---|---|---|---|
| Arm | 66.22 | <0.0001 | NP vs P | −8.62 |
| Load Level |
11.73 | 0.0008 | 25% vs 50% | 3.63 |
| Dataset | 16.31 | <0.0001 | Comb vs LC EMG vs LC |
−4.09 3.14 |
TABLE II.
CONTROL VS NON-PARETIC LMEM RESULTS
| C vsNP | F | p-value | Compare | Δ coef |
|---|---|---|---|---|
| Arm | 9.8 | 0.0049 | C vs NP | −4.52 |
| Load Level |
8.22 | 0.0049 | 25% vs 50% | 2.00 |
| Dataset | 12.69 | <0.0001 | Comb vs LC EMG vs LC |
−2.45 0.87 |
B. Classifier Performance
Table III shows the confusion matrix for the combined dataset of the participants with stroke for the 50% load level condition. Each row represents what the user intended (actual) while the predicted classes are represented by the columns. Grey shading indicates single-DOF conditions.
TABLE III.
CONFUSION MATRIX FOR PARETIC, 50% LIFT, COMBINED DATASET
|
|
AD = adduction, AB = abduction, ER = external rotation, IR = internal rotation, Mvt = movement.
Group averages of classification error rate for the 25% lift condition are presented in Table IV and the 50% lift condition in Table V, with standard deviations presented within parentheses (n=12 for each group and condition).
TABLE IV.
AVERAGE CLASSIFICATION ERROR FOR 25% LIFT CONDITION
| 25% | EMG | LC | Comb | Avg. |
|---|---|---|---|---|
| P | 24.2(13.7) | 20.0(11.6) | 14.0(11.3) | 19.4 (12.6) |
| NP | 11.3 (6.7) | 11.0(10.4) | 4.9 (4.1) | 9.1 (7.9) |
| C | 5.1 (3.1) | 6.4 (7.9) | 2.3 (2.1) | 4.6 (5.2) |
| Avg. | 13.5(11.9) | 12.4(11.3) | 7.0 (8.5) |
P = paretic, NP= non-paretic, C = Control arms; LC = load cell data, Comb = combined data; Avg. = average.
TABLE V.
AVERAGE CLASSIFICATION ERROR FOR 50% LIFT CONDITION
| 50% | EMG | LC | Comb | Avg. |
|---|---|---|---|---|
| P | 18.3 (10.3) | 14.1 (9.3) | 9.7 (7.0) | 14.1 (9.4) |
| NP | 8.4 (4.2) | 8.3 (5.3) | 4.7 (3.5) | 7.1 (4.7) |
| C | 1.9 (1.0) | 4.0 (3.9) | 1.7 (2.6) | 2.5 (2.9) |
| Avg. | 9.5 (9.3) | 8.8 (7.6) | 5.4 (5.8) |
P = paretic, NP= non-paretic, C = Control arms; LC = load cell data, Comb = combined data; Avg. = average.
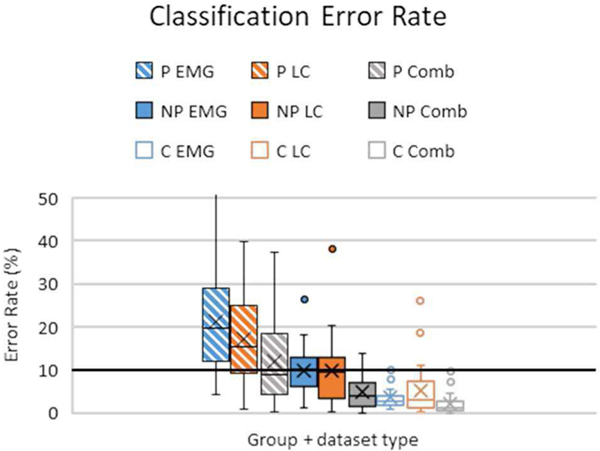
Averaged results from each group and dataset are presented in Fig 3. Although the 50% lift condition was significantly better than the 25% condition, the two levels are averaged for figure clarity since their differences were relatively small.
Fig. 3.
Summary of classifier error rates for all groups and dataset types. Rectangular boxes indicate 1st − 3rd interquartile range, with ‘X’ indicating means, horizontal bars are medians, and error bars indicate standard deviation. The bold horizontal line denotes a 10% error rate controllability cutoff for myoelectric devices [44]. P = paretic, NP = non-paretic, C = Control arms; LC = load cell data, Comb = combined data.
Post-hoc comparisons of dataset performance revealed that the combined dataset performed significantly better than both the load cell and EMG dataset (p=0.0005, p<0.0001 respectively for paretic vs non-paretic and p<0.0001, p=0.0005 respectively for control vs non-paretic). Performance of load cell and EMG datasets alone were not significantly different from each other (p=0.212 for paretic vs non-paretic and p=0.679 for control vs non- paretic).
IV. DISCUSSION
The aim of our study was to investigate the feasibility of determining user intent for movement of the shoulder of the paretic limb in individuals with stroke. This approach could allow future development of an exoskeleton to provide abduction support for the paretic arm. In particular, we were interested in the relationship between controlling internal/external rotation and shoulder abduction/adduction simultaneously. Overall, we found that using a pattern recognition system based on the combination of EMG and load cell information in individuals with stroke resulted in an average error rate as low as 9.7%. These results are promising as related work in controlling myoelectric prostheses has shown that systems with error rates in the range of 0 – 10% allow good control of a device [46]. Control system error rates as high as 35% can be used, but result in greater variability in performance between individuals. Thus, our next phase of research is to implement the combined control system on an embedded system to allow real-time control of a lab-based robot.
We hypothesized that classification of control limb data would be better than non-paretic arm data, which would be better than paretic arm data. Our results support this hypothesis. Differences in classifier error rate between paretic and non-paretic arms are likely due to stroke sequelae (weakness, spasticity, etc) caused by the damage to the cortical neurons. Additional weakness could be attributed to limb disuse.
Classification accuracies using data from non-paretic limbs were significantly lower than from control arm data, although still within a useable range. We speculate that the difference may be related to damage due to stroke, possibly in structures that are bilaterally activated. We did not use imaging to locate stroke-related damage nor did we control for stroke location. While the FMA-UE confirmed hemiparesis within a specified range, this test has a known ceiling effect for detecting deficits in a non-paretic limb [47]. A more detailed evaluation of the non-paretic limb was beyond the scope of this study; however, we acknowledge that the “less affected” limb is known to have subtle impairments after stroke [40] that may have reduced classifier accuracy compared to controls.
Abnormal flexion synergy that presents proportional to proximal shoulder effort [9] and represents the primary impairment contributing to reaching dysfunction [48], did not seem to explain any error since greater shoulder effort caused an improvement in classification accuracy similar to the other groups. In fact, the 50% lift condition was significantly better than the 25% condition. Thus, we reject our hypothesis that the greater loading condition (50%) has a negative effect on classification accuracy. It may be that even at low loading levels, abnormal synergy is fully expressed in the shoulder DOFs, thus higher loads do not cause a further decrease in classification accuracy. Evaluation of torque coupling within the shoulder was beyond the scope of this discussion but may shed light on the possibility. These results are intuitive in retrospect, if we ignore the possible effects of abnormal synergy, due to the inner workings of an LDA. An LDA linearly separates groups by maximizing distance between means and minimizing overlap of variance. A higher load level would create greater distance between data clusters, which would result in better classification accuracy. The paretic arms were weaker, as expected post-stroke, thus reducing separation between the classes. Additionally, the no-movement class was based on 0% effort which may have some amount of torque generated in other directions and/or co-contraction with concurrent EMG generation. Greater abduction and adduction effort required in the 50% condition generate greater distance between data clusters from the 0% condition compared to the 25% condition, thus increasing classification accuracy.
Classifier performance using load cell data was not statistically different than using EMG data; however, the combination of both types of data performed significantly better. Therefore, we can accept the hypothesis that the combined data set provided the best classification accuracy yet reject the hypothesis that load cell data would perform better than EMG. This indicates that the load cell and the EMG sensors provide some complementary information as Huang et al also found [49]. We expected that the load cell data would outperform EMG because EMG signals are noisy and difficult to measure from small or deep muscles involved in movement while load cells are sensitive and accurate to all changes in forces and moments. Since we used 11 EMG sensors on the back, shoulder, and arm, it is possible that we were able to overcome these disadvantages. Unlike the muscles in the forearm or arm which are commonly used to control many degrees of freedom of the hand, wrist, and elbow after amputation, the muscles that accomplish the actions tested in this study are larger and have a greater amount of separation possibly enabling higher levels of classification accuracy. Surface EMG sensors are inexpensive, non-invasive, and easy to apply but come with their own disadvantages including sensitivity to sweat, movement, and fatigue and must be applied generally in the correct area and orientation [50]. Implantable EMG sensors could be a solution to these disadvantages and is a promising avenue of exploration. Further analysis is needed to determine if common muscle sites provided the most useful information across participants or groups and how much the number of sensors could be reduced. We suspect there is an optimal subset of surface EMG channels, maybe 4 to 6, for each individual that may not be shared across groups or participants similar to the results found in Hargrove 2007 [51]. A detailed analysis extends beyond the scope of the present study.
While mounting a load-cell to the ACT3D robot was straightforward, incorporating a load cell to measure forces and moments from a paretic limb in a portable wearable device has challenges. Although not considered in this study, data from other sensors could be incorporated into a pattern recognition approach. For example, force sensitive resistors used to measure interface pressures or inertial sensors to measure joint orientation could provide valuable information. Classification error rates were higher using single sources of information compared to using combined EMG and load cell data, but still showed promise. EMG data may be sufficient on its own but classification accuracy could be improved using other sensors that would be easier to implement than a load cell [49, 50].
As depicted by the confusion matrix of paretic arm data in Table III there were increased errors between the single task motions, internal and external rotation, and the no movement class. Additional significant error existed between the external rotation dual-tasks and their corresponding 1-DOF lift condition counterpart (e.g. adduction + external rotation being confused as pure adduction). Determining the underlying cause of these errors is outside the scope of this study but we offer two hypotheses to explain them.
These sources of error could be explained by arm weakness; participants may not generate enough torque or EMG during these tasks to allow these classes to be distinguished from others. In addition to rotating the humerus, rotator cuff muscles are also used to maintain dynamic stability within the glenohumeral joint [52]. Additionally, muscles that adduct (latissimus dorsi, pectoralis major, and teres major) also internally rotate the humerus [53]. Together, these factors may limit the amount of external rotation torque that can be generated during adduction. These anatomical/neuromuscular phenomenon are present in all arms/populations but may only affect the classification accuracies of the paretic arm due to its underlying weakness.
Additionally, a loss of independent DOF control within the shoulder joint, similar to that observed between joints (shoulder, elbow, and hand) [7, 54, 55], could account for diminished classification accuracies in the paretic arm. In the present example, a reduced ability to produce external rotation torque during adduction would be considered a “task-dependent weakness” in the context of loss of independent joint/DOF control [56], further reducing the distance between means and increasing classification error in an LDA. In fact, abnormal co-contraction of each part of the deltoid has also been found in paretic arms of individuals with stroke [57].
We performed an offline analysis to compute the classification error rate of the collected data. The relationship between offline analysis and real-time performance is an open topic with some studies showing weak or no correlation [58], with others showing positive correlation within certain ranges of the classification error rate metric [46]. One drawback of offline analyses of pattern recognition for amputees is that they do not have proprioceptive or visual feedback of their attempted movements. In our study, participants had force and proprioceptive feedback from their limbs (although these feedback mechanisms may have been altered as a result of the stroke) and real-time visual feedback from their arm and the graphical user interface. Thus while our analysis was performed offline, the participants were attempting to perform real-time movements and did have access to feedback. Future work controlling an actual device will investigate how users’ response to generated errors affects their performance.
This study is limited by the small sample size for the number of conditions tested, the minimal amount of movement permitted only in one degree of freedom, and the use of a large amount of sensors, possibly limiting the generalizability of these findings to an entire population or to a future control scheme. Despite these limitations, this work is a logical and necessary step towards understanding user-in-the-loop control of a device that can minimize expression of abnormal synergy within the paretic population.
This work extends our prior work [38] by comparing the non-paretic arm and healthy control arms with the paretic arm. Additionally, instead of a pure isometric 1-DOF task we used a mixed, partly-dynamic partly-isometric task enabling intuitive control of an additional DOF. In our previous study, participants were asked to generate their maximum strength in one of eight directions without regard to what torques were being generated in the other directions. This made it impossible to determine if classification errors were due to limitations caused by the stroke or by selection of a strategy or preference in completing the task. By imposing a dual task paradigm, we ensured that each participant was controlling what was happening in the directions that were most commonly confused in the previous study (abduction/adduction and internal/external rotation). By incorporating a mixed dynamic and isometric task, we moved towards a less constrained task and enabled intuitive control of torque generation in one degree of freedom while measuring maximum torque generation in the other. As a result, we believe that the higher error rates in our prior work were caused by the strategy selected by certain participants to achieve the task rather than their impairments due to stroke or the capability of a pattern recognition system to classify the data.
V. CONCLUSION
Using a testing paradigm that enabled control of the level of effort in one DOF via dynamic movement along with inherent proprioceptive and visual feedback enabled us to begin to better discern the ability of an LDA-based classifier to determine user intent in single- and dual- shoulder tasks after a stroke. Using linear mixed-effects models, estimates of contribution to classifier accuracy, in addition to group differences were obtained. This provided insight not only into which groups were different but in which direction and by how much. Although the classifier trained with paretic arm data performed the worst, sufficient classification accuracies suggest that future work is warranted to examine active control of a robotic device using LDA-based classification for this population.
ACKNOWLEDGMENT
We would like to acknowledge the continued outstanding support provided by the staff at the Dept. of Physical Therapy and Human Movement Sciences, Northwestern University Feinberg School of Medicine and at the Center for Bionic Medicine, Shirley Ryan AbilityLab. Additionally, we acknowledge Ann Barlow who made significant improvements to our manuscript through her editing.
REFERENCES
- [1].Benjamin EJ et al. , “Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association,” Circulation, p. CIR0000000000000659, January 31 2019, doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [2].Kwakkel G, Kollen BJ, and Krebs HI, “Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review,” Neurorehabil Neural Repair, vol. 22, no. 2, pp. 111–21, Mar-Apr 2008, doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anderson CS, Linto J, and Stewartwynne EG, “A Population-Based Assessment of the Impact and Burden of Caregiving for Long-Term Stroke Survivors,” (in English), Stroke, vol. 26, no. 5, pp. 843–849, May 1995, doi: Doi 10.1161/01.Str.26.5.843. [DOI] [PubMed] [Google Scholar]
- [4].Dewald JP, Pope PS, Given JD, Buchanan TS, and Rymer WZ, “Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects,” Brain, vol. 118 ( Pt 2), pp. 495–510, April 1995. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/7735890. [DOI] [PubMed] [Google Scholar]
- [5].Ellis MD, Sukal T, DeMott T, and Dewald JP, “Augmenting clinical evaluation of hemiparetic arm movement with a laboratory-based quantitative measurement of kinematics as a function of limb loading,” Neurorehabil. Neural Repair, vol. 22, no. 4, pp. 321–9, Jul-Aug 2008, doi: 10.1177/1545968307313509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McPherson JG, Chen A, Ellis MD, Yao J, Heckman CJ, and Dewald JPA, “Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke,” J Physiol, February 19 2018, doi: 10.1113/JP274968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller LC and Dewald JP, “Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke,” Clin. Neurophysiol, vol. 123, no. 6, pp. 1216–25, June 2012, doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].O’Sullivan SB and Schmitz TJ, Physical rehabilitation. Philadelphia: F.A. Davis; (in English), 2007. [Google Scholar]
- [9].Sukal TM, Ellis MD, and Dewald JP, “Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications,” Exp Brain Res, vol. 183, no. 2, pp. 215–23, November 2007, doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ellis MD, Sukal-Moulton T, and Dewald JPA, “Progressive Shoulder Abduction Loading is a Crucial Element of Arm Rehabilitation in Chronic Stroke,” (in English), Neurorehab Neural Re, vol. 23, no. 8, pp. 862–869, October 2009, doi: 10.1177/1545968309332927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ellis MD, Carmona C, Drogos J, and Dewald JPA, “Progressive Abduction Loading Therapy with Horizontal-Plane Viscous Resistance Targeting Weakness and Flexion Synergy to Treat Upper Limb Function in Chronic Hemiparetic Stroke: A Randomized Clinical Trial,” Front Neurol, vol. 9, p. 71, 2018, doi: 10.3389/fneur.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Simpson CS, Okamura AM, and Hawkes EW, “Exomuscle: An inflatable device for shoulder abduction support,” in 2017 IEEE International Conference on Robotics and Automation (ICRA), 29 May-3 June 2017 2017, pp. 6651–6657, doi: 10.1109/ICRA.2017.7989785. [DOI] [Google Scholar]
- [13].Neill CTO, Phipps NS, Cappello L, Paganoni S, and Walsh CJ, “A soft wearable robot for the shoulder: Design, characterization, and preliminary testing,” in 2017 International Conference on Rehabilitation Robotics (ICORR), 17–20 July 2017 2017, pp. 1672–1678, doi: 10.1109/ICORR.2017.8009488. [DOI] [PubMed] [Google Scholar]
- [14].Lenzi T. et al. , “The neuro-robotics paradigm: NEURARM, NEUROExos, HANDEXOS,” in 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 3–6 Sept. 2009 2009, pp. 2430–2433, doi: 10.1109/IEMBS.2009.5334957. [DOI] [PubMed] [Google Scholar]
- [15].“Exoskeleton Report: Arm Support.” https://exoskeletonreport.com/product-category/exoskeleton-catalog/industrial/shoulder-support-exoskeleton-for-work-and-industry/ (accessed 06 May, 2019). [Google Scholar]
- [16].“MATE Exoskeleton by Comau Boosts Workers’ Strength and Performance without Using Electric Power.” https://www.wearabletechnologies.com/2018/10/mate-exoskeleton-by-comau-boosts-workers-strength-and-performance-without-using-electric-power/ (accessed 06 May, 2019). [Google Scholar]
- [17].Novak D. and Riener R, “A survey of sensor fusion methods in wearable robotics,” (in English), Robot Auton Syst, vol. 73, pp. 155–170, November 2015, doi: 10.1016/j.robot.2014.08.012. [DOI] [Google Scholar]
- [18].Kuiken TA, Miller LA, Turner K, and Hargrove LJ, “A Comparison of Pattern Recognition Control and Direct Control of a Multiple Degree-of-Freedom Transradial Prosthesis,” IEEE J Transl Eng Health Med, vol. 4, p. 2100508, 2016, doi: 10.1109/JTEHM.2016.2616123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hargrove LJ, Lock BA, and Simon AM, “Pattern recognition control outperforms conventional myoelectric control in upper limb patients with targeted muscle reinnervation,” vol. 2013, pp. 1599–602, 2013. [DOI] [PubMed] [Google Scholar]
- [20].Hargrove LJ, Simon AM, Lipschutz RD, Finucane SB, and Kuiken TA, “Real-time myoelectric control of knee and ankle motions for transfemoral amputees,” vol. 305, no. 15, pp. 1542–4, April 20 2011. [DOI] [PubMed] [Google Scholar]
- [21].Hargrove LJ et al. , “Intuitive control of a powered prosthetic leg during ambulation: a randomized clinical trial,” vol. 313, no. 22, pp. 2244–52, June 09 2015. [DOI] [PubMed] [Google Scholar]
- [22].Hargrove LJ et al. , “Robotic leg control with EMG decoding in an amputee with nerve transfers,” vol. 369, no. 13, pp. 1237–42, September 26 2013. [DOI] [PubMed] [Google Scholar]
- [23].Geethanjali P, “Myoelectric control of prosthetic hands: state-of-the-art review,” Med Devices (Auckl), vol. 9, pp. 247–55, 2016, doi: 10.2147/MDER.S91102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scheme E. and Englehart K, “Electromyogram pattern recognition for control of powered upper-limb prostheses: state of the art and challenges for clinical use,” J Rehabil Res Dev, vol. 48, no. 6, pp. 643–59, 2011. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/21938652. [DOI] [PubMed] [Google Scholar]
- [25].Hakonen M, Piitulainen H, and Visala A, “Current state of digital signal processing in myoelectric interfaces and related applications,” (in English), Biomed Signal Proces, vol. 18, pp. 334–359, April 2015, doi: 10.1016/j.bspc.2015.02.009. [DOI] [Google Scholar]
- [26].“Coapt Engineering Technology.” https://www.coaptengineering.com/technology.html (accessed 06 May, 2019). [Google Scholar]
- [27].“IBT Products.” https://www.i-biomed.com/products.html (accessed 06 May, 2019). [Google Scholar]
- [28].Nam KY, Kim HJ, Kwon BS, Park JW, Lee HJ, and Yoo A, “Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review,” J Neuroeng Rehabil, vol. 14, no. 1, p. 24, March 23 2017, doi: 10.1186/s12984-017-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blank AA, French JA, Pehlivan AU, and O’Malley MK, “Current Trends in Robot-Assisted Upper-Limb Stroke Rehabilitation: Promoting Patient Engagement in Therapy,” Curr Phys Med Rehabil Rep, vol. 2, no. 3, pp. 184–195, September 2014, doi: 10.1007/s40141-014-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rivela D, Scannella A, Pavan EE, Frigo CA, Belluco P, and Gini G, “Analysis and Comparison of Features and Algorithms to Classify Shoulder Movements From sEMG Signals,” IEEE Sensors Journal, vol. 18, no. 9, pp. 3714–3721, 2018, doi: 10.1109/JSEN.2018.2813434. [DOI] [Google Scholar]
- [31].Buerkle VR, Englehart K, and Hudgins B, “Pattern recognition of single and combined motions from the shoulder complex,” Conf Proc IEEE Eng Med Biol Soc, vol. 1, pp. 3419–22, 2006, doi: 10.1109/IEMBS.2006.260392. [DOI] [PubMed] [Google Scholar]
- [32].Lan YY, Yao J, and Dewald JPA, “The Impact of Shoulder Abduction Loading on Volitional Hand Opening and Grasping in Chronic Hemiparetic Stroke,” (in English), Neurorehab Neural Re, vol. 31, no. 6, pp. 521–529, June 2017, doi: 10.1177/1545968317697033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lan YY, Yao J, and Dewald JPA, “The Impact of Shoulder Abduction Loading on EMG-based Intention Detection of Hand Opening and Closing After Stroke,” (in English), 2011 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc), pp. 4136–4139, 2011. [Online]. Available: < Go to ISI>://WOS:000298810003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang X. and Zhou P, “High-Density Myoelectric Pattern Recognition Toward Improved Stroke Rehabilitation,” (in English), Ieee T Bio-Med Eng, vol. 59, no. 6, pp. 1649–1657, June 2012, doi: 10.1109/Tbme.2012.2191551. [DOI] [PubMed] [Google Scholar]
- [35].Lu Z, Tong R. K. y., Zhang X, Li S, and Zhou P, “Myoelectric Pattern Recognition for Controlling a Robotic Hand: A Feasibility Study in Stroke,” Ieee T Bio-Med Eng, pp. 1–1, 2018, doi: 10.1109/TBME.2018.2840848. [DOI] [PubMed] [Google Scholar]
- [36].Zhang SQ, Zhang X, Cao S, Gao XP, Chen X, and Zhou P, “Myoelectric Pattern Recognition Based on Muscle Synergies for Simultaneous Control of Dexterous Finger Movements,” (in English), Ieee T Hum-Mach Syst, vol. 47, no. 4, pp. 576–582, August 2017, doi: 10.1109/Thms.2017.2700444. [DOI] [Google Scholar]
- [37].Cesqui B, Tropea P, Micera S, and Krebs HI, “EMG-based pattern recognition approach in post stroke robot-aided rehabilitation: a feasibility study,” (in English), J Neuroeng Rehabil, vol. 10, July 15 2013, doi: Artn 75 10.1186/1743-0003-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kopke JV, Hargrove LJ, and Ellis MD, “Applying LDA-based pattern recognition to predict isometric shoulder and elbow torque generation in individuals with chronic stroke with moderate to severe motor impairment,” (in English), J Neuroeng Rehabil, vol. 16, March 5 2019, doi: ARTN 35 10.1186/s12984-019-0504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kopke JV, Hargrove LJ, and Ellis MD, “Application of an LDA Classifier for Determining User-Intent in Multi-DOF Quasi-Static Shoulder Tasks in Individuals with Chronic Stroke: Preliminary Analysis,” Conf Proc IEEE Eng Med Biol Soc, vol. 2018, pp. 2312–2315, July 2018, doi: 10.1109/EMBC.2018.8512787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pohl PS and Winstein CJ, “Practice effects on the less-affected upper extremity after stroke,” Arch Phys Med Rehabil, vol. 80, no. 6, pp. 668–75, June 1999. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/10378493. [DOI] [PubMed] [Google Scholar]
- [41].Perotto A. and Delagi EF, Anatomical guide for the electromyographer : the limbs and trunk, 3rd ed. Springfield, Ill., USA: Charles C. Thomas, 1994, pp. xvii, 309 p. [Google Scholar]
- [42].Sukal TM, Ellis MD, and Dewald JPA, “Evaluation and intervention in the paretic upper extremity following hemiparetic stroke using the ACT (3D) system,” (in English), P Ieee Ras-Embs Int, pp. 809-+, 2006. [Online]. Available: < Go to ISI>://WOS:000244445100139. [Google Scholar]
- [43].Ellis MD, Lan YY, Yao J, and Dewald JPA, “Robotic quantification of upper extremity loss of independent joint control or flexion synergy in individuals with hemiparetic stroke: a review of paradigms addressing the effects of shoulder abduction loading,” (in English), J Neuroeng Rehabil, vol. 13, October 29 2016, doi: ARTN 95 10.1186/s12984-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hudgins B, Parker P, and Scott RN, “A New Strategy for Multifunction Myoelectric Control,” (in English), Ieee T Bio-Med Eng, vol. 40, no. 1, pp. 82–94, January 1993, doi: Doi 10.1109/10.204774. [DOI] [PubMed] [Google Scholar]
- [45].Englehart K. and Hudgins B, “A robust, real-time control scheme for multifunction myoelectric control,” (in English), Ieee T Bio-Med Eng, vol. 50, no. 7, pp. 848–854, July 2003, doi: 10.1109/Tmbe.2003.813539. [DOI] [PubMed] [Google Scholar]
- [46].Young AJ, Hargrove LJ, and Kuiken TA, “The effects of electrode size and orientation on the sensitivity of myoelectric pattern recognition systems to electrode shift,” IEEE Trans Biomed Eng, vol. 58, no. 9, pp. 2537–44, September 2011, doi: 10.1109/TBME.2011.2159216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, and Piacentino A, “Assessing Wolf Motor function Test as outcome measure for research in patients after stroke,” (in English), Stroke, vol. 32, no. 7, pp. 1635–1639, July 2001, doi: Doi 10.1161/01.Str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- [48].Ellis MD, Schut I, and Dewald JPA, “Flexion synergy overshadows flexor spasticity during reaching in chronic moderate to severe hemiparetic stroke,” (in English), Clin Neurophysiol, vol. 128, no. 7, pp. 1308–1314, July 2017, doi: 10.1016/j.clinph.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huang H, Zhang F, Hargrove LJ, Dou Z, Rogers DR, and Englehart KB, “Continuous Locomotion-Mode Identification for Prosthetic Legs Based on Neuromuscular-Mechanical Fusion,” (in English), Ieee T Bio-Med Eng, vol. 58, no. 10, pp. 2867–2875, October 2011, doi: 10.1109/Tbme.2011.2161671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Young AJ, Kuiken TA, and Hargrove LJ, “Analysis of using EMG and mechanical sensors to enhance intent recognition in powered lower limb prostheses,” J Neural Eng, vol. 11, no. 5, p. 056021, October 2014, doi: 10.1088/1741-2560/11/5/056021. [DOI] [PubMed] [Google Scholar]
- [51].Hargrove LJ, Englehart K, and Hudgins B, “A comparison of surface and intramuscular myoelectric signal classification,” (in English), Ieee T Bio-Med Eng, vol. 54, no. 5, pp. 847–853, May 2007, doi: 10.1109/Tbme.2006.889192. [DOI] [PubMed] [Google Scholar]
- [52].Wilk KE, Arrigo CA, and Andrews JR, “Current concepts: The stabilizing structures of the glenohumeral joint,” (in English), J Orthop Sport Phys, vol. 25, no. 6, pp. 364–379, June 1997, doi: DOI 10.2519/jospt.1997.25.6.364. [DOI] [PubMed] [Google Scholar]
- [53].Ackland DC and Pandy MG, “Moment Arms of the Shoulder Muscles during Axial Rotation,” (in English), J Orthop Res, vol. 29, no. 5, pp. 658–667, May 2011, doi: 10.1002/jor.21269. [DOI] [PubMed] [Google Scholar]
- [54].Dewald JP, Sheshadri V, Dawson ML, and Beer RF, “Upper-limb discoordination in hemiparetic stroke: implications for neurorehabilitation,” Top Stroke Rehabil, vol. 8, no. 1, pp. 1–12, Spring 2001, doi: 10.1310/WA7K-NGDF-NHKK-JAGD. [DOI] [PubMed] [Google Scholar]
- [55].Ellis MD, Sukal T, DeMott T, and Dewald JPA, “ACT(3D) exercise targets gravity-induced discoordination and improves reaching work area in individuals with stroke,” (in English), Int C Rehab Robot, pp. 890–895, 2007, doi: Doi 10.1109/Icorr.2007.4428529. [DOI] [Google Scholar]
- [56].Beer RF, Given JD, and Dewald JPA, “Task-dependent weakness at the elbow in patients with hemiparesis,” (in English), Arch Phys Med Rehab, vol. 80, no. 7, pp. 766–772, July 1999, doi: Doi 10.1016/S0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- [57].Roh J, Rymer WZ, Perreault EJ, Yoo SB, and Beer RF, “Alterations in upper limb muscle synergy structure in chronic stroke survivors,” (in English), J Neurophysiol, vol. 109, no. 3, pp. 768–781, February 2013, doi: 10.1152/jn.00670.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jiang N, Vujaklija I, Rehbaum H, Graimann B, and Farina D, “Is Accurate Mapping of EMG Signals on Kinematics Needed for Precise Online Myoelectric Control?,” Ieee T Neur Sys Reh, vol. 22, no. 3, pp. 549–558, 2014, doi: 10.1109/TNSRE.2013.2287383. [DOI] [PubMed] [Google Scholar]