Abstract
In the light of thousands of infections and deaths, the World Health Organization (WHO) has declared the outbreak of coronavirus disease (COVID-19) a worldwide pandemic. It has spread to about 22 million people worldwide, with a total of 0.45 million expiries, limiting the movement of most people worldwide in the last 6 months. However, COVID-19 became the foremost health, economic, and humanitarian challenge of the twenty-first century. Measures intended to curb the pandemic of COVID-19 included travel bans, lockdowns, and social distances through shelter orders, which will further stop human activities suddenly and eventually impact the world and the national economy. The viral disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). After SARS-CoV-2 virus and Middle East respiratory syndrome (MERS)-related CoV, COVID-19 is the third most significant lethal disease to humans. According to WHO, COVID-19 mortality exceeded that of SARS and MERS since COVID-19 was declared an international public health emergency. Genetic sequencing has recently established that COVID-19 is close to SARS-CoV and bat coronavirus which has not yet been recognized as the key cause of this pandemic outbreak, its transmission, and human pathogen mechanism. This review focuses on a brief introduction of novel coronavirus pathogens, including coronavirus in humans and animals, its taxonomic classification, symptoms, pathogenicity, social impact, economic impact, and potential treatment therapy for COVID-19.
Keywords: Emerging pathogens, Novel coronavirus, Social impact, Economy, Potential treatment, COVID-19
Introduction
In the mid of 1960s, coronaviruses were found to infect humans and livestock, including birds and mammals (Al Naggar et al. 2020). A novel coronavirus, a severe acute respiratory syndrome, coronavirus-2 (SARS-CoV-2), has been developed in recent times and has sparked a pandemic in several countries and territories, creating global health havoc (Rodriguez-Morales et al. 2020). Globally, according to the WHO, there were more than 21,867,420 confirmed cases of COVID-19 with over 4,507,837 death cases and 5,019,907,027 doses administered by 30 August 2021 (WHO Data August 2021). The pandemic of COVID-19 comes from the successful adaptation of SARS-CoV-2 to human transmission through zoonotic beta-coronaviruses (X. Li et al. 2020a, b). Travel bans, lockdowns, and social distance, also under “shelter in place” orders, are often used to curb this unprecedented challenge (Gu et al. 2020), which involve abrupt shifts in human activities and seriously affect the economy subsequently. Therefore, the social and economic impacts of the COVID-19 pandemic are also expected to vary between countries. The countries or regions with longer social distances or greater reliance upon industries such as tourism or automotive industry that are most severely affected and face major challenges are expected to recover in China, where COVID-19 originated, earlier than Europe and North America.
It was understood that COVID-19 was involved in damage to multi-organ systems. The SARS-CoV-2 virus affects the lungs, heart, nerves, brain, vessels, kidneys, and skin (M. F. Hossain et al. 2020a, 2020b). Coronaviruses are one of the biggest groups of sense RNA viruses with spiky projections on their surface and have a broad, uncomplicated genome with a special, high degree of mutation and self-replicating recombination phenomenon. This virus will cross the boundary of this species and find a new host to live in and reproduce due to these special characteristics as examples of animal viruses that are of great importance in veterinary research methodology. Susan and Sonia have documented avian infectious bronchitis virus (IBV), bovine coronavirus (BCoV), and porcine transmissible gastroenteritis virus (TGEV) (Weiss et al. 2005). However, SARS-CoV, porcine epidemic diarrhea virus (PEDV), and Middle East respiratory syndrome coronavirus (MERS-CoV) are the best examples of CoV causing epidemic syndromes in animals with major economic losses, according to a study published in 2015 (Lau & Chan 2015).
The virus is widely adaptable to the host and is capable of causing serious illnesses in humans, rodents, dogs, cats, camels, pigs, chickens, and bats (Pal et al. 2020). BetaCoV conserved open reading frame 1a/b is less than 90% identity (Sharun et al. 2020a,b,c). In the internal environment, the infection was shown by dogs and cats with moderate to extreme symptoms (Mykytyn et al. 2020). Foodborne and zoonotic pathogens are an important threat in the poultry industry for eradication, elimination, and/or control (H. M. Hafez et al. 2020). With respect to novelty, SARS-CoV-2 differs from the two other zoonotic coronaviruses, SARS-CoV and MERS-CoV, introduced to humans earlier in the twenty-first century. A decade later, in 2012, MERS-CoV was also replicated as a zoonotic infection that contributed to a continuing outbreak and intermittent spread in the Arab Peninsula (Alluwaimi et al. 2020). SARS-CoV-2 is a new human CoV that, as of 5 August 2020, contributed to a worldwide current virus that claimed over 710,110 lives and infected over 18,895,712 individuals (of the International 2020). It is artistic with the potential to infect other animal species due to superior host adaptability compared to previous zoonotic coronaviruses (Tiwari et al. 2020). Chickens, ducks, and pigs, on the other hand, are not vulnerable to SARS-CoV-2 infection (Y. Zhao et al. 2020a,b,c). At present, health professionals worldwide are seeking to monitor more outbreaks of disease triggered by the new CoV (originally named 2019-nCoV). Pharmacokinetic models show that in inhibiting SARS-CoV-2 in vitro, hydroxychloroquine sulfate is substantially superior (5 days prior) to chloroquine phosphate (Yao et al. 2020) and was demonstrated to be much less (40%) toxic than chloroquine in animals (McChesney 1983). In the current review, we focus on a brief introduction to novel coronavirus pathogens, including coronavirus in humans and animals, its taxonomic classification, symptoms, pathogenicity, social impact, economic impact, and potential treatment therapy for COVID-19.
Symptoms
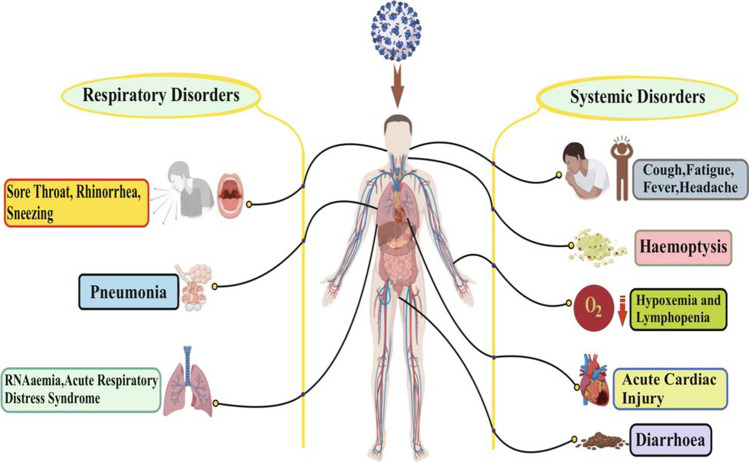
The signs of COVID-19-infected persons are seen from 2 to 14 days, but this disease also prevails for 27 days. However, a group of Chinese researchers has shown the average incubation period is around 5.2 days (Pan et al. 2020). Time relies on the age and immune system of a patient. The patients were below the age of 70 years (W. Wang et al. 2020a,b,c,d). The most common signs of COVID-19 are fever, cough, and tiredness, while other signs are dry coughing, headache, hemoptysis, diarrhea, and lymphopenia (Carlos et al. 2020; Huang et al. 2020; Ren et al. 2020). Growing evidence indicates that environmental conditions might influence the current outbreak of COVID-19 (Hasana et al.). However, irregular characteristics, including RNAaemia, acute syndrome of respiratory failure, acute heart injury, and the occurrence of ground-glass opacities leading to death, were seen via a chest CT scan showing pneumonia (Huang et al. 2020). BoCoVs have zoonotic potential in large animals as isolated from asymptomatic kids and have also been found to affect numerous domestic and wild ruminants, in which calf diarrhea in neonates, bloody diarrhea in adult cattle, and respiratory shipping fever are universal consequences in all age groups of cattle (Tiwari et al. 2020).
While CoVs have a prospective to be infectious from several days to several weeks on food or food packaging, the potency of infectivity decreases as time passes (Yekta et al. 2020). For their zoonotic origin, various investigations in China, Southeast Asia, and other constituencies targeting bats, wild and arrested pangolins, and other wildlife species will aid in better understanding (Hu et al. 2021). SARS-CoV-2 is endowed with the potential to infect other animal species due to superior host adaptability compared to previous zoonotic coronaviruses (Tiwari et al. 2020). It is believed that it has initiated from bats, just like SARS-CoV and MERS-CoV. They were spread to humans via civets and dromedary camels as intermediate hosts of SARS- and MERS-CoV, respectively (Park et al. 2020). It is considered that chickens, ducks, and pigs are not susceptible to this infection (Zhao et al. 2020a,b,c). In Southeast Asia, it was observed in bats and pangolins as SARS-CoV-2-related viruses have earlier been identified in these creatures. Sequencing and PCR-based techniques offer more conclusive statistics; historical data revealed that serological studies can be superior and have a greater opportunity of victory for identification of virus origin because of the fact those virus-specific antibodies are retained longer than viral genetic material in diseased animals (Wacharapluesadee et al. 2021). In certain cases, some peripheral ground-glass opacity was found in subpleural portions of each of the lungs, which presumably caused systemic as well as localized immune responses contributing to increased inflammation (Lei et al. 2020) (Fig. 1). It should be noted that signs of COVID-19 are identical to early beta-coronavirus, such as fever, dry cough, and dyspnea as well as bilateral chest CT opacity in the ground-glass region (Huang et al. 2020). COVID-19 has shown some unusual clinical characteristics that show symptoms of the lower airways such as rhinorrhea, sneezing, and sore throat (Assiri et al. 2013; N. Lee et al. 2003). Some instances suggest that, after admission, the upper lung lobe has an infiltrate associated with increasing hypoxemia dyspnea (Phan et al. 2020).
Fig. 1.
The symptoms of SARS-CoV-2
Pathogenesis and transmission of COVID-19
The serious symptoms of COVID-19 are linked to a growing number and rate of deaths in an epidemic area of China in particular. The Chinese National Health Commission declared the first 17 deaths on 22 January 2020, and on 25 January 2020, it grew to 56 deaths (W. Wang et al. 2020a,b,c,d). Of 2684 registered cases of COVID-19, the death rate was about 2.84% as of 25 January 2020, and the average death age was about 75 (range of 48–89) years (W. Wang et al. 2020a,b,c,d). Patients with the COVID-19 count of 11,000 leucocytes/mm3 have been classified by their full blood cell count as patients with increased leucocyte count (K. Zhao et al. 2020a,b,c). The source of COVID-19 is SARS-CoV-2, a zoonosis that is possibly obtained by the ingestion of wild animals in foodstuffs or by the contact between rural and wildlife residents in those regions (Córdoba-Aguilar et al. 2020). Bats have been accused of evolving viruses since the advent of SARS-CoV and MERS-CoV. Recently, several studies have documented detecting pandemic coronaviruses (El Zowalaty & Jarhult 2020; Zhou et al. 2018). The clinical symptoms of nCOVID-19 are varied, such as cough, fever, tiredness, the radiographic indication of pneumonia, dyspnea, reduced or normal counts of leukocyte, and myalgia (Kabir et al. 2020). The SARS-CoV-2 transmission to mink caused theviral spread between farm animals and human spillover, resulting in mass abrasion of mink to restrict the propagation of the virus. SARS-CoV-2 has been transmitted to mink, which has triggered viral spreading between animals and spilling into humans, leading to the mass exploitation of mink to reduce virus spread (Mykytyn et al. 2020). MERS-CoV has been documented as a newly developed zoonotic disease in dromedary camels (Alluwaimi et al. 2020). A genetic evolutionary study of SARS-CoV-2 has shown that two bat coronaviruses are genetically related to this virus (El Zowalaty & Jarhult, 2020). Because of numerous factors such as the flowering of trade and travel, variation in climate, quickly developing pathogens, population explosion, altering human habits and behaviors, intensive integrated animal farming, and others, the risk of evolving and re-emerging zoonoses has now improved worldwide (Bonilla-Aldana et al. 2020). Primary transmission occurs through close interaction between dromedary camels and human beings (Fung et al. 2020; Kaur et al. 2021). Older and comorbid patients have an elevated risk of serious COVID-19 disease (Fung et al. 2020). In an attempt to avoid more public health risks associated with foodborne zoonotic diseases, SARS-CoV-2 supports a debate on permanently banning the eating of wildlife (Jacob et al. 2020). There was increasing interest in investigating the coronavirus in bats following the SARS outbreak in 2002 (Jacob et al. 2020). Apart from this, SARS-CoV-2 infection has been identified with animals from zoos such as tigers and lions and shows clinical symptoms includingvomiting, diarrhea, dry cough, trouble breathing, and wheezing (Dhama et al. 2020a,b). The new MERS-CoV is enzootic (mostly Camelus dromedarius) in camels and contributes to a frequent human spill infection (Jo et al. 2020). Its zoonotic links and spillover and transmission to humans have been implicated in this infection as also reported with SARS and MERS (Dhama et al. 2020a,b).
Laboratory studies showed leucopenia (white blood cell count: 2.91 × 109/L). Furthermore, a blood-C-reactive protein value more than the normal range (0–10 mg/L) was found to be 16.16 mg/L. Also, a high rate of sedimentation for erythrocytes and D-dimers was observed (Lei et al. 2020). Because of the high genetic diversity, the occurrence, and frequent recombination in a wide variety of species worldwide, CoV will reoccur from its reservoir to a new population and thus appear in humans (M. F. Hossain et al. 2020a,b). The key pathogenesis for COVID-19 infection, such as the respiratory systems, was severe pneumonia, RNAaemia, and acute cardiac damage combined with opacity in ground glass (Huang et al. 2020). In addition, the laboratory analyses of the plasma in relation to disease severity were also shown to be high concentration of other inflammatory cytokines including interleukin-2 (IL-2), interleukin-7 (IL-7), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) (Guo et al. 2020; Rothan et al. 2020; Huang et al. 2020). Studies in bats found the possible vector for human infections to be viruses originating in this genus. For example, there is a higher risk of horizontal (intra-species) transmission of these viruses because bats live in colonies. Further proof of bat to human indirect transfer comes from spike protein interactions with COVID-19 and the ACE2 receptor (W. Li et al. 2006). SARS-CoV and MERS-CoV are highly pathogenic coronaviral strains that have been found both in bat and human transmitting species (Docea et al. 2020). Interestingly, the first highly pathogenic SARS-CoV-1 strain of coronavirus has a poor genetic resemblance with other identified coronaviruses (Drosten et al. 2003). Three comparative experiments with pangolin strains have recently been performed. In the first (18 February 2020), a sequence of 85.5–91.4% compared COVID-19 to coronaviruses in trafficked pangolins (Lam et al. 2020), with the following papers showing a sequence of similarities of 90.23 and 91.02%, respectively, with pangolin coronaviruses (Xiao et al. 2020; Zhang et al. 2020). Although, SARS-CoV-2 has 96% genetic resemblance (whole genome) with coronavirus isolated in Chinese bat species, α-coronavirus strains that caused human diseases were originally described in the bats (P. Zhou et al. 2020a,b). Another main problem is, why China? China is the world’s third largest surface economy and most populated country. Its different soil features and climate support enormous biodiversity, which allows for virus transmission among animal populations. However, the close coexistence of animals and people and food traditions that involve several native animals such as wild animals (bats) result in high risk of vertical transmission across the food supply chain. Live-bird markets like those in China, near contact, and sometimes living in African rural areas with virtually no wild-environment barriers remain very common to humans and animals (Docea et al. 2020).
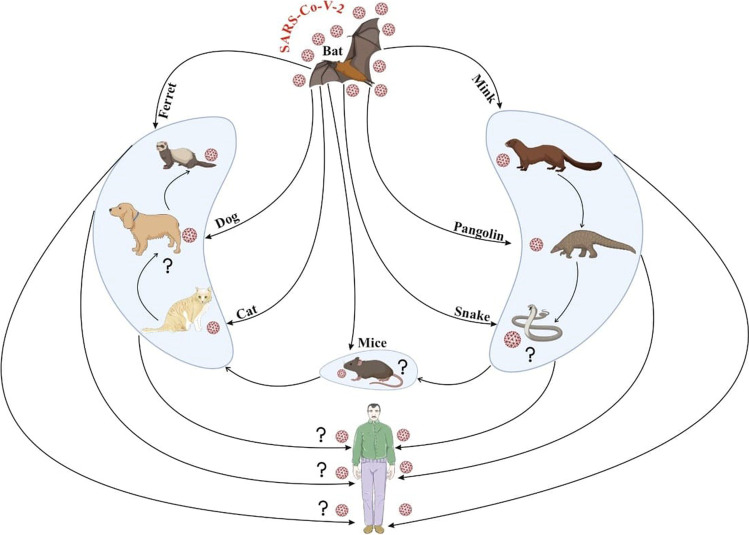
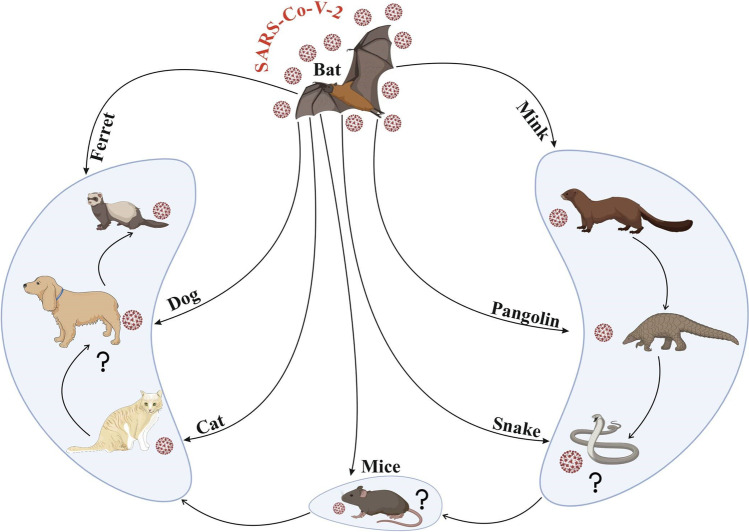
In another investigation, researchers demonstrated that ferrets, cats, and dogs could be infected experimentally with SARS-CoV-2 through the intranasal route (Tazerji et al. 2020). Mink such as ferrets belong to the Mustelidae family, and they have good susceptibility to SARS-CoV-2; therefore, mink can be utilized as an animal model for SARS-CoV-2 infection (Nadeem et al. 2020). It has been documented that efficient transmission among ferrets has been reported via direct contact with infectious ferrets and indirect airborne spread (Spiteri et al. 2020). The European Centre has reported for Disease Prevention and Control (ECDPC) that Europe owns about 2750 mink farms, producing greater than 27 million pelts each year. As we know, farmed minks are assembled in many groups in pens in wire cages placed with bedding that produces a greater amount of dust; therefore, there is boundless opening for transmission of infection among minks. Once the virus is introduced, this might ignore this spread because infected farms have been identified through serosurveys that suggest that disease might be mild, although upper and lower respiratory tract infection symptoms have also been reported (Oreshkova et al. 2020). In addition to this, it is also observed through the experience done from Denmark and the Netherlands that once the virus is introduced then it might be very problematic to halt transmission of the virus. There has been constant agricultural-to-farm transmission and numerous studies explore the methods of transmission between farms (Huo et al. 2020). Although it is also documented that adaptation to mink would lessen possibility of transmission among humans, a major concern is the accumulation of the virus in non-human reservoirs and from there viruses could be re-introduced, once spreading of SARS-CoV-2 in humans is suppressed or even immobile. In the Netherlands, a total of more than 2.7 million mink were registered on confirmation of SARS-CoV-2, which is 6.5-fold higher than total human cases. It is not clarified how this transmission of the virus has occurred between farms, and the role of humans was considered in the transmission of this virus infection (Letko et al. 2020). These disclosed statistics have fostered discussions on the function of “herd immunity” in natural exposure to emergent tropical or maybe rural zoonoses. Notice that comparable threats have been reported with viruses that are not usually connected to highly biodiverse geographies or wild meat feeding. In particular, the 2012 MERS-CoV outbreak in the Middle East and Arab Peninsula has revealed that local and foreign city residents are not previously exposed to natural camelid hosts, who continue to have close ties with rural society (Azhar et al. 2014; Haagmans et al. 2014). As shown in COVID-19, the direct impact of zoonoses on individual health and indirect effects on healthcare systems emphasize the increased risk of human transmission in areas where there are blurred wildlife environments even among developed countries, despite past exposition and indeed immunity. The most common pathetic conditions underlying the disease are, i.e., pneumonia, mostly due to fever, tobacco, sore throat, exhaustion, headache, and shortness of breath. SARS-CoV-2 can lead to a severe immune reaction in the host body, leading to massive tissue damage in some cases. Interleukin-6 (IL-6), which triggered a series of inflammatory events, is one of the leading actors of this reaction. Viral pneumonia usually occurs bilaterally and affects primarily the lower lobes and is more serious in geriatric and comorbidity patients older than 70 years (Di Gennaro et al. 2020). Nevertheless, most children and young individuals suffering from SARS-CoV-2 have mild to moderate flu-like signs with an outstanding prognostic. In addition to the health conditions described above, several patients have complained of gastrointestinal issues including vomiting and diarrhea (Guo et al. 2020). SARS-CoV-2 has been conjectured to have initiated from bats and pangolins, although other species have been examined to establish some intermediate host. Its current detections in farms (mink), and domesticated (cats and dogs) and wild animals (tigers, pumas, and lions) have raised questions about the zoonotic origins of COVID-19 (Sharun et al. 2020a,b,c). In a subsequent SARS-CoV-2 natural infection investigation in farmed mink, a serological survey was executed among stray cats/feral cats found in the Netherlands around mink farms (Oreshkova et al. 2020; Sharun et al. 2020a,b,c). The illustrations of mink as a possible reservoir host of SARS-CoV-2 and transmission CoV strain are shown in Fig. 2. Viruses can come into the body via mucosa, especially nasal mucosa and larynx mucosa, pass through airways, reach the lungs, and quickly replicate (Li et al. 2020; 2021). While various transmission methods are available, the main route for the dissemination of the virus is through the propagation of breathing or sneezing which can also be done by asymptomatic persons (Jin et al. 2020; Morawska & Cao 2020). SARS-CoV-2 can also be transmitted via fecal–oral transmission because this virus has been found in patients’ stools and urines (Jin et al. 2020). If the virus has reached the body, angiotensin-converting enzymes 2 (ACE2) will potentially invade target tissues such as the liver, heart, kidney, and gastrointestinal tract (Jin et al. 2020).
Fig. 2.
The illustrations of mink as a possible reservoir host of SARS-CoV-2 and transmission of CoV strain
Taxonomic classification and structure
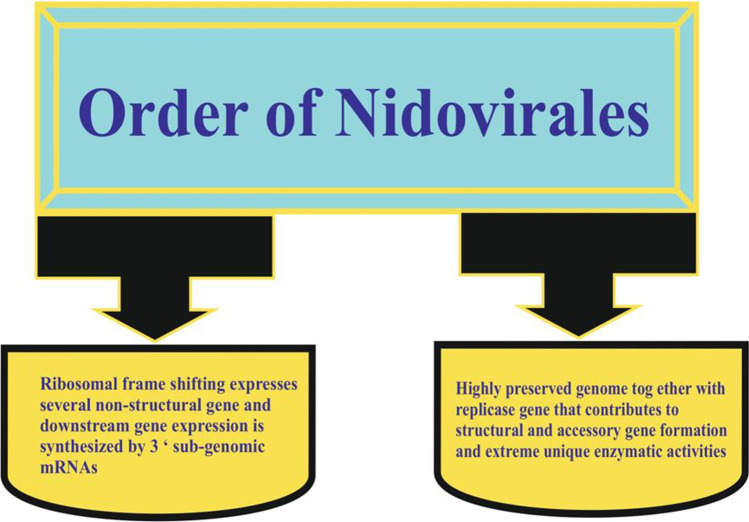
Nidovirus order consists of the Coronaviridae family (SARS, PEDV), Arteriviridae (spinal and equine pathogens), and Roniviridae family (invertebrate virus) (Cowley et al. 2000). Figure 3 shows other common Nidovirales features. The key variances in the Nidovirus family are the number, size, and form of structural proteins that cause the virus structure’s morphologic and structural changes. Still under debate, coronavirus is in SARS-CoV (Goebel et al. 2004; Gorbalenya et al. 2004). The reverse transcript, phylogenetic replication, and nucleocapsid sequences of the coronavirus sequence of feral pigeons, graylag gulls, and mallards have been used for cellular receptors (Cavanagh et al. 2002; Jonassen et al. 2005). The current taxonomy of viruses users usually organize all diversity of viruses into a hierarchical structure in terms of the order, family, genus, and species (Alluwaimi et al. 2020). The coronavirus of about 80–125 nm in size is rounded, enveloped, and non-segmented. It contains positive sense RNA with a genome size of 30 kb and includes four structural proteins encoded within the viral genome’s 3″ end (Lee et al. 1991). The virus also includes membrane proteins, a small membrane protein, and a short N-terminal ectodomain, along with a cytoplasmic tail and a strongly lipophilic protein that stretches three times to the outer surface as shown in Fig. 4 (Delmas et al. 1990). Spike glycoprotein mediates the entry of coronaviruses into host cells (S protein). Overall, the SARS-CoV-2 S protein structure resembles that of the closely related SARS-CoV S protein. The S1 and S2 subunits remain non-covalently bound in a prefusion conformation. To identify various entry receptors, different types of coronaviruses use special domains in the S1 subunit (Wang et al. 2020a,b,c,d). Coronaviridae family members consist of large, enveloped RNA viruses with a single stranding. It is the most known RNA virus with genomes between 25 and 32 kb and virions with a diameter of 118–140 nm. Within the subfamily, Coronavirinae has four genera, the alpha-, beta-, gamma-, and deltacoronaviruses (Payne 2017). Both family members have the same special mRNA synthesis strategy in which the complex polymerase leaps from one region of the template into a distant region. Viruses are approximately spherical and notable for the large (S) spike glycoprotein, 16–21 nm from the envelope of the virus (Payne 2017). Table 1 offers a thorough classification of the coronavirus community with its CoV host, virus, disease, and cellular response. The structure of the human coronavirus causing respiratory syndrome is shown in Fig. 5.
Fig. 3.
Characteristics of Nidovirales
Fig. 4.
Structure of the coronavirus: (1) spike (S), (2) membrane protein (M), (3) envelope protein (E), (4) lipid bilayer, (5) nucleocapsid (N)
Table 1.
Classification of host, virus, disease, and cellular response classes of coronavirus
| Group | Host category | Name of the virus | Cellular responses | Types of disease | Reference |
|---|---|---|---|---|---|
| I (Animal pathogens) | Cat | Canine coronavirus, FeCoV, FIPV | Canine and feline APN | Respiratory, enteric and neurologic infection, and hepatitis | (Sood et al. 2020) |
| Pig | TGEV, PRCoV | Porcine APN | Respiratory and enteric infection | (Sood et al. 2020) | |
| Human | 229E and NL-63 | Human APN and ACE2 | Respiratory infections | (Ijaz et al. 1985) | |
| II (Veterinary pathogens) | Cow | BCoV | Neu5,9Ac2-containing moiety | Enteric infection | (Sood et al. 2020) |
| Human | OC43, HKU1, and SARS-CoV | Neu5,9Ac2-containing moiety | Respiratory infection, possibly enteric infection | (Saletti et al. 2020) | |
| Pig | Hemagglutinating encephalomyocarditis | Neu5,9Ac2-containing moiety | Respiratory, enteric and neurologic infection, and hepatitis | (Sood et al. 2020) | |
| Mouse | MHV | Murine CEACAM1 | Enteric and neurologic infection and hepatitis | (Miura et al. 2004) | |
| III (Avian pathogens) | Chicken | IBV | Not determined | Respiratory infection, hepatitis | (Dai & Gao 2020) |
| Turkey | Turkey coronavirus | Not determined | Respiratory and enteric infection | (Bayram et al. 2020) |
Fig. 5.
Illustration of coronavirus causing severe acute respiratory syndrome in animals
Social impact
A combination of aspects like very high density, insufficient access to fundamental infrastructure services, and unstable living style makes it hard, if not difficult, to contain the spread of COVID-19 in slums via encouraging social distance and quarantine actions. These concerns have already been facts across various cities of Asia, South Africa, South America, and so on. Quarantine and social distancing could have a significant effect on the psychological impact on the population’s health. Partitioning from dear ones, the loss of liberty, ambiguity over ailment status, and tediousness can, on occasion, create dramatic effects. Suicide has been reported, significant annoyance caused, and grievances fetch following the implementation of quarantine in earlier outbreaks (Zeidner et al. 2016). In reviewed investigations, economic loss because of quarantine produced severe socioeconomic distress and was reported to be a risk aspect for indications of psychological disorders, and both anger and anxiety continue months after quarantine. While quarantine and isolation are used to protect the physical health of individuals from infectious diseases, the mental health effects of these steps should also be taken into consideration for those with these limitations (Hossain et al. 2020a,b). Suicidal thoughts are also reported among people affected by COVID-19 (Hodžić et al. 2020). Lockdown severely affected schools’ and colleges’ regular schedule of education because all educational institutes were closed during this lockdown period and exams were also postponed. In addition to this, many research scholars put their research work on hold. Fear of infection, economic reduction, short supplies, and stigmatization are the major issues that resulted in mental health problems during the COVID-19 era (Brooks et al. 2020). Suicidal cases were also identified among COVID-19-affected patients (Rogers et al. 2020). Another acute problem is the impact of COVID-19 on the psychological behavior of healthcare workers. Spoorthy et al. reported that healthcare employees across the globe had faced a significant degree of anxiety, stress, and insomnia during this pandemic era (Spoorthy et al. 2020). It can be summarized that the core of the COVID-19 pandemic is socioeconomic demographics. It significantly affects industrial and economic activities and ensures that post-COVID economic and social objectives are in stark contrast with the pre-COVID-19 timeline by introducing a full and minimal lockdown strategy.
According to data published on 20 May 2021 by Tencent (https://www.tencent.com/zh-cn/investors.html), in WeChat (WeiXin in Chinese), as a trending social media app for Chinese people’s daily work and life, every month there were > 1.2 billion active users globally. This app was not used for conducting commercial transactions, working, and learning but used for shopping, playing games, and communication. Additionally, it was also used to prevent infection with the COVID-19 virus and for close observation in China (Li et al. 2021).
Exercising at home may result in relief from stress, retaining fitness, and defending their life satisfaction. The latest study also revealed that people doing workouts regularly during this pandemic have the best mood (Brand et al. 2020). This virus hit almost all nations with wildly different levels due to differences in cultural norms and heathcare system, as well as mitigation events such as social distancing, resulting in closure of many public institutions (Okorie et al. 2021).
Economic impact
COVID-19 resulted in considerable chaos of the global economy, mainly in transport, the vacation industry, and global supply chain. The World Bank declared that gross domestic product worldwide contracted by 5.2% in 2020, the most significant decline in decades. The economic growth was diminished in all continents, namely the Middle East and North Africa (4.2%), East Asia and Pacific (0.5%), Sub-Saharan Africa (2.8%), South Asia (2.7%), Europe and Central Asia (4.7%), and Latin America (7.2%) (Pitoyo et al. 2021). After the highly integrated global capital outbreak, the influence of a new strain of coronavirus and mortality and morbidity has become evident. The global blocking was related to production interruptions as the global economy slowed down. The global supply chain, which contributes to a significant socioeconomic increase, was also interrupted. The findings show that the increasing number of lockdown days, fiscal arrangements, and travel constraints tremendously affected the degree of financial growth, and end, opening, minimal, and most notable stock expenses in large stock exchanges.
The impact of COVID-19 also affected the informal sector. The International Labour Organization (ILO) declared that more than 1.6 billion workers in informal sectors were affected during this pandemic (ILO 2020a). Moreover, other concerns resulting from COVID-19 are the rise in unemployment and underemployment rates (ILO 2020b). More than 436 million informal businesses were highly affected economically throughout the pandemic, like manufacturing (111 million), housing and food (51 million), and other businesses (42 million) (ILO 2020a,b). The informal workers faced helplessness (Tim Forbil Institute and IGPA 2020). This pandemic resulted in skyrocketing food uncertainty in low-income continents, responsible for increment of susceptibility to this. The COVID-19 outbreak has severely impacted the economies worldwide. Varona et al. found that the leading economies dropped to an average of − 5% in the year’s first quarter. The USA and the European Union economies are projected to drop − 7.1% and − 9.3%, respectively, for the year 2020 (Varona et al. 2021). The urban economy is decreased due to long-term economic shutdowns. Early research revealed that the outbreak had a considerable impact on city tax revenues, citizens’ income, tourism, unemployment, small and medium-sized businesses, urban food supply chains, and migrant workers (Sharifi et al. 2020). During the lockdown period, the traveling and transportation industries had fallen to almost zero. Economic institutes have relieved spur packages of > $6 trillion for solving this issue (Holmes et al. 2020). Agriculture, education, and healthcare sectors also have several negative impacts, but a drastic improvement in the air quality index of urban centers has been recorded during lockdowns (Kousi et al. 2021). The period of lockdown disrupted the mood of sports lovers also. Several sports events were canceled worldwide that not only affected the mood of sports lovers but also disrupted the financial status of sportspersons and organizations (Kousi et al. 2021). In one report on Greece, it was found that there was a decline in mobility trends in retail and recreation places (85%), parks (− 70%), and transit stations (− 80%). There was also a decline in movement near grocery and pharmacist shops (− 45%), as well as working places (− 55%) (Kousi et al. 2021).
On the other hand, the restriction on domestic development and higher spending of the money approach positively affected financial growth, although the increasing number of reported coronavirus cases had no significant impact on economic growth (Ozili et al. 2020). Global businesses, regardless of scale, have begun to face production and export cuts due to the further downturn in bounded transportation activities in confined countries. The tertiary sector has also had an impact on COVID-19. In addition to the impact on education for undergraduates, research into many non-COVID-related topics often impacts most strongly on the postgraduate research culture. In the UK, the national health research funding agency stops all non-COVID research so that qualified, usually academic, staff can go back to the frontline (Thornton 2020). Time, sales, and direct financial harm of the medical bears and related facilities are extra to the quantitative evaluation of the economic losses caused by COVID-19. India has been described as a tourism powerhouse and the world’s largest market in the Southern Asian economy. Today, it is the 8th largest nation to contribute to GDP (Sundaram et al. 2020). Although the COVID-19 pandemic is constantly spreading with little indication of containment as of 15 April 2020, it will probably have a very significant negative effect on economic growth in the region. The UN has warned of a coronavirus pandemic with a major adverse impact on the global economy, and India’s GDP growth is most importantly predicted to fall to 4.8% in the current economy (Neves et al. 2020). The decrease of social distance, travel constraints, and self-isolation reduced economic sectors around the world and caused the loss of a large number of educational institutions. In addition, various scientific conferences have been canceled or postponed. These conferences are important to scientific investigation in a wide range of fields which enable research to be distributed and provide collaborative and job search networking opportunities. Therefore, by taking into account the physical risk and social and physical distancing policies, the countrywide lockdown has enforced tremendous stress on the population below the poverty line. In the meantime, webinars played a significant role in providing people with recent information by increasing knowledge among them by claiming that they are in a pandemic situation at home.
Potential treatments
As a deadly new process and wreaking havoc have reached the COVID-19 pandemic, the world is seeking ways to slow the spread of new strains and discover viable drugs. It would promote the reduction of the burden on a nation’s healthcare system by limiting various critically ill people with COVID-19 and decreasing the length of the carriage of infection to reduce the transmission of the network (Colson et al. 2020). There is also a significant need for SARS-CoV-2 therapy. It is appalling that the FDA has not yet approved medicines for COVID-19; however, the coronavirus treatment acceleration program has created a single crisis program for potential therapies. It uses every method to transfer new drugs to patients as quickly as possible under the circumstances and at the same time testing for their utility or damage (FDA 2020). Currently, therapy is mainly symptomatic and organ support is given for critically ill individuals (Zumla et al. 2020). The use of old antiviral drugs will be a fascinating technique because of safety profiles, reactions, and phonology, and contact of medicines is remarkable (Colson et al. 2016). Here, we discuss a part of the essential medicinal products that can help combat COVID-19. Recent papers have revealed the inhibitory effect for the production of SARS-CoV-2 in vitro by remdesivir and chloroquine (M. Wang et al., 2020). Thus, 20 clinical exams were carried out in many Chinese emergency clinics following the in vitro findings (Jie et al. 2020). The results show that chloroquine 500 mg was predominant twice a day for 10 days compared with control group therapy, as long as pneumonia, indicator duration, and viral liberty were decreased, all without extreme reactions (Gao et al. 2020). Similarly, it has been demonstrated that hydroxychloroquine (chloroquine analog) has an effect on in vitro SARS-CoV operation (Yao et al. 2020). A series of further in vitro preclinical studies indicate that both chloroquine and HIC have activity against SARS-CoV-2 despite an in vitro inquiry which suggests that HIC may be better than chloroquine and has a higher antiviral in vitro effect than chloroquine (Dong et al. 2020; Yao et al. 2020). The clinical benefit profile of hydroxychloroquine is better than that of chloroquine (when long-term use is required) and provides higher daily doses (Marmor et al. 2016). Both drugs are responsible for hindering large viral enzyme replication, such as DNAand RNA,and processes like glycosylation, assembly of viruses, the transport of new infection molecules, and discharge of infections. Some reports indicate that these drugs can lead to ACE2 receptor inhibition, surface acidifications of the membrane impeding virus fusion, and cytokine release immunomodulation (Ben-Zvi et al. 2012; Colson et al. 2020). Remdesivir is an antiviral agent with a wide variety of nucleosides (adenosine). Gilead Sciences developed it for Ebola infection in 2017. Remdesivir, for example, can repress SARS-CoV and MERS-CoV replication in in vitro studies (Rong et al. 2021; Agostini et al. 2018). Remdesivir represses movement using human CoV receptors of bat CoV, SARS-CoV, and MERS-CoV strains, which can replicate in human epithelial cells and function like intermediate channels. Remdesivir suggested prophylactic and remedial suitability for 2002 SARS-CoV (Agostini et al. 2018; Sheahan et al. 2017). Remdesivir was suggested as a possibility for treating COVID-19 patients (W. Liu et al. 2020a,b). Remdesivir has shown remarkable coronavirus activity and a great genetic barrier to resilience in preclinical trials (Agostini et al. 2018). The assessment of safety and effectiveness of reconstruction in hospitalized patients with mild or moderate respiratory disease COVID-19 will be performed by a randomized controlled, double-visual preliminary trial (Qu et al. 2020). Remdesivir treatment for a patient with COVID-19 was initiated intravenously on the 7th day. A total of 68% of patients were seen to benefit clinically with remdesivir when treated (Holshue et al. 2020). Remdesivir acts as an RNA-dependent polymerase inhibitor. Adenosine triphosphate is reportedly used to be inserted into the newer viral RNAchains. As no fast chain ends exist in RDV-TP (three additional nucleotides were linked after RDV-TP), the drug tends to resist viral exoribonuclease proofreading (Colson et al. 2016; Gordon et al. 2020; Warren et al. 2016; H. Zhao et al. 2020a,b,c). In the lopinavir- and ritonavir-treated patients, however, there was no difference between the length of viral shedding following clinical treatment improvement (Cao et al. 2020). These two medicaments have been found to bind with Mpro (key enzyme for the replication of virus) and to help prevent coronavirus action (W. Liu et al. 2020a,b; X. Liu et al. 2020a,b). Favipiravir, another commonly used antiviral medication popular in vitro for treating SARS-CoV-2 diseased RNA viruses, is clinically examined (Peking University 2020) (Shiraki et al. 2020). Favipiravir is a polymerase inhibitor of RNA, which suppresses RNAsynthesis (Shiraki et al. 2020). Azithromycin and hydroxychloroquine were also used in a clinical trial to prevent bacterial pollination in COVID-19 patients (Gautret et al. 2020). At first, the possible value of azithromycin as additional therapy is apparent. Azithromycin was used as an adjunct therapy for MERS-CoV (Arabi et al. 2019). Teicoplanin, another glycopeptide for the treatment of bacterial infection used regularly, has been considered a SARS-CoV in vitro dynamic and combined with the rundown of particles that could have been used as a restraining agent against COVID-19 (Baron et al. 2020). This antibiotic has recently proven to be effective for various infections such as Ebola, flu virus, flavivirus, hepatitis C virus, HIV virus, and coronavirus, in particular, in staphylococcal infections (MERS-CoV and SARS-CoV) (Colson et al. 2016). The extended plasma junctions of inflammatory cytokines, such as TNF-α and IL-2, 7, and 10, have been seen in patients with COVID-19, in particular for ICU which indicated that a storm occurred (Huang et al. 2020). Based on these research findings, COVID-19 could prove effective by an inhibitor IL-6 monoclonal antibody, called tocilizumab. Of the 21 patients, 20 were recovered and released within 14 days of treatment after tocilizumab (Xu et al. 2020). Additional clinical feasibility knowledge for COVID-19 is evaluated (Zhao et al. 2020a,b,c). Tocilizumab prevents intercom passed IL-6 movement by binding IL-6 receivers both solvent and film-bound (Shetty et al. 2014). For COVID-19, other monoclonal IL-6 receptor inhibitors (sarilumab and siltuximab, namely) are suggested as they are responsible for cytokine discharge dysfunction that is a part of the severe cases in COVID-19 patients (Zhao et al. 2020a,b,c). The research was performed on 21 patients who were treated with siltuximab with COVID-19 pneumonia/ARDS (Gritti et al. 2020). In extreme COVID-19 patients, including IL-2, IL-4, IL-6, IL-7, IL-10, TNF-α, and IFN-γ, an elevated serum level of proinflammatory cytokines was recorded. Among these, a different intracellular signaling pathway regulated by Janus kinases (JAKs) is used by many cytokines (Luo et al. 2020). Another monoclonal antibody, leronlimab, responded by assessing cytokine tempests for possible treatment of COVID-19 to intensify the insensitive reaction (Muslim et al. 2020). The use of COVID-19 convalescent plasma for treatment of patients with severe or immuno-life-threatening diseases is to be tested by clinical trials. The prevention of the disease is not predicted with COVID-19 recovery plasma. The plasma collected from those recovering from COVID-19 may have SARS-CoV-2 antibodies to clinical preliminaries in clinical patients (Caly et al. 2020). In patients administered convalescent plasma, rousing results were obtained. The FDA is also investigating the use of NSAIDs in patients suffering from COVID-19 (Caly et al. 2020). Worries have been suggested for the possible deterioration of COVID-19 symptoms, but no significant clinical evidence is available (Loutfy et al. 2003). The work of safe changes in COVID-19 care or prevention is unclear. Some improvements for both care and prophylaxis (for example zinc, nutrient C, and nutrient D) are under consideration in conjunction with other treatment approaches (Sood et al. 2020). Since COVID-19 disease is not licensed and investments in potential vaccine developments have to be made, it is ideal for everyone to unite their hands in combatting coronavirus by rehearsing self-cleanliness and social distance. Remdesivir efficacy and safety is being tested in several clinical trials for COVID-19 therapy. The results were published in the latest clinical trial on COVID-19 by Gilead Sciences on remdesivir effectiveness (Grein et al. 2020). Ivermectin antiviral function is also found to be correlated with other pathways. Ivermectin has been reported to suppress pseudorabies virus replication by obstructing nuclear imports of UL422 (Sharun et al. 2020a,b,c). The efficacy of oseltamivir in treating SARS-CoV-2 infection is still being tested in many clinical trials. Oseltamivir is also used in some combinations such as with chloroquine and favipiravir, in clinical research (Rosa et al. 2020The prodrug favipiravir first reaches the infected cells by endocytosis and is then converted by phosphoribosylation and phosphorylation into active favipiravir ribofuranosyl phosphates (Furuta et al. 2017). Antiviral activity, however, is shown by selectively targeting conservative RNA-dependent RNApolymerase catalytic domain, interfering with the process of nucleotide addition during replication of viral RNA(Wu et al., 2020). Later, umifenovir was shown to have an in vitro antiviral effectiveness in the widespread virus strains including Ebola, human herpesvirus 8, hepatitis C virus, and arenavirus of Tacaribe (Pécheur et al. 2016). China is examining efficiency and safety of umifenovir for COVID-19 by executing two randomized and open-label trials (Wu et al. 2020). In this review, the utility of remdesivir was generally discussed. The sample size of this study was reasonably low for COVID-19 therapy; however, inefficiency and determining efficacy should be examined (Table 2) (Rahman et al. 2020a, 2020b). Immunotherapy, an effective way to treat infectious illnesses clinically, has been suggested (Cutino-Moguel et al. 2017). A new perspective on infectious disease control is the use of monoclonal antibodies. Monoclonal antibody is used to bind to a particular body material. This binding is very flexible and can imitate, block, or trigger change in precise mechanisms and provide an efficient treatment for diseases (Lu et al. 2020). Based on similarity among SARS-CoV-2 and SARS-CoV, multiple studies have shown that SARS monoclonal antiviral antibodies have been used in patients suffering from SARS-CoV-2 (W. Li et al. 2003). Dexamethasone, a broad-spectrum immunosuppressor agent, belongs to the synthetic corticosteroid class with greater activity and longer duration of action than cortisone (Huang et al. 2020; Theoharides & Conti 2020). It acts via different mechanisms of action; therefore, it can affect various systems of the body. Dexamethasone exhibits anti-inflammatory activity due to its ability to decrease the gene transcription of many pro-inflammatory cytokines, chemokines, and adhesion molecules (Rhen et al. 2005). Based on this fact, dexamethasone can be utilized to treat COVID-19 patients because of its property to inhibit the release of cytokines that further reduces their devastating effects. Therefore, this drug may be useful to curb the cytokine storm associated with COVID-19 (Huang et al. 2020). Moreover, it has been described by some studies that short-term use of dexamethasone reduced the severity of inflammation through inhibition of the cytokine storm and hyper-inflammatory phase in COVID-19 patients who developed pneumonia (Miga et al. 2020). These outcomes were further substantiated by a large randomized controlled trial (RECOVERY) that showed the promising effects of dexamethasone therapy on COVID-19 patients at the dose of 6 mg four times a day for 10 days. This RECOVERY trial reported that this treatment approach gave significant improvements in the patients with hypoxemia and who are under invasive and non-invasive respiratory support, but this study did not show any improvement sign in patients without hypoxemia (Verity et al. 2020; C. Wang et al. 2020a,b,c,d). On the other side, dexamethasone can also prevent the B-cell-mediated antibody production that further reduces the beneficial effects of T cells and eventually prevents the clearance of apoptotic cells mediated via macrophages that further increases the risk of secondary infections (Huang et al. 2020; Theoharides & Conti 2020). A national plan must be formulated and enforced internationally to contain the recent outbreak. The key lessons, however, are probably the fact that natural immunity is the first line of protection and that global health needs to be seen as a unit that cannot be easily dispelled. One of the most helpful immune therapies is the vaccine since it is able to facilitate defense against infectious diseases through the targeted path of the immune system (H. Hafez et al. 1999). A valuable, permanent vaccine and/or antiviral therapy for this will take time to grow (T. Rahman et al. 2020a,b). Human vaccines are vital for life-saving and well-being, and thus much more important than the invention of farm animal vaccines. The global spread of SARS-CoV does not appear to threaten poultry production (Mykytyn et al. 2020). During the COVID-19 pandemic, the production of vaccines was highly regarded as the most powerful method for protecting human and animal health and eradicating health threats (H. M. Hafez et al. 2020). Investigations for the development of new vaccines and to monitor improvements in RNAand epidemiological diseases can continue to prevent risks of zoonotic disease (H. Hafez et al. 1999). CoV infection, however, is routinely monitored by vaccination on various domestic animals like cats, dogs, pigs, livestock, and poultry. The bovine coronavirus (BCoV) vaccines protect young calves from enteric and respiratory diseases (Alluwaimi et al. 2020).
Table 2.
Antiviral medicines for COVID-19
| Intervention | Class | Type and mechanisms of action | Recommendations | Reference |
|---|---|---|---|---|
| Remdesivir | Antiviral | Remdesivir is a drug contact to combat the invasion of RNA polymerases | Tests in clinical studies | (M. F. Hossain et al. 2020a,b) |
| Favipiravir | Antiviral | Favipiravir blocks viral polymerase RNA and thus stops viral replication | Completed clinical studies proved efficacy | (M. H. Rahman et al. 2020a,b) |
| Convalescent plasma | Antiviral | Curated plasma contains defensive antibodies against SARS-CoV-2 | Confirmed effectiveness | (M. F. Hossain et al. 2020a,b; M. H. Rahman et al. 2020a,b) |
| Lopinavir | Antiviral | HIV-approved protease inhibitors are lopinavir/ritonavir | Inconsistency in clinical trial findings | (Horby et al. 2020) |
| EIDD-2801 | Antiviral | Drives mutagenesis and impedes viral replication integration throughout RNA synthesis EDI-2801 | Clinical testing prepared | (M. H. Rahman et al. 2020a,b) |
| Ritonavir | Antiviral | Ritonavir is HIV-approved protease inhibitor | Inconsistency in clinical trial findings | (Owa et al. 2020) |
| Ivermectin | Antiviral | To suppress the replication of the pseudorabies virus by inhibiting nuclear import of UL42 | Confirmed effectiveness | (Sharun, Dhama, et al. 2020) |
| Oseltamivir | Antiviral | Antiviral action is showed through selectively targeting conservative catalytic domain of RNA-dependent RNA polymerase (RdRp) | Completed clinical studies proved efficacy | (Rosa et al. 2020) |
| Umifenovir | Antiviral | To block the virus-cell membrane fusion | Completed clinical studies proved efficacy | (Pécheur et al. 2016) |
| Favipiravir | Antiviral | Antiviral action is showed through selectively targeting conservative catalytic domain of RdRp, interrupting nucleotide incorporation process amid viral RNA replication | Confirmed effectiveness | (Furuta et al. 2017) |
| GC376 | Protease inhibitor | Blocks virus replication | Confirmed effectiveness | (Sharun et al. 2021) |
| GC373 | Protease inhibitor | Blocks virus replication | Confirmed effectiveness | (Sharun et al. 2021) |
For example, infectious bronchitis virus (IBV) was the first approved vaccine in chicks to prevent upper CoV infection in the respiratory tract (Alluwaimi et al. 2020). A majority of veterinary vaccines were developed to prevent shipping fever on young calves for CoVs, for example, canine enteric coronavirus (CECoV) infection in dogs, porcine epidemic diarrhea virus (PEDV), and transmissible gastroenteritis virus (TGEV) infections in pigs, and BCoV in cattle (Gerdts et al. 2017). Recombinant vaccines have been promoted by the use of reverse genetics as a prevention strategy (Tizard 2020). Searching for a suitable vaccine by InovioPharmaceuticals and other firms, including CanSino Biologics, Sinovac Biotech Ltd., and Johnson&Johnson, for the SARS-CoV-2 mRNA-based vaccine (mRNA-1273) (NASDAQ: MRNA), is underway and the clinical trials are finalized. The clinical review involves 29 vaccine candidates and 138 are preclinical evaluations as of 13 August 2020 (Rabaan et al. 2020). In addition, SARS-CoV-2 S-protein vaccine candidate is in the final step using mRNA vaccine technology (Rabaan et al. 2020). INOVIO Pharmaceuticals has developed and reportedly induces intradermal inoculation to induce immune cell activation through DNA plasmid–dependent vaccine aiming at SARS-CoV-2 protein (INO-4800) (Kim et al. 2020; Rabaan et al. 2020). In comparison, recombinant vaccines based on DNA and mRNA are more available and quicker for clinical trials. DNA, chimeric viral, and membrane vesicle vaccines are valuable options (Shang et al. 2020).
Various efforts are ongoing for its treatment. Various vaccines have been developed but failed in clinical trials. But some of the vaccines got the approval of their treatment such as Covishield, Covaxin, Sputnik V, Moderna vaccine, Pfizer-BioNTech COVID-19 Vaccine, Johnson&Johnson’s, and Jab.
Covishield and Covaxin are mostly vaccinated vaccines in India. India has also approved Johnson&Johnson’s single-dose vaccine for emergency usage. Jab is India’s second foreign vaccine to be granted emergency use authorization (Emergency approved vaccine 2021). Table 3 includes a list of vaccines approved for their emergency use.
Table 3.
List of vaccines approved for their emergency use
| Vaccine | Type | Shot | Age group | Adverse effects |
|---|---|---|---|---|
| Pfizer-BioNTech | mRNA vaccine | 2 shots, 21 days apart | 16 and older | Chills, pain, headache, tiredness, and swelling at the injection site |
| Moderna | mRNA | 2 shots, 28 days apart | Adults 18 and older | Chills, pain, headache, tiredness, and swelling at injection site |
| Janssen | Viral vector | Single shot | Adults 18 and older | Fatigue, fever headache, injection site pain, or myalgia |
| Oxford-AstraZeneca | Viral vector | 2 shots, 4–12 weeks apart | Adults 18 and older | Tenderness, redness, pain, warmth, itching, swelling at the injection site |
| Novavax | Protein adjuvant | 2 shots, 3 weeks apart | 12–84 years old | Pain at the site of injection, fatigue, and fever |
The Sputnik V (uses adenoviruses) is also an approved vaccine that shuttles genetic material of SARS-CoV-2 into cells of vaccinated individuals. The mRNA-based Moderna vaccine spurs an immune response for at least half a year (Approved vaccine 2021). USFDA approved the first vaccine for COVID-19 called Pfizer-BioNTech and will now be commercially available as Comirnaty (koe-mir’-na-tee) for prevention of COVID-19 in persons 16 years and older (USFDA approved vaccine 2021).
Conclusion
In 2002, in Guangdong, China, the SARS-CoV-1 epidemic was a tragedy but in December 2019, in Wuhan, China, SARS-CoV-2 was a new coronavirus that triggered epidemics worldwide. This virus has spread so exponentially that more than hundreds of thousands of people worldwide have been infected. Although the source, nature, and transmission mechanism of coronaviruses are not yet clear, preventative action is recognized worldwide such as social distancing, hand washing, and hand sanitation. Researchers study this problem quickly and help people solve this pandemic. Doctors, intelligence agents at the border, military personnel, police officers, nurses, and local staff are all working hard to save the world from this epidemic. Quarantine and social distancing could substantially affect the psychological impact on the health of the population. Partitioning from dear ones, loss of liberty, ambiguity over ailment status, and tediousness can sometimes produce dramatic effects. Economic loss because of quarantine and severe socioeconomic distress were risk aspects for symptoms of psychological disorders, and both anger and anxiety continue months after quarantine. COVID-19 resulted in considerable chaos of the global economy, mainly in transport, the vacation industry, and global supply chain. The World Bank declared that gross domestic product worldwide contracted by 5.2% in 2020, the most significant decline in decades. Agriculture, education, healthcare, and informal sectors also have several negative impacts, but a drastic improvement in the air quality index of urban centers has been recorded during lockdowns. The global supply chain, which contributes to a big socioeconomic increase, was also interrupted.
To date, in large-scale clinical trials, most vaccine candidates have entered the final stages of vaccine safety and protection effectiveness, with very recent announcements by BioNTech/Pfizer and Moderna/NIAID claiming safety and very high levels of protection effectiveness for their leading candidates for mRNA vaccine. Covishield, Covaxin, and Sputnik V are the most used vaccines for vaccinations. India has also approved Johnson&Johnson’s single-dose vaccine for emergency usage. The Jab is the second foreign vaccine to be granted emergency use authorization in India. Different antiviral products have meanwhile been used primarily for short-term benefits, combined with azithromycin. Finally, this is a lesson for any possible pandemic like this to be learned based on this viral tragedy in terms of global and public health.
Author contribution
Idea: MHR and DK; Writing draft: RA; Review and editing; TB, VM, JP, KD, TK and PT. All authors read the final version of this manuscript and approved it for submission.
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/19/2022
A Correction to this paper has been published: 10.1007/s11356-022-18766-2
Contributor Information
Md. Habibur Rahman, Email: pharmacisthabib@gmail.com.
Deepak Kaushik, Email: deepkaushik1977@gmail.com.
References
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR, Jordan RJM (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. 9(2) [DOI] [PMC free article] [PubMed]
- Al Naggar Y, Giesy JP, Abdel-Daim MM, Javed Ansari M, Al-Kahtani SN, Yahya G (2020) Fighting against the second wave of covid-19: Can honeybee products help protect against the pandemic? 28(3), 1519–1527 [DOI] [PMC free article] [PubMed]
- Alluwaimi AM, Alshubaith IH, Al-Ali AM, Abohelaika S (2020) The coronaviruses of animals and birds: their zoonosis, vaccines, and models for SARS-CoV and SARS-CoV2. 7, 655 [DOI] [PMC free article] [PubMed]
- Approved vaccine (2021) https://www.nature.com/articles/d41586-020-00502-w. Accesed 1 Aug 2021
- Arabi YM, Deeb AM, Al-Hameed F, Mandourah Y, Almekhlafi GA, Sindi AA, Al-Omari A, Shalhoub S, Mady A, Alraddadi B, Almotairi A, Al Khatib K, Abdulmomen A, Qushmaq I, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Al Harthy A, Kharaba A, Jose J, Dabbagh T, Fowler RA, Balkhy HH, Merson L, Hayden FG; Saudi Critical Care Trials group (2019) Macrolides in critically ill patients with Middle East Respiratory Syndrome. 81, 184–190 [DOI] [PMC free article] [PubMed]
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ, Zumla AI, Memish ZA (2013) Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. 13(9), 752–761 [DOI] [PMC free article] [PubMed]
- Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA (2014) Evidence for camel-to-human transmission of MERS coronavirus. 370(26), 2499–2505 [DOI] [PubMed]
- Baron SA, Devaux C, Colson P, Raoult D, Rolain JM (2020) Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19. 55(4), 105944 [DOI] [PMC free article] [PubMed]
- Bayram H, Köktürk N, Elbek O, Kılınç O, Sayıner A, Dağlı E; Turkish Thoracic Society (2020) Interference in scientific research on COVID-19 in Turkey. 396(10249), 463–464 [DOI] [PMC free article] [PubMed]
- Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y (2012) Hydroxychloroquine: from malaria to autoimmunity. 42(2), 145–153 [DOI] [PMC free article] [PubMed]
- Bonilla-Aldana DK, Dhama K, Rodriguez-Morales AJ (2020) Revisiting the one health approach in the context of COVID-19: a look into the ecology of this emerging disease 8(3), 234–237
- Brand R, Timme S, Nosrat S (2020) When pandemic hits: exercise frequency and subjective well-being during COVID-19 pandemic.11, 570567. 10.3389/fpsyg.2020.570567 [DOI] [PMC free article] [PubMed]
- Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin G (2020) El Impacto Psicológico De La Cuarentena y Cómo Reducirla: Revisión Rápida De Las Pruebas 395:912–920
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. 104787 [DOI] [PMC free article] [PubMed]
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C (2020) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. 7, 382(19), 1787–1799 [DOI] [PMC free article] [PubMed]
- Carlos WG, Dela Cruz CS, Cao B, Pasnick S, Jamil S (2020) Novel Wuhan (2019-nCoV) coronavirus. P7-P8 [DOI] [PubMed]
- Cavanagh D, Mawditt K, Welchman Dde B, Britton P, Gough RE (2002) Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. 31(1), 81–93 [DOI] [PubMed]
- Colson P, Raoult D (2016) Fighting viruses with antibiotics: an overlooked path. 48(4), 349 [DOI] [PMC free article] [PubMed]
- Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. 105932(10.1016) [DOI] [PMC free article] [PubMed]
- Córdoba-Aguilar A, Ibarra-Cerdeña CN, Castro-Arellano I, Suzan G (2020) Tackling zoonoses in a crowded world: lessons to be learned from the COVID-19 pandemic. 214, 105780 [DOI] [PMC free article] [PubMed]
- Cowley JA, Dimmock CM, Spann KM, Walker PJ (2000) Gill-associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri-and coronaviruses. 81(6), 1473–1484 [DOI] [PubMed]
- Cutino-Moguel MT, Eades C, Rezvani K, Armstrong-James D (2017) Immunotherapy for infectious diseases in haematological immunocompromise. 177(3), 348–356 [DOI] [PubMed]
- Dai L, Gao GF (2020) Viral targets for vaccines against COVID-19. 1–10 [DOI] [PMC free article] [PubMed]
- Delmas B, Laude H (1990) Assembly of coronavirus spike protein into trimers and its role in epitope expression. 64(11), 5367–5375 [DOI] [PMC free article] [PubMed]
- Dhama K, Patel SK, Pathak M, Yatoo MI, Tiwari R, Malik YS, Singh R, Sah R, Rabaan AA, Bonilla-Aldana DK, Rodriguez-Morales AJ (2020a) An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. 101755 [DOI] [PMC free article] [PubMed]
- Dhama K, Patel SK, Sharun K, Pathak M, Tiwari R, Yatoo MI, Malik YS, Sah R, Rabaan AA, Panwar PK, Singh KP, Michalak I, Chaicumpa W, Martinez-Pulgarin DF, Bonilla-Aldana DK, Rodriguez-Morales AJ (2020b) SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. 101830 [DOI] [PMC free article] [PubMed]
- Di Gennaro F, Pizzol D, Marotta C, Antunes M, Racalbuto V, Veronese N, Smith L (2020) Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. 17(8), 2690 [DOI] [PMC free article] [PubMed]
- Docea AO, Tsatsakis A, Albulescu D, Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou M, Drakoulis N, Dumanov JM, Tutelyan VA, Onischenko GG, Aschner M, Spandidos DA, Calina D (2020) A new threat from an old enemy: re‑emergence of coronavirus. 45(6), 1631–1643 [DOI] [PMC free article] [PubMed]
- Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019 (COVID-19). 14(1), 58–60 [DOI] [PubMed]
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. 348(20), 1967–1976 [DOI] [PubMed]
- El Zowalaty ME, Järhult JD (2020) From SARS to COVID-19: a previously unknown SARS-related coronavirus (SARS-CoV-2) of pandemic potential infecting humans. 9, 100124 [DOI] [PMC free article] [PubMed]
- Emergency approved vaccine (2021) https://www.bbc.com/news/world-asia-india-55748124
- FDA (2020) https://www.fda.gov/news-events/fda-voices/path-forward-coronavirus-treatment-acceleration-program
- Fung M, Otani I, Pham M, Babik J (2020) Zoonotic coronavirus epidemics: Severe acute respiratory syndrome, Middle East respiratory syndrome, and coronavirus disease 2019. 126(4), 321–337 [DOI] [PMC free article] [PubMed]
- Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. 93(7), 449–463 [DOI] [PMC free article] [PubMed]
- Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. 16, 14(1), 72–73 [DOI] [PubMed]
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. 105949 [DOI] [PMC free article] [PubMed]
- Gerdts V, Zakhartchouk A (2017) Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. 206, 45–51 [DOI] [PMC free article] [PubMed]
- Goebel SJ, Taylor J, Masters PS (2004) The 3′ cis-acting genomic replication element of the severe acute respiratory syndrome coronavirus can function in the murine coronavirus genome. 78(14), 7846–7851 [DOI] [PMC free article] [PubMed]
- Gorbalenya AE, Snijder EJ, Spaan WJ (2004) Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. 78(15), 7863–7866 [DOI] [PMC free article] [PubMed]
- Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M (2020) The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. 295(15), 4773–4779 [DOI] [PMC free article] [PubMed]
- Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T (2020) Compassionate use of remdesivir for patients with severe Covid-19. 382(24), 2327–2336
- Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, Frigeni M, Damiani M, Micò C, Fagiuoli S, Cosentini R, Lorini FL, Fabretti F, Morgan J, Owens BMJ, Kanhai KJ, Cowburn J, Rizzi M, Marco FD, Rambaldi A (2020). Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. 10.1101/2020.04.01.20048561
- Gu T, Wang XH, Pung HK, Zhang DQ (2020) An ontology-based context model in intelligent environments. arXiv:2003.05055 [cs.DC]
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. 7(1), 1–10 [DOI] [PMC free article] [PubMed]
- Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP (2014) Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. 14(2), 140–145 [DOI] [PMC free article] [PubMed]
- Hafez H, Hess MJ (1999) Modern techniques in diagnosis of poultry diseases
- Hafez HM, Attia YA (2020) Challenges to the poultry industry: current perspectives and strategic future after the COVID-19 outbreak. 26(7), 516 [DOI] [PMC free article] [PubMed]
- Hasana S, Hossain MF, Jalouli M, Kabir MT, Uddin MG, Wahed MII, Behl T, Bin-Jumah MN, Abdel-Daim MM, Aleya L, Uddin MS (2021) Genetic diversity of SARS-CoV2 and environmental settings: possible association with neurological disorders. 1–15. [DOI] [PMC free article] [PubMed]
- Hodžić N, Hasanović M, Pajević I (2020) COVID-19 affected mental health of at-risk groups of psychiatric patients: two case reports. 32(2), 294–299 [DOI] [PubMed]
- Holmes EA, O'Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, Ballard C, Christensen H, Cohen Silver R, Everall I, Ford T, John A, Kabir T, King K, Madan I, Michie S, Przybylski AK, Shafran R, Sweeney A, Worthman CM, Yardley L, Cowan K, Cope C, Hotopf M, Bullmore E (2020). Multidisciplinary research priorities for the COVID- 19 pandemic: a call for action for mental health science. 7(6), 547–560 [DOI] [PMC free article] [PubMed]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H (2020) First case of 2019 novel coronavirus in the United States. 382, 929–936 [DOI] [PMC free article] [PubMed]
- Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ (2020) Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 396(10259), 1345–1352 [DOI] [PMC free article] [PubMed]
- Hossain MF, Hasana S, Mamun AA, Uddin MS, Wahed MII, Sarker S, Behl T, Ullah I, Begum Y, Bulbul IJ, Amran MS, Rahman MH, Bin-Jumah MN, Alkahtani S, Mousa SA, Aleya L, Abdel-Daim MM (2020) COVID-19 outbreak: Pathogenesis, current therapies, and potentials for future management. 16(11), 563478 [DOI] [PMC free article] [PubMed]
- Hossain MM, Sultana A, Purohit N (2020) Mental health outcomes of quarantine and isolation for infection prevention: A systematic umbrella review of the global evidence. 42, e2020038 [DOI] [PMC free article] [PubMed]
- Hu B, Guo H, Zhou P, & Shi ZL (2021) Characteristics of SARS-CoV-2 and COVID-19. 19(3), 141–154. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, . . . Gu XJTL (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. 395(10223), 497-506 [DOI] [PMC free article] [PubMed]
- Huo J, Le Bas A, Ruza RR, Duyvesteyn HME, Mikolajek H, Malinauskas T, Tan TK, Rijal P, Dumoux M, Ward PN, Ren J, Zhou D, Harrison PJ, Weckener M, Clare DK, Vogirala VK, Radecke J, Moynié L, Zhao Y, Gilbert-Jaramillo J, Knight ML, Tree JA, Buttigieg KR, Coombes N, Elmore MJ, Carroll MW, Carrique L, Shah PNM, James W, Townsend AR, Stuart DI, Owens RJ, Naismith JH (2020) Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. 27(9), 846–854 [DOI] [PubMed]
- Ijaz MK, Brunner AH, Sattar SA, Nair RC, Johnson-Lussenburg CM (1985) Survival characteristics of airborne human coronavirus 229E. 66(12), 2743–2748 [DOI] [PubMed]
- ILO (2010) Social Security for Informal Economy Workers in Indonesia; Looking for flexible and highly targeted programmes. Jakarta: ILO
- ILO . ILO Monitor: COVID19 and the world of work. Third edition updated estimates and analysis. Geneva: ILO; 2020. [Google Scholar]
- ILO (2020b) COVID19 crisis and the informal economy: immediate responses and policy challenges (ILO brief). https://www.ilo.org/global/topics/employment-promotion/informal-economy/publications/WCMS_743623/lang--en/index.htm. Accessed 12 Oct 2020
- Jacob MCM, Feitosa IS, & Albuquerque UPJPHN (2020) Animal-based food systems are unsafe: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) fosters the debate on meat consumption. 23(17), 3250–3255 [DOI] [PMC free article] [PubMed]
- Jie Z, He H, Xi H, Zhi ZJZJHHHXZZ. Expert Consensus on Chloroquine Phosphate for the Treatment of Novel Coronavirus Pneumonia. 2020;43(3):185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G (2020) Virology, epidemiology, pathogenesis, and control of COVID-19. 12(4), 372 [DOI] [PMC free article] [PubMed]
- Jo WK, de Oliveira-Filho EF, Rasche A, Greenwood AD, Osterrieder K, Drexler JF (2020) Potential zoonotic sources of SARS‐CoV‐2 infections. 68(4), 1824–1834 [DOI] [PMC free article] [PubMed]
- Jonassen CM, Kofstad T, Larsen IL, Løvland A, Handeland K, Follestad A, Lillehaug A (2005) Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). 86(6), 1597–1607 [DOI] [PubMed]
- Kabir MT, Uddin MS, Hossain MF, Abdulhakim JA, Alam MA, Ashraf GM, Bungau SG, Bin-Jumah MN, Abdel-Daim MM, Aleya L (2020) nCOVID-19 pandemic: from molecular pathogenesis to potential investigational therapeutics. 10(8), 616 [DOI] [PMC free article] [PubMed]
- Kaur A, Bhalla V, Salahuddin M, Rahman SO, Pottoo FH (2021) COVID-19 infection: epidemiology, virology, clinical features, diagnosis and pharmacological treatment. 11 [DOI] [PubMed]
- Kim YC, Dema B, Reyes-Sandoval A (2020) COVID-19 vaccines: breaking record times to first-in-human trials. 5(1), 1–3 [DOI] [PMC free article] [PubMed]
- Kousi T, Mitsi LC, Simos J (2021) The Early stage of COVID-19 outbreak in Greece: a review of the national response and the socioeconomic impact. 18(1), 322 [DOI] [PMC free article] [PubMed]
- Lam TT, Jia N, Zhang YW, Shum MH, Jiang JF, Zhu HC, Tong YG, Shi YX, Ni XB, Liao YS, Li WJ, Jiang BG, Wei W, Yuan TT, Zheng K, Cui XM, Li J, Pei GQ, Qiang X, Cheung WY, Li LF, Sun FF, Qin S, Huang JC, Leung GM, Holmes EC, Hu YL, Guan Y, Cao WC (2020) Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. 583(7815), 282–285 [DOI] [PubMed]
- Lau SK, Chan JF (2015) Coronaviruses: Emerging and re-emerging pathogens in humans and animals. 12, 209 [DOI] [PMC free article] [PubMed]
- Lee HJ, Shieh CK, Gorbalenya AE, Koonin EV, La Monica N, Tuler J, Bagdzhadzhyan A, Lai MM (1991) The complete sequence (22 kilobases) of murine coronavirus gene 1 encoding the putative proteases and RNA polymerase. 180(2), 567–582 [DOI] [PMC free article] [PubMed]
- Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. 348(20), 1986–1994 [DOI] [PubMed]
- Lei J, Li J, Li X, Qi X (2020) CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. 295(1), 18–18 [DOI] [PMC free article] [PubMed]
- Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ (2020) Bat-borne virus diversity, spillover and emergence. 18(8), 461–471 [DOI] [PMC free article] [PubMed]
- Li H, Liu SM, Yu XH, Tang SL, Tang CK (2020a) Coronavirus disease 2019 (COVID-19): current status and future perspective. 105951 [DOI] [PMC free article] [PubMed]
- Li X, Song Y, Wong G, Cui JJSCLS. Bat Origin of a New Human Coronavirus: There and Back Again. 2020;63(3):461–462. doi: 10.1007/s11427-020-1645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhou L, Van Der Heijden B, Li S, Tao H, & Guo Z (2021) Social Isolation, loneliness and well-being: the impact of WeChat use intensity during the COVID-19 pandemic in China. 12, 707667. 10.3389/fpsyg.2021.707667 [DOI] [PMC free article] [PubMed]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. 426(6965), 450–454 [DOI] [PMC free article] [PubMed]
- Li W, Wong SK, Li F, Kuhn JH, Huang IC, Choe H, Farzan M (2006) Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. 80(9), 4211–4219 [DOI] [PMC free article] [PubMed]
- Liu W, Morse JS, Lalonde T, Xu S (2020) Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV [DOI] [PMC free article] [PubMed]
- Liu X, Wang XJ (2020) Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. 47(2), 119 [DOI] [PMC free article] [PubMed]
- Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Pham DH, Deif H, LaMere EA, Chang M, Kain KC, Farcas GA, Ferguson P, Latchford M, Levy G, Dennis JW, Lai EK, Fish EN (2003) Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. 290(24), 3222–3228 [DOI] [PubMed]
- Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC (2020) Development of therapeutic antibodies for the treatment of diseases. 27(1), 1–30 [DOI] [PMC free article] [PubMed]
- Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW (2020) Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. 41(8), 531–543 [DOI] [PMC free article] [PubMed]
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF; American Academy of Ophthalmology (2016) Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). 123(6), 1386–1394 [DOI] [PubMed]
- McChesney EW (1983) Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. 75(1), 11–18 [DOI] [PubMed]
- Miga KH, Koren S, Rhie A, Vollger MR, Gershman A, Bzikadze A, Brooks S, Howe E, Porubsky D, Logsdon GA, Schneider VA, Potapova T, Wood J, Chow W, Armstrong J, Fredrickson J, Pak E, Tigyi K, Kremitzki M, Markovic C, Maduro V, Dutra A, Bouffard GG, Chang AM, Hansen NF, Wilfert AB, Thibaud-Nissen F, Schmitt AD, Belton JM, Selvaraj S, Dennis MY, Soto DC, Sahasrabudhe R, Kaya G, Quick J, Loman NJ, Holmes N, Loose M, Surti U, Risques RA, Graves Lindsay TA, Fulton R, Hall I, Paten B, Howe K, Timp W, Young A, Mullikin JC, Pevzner PA, Gerton JL, Sullivan BA, Eichler EE, Phillippy AM (2020) Telomere-to-telomere assembly of a complete human X chromosome. 585(7823), 79–84 [DOI] [PMC free article] [PubMed]
- Miura HS, Nakagaki K, Taguchi F (2004) N-terminal domain of the murine coronavirus receptor CEACAM1 is responsible for fusogenic activation and conformational changes of the spike protein. 78(1), 216–223 [DOI] [PMC free article] [PubMed]
- Morawska L, Cao J (2020) Airborne transmission of SARS-CoV-2: the world should face the reality. 105730 [DOI] [PMC free article] [PubMed]
- Muslim S, Nasrin N, Alotaibi FO, Prasad G, Singh SK, Alam I, Mustafa G (2020) Treatment options available for COVID-19 and an analysis on possible role of combination of rhACE2, angiotensin (1-7) and angiotensin (1-9) as effective therapeutic measure. 22, 1–6 [DOI] [PMC free article] [PubMed]
- Mykytyn AZ, Lamers MM, Okba NMA, Breugem TI, Schipper D, van den Doel PB, van Run P, van Amerongen G, de Waal L, Koopmans MPG, Stittelaar KJ, van den Brand JMA, Haagmans BL (2020) Susceptibility of rabbits to SARS-CoV-2. 1–17 [DOI] [PMC free article] [PubMed]
- Nadeem S, Abbas N, Elmasry Y, Malik MY (2020) Numerical analysis of water based CNTs flow of micropolar fluid through rotating frame. 186, 105194 [DOI] [PubMed]
- Neves R, Cho H, Zhang J (2020) State of the nation: customizing energy and finances for geothermal technology in the United States residential sector. 110463
- of the International- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. 5(4), 536 [DOI] [PMC free article] [PubMed]
- Okorie IE, Afuecheta E, Alaebo CG, Nadarajah S (2021) Socio-economic and demographic impacts on the full awareness of the methods for controlling/preventing the spread of COVID-19 among social media users in some African countries at the onset of the pandemic. 14(1), 331. 10.1186/s13104-021-05736-z [DOI] [PMC free article] [PubMed]
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema RS, Tacken MG, de Rooij MM, Weesendorp E, Engelsma MY, Bruschke CJ, Smit LA, Koopmans M, van der Poel WH, Stegeman A (2020) SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. 25(23), 2001005 [DOI] [PMC free article] [PubMed]
- Owa AB, Owa OT (2020) Lopinavir/ritonavir use in COVID-19 infection: is it completely non-beneficial? , 53(5), 674–675 [DOI] [PMC free article] [PubMed]
- Ozili PK, Arun TJ (2020) Spillover of COVID-19: impact on the Global Economy. Available at SSRN: https://ssrn.com/abstract=3562570 or 10.2139/ssrn.3562570. Accessed 27 Mar 2020
- Pal M, Berhanu G, Desalegn C, Kandi V (2020) Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. 12(3):e7423 [DOI] [PMC free article] [PubMed]
- Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. 30(6), 3306–3309 [DOI] [PMC free article] [PubMed]
- Park M, Thwaites RS, Openshaw PJM (2020) COVID-19: lessons from SARS and MERS. 50, 308–311
- Payne S (2017) Family Coronaviridae. 149–158. 10.1016/B978-0-12-803109-4.00017-9
- Pécheur EI, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW, Polyak SJ (2016) The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. 90(6), 3086–3092 [DOI] [PMC free article] [PubMed]
- Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD (2020) Importation and human-to-human transmission of a novel coronavirus in Vietnam. 382(9), 872–874 [DOI] [PMC free article] [PubMed]
- Pitoyo AJ, Aditya B, Amri I, Rokhim AA (2021) Impacts and strategies behind COVID-19-induced economic crisis: evidence from informal economy. 1–21. Advance online publication. 10.1007/s41027-021-00333-x [DOI] [PMC free article] [PubMed]
- Qu JM, Wang C, Cao B; Chinese Thoracic Society and Chinese Association of Chest Physicians (2020). Guidance for the management of adult patients with coronavirus disease 2019. 133(13), 1575 [DOI] [PMC free article] [PubMed]
- Rabaan AA, Al-Ahmed SH, Sah R, Tiwari R, Yatoo MI, Patel SK, Pathak M, Malik YS, Dhama K, Singh KP, Bonilla- Aldana DK, Haque S, Martinez-Pulgarin DF, Rodriguez-Morales AJ, Leblebicioglu H (2020) SARS-CoV-2/COVID-19 and advances in developing potential therapeutics and vaccines to counter this emerging pandemic. 19(1), 1–37 [DOI] [PMC free article] [PubMed]
- Rahman MH, Akter R, Behl T, Chowdhury MAR, Mohammed M, Bulbul IJ, Elshenawy SE, Kamal MA (2020a) COVID-19 outbreak and emerging management through pharmaceutical therapeutic strategy. 26(41), 5224–5240 [DOI] [PubMed]
- Rahman MT, Sobur MA, Islam MS, Toniolo A, Nazir KHMNH (2020b) Is the COVID-19 pandemic masking dengue epidemic in Bangladesh? , 7(2), 218–219 [DOI] [PMC free article] [PubMed]
- Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Jiang YZ, Xiong Y, Li YJ, Li XW, Li H, Fan GH, Gu XY, Xiao Y, Gao H, Xu JY, Yang F, Wang XM, Wu C, Chen L, Liu YW, Liu B, Yang J, Wang XR, Dong J, Li L, Huang CL, Zhao JP, Hu Y, Cheng ZS, Liu LL, Qian ZH, Qin C, Jin Q, Cao B, Wang JW (2020) Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. 133(9):1015–1024. 10.1097/CM9.0000000000000722 [DOI] [PMC free article] [PubMed]
- Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. 353(16), 1711–1723 [DOI] [PubMed]
- Rodriguez-Morales AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, Rabaan AA, Sah R, Paniz-Mondolfi A, Pagliano P, Esposito S (2020) History is repeating itself: probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. 28(1), 3–5 [PubMed]
- Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. 7(7), 611–627. 10.1016/S2215-0366(20)30203-0 [DOI] [PMC free article] [PubMed]
- Rosa SGV, Santos WC (2020) Clinical trials on drug repositioning for COVID-19 treatment. 44, e40 [DOI] [PMC free article] [PubMed]
- Rong X, Jiang L, Qu M, Liu Z. Enhancing therapeutic efficacy of donepezil by combined therapy: a comprehensive review. Curr Pharm Des. 2021;27(3):332–344. doi: 10.2174/1381612826666201023144836. [DOI] [PubMed] [Google Scholar]
- Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. 102433 [DOI] [PMC free article] [PubMed]
- Saletti G, Gerlach T, Jansen JM, Molle A, Elbahesh H, Ludlow M, Li W, Bosch BJ, Osterhaus ADME, Rimmelzwaan GF (2020) Older adults lack SARS CoV-2 cross-reactive T lymphocytes directed to human coronaviruses OC43 and NL63. 10(1), 1–10 [DOI] [PMC free article] [PubMed]
- Shang W, Yang Y, Rao Y, Rao X (2020) The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. 5(1), 1–3 [DOI] [PMC free article] [PubMed]
- Sharifi A, Khavarian-Garmsir AR (2020) The COVID-19 pandemic: Impacts on cities and major lessons for urban planning, design, and management. 749, 142391 [DOI] [PMC free article] [PubMed]
- Sharun K, Dhama K, Patel SK, Pathak M, Tiwari R, Singh BR, Sah R, Bonilla-Aldana DK, Rodriguez-Morales AJ, Leblebicioglu H (2020a) Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. 19(1), 23 [DOI] [PMC free article] [PubMed]
- Sharun K, Tiwari R, Natesan S, Dhama K (2020b) SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. 1–14 [DOI] [PMC free article] [PubMed]
- Sharun K, Tiwari R, Dhama K (2021) Protease inhibitor GC376 for COVID-19: Lessons learned from feline infectious peritonitis. 61, 122–125 [DOI] [PMC free article] [PubMed]
- Sharun K, Tiwari R, Patel SK, Karthik K, Iqbal Yatoo M, Malik YS, Singh KP, Panwar PK, Harapan H, Singh RK, Dhama K (2020c) Coronavirus disease 2019 (COVID-19) in domestic animals and wildlife: advances and prospects in the development of animal models for vaccine and therapeutic research. 1–12 [DOI] [PMC free article] [PubMed]
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS (2017). Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. 9(396), eaal3653 [DOI] [PMC free article] [PubMed]
- Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, Sawaqed R, Perkins D, Shahrara S, Sweiss NJ (2014). Tocilizumab in the treatment of rheumatoid arthritis and beyond. 8, 349 [DOI] [PMC free article] [PubMed]
- Shiraki K, Daikoku T (2020) Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. 107512 [DOI] [PMC free article] [PubMed]
- Sood S, Aggarwal V, Aggarwal D, Upadhyay SK, Sak K, Tuli HS, Kumar M, Kumar J, Talwar S (2020) COVID-19 pandemic: from molecular biology, pathogenesis, detection, and treatment to global societal impact. 1–16 [DOI] [PMC free article] [PubMed]
- Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, Bella A, Sognamiglio P, Sierra Moros MJ, Riutort AN, Demina YV, Mahieu R, Broas M, Bengnér M, Buda S, Schilling J, Filleul L, Lepoutre A, Saura C, Mailles A, Levy-Bruhl D, Coignard B, Bernard-Stoecklin S, Behillil S, van der Werf S, Valette M, Lina B, Riccardo F, Nicastri E, Casas I, Larrauri A, Salom Castell M, Pozo F, Maksyutov RA, Martin C, Van Ranst M, Bossuyt N, Siira L, Sane J, Tegmark-Wisell K, Palmérus M, Broberg EK, Beauté J, Jorgensen P, Bundle N, Pereyaslov D, Adlhoch C, Pukkila J, Pebody R, Olsen S, Ciancio BC (2020) First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. 25(9), 2000178 [DOI] [PMC free article] [PubMed]
- Spoorthy MS, Pratapa SK, Mahant S (2020) Mental health problems faced by healthcare workers due to the COVID-19 pandemic–A review. 51, 102119 [DOI] [PMC free article] [PubMed]
- Sundaram S, Nair SS, Jaganmohan D, Unnikrishnan G, Nair MJMSJ. Relapsing Lumbosacral Myeloradiculitis: an Unusual Presentation of MOG Antibody Disease. 2020;26(4):509–511. doi: 10.1177/1352458519840747. [DOI] [PubMed] [Google Scholar]
- Tazerji SS, Magalhães Duarte P, Rahimi P, Shahabinejad F, Dhakal S, Singh Malik Y, Shehata AA, Lama J, Klein J, Safdar M, Rahman MT, Filipiak KJ, Rodríguez-Morales AJ, Sobur MA, Kabir F, Vazir B, Mboera L, Caporale M, Islam MS, Amuasi JH, Gharieb R, Roncada P, Musaad S, Tilocca B, Koohi MK, Taghipour A, Sait A, Subbaram K, Jahandideh A, Mortazavi P, Abedini MA, Hokey DA, Hogan U, Shaheen MNF, Elaswad A, Elhaig MM, Fawzy M (2020) Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review. 18(1), 1–11 [DOI] [PMC free article] [PubMed]
- Tencent (2021) https://www.tencent.com/zh-cn/investors.html
- Theoharides TC, Conti P (2020) Dexamethasone for COVID-19? Not so fast. 34(3), 10.23812 [DOI] [PubMed]
- Thornton J (2020) Clinical trials suspended in UK to prioritise COVID-19 studies and free up staff. 368, m1172 [DOI] [PubMed]
- Tim Forbil Institute and IGPA . Pekerja informal di tengah pandemi COVID-19. In: Mas’udi W, Winanti PS, editors. Tata kelola penanganan COVID-19 di Indonesia: Kajian Awal pp 238–252. Yogyakarta: Gadjah Mada University Press; 2020. [Google Scholar]
- Tiwari R, Dhama K, Sharun K, Iqbal Yatoo M, Malik YS, Singh R, Michalak I, Sah R, Bonilla-Aldana DK, Rodriguez- Morales AJ (2020) COVID-19: animals, veterinary and zoonotic links. 40(1), 169–182 [DOI] [PMC free article] [PubMed]
- Tizard IRJV. Vaccination against Coronaviruses in Domestic Animals. 2020;38(33):5123. doi: 10.1016/j.vaccine.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USFDA approved vaccine (2021) https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine. Accessed 1 Aug 2021
- Varona L, Gonzales JR (2021) Dynamics of the impact of COVID-19 on the economic activity of Peru. 16(1), e0244920 [DOI] [PMC free article] [PubMed]
- Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM (2020). Estimates of the severity of coronavirus disease 2019: a model-based analysis. 20(6), 669–677 [DOI] [PMC free article] [PubMed]
- Wacharapluesadee S, Tan CW, Maneeorn P, Duengkae P, Zhu F, Joyjinda Y, Kaewpom T, Chia WN, Ampoot W, Lim BL, Worachotsueptrakun K, Chen VC, Sirichan N, Ruchisrisarod C, Rodpan A, Noradechanon K, Phaichana T, Jantarat N, Thongnumchaima B, Tu C, Crameri G, Stokes MM, Hemachudha T, Wang LF (2021) Author Correction: Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. 12(1), 1430. 10.1038/s41467-021-21768-2
- Wang C, Horby PW, Hayden FG, Gao GF (2020a) A novel coronavirus outbreak of global health concern. 395(10223), 470–473 [DOI] [PMC free article] [PubMed]
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020b) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. 30(3), 269–271 [DOI] [PMC free article] [PubMed]
- Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM (2020c) SARS-CoV-2: structure, biology, and structurebased therapeutics development. 10, 587269 [DOI] [PMC free article] [PubMed]
- Wang W, Tang J, Wei F (2020d) Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan. China 92(4):441–447 [DOI] [PMC free article] [PubMed]
- Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. 531(7594), 381–385 [DOI] [PMC free article] [PubMed]
- Weiss SR, Navas-Martin S (2005) Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. 69(4), 635–664 [DOI] [PMC free article] [PubMed]
- WHO Data August (2021) https://www.who.int/emergencies/diseases/novel-coronavirus2019?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQjwpreJBhDvARIsAF1_BU3dirzdhys7bNegHEPWXYPjICMX6U9hTeNmvrVGgzRsSX6WjVOqKUaAi-AEALw_wcB. Accessed 15 Aug 2021
- World Bank (2020a) Global economic prospects. International Bank for Reconstruction and Development. Washington, DC: World Bank
- World Bank (2020b) The global economic outlook during the COVID-19 pandemic: A changed world. www.worlbank.org/en/news/feature/2020/06/08/the-Global-Economic-Outlook-During-the-covid-19-pandemi-achanged-world. Accessed 15 Aug 2021
- Wu R, Wang L, Kuo HD, Shannar A, Peter R, Chou PJ, Li S, Hudlikar R, Liu X, Liu Z, Poiani GJ, Amorosa L, Brunetti L, Kong AN (2020) An update on current therapeutic drugs treating COVID-19. 1–15 [DOI] [PMC free article] [PubMed]
- Xiao H, Zhang Y, Kong D, Li S, Yang N (2020) The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. 26, e923549–923541 [DOI] [PMC free article] [PubMed]
- Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H (2020) Effective treatment of severe COVID-19 patients with tocilizumab. 117(20), 10970–10975 [DOI] [PMC free article] [PubMed]
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). 71(15), 732–739 [DOI] [PMC free article] [PubMed]
- Yekta R, Vahid-Dastjerdi L, Norouzbeigi S, Mortazavian AM (2020) Food Products as Potential Carriers of SARSCoV- 2. 123, 107754 [DOI] [PMC free article] [PubMed]
- Zeidner M, Matthews G, Shemesh D (2016) Cognitive-social sources of wellbeing: differentiating the roles of coping style, social support and emotional intelligence. 17(6), 2481–2501
- Zhang Z, Wu Q, Zhang T (2020) Pangolin homology associated with 2019-nCoV. bioRxiv 2020.02.19.950253
- Zhao H, Zhu Q, Zhang C, Li J, Wei M, Qin Y, Chen G, Wang K, Yu J, Wu Z, Chen X, Wang G (2020a) Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. 133, 110825 [DOI] [PMC free article] [PubMed]
- Zhao K, Li R, Wu X, Zhao Y, Wang T, Zheng Z, Zeng S, Ding X, Nie H (2020b) Clinical features in 52 patients with COVID-19 who have increased leukocyte count: a retrospective analysis. 39(12), 2279–2287 [DOI] [PMC free article] [PubMed]
- Zhao Y, Wang J, Kuang D, Xu J, Yang M, Ma C, Zhao S, Li J, Long H, Ding K, Gao J, Liu J, Wang H, Li H, Yang Y, Yu W, Yang J, Zheng Y, Wu D, Lu S, Liu H, Peng X (2020c) Susceptibility of tree shrew to SARS-CoV-2 infection. 10(1), 1–9 [DOI] [PMC free article] [PubMed]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020a) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. 395(10229), 1054–1062 [DOI] [PMC free article] [PubMed]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020b) A pneumonia outbreak associated with a new coronavirus of probable bat origin. 579(7798), 270–273 [DOI] [PMC free article] [PubMed]
- Zhou P, Fan H, Lan T, Yang XL, Shi WF, Zhang W, Zhu Y, Zhang YW, Xie QM, Mani S, Zheng XS, Li B, Li JM, Guo H, Pei GQ, An XP, Chen JW, Zhou L, Mai KJ, Wu ZX, Li D, Anderson DE, Zhang LB, Li SY, Mi ZQ, He TT, Cong F, Guo PJ, Huang R, Luo Y, Liu XL, Chen J, Huang Y, Sun Q, Zhang XL, Wang YY, Xing SZ, Chen YS, Sun Y, Li J, Daszak P, Wang LF, Shi ZL, Tong YG, Ma JY (2018) Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. 556(7700), 255–258 [DOI] [PMC free article] [PubMed]
- Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M (2020) Reducing mortality from 2019-nCoV: host-directed therapies should be an option. 395(10224), e35–e36 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.