Abstract
Introduction
Single-agent belantamab mafodotin (belamaf; BLENREP) demonstrated deep and durable responses in patients with relapsed/refractory multiple myeloma and ≥ 3 prior lines of therapy, including an immunomodulatory agent, proteasome inhibitor, and anti-CD38 antibody (DREAMM-2; NCT03525678).
Methods
At the time of this study, STORM Part 2, NCT02336815 (selinexor plus low-dose dexamethasone; sel + dex) was systematically identified as the only feasible comparator to the DREAMM-2 cohort. Matching-adjusted indirect comparisons (MAIC) evaluated efficacy and safety of belamaf (2.5 mg/kg; n = 97) versus sel + dex (80 mg + 20 mg, respectively; n = 123). Populations were weighted for clinically validated effect modifiers and prognostic factors. Outcomes included overall survival (OS), progression-free survival (PFS), duration of response (DoR), overall response rate (ORR), time to response (TTR), and safety. The relative efficacy of belamaf versus standard of care (SoC) on OS was estimated by a Bucher indirect treatment comparison using the MAIC-adjusted hazard ratios (HR) for OS of belamaf (DREAMM-2) versus sel + dex (STORM Part 2) and a HR adjusted for refractoriness to carfilzomib and high-risk cytogenetics of sel + dex (STORM) versus SoC (MAMMOTH).
Results
Belamaf demonstrated improved OS (HR 0.53; 95% confidence interval 0.34, 0.83; p = 0.005) and DoR (0.41; 0.21, 0.83; p = 0.013) versus sel + dex. There were no statistically significant differences in ORR, TTR, and PFS. Belamaf had a favorable safety profile for most evaluable hematologic (any-grade, Grade 3–4) and non-hematologic (any-grade) adverse events versus sel + dex. Significantly improved OS was observed with belamaf versus SoC (0.29; 0.16, 0.54; p < 0.001).
Conclusion
Single-agent belamaf represents a new treatment option for triple-class refractory patients with RRMM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01884-7.
Keywords: Belamaf, Indirect treatment comparison, MAIC, MAMMOTH, Matching-adjusted, RRMM, Selinexor, Survival
Key Summary Points
| There is a high unmet need for treatment for triple-class refractory patients with relapsed refractory multiple myeloma (RRMM); belantamab mafodotin was recently approved for clinical use in this patient population |
| Comparative effectiveness and safety of belantamab mafodotin versus relevant therapies and standard of care (SoC) in RRMM have not yet been established |
| In the absence of head-to-head comparisons, data from separate studies with similar designs, definitions, and patient populations can be evaluated via indirect treatment comparisons such as matching-adjusted indirect comparison |
| At the time of the study, the indirect treatment comparison revealed selinexor + dexamethasone in the STORM Part 2 study as the only feasible comparator to belantamab mafodotin in the DREAMM-2 study |
| The analyses demonstrated improved overall survival (OS) and duration of response as well as favorable safety profile for most evaluable adverse events with belantamab mafodotin versus selinexor + dexamethasone and a significantly improved OS with belantamab mafodotin versus SoC |
Introduction
Multiple myeloma (MM) accounts for approximately 1% of all cancers and 15% of hematologic malignancies with an annual incidence of 86,000 new cases globally [1, 2]. Despite major advances in treatment, MM remains an incurable disease, which requires multiple lines of therapy due to relapse [2, 3]. Available treatments for relapsed refractory MM (RRMM) include immunomodulatory drugs (e.g., lenalidomide and pomalidomide), proteasome inhibitors (PIs; e.g., carfilzomib and ixazomib), monoclonal antibodies (mAbs) targeting CD38 (e.g., daratumumab), and signaling lymphocytic activation molecule F7 (SLAM7; e.g., elotuzumab), the nuclear export protein inhibitors (XPO1; selinexor), alkylators, and steroids [2–4]. After initial response, patients eventually relapse, and each subsequent relapse is associated with cumulative treatment toxicity and a shorter duration of response, as patients develop refractory disease due to multiple drug resistance mechanisms [2, 5–7]. Patients with RRMM that is refractory to immunomodulatory agents, PIs, and an anti-CD38 antibody have a particularly poor prognosis [2, 8]. Therefore, novel therapies with alternative modes of action are needed for this population with a high unmet need.
Belantamab mafodotin (belamaf; BLENREP; GSK2857916) is a first-in-class antibody–drug conjugate (ADC) that targets B-cell maturation antigen (BCMA) [9, 10]. It comprises a humanized afucosylated anti-BCMA mAb conjugated to a cytotoxic payload monomethyl auristatin F (MMAF) by a protease-resistant mc linker [9]. Belamaf binds to BCMA and eliminates MM cells by a multimodal mechanism of action, including delivery of MMAF to MM cells, immune-independent ADC mediated apoptosis, and release of markers characteristic of immunogenic cell death as well as immune-dependent mechanisms of action such as antibody-directed cellular cytotoxicity/phagocytosis [9, 10].
In the Phase II, single-arm DRiving Excellence in Approaches to Multiple Myeloma 2 (DREAMM-2 study; NCT03525678), multiply relapsed patients who received single-agent belamaf 2.5 mg/kg every 3 weeks showed an overall response rate (ORR) of 32%, estimated median duration of response (DoR) of 11 months, overall survival (OS) of 13.7 months, and median progression-free survival (PFS) of 2.8 months at a median follow-up of 13 months [9, 11]. On the basis of the DREAMM-2 study, single-agent belamaf (2.5 mg/kg) was recently approved in the USA and European Union for the treatment of adult patients with RRMM who have received at least four prior therapies including an anti-CD38 mAb, a PI, and an immunomodulatory agent [12, 13].
Demonstration of added value is important for novel treatments through comparative evaluations of efficacy and safety, which will inform on cost-effectiveness and enable decisions on payer interactions, clinical care, and reimbursement coverage. In the absence of head-to-head comparisons, data from separate studies can be evaluated via indirect treatment comparisons (ITC). ITC through network meta-analysis is not feasible for single-arm studies because of the network of evidence being disconnected [14]. Instead, population-adjusted ITCs are applicable in this setting, as recommended by the National Institute for Health and Care Excellence (NICE) Decisions Support Unit (DSU) [14].
Matching-adjusted indirect comparisons (MAIC) are a form of population-adjusted ITC that can be used to compare trials with similar designs, definitions, and patient populations. The MAIC method relies on weights assigned to patients in the trial, for which individual patient-level data are available, to match aggregate baseline data from comparator trials, thereby removing population differences that could bias comparisons of treatment outcomes. This provides important information to contextualize data from single-arm studies.
The goal of this post-hoc analysis of the DREAMM-2 study was to conduct ITC of belamaf versus relevant comparators and standard of care (SoC) in similar patient populations. A systematic literature review (SLR) was conducted to identify relevant comparator studies and is being submitted for publication [15]. Results of the SLR were used to assess the feasibility of conducting an ITC using the MAIC method to compare the efficacy and safety of belamaf versus selinexor data from Selinexor Treatment of Refractory Myeloma (STORM) Part 2 [16]. A Bucher ITC analysis was then conducted using the MAIC results for the OS of SoC in a subset of patients in the Monoclonal Antibodies in Multiple Myeloma: Outcomes after Therapy Failure (MAMMOTH) study who were refractory to a PI, an immunomodulatory agent, and daratumumab [8, 17, 18].
Methods
Inclusion Criteria, Study Selection, and Data Sources
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The DREAMM-2 study enrolled patients with RRMM treated with ≥ 3 prior lines of therapy, who were refractory to an immunomodulatory agent and PI, and refractory and/or intolerant to an anti-CD38 mAb [9]. In this analysis, data from the 13-month follow-up of the DREAMM-2 study were used (cutoff date: January 31, 2020) [9]. An SLR was conducted in Embase, Medline, and MEDLINE In-Process, Cochrane Collection Central Register of Clinical Trials (CENTRAL), the Database of Abstracts of Reviews of Effects (DARE), Cochrane Database of Systematic Reviews (CDSR), and National Institute for Health Research-health technology assessment (NIHR-HTA) to identify suitable evidence for comparator treatments. Studies, including reports from previously published SLR, randomized clinical trials, single-arm studies, or observational studies conducted on prospective or retrospective evidence, published between January 2008 and March 2019, that enrolled patients with RRMM who received ≥ 3 prior lines of therapy were included in the review (Supplementary Methods).
Of the studies identified by the SLR, only the STORM (NCT02336815) Part 2 study [16] was comparable with the DREAMM-2 study in study design, baseline patient and disease characteristics, including prior anti-CD38 therapy exposure, and definitions of outcomes (as per the International Myeloma Working Group [IMWG] 2016 efficacy criteria [19]).
STORM was a multicenter, open-label, Phase II study of selinexor (sel; XPOVIO[R]) 80 mg (orally [PO]) plus dexamethasone (dex) 20 mg PO, both administered twice weekly for 4-week cycles [16, 20]. The study was conducted in patients with RRMM that were refractory to at least one PI, one immunomodulatory agent, and daratumumab, glucocorticoids, and last treatment. Although in DREAMM-2, the inclusion criteria specified refractory and/or intolerant status to an anti-CD38 mAb, all patients who enrolled in the 2.5 mg/kg group were refractory to an anti-CD38 mAb at baseline. Patient-level data from the DREAMM-2 study [9, 11] (data on file, 13-month follow-up manuscript in preparation) and multiple sources of efficacy and safety data for STORM Part 2 were used in this evaluation (Table 1) [9, 16, 20–23].
Table 1.
| Characteristics | DREAMM-2 2.5 mg/kg cohort |
STORM Part 1 | STORM Part 1, penta-refractory cohort | STORM Part 2 | MAMMOTH, main population in Gandhi et al. 2019 | MAMMOTH, penta-refractory cohort in Gandhi et al. 2019 | MAMMOTH, population in Costa et al. 2019 |
|---|---|---|---|---|---|---|---|
| Population | 97 | 79 | 31 | 123 | 275 | 70 | 128 |
| Phase | Phase II | Phase II | Phase II | Phase II | Observational study | Observational study | Observational study |
| Method | Single arm | Single arm | Single arm | Single arm | Not applicable | Not applicable | Not applicable |
| Design | Open-label | Open-label | Open-label | Open-label | Not applicable | Not applicable | Not applicable |
| Number of prior lines of therapy | At least 3 | At least 3 | At least 3 | At least 3 | No requirement | No requirement | At least 3 |
| Prior PI/immunomodulatory agent use | Refractory to PI and immunomodulatory agent | Refractory to PI and immunomodulatory agent | Refractory to PI and immunomodulatory agent | Refractory to PI and immunomodulatory agent | No requirement | Refractory to PI and immunomodulatory agent | Refractory to PI and immunomodulatory agent |
| Exposure to anti-CD38 | Refractory | Not required | Refractory (required) | Refractory (required) | Refractory (required) | Refractory (required) | Refractory (required) |
| Patients received active therapy | Yes | Yes | Yes | Yes | No requirement | No requirement | Yes |
| Index time for time-to-event outcomes | Time of randomization | Time of initiation of therapy | Time of initiation of therapy | Time of initiation of therapy | Time of refractoriness to prior anti-CD38 therapy | Time of refractoriness to prior anti-CD38 therapy | Time of initiation of therapy |
| Response criteria used | IMWG 2016 | IMWG 2014 | IMWG 2014 | IMWG 2016 | Not reported | Not reported | Not reported |
| Outcomes available for analyses | OS, PFS-IRC, ORR-IRC, TTR-IRC, DoR-IRC, safety | OS, PFS-IRC, ORR-IRC, TTR-IRC, DoR-IRC, safety | ORR-IRC | OS, PFS-IRC, ORR-IRC, TTR-IRC, DoR-IRC, safety | OS | OS | OS, ORR |
DoR, duration of response; IMWG, International Myeloma Working Group; IRC, independent review committee; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PI, proteasome inhibitor; TTR, time to response
At the time of the SLR, no additional studies were found that were comparable with the DREAMM-2 population. However, a search for updated results on the STORM Part 2 study conducted after the SLR identified a publication comparing a cohort of the STORM Part 2 study versus a real-world cohort from the MAMMOTH study [17], which could facilitate an ITC of belamaf versus SoC. The MAMMOTH study was a retrospective study of patients with disease refractory to anti-CD38 [8]. The inclusion criteria of the subcohort of the MAMMOTH study reported in Costa et al. (2019) were similar to those of STORM Part 2 (Table 1), facilitating comparison between sel + dex and SoC [17]. Given the available published information, a Bucher ITC using the MAIC-adjusted hazard ratios (HR) for OS of belamaf versus sel + dex, and an HR adjusted for refractoriness to carfilzomib and high-risk cytogenetics of sel + dex versus SoC, was used to determine the relative efficacy of belamaf versus SoC from the MAMMOTH study on OS [17]. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
MAIC and Bucher ITC Methodology
Due to the absence of a connected network for these single-arm randomized studies, unanchored MAIC of belamaf versus sel + dex was performed by following guidelines from the NICE DSU for population-adjusted ITC [14]. Propensity score-like regression was used to calculate for each patient in DREAMM-2 a predicted probability of inclusion in the STORM Part 2 study based on patient characteristics (Table 2). These estimated probabilities were then used as statistical weights and applied to the DREAMM-2 population to balance DREAMM-2 and STORM Part 2 on the factors included in the regression model (by weighting the DREAMM-2 population). These weights were also used to calculate the effective sample size (ESS) corresponding to population size of the weighted cohort of patients who received belamaf.
Table 2.
Prognostic factors and treatment-effect modifiers included in the MAIC analysis
| Factors | Prognostic factor according to clinical experts | Effect-modifier according to clinical experts | Prognostic factors in the DREAMM-2 data | Available for comparison in STORM Part 2 | Included in the MAIC models |
|---|---|---|---|---|---|
| Age | ✓ | ✓ | ✓ | ✓ | |
| Sex | ✓ | ✓ | ✓ | ||
| ECOG status | ✓ | ✓ | ✓ | ✓ | |
| Comorbidities (renal, liver, or frailty index) | ✓ | ✓ | ✓ | ||
| Cytogenetic factors | ✓ | ✓ | ✓ | ✓ | ✓ |
| R-ISS stage | ✓ | ✓ | ✓ | ✓ | ✓ |
| Extramedullary disease | ✓ | ✓ | |||
| Serum BCMA levels | ✓ | ||||
| Number of prior lines of therapy | ✓ | ✓ | ✓ | ✓ | ✓ |
| Refractory status | ✓ | ✓ | ✓ | ✓ | |
| Lytic bone lesions at baseline | ✓ |
BCMA, B-cell maturation antigen; ECOG, Eastern Cooperative Oncology Group; R-ISS, revised international staging system; MAIC, matching-adjusted indirect comparison
Adjustments were made for imbalances of known treatment-effect modifiers identified through independent clinical expert opinion and prognostic factors identified by exploratory analyses. Specifically, DREAMM-2 data were analyzed using Cox proportional hazards models to investigate the prognostic ability of several factors on OS, PFS, DoR, and TTR. Similarly, logistic regression models were used for ORR (Table 2). The base case MAIC model adjusted for between-study population differences in the following factors: age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, creatinine clearance, revised international staging system, high cytogenetic risk (defined by either t[4;14], t[14;16], 17p13del, or 1q21+), number of prior lines of therapy, and refractory status to last line of therapy. Two sensitivity MAICs were also carried out by repeating the MAIC after adjusting for a different parametrization of ECOG (Sensitivity Model 1) or by adjusting for differences in the proportion of patients who were refractory to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab (Sensitivity Model 2).
Study Populations Included in ITC
The comparative efficacy and safety of belamaf versus sel + dex were estimated using the weighted DREAMM-2 and the STORM Part 2 patient populations. For both the efficacy and safety analyses, MAIC weights were derived from the intention-to-treat (ITT) population of DREAMM-2 who received belamaf 2.5 mg/kg (n = 97) and who had creatinine levels reported (n = 95). The reported data for the ITT (n = 122) and safety (n = 123) populations of STORM Part 2 were used for population weighting [9, 16].
Patients from the MAMMOTH study were observed to receive SoC regimens, including combinations of daratumumab with an immunomodulatory drug or PI, elotuzumab with an immunomodulatory drug, carfilzomib with an immunomodulatory drug or an alkylator, or chemotherapy [8]. An ITC analysis was previously conducted using data from a subset of the MAMMOTH study (n = 128) [17]. This subset was selected to include only patients comparable with the STORM Part 2 study population, i.e., patients refractory to a PI, an immunomodulatory drug, and an anti-CD38 mAb who received anti-MM treatment other than sel + dex and were comparable with the STORM Part 2 study population [17].
Outcome Measures
Efficacy outcomes included: ORR, time to response (TTR), DoR, PFS, and OS of belamaf versus sel + dex or SoC. Tumor assessment-based efficacy endpoints, such as ORR, TTR, DoR, PFS, and OS, were determined according to the IMWG criteria by an independent review committee in the DREAMM-2 trial.
Safety outcomes included any-grade treatment-emergent adverse events (TEAEs) in ≥ 5% of patients or Grade 3–4 AEs in ≥ 5% of patients in either study. The proportions of patients who experienced certain TEAEs were compared between the DREAMM-2 and STORM Part 2 studies. These TEAEs included: thrombocytopenia, anemia, neutropenia, lymphopenia, leukopenia, fatigue, nausea, hyponatremia, pneumonia, diarrhea, hypokalemia, hyperglycemia, sepsis, mental status changes, or decreased appetite.
Statistical Analyses
HRs of belamaf versus sel + dex were derived using Cox regression model for time-to-event outcomes. Odds ratios (OR) of belamaf versus sel + dex were derived using logit models for overall response and safety outcomes. Statistical significance was assumed at p < 0.05. Robust estimates of variance were used.
Comparative efficacy estimates of belamaf versus SoC were derived using a Bucher ITC using the HR reported in Costa et al.[17] (in which population differences were addressed by covariate adjustment) and the HR of belamaf versus sel + dex estimated in the MAIC.
Results
Systematic Literature Review
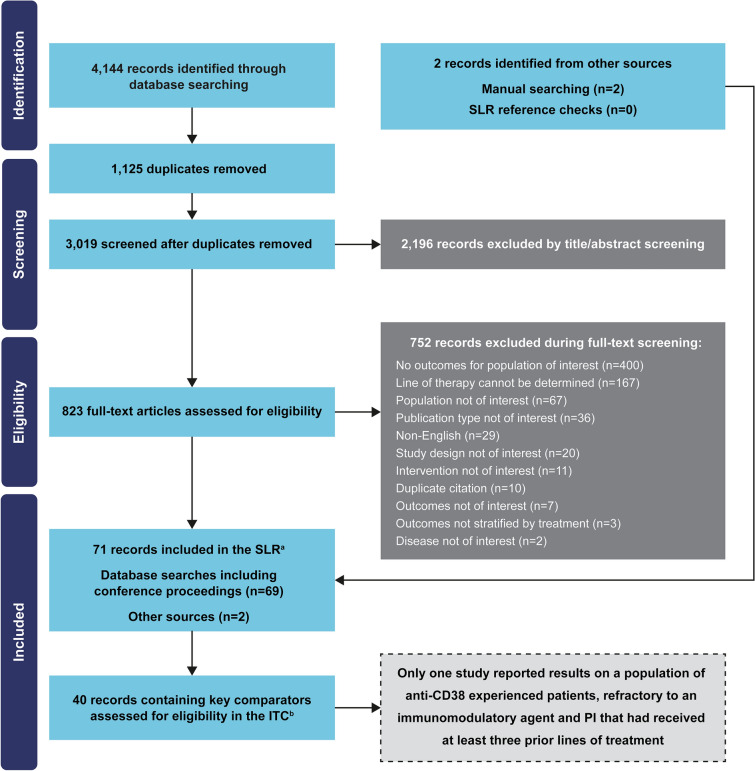
The SLR identified 40 publications that reported data from 22 studies with late-line RRMM (seven randomized controlled trials, eight single-arm studies, four observational studies, and three pooled analyses of randomized controlled trials; Fig. 1). However, only the STORM (NCT02336815) Part 2 study [16] matched the inclusion criteria of the DREAMM-2 study and had similar study design and definitions of outcomes (as per IMWG 2016 efficacy criteria) [19].
Fig. 1.
PRISMA diagram. aThe scope of the SLR was wider than the inclusion criteria of the DREAMM-2 study in order to provide an overview of the treatment landscape. bKey comparators to belantamab mafodotin included the following treatments administered as mono- or combination therapies: bortezomib, carfilzomib, daratumumab, dexamethasone, elotuzumab, ixazomib, lenalidomide, pomalidomide, and selinexor. PI, proteasome inhibitor; RRMM, relapsed or refractory multiple myeloma; SLR, systematic literature review; ITC, indirect treatment comparisons
Population Weighting
MAIC weights were derived separately for the efficacy and safety analyses of belamaf versus sel + dex. However, no weights could be derived for the two patients from the ITT population not included in the DREAMM-2 safety population because of missing values on some of the matching factors. Therefore, the MAIC weights produced for both the efficacy and safety analyses were identical. The baseline characteristics of the patients enrolled in DREAMM-2 before and after applying the MAIC weights and the corresponding characteristics for the STORM Part 2 patient population are presented in Table 3. Population adjustment was successful as the baseline characteristics of the weighted DREAMM-2 cohort matched the reported characteristics of the STORM Part 2 cohort for all the factors included in the MAIC. Following the MAIC adjustment, the ESS size was 63.46 patients, which corresponded to 65% of the original ITT population size.
Table 3.
Baseline characteristics in the DREAMM-2 trial before and after MAIC adjustment plus baseline characteristics in the STORM Part 2 trial
| Factor | Level | Population | ||
|---|---|---|---|---|
| DREAMM-2 unadjusted data | DREAMM-2 after MAIC weighting | sel + dex observed in STORM Part 2 | ||
| Age, years | Median | 65 | 64.4 | 65 |
| 51–64 | 37.1% | 39.8% | 42.6% | |
| 65–74 | 40.2% | 36.1% | 36.1% | |
| ≥ 75 | 13.4% | 14.8% | 14.8% | |
| Sex | Male | 52.6% | 58.2% | 58.2% |
| Race | White | 80.0%a | 76.7% | 69.9% |
| ECOG status | 1 | 50.0%a | 53.4% | 58.2% |
| 2 | 16.7%a | 13.8% | 9.0% | |
| 1 or 2 | 66.7%a | 67.2% | 67.2% | |
| R-ISS stage | II | 60.8% | 63.9% | 63.9% |
| III | 24.7% | 18.9% | 18.9% | |
| II or III | 85.6% | 82.8% | 82.8% | |
| Cytogenetic risk | High-riskb | 42.3% | 53.3% | 53.3% |
| Extramedullary plasmacytomas | ≥ 1 | 22.7% | 21.5% | Not reported |
| Lytic bone lesion | Yes | 71.1% | 67.0% | Not reported |
| Creatinine clearance | ≥ 60 ml/min | 72.0%a | 66.4% | 66.4% |
| Number of prior lines of therapy | ≥ 5 | 83.5% | 87.8% | 87.8% |
| ≥ 9 | 17.5% | 29.3% | 29.3% | |
| Prior ASCT | Yes | 75.3% | 75.9% | 82.9% |
| Refractory status | To CFZ-POM-DARA | 59.8% | 63.4% | 95.9% |
| To BTZ-CFZ-POM-DARA | 47.4% | 51.7% | 77.0% | |
| To LD-CFZ-POM-DARA | 54.6% | 55.8% | 82.8% | |
| To BTZ-CFZ-LD-POM-DARA | 42.3% | 44.1% | 68.0% | |
| To last line of therapy | 95.7%a | 100.0% | 100.0% | |
| Myeloma subtype | IgG | 67.0% | 67.1% | 63.4% |
| Other heavy chain Ig | 24.7% | 24.2% | 14.6% | |
| Bone marrow percent plasma cells | ≥ 50% | 27.5%a | 26.0% | 22.8% |
Bold font indicates characteristics included in the base case population-matching model
ASCT, autologous stem cell transplant; BTZ, bortezomib; CFZ, carfilzomib; DARA, daratumumab; ECOG, Eastern Cooperative Oncology Group; DoR, duration of response; Ig, immunoglobulin; LD, lenalidomide; MAIC, matching-adjusted indirect comparison; POM, pomalidomide; R-ISS, revised international staging system; sel + dex, selinexor plus dexamethasone; TTR, time to response
aOf non-missing observations
bThe definition used to identify patients with high-risk cytogenetics was similar in both studies, i.e., patients with t(4;14), t(14;16), 17p13del or 1q21+
Efficacy
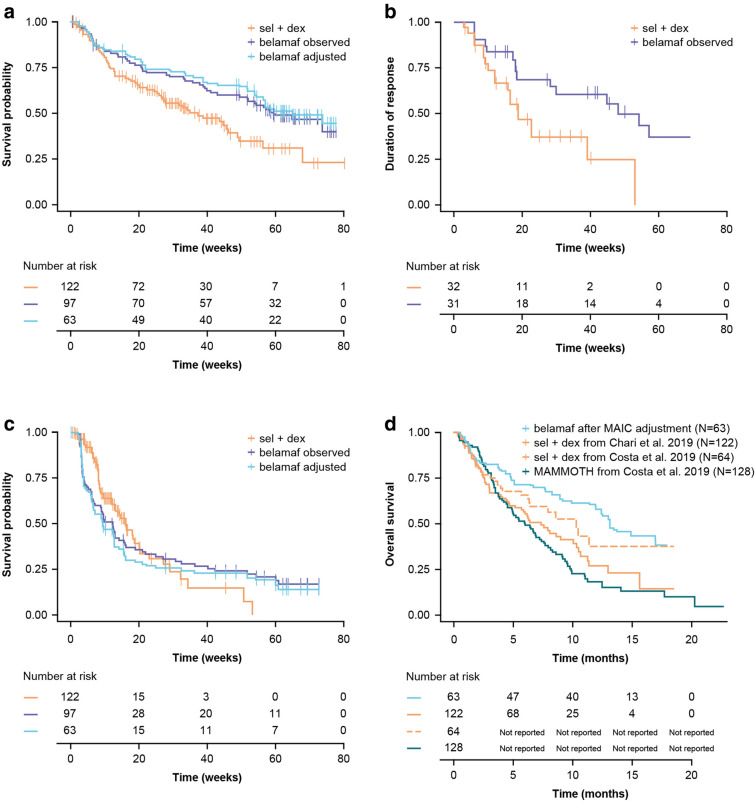
In the naive comparison, belamaf had a superior OS to sel + dex (HR 0.60; 95% confidence interval [CI] 0.41, 0.88; p = 0.010; Table 4). Following population weighting, the OS curve of the re-weighted belamaf cohort was shifted upwards (Fig. 2A), and the HR of belamaf versus sel + dex for OS improved to 0.53 (95% CI 0.34, 0.83; p = 0.005; Table 4).
Table 4.
MAIC of efficacy outcomes for belamaf versus sel + dex
| Outcomea | Naive estimates (95% CI) [p value] | Base case estimates (95% CI) [p value] |
|---|---|---|
| ORRb | OR: 1.32 (0.73, 2.38) [0.355] | OR: 1.00 (0.52, 1.91) [0.996] |
| DoR | HR: 0.41 (0.21, 0.83) [0.013] | NA |
| TTRc | HR: 0.65 (0.39, 1.10) [0.110] | HR: 0.71 (0.43, 1.15) [0.165] |
| PFSc,d | HR: 1.15 (0.80, 1.66) [0.438] | HR: 1.29 (0.87, 1.92) [0.199] |
| OSc | HR: 0.60 (0.41, 0.88) [0.010] | HR: 0.53 (0.34, 0.83) [0.005] |
Bold font indicates outcomes for which belamaf was significantly (p < 0.05) more efficacious than sel + dex
Belamaf, belantamab mafodotin; CI, confidence interval; DoR, duration of response; HR, hazard ratio; MAIC, matching-adjusted indirect comparison; NA, not applicable; OR, odds ratio; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; sel + dex, selinexor plus dexamethasone; TTR, time to response
aHR < 1 (except for TTR, HR > 1) and OR > 1 favor belamaf
bORR was defined as achieving partial response or above
cHR should be interpreted with caution due to the crossing of the curves
dSuspicion of assessment-time bias
Fig. 2.
OS (A), DoR (B) and PFS (C) Kaplan-Maier plots for belamaf 2.5 mg/kg (DREAMM-2) observed and MAIC adjusted versus sel + dex (STORM Part 2). (D) OS versus SoC from the MAMMOTH study (overlay of the estimates from different sources). Belamaf, belantamab mafodotin; DoR, duration of response; MAIC, matching-adjusted indirect comparison; OS, overall survival; PFS, progression-free survival; sel + dex, selinexor plus dexamethasone
Both before and after the population adjustment, patients treated with belamaf were found to achieve significantly longer DoR compared with sel + dex (Fig. 2B and Table 4). In the naive comparison, belamaf had a longer DoR compared with sel + dex (HR 0.41; 95% CI 0.21, 0.83; p = 0.013; Table 4). As DoR is measured from time of response rather than time from baseline, and DoR is interpretation based only on patients who respond to treatment rather than the full trial population, a MAIC conducted with weights that match full populations at baseline may be inappropriate. Acknowledging this limitation, an exploratory MAIC analysis was conducted and provided similar conclusions (HR 0.32; 95% CI 0.13, 0.75; p = 0.009; Supplementary Table 1).
The difference in PFS (Fig. 2C) and TTR between treatments was not statistically significant although numerically favorable HRs for sel + dex were observed. The HR for PFS was 1.29 (95% CI 0.87, 1.92; p = 0.199) and for TTR was 0.71 (95% CI 0.43, 1.15; p = 0.165).
Belamaf had a superior OS to SoC in MAMMOTH (Fig. 2D) in both Bucher analyses (i.e., with and without population matching in the comparison of belamaf versus sel + dex). The Bucher HR of belamaf versus sel + dex (using the MAIC adjusted HR versus sel + dex and covariate-adjusted HR of sel + dex versus MAMMOTH) was 0.29 (95% CI 0.16, 0.54; p < 0.001) favoring belamaf. This was improved from 0.33 (95% CI 0.18, 0.54; p < 0.001) in the Bucher analysis without population weighting in the comparison of belamaf versus sel + dex.
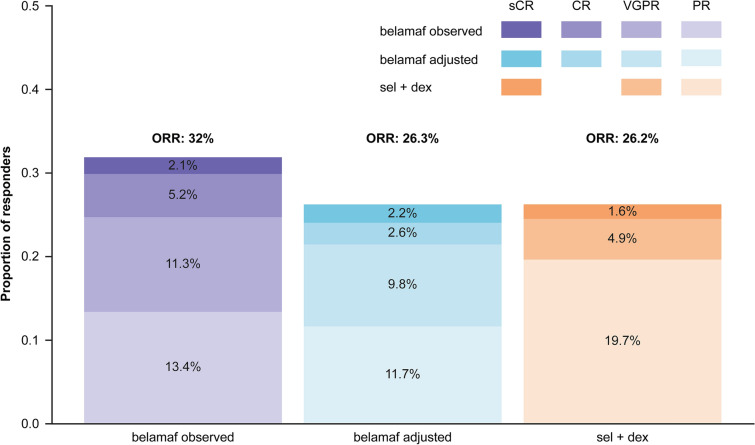
ORR values were not significantly different between the two treatments, with equivalent response rates found between belamaf and sel + dex (Fig. 3; Table 4). The adjusted OR was 1.00 (95% CI 0.52, 1.91; p = 0.996). Overall, 56% of responders had ≥ very good partial response with belamaf compared with 25% responders who were treated with sel + dex; p = 0.065.
Fig. 3.
Breakdown of patients per response type in the belamaf cohort before and after base case population adjustment from DREAMM-2 and in the observed sel + dex cohort from STORM Part 2. Belamaf, belantamab mafodotin; CR, complete response; ORR, overall response rate; OS, overall survival; PR, partial response; sCR, stringent complete response; sel + dex, selinexor plus dexamethasone; VGPR, very good partial response
Results across sensitivity analyses were consistent with the base case (Supplementary Table 1).
Safety
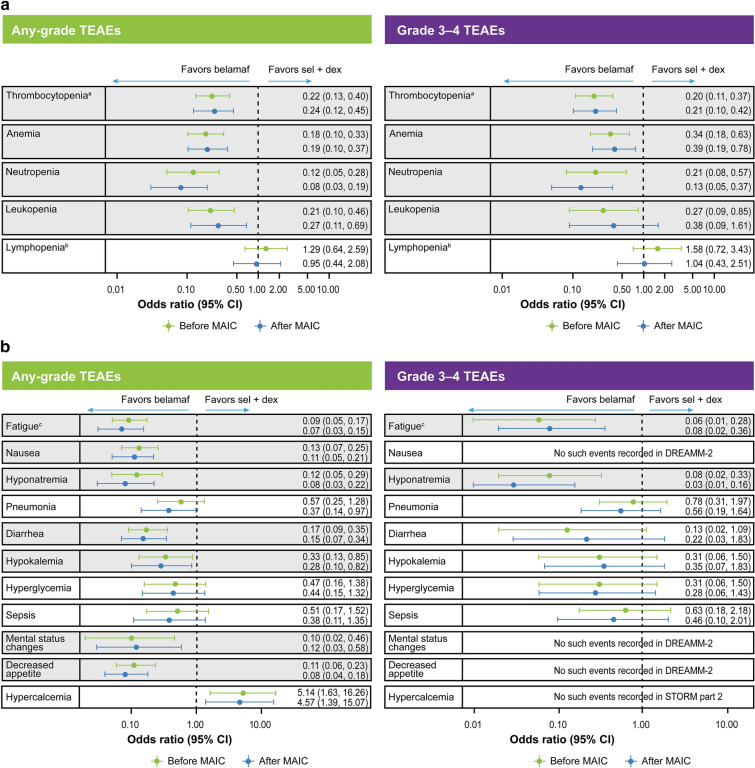
Compared with sel + dex, belamaf was found to have a significantly (p < 0.05) lower risk for most hematologic TEAEs, including any-grade and Grade 3–4 thrombocytopenia, anemia, and neutropenia as well as any-grade leukopenia (Fig. 4A). Belamaf and sel + dex were found to be equivalent in terms of risk for any-grade or Grade 3–4 lymphopenia.
Fig. 4.
Summary of the comparative safety of belamaf versus sel + dex before and after MAIC adjustment using all MAIC models. A Hematologic and B non-hematologic adverse events. aIncludes the preferred terms thrombocytopenia, platelet count decreased; bincludes the preferred terms lymphopenia, lymphocyte count decreased; cincludes the preferred terms fatigue and asthenia. OR < 1 favors belamaf; OR < 0.5, risk 50% lower with belamaf. TEAEs highlighted in gray boxes were significantly different. Belamaf, belantamab mafodotin; CI, confidence interval; MAIC, matching adjusted indirect comparison; sel + dex, selinexor plus dexamethasone; TEAE, treatment-related adverse events
ORs favored belamaf over sel + dex for most non-hematologic TEAEs (Fig. 4B). This difference was statistically significant (p < 0.05) for most TEAEs of any grade, with the exception of hyperglycemia and sepsis, which had a numerically lower (though not statistically significant) risk with belamaf. Hypercalcemia of any grade was the only TEAE that was significantly more frequent with belamaf than with sel + dex. Patients treated with belamaf were also at significantly lower risk of developing Grade 3–4 fatigue, asthenia, or hyponatremia. Risk of Grade 3–4 pneumonias, diarrhea, hypokalemia, hyperglycemia, or sepsis was numerically (but not statistically) lower with belamaf. The sensitivity analysis results were similar to and supported the base case results (Supplementary Table 2).
Keratopathy (microcyst-like epithelial changes [MECs] defined as corneal epithelium changes identified on eye examination, with or without symptoms) was the most frequent TEAE in DREAMM-2 (any-grade keratopathy was observed in 72% of patients and Grade 3–4 in 46% of patients who received belamaf 2.5 mg/kg; full details of keratopathy are described elsewhere[24]) but no such event has been reported in the STORM Part 2 study; therefore, an OR could not be derived. Similarly, no hypophosphatemia events were reported in the STORM Part 2 study, and therefore no comparative safety estimate could be derived.
Discussion
The MAIC of belamaf (DREAMM-2) with sel + dex (STORM Part 2) was conducted following a SLR and searching of all relevant evidence. At the time of this research, STORM Part 2 was systematically identified as the only feasible comparator to the DREAMM-2 cohort. However, with the continuous development of new experimental therapies, more treatments may become available in the future requiring additional comparisons. The results of the MAIC analysis suggested that belamaf has a more favorable safety profile for most TEAEs, and patients treated with belamaf experienced a longer OS and DoR than those treated with sel + dex. It has been demonstrated that patients with RRMM typically experience shorter DoR with each subsequent therapy [6]. Therefore, sustaining longer responses with belamaf compared with sel + dex and SoC is particularly encouraging in patients who received ≥ 3 prior therapies and whose MM was triple-class refractory to an immunomodulatory agent, a PI, and an anti-CD38 mAb. Response rates were found to be equivalent in terms of ORR between belamaf and sel + dex. TTR had a numerically worse efficacy profile with belamaf compared with sel + dex. However, the difference was not statistically significant. The steeper decline of the belamaf PFS curve around 4 weeks compared with sel + dex, combined with the similar response rates observed between belamaf and sel + dex, may suggest a faster progression among non-responding patients in the DREAMM-2 versus those in the STORM Part 2 study. However, this could also be attributed to differences in the time schedule of assessment; by trial design, the initial assessment for progressive disease (PD) happened earlier in the DREAMM-2 than the STORM, and therefore PD events were captured earlier in the DREAMM-2 compared with the STORM study.
In a single-arm study, OS, which measures death from any cause, can potentially be driven by other factors including subsequent treatments. The median PFS in the DREAMM-2 trial was 2.8 months (95% CI 1.6, 3.6) and median OS was 13.7 months (95% CI 9.9, not reached [NR]) at the time of the January 2020 data cutoff [9, 11]. In the 2.5 mg/kg cohort of the DREAMM-2 study, median PFS for the 35% of patients who had PD/not established (NE) response was 0.8 months (95% CI 0.7, 0.8), and for the 31% of patients who achieved SD, median PFS was 2.9 months (95% CI 2.1, 3.0). As displayed in Supplementary Fig. 1, this can be contrasted with median PFS NR (95% CI 7.1, NR) in 15% of patients who had a ≥ minimal response (MR)/partial response (PR). Overall, 38 (39%) patients received subsequent anticancer therapy (of these, only 2 received sel). The difference in OS outcomes by responder group is shown in Supplementary Fig. 2. Median OS was 8.7 months (95% CI 1.9, 13.1.9) in patients who had PD/NE and 7.7 months (95% CI 4.7, 13.4) in those who achieved SD. The median OS among patients with ≥ MR/PR was NR (95% CI NR, NR). It is possible that some aspects of the observed survival benefit were driven by post-progression treatments. However, given the proportion of patients receiving subsequent anticancer treatments in the belamaf cohort and the magnitude of differences in outcomes between non-responders and responders, the difference seen in OS is likely to be driven by patients responding to belamaf treatment.
In general, belamaf had a more favorable safety profile than sel + dex for most evaluable hematologic and non-hematologic AEs, with the exception of hypercalcemia. These results were consistent across all models. As hypercalcemia is commonly reported in patients with MM, the difference in incidence between belamaf and sel + dex may be related to disease progression rather than treatment [19, 25]. In addition, dexamethasone used in the sel + dex combination could have had a calcium reduction effect via decreased intestinal calcium absorption. Similarly, dexamethasone may have contributed to the higher hyperglycemia rate in sel + dex. It should be noted that keratopathy was the most frequent treatment-associated AE in DREAMM-2, with 1% of patients in the 2.5 mg/kg cohort discontinuing treatment as a result [11]. Keratopathy was managed with dose modification (47% of patients had dose delays and 25% had dose reductions in the 2.5 mg/kg cohort). Ocular events are known side effects of MMAF-containing ADCs [26]. No keratopathy and hypophosphatasemia events were reported in the STORM Part 2 study so no statistical comparison could be made between the two treatments [9, 16, 24].
In the absence of head-to-head randomized controlled trials, population-adjusted ITCs can be valuable tools to compare efficacy and safety of treatments from separate studies to inform clinical practice and value analyses. However, it is crucial that the included clinical trials have similar patient populations, design, and definitions. In this study, the weighting process for all of these aspects was successful. The ESS achieved was considered satisfactory (65% of original sample size), and there were no extreme MAIC weights, which ensured that the results were not affected disproportionately by only a few patients. This notion was further supported by the similar trend of time to events both before and after the population adjustment.
The current MAIC analyses are subject to potential limitations relating to the comparability of studies. Although both studies had similar trial designs, and population characteristics were weighted successfully, differences in the frequency of assessment for PD between the two studies may have introduced bias in these unanchored ITC. In the STORM Part 2 study, the response and PD assessments were performed on a 4-weekly schedule, while in the DREAMM-2 study, patients were monitored on a 3-weekly schedule. As PFS and TTR were recorded at different scheduled monitoring visits in each study, unanchored comparisons of PFS and TTR may be subject to assessment time bias [27].
Additionally, differences in unobserved patient baseline characteristics can confound comparisons despite matching populations on observed characteristics. Limited data were available for the STORM Part 2 study population on frailty of patients at baseline. Furthermore, certain prognostic factors were not reported in STORM Part 2 (extramedullary disease at baseline, BCMA levels, presence of lytic bone lesions at baseline) and could not be included in the MAIC models (Supplementary Table 1). The two studies could not be balanced for time since diagnosis or mutation-specific factors because of missing data. Different levels of prognostic factors with similar prognosis of outcomes were combined to increase ESS after MAIC weighting, as matching distributions at a more granular level required a larger reduction in the effective sample. Additionally, as the proportion of patients that were refractory to a variety of combinations of active drugs was higher in STORM Part 2 compared with the DREAMM-2 study, the results should be interpreted with caution. However, a sensitivity analysis in which the proportion of patients with penta-refractory disease at baseline were matched across both studies provided similar results (Supplementary Table 1). Furthermore, there was a single trial to inform the comparison between belamaf and sel + dex. If more trials were available for MAIC, HRs for each comparator could have been pooled. Despite these limitations, the MAIC methodology was successfully applied to compare belamaf versus sel + dex and suggests a significant difference in OS, DoR, and most AEs in favor of belamaf.
The ITC of belamaf versus SoC suggests that belamaf significantly prolongs OS over SoC. This analysis relies on the assumption that the two HRs that were compared, i.e., the HR comparing sel + dex versus SoC in Costa et al. [17] and the HR comparing belamaf versus sel + dex after population weighting of the DREAMM-2 and STORM Part 2 populations, are independent from the population in which they have been measured and can, therefore, be compared. We could find no evidence suggesting that this assumption does not hold. In addition, the adjusted HR of sel + dex versus SoC from the MAMMOTH study could be confounded by the use of real-world studies in the comparison. A final consideration is that patients included in the MAMMOTH study may have been excluded from participation in clinical trials because of their fragile health status.
Conclusion
In conclusion, single-agent belamaf represents a new treatment option for multiply relapsed patients with RRMM. In these analyses, the ITC that used MAIC based on the 13-month follow-up of the DREAMM-2 study found belamaf to be significantly more efficacious than sel + dex in terms of OS and DoR. A significantly prolonged OS was also estimated for belamaf compared with SoC, as observed in the MAMMOTH study. The results also revealed a more favorable safety profile for belamaf than sel + dex, as demonstrated by significantly lower incidence of any-grade and Grade 3–4 hematologic AEs (with the exception of lymphopenia) and of most any-grade non-hematologic AEs including fatigue, nausea, hyponatremia, pneumonia, diarrhea, hypokalemia, mental status changes, and decreased appetite. Keratopathy (MECs) was the most common TEAE in DREAMM-2 but was not reported in STORM Part 2. Further comparisons of efficacy and safety can be carried out if suitable data become available.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
We thank the participants of the study. This study was funded by GlaxoSmithKline (GSK; 207147). GSK contributed to the study design, implementation, data collection, interpretation, and analysis.
Medical Writing and Editorial Assistance
Medical writing support was provided by Joanna Nikitorowicz-Buniak, PhD, of Fishawack Indicia Ltd., UK, and funded by GSK. GSK is also funding the Open Access Fee for this manuscript. Drug linker technology was licensed from Seagen Inc (Bothell, WA, USA), and the monoclonal antibody was produced with POTELLIGENT Technology licensed from BioWa (Princeton, NJ, USA). RP is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. AS is supported by the Veterans Affair merit award (BX004514).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; were responsible for drafting the work or revising it critically for important intellectual content; provided final approval of the version to be published.
Prior Presentation
Part of the data reported in this manuscript were presented as an abstract at the ASCO 2020 Congress and as posters at the European Haematology Association (EHA) 2020 Congress, annual meeting of the Society of Hematologic Oncology (SOHO) 2020, and the European Society for Medical Oncology (ESMO) virtual congress 2020.
Disclosures
Thibaud Prawitz, Rachel Hughes, and Venediktos Kapetanakis are employees of Evidera, which received research funding from GSK. Rakesh Popat has received consulting fees from AbbVie, Celgene, GSK, and Takeda; personal fees from GSK, Janssen, and Takeda; honoraria from Celgene, GSK, Janssen, and Takeda, and research funding from Takeda. Attaya Suvannasankha has received consulting fees from GSK, Janssen, and Karyopharm Therapeutics; research funding from Bristol-Myers Squibb, Celgene, GSK, and Janssen; and personal fees from GSK and Janssen. Grammati Sarri was an employee of Evidera at the time of study design and initiation. Feng Wang, Cosmina Hogea, Boris Gorsh, and Jenny Willson are employees of and have stocks and shares in GSK. Shannon Allen Ferrante is an employee of and has stocks and shares in GSK, and has shares in Merck.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to http://www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
References
- 1.Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol. 2016;43:676–681. doi: 10.1053/j.seminoncol.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robak P, Drozdz I, Szemraj J, Robak T. Drug resistance in multiple myeloma. Cancer Treat Rev. 2018;70:199–208. doi: 10.1016/j.ctrv.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–119. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podar K, Shah J, Chari A, Richardson PG, Jagannath S. Selinexor for the treatment of multiple myeloma. Expert Opin Pharmacother. 2020;21:399–408. doi: 10.1080/14656566.2019.1707184. [DOI] [PubMed] [Google Scholar]
- 5.Sonneveld P, De Wit E, Moreau P. How have evolutions in strategies for the treatment of relapsed/refractory multiple myeloma translated into improved outcomes for patients? Crit Rev Oncol Hematol. 2017;112:153–170. doi: 10.1016/j.critrevonc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 7.Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. 2016;175:252–264. doi: 10.1111/bjh.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–2275. doi: 10.1038/s41375-019-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–221. doi: 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 10.Tai YT, Anderson KC. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy. 2015;7:1187–1199. doi: 10.2217/imt.15.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonial S, Lee H, Badros A, et al. The American Society for Clinical Oncology (ASCO) Congress; May 29–31, 2020; Virtual Format: ASCO; 2020.
- 12.GlaxoSmithKline, Research Triangle Park, NC. BLENREP prescribing information. 2020. https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Blenrep/pdf/BLENREP-PI-MG.PDF.
- 13.European Medicines Agency (EMA). BLENREP 100 mg powder [Summary of Product Characteristics]; 2020. https://www.ema.europa.eu/en/documents/product-information/blenrep-epar-product-information_en.pdf.
- 14.Phillippo DM, AE A, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. 2016. http://www.nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf. Accessed 2 Apr 2020.
- 15.Hughes R, Sarri G, Kapetanakis V, et al. Clinical outcomes in late-line relapsed/refractory multiple myeloma: systematic literature review [unpublished manuscript]. Evidera, San Francisco, CA, USA 2021.
- 16.Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381:727–738. doi: 10.1056/NEJMoa1903455. [DOI] [PubMed] [Google Scholar]
- 17.Costa LJ, Hari P, Kumar SK, et al. Overall survival of triple class refractory, penta-exposed multiple myeloma (MM) patients treated with selinexor plus dexamethasone or conventional care: a combined analysis of the STORM and mammoth studies. Blood. 2019;134:3125. doi: 10.1182/blood-2019-124991. [DOI] [Google Scholar]
- 18.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 20.Vogl DT, Dingli D, Cornell RF, et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:859–866. doi: 10.1200/JCO.2017.75.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chari A, Vogl DT, Dimopoulos M, et al., editors. Results of the pivotal STORM study (Part 2): deep and durable responses with oral selinexor plus low dose dexamethasone in patients with penta-exposed and triple class refractory MM. 62nd American Society of Hematology Annual Meeting & Exposition 2018; San Diego, CA, USA.
- 22.Jagannath S, Vogl DT, Dimopoulos M, et al., editors. Efficacy of oral selinexor plus low dose dexamethasone (Sd) in patients with penta-refractory myeloma: results of the pivotal STORM Part II Study. 6th Annual Meeting: Society of Hematologic Oncology; 2018; Houston, TX, USA.
- 23.Food and Drug Administration (FDA). Multi-discipline review of selinexor (proposed trade name: XPOVIO®) application number 212306Orig1s000; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212306Orig1s000MultidisciplineR.pdf. Accessed Apr 2020.
- 24.Farooq AV, Degli Esposti S, Popat R, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9(4):889–911. doi: 10.1007/s40123-020-00280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5:2091–2100. doi: 10.1002/cam4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31:589–604. doi: 10.1089/jop.2015.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapetanakis V, Prawitz T, Schlichting M, et al. Assessment-schedule matching in unanchored indirect treatment comparisons of progression-free survival in cancer studies. Pharmacoeconomics. 2019;37:1537–1551. doi: 10.1007/s40273-019-00831-3. [DOI] [PubMed] [Google Scholar]
- 28.Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. Am Soc Clin Oncol Educ Book. 2016;35:e418–e423. doi: 10.1200/EDBK_159009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to http://www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.